Abstract
Background
Chronic inflammation is known to facilitate cancer progression and metastasis. Less is known about the effect of acute inflammation within the tumor microenvironment, resulting from standard invasive procedures. Recent studies in mouse models have shown that the acute inflammatory response triggered by a biopsy in mammary cancer increases the frequency of distal metastases. Although tumor biopsies are part of the standard clinical practice in breast cancer diagnosis, no studies have reported their effect on inflammatory response. The objective of this study is to 1) determine whether core needle biopsies in breast cancer patients trigger an inflammatory response, 2) characterize the type of inflammatory response present, and 3) evaluate the potential effect of any acute inflammatory response on residual tumor cells.
Methods
The biopsy wound site was identified in the primary tumor resection tissue samples from breast cancer patients. The inflammatory response in areas adjacent (i.e. immediately around previous biopsy site) and distant to the wound biopsy was investigated by histology and immunohistochemistry analysis. Proliferation of tumor cells was also assayed.
Results
We demonstrate that diagnostic core needle biopsies trigger a selective recruitment of inflammatory cells at the site of the biopsy and they persist for extended periods of time. While macrophages were part of the inflammatory response, an unexpected accumulation of eosinophils at the edge of the biopsy wound was also identified. Importantly, we show that biopsy causes an increase in the proliferation rate of tumor cells located in the area adjacent to the biopsy wound.
Conclusions
Diagnostic core needle biopsies in breast cancer patients do induce a unique acute inflammatory response within the tumor microenvironment and have an effect on the surrounding tumor cells. Therefore biopsy-induced inflammation could have an impact on residual tumor cell progression and/or metastasis in human breast cancer. These findings may carry relevance in the clinical management of breast cancer.
Keywords: breast cancer, metastasis, biopsy, inflammation, eosinophils
INTRODUCTION
Despite the progress made in early detection and treatment, breast cancer remains the second leading cause of cancer death in women. In 2015, over forty thousand women were estimated to die from breast cancer [1]. Mortality in breast cancer is primarily due to the development of metastasis. While localized breast cancer has an excellent 5-year survival rate of 98.5%, it does decrease to 25.0% for metastatic disease [1]. Identifying factors that can increase the risk of metastases, and developing clinical treatments that reduce this risk are essential steps in decreasing mortality from breast cancer. It is becoming evident that progression from non-invasive to invasive breast cancer or progression to metastasis is highly influenced by the tumor microenvironment. Changes in the tumor microenvironment can radically affect the properties of tumor cells. Although natural immunosurveillance plays an important role in prevention of carcinogenesis, evidence has demonstrated that the immune response within the tumor microenvironment can also be detrimental and promote cancer progression, including breast cancer [2–5].
Chronic inflammation has been extensively studied in regards to its effect on cancer progression [6–9]; however, the role of acute inflammation within tumors in cancer progression and metastasis is less well understood [10]. Given that chronic inflammation (with only low levels of cytokines) has demonstrated effect on tumorigenesis, it is hypothesized that the presence of an acute inflammatory response in the tumor microenvironment could also be detrimental by supporting tumor cell proliferation and, more importantly, metastasis. The presence of acute inflammation in the tumor can lead to the production of cytokines that may act directly on cancer cells, or affect the microenvironment to facilitate cancer cell migration. The acute inflammatory response in the tumors can be triggered by invasive procedures such as core needle biopsies and surgical excision. Core needle biopsy is a standard procedure in breast cancer diagnosis. Although it is considered a safe and reliable procedure, it may trigger the recruitment of inflammatory cells at the biopsy site as a normal component of the wound healing response. Wound healing is a complex process characterized by the sequential recruitment of inflammatory cells that secrete different cytokines (e.g. IL-6, IL-8), and some of these cytokines contribute to the proliferation of epithelial cells and tissue remodeling. Thus, wound healing-induced inflammation within the breast tumor may promote tumor progression and/or metastasis [11, 12]. The period from the time the diagnostic biopsy is obtained until subsequent surgery (or other cancer treatment is initiated) may span from a number of days to weeks, providing time for proliferation of residual tumor cells and possible lymphovascular migration into the systemic circulation promoted by the immune response. The risk that needle biopsies of breast cancer may have on breast cancer progression or recurrence is a controversial topic [13–18]. While there is little evidence to support the potential risk of tumor displacement with biopsies, the effect of inflammation caused by the biopsy on the potential risk for metastases has not been investigated.
Studies using a mammary tumor mouse model for breast cancer have shown that a biopsy of the mammary tumor has only a marginal impact on the rate of growth of the tumor in mice that underwent biopsy compared with control mice, but it significantly increases the frequency of distant metastases [19]. Core needle biopsies in these studies caused the recruitment of inflammatory cells to the site of biopsy in the mammary tumor and increased the proliferation rate of the cancer cells adjacent to the biopsy wound [19]. More importantly, these studies demonstrate that treatment with common anti-inflammatory medications can reduce the risk of distant metastases associated with biopsies [19]. Thus, based on mouse models, inflammation caused by mammary tumor biopsies can increase the risk of metastasis.
Unlike mice, the immune response in humans is highly diverse. It is therefore also plausible that the acute immune response triggered by breast cancer biopsies at the tumor site is also heterogeneous. The clinical implications are that a subset of cancer patients may be more vulnerable to inflammation-induced tumor progression or risk for early local recurrence and distant metastasis. No studies have previously addressed the impact of biopsies on the acute inflammatory response in tumors of breast cancer patients, nor have there been studies evaluating the type of inflammatory response and the duration of this inflammatory response. In this study we show that breast cancer biopsies induce the recruitment of inflammatory cells at the site of the biopsy wound, and this inflammatory response remains at or near the residual tumor for extended periods of time. We identified for the first time the presence of eosinophils selectively at the site of the biopsy wound. More importantly, our study reveals an increase in the tumor cell proliferation rate after performing a core needle biopsy. Consequently, the acute inflammatory response associated with breast biopsies could have a profound clinical impact by stimulating residual cancer cells in certain cases. Furthermore, understanding the potential mechanism of acute inflammation on the tumor microenvironment may lead to new strategies to mitigate this mechanism through the use of anti-inflammatory medication at the time of the diagnostic biopsy.
METHODS
Study Subjects and Materials
In order to be eligible for our study, we required samples from patients who had undergone breast cancer biopsy followed by surgical resection of invasive carcinoma or ductal carcinoma in situ (DCIS) between years 2007 and 2012. We reviewed histological sections from paraffin embedded breast tumors of patients who did not receive neoadjuvant treatment prior to surgery. In addition, visualization of the original biopsy site was required within each surgical resection. We identified 44 formalin-fixed paraffin-embedded histological samples that met these criteria. The distribution of breast cancer types and subtypes, age, grade, differentiation stage, LVI (lymphovascular invasion) status, and the surgical procedure for breast cancer patients is provided in Table I. For the studies regarding Ki67 in cancer cells, only invasive breast samples were included. Since the natural progression of inflammatory response following a biopsy was anticipated to change over time, information on the time (number of days) between the biopsy and subsequent surgical resection was also recorded. The distribution of the histological samples according to the time between biopsy and surgical resection is provided in Table I where samples are stratified in 7 day-intervals. This study was approved by the Institutional Review Board at the University of Vermont. No consent was needed for these studies.
Table I.
Clinical characteristics of study participants
| Characteristic | Number of patients | |
|---|---|---|
| Age | mean | (57.7 years) (46–90 years) |
| range | ||
| median | (56 years | |
| Tumor type | 1DCIS | 11 |
| Invasive | 33 | |
| Differentiation | well | 8 |
| moderate | 16 | |
| poor | 11 | |
| 2NA (DCIS) | 9 | |
| Nuclear grade | I | 0 |
| II | 26 | |
| III | 18 | |
| 3ER | Positive | 36 |
| Negative | 8 | |
| 4PR | Positive | 33 |
| Negative | 11 | |
| 5Her2 | Positive | 4 |
| Negative | 29 | |
| NA (DCIS) | 11 | |
| 6LVI | Positive | 11 |
| Negative | 33 | |
| 7Partial mastectomy | 37 | |
| Total mastectomy | 7 | |
| 8Time: biopsy to excision | ||
| < 7 days | 0 | |
| 7–14 days | 10 | |
| 15–21 days | 8 | |
| 22–28 days | 12 | |
| 29–35 days | 8 | |
| >35 days | 6 |
DCIS, ductal carcinoma in situ
NA, not applicable
ER, Estrogen Receptor
PR, Progesterone Receptor
Her2, Human epidermal growth factor receptor 2
LVI, lymphovascular invasion
Surgical excision procedure
Time lag from the core biopsy to the surgical excision
Immunohistochemistry (IHC)
Surgical pathology specimens were fixed in a 10% formalin solution, then dehydrated, embedded in paraffin wax, and 3-μm sections were placed on SuperFrost Plus microscope slides. Before immunohistochemistry, routine histology of all tissue samples was carried out after H&E staining of the sections. Cell counts for eosinophils and neutrophils were also collected from H&E stained tissue sections. Neutrophils are characterized by the presence of a single nucleus which is multilobed (2–5 lobes) and contains highly condensed chromatin. Unlike eosinophils, neutrophils have few granules and they do not display a highly eosinophilic cytoplasm (not a strong pink stain). Human eosinophils have a single nucleus with only two well-defined lobes. Because of nuclear position in the cells, sometimes the two lobes could be visualized as a single round smaller nucleus (one lobe on top of the other). Eosinophils are also characterized by the strong eosinophilic cytoplasmic staining (strong pink color on the cytoplasm) due to the accumulation in the cytoplasm of acidophilic specific granules. Slides were deparaffinized in two changes of fresh xylene, and rehydrated in a descending alcohol series. For IHC analysis we used markers and antibodies that are standardly used for patient diagnosis at the University of Vermont Medical Center Histology facility. For quantification of overall inflammation, IHC for CD45 as a marker of all leukocytes (leukocyte common antigen) was performed using a polyclonal rabbit anti-human CD45 antibody (LCA, PD7/26/16+2B11, Thermo Scientific). For quantification of macrophages, IHC for CD68 was performed using a monoclonal rabbit anti-human CD68 antibody (KP1, Dako). CD68 is cell plasma membrane (also endosome/lysosome membrane) protein and is clinically used as marker for monocytes, macrophages and myeloid cells. For quantification of proliferating cells, IHC for Ki67 antigen using a polyclonal rabbit anti-human Ki67 antibody (SP6, Thermo Scientific) was performed. Ki67 is a protein strictly associated with cell proliferation and is detected exclusively in the nucleus during the interphase and during mitosis segregates with the chromosomes. Ki67 is standardly used in clinical tests. To verify that tumor cells identified histologically were tumor cells, tissue sections were stained anti-pan cytokeratin monoclonal antibodies (AE1/AE3), used as marker for epithelial cells and abundantly present in breast cancer cells. The AE1 and AE3 monoclonal antibodies (Thermo Scientific) recognize cytokeratins 1, 2, 3, 4, 5, 6, 7 and 8 (AE3), and 10, 14, 15, 16 and 19 (AE1).
Antigen retrieval involved treatment with a protease (Antigen Retrieval 1:10; Dako) for 30 min in a steam bath. Before immunohistochemical staining, the sections were washed with TBS and blocked with protein blocking serum (Dako) in TBS for 30 min to reduce nonspecific antibody binding. Primary antibody was then applied at appropriate dilutions overnight at 4°C. Polyclonal Rabbit Anti-mouse Immunoglobulins (1:20; Dako) secondary antibody was incubated for 30 min in accordance with the manufacturer’s instructions.
Color was developed with a solution of chromagen (Dako). Slides were counterstained with hematoxylin, dehydrated in an ascending alcohol series, and covered with a coverslip (Permount mounting medium; Sigma-Aldrich).
Scoring System
One slide for each sample was scored in most cases. A quantitative score was used. We defined “adjacent” region as the areas adjacent or proximal to the biopsy wound (within 500 μm). For “adjacent” biopsy cell counts, we chose fields just around the biopsy site, avoiding necrosis, vasculature and lysis, and we focused on areas with the highest density of staining. We defined “distant” region as the area more distal to the biopsy wound (> 5 mm). For distant biopsy sites, we chose areas where the breast architecture was intact and focused on areas with the highest density of staining. Distant cell counts were taken from the same slide the majority of the time and or from an adjacent tissue sample determined from the pathology report for cases, which the biopsy site was present near the edge of the tissue sample.
For each slide, a total of 100 cells within 3 separate fields of the slide were counted for the “adjacent” region, and a total of 100 cells within 3 separate fields of the slide were counted for the “distant” region. A 400x magnification on the light microscope (EVO XL Cell Imaging System, Life Technologies) was used for cell counting in the different fields. 400x images were captured with the light microscope and counting was performed at the computer on the saved images. For quantification of the percentage of neutrophils, eosinophils, total leukocytes (CD45 positive cells) and macrophages (CD68 positive cells), the 100 cells counted among the three different fields refer to all nucleated cells. For quantification of the percentage of tumor proliferating cells (Ki67 positive), only cancer cells (identified by morphology and verified by AE1/AE3 staining) were counted. When counting tumor cells counting the ductal carcinoma in situ (DCIS) areas were avoided. We calculated the mean number of positively stained cells for three fields in the adjacent and distant areas to the biopsy wound site for each patient. Slides of samples were unable to be scored blindly as the biopsy site is visible in the areas of interest for adjacent cell counts. At least five slides for each group of adjacent and distant biopsy locations were scored by two independent observers, and the results were compared to test reproducibility of scoring. When the staining scores were discordant or unclear, an agreement was reached by the two observers and confirmed by the pathologist using a multi-head light microscope or by reviewing slide images from which the counts were taken. All the images used for cell counts were recorded and kept as electronic files. Microscopy images for the representative figures were taken with an Olympus BX50 microscope using a 4x (40x magnification) or 40x (400x magnification) objective. Scale bars are shown in the figures.
Statistical Data Analysis
The difference in mean staining scores of inflammatory and proliferative cell populations between the adjacent and distant biopsy locations was compared using a paired Student’s t test or a Wilcoxon Signed Ranks Tests when the normality assumption was not met. Regression analysis was used to examine the relationship between mean staining scores and number of days between biopsy and surgical resection. A p-value of <5% was considered statistically significant.
RESULTS
Human breast cancer biopsy triggers an acute inflammatory response at the tumor biopsy site
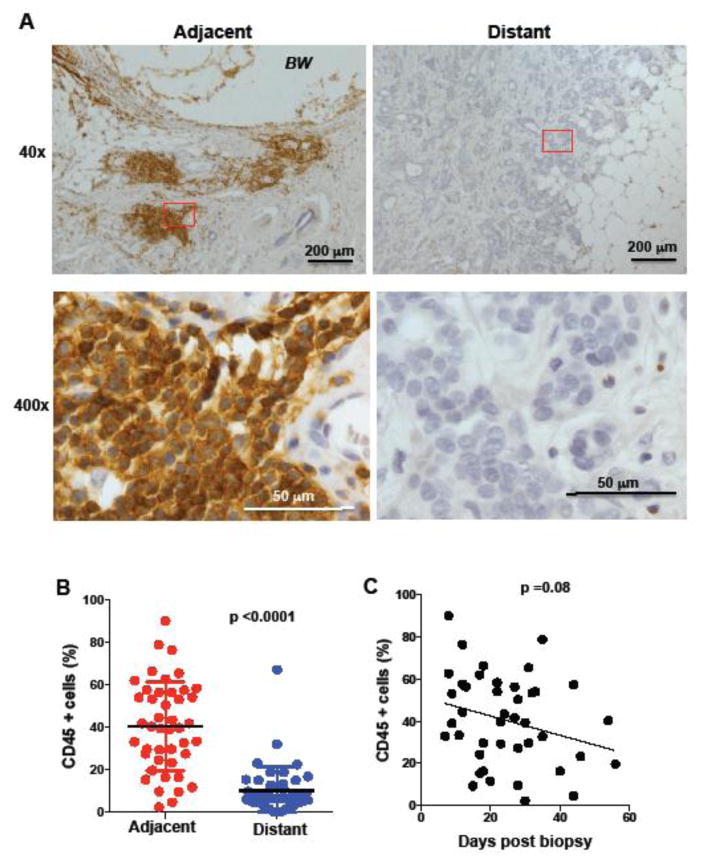
No previously published studies have characterized the acute inflammatory response resulting from core needle biopsies in the clinical breast cancer specimens. We performed a retrospective study with breast cancer samples where the biopsy site was identified. The breast cancer patients selected had not received any treatment (neoadjuvant systemic therapy or radiotherapy) from the time of the biopsy to the time of surgical excision of the tumor. The biopsy site wound was identified on H&E tissue sections from archival specimens. Inflammation at the area adjacent to the biopsy wound was readily detected in the H&E sections (Fig. 1A). However for better identification and quantification we performed (immunohistochemistry) IHC analysis for CD45, a pan leukocyte marker present in all inflammatory cells. For all the studies described here, we quantified the percentage of positive cells in the region adjacent or proximal to the biopsy wound (defined as “adjacent”), and a region distant from the biopsy wound but in the same slide (defined as “distant”) (Fig. 1A). The results show a preferential accumulation of CD45 cells in the proximity of the biopsy wound relative to the presence of CD45 cells in the tumor in a region distant to the biopsy (p <0.0001) (Fig. 1B).
Figure 1. Accumulation of inflammatory cells at the region adjacent to the biopsy wound.
(A) IHC of human breast tissue for CD45 marker in regions of the tumor adjacent to the biopsy wound (adjacent) and regions far away from the biopsy wound (distant). The images 40x magnification (upper panels) and 400x magnification (lower panels) of the 40x-red labeled areas are shown. Scale bars are shown. BW, biopsy wound. (B) Percentage of CD45 positive cells (relative to all nucleated cells in the analyzed fields) in breast cancer samples (n= 44) in regions adjacent and distant to the biopsy wound, p<0.0001 using a paired student’s t test analysis. (C) Percentage of CD45 positive cells in in the region adjacent to the biopsy as a function of time (days) between the core needle biopsy procedure and surgical excision of the breast tumor tissue (n=44), p=0.08 as determined by regression analysis.
The time lag from the day the biopsy was taken to the day excision surgery of the tumor was performed is highly variable (7 to 50 days) since patients require consultation and decision-making (Table I). In our study, there were few cases (n=10) where this time lag was less than 15 days (Table I), but there were some cases (n=6) where a time lag was more than 35 days (Table I). The inflammatory phase in injured normal tissues is limited to 3–14 days. Although there was a trend for the overall inflammation to decrease over time (p= 0.08), surprisingly the accumulation of CD45 positive cells at the adjacent region to the biopsy remained present even 40 days after the biopsy (Fig. 1C). Therefore, the biopsy process in breast cancer specimens triggers a local inflammatory response in the tumor that lasts for an extended period of time at the site of the biopsy wound.
Preferential accumulation of macrophages at the site of the biopsy wound in the breast cancer patients
To characterize the type of inflammatory response triggered by the biopsy in breast cancer, we first examined the presence of neutrophils since they are rapidly mobilized in response to tissue damage and recruited to the tissue. Neutrophils can be easily identified by their segmented nuclear morphology, and the lack of eosinophilic staining in H&E stained tissue slides (Fig. 2A and Supplementary Fig. S1A). Although the presence of neutrophils in the region adjacent to the biopsy wound was significantly greater than the accumulation in the areas of the tumor distant to the biopsy wound (p =0.019), in only a few cases neutrophils were detected in the adjacent region (Fig. 2B). Most likely neutrophils are released very rapidly as part of the innate immune response and are recruited to the site of the biopsy, but by the time the tumor is surgically excised (at least 7 days after the biopsy) they are no longer present (Fig. 2C). Accordingly, correlation analysis of neutrophils at the site of the biopsy with the time lag from biopsy to the surgical excision of the tumor indicated that the presence of neutrophils decreased with time lag (p =0.0086) (Fig. 2C). Thus, although neutrophils are most likely recruited to the site of the biopsy, they do not persist within the tumor.
Figure 2. Neutrophils are not frequently detected at the region adjacent to the biopsy wound.
(A) H&E staining of human breast tissue in the region adjacent to (adjacent) and a region far away (distant) from to the biopsy wound. The images from 40x magnification (upper panels) and 400x magnification (lower panels) of the 40x-red labeled area are shown. Scale bars are shown. BW, biopsy wound. (B) Percentage of neutrophils (relative to all nucleated cells in the analyzed fields) in the regions adjacent and distant to the biopsy wound (n=44), p=0.019 using a Wilcoxon Signed-Rank test. (C) Percentage of neutrophils in the region adjacent to the biopsy wound, as a function of days between the core needle biopsy procedure and the surgical excision of the tumor tissue (n=44), p=0.0086 using a regression analysis based on ranks.
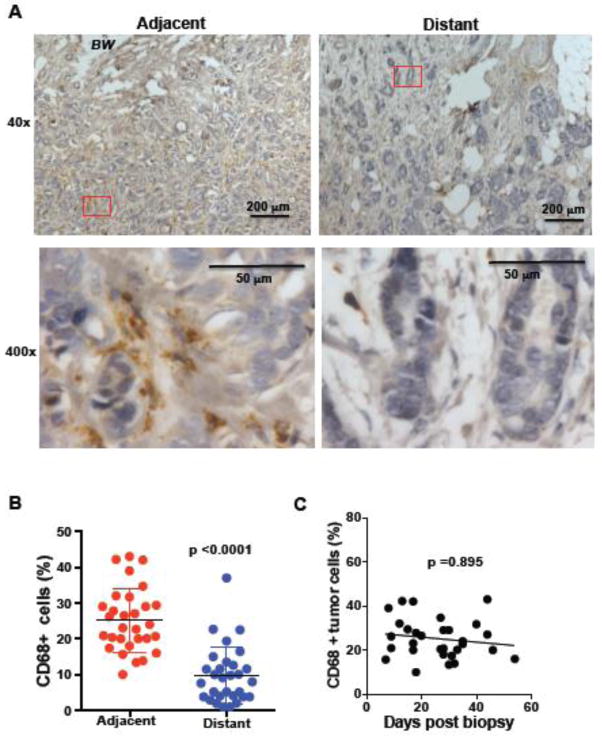
Unlike most other inflammatory cells, macrophages are commonly found in the tumor in breast cancer patients, have been defined as “tumor associated macrophages” (TAM) and are considered to be a factor that contributes to tumor progression or metastasis [20–22]. We therefore examined the presence of macrophages at the site of biopsy in our breast cancer samples by immunostaining for CD68, a standard clinical marker for macrophages. According to previous studies, macrophages could be found in the regions of the tumor distant from the biopsy (Fig. 3A and Supplementary Fig. S1B), but there was a significantly greater accumulation of macrophages at the site of the biopsy (p <0.0001) (Fig. 3A and 3B). In contrast to neutrophils, the preferential accumulation of macrophages at the region of the tumor adjacent to the biopsy lasts for longer periods of time after biopsy and no significant decrease over time could be detected (p= 0.895) (Fig. 3C). Thus, breast cancer biopsies trigger relatively rapid (observed already at 12 days after biopsy) recruitment of macrophages to the biopsy site, where they remain for long periods of time (more than 40 days).
Figure 3. Accumulation of macrophages adjacent to the biopsy wound.
(A) CD68 staining for macrophages in breast tissue in the region near (adjacent) and a region far away (distant) from to the biopsy wound. The images from 40x magnification (upper panels) and 400x magnification (lower panels) of the 40x-red labeled area are shown. Scale bars are shown. BW, biopsy wound. (B) Percentage of macrophages (relative to all nucleated cells in the analyzed fields) in the regions adjacent and distant to the biopsy wound (n=30), p<0.00001 using a Wilcoxon Signed-Rank test. (C) Percentage of macrophages in the region adjacent to the biopsy wound, as a function of days between core needle biopsy and surgical excision of the tumor tissue (n=30), p=0.895 using a regression analysis based on ranks.
Recruitment of eosinophils to the site of the biopsy wound in human breast cancer
While studying the H&E tissue slides, we identified an unexpected immune cell type that is well characterized by its bi-lobular nucleus and by the eosinophilic cytoplasm - eosinophils (Fig. 4A and Supplementary Fig. S1C). No studies have reported the presence of eosinophils within the tumor in breast cancer patients. Accordingly, we could not find eosinophils in the regions distant to the biopsy (Fig. 4B). In contrast, there was a pronounced accumulation of eosinophils at the region of the tumor adjacent to the biopsy wound (p <0.0001) (Fig. 4B). Interestingly, eosinophils were detected primarily at the edge of the biopsy wound (Fig. 4A). These results indicate that breast cancer biopsies trigger the recruitment of eosinophils specifically at the site where the biopsy was taken. In addition, we found that the presence of eosinophils at the site adjacent to the biopsy correlated with the time lag between the biopsy and the surgical excision of the tumor (p= 0.019) (Fig. 4C). To address whether the greater accumulation of eosinophils at the biopsy wound site (Fig. 4A) was preferentially found in those patients with the longer time lag to surgery, we defined two groups according to the time: a) a group where surgery was performed within the month after the biopsy (< 30d) (, b) a group where surgery was performed 30 days or more after the biopsy. The average number of eosinophils at the biopsy wound in the ≥30d group was about 4-fold higher than the average eosinophils in the <30d group (p= 0.002) (Fig. 4D). Thus, the longer the period of time between the biopsy and the surgical excision of the primary tumor, the higher the recruitment of the eosinophils to the biopsy wound occurred.
Figure 4. Core needle biopsy of breast tumors trigger the recruitment of eosinophils to the biopsy wound.
(A) H&E staining of human breast tissue in the region adjacent to the biopsy (Adjacent) and a region far away (Distant) from to the biopsy wound. The images from 40x magnification (upper panels) and 400x magnification (lower panels) of the 40x-red labeled area are shown. Scale bars are shown. BW, biopsy wound. (B) Percentage of eosinophils (relative to all nucleated cells in the analyzed fields) in the regions adjacent and distant to the biopsy wound (n=43). p<0.0001, using a paired student’s t test analysis. (C) Percentage of eosinophils in the region adjacent to the biopsy wound, as a function of days between the core needle biopsy procedure and the surgical excision of the tumor tissue (n=43), p=0.019 using a regression analysis. (D) Percentage of eosinophils in the region adjacent to the biopsy wound in patients where surgical excision was performed within < 30 days (n= 29) or ≥ 30 days (n= 14) of the biopsy, p=0.013 using a Wilcoxon Rank Sums test. (E) Correlation of percentage of eosinophils and CD45+ cells in the < 30 days and ≥ 30 days groups. Within the ≥30d group ρ = 0.52 (p for simple effects= 0.0017), and within the <30d group ρ = −0.038 (p for simple effects= 0.91), as determined by regression analysis, including the time-by-CD45+ level interaction as a predictor (p for interaction=0.009).
We then investigated whether the accumulation of eosinophils at the site of the biopsy could be associated with the magnitude of the inflammatory response or whether it was determined by individual differences in the type of immune response. We examined the interaction of eosinophil accumulation and overall inflammation determined by the presence of CD45 cells at the site adjacent to the biopsy wound (p for interaction= 0.009). Within the ≥30d group, the accumulation of eosinophils correlated with the overall presence of CD45 cells (p= 0.0017) (Fig. 4E). In contrast, within the <30d group the presence of eosinophils at the biopsy wound was independently variable from the magnitude of the inflammatory response (p= 0.91) (Fig. 4E) indicating that individual difference in the immune response may account for the presence of eosinophils in cases where these cells are detected relatively early after the biopsy.
Higher rate of proliferation of the breast cancer cells at the site of the biopsy compared with the rate at a distant site of the tumor
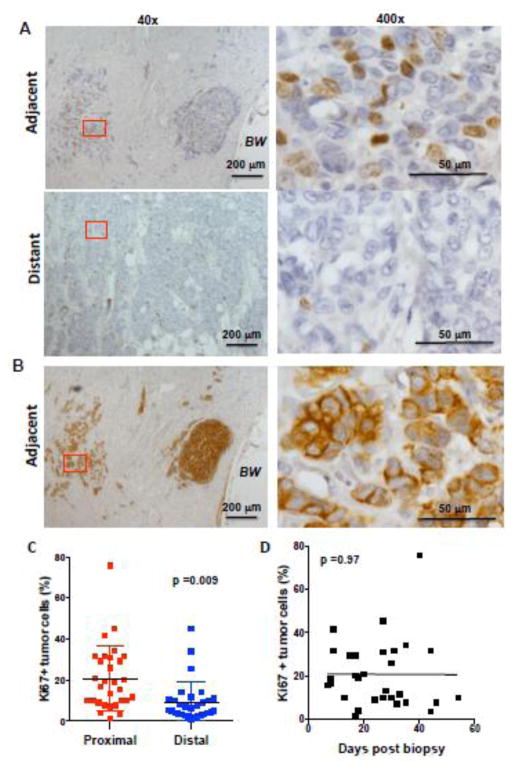
To investigate whether diagnostic core biopsy could have an effect on the tumor cells that remain in the proximity of the biopsy site, we performed immunostaining for Ki67 (Fig. 5A), a standard maker for cell proliferation characterized by its nuclear accumulation in proliferating cells (Supplementary Fig. S2A). Identification of tumor cells by H&E staining was also verified by immunostaining for cytokeratin (AE1/AE3), a marker clinically used to identify epithelial origin of cells (Fig. 5B and Supplementary Fig. S2B). The results showed higher frequency of Ki67 positive tumor cells in the proximity of the biopsy wound compare to the frequency in areas of the tumor distant to the biopsy (p=0.009) (Fig. 5C). We also investigated the rate of proliferation at the site of the biopsy over time from the core needle biopsy to surgical excision. High rate of proliferation at the site of the biopsy could be observed at early times post-biopsy and longer lag time did not further increase the proliferation rate (p= 0.97) (Fig. 5D). These results suggested that biopsies could have an impact in the tumor surrounding the biopsy wound.
Figure 5. Higher frequency of proliferating tumor cells at the region adjacent to the biopsy wound than in distant areas of the tumor.
(A) IHC of human breast tissue for Ki67 marker in the region adjacent to the biopsy wound (adjacent) and the region distant to the biopsy wound (distant). The images from 40x magnification (left panels) and 400x magnification (right panels) of the 40x-red labeled area are shown. Scale bars are shown. BW, biopsy wound. (B) IHC of the same tissue sample from (A) with AE1/AE3 antibody as a marker for cytokeratin in the region adjacent to the biopsy (adjacent). The images from 40x magnification (left panels) and 400x magnification (right panels) of the 40x-red labeled area are shown. (C) Percentage of Ki67 positive cells (relative to all cancer cells present in the analyzed fields) in invasive tumor in breast cancer samples in regions adjacent and distant to the biopsy wound (n= 30), p=0.009, using a paired student’s t test analysis. (D) Percentage of Ki67 positive tumor cells in the tumor in the region adjacent to the biopsy as a function of time (days) between the core needle biopsy procedure and surgical excision of the breast tissue (n=30), p=0.97, using a regression analysis.
Breast cancer core biopsy increases proliferation rate of the tumor cells adjacent to the biopsy wound
To investigate whether the higher rate of proliferation in tumor cells found at the site of the biopsy wound was caused by the diagnostic core biopsy, we examined the proliferation of the tumor cells within the original core needle biopsy (pre-excision) in the same cohort of patients by Ki67 immunostaining (Fig. 6A and Supplementary Fig. S3). We compared the frequency of proliferating tumor cells within the core needle biopsy specimen (the area of the tumor where the biopsy was obtained but prior biopsy excision) and the frequency of proliferating tumor cells in an area of the excised tumor distant from the biopsy (post-surgical excision). No significant difference (p= 0.18) was observed between the frequency of Ki67 positive tumor cells in the core needle biopsy and the frequency in a distant region of the tumor (Fig. 6B). Thus, the proliferation rate in the tumor adjacent to the biopsy wound prior to the biopsy was comparable to the proliferation rate in areas distant from the biopsy after surgical excision. We then compared the frequency of proliferating tumor cells in an area of the tumor adjacent to the biopsy wound with the frequency of proliferating cells within the core biopsy tissue. The results revealed an increased proliferative rate in the areas adjacent to the biopsy wound relative to the rate found in the core needle biopsy itself (p< 0.0001) (Fig. 6C). Thus, core needle biopsies have an impact on the proliferation rate of the tumor cells that remain at the site of the tumor.
Figure 6. Increased proliferative rate of tumor cells in the area adjacent to the biopsy wound is secondary to the biopsy.
(A) IHC of human breast tissue for Ki67 marker in the breast cancer core needle biopsy (Core Biopsy) and in the region adjacent to the biopsy wound after surgical excision (Adjacent). The images from 400x magnification are shown. Scale bars are shown. BW, biopsy wound. (B) Percentage of Ki67 positive tumor cells (relative to all cancer cells present in the analyzed fields) in the original core biopsy tissue (pre-biopsy) compared with the percentage in a tumor region distant to the biopsy wound in the excised tumor (distant region in post-biopsy). (C) Percentage of Ki67 positive tumor cells in the original core needle biopsy compared with the percentage in a tumor region adjacent to the biopsy wound in the excised tumor (post-biopsy samples (n=25), p<0.0001 using a paired student’s t test analysis.
DISCUSSION
A number of studies have shown an association between chronic inflammation and cancer development, including breast cancer [9, 23]. Recently, specific inflammatory markers have been singled out, such as tumor associated macrophages (TAMs), matrix metalloproteinases (MMPs), sphingosine 1-phosphate (S1P), and C-reactive protein (CRP), as well as IL6 and IL8. Importantly, novel therapies targeting TAM (CSF-1R inhibitors) are currently being tested in clinical trials for glioblastoma and Hodgkin lymphoma [24, 25]. In contrast, the role of acute inflammation within the tumor on cancer progression and metastasis has not been extensively addressed in mouse models or in human breast cancer patients. The limited interest in addressing this question is probably due to the fact that acute inflammatory responses in tumors are not frequent in a natural setting. However, invasive diagnostic and therapeutic procedures in the tumor can cause acute inflammatory responses in the remaining tumor cells. In this study, we have investigated the immune response in breast tumors triggered by core needle biopsies. It is generally believed that core needle biopsies do not cause marked inflammatory response in the tumor microenvironment [26]. However, our current study shows a clear accumulation of CD45 positive cells selectively in the proximity of the biopsy wound. Interestingly, in a wound healing response in normal tissue inflammation does not last more that 7–14 days, but inflammation at the biopsy wound in breast cancer can be detected even up to 40 days after the procedure. It is possible that once the inflammatory cells are recruited to the biopsy wound, survival signals generated by the tumor microenvironment retain some of the inflammatory cells at the wound.
With a few exceptions, no neutrophils were identified at the area adjacent to the biopsy wound. This is probably due to the fact that neutrophils are one of the first inflammatory cells recruited during the wound healing response to scavenge debris and bacteria, as well as secrete chemokines that promote the recruitment of macrophages and lymphocyte to the wound. Since in many cases the lag time between biopsy and surgical excision is more than 7 days, the initial neutrophil infiltrate is probably resolved by the time of surgery. However, recruitment of neutrophils at the site of the biopsy within breast tumor could have an impact in potential metastasis. Neutrophils have been associated with poor outcome in breast cancer [27] and they have been shown to contribute to tumor angiogenesis [28]. Recent studies in mouse models have shown that neutrophils contribute to the spread of melanoma cells and lung metastasis after exposure of melanoma to ultraviolet radiation [29].
Unexpectedly, our studies reveal a frequent accumulation of eosinophils at the area adjacent to the biopsy wound in the breast tumor. Eosinophils are not commonly found during a normal immune response against pathogens with the exception of helminthes [30]. They are abundant in the context of specific pathological situations and are hallmark signs of allergy, asthma and eosinophilic gastrointestinal disorders [31]. Importantly, after tissue injury, eosinophils are part of the inflammatory response during wound healing and contribute to tissue regeneration by secretion of a variety of cytokines that store in granules [32]. Little is known about eosinophils in breast cancer, and while prior studies have reported the presence of mast cells in the stroma of invasive breast cancer, previously no eosinophils were found [33]. However, eosinophils are also known to be essential in mammary gland development, including angiogenesis [9]. Our results here further confirm the absence of eosinophils in breast cancer except the area adjacent to the biopsy wound. Interestingly, within the area adjacent to the biopsy wound, eosinophils primarily accumulate at the edge of the wound. Considering the established role of eosinophils in normal wound healing and angiogenesis, most likely eosinophils are recruited at the site of biopsy for tissue repair. Thus, eosinophils may affect the proliferative and/or migratory activity of tumor cells. Interestingly, not all patients have eosinophils at the biopsy site, posing the question whether individual variations in the immune response may account for the differential presence of these cells at the biopsy site. Similar to other immune-related disorders such as the asthma or allergy conditions, there may be specific individuals who develop this type of response. Detection of eosinophils at the site of the biopsy wound is a novel finding that needs further investigation to determine a potential correlation with prognosis.
The risk that core biopsies of breast cancer may have on breast cancer progression or recurrence is still a controversial topic [13–15]. Our results revealed an increased frequency of proliferating tumor cells in the proximity of the biopsy wound compared to the distant areas in the tumor. More importantly, this increased frequency of proliferating cells is exclusively identified in the surgical specimen and not observed in the diagnostic core biopsy, indicating that it is a consequence of the core biopsy procedure. In addition, we show the presence of a prolonged inflammatory response that, as part of normal wound healing, can affect the migratory capacity of tumor cells and, consequently, may have an impact on local or metastatic recurrence. These findings may have implications for the clinical management of breast cancer. The effect of surgery (i.e. surgery-induced inflammation) and wound healing in breast cancer and metastasis, as well as its clinical implications are emerging as important areas of consideration [11, 12]. Certain patients undergoing a core needle biopsy for the diagnosis of breast cancer may be at risk of accelerated tumor migration as a result of the acute inflammatory process and changes in the tumor microenvironment. This may only hold true for a subset of patients with a specific immune response to acute inflammation, and those who have a longer time interval between diagnosis and treatment (e.g. surgical excision or chemotherapy). Furthermore, these findings may have implications for patients undergoing breast-conserving surgery who are found to have positive margins/residual tumor following an initial partial mastectomy procedure. If, as suggested by the findings of this study, residual tumor cells are at greater risk for metastasis due to changes in the tumor microenvironment resulting from invasive procedures, then both the time to therapy and the completeness of initial surgical excision may take on even greater clinical importance in the management of breast cancer.
Using mouse models, we have shown that a biopsy of mammary tumor increases the proliferation of cancer cells at the site of the biopsy wound, and drastically increases the risk of lung metastases [19]. Furthermore, we have shown in the same mouse model that treatment with anti-inflammatory medication (ibuprofen) at the time of the biopsy and for three subsequent days decreases the risk of metastases following biopsy procedures [19]. Thus, the inflammation associated with biopsies appears to contribute to the risk of metastases in mouse models. The presence of inflammatory cells at the biopsy site could affect the proliferative or migratory capacity of the tumor cells by secreting cytokines, chemokines or factors that alter the matrix in the stroma. In this regard, our previous research observed that the number of metastases triggered by acute inflammation is substantially lower in IL-6 deficient mice [19]. The pro-inflammatory cytokine IL-6 is now emerging as a key cytokine that contributes to breast cancer progression and metastasis [34, 35], as well to the response to chemotherapy [36]. Unlike mice, the immune response in humans is highly diverse and this leads to major differences in the response to infections, vaccines and allergens. It is therefore possible that the inflammation caused by biopsies within the tumor could also have a different impact on tumor cells depending on the type or extent of inflammatory response generated by the patient. Obtaining a greater understanding of this variable immune response may shed light into the greater mechanism of acute inflammatory mediated tumor cell progression and help identify subsets of patients at greatest risk.
CONCLUSIONS
In summary, our study for the first time demonstrates that core needle biopsies trigger a lasting inflammatory response at the site of the core biopsy wound, with eosinophils being an unexpected immune cell recruited to the tumor microenvironment. In addition, our studies also reveal a stimulatory effect on tumor cells adjacent to the biopsy site. These findings may help further uncover mechanisms of metastasis related to the acute inflammatory response in the tumor, and lead to mitigating strategies using anti-inflammatory medications at the time of diagnostic biopsy.
Supplementary Material
Supplementary Figure S1. (A and C) Enlarged images displaying the characteristic cytomorphology of neutrophils (A) and eosinophils (C) obtained by H&E staining. (B) Enlarged image of macrophages stained with anti-CD68 antibody. The images represent enlarged areas within the 400x images (adjacent) provided in Fig. 2 (neutrophils), Fig. 3 (CD68) and Fig. 4 (eosinophils). Arrows point to a representative cell within the area.
Supplementary Figure S2. Enlarged images displaying the characteristic nuclear localization of Ki67 (A) or cytoplasmic localization of cytokeratins after staining with the AE1/AE3 Ab (B). The images represent enlarged areas within the 400x images (adjacent) provided in Fig. 5. Arrows point to a representative positive cell within the area.
Supplementary Figure S3. Enlarged image displaying the characteristic nuclear localization of Ki67. The image represent an enlarged area within the 400x image (adjacent) provided in Fig. 6. Arrows point to a representative positive cell within the area.
Acknowledgments
We wish to thank the staff of the Pathology Facility at the University of Vermont Medical Center for their assistance in retrieving archives tissue samples and providing the unstaining tissue slide section. We also thank Phani Gummadidala for technical support. This work was funded by the Virginia Wellington Cabot Foundation (M.R) and Lake Champlain Cancer Research Organization (LLCRO) (T.J.).
LIST OF ABBREVIATIONS
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- CD (from CD45 or CD68)
Cluster of differentiation (cluster of differentiation 45 and cluster of differentiation 68)
Footnotes
COMPETING INTERESTS
The authors declare no conflicts of interest
AUTHOR’S CONTRIBUTIONS
G.S and A.O, contribute to the performance of experiments (e.g. immunostaining, identification of the biopsy area, manual quantification of number of cells in each area etc), the design of the study, acquisition of data, analysis and interpretation of the results, and involved in the drafting of the manuscript.
B.S and J.W. contribute to the performance of experiments (e.g. immunostaining, identification of the biopsy area, manual quantification of number of cells in each area etc), the design of the study, acquisition of data, analysis and interpretation of the results.
J.B. performed all final statistical analysis in the manuscript, and as involved in the drafting of the manuscript.
A.A. (pathologist), he identified the eligible patients, verified the identification of the biopsy wound or identified the wound, verify the results from the immunostaining, participated in the interpretation of the data, and involved in the drafting of the manuscript.
T.J. contributed to the design of the study, evaluated the results and involved in the drafting of the manuscript.
M.R. contributed the design of the study, wrote the IRB protocol, participate in the identification of the biopsy area, evaluate the immunostaining results, participate in the analysis and interpretation of the results, and did most of the writing of the manuscript.
Contributor Information
Gabriela Szalayova, Email: gabi.szalayova@gmail.com.
Aleksandra Ogrodnik, Email: Aleksandra.Ogrodnik@wchn.org.
Brianna Spencer, Email: brianna.spencer@uvm.edu.
Jacqueline Wade, Email: jacqueline.wade@uvm.edu.
Janice Bunn, Email: janice.bunn@uvm.edu.
Abiy Ambaye, Email: abiy.ambaye@uvm.edu.
Ted James, Email: ted.james@uvm.edu.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; Bethesda, MD: Apr, 2015. http://seercancergov/csr/1975_2012/, . 2015; based on November 2014 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev. 2011;25:2559–72. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125:3347–55. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatelia K, Singh K, Singh R. TLRs: linking inflammation and breast cancer. Cellular signalling. 2014;26:2350–7. doi: 10.1016/j.cellsig.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, Trinchieri G, Dubinett SM, Mao JT, Szabo E, et al. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33:335–51. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70(Suppl 1):i104–8. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 8.Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Frontiers in immunology. 2011;2:98. doi: 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coussens LM, Pollard JW. Leukocytes in mammary development and cancer. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szalayova G, James TA, Rincon M. A framework for the role of acute inflammation in tumor progression. Breast Cancer Res Treat. 2015;151:235–8. doi: 10.1007/s10549-015-3392-5. [DOI] [PubMed] [Google Scholar]
- 11.Arnold KM, Opdenaker LM, Flynn D, Sims-Mourtada J. Wound healing and cancer stem cells: inflammation as a driver of treatment resistance in breast cancer. Cancer growth and metastasis. 2015;8:1–13. doi: 10.4137/CGM.S11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceelen W, Pattyn P, Mareel M. Surgery, wound healing, and metastasis: recent insights and clinical implications. Critical reviews in oncology/hematology. 2014;89:16–26. doi: 10.1016/j.critrevonc.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Fitzal F, Sporn EP, Draxler W, Mittlbock M, Taucher S, Rudas M, Riedl O, Helbich TH, Jakesz R, Gnant M. Preoperative core needle biopsy does not increase local recurrence rate in breast cancer patients. Breast Cancer Res Treat. 2006;97:9–15. doi: 10.1007/s10549-005-6935-3. [DOI] [PubMed] [Google Scholar]
- 14.Chen AM, Haffty BG, Lee CH. Local recurrence of breast cancer after breast conservation therapy in patients examined by means of stereotactic core-needle biopsy. Radiology. 2002;225:707–12. doi: 10.1148/radiol.2253011698. [DOI] [PubMed] [Google Scholar]
- 15.King TA, Hayes DH, Cederbom GJ, Champaign JL, Smetherman DH, Farr GH, Bolton JS, Fuhrman GM. Biopsy technique has no impact on local recurrence after breast-conserving therapy. Breast J. 2001;7:19–24. doi: 10.1046/j.1524-4741.2001.007001019.x. [DOI] [PubMed] [Google Scholar]
- 16.Hoorntje LE, Schipper ME, Kaya A, Verkooijen HM, Klinkenbijl JG, Borel Rinkes IH. Tumour cell displacement after 14G breast biopsy. Eur J Surg Oncol. 2004;30:520–5. doi: 10.1016/j.ejso.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Thurfjell MG, Jansson T, Nordgren H, Bergh J, Lindgren A, Thurfjell E. Local breast cancer recurrence caused by mammographically guided punctures. Acta Radiol. 2000;41:435–40. doi: 10.1080/028418500127345884. [DOI] [PubMed] [Google Scholar]
- 18.Chao C, Torosian MH, Boraas MC, Sigurdson ER, Hoffman JP, Eisenberg BL, Fowble B. Local recurrence of breast cancer in the stereotactic core needle biopsy site: case reports and review of the literature. Breast J. 2001;7:124–7. doi: 10.1046/j.1524-4741.2001.007002124.x. [DOI] [PubMed] [Google Scholar]
- 19.Hobson J, Gummadidala P, Silverstrim B, Grier D, Bunn J, James T, Rincon M. Acute inflammation induced by the biopsy of mouse mammary tumors promotes the development of metastasis. Breast Cancer Res Treat. 2013;139:391–401. doi: 10.1007/s10549-013-2575-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–30. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–9. [PubMed] [Google Scholar]
- 22.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 23.Ham M, Moon A. Inflammatory and microenvironmental factors involved in breast cancer progression. Archives of pharmacal research. 2013;36:1419–31. doi: 10.1007/s12272-013-0271-7. [DOI] [PubMed] [Google Scholar]
- 24.Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, Cloughesy TF, Marimuthu A, Haidar S, Perry A, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro-oncology. 2015 doi: 10.1093/neuonc/nov245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Tresckow B, Morschhauser F, Ribrag V, Topp MS, Chien C, Seetharam S, Aquino R, Kotoulek S, de Boer CJ, Engert A. An Open-Label, Multicenter, Phase I/II Study of JNJ-40346527, a CSF-1R Inhibitor, in Patients with Relapsed or Refractory Hodgkin Lymphoma. Clin Cancer Res. 2015;21:1843–50. doi: 10.1158/1078-0432.CCR-14-1845. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YJ, Wei L, Li J, Zheng YQ, Li XR. Status quo and development trend of breast biopsy technology. Gland surgery. 2013;2:15–24. doi: 10.3978/j.issn.2227-684X.2013.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, Widmann WD. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–24. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 28.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–8. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Homig-Holzel C, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507:109–13. doi: 10.1038/nature13111. [DOI] [PubMed] [Google Scholar]
- 30.Ravin KA, Loy M. The Eosinophil in Infection. Clinical reviews in allergy & immunology. 2015 doi: 10.1007/s12016-015-8525-4. [DOI] [PubMed] [Google Scholar]
- 31.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer LA, Bonjour K, Melo RC, Weller PF. Eosinophil secretion of granule-derived cytokines. Frontiers in immunology. 2014;5:496. doi: 10.3389/fimmu.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amini RM, Aaltonen K, Nevanlinna H, Carvalho R, Salonen L, Heikkila P, Blomqvist C. Mast cells and eosinophils in invasive breast carcinoma. BMC Cancer. 2007;7:165. doi: 10.1186/1471-2407-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dethlefsen C, Hojfeldt G, Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat. 2013;138:657–64. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- 35.Tawara K, Oxford JT, Jorcyk CL. Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies. Cancer Manag Res. 2011;3:177–89. doi: 10.2147/CMR.S18101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conze D, Weiss L, Regen PS, Bhushan A, Weaver D, Johnson P, Rincon M. Autocrine production of interleukin 6 causes multidrug resistance in breast cancer cells. Cancer Res. 2001;61:8851–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. (A and C) Enlarged images displaying the characteristic cytomorphology of neutrophils (A) and eosinophils (C) obtained by H&E staining. (B) Enlarged image of macrophages stained with anti-CD68 antibody. The images represent enlarged areas within the 400x images (adjacent) provided in Fig. 2 (neutrophils), Fig. 3 (CD68) and Fig. 4 (eosinophils). Arrows point to a representative cell within the area.
Supplementary Figure S2. Enlarged images displaying the characteristic nuclear localization of Ki67 (A) or cytoplasmic localization of cytokeratins after staining with the AE1/AE3 Ab (B). The images represent enlarged areas within the 400x images (adjacent) provided in Fig. 5. Arrows point to a representative positive cell within the area.
Supplementary Figure S3. Enlarged image displaying the characteristic nuclear localization of Ki67. The image represent an enlarged area within the 400x image (adjacent) provided in Fig. 6. Arrows point to a representative positive cell within the area.