Patients with immunoglobulin G4–related disease (IgG4-related disease) can present to any clinical specialty. It is an uncommon, immune-mediated, fibroinflammatory process that can progress to organ failure and death if untreated.
Presentation is protean and can mimic other conditions, including systemic diseases (e.g., sarcoidosis), single-organ disorders (e.g., primary sclerosing cholangitis) and malignant disease (i.e., hematologic [lymphoma] and mass lesions [pancreatic cancer]). However, IgG4-related disease is eminently manageable; early treatment prevents organ damage and long-term morbidity. Nevertheless, recognition of IgG4-related disease is challenging and requires clinicians to have an awareness of the disease.
We provide an overview of current understanding of the cause, pathogenesis, diagnosis and management of IgG4-related disease, and we outline challenges to be addressed to improve care for patients (Box 1).
Box 1: Search strategy.
We searched publications in MEDLINE and PubMed from 2001 onwards (when the multisystem nature of the disease was recognized) using the key phrases “IgG4,” “IgG4-related,” “IgG4-associated,” “IgG4 pancreatitis” and “autoimmune pancreatitis.” Recommendations are based on information from international consensus guidelines.
What is IgG4-related disease?
Immunoglobulin G4–related disease is a systemic immune-mediated fibroinflammatory disease that presents as organ dysfunction or mass lesions with lymphoplasmacytic infiltration in single or multiple organs. It can result in organ failure or death if untreated. This disease has been recognized as a distinct clinical entity since the beginning of the 21st century,1 when investigators in Japan reported that extrapancreatic manifestations of autoimmune sclerosing pancreatitis shared a distinct histopathologic signature with the parent disease.2 Since then, the histologic features of infiltrative IgG4-positive plasma cells, storiform fibrosis and obliterative phlebitis have been reported in almost every organ (Table 1)3–14 and share similar features with apparently unrelated pathologic entities, such as dacryoadenitis (Mikulicz disease) to retroperitoneal fibrosis (Ormond disease).
Table 1:
Manifestations of IgG4-related disease in different organ systems
| Organ system | Common clinical presentation | Preferred name3 |
|---|---|---|
| Pancreas | Painless obstructive jaundice and endocrine failure (secondary diabetes mellitus); frequently associated with IgG4-related sclerosing cholangitis4 | Type 1 autoimmune pancreatitis* |
| Lacrimal and salivary glands | Mikulicz disease:5 bilateral dacryoadenitis, and enlargement of the parotid and submandibular glands with associated xerophthalmia and xerostomia Küttner tumour/chronic sclerosing sialadenitis:6 hard indurated masses of submandibular (or parotid) glands; associated xerostomia is common |
IgG4-related dacryoadenitis and IgG4-related sialadenitis |
| Orbits | Proptosis due to dacryoadenitis, local myositis or orbital pseudotumours; scleritis, uveitis and locoregional neuronal damage can occur from mass effect7,8 | IgG4-related ophthalmic disease |
| Lungs | Dyspnea, wheeze, cough Mild stridulous symptoms can be associated with upper respiratory tract (e.g., pharynx, trachea) inflammation; often diagnosed incidentally on imaging9 |
IgG4-related lung disease |
| Thyroid gland | Riedel thyroiditis:10 stony goiter — usually euthyroid or subclinical hypothyroid profile | IgG4-related thyroid disease |
| Lymph nodes | Nontender generalized lymphadenopathy or localized disease near other affected organs; often asymptomatic and diagnosed incidentally on imaging | IgG4-related lymphadenopathy |
| Arterial system | Aortitis of either the thoracic or abdominal aorta; aneurysmal disease can present with pain or vascular insufficiency but can also be acute and catastrophic.11 Coronary arteritis may be associated with ischemic heart disease12 | IgG4-related aortitis or periaortitis |
| Retroperitoneum | Ormond disease: poorly localized lower back pain and chronic renal failure Ureteric obstruction, hydronephrosis and renal failure | IgG4-related retroperitoneal fibrosis |
| Kidneys | Diffuse renal enlargement and chronic renal failure;13 commonly, tubulointerstitial nephritis associated with a profound hypocomplementemia Proteinuria due to coexistent glomerulonephritis (membranous) is common |
IgG4-related kidney disease |
| Biliary tree | Jaundice, weight loss and abdominal pain that closely mimicks PSC or cholangiocarcinoma; associated cholecystitis is usually asymptomatic | IgG4-related sclerosing cholangitis |
| Meninges | Headache, nerve palsies and radiculomyelopathy | IgG4-related pachymeningitis |
Note: PSC = primary sclerosing cholangitis. Other involvement includes IgG4-related skin disease, prostatitis, mastitis, mesenteritis, mediastinitis and hypophysitis.
Should not be confused with type 2 autoimmune pancreatitis. Type 2 autoimmune pancreatitis is also called idiopathic duct centric pancreatitis and is not related to IgG4-RD, affects younger patients with no sex preponderance,14 is less common than type 1 but is also treated with steroids.
What pathophysiologic mechanisms are involved in IgG4-related disease?
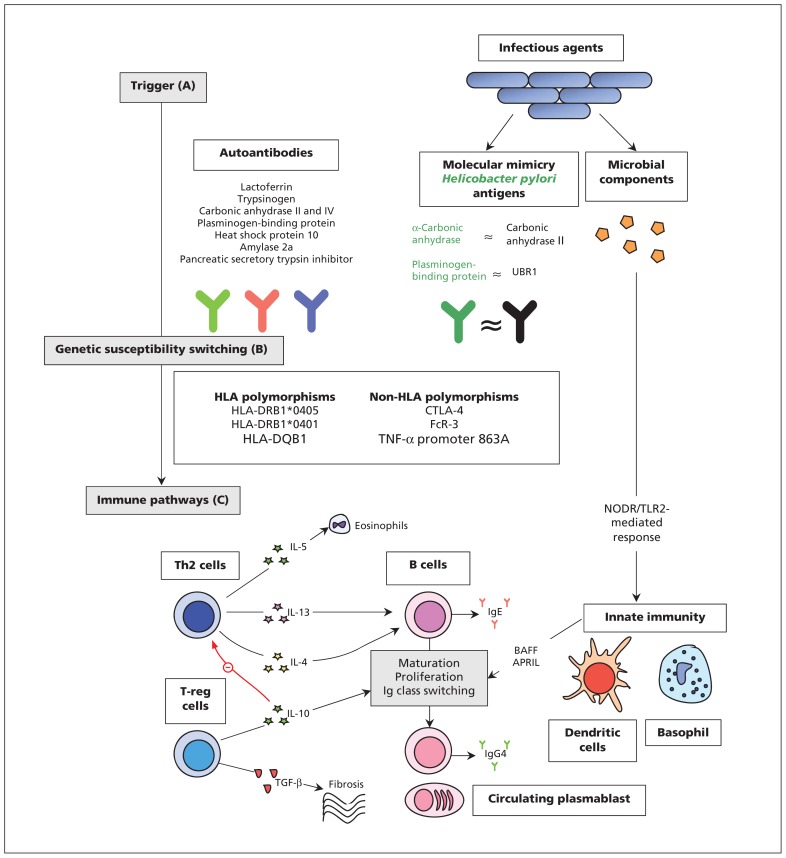
The pathophysiology of IgG4-related disease is uncertain. Both genetic predisposition and environmental triggers may prompt aberrant immune pathways to perpetuate the disease.15 An overview of current understanding of the disease is shown in Figure 1.15–23
Figure 1:
Mechanisms of pathogenesis for IgG4-related disease. (A) Antibodies versus antigens commonly found in exocrine organs may drive Th2-cell response. Most have only been investigated in type 1 AIP, are nonspecific, and none have been consistently found in active disease.16–18 Molecular mimicry of Helicobacter pylori antigens with human counterparts may be a trigger for type 1 AIP.19,20 Microbial components may stimulate innate immune mechanisms by activating NODR and TLR2 to produce BAFF and APRIL which lead to changes to B cells in a T cell–independent manner.21 (B) Polymorphisms of both HLA and non-HLA antigens have been implicated in the development of type 1 AIP.15 (C) A dominant Th2-cell (and associated cytokine) response occurs systemically and within affected organs. An expansion in T-reg cells may contribute to both B-cell Ig class switching and fibrosis.22 A treatment-sensitive expansion in circulating plasmablasts is present in active disease,23 although their exact role in pathogenesis remains unclear. Elevated levels of IgG4 in serum is a hallmark of the disease and is a consequence of a modified Th2-cell response.15 Note: AIP = autoimmune pancreatitis, APRIL = a proliferation-inducing ligand, BAFF = B cell–activating factor belonging to the TNF family, B cell = beta cell, HLA = human leucocyte antigens, Ig = immunoglobulin, NODR = nucleotide-binding oligomerization domain receptor, T cell = T lymphocyte cell, TGF = transforming growth factor, Th2 = type 2 T helper, TLR2 = toll-like receptor 2, T-reg = regulatory T cell, TNF = tumour necrosis factor, UBR1 = ubiquitin-protein ligase E3 component n-recognin 1.
The pathology seems to be primarily affected by B cells. Patients with IgG4-related disease carry dominantly expanded clones of tissue B cells that produce immunoglobulins with greater antigen affinity and, preferentially, make more IgG4. However, the pathogenic role of IgG4 remains a contentious issue in view of its immune-modulating properties.15,24 Expansion of regulatory T cells is observed,22 which may contribute to fibrosis. Furthermore, a modified type 2 T-helper (Th2) cellular response25 augments B-cell changes.26,27 Maturation of B cells may also be directly stimulated by microbial components.28
Certain serotypes of human leukocyte antigens (HLA) and non-HLA polymorphisms of immune-related genes may confer genetic predisposition to type-1 autoimmune pancreatitis, the pancreatic manifestation of IgG4-related disease.29–32 However, the trigger mechanisms remain elusive. Molecular mimicry of Helicobacter pylori antigens with human counterparts19 may act as a trigger — most patients with autoimmune pancreatitis have antibodies against the plasminogen-binding protein of H. pylori20 — although this theory still requires confirmation. Only half of patients with autoimmune pancreatitis express autoantibodies.16,33 (Figure 1).
How does IgG4-related disease present?
A detailed understanding of the geoepidemiology of IgG4-related disease will require the introduction and widespread use of easily accessible diagnostic tools or biomarkers. Studies of autoimmune pancreatitis involving patients in Japan showed that it is an uncommon disease, with an incidence and prevalence of 0.14 and 0.46 per 1 000 000 population, respectively.15,34 Patients usually present between the ages of 50 and 70 years and are more likely to be men (3:1).35 However, this can vary between disease sites: a sex ratio of 1:1 has been reported for disease occuring in the head and neck.36,37
IgG4-related disease can present as a consequence of organ involvement anywhere in the body. Therefore, it is within the remit of all adult medical and surgical specialties, although the organs most frequently involved are the pancreas, salivary and lacrimal glands, biliary tree, kidneys, thyroid and lungs.38
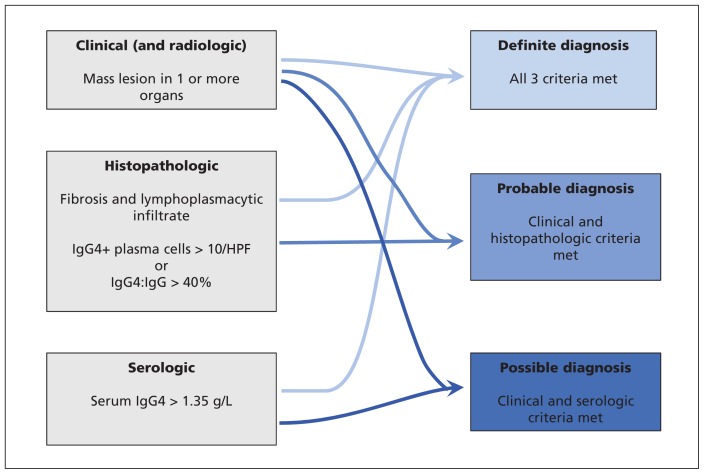
The rarity, diverse presentation and lack of a gold standard test often result in delayed diagnosis, usually after investigation for malignant growths has occurred.39,40 Thus, an understanding of the clinical, radiologic, laboratory tests (in particular serum concentrations of IgG4) and a focused histopathology examination are required to ensure that IgG4-related disease has been considered in the differential diagnosis.41,42 Organ-specific guidelines, such as the HISORt (histology, imaging, serology, other organ involvement, response to steroid therapy) criteria for IgG4-related sclerosing cholangitis, facilitate this multimodal diagnostic approach but may blind specialists to clinical manifestations outside their area of interest.41 In recognition of this, an international panel of experts described comprehensive consensus recommendations to aid clinicians in the management and treatment of IgG4-related disease (summarized in Figure 2).43,44 The evolving nature of this novel disease means the research to guide the consensus was limited, and recommendations to instruct a diagnostic approach were supported by expert opinion and limited data. Organ-specific diagnostic criteria, such as those for IgG4-related pancreatitis, cholangiopathy, kidney disease and dacryoadenitis, should be used concurrently (Table 1 and Figure 2).
Figure 2:
Diagnostic criteria for IgG4-related disease. HPF = high-powered field. (Adapted from Khosroshahi A, Wallace ZS, Crowe JL, et al. Arthritis Rheumatol 2015;67:1688-99).43
Signs and symptoms
Systemic symptoms can develop insidiously over several months; asthenia (26%) and weight loss (21%) were common in the patient populations of studies conducted in France45 and China46. Although most patients with IgG4-related disease have multiorgan involvement, 40% of patients have single-organ involvement at the time of diagnosis.38
Abdominal symptoms are common: in a review of relevant articles published between Jan. 1, 2000, and Nov. 1, 2014, Stone and colleagues reported that pain, jaundice and diarrhea occurred in 40%, 23% and 6% of patients, respectively.47 Sicca syndrome and respiratory symptoms were reported in 13%–15% of patients involved in the studies conducted in France and China.45,46 Stone and colleagues reported that patients with head and neck disease usually presented with organ swelling, and they noted that swelling of the salivary and lacrimal glands, and lymphadenopathy were common sentinel signs.47
Investigations
Routine blood tests
Routine blood tests may direct attention to the organs involved (e.g., abnormal results for liver function tests would prompt focused investigation of the liver and pancreas). However, such testing gives little indication to suggest an underlying diagnosis of IgG4-related disease. A study in Massachusetts involving patients with IgG4-related disease reported elevated counts for peripheral eosinophilia in 27% of these patients (mean 1062 cells/μL; normal count < 500 cells/μL).48 Elevated levels of inflammatory markers (i.e., C-reactive protein and erythrocyte sedimentation rate) were reported in one-fifth of cases in a case series study conducted in Japan.49
Immunologic blood tests
Stone and colleagues reported that polyclonal hypergammaglobulinemia was detected in the sera of 80% of patients and an elevated level of IgE in 60% of patients.47 One-third of patients have hypocomplementemia; this finding may be particularly associated with IgG4-related kidney disease,13 presumably as a consequence of proteinuria. A systematic review that examined the clinical features of patients with a diagnosis of IgG4-related disease showed that only 32% and 20% of patients had detectable antinuclear antibodies and rheumatoid factor, respectively.47,50 The presence of specific autoantibodies should prompt consideration of alternative diagnoses.47
Serum IgG4 levels are elevated in as many as 84% of patients47,50 with IgG4-related disease, and this is an important diagnostic tool; however, clinicians should be aware of the limitations of the test.51 The reported sensitivity (80%–90%) of an elevated level of IgG4 in serum is likely to be inflated by selection bias.51,52 In a study that required histologic proof of IgG4-related disease as an inclusion criterion, only 50% of the patients had elevated levels of serum IgG4 before treatment.53 Elevated levels of IgG4 in serum can occur in many other conditions, including some pancreatobiliary diseases (e.g., primary sclerosing cholangitis, cholangiocarcinoma), hematologic malignant disease and infectious diseases (e.g., chronic sinusitis, recurrent pneumonia, aspergillosis).51
An elevated IgG4 level in serum (upper limit of normal = 1.35 g/L) has low specificity and a positive predictive value under 40%.52 Nevertheless, higher serum concentrations may suggest a positive diagnosis (mean = 6.70 g/L) over alternatives;51 a threshold of four times the upper limit of normal provides a positive predictive value of 100% for the investigation of IgG4-related sclerosing cholangitis.52 For moderately elevated serum IgG4 concentrations (up to 2 times the upper limit of normal; positive predictive value = 28%), the IgG1:IgG4 ratio in serum (> 0.24) may improve diagnostic yield for IgG4-related sclerosing cholangitis (positive predictive value = 55%; negative predictive value = 90%), although the use of IgG1:IgG4 ratios in serum has not been validated outside this setting.52
Higher concentrations of IgG4 in serum are associated with multiorgan involvement (mean concentration: 6.99 g/L in multiorgan v. 2.33 g/L in single-organ disease) or a greater burden of localized disease that is prone to relapse after initial treatment.51,54,55
Imaging studies
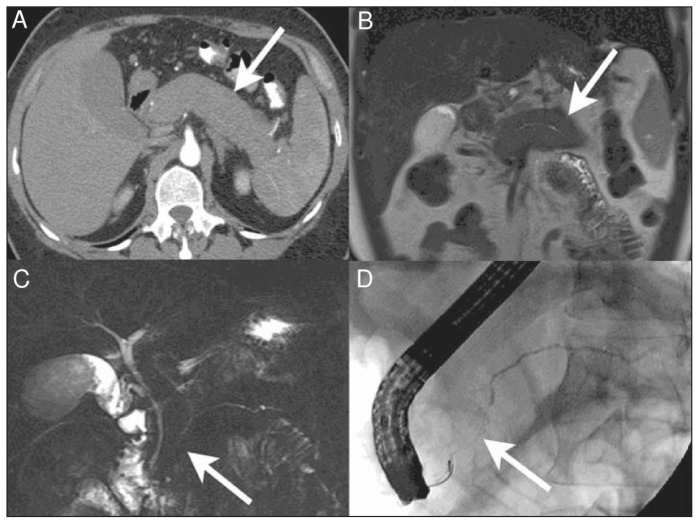
Computed tomography (CT) may delineate a mass and identify the extent of multiorgan involvement; however, the assessment of multiorgan involvement may be better served by 18F-fluorodeoxyglucose positron emission tomography/CT.56 Magnetic resonance imaging of affected areas generates a low signal on T2-weighted imaging.57 An overview of the typical radiographic findings in different organs is described in Table 2.8,9,58–67 Of these, only the classical CT findings of autoimmune pancreatitis (i.e., a diffusely enlarged, sausage-shaped pancreas with an enhancing rim; Figure 3)68 are pathognomonic, and may forego the need for histologic assessment44 (Figure 3 and Table 2).
Table 2:
Radiographic findings in IgG4-related disease
| Organ system | Typical findings |
|---|---|
| Pancreas58 | CT: focal (more common) or diffuse pancreatic enlargement with delayed enhancement and a low-density “halo”; pancreatic atrophy is uncommon Cholangiography: diffuse irregular narrowing of the pancreatic duct |
| Salivary gland | CT: swelling is often bilateral and preferentially involves the submandibular glands US:59 multiple hypoechoic lesions in affected glands MRI:60 homogenous enhancement in hypointense or isointense T2-weighted imaging |
| Orbits61 | CT: involvement of any surrounding structures including lacrimal glands, nerves, extraocular muscles and maxillary and frontal bony structures |
| Lungs9 | CT: four major categories of findings (solid nodular masses, localized ground glass opacities, diffuse ground glass opacities associated with honeycomb lung and bronchovascular thickening); mediastinal lymphadenopathy is common Diffuse tracheal inflammation and subglottic stenoses may also be seen |
| Arterial system62 | CT: adventitial sclerosing inflammation characterized by diffuse wall thickening and late-phase enhancement |
| Retroperitoneum63 | CT: perivascular fibrosis concentrates around the aorta, iliac vessels and vena cava. Occasionally, fibrotic disease will dominate perirectal and retrovesicular spaces. Associated lymphadenopathy and aortitis is common. |
| Kidneys64 | CT: abnormalities noted in 70% of patients with renal disease include bilateral diffuse enlargement, solitary nodules and atrophy MRI: low-density lesions with T2-weighting hypointensity, with progressive enhancement pattern |
| Biliary tree | Cholangiography: stricturing disease is difficult to differentiate from other causes of sclerosing cholangitis. Common bile duct thickness of > 2.5 mm and continuous strictures can be suggestive of IgG4-RD rather than PSC;65 4 patterns of stricturing disease have been described, but the clinical relevance of the different types is not obvious66 Cholangioscopy: direct mucosal visualization may give diagnostic clues, although this requires thorough validation67 |
| Meninges and brain8 | CT: diffuse dural thickening or perineural masses as large as 3 cm in diameter; pituitary lesions require MRI |
Note: CT = computed tomography, IgG4-RD = immunoglobulin G4–related disease, MRI = magnetic resonance imaging, PSC = primary sclerosing cholangitis, US = ultrasonography.
Figure 3:
Diffuse autoimmune pancreatitis.68 (A) Axial computed tomography (CT) image in the pancreatic parenchymal phase of the typical enlarged and poorly enhanced pancreas in a patient with diffuse autoimmune pancreatitis68 (arrow). Note the lack of inflammatory change around the organ, which differentiates the disease from acute pancreatitis and necrosis. (B) View of the pancreas using coronal T2-weighted magnetic resonance imaging (MRI) that shows low signal intensity in the pancreas (arrow) because of the diffuse fibrosis in the gland. (C) Coronal magnetic resonance cholangiopancreatography image showing a diffusely irregular pancreatic duct with stenosis distally in the pancreatic head (arrow). (D) Endoscopic retrograde cholangiopancreatography image that confirms the MRI findings, including ductal stenosis (arrow). Images reproduced from reference 68 under Creative Commons licence 2.0 (http://creativecommons.org/licenses/by/2.0/legalcode).
Histologic assessment
The purpose of histologic assessment of affected organs is to exclude other differential diagnoses (notably malignant disease) and to provide evidence of a diagnosis of IgG4-related disease before treatment starts.43 Tissue procurement can be difficult with deep-seated disease, although modern techniques, such as endoscopic ultrasonography with fine-needle aspiration for pancreatic and biliary disease, have overcome some of these challenges. Nevertheless, needle biopsy and fine-needle aspiration often deliver insufficient tissue for assessment and can miss patchy disease.69
Gross morphologic changes are often organ specific: a tumefactive mass is common in pancreatic involvement, with diffuse enlargement of the entire organ;58 in organs such as salivary glands, discrete areas of disease are seen on a background of unaffected tissue even though no discernible difference in tissue constituency can be observed.70
Lymphoplasmacytic infiltrate laden with IgG4-positive plasma cells on a background of storiform fibrosis and obliterative phlebitis describes the distinctive histopathology that unifies the diagnosis of IgG4-related disease.71 Thus, a comprehensive and meaningful assessment of tissue in this setting will require IgG4 immunostaining. In glandular organs, the infiltrate will typically concentrate around major ductal (biliary and bronchiolar) structures and can manifest as long regions of wall thickening.71,72 Lymphocytes are prominent, with T cells distributed throughout and B cells organized in germinal centres. Although diffusely infiltrating IgG4-positive plasma cells are not specific to IgG4-related disease, they are a prerequisite for histopathologic diagnosis.43,71 High relative density of IgG4-positive plasma cells has high diagnostic utility; some researchers have adopted an IgG4:IgG plasma cell ratio of greater than 40% (50% for aortic disease73) as highly discriminatory when supported by clinical and pathologic features — an approach supported in the consensus statement on the pathology of IgG4-related disease.71
Fibrosis is an early feature of IgG4-related disease and is arranged in radial fibres that interlace in a storiform pattern throughout the tissue. This storiform pattern may be absent in IgG4-related dacryoadenitis (although fibrosis is often dense) and lymphadenopathy, making the diagnosis in isolated lymph node disease challenging.71,74
Obliterative phlebitis describes a partial or complete obliteration of medium-sized veins, which may look like an inflammatory nodule, in proximity to an unaffected artery. This is invariably present in pancreatic or submandibular disease but scarce in lacrimal gland disease.71
In addition to the classical triad, an eosinophilic infiltration is common and can be dense.72,75,76 Neutrophilic infiltration and granulomata are rare.74
The phase of the disease may determine the predominant pathologic findings and predict response to treatment. Retroperitoneal disease is often diagnosed late, and histology shows a relatively paucicellular and dense fibrosis that does not respond well to glucocorticoid therapy.77 End-stage or “burnt-out” disease of this type may manifest in different organ systems (e.g., cryptogenic cirrhosis, honeycomb lung, chronic pancreatitis).34
How is IgG4-related disease treated?
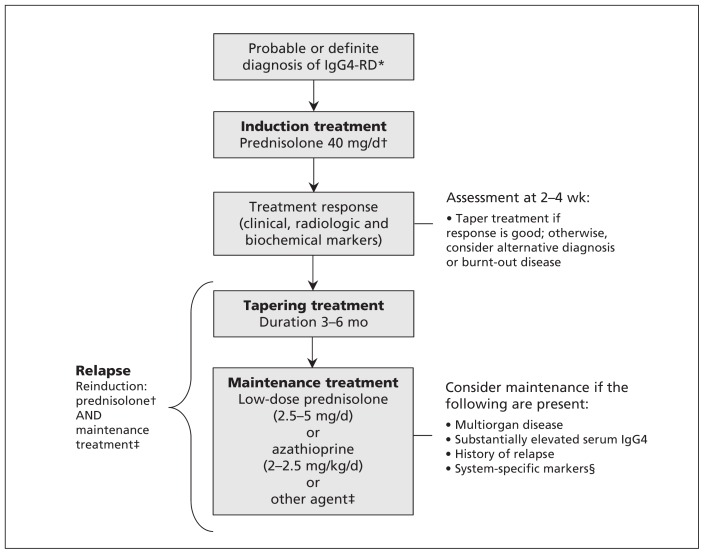
Currently, there are no robust data available to inform treatment guidelines, and recommendations are made based on expert opinion and limited data. A suggested treatment schematic based on the international consensus guidance statement on the management and treatment of IgG4-related disease is shown in Figure 4.43
Figure 4:
Treatment schematic for IgG4-related disease. *As diagnosed by international consensus guidance.43 †Consider B-cell depletion if the patient is resistant to or intolerant of glucocorticoids and therapies available to the clinician. ‡Other agents include calcineurin inhibitors, cyclophosphamide, mycophenolate mofetil and methotrexate. B-cell depletion could be considered. §Predictors of relapse in IgG4-related sclerosing cholangitis or autoimmune pancreatitis include coexisting diabetes, or a high burden of biliary involvement. Note: IgG4-RD = IgG4-related disease.
The treatment model is to induce suppression of the inflammation and maintain the disease in a quiescent state. It should be started early in patients with clinical, biochemical or radiologic evidence of major organ involvement and/or symptomatic disease.43 However, asymptomatic indolent disease in noncritical systems, such as IgG4-related lymphadenopathy, may only need monitoring.15 At the other end of the spectrum, burnt-out fibrotic disease, as in some cases of retroperitoneal fibrosis, may be resistant to available treatments.15 Occasionally, mechanical biliary drainage may be needed for urgent cases of autoimmune pancreatitis or cholangiopathy.43
Induction
IgG4-related disease is usually sensitive to glucocorticoid therapy, which is the recommended first-line treatment; a weight-based dosing regimen for prednisolone of 0.6 mg/kg body weight has been suggested in the treatment of autoimmune pancreatitis.78 A typical starting dose for prednisolone taken orally is 40 mg;43 however, a nationwide survey in Japan reported no significant differences in outcomes among patients treated with 30 or 40 mg of prednisolone.79 Glucocorticoid tapering and discontinuation can be associated with disease relapse; however, there is little consistent guidance on how to taper glucocorticoids. Centres in North America aim to stop treatment with glucocorticoids within 11 weeks,14 whereas some centres in Japan continue low-dose (5 mg) prednisolone for as much as three years.78 International consensus is to take a pragmatic approach: start to taper the drug after two to four weeks of induction dosing and aim to stop treatment within three to six months, dependent on patient response.78
Depletion of B cells by rituximab has been shown to be highly effective at inducing and maintaining remission in early phase two trials, and it may serve as an alternative for those with steroid-refractory disease.43,80 However, B-cell depletion treatment is not readily available everywhere, and some specialists have little or no experience with its use. Thus, the consensus guidance statement suggested its use should be at the discretion and comfort of the prescribing physician.43 The short- and medium-term safety of rituximab is well established for other autoimmune and hematologic malignant diseases.81–83 However, long-term safety data are lacking, and concerns remain about long-term immunosuppression in certain populations, particularly among children.84
There is little evidence to support the use of other induction agents.21
Following treatment, relapsing disease occurs in about 30%–54% of patients and requires reinduction therapy.35,78,79 The practice of adding a second agent (e.g., azathioprine, 6-mercaptopurine or mycophenolate mofetil) is still common, but there is yet little evidence to suggest a relapse-free survival advantage.85
Treatment response
At present, there are no standardized outcomes by which to measure response to treatment. Serum IgG4 levels do not reliably reflect disease activity,86 and the utility of serum IgG4-positive plasmablast concentrations23 and the IgG4-responder index87 require validation studies. Clinicians must use clinical, laboratory and radiologic markers on a case-by-case basis to gauge response to treatment.
Response is usually seen within two to four weeks.88,89 A lack of improvement within this time frame should prompt a review of the diagnostic evidence and consideration of burnt-out disease77 or other diagnoses, especially cancer.
Maintenance
The aim of maintenance treatment is to prevent recurrence of disease following remission. The risk factors for relapse are not well described, although anecdotal evidence suggests multiorgan disease, substantially elevated serum IgG4 concentrations and history of disease relapse are associated with recurrence after remission.43 A higher burden of biliary involvement or coexisting diabetes in patients with autoimmune pancreatitis or IgG4-related sclerosing cholangitis predicts a higher relapse rate.78
Studies investigating the efficacy of low-dose prednisolone (2.5–5 mg/d) treatment showed a reduction in relapse rate from 34% to 23% compared with patients who were not receiving maintenance immunosuppression.90 It is unclear how long to continue maintenance treatment with low-dose steroids, and risks related to long-term glucocorticoid treatment in older patients are not insignificant.91 These issues will need further evaluation before any firm recommendations can be made.
Several agents have been used as steroid-sparing maintenance treatment85,92,93 after remission has been achieved. The use of azathioprine (2–2.5mg/kg body weight) is suggested,94 although data to support the use of one agent over another are limited.85 The use of rituximab as maintenance treatment for patients who are at high risk of relapse has been acknowledged in the international consensus guidance statement; however, the optimal frequency and duration of treatment with the drug are subject to further evaluation,23,36,80,85,95,96 and its practical use is under the aforementioned caveats of physician preference and sparse long-term safety data (Figure 4).
Conclusion
In just over a decade since its recognition as a clinical entity, the diverse clinical presentation of IgG4-related disease has been mapped, and recent efforts have been geared to optimize its diagnosis and management. However, shortcomings in our understanding and approach persist (Box 2)23,80,87,91 and need to be addressed. Awareness of the potential for IgG4-related disease to mimic a range of disorders and an understanding of how to investigate and manage treatment in patients by the current criteria will lead to earlier treatment and better outcomes (Box 2).
Box 2: Future perspectives and unanswered questions.
Promising noninvasive diagnostic markers (e.g., flow cytometry for circulating plasmablasts) may circumvent the need for tissue diagnosis and skilled histopathologic assessment.23,80,97
There is an urgent clinical imperative for validated specific outcome measures that will allow prognosis and treatment response to be monitored. Circulating plasmablast levels may fulfil this role, and an IgG4-responder index is currently under validation.87 This will need to be linked to long-term studies to ascertain the optimal maintenance strategy for those patients who achieve remission.
The pathophysiology of IgG4-related disease is elusive, and the links between the serologic and histologic findings are unclear. Treatment response (especially with B-cell depletion) and laboratory modelling of the disease may provide invaluable insights into the disease and interplay between immune pathways in other immune-mediated diseases.
Key points
IgG4-related disease is a multisystem fibroinflammatory disorder that can present to any clinical specialty and can lead to organ failure if left untreated.
The pathophysiology is unclear but is likely to be affected by B-cell lineage with a modified type 2 T-helper cell response, supporting and contributing to fibrosis.
The diagnosis requires prior awareness of the disease and a skillful correlation of clinical, biochemical, radiologic and histopathologic clues.
IgG4-related disease is sensitive to glucocorticoid therapy, but the best way to maintain remission remains unclear.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Debashis Haldar and Gideon Hirschfield contributed substantially to conception and design of the article. All of the authors drafted the article, reviewed it critically for intellectual content, gave final approval of the version to be published and agreed to act as guarantors of the work.
Funding: Debashis Haldar was supported by a Wellcome Trust Clinical Research Fellowship Program. Debashis Haldar and Gideon Hirschfield were supported by the National Institute for Health Research (NIHR) Birmingham Liver Biomedical Research Unit. This article presents independent research funded by the NIHR. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
References
- 1.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001;344:732–8. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol 2003;38:982–4. [DOI] [PubMed] [Google Scholar]
- 3.Stone JH, Khosroshahi A, Deshpande V, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum 2012;64:3061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zen Y, Harada K, Sasaki M, et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: Do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol 2004;28:1193–203. [DOI] [PubMed] [Google Scholar]
- 5.Himi T, Takano K, Yamamoto M, et al. A novel concept of Mikulicz’s disease as IgG4-related disease. Auris Nasus Larynx 2012;39:9–17. [DOI] [PubMed] [Google Scholar]
- 6.Geyer JT, Ferry JA, Harris NL, et al. Chronic sclerosing sialadenitis (Küttner Tumor) Is an IgG4-associated disease. Am J Surg Pathol 2010;34:202–10. [DOI] [PubMed] [Google Scholar]
- 7.Ohno K, Sato Y, Ohshima K-I, et al. IgG4-related disease involving the sclera. Mod Rheumatol 2014;24:195–8. [DOI] [PubMed] [Google Scholar]
- 8.Inoue D, Zen Y, Sato Y, et al. IgG4-related perineural disease. Int J Rheumatol 2012:401890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue D, Zen Y, Abo H, et al. Immunoglobulin G4-related lung disease: CT findings with pathologic correlations. Radiology 2009;251:260–70. [DOI] [PubMed] [Google Scholar]
- 10.Dahlgren M, Khosroshahi A, Nielsen GP, et al. Riedel’s thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis Care Res (Hoboken) 2010;62:1312–8. [DOI] [PubMed] [Google Scholar]
- 11.Zen Y, Kasashima S, Inoue D. Retroperitoneal and aortic manifestations of immunoglobulin G4-related disease. Semin Diagn Pathol 2012;29:212–8. [DOI] [PubMed] [Google Scholar]
- 12.Inokuchi G, Hayakawa M, Kishimoto T, et al. A suspected case of coronary periarteritis due to IgG4-related disease as a cause of ischemic heart disease. Forensic Sci Med Pathol 2014;10:103–8. [DOI] [PubMed] [Google Scholar]
- 13.Pradhan D, Pattnaik N, Silowash R, et al. IgG4-related kidney disease — a review. Pathol Res Pract 2015;211:707–11. [DOI] [PubMed] [Google Scholar]
- 14.Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut 2013;62:1771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 2012;366:539–51. [DOI] [PubMed] [Google Scholar]
- 16.Aparisi L, Farre A, Gomez-Cambronero L, et al. Antibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: relevance for diagnosis of autoimmune pancreatitis. Gut 2005;54:703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asada M, Nishio A, Uchida K, et al. Identification of a novel autoantibody against pancreatic secretory trypsin inhibitor in patients with autoimmune pancreatitis. Pancreas 2006;33:20–6. [DOI] [PubMed] [Google Scholar]
- 18.Nishimori I, Miyaji E, Morimoto K, et al. Serum antibodies to carbonic anhydrase IV in patients with autoimmune pancreatitis. Gut 2005;54:274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guarneri F, Guarneri C, Benvenga S. Helicobacter pylori and autoimmune pancreatitis: Role of carbonic anhydrase via molecular mimicry? J Cell Mol Med 2005;9:741–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frulloni L, Lunardi C, Simone R, et al. Identification of a novel antibody associated with autoimmune pancreatitis. N Engl J Med 2009;361:2135–42. [DOI] [PubMed] [Google Scholar]
- 21.Islam AD, Selmi C, Datta-Mitra A, et al. The changing faces of IgG4-related disease: clinical manifestations and pathogenesis. Autoimmun Rev 2015;14:914–22. [DOI] [PubMed] [Google Scholar]
- 22.Zen Y, Fujii T, Harada K, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 2007;45:1538–46. [DOI] [PubMed] [Google Scholar]
- 23.Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis 2015;74:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maillette de Buy Wenniger LJ, Beuers U. Immunoglobulin G4-related cholangiopathy: clinical and experimental insights. Curr Opin Gastroenterol 2015;31:252–7. [DOI] [PubMed] [Google Scholar]
- 25.Jeannin P, Lecoanet S, Delneste Y, et al. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol 1998;160:3555–61. [PubMed] [Google Scholar]
- 26.Deshpande V, Chicano S, Chiocca S, et al. Autoimmune pancreatitis: a systemic immune complex mediated disease. Am J Surg Pathol [published erratum in Am J Surg Pathol 2007;31:328]. 2006;30:1537–45. [DOI] [PubMed] [Google Scholar]
- 27.Detlefsen S, Bräsen JH, Zamboni G, et al. Deposition of complement C3c, immunoglobulin (Ig)G4 and IgG at the basement membrane of pancreatic ducts and acini in autoimmune pancreatitis. Histopathology 2010;57:825–35. [DOI] [PubMed] [Google Scholar]
- 28.Umehara H, Nakajima A, Nakamura T, et al. IgG4-related disease and its pathogenesis-cross-talk between innate and acquired immunity. Int Immunol 2014;26:585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawa S, Ota M, Yoshizawa K, et al. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology 2002;122:1264–9. [DOI] [PubMed] [Google Scholar]
- 30.Chang M-C, Chang Y-T, Tien Y-W, et al. T-cell regulatory gene CTLA-4 polymorphism/haplotype association with autoimmune pancreatitis. Clin Chem 2007;53:1700–5. [DOI] [PubMed] [Google Scholar]
- 31.Umemura T, Ota M, Hamano H, et al. Association of autoimmune pancreatitis with cytotoxic T-lymphocyte antigen 4 gene polymorphisms in Japanese patients. Am J Gastroenterol 2008;103:588–94. [DOI] [PubMed] [Google Scholar]
- 32.Umemura T, Ota M, Hamano H, et al. Genetic association of Fc receptor-like 3 polymorphisms with autoimmune pancreatitis in Japanese patients. Gut 2006;55:1367–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okazaki K, Uchida K, Fukui T. Recent advances in autoimmune pancreatitis: concept, diagnosis, and pathogenesis. J Gastroenterol 2008;43:409–18. [DOI] [PubMed] [Google Scholar]
- 34.Kamisawa T, Zen Y, Pillai S, et al. IgG4-related disease. Lancet 2015;385:1460–71. [DOI] [PubMed] [Google Scholar]
- 35.Raina A, Yadav D, Krasinskas AM, et al. Evaluation and management of autoimmune pancreatitis: experience at a large US center. Am J Gastroenterol 2009;104:2295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto M, Yajima H, Takahashi H, et al. Everyday clinical practice in IgG4-related dacryoadenitis and/or sialadenitis: results from the SMART database. Mod Rheumatol 2015;25: 199–204. [DOI] [PubMed] [Google Scholar]
- 37.Guma M, Firestein GS. IgG4-related diseases. Best Pract Res Clin Rheumatol 2012;26:425–38. [DOI] [PubMed] [Google Scholar]
- 38.Brito-Zerón P, Ramos-Casals M, Bosch X, et al. The clinical spectrum of IgG4-related disease. Autoimmun Rev 2014;13:1203–10. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Bae H, Choi M, et al. Isolated mass-forming IgG4-related cholangitis as an initial cinical presentation of systemic IgG4-related disease. J Pathol Transl Med 2016;50:300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park HG, Kim KM. IgG4-related inflammatory pseudotumor of the renal pelvis involving renal parenchyma, mimicking malignancy. Diagn Pathol 2016;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic’s HISORt criteria. J Gastroenterol 2007;42(Suppl 18):39–41. [DOI] [PubMed] [Google Scholar]
- 42.van Heerde MJ, Buijs J, Rauws EA, et al. A comparative study of diagnostic scoring systems for autoimmune pancreatitis. Pancreas 2014;43:559–64. [DOI] [PubMed] [Google Scholar]
- 43.Khosroshahi A, Wallace ZS, Crowe JL, et al. International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheumatol 2015;67:1688–99. [DOI] [PubMed] [Google Scholar]
- 44.Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 2012;22:21–30. [DOI] [PubMed] [Google Scholar]
- 45.Ebbo M, Daniel L, Pavic M, et al. IgG4-related systemic disease. Medicine (Baltimore) 2012;91:49–56. [DOI] [PubMed] [Google Scholar]
- 46.Chen H, Lin W, Wang Q, et al. IgG4-related disease in a Chinese cohort: a prospective study. Scand J Rheumatol 2014;43:70–4. [DOI] [PubMed] [Google Scholar]
- 47.Stone JH, Brito-Zerón P, Bosch X, et al. Diagnostic approach to the complexity of IgG4-related disease. Mayo Clin Proc 2015;90: 927–39. [DOI] [PubMed] [Google Scholar]
- 48.Della Torre E, Mattoo H, Mahajan VS, et al. Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-related disease. Allergy 2014;69:269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamisawa T, Anjiki H, Egawa N, et al. Allergic manifestations in autoimmune pancreatitis. Eur J Gastroenterol Hepatol 2009;21:1136–9. [DOI] [PubMed] [Google Scholar]
- 50.Brito-Zerón P, Ramos-Casals M, Bosch X, et al. The clinical spectrum of IgG4-related disease. Autoimmun Rev 2014;13:1203–10. [DOI] [PubMed] [Google Scholar]
- 51.Carruthers MN, Khosroshahi A, Augustin T, et al. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 2015;74:14–8. [DOI] [PubMed] [Google Scholar]
- 52.Boonstra K, Culver EL, de Buy Wenniger LM, et al. Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology 2014;59:1954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in 125 patients. Arthritis Rheumatol 2015;67:2466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsubayashi H, Sawai H, Kimura H, et al. Characteristics of autoimmune pancreatitis based on serum IgG4 level. Dig Liver Dis 2011;43:731–5. [DOI] [PubMed] [Google Scholar]
- 55.Kawa S, Ito T, Watanabe T, et al. The utility of serum IgG4 concentrations as a biomarker. Int J Rheumatol 2012;2012:198314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J, Chen H, Ma Y, et al. Characterizing IgG4-related disease with 18F-FDG PET/CT: a prospective cohort study. Eur J Nucl Med Mol Imaging 2014;41:1624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toyoda K, Oba H, Kutomi K, et al. MR imaging of IgG4-related disease in the head and neck and brain. AJNR Am J Neuroradiol 2012;33:2136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sahani DV, Kalva SP, Farrell J, et al. Autoimmune pancreatitis: imaging features. Radiology 2004;233:345–52. [DOI] [PubMed] [Google Scholar]
- 59.Takagi Y, Nakamura H, Origuchi T, et al. IgG4-related Mikulicz’s disease: ultrasonography of the salivary and lacrimal glands for monitoring the efficacy of corticosteroid therapy. Clin Exp Rheumatol 2013;31:773–5. [PubMed] [Google Scholar]
- 60.Katsura M, Mori H, Kunimatsu A, et al. Radiological features of IgG4-related disease in the head, neck, and brain. Neuroradiology 2012;54:873–82. [DOI] [PubMed] [Google Scholar]
- 61.Song YS, Choung H-K, Park S-W, et al. Ocular adnexal IgG4-related disease: CT and MRI findings. Br J Ophthalmol 2013;97:412–8. [DOI] [PubMed] [Google Scholar]
- 62.Inoue D, Yoshida K, Yoneda N, et al. IgG4-related disease. Medicine (Baltimore) 2015;94:e680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khosroshahi A, Carruthers MN, Stone JH, et al. Rethinking Ormond’s disease: “idiopathic” retroperitoneal fibrosis in the era of IgG4-related disease. Medicine (Baltimore) 2013;92:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahashi N, Kawashima A, Fletcher JG, et al. Renal involvement in patients with autoimmune pancreatitis: CT and MR imaging findings. Radiology 2007;242:791–801. [DOI] [PubMed] [Google Scholar]
- 65.Tokala A, Khalili K, Menezes R, et al. Comparative MRI analysis of morphologic patterns of bile duct disease in IgG4-related systemic disease versus primary sclerosing cholangitis. AJR Am J Roentgenol 2014;202:536–43. [DOI] [PubMed] [Google Scholar]
- 66.Nakazawa T, Ohara H, Sano H, et al. Schematic classification of sclerosing cholangitis with autoimmune pancreatitis by cholangiography. Pancreas 2006;32:229. [DOI] [PubMed] [Google Scholar]
- 67.Okano N, Igarashi Y, Kishimoto Y, et al. Case of immunoglobulin G4-related cholangitis accompanying autoimmune pancreatitis: diagnosis by peroral cholangioscopy and treatment by endoscopic biliary stenting. Dig Endosc 2012;24(Suppl 1):62–6. [DOI] [PubMed] [Google Scholar]
- 68.Patel H, Khalili K, Kyoung KT, et al. IgG4 related disease — a retrospective descriptive study highlighting Canadian experiences in diagnosis and management. BMC Gastroenterol 2013;13:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morishima T, Kawashima H, Ohno E, et al. Prospective multicenter study on the usefulness of EUS-guided fine-needle aspiration biopsy for the diagnosis of autoimmune pancreatitis. Gastrointest Endosc 2016. January 14 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 70.Asai S, Sato S, Okami K, et al. Sonographic evaluation of the treatment response in patients with immunoglobulin G4-related disease of the submandibular glands. J Ultrasound Med 2015;34:783–8. [DOI] [PubMed] [Google Scholar]
- 71.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012;25:1181–92. [DOI] [PubMed] [Google Scholar]
- 72.Deshpande V, Gupta R, Sainani N, et al. Subclassification of autoimmune pancreatitis: a histological classification with clinical significance. Am J Surg Pathol 2011;35:26–35. [DOI] [PubMed] [Google Scholar]
- 73.Stone JR. Aortitis, periaortitis, and retroperitoneal fibrosis, as manifestations of IgG4-related systemic disease. Curr Opin Rheumatol 2011;23:88–94. [DOI] [PubMed] [Google Scholar]
- 74.Zen Y, Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol 2010;34:1812–9. [DOI] [PubMed] [Google Scholar]
- 75.Deshpande V, Khosroshahi A, Nielsen GP, et al. Eosinophilic angiocentric fibrosis is a form of IgG4-related systemic disease. Am J Surg Pathol 2011;35:701–6. [DOI] [PubMed] [Google Scholar]
- 76.Zamboni G, Lüttges J, Capelli P, et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch 2004;445:552–63. [DOI] [PubMed] [Google Scholar]
- 77.Shimizu Y, Yamamoto M, Naishiro Y, et al. Necessity of early intervention for IgG4-related disease — delayed treatment induces fibrosis progression. Rheumatology (Oxford) 2013;52:679–83. [DOI] [PubMed] [Google Scholar]
- 78.Kamisawa T, Okazaki K, Kawa S, et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 III. Treatment and prognosis of autoimmune pancreatitis. J Gastroenterol 2014;49:961–70. [DOI] [PubMed] [Google Scholar]
- 79.Kanno A, Nishimori I, Masamune A, et al. ; Working Committee of the Japan Pancreas Society and the Research Committee for Intractable Pancreatic Disease supported by the Ministry of Health, Labour and Welfare of Japan. Nationwide epidemiological survey of autoimmune pancreatitis in Japan. Pancreas 2012;41:835–9. [DOI] [PubMed] [Google Scholar]
- 80.Carruthers MN, Topazian MD, Khosroshahi A, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis 2015;74:1171–7. [DOI] [PubMed] [Google Scholar]
- 81.Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2011;(2):CD008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bauer K, Rancea M, Roloff V, et al. Rituximab, ofatumumab and other monoclonal anti-CD20 antibodies for chronic lymphocytic leukaemia. Cochrane Database Syst Rev 2012;(11): CD008079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lopez-Olivo MA, Amezaga Urruela M, McGahan L, et al. Rituximab for rheumatoid arthritis. Cochrane Database Syst Rev 2015;(1):CD007356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.LaBarba S, Kaplan BM, Eberhard B. Prolonged immune suppression after rituximab use in children. J Allergy Clin Immunol 2016;137:AB218. [Google Scholar]
- 85.Hart PA, Topazian MD, Witzig TE, et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut 2013;62:1607–15. [DOI] [PubMed] [Google Scholar]
- 86.Sah RP, Chari ST. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr Opin Rheumatol 2011;23:108–13. [DOI] [PubMed] [Google Scholar]
- 87.Carruthers MN, Stone JH, Deshpande V, et al. Development of an IgG4-RD responder index. Int J Rheumatol 2012;2012:259408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamisawa T, Egawa N, Inokuma S, et al. Pancreatic endocrine and exocrine function and salivary gland function in autoimmune pancreatitis before and after steroid therapy. Pancreas 2003;27:235–8. [DOI] [PubMed] [Google Scholar]
- 89.Ko SBH, Mizuno N, Yatabe Y, et al. Corticosteroids correct aberrant CFTR localization in the duct and regenerate acinar cells in autoimmune pancreatitis. Gastroenterology 2010;138:1988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kamisawa T, Shimosegawa T, Okazaki K, et al. Standard steroid treatment for autoimmune pancreatitis. Gut 2009;58:1504–7. [DOI] [PubMed] [Google Scholar]
- 91.Hirano K, Tada M, Isayama H, et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut 2007;56:1719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buechter M, Klein CG, Kloeters C, et al. Tacrolimus as a reasonable alternative in a patient with steroid-dependent and thiopurine-refractory autoimmune pancreatitis with IgG4-associated cholangitis. Z Gastroenterol 2014;52:564–8. [DOI] [PubMed] [Google Scholar]
- 93.Bosco JJ, Suan D, Varikatt W, et al. Extra-pancreatic manifestations of IgG4-related systemic disease: a single-centre experience of treatment with combined immunosuppression. Intern Med J 2013;43:417–23. [DOI] [PubMed] [Google Scholar]
- 94.Sah RP, Chari ST. Autoimmune pancreatitis: an update on classification, diagnosis, natural history and management. Curr Gastroenterol Rep 2012;14:95–105. [DOI] [PubMed] [Google Scholar]
- 95.Khosroshahi A, Bloch DB, Deshpande V, et al. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum 2010;62:1755–62. [DOI] [PubMed] [Google Scholar]
- 96.Mattoo H, Mahajan VS, Della-Torre E, et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol 2014;134: 679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Della-Torre E, Feeney E, Deshpande V, et al. B-cell depletion attenuates serological biomarkers of fibrosis and myofibroblast activation in IgG4-related disease. Ann Rheum Dis 2015; 74:2236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]