Abstract
Metabolite carrier proteins of the mitochondrial inner membrane share homology in their transmembrane domains, which also carries their targeting information. In addition, some carriers have cleavable presequences which are not essential for targeting, but have some other function before import. The cytosolic chaperones Hsc70 (heat-shock cognate 70) and Hsp90 (heat-shock protein 90) complex with carrier precursors and interact specifically with the Tom (translocase of the mitochondrial outer membrane) 70 import receptor to promote import. We analysed how the presequences of the PiC (phosphate carrier) and CIC (citrate carrier) relate to the mechanisms of chaperone-mediated import. Deletion of the PiC presequence reduced the efficiency of import but, notably, not by causing aggregation. Instead, binding of the protein to Hsc70 was reduced, as well as the dependence on Hsc70 for import. Hsp90 binding and function in import was not greatly affected, but it could not entirely compensate for the lack of Hsc70 interaction. Deletion of the presequence from CIC was shown to cause its aggregation, but had little effect on the contribution to import of either Hsc70 or Hsp90. The presequence of PiC, but not that of CIC, conferred Hsc70 binding to dihydrofolate reductase fusion proteins. In comparison, OGC (oxoglutarate carrier) lacks a presequence and was more soluble, though it is still dependent on both Hsc70 and Hsp90. We propose that carrier presequences evolved to improve targeting competence by different mechanisms, depending on physical properties of the precursors in the cytosolic targeting environment.
Keywords: chaperone, heat-shock cognate 70 (Hsc70), heat-shock protein 90 (Hsp90), metabolite carrier protein, mitochondrial import, translocase of the mitochondrial outer membrane 70 (Tom70)
INTRODUCTION
The essential cellular function of mitochondria to generate energy through oxidative phosphorylation also involves the controlled transport of metabolites into and out of the organelle. Most small molecule metabolites are brought across the mitochondrial inner membrane by the metabolite carrier proteins, which are a structurally related family [1,2]. Like the majority of mitochondrial proteins, the carriers are encoded by nuclear genes, translated by cytosolic ribosomes and imported into the organelle. The import system also sorts the proteins into their final compartment: outer membrane, intermembrane space, matrix, and, for the carrier proteins, the inner membrane. The carrier proteins are thought to follow a defined mitochondrial import pathway. Carriers contain their targeting signals within sequences that eventually form the native structure of six transmembrane helices. Import of the carriers requires components of the outer membrane translocase machinery, including the Tom (translocase of the mitochondrial outer membrane) 70 import receptor and the Tom40 general import pore. The subsequent insertion of the carriers into the inner membrane requires the Tim (translocase of the mitochondrial inner membrane) 22 inner membrane translocase complex and the electrochemical potential across that membrane [3–5].
Although the broad outlines of this import pathway appear to be conserved throughout eukaryotes, the early steps are still under investigation. In the cytosol before import, hydrophobic mitochondrial precursor proteins cannot fold, but are kept soluble by the molecular chaperone proteins Hsc70 (heat-shock cognate 70) and Hsp90 (heat-shock protein 90). These ATP-dependent chaperones are responsible for the folding of proteins in the cell, but also fulfil additional functions, including protein transport [6,7]. Hsc70 and Hsp90 form specific interactions with the Tom70 import receptor in a key step of carrier protein import. Studies of the PiC (phosphate carrier) and AAC (ADP/ATP carrier) found that their import depended on the Tom70-targeting function of Hsc70 and Hsp90 [8,9]. The strong conservation of this pathway in eukaryotes suggests that it is the general mechanism of carrier protein import. The pathway also appears to be important for some proteins unrelated to the carriers, such as Tom40 itself [10].
Cytosolic chaperone complexes with these carriers were also found to include the co-chaperones DJA1 (DNAJA1/Hdj2/HSDJ), DJA2 (DNAJA2/Hdj3/Rdj2), Hop, TPR (tetratricopeptide repeat domain) 2 and p23, which regulate the function of Hsc70 and Hsp90. However, the chaperone components do not specifically recognize the targeting signals of the carriers; instead, sorting of carriers is thought to be performed by the Tom70 import receptor. Tom70 has an N-terminal membrane anchor, a central chaperone-interacting TPR and a C-terminal domain proposed to recognize carrier proteins. Chaperone complexes with carrier protein precursors dock on to the Tom70 TPR domain in the first step. Interactions between Tom70 and precursors are then thought to select carrier proteins to be imported. ATPase cycling of chaperones then promotes the translocation of carriers across the outer membrane [8,9,11,12].
Some carrier proteins are translated as precursors with N-terminal presequences that are proteolytically cleaved after import (Figure 1). Unlike the matrix-targeting sequences, carrier presequences are not necessary for import, but still assist import in some other way. Also unlike matrix import signals, carrier presequences do not follow a clear pattern of length or hydrophobicity/polarity. mPiC (mature PiC) lacking the 49-aminoacid-residue presequence could still be imported into the inner membrane similar to full-length pPiC (precursor PiC), but the presequence supported only marginal import when fused to an unrelated protein [13,14]. The 13-amino-acid-residue presequence of CIC (citrate carrier) was also not required for the import of mCIC (mature CIC), but increased the population of soluble pCIC (precursor CIC) available for import. Curiously, aberrant interactions of pCIC and pPiC presequences with Tom20, the matrix signal receptor, can even interfere with import [15,16]. The role of presequences in the context of chaperone-mediated targeting is as yet unexplored. Hsc70 and Hsp90 belong to different structural families, and their respective ATPase cycles regulate binding to polypeptide substrates. Hsc70 is thought to preferentially bind hydrophobic sequences with an extended conformation. The features recognized by Hsp90 are less clear, but it has been proposed to bind to surfaces of partially folded proteins [17,18]. How these modes of interaction apply to carrier protein precursors and presequences in particular remains undefined.
Figure 1. The N-termini of carrier proteins studied.

The sequences of bovine PiC (NP_777082), rat CIC (NP_059003), bovine OGC (NP_777096) and mouse AAC (NP_031477) were aligned using T-COFFEE and the N-terminal region is shown. The cleaved presequences of PiC and CIC are shown as white text on a black background. The predicted first transmembrane helix of AAC is underlined. Absolutely conserved residues are marked with a star, and those of high and low similarity with double (:) and single dots (.).
In the present study, we examined chaperone interactions of the newly translated mature and precursor forms of PiC and CIC, and for comparison, OGC (oxoglutarate carrier), which naturally lacks a presequence [19]. The binding of each protein to Hsc70 and Hsp90 was tested in relation to solubility before import. The function of chaperones in import was analysed by inhibition of Hsc70, and by competition of Tom70 targeting using an Hsp90 fragment. The presequences appeared to compensate for different deficiencies of the mPiC and mCIC polypeptides that were not present in OGC.
MATERIALS AND METHODS
Materials
The expression vectors pGEM4 and pGM4Z were purchased from Promega, and pCRII and pPROEXHTa were from Invitrogen. TNT™-coupled reticulocyte lysate system was from Promega, [35S]methionine/cysteine labelling mix was from GE Healthcare and the Bradford protein assay kit was purchased from Bio-Rad. BSA, sodium succinate, PMSF, proteinase K and Coomassie Brilliant Blue G-250 were from Sigma. All other reagents were of analytical grade.
Animals
Male Wistar rats (150–200 g) were obtained from Harlan (Carezzana, Italy) and housed in animal cages at a temperature of 22 ± 1 °C. Experiments were carried out in accordance with local and national guidelines regarding animal experiments.
Preproteins
Plasmids encoding the bovine PiC, rat CIC, murine AAC and bovine OGC preproteins were constructed as previously described [11,13–15,19]. The preparation of the plasmids coding for preCIC–DHFR (dihydrofolate reductase) (where preCIC is the presequence of CIC), containing the presequence of rat liver CIC fused to DHFR, including three N-terminal amino acid residues of mCIC, and prePiC–DHFR (where prePiC is the presequence of PiC) has been described previously [13–15]. The carrier proteins were expressed by coupled transcription and translation in vitro in rabbit reticulocyte lysate in the presence of [35S]methionine/ cysteine. In the solubility assay, the reticulocyte lysate, containing the radiolabelled proteins, was centrifuged at 40 000 rev./min for 45 min at 2°C using a Ti50 rotor (Beckman).
Protein purification
The His-tagged TPR1 (residues 1–118) and TPR2A (residues 223–352) domains of human Hop, C-Bag [C-terminal fragment of Bag1M (Bcl-2-associated athanogene 1M)] (residues 151–263) and C-90 (C-terminal fragment of Hsp90α) (residues 566–732) were purified as described previously [8,20,21]. The proteins were expressed in BL21(DE3) cells induced with 1 mM isopropyl β-D-thiogalactopyranoside, and the cells were lysed with a French Press and cleared by ultracentrifugation at 28 000 rev./min for 30 min in a Ti50.2 rotor (Beckman). Purification was on nickel–Sepharose HP (GE Healthcare) equilibrated in 20 mM KH2PO4 (pH 7.5), 500 mM NaCl and 20 mM imidazole, and eluted with 20 mM KH2PO4 (pH 7.5) and 300 mM imidazole, followed by gel filtration on Superdex 200 (GE Healthcare) in buffer CG [20 mM Hepes/KOH (pH 7.5), 100 mM potassium acetate and 5 mM magnesium acetate].
Chaperone-binding experiments
Co-precipitation with the chaperone-binding domains of Hop was tested as described previously [8,11]. Radiolabelled proteins were diluted 10-fold with buffer CG containing 0.1 % Triton X100 and 2 mg/ml BSA. His-tagged TPR1 or TPR2A domains of Hop were added to the reactions at a final concentration of 10 μM. The protein complexes were recovered by chromatography on Ni-NTA (Ni2+-nitrilotriacetate)–agarose. The beads were washed with buffer CG containing 20 mM imidazole and 0.1 % Triton X100 and then analysed by SDS/PAGE.
Mitochondrial import assays
Mitochondria from rat liver were isolated by differential centrifugation as described previously [13]. Briefly, the livers were rapidly excised and placed into STE (sucrose, Tris and EDTA) medium containing 10 mM Tris/HCl (pH 7.2), 250 mM sucrose and 1 mM EDTA at 4 °C. Tissue samples were homogenized on ice using a Teflon Potter homogenizer. The homogenates were centrifuged at 1000 g for 8 min, and the supernatants were centrifuged for 10 min at 8000 g to obtain mitochondrial pellets. The resulting pellets were washed twice with STE medium and centrifuged for 10 min at 8000 g.
Rabbit reticulocyte lysate containing the radiolabelled proteins and mitochondria (75 μg of protein) were incubated in BSA buffer [10 mM Mops/KOH (pH 7.2), 3 %(w/v) BSA, 250 mM sucrose, 80 mM KCl and 5 mM MgCl2] in a final volume of 100 μl at 25 °C. Then sodium succinate (10 mM final concentration) was added. Inhibition of import by C-90 and C-Bag was assayed by adding purified C-90 and C-Bag to reactions at a final concentration of 20 and 5 μM respectively, as previously reported [8]. After the import reaction, the samples were cooled to 0 °C, incubated with proteinase K (50 μg/ml) for 20 min at 0 °C and then with 1.5 mM PMSF for 5 min at 0 °C to stop the digestion. The mitochondria were re-isolated by centrifugation (10 min, 16 000 g) and analysed by SDS/PAGE, fluorography and densitometry.
Other methods and statistical analysis
Protein concentration was determined by using the Bradford reagent, with BSA used as the standard protein, or by absorbance at 280 nm. Polyacrylamide-gel electrophoresis was performed in the presence of 0.1 % SDS (SDS/PAGE) according to standard procedures. Experimental results are presented as the mean ± S.D.
RESULTS AND DISCUSSION
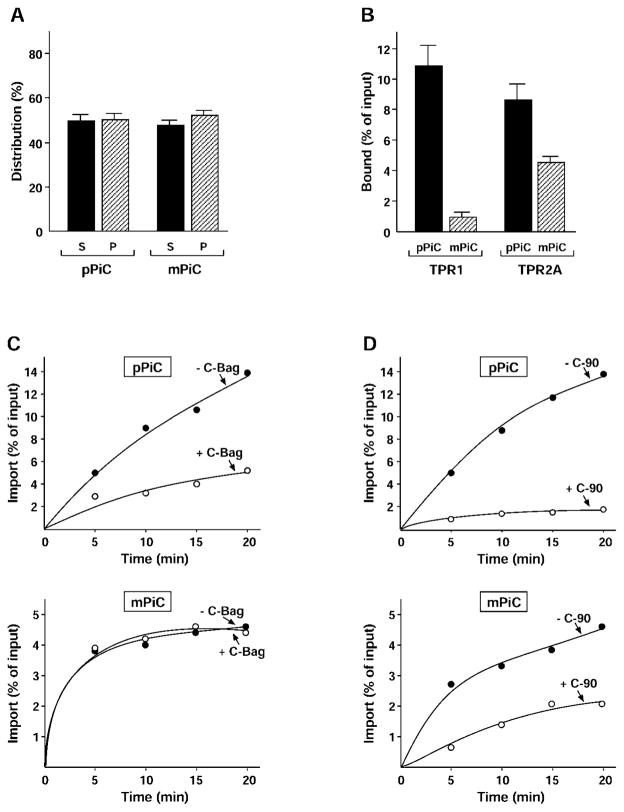
Chaperones are thought to have a dual role in carrier import: a passive function in maintaining the solubility of the polypeptides, and an active function in targeting to Tom70 and subsequent translocation across the outer membrane. Both pPiC and mPiC were analysed for these aspects. The polypeptides were translated and radiolabelled in reticulocyte lysate, an established model of chaperone activity. Aggregation of the polypeptides was tested by centrifugation after termination of the translation [15]. Approximately half of the pPiC and mPiC present was found in the pellet (Figure 2A and Supplementary Figure S1 at http://www.BiochemJ.org/bj/419/bj4190369add.htm). Solubility in the lysate was expected to depend on both the intrinsic physical properties of the polypeptides, for example hydrophobicity and flexibility, and the ability of chaperones to bind and prevent aggregation. Binding of substrates by the chaperones is itself determined by physical features; Hsc70 is thought to prefer extended peptide stretches, whereas Hsp90 may bind more native-like features. So the aggregation behaviour of pPiC and mPiC may stem from different combinations of intrinsic solubility and chaperone function.
Figure 2. The PiC presequence determines Hsc70 interactions.
(A) pPiC and mPiC were radiolabelled by cell-free translation in reticulocyte lysate, reactions were terminated and separated by centrifugation. The supernatants (S) and pellets (P) were analysed by SDS/PAGE, fluorography and densitometry, and the distribution as a percentage of total protein is shown. Results are means ± S.D. (n ≥3). (B) pPiC and mPiC were radiolabelled and incubated with purified His-tagged Hop TPR1 and TPR2A fragments. The fragments were recovered with nickel–Sepharose and the co-precipitated protein was analysed. The amount of binding is shown as a percentage of input translation. Results are means ± S.D. (n ≥3). (C) pPiC and mPiC were radiolabelled and incubated with isolated rat liver mitochondria for the indicated times to allow import. Mitochondria were re-isolated and treated with proteinase K, and the protease-resistant imported material was analysed. Reactions were conducted in the absence (− C-Bag, ●) or presence of C-Bag fragment (+ C-Bag, ○). Import is shown as a percentage of input translation. (D) The import of pPiC and mPiC was assayed as stated for (C), except that reactions contained either no addition (− C-90, ●) or purified C-90 fragment (+ C-90, ○).
The interaction of the polypeptides with Hsc70 and Hsp90 was therefore tested with an established co-precipitation assay [8,11]. The co-chaperone Hop contains two chaperone-specific TPR domains: the N-terminal TPR1 domain recognizes Hsc70, whereas the central TPR2A domain binds Hsp90. Both Hsc70 and Hsp90 can be bound by these TPR domains without disturbing their interaction with their substrate, as full-length Hop coordinates the chaperones into a larger substrate-binding complex [20]. Radiolabelled cell-free translated carriers were incubated with purified His-tagged Hop TPR1 and TPR2A, and chaperone-bound fractions were recovered using nickel–Sepharose. Previously, pPiC was found to co-precipitate similarly with TPR1 and TPR2A, indicating similar binding to both Hsc70 and Hsp90. In the present study, we observed that mPiC without a presequence co-precipitated very poorly with TPR1 compared to pPiC (Figure 2B and Supplementary Figure S1). In contrast, mPiC co-precipitation with TPR2A was only mildly reduced compared to pPiC. These results suggested that the PiC presequence provides the major binding sites for Hsc70, whereas the main sites of Hsp90 binding are in the mature portion of the carrier. The 49-amino-acid-residue presequence could contain more than one binding site for Hsc70, which is thought to bind to short hydrophobic sequences. Because mPiC did not aggregate more than pPiC, Hsc70 binding to the presequence may not contribute greatly to the solubility of the carrier before import. Hsp90 binding appears to be sufficient to prevent aggregation, and perhaps for targeting and import.
To test these ideas, the importance of Hsc70 for pPiC and mPiC import was examined. As established, the import of mPiC into rat liver mitochondria was clearly observed at approximately half the efficiency of pPiC. To disrupt Hsc70 activity, an excess of the purified Bag1 nucleotide exchange factor domain C-Bag was added to import reactions, to trigger release of substrate polypeptides from Hsc70 [8,10,21]. Similar to previous results, C-Bag significantly reduced the import of pPiC compared to untreated control reactions. Remarkably, C-Bag had no effect at all on the import of mPiC (Figure 2C and Supplementary Figure S1). This was consistent with the lack of interaction between mPiC and Hsc70, and a major role of the presequence as an Hsc70-binding site.
The partially reduced import of mPiC compared to pPiC suggested that Hsp90 could not fully compensate for the lack of Hsc70 binding. However, Hsp90 could still be functional in targeting to Tom70. To test this, we analysed the effect on pPiC and mPiC import of a competitor of chaperone–Tom70 docking. C-90 was known to compete both Hsp90 and Hsc70 from Tom70, and to inhibit the import of pPiC [8,9]. Unlike C-Bag, an excess of C-90 was now observed to inhibit mPiC import (Figure 2D and Supplementary Figure S1), in agreement with the Hsp90 interaction. Furthermore, the results suggest that Hsc70 binding to the presequence is more important for targeting of the carrier than for preventing its aggregation. In this function, Hsc70 most probably does not read out the presequence as a targeting signal, but, along with Hsp90, serves to connect the carrier polypeptide to Tom70.
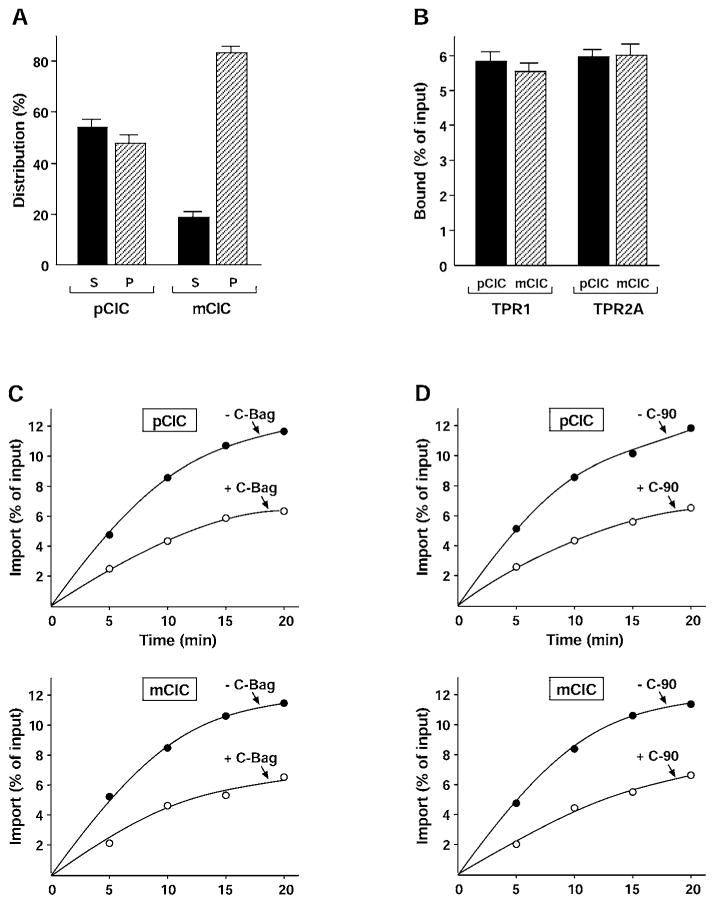
To extend the study, pCIC and mCIC were compared with the above tests. The 13-amino-acid-residue pCIC presequence is much shorter and less hydrophobic than that of pPiC [15]. Newly translated pCIC was soluble at similar levels to pPiC, but removal of the presequence caused marked aggregation of mCIC, as noted previously (Figure 3A). Binding of the polypeptides to Hsc70 and Hsp90 was next assayed by co-precipitation with Hop TPR1 and TPR2A. The pCIC polypeptide was bound to both chaperones at similar levels to pPiC. Surprisingly, the interaction of mCIC with the chaperones was almost identical to that of pCIC (Figure 3B), despite the very different aggregation behaviour. Thus, unlike for pPiC, the CIC presequence does not provide a chaperone binding site. The increased solubility provided by the presequence may instead be due to its physical characteristics.
Figure 3. The function of the CIC presequence is independent of chaperone binding.
(A) pCIC and mCIC were radiolabelled and separated by centrifugation as stated in the legend to Figure 2(A). The supernatants (S) and pellets (P) were analysed. Results are means ± S.D. (n ≥3). (B) The binding of radiolabelled pCIC and mCIC to TPR1 and TPR2A was assayed as stated in the legend to Figure 2(B). Results are means ± S.D. (n ≥3). (C and D) The mitochondrial import of radiolabelled pCIC and mCIC was assayed as stated in the legend to Figure 2(C) in the presence (○) or absence (●) of the C-Bag (C) or C-90 (D) fragments.
This idea suggested that pCIC and mCIC should be similar in their dependence on chaperones for targeting and import. So, we tested the effect on import of Hsc70 disruption by C-Bag. Addition of C-Bag partially but significantly reduced the import of pCIC, similar to the effect on pPiC import (Figure 3C). The import of mCIC was similar to that of pCIC, confirming that the pre-sequence does not carry targeting information. As predicted, the import of mCIC was inhibited by C-Bag to the same extent as pCIC. Partial inhibition of pCIC import was also observed with the C-90 competitor of Hsp90, and again, the import of mCIC was similarly reduced (Figure 3D). Our results thus show that the chaperones bind in the mature region of CIC, and that this is sufficient to mediate targeting. The strong aggregation of mCIC suggests that chaperone binding in this case is less effective at maintaining solubility. Therefore, as postulated for Hsc70 binding to the PiC presequence, chaperone binding to CIC may be more important for targeting than for aggregation prevention. The latter function is performed by the presequence of CIC.
As a further test, we examined the presequences of pPiC and pCIC fused to an unrelated protein, DHFR. This protein is not normally imported into mitochondria and carries no localization signals; DHFR is also small and folds spontaneously into the native state without requiring chaperones [22]. The fusion proteins prePiC–DHFR and preCIC–DHFR were constructed, bearing the presequences of pPiC and pCIC respectively, and tested for binding to Hsc70 and Hsp90. Co-precipitation with Hop TPR1 showed that prePiC–DHFR was clearly bound by Hsc70, whereas preCIC–DHFR was not bound above background levels (Figure 4). In contrast, neither fusion protein was bound by Hsp90, as shown by Hop TPR2A co-precipitation. Thus the pPiC presequence is sufficient for the interaction with Hsc70 but does not confer an Hsp90-binding site, and the pCIC presequence does not interact with either chaperone, consistent with a chaperone-independent function.
Figure 4. The PiC presequence is sufficient for Hsc70 interaction.
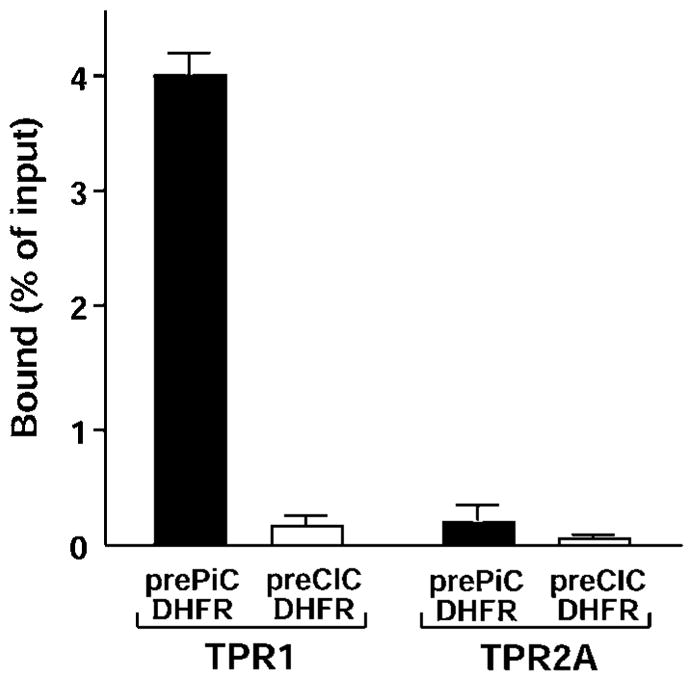
The presequences of PiC and CIC were fused to DHFR to form prePiC–DHFR and preCIC–DHFR respectively. The binding of prePiC–DHFR and preCIC–DHFR to TPR1 and TPR2A was assayed as stated in the legend to Figure 2(A).
The presequences of PiC and CIC appear to use different mechanisms to achieve the same goal of improving import. Nevertheless, many other carrier proteins do not have such presequences. Following our hypothesis, these carriers have not developed presequences in evolution because they already have suitable chaperone-binding sites, and are sufficiently soluble in chaperone complexes for import. Some evidence of this was observed with AAC, which has no presequence. Both Hsc70 and Hsp90 were known to bind AAC, maintaining it in a high-molecular-mass cytosolic complex. Experiments with C-Bag and C-90 showed that the chaperones also functioned in the import of AAC [9]. To verify this, we investigated another carrier lacking a presequence, OGC.
Newly translated OGC showed a similar level of aggregation as pPiC and pCIC, approximately half of the population, and comparable to AAC (Figure 5A). Co-precipitation of OGC with the chaperones was assayed, and slightly more OGC was found to co-precipitate with Hop TPR1 than with TPR2A (Figure 5B). This suggests that OGC may be bound more by Hsc70 than Hsp90, whereas pPiC and pCIC were bound equally by both chaperones. When the import of OGC into mitochondria was tested, disruption of Hsc70 by C-Bag caused a marked inhibition of import to less than half of the control (Figure 5C). This agrees with a function of Hsc70 in the targeting of OGC. The C-90 competitor of Hsp90 inhibited OGC import even more strongly, suggesting that the combined functions of Hsc70 and Hsp90 are important to target OGC to mitochondria. Thus OGC, like AAC, has sufficient chaperone-binding sites to support its import, making the evolutionary development of a presequence unnecessary.
Figure 5. Solubility and import of OGC lacking a presequence.
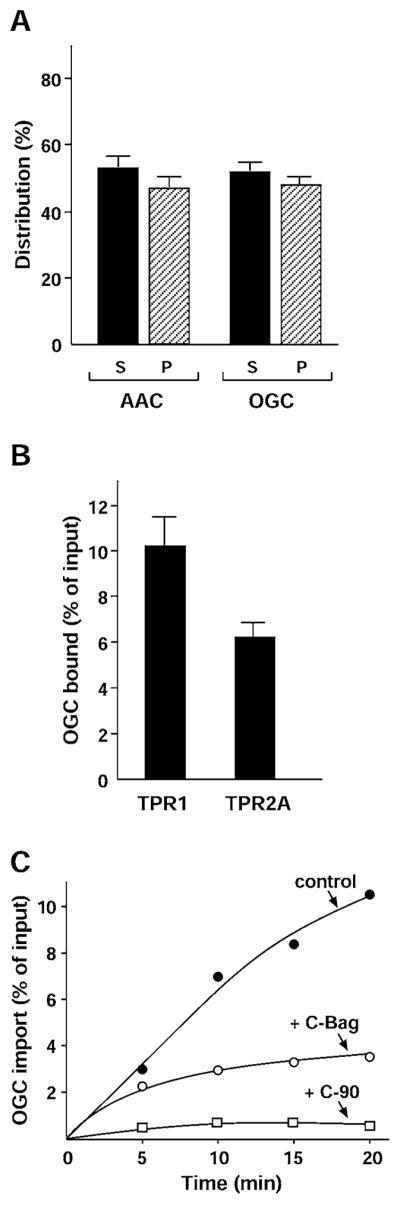
(A) AAC and OGC were radiolabelled and separated by centrifugation as stated in the legend to Figure 2(A). The supernatants (S) and pellets (P) were analysed. Results are means ± S.D. (n ≥3). (B) The binding of radiolabelled OGC to TPR1 and TPR2A was assayed as stated in the legend to Figure 2(B). Results are means ± S.D. (n ≥3). (C) The mitochondrial import of radiolabelled OGC was assayed as stated in the legend to Figure 2(C) in the absence (●) or presence of the C-Bag (○) or C-90 (□) fragments.
Overall, the results of the present study suggest that the presequences of PiC and CIC improve import competence by different mechanisms, with PiC by providing a binding site for a particular chaperone, Hsc70, and CIC reducing the aggregation of the polypeptide independent of any external chaperone activity. In the case of PiC, the presequence may also provide some targeting information, but this is minimal. The PiC presequence carries a net positive charge, but has sufficient hydrophobic character to be consistent with Hsc70 binding. It may seem surprising that few Hsc70-binding sites are found in the rest of the PiC sequence, considering that it is mostly transmembrane and hydrophobic. However, other potential Hsc70 sites may be blocked by Hsp90 binding to overlapping sites. The substrate-binding mode of Hsp90 is still in question, but it is expected to bind to a larger surface of a polypeptide, as suggested by its extended dimeric structure. The PiC presequence may protrude enough from an Hsp90-bound complex to form a handle for Hsc70 binding. The CIC presequence is positively charged and this extra polar character may balance the hydrophobicity of the remaining sequence enough to increase solubilization, a detergent effect. Alternatively, the presequence may interact with parts of the unfolded mature region to block hydrophobicity, slowing aggregation and acting as an internal chaperone.
Many carrier proteins do not have presequences, and must have internal chaperone-binding sites to follow the chaperone-mediated import pathway. However, sequence conservation between the carriers is not high. For example, the first transmembrane helix contains the highest homology between carriers [23], but with notably few identical residues (Figure 1). With such divergence, it is possible that each carrier has different chaperone-binding sites, and may have a different dependence on Hsc70 compared to Hsp90. Indeed, much more Hsc70 than Hsp90 seems to bind AAC, whereas the difference for OGC observed in the present study is less extreme. Moreover, there is significant sequence divergence at the N-termini of carriers, and some have extended sequences before their known or predicted transmembrane helices. Some of these sequences may in fact possess the same chaperone-binding or solubility functions as the cleaved presequences, to increase competence for import, although they are not cleaved in the native protein.
Finally, other roles for cleaved presequence and N-terminal extensions during import cannot be ruled out and remain to be addressed. Precursors without cleaved presequences, such as AAC and Tim23 protein, are thought to translocate through the outer membrane pore in a loop conformation, with both polypeptide termini trailing in the cytosol while the central region reaches the intermembrane space [24,25]. It is interesting to speculate that the presequences or N-terminal extensions of the metabolite carriers aid formation of the translocation loop state. Although the conformation of a precursor before translocation is not known, one AAC polypeptide can be complexed with three Tom70 homodimers on the outer membrane, with multiple chaperones probably also bound [11,12,24]. Release from these complexes upon translocation might be more efficient for the central parts of the polypeptide, while presequences (or extensions) might be slower to translocate because of interactions with chaperones, Tom70 or the aqueous solvent. So there might be several biophysical and biochemical mechanisms by which the sequence of polypeptides influences their targeting efficiency.
Supplementary Material
Acknowledgments
We thank Michael J.H. Wong for technical assistance.
FUNDING
This work was supported in part by the Ministero dell’Istruzione, dell’Università e della Ricerca [grant numbers PRIN 2006, PRIN 2007] and by the Canadian Institutes of Health Research [grant number MOP-68825]. J. C. Y. holds a Canada Research Chair in Molecular Chaperones.
Abbreviations used
- AAC
ADP/ATP carrier
- Bag1M
Bcl-2-associated athanogene 1M
- C-Bag
C-terminal fragment of Bag1M
- CIC
citrate carrier
- mCIC
mature CIC
- pCIC
precursor CIC
- DHFR
dihydrofolate reductase
- Hsc70
heat-shock cognate 70
- Hsp90
heat-shock protein 90
- C-90
C-terminal fragment of Hsp90α
- OGC
oxoglutarate carrier
- PiC
phosphate carrier
- mPiC
mature PiC
- pPiC
precursor PiC
- STE
sucrose, Tris and EDTA
- Tim
translocase of the mitochondrial inner membrane
- Tom
translocase of the mitochondrial outer membrane
- TPR
tetratricopeptide repeat
References
- 1.Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch. 2004;447:689–709. doi: 10.1007/s00424-003-1099-7. [DOI] [PubMed] [Google Scholar]
- 2.Palmieri F. Diseases caused by defects of mitochondrial carriers: a review. Biochim Biophys Acta. 2008;1777:564–578. doi: 10.1016/j.bbabio.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Rehling P, Brandner K, Pfanner N. Mitochondrial import and the twin-pore translocase. Nat Rev Mol Cell Biol. 2004;5:519–530. doi: 10.1038/nrm1426. [DOI] [PubMed] [Google Scholar]
- 4.de Marcos-Lousa C, Sideris DP, Tokatlidis K. Translocation of mitochondrial inner-membrane proteins: conformation matters. Trends Biochem Sci. 2006;31:259–267. doi: 10.1016/j.tibs.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 6.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 7.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- 8.Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. [Google Scholar]
- 9.Bhangoo MK, Tzankov S, Fan AC, Dejgaard K, Thomas DY, Young JC. Multiple 40-kDa heat-shock protein chaperones function in Tom70-dependent mitochondrial import. Mol Biol Cell. 2007;18:3414–3428. doi: 10.1091/mbc.E07-01-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humphries AD, Streimann IC, Stojanovski D, Johnston AJ, Yano M, Hoogenraad NJ, Ryan MT. Dissection of the mitochondrial import and assembly pathway for human Tom40. J Biol Chem. 2005;280:11535–11543. doi: 10.1074/jbc.M413816200. [DOI] [PubMed] [Google Scholar]
- 11.Fan AC, Bhangoo MK, Young JC. Hsp90 functions in the targeting and outer membrane translocation steps of Tom70-mediated mitochondrial import. J Biol Chem. 2006;281:33313–33324. doi: 10.1074/jbc.M605250200. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Sha B. Crystal structure of yeast mitochondrial outer membrane translocon member Tom70p. Nat Struct Mol Biol. 2006;13:589–593. doi: 10.1038/nsmb1106. [DOI] [PubMed] [Google Scholar]
- 13.Zara V, Rassow J, Wachter E, Tropschug M, Palmieri F, Neupert W, Pfanner N. Biogenesis of the mitochondrial phosphate carrier. Eur J Biochem. 1991;198:405–410. doi: 10.1111/j.1432-1033.1991.tb16029.x. [DOI] [PubMed] [Google Scholar]
- 14.Zara V, Palmieri F, Mahlke K, Pfanner N. The cleavable presequence is not essential for import and assembly of the phosphate carrier of mammalian mitochondria but enhances the specificity and efficiency of import. J Biol Chem. 1992;267:12077–12081. [PubMed] [Google Scholar]
- 15.Zara V, Ferramosca A, Palmisano I, Palmieri F, Rassow J. Biogenesis of rat mitochondrial citrate carrier (CIC): the N-terminal presequence facilitates the solubility of the preprotein but does not act as a targeting signal. J Mol Biol. 2003;325:399–408. doi: 10.1016/s0022-2836(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 16.Zara V, Ferramosca A, Papatheodorou P, Palmieri F, Rassow J. Import of rat mitochondrial citrate carrier (CIC) at increasing salt concentrations promotes presequence binding to import receptor Tom20 and inhibits membrane translocation. J Cell Sci. 2005;118:3985–3995. doi: 10.1242/jcs.02526. [DOI] [PubMed] [Google Scholar]
- 17.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Pearl LH, Prodromou C. Structure and mechanism of the hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 19.Palmisano A, Zara V, Honlinger A, Vozza A, Dekker PJ, Pfanner N, Palmieri F. Targeting and assembly of the oxoglutarate carrier: general principles for biogenesis of carrier proteins of the mitochondrial inner membrane. Biochem J. 1998;333:151–158. doi: 10.1042/bj3330151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- 21.Sondermann H, Scheufler C, Schneider C, Hohfeld J, Hartl FU, Moarefi I. Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science. 2001;291:1553–1557. doi: 10.1126/science.1057268. [DOI] [PubMed] [Google Scholar]
- 22.Rassow J, Guiard B, Wienhues U, Herzog V, Hartl FU, Neupert W. Translocation arrest by reversible folding of a precursor protein imported into mitochondria. A means to quantitate translocation contact sites. J Cell Biol. 1989;109:1421–1428. doi: 10.1083/jcb.109.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trezeguet V, Lauquin GJ, Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]
- 24.Wiedemann N, Pfanner N, Ryan MT. The three modules of ADP/ATP carrier cooperate in receptor recruitment and translocation into mitochondria. EMBO J. 2001;20:951–960. doi: 10.1093/emboj/20.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curran SP, Leuenberger D, Schmidt E, Koehler CM. The role of the Tim8p-Tim13p complex in a conserved import pathway for mitochondrial polytopic inner membrane proteins. J Cell Biol. 2002;158:1017–1027. doi: 10.1083/jcb.200205124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.