Abstract
Objective
Clinical and animal studies demonstrate that alcohol intoxication at the time of injury worsens post-burn outcome. The purpose of this study was to determine the role and mechanism of Kupffer cell derangement in exacerbating post-burn end organ damage in alcohol exposed mice.
Design
Interventional study.
Setting
Research Institute.
Subjects
Male C57BL/6 mice.
Interventions
Alcohol administered 30 minutes before a 15% scald burn injury. Antecedent Kupffer cell depletion with clodronate liposomes (0.5 mg/kg). p38 mitogen-activated protein kinase (MAPK) inhibition via SB203580 (10 mg/kg).
Measurements and Main Results
Kupffer cells were isolated 24 hours after injury and analyzed for p38 activity and IL-6 production. Intoxicated burned mice demonstrated a 2-fold (p<0.05) elevation of Kupffer cell p38 activation relative to either insult alone and this corresponded to a 43% (p<0.05) increase in IL-6 production. Depletion of Kupffer cells attenuated hepatic damage as seen by decreases of 53% (p<0.05) in serum ALT and 74% (p<0.05) in hepatic triglycerides, as well as a 77% reduction (p<0.05) in serum IL-6 levels compared to matched controls. This mitigation of hepatic damage was associated with a 54% decrease (p<0.05) in pulmonary neutrophil infiltration and reduced alveolar wall thickening by 45% (p<0.05). In vivo p38 inhibition conferred nearly identical hepatic and pulmonary protection after the combined injury as mice depleted of Kupffer cells.
Conclusions
Intoxication exacerbates post-burn hepatic damage through p38-dependent IL-6 production in Kupffer cells.
Keywords: Trauma, Burns, p38 MAPK, Intoxication, Alcohol, Inflammation
Introduction
Severe burns are a devastating injury affecting every major organ system, with the degree of systemic inflammation correlating to the size of the burn (1). Nearly 50% of patients admitted for burns have a positive blood alcohol content (BAC) and have worse clinical outcomes than individuals who sustain similar injuries not under the influence of alcohol (2–5). Specifically, they are twice as likely to acquire an infection, require more surgical procedures and have a longer duration of stay in the intensive care unit than their nonintoxicated counterparts (2, 6). With 1 million burn injuries in the US each year, resulting in 40,000 hospitalizations (7), this represents a substantial burden of morbidity, mortality and socioeconomic cost for which there is currently no specific treatments. Recent studies suggest the majority of intoxicated trauma patients are binge drinkers without evidence of chronic alcoholism (8), consistent with the majority of alcohol consumption in the US (9) and the design of the enclosed animal study.
Previous work in this animal model has demonstrated that the presence of alcohol at the time of a burn alters the gut-liver axis (10) leading to increased interleukin-6 (IL-6) which drives pulmonary inflammation (11). We therefore sought to examine the role of Kupffer cells in the response to the combined insult of intoxication and burn with the aim of identifying a mechanistic target for this aberrant response. We describe within novel findings that demonstrate intoxication augments Kupffer cell IL-6 production through increased p38 activation after burn, suggesting that specifically targeting Kupffer cell p38 activity may have therapeutic value in this at-risk patient population.
Materials and Methods
Animals
Male C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in sterile micro-isolator cages under pathogen-free conditions at Loyola University Chicago. All experiments were conducted with approval of the Loyola Institutional Animal Care and Use Committee, performed with mice weighing between 23–25 g, and during the hours of 8–10 AM to avoid confounding factors related to circadian rhythms.
Murine model of intoxication and burn
A murine model of ethanol exposure and burn injury was employed as described previously (12) with minor modifications. Mice were given a single dose of ethanol (1.12 g/kg) by oral gavage that resulted in a BAC of 180 mg/dl at 30 minutes (13). The mice were anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine), their dorsum shaved and they were placed in a plastic template exposing 15% of the total body surface area and subjected to a scald injury in 92ºC water or a sham injury in room-temperature water. The scald injury resulted in an insensate, full-thickness burn (14). All mice were resuscitated with 1.0 ml of 0.9% normal saline and placed on warming pads until recovered from anesthesia. Mice were euthanized by CO2 narcosis followed by exsanguination 24 hours after injury. For experiments involving in vivo p38 inhibition, the intoxicated, burn injured mice were split into two groups and at 30 minutes after injury, given an intraperitoneal injection of the selective p38 mitogen-activated protein kinase (MAPK) inhibitor, SB203580 (InvivoGen, 10 mg/kg) or vehicle control (saline). For experiments involving antecedent Kupffer cell depletion, clodronate liposomes (Encapsula NanoSciences, 0.5 mg/kg) or vehicle control (empty liposomes) were administered via tail vein injection. The control arms of sham vehicle, sham ethanol, and burn vehicle are presented herein for the clodronate and p38i experiments to demonstrate baseline results and provide injury context for the assessed parameters. The data from these control groups can be found in the manuscript figures and are consistent with previously reported results in the liver (10, 13, 15), and lungs (11, 13, 16). As this study examines the role of Kupffer cells in the established consequences of intoxication and burn injury in this animal model, the discussion of the in vivo studies will focus on the animals given the combined injury of ethanol and burn, with and without treatments. Furthermore, as the treatment vehicle controls given to intoxicated and burn injured mice (saline and empty liposomes for p38 inhibition and clodronate, respectively) did not differ from intoxication and burn without vehicle controls, only one set of results are shown for clarity.
Serum measurements
At 24 hours post injury, blood was collected via cardiac puncture, an aliquot was placed into a microcapillary tube, and read for a complete blood count with differential by Hemavet (Drew Scientific, Dallas, TX). The remaining blood was harvested for serum by centrifugation after clotting. Serum aliquots were used to measure IL-6 by ELISA (BD Biosciences, Franklin Lakes, NJ) or liver transaminase levels using a DRI-CHEM 7000 (HESKA, Loveland, CO) as described previously (13).
Liver histology
The whole liver was removed at the time of sacrifice, weighed and a portion embedded in OCT or snap frozen in liquid nitrogen. Frozen sections were cut at 7 μm, stained with Oil Red O, and examined for the presence of fat droplets as described previously (17). Sectioned tissue was rinsed in isopropyl alcohol and placed in a filtered working solution of Oil Red O for 20 minutes. The slides were rinsed in isopropyl alcohol, followed by tap water and counterstained with hematoxylin for 1 minute. The slides were serially rinsed in tap water, ammonia water (lithium carbonate), and finally tap water. Representative images were taken at 400x magnification.
Liver tissue measurements
Liver tissue was homogenized in 1 ml of BioPlex cell lysis buffer according to manufacturer’s instructions (BioRad, Hercules, CA) and analyzed for cytokine production using an ELISA for IL-6 (BD Biosciences, Franklin Lakes, NJ). The results were normalized to total protein using the BioRad protein assay (BioRad, Hercules, CA). A portion of the frozen liver was used in a Triglyceride Quantification Kit according to manufacturer instruction (Abcam, Cambridge, MA).
Kupffer cell isolation
Kupffer cells were isolated 24 hour after injury as described by Kuriakose et al. (18) with minor modifications. Rinsed liver tissue was incubated at 37°C for 30 minutes in collagenase IV (5000 CDU/mL), run through a gentleMACS Dissociator (Miltenyi Biotec, Auburn, CA), and passed through a sterile 100 um strainer into phosphate-buffered saline (Miltenyi Biotec, Auburn, CA) containing 0.5% bovine serum albumin. The hepatocytes were removed by centrifugation at 20xg for 4 minutes, and the residual cell suspension was centrifuged twice at 300xg for 10 minutes at 4°C with the cell pellet washed with Red Blood Cell Lysis Solution between centrifugations. CD11b+ cells were enriched by positive selection using AUTOMACS (Miltenyi Biotec Auburn, CA). Enriched liver CD11b+ cells isolated by this method have been shown to be greater than 96% positive for F4/80 expression as assessed by flow cytometry (18).
Cell culture
Isolated Kupffer cells were cultured at a concentration of 3×105 per well in RPMI medium supplemented with 10% fetal bovine serum and 50μg/ml gentamicin for 2 hours. Cells were pretreated with SB203580 (10 μM) for 1 hour before stimulation with lipopolysaccharide (LPS) (100 ng/mL) followed by incubation at 37°C with 5% CO2 and 95% humidity for 18 hours. At the concentration used, SB203580 is highly selective for p38 (19). Supernatants were removed and analyzed for cytokine production using an enzyme-linked immunosorbent assay (ELISA) for interleukin-6 (IL-6; BD Biosciences, Franklin Lakes, NJ). Activation of p38 was determined by cell-based ELISA (RayBiotech Inc, Norcross, GA) using primary antibodies against p38 and phospho-p38 (Thr180/Tyr182) as specified per manufacturer instructions.
Lung histology
The upper right lobe of the lung was inflated with 10% formalin and fixed overnight as described previously (20). The lung was embedded in paraffin, sectioned at 5μm, and stained with hematoxylin and eosin (H&E). The sections were analyzed microscopically in a blinded fashion for the number of neutrophils in 10 high power fields (HPF). Representative images were taken at 400x magnification. Ten HPF (400X) per animal were analyzed using ImageJ (National Institutes of Health, Bethesda, MD). The images were converted to binary to differentiate lung tissue from air space and analyzed for the percent area covered by lung tissue in each field of view as described previously (11).
Statistical analysis
Statistical comparisons (GraphPad Instat, La Jolla, CA) were made between the sham vehicle, sham ethanol, burn vehicle, and burn ethanol animals, resulting in four total groups analyzed treated as an independent variable with one factor. One-way analysis of variance (ANOVA) was used to determine differences between groups, and Tukey’s post hoc test once significance was achieved (p<0.05). Statistical comparisons made between intoxicated burned mice given saline and intoxicated burned mice given a treatment (clodronate or SB203580) were performed using a Student’s t-test (p<0.05). Data are reported as mean values ± the standard error of the mean (SEM).
Results
Kupffer cell p38 activation and IL-6 production
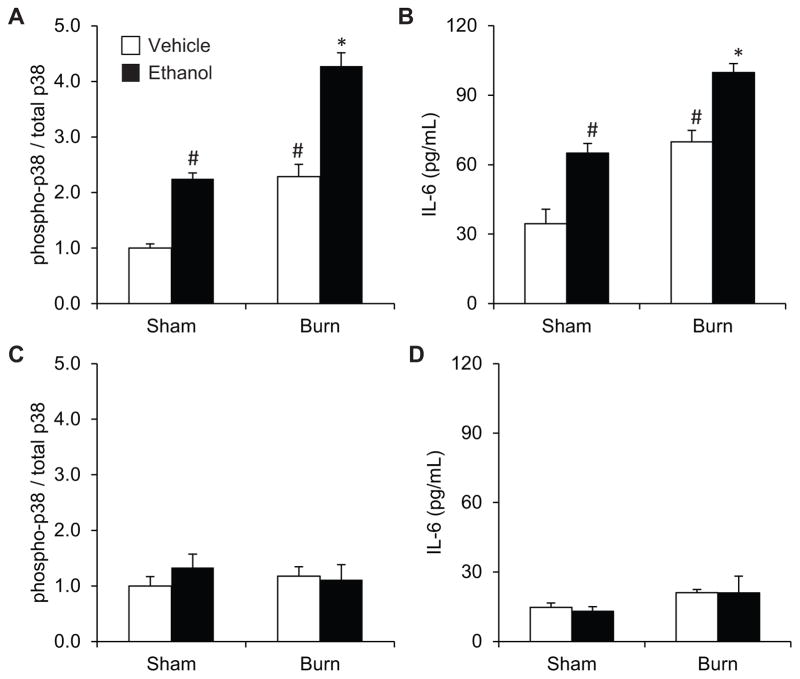
Total p38 was detectable in all samples and did not change between treatment groups. The combination of ethanol and burn injury led to a 4-fold (p<0.05) increase in p38 activation relative to sham, which was 2-fold (p<0.05) greater than either insult alone (Figure 1A). IL-6 levels paralleled p38 activation with the combined injury demonstrating a 3-fold (p<0.05) and 1.5-fold (p<0.05) increase over sham injury or isolated intoxication or burn, respectively (Figure 1B). Ex vivo p38 inhibition abrogated the increases in LPS-induced p38 activation (Figure 1C) or IL-6 production (Figure 1D) in Kupffer cells from any injury group.
Figure 1. Kupffer cell p38 activation is increased when intoxication precedes burn.
Kupffer cells were isolated 24 hours after injury and assessed for (A) p38 activation and (B) IL-6 production after LPS-stimulation, which were also assessed (C and D) in the presence of the p38 inhibitor, SB203580. *p<0.05 by compared to all groups by one-way ANOVA with Tukey’s post hoc. #p<0.05 compared to Sham Vehicle by one-way ANOVA with Tukey’s post hoc. N=4–6 animals per group.
Hepatic damage
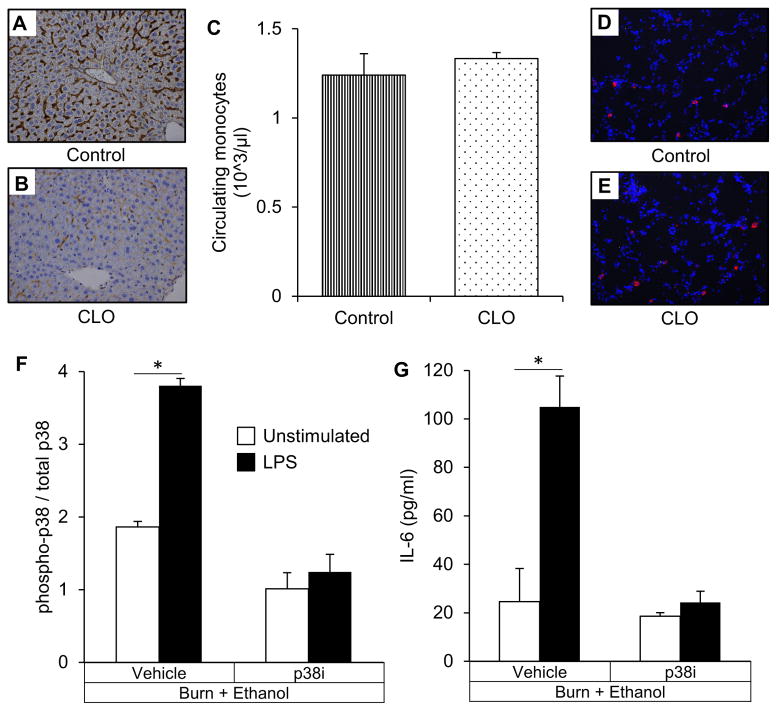
The clodronate dose and administration route used is a well-established method to achieve relatively selective depletion of Kupffer cells ((21), Figure 2A&B), while leaving other macrophage populations such as the circulating monocytes (Figure 2C) and alveolar macrophages (Figure 2D&E.) intact. The dosage of p38i used in the present study has been shown to inhibit p38 activation in Kupffer cells 24 hours after burn (22) which we confirm through decreased p38 phosphorylation (Figure 2F) and IL-6 production (Figure 2G) after LPS stimulation.
Figure 2. Selective depletion of Kupffer cells and in vivo Kupffer cell p38 inhibition.
(A) Liver sections immunostained for CD68 from mice receiving empty liposomes. (B) Liver sections immunostained for CD68 from mice receiving clodronate liposomes. (C) Peripheral blood monocytes. (D) Lung sections stained for alveolar macrophage marker SiglecF from mice receiving empty liposomes. (E) Lung sections stained for alveolar macrophage marker SiglecF from mice receiving clodronate liposomes. (F) p38 activation in isolated Kupffer cells from animals of the Burn + Ethanol Subgroup with and without LPS stimulation. Within the subgroup, animals given in vivo SB203580 (p38i) or vehicle control are shown. (G) IL-6 production in isolated Kupffer cells from animals of the Burn + Ethanol Subgroup with and without LPS stimulation. *p<0.05 by Student’s T test. N=3–6 animals per group.
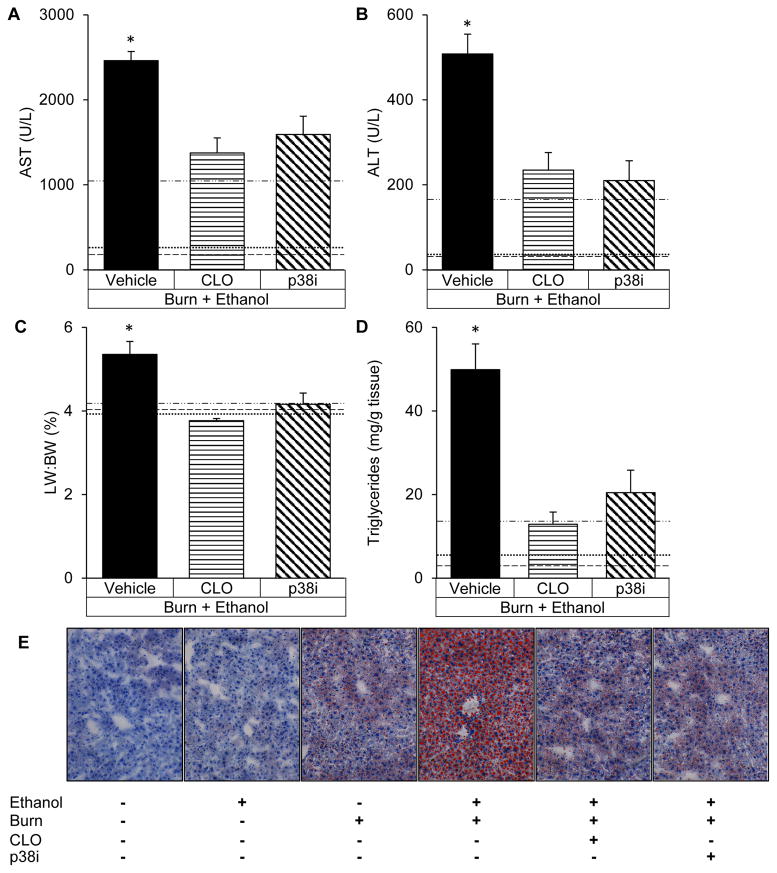
Inhibition of p38 after the combined insult alleviated hepatic damage as measured by a 35% reduction (p<0.05) in serum AST, a 58% decrease (p<0.05) in serum ALT and a 22% lower (p<0.05) liver weight to body weight ratio compared to intoxicated and burned animals given saline (Figure 3A–C). Analogous to SB203580 treatment, antecedent Kupffer cell depletion reduced serum AST by 44% (p<0.05), ALT by 53% (p<0.05) and liver weight to body weight ratio by 28% (p<0.05), relative to animals given saline after ethanol and burn (Figure 3A–C). The decreased hepatic damage after p38 inhibition or the absence of Kupffer cells paralleled decreases in hepatic triglycerides of 59% (p<0.05) and 74% (p<0.05), respectively (Figure 3D) which can also be appreciated upon histologic examination (Figure 3E).
Figure 3. The absence of Kupffer cells or p38 inhibition equally attenuate hepatic damage.
(A) Serum levels of aspartate aminotransferase (AST) after treatment with vehicle, clodronate liposomes (CLO) or SB203580 (p38i). (B) Serum levels of alanine aminotransferase (ALT). (C) Liver weight to total body weight ratio (LW:BW). (D) Triglycerides in liver homogenates after intoxication and burn. (E) Liver sections stained with Oil Red O to visualize triglyceride accumulation at 400x magnification. *p<0.05 compared to CLO and p38i by Student’s T test. N=4–6 animals per group.
 represents Sham Vehicle,
represents Sham Vehicle,
 represents Sham Ethanol, and
represents Sham Ethanol, and
 represents Burn Vehicle.
represents Burn Vehicle.
Hepatic and systemic levels of IL-6
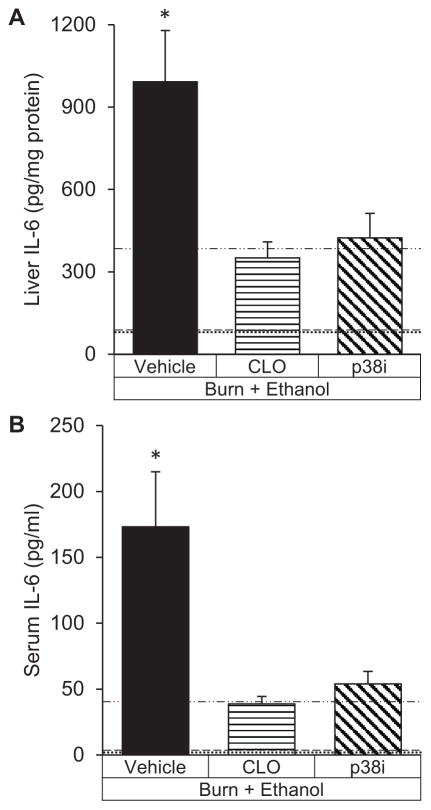
After the combined insult of intoxication and burn, p38 inhibition led to a 57% reduction (p<0.05) in hepatic IL-6 levels (Figure 4A) which corresponded to a 68% decrease (p<0.05) in serum IL-6 (Figure 4B). Similarly, IL-6 levels in mice that lacked Kupffer cells at the time of injury had reduced hepatic IL-6 by 65% (p<0.05) and serum IL-6 by 77% (p<0.05) compared to mice undergoing intoxication and burn with intact Kupffer cells.
Figure 4. Interleukin-6 production is decreased with clodronate liposomes (CLO) or SB203580 (p38i) after intoxication and burn.
(A) Interleukin-6 (IL-6) protein levels in liver homogenates. (B) Serum IL-6 levels. *p<0.05 compared to CLO and p38i by Student’s T test. N=4–6 animals per group.
 represents Sham Vehicle,
represents Sham Vehicle,
 represents Sham Ethanol, and
represents Sham Ethanol, and
 represents Burn Vehicle.
represents Burn Vehicle.
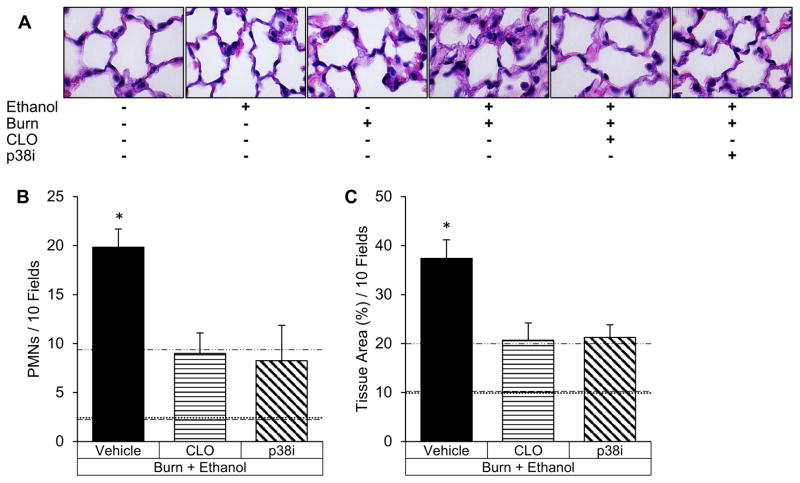
Pulmonary inflammation
Clodronate or p38 inhibition lessened pulmonary congestion and cellularity upon visual examination (Figure 5A). Consistent with visual findings, p38 inhibition decreased alveolar wall thickness by 43% (p<0.05) compared to matched controls and similarly, Kupffer cell depletion lessened alveolar wall thickness by 45% (p<0.05) (Figure 5C). Neutrophil accumulation was reduced by 58% (p<0.05) with SB203580 treatment and 54% (p<0.05) with clodronate compared to matched controls (Figure 5B).
Figure 5. Pulmonary inflammation after intoxication and burn is attenuated by Kupffer cell depletion or p38 inhibition.
(A) Lung histology (hematoxylin and eosin at 400x) of intoxicated and burn injured mice receiving clodronate liposomes (CLO) or SB203580 (p38i). (B) Neutrophil (PMN) quantification in 10 high power fields of view of intoxicated and burn-injured mice. (C) Pulmonary congestion quantified using imaging software to calculate the lung tissue area in 10 fields of view. *p<0.05 compared to CLO and p38i by Student’s T test. N=4–6 animals per group.
 represents Sham Vehicle,
represents Sham Vehicle,
 represents Sham Ethanol, and
represents Sham Ethanol, and
 represents Burn Vehicle.
represents Burn Vehicle.
Discussion
Animal studies suggest hepatic derangement is dependent on the size of the burn with a threshold beyond which a qualitatively different response occurs (23). Work from our lab and others indicates that the presence of alcohol intoxication at the time of injury lowers the size of burn required to cause liver damage (10, 15, 24), though the mechanisms of how this ensues is currently unknown. One possibility is that alcohol and burn act synergistically on Kupffer cells to cause excessive cytokine production. Existing literature suggests alcohol consumption (25) or an isolated burn (26) can independently increase Kupffer cell sensitization to LPS, a potent stimulator of Kupffer cell activation. We now report that the combined insult of intoxication and burn leads to greater Kupffer cell activity after LPS stimulation than either insult alone. Taken together, this novel finding combined with previous work showing increased bacterial translocation after the combined injury (10), indicates that alcohol augments post-burn IL-6 through increasing both the stimulus and response to LPS. This is of clinical importance as elevated IL-6 correlates with mortality risk in burn patients (27), possibly explaining the worsened outcomes of patients who are intoxicated at the time of a burn (2). While it remains controversial if IL-6 in human burn patients plays a contributory role or is simply a biomarker of mortality risk, animal studies have conclusively demonstrated a causative role for IL-6 in end-organ damage after intoxication and burn (11, 28). We therefore sought to elucidate the signaling mechanisms responsible for Kupffer cell hyperactivity in this setting.
Kupffer cells produce IL-6 when LPS binds to Toll-like receptor 4 (TLR4), initiating a variety of potential down-stream signaling pathways such as the mitogen-activated protein kinase (MAPK) pathway. A member of the MAPK family, p38 has a well-established role in post-burn remote organ damage (29–34) and enhances TLR4 reactivity following injury (35). Our observations from isolated Kupffer cells establish that ethanol augments p38 activation in Kupffer cells after burn, which corresponds to heightened IL-6 production and end organ damage. Furthermore, ex vivo inhibition of p38 abrogated LPS-induced IL-6 production suggesting that the increased IL-6 after intoxication and burn is produced in a p38-dependent manner.
Global p38 inhibition attenuated hepatic damage to an extent equal to that of antecedent Kupffer cell depletion, suggesting Kupffer cell p38 may be responsible for the increased hepatic damage of the combined injury. Furthermore, both treatments reduced hepatic damage and steatosis to the extent seen after a burn alone, implying that alcohol may potentiate post-burn hepatic damage through Kupffer cell p38 activity. These findings do not rule out the possible influence of other etiologies of hepatic damage, such as oxidative stress (36) or changes in fat metabolism (37). However our ex vivo results of increased LPS-induced Kupffer cell activity and our in vivo findings of elevated p38-dependent IL-6 production allude to a more prominent role for Kupffer cell IL-6 in causing the hepatotoxicity of this setting. Indeed, excess IL-6 can be detrimental to hepatocytes (38) and promote steatosis (39).
Increased serum IL-6 is linked to poor survival in patients with acute respiratory distress syndrome (ARDS) (40), a common complication after a burn (41) which develops independently of inhalation injury (42). ARDS is characterized by inflammation and edema in the lung parenchyma leading to impaired gas exchange which is exacerbated when alcohol precedes burn (11, 13, 43, 44). The degree of reduction in pulmonary inflammation observed with p38i treatment matches the level of reduction found in studies of IL-6 deficient mice undergoing the same combined injury (11), suggesting the pulmonary benefit in p38i-treated animals may be related to decreased IL-6 levels. The parallel results in clodronate-treated mice imply the pulmonary benefits of p38i treatment stem from its effect on Kupffer cells and not a direct action of p38 inhibition in the lungs.
Conclusions
The studies above delineate a clear and reversible role for Kupffer cell p38 MAPK in the hepatic response to intoxication and burn. The ability to manipulate hepatic IL-6 production through specifically targeting Kupffer cell p38 may be a more viable clinical approach than global p38 inhibition or impairment of Kupffer cell function which work at the expense of other critical functions of this pathway and cell, respectively. Inhibition of p38 in animal models has been shown to benefit local wound inflammation and remote organ damage after a burn when applied topically (45) or given systemically (29–33). However, clinical trials of p38 inhibitors for chronic inflammatory conditions have been disappointing in efficacy and fraught with adverse events, likely due to the ubiquitous and multi-functional nature of p38. Therefore, specifically targeting Kupffer cell p38, perhaps through packaging into liposomes, may be a superior treatment for patients intoxicated at the time of a burn.
Acknowledgments
Funding received for this work includes: NIH AA012034 (EJK), R01GM115257 (EJK), T32 AA013527 (EJK), F30 AA022856 (MMC), F31 AA022566 (JAI), Department of Defense. USAMRAA. W81XWH-11-1-0559, an Illinois Excellence in Academic Medicine Grant, The Margaret A. Baima Endowment Fund for Alcohol Research, and the Dr. Ralph and Marian C. Falk Medical Research Trust.
This research was supported by the National Institute of Alcohol Abuse and Alcoholism and the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under award numbers R01AA012034 (EJK), R01GM115257 (EJK), F30AA022856 (MMC), T32AA013527 (EJK), F31AA022566 (JAI), Department of Defense USAMRAA. W81XWH-11-1-0559, the Illinois Excellence in Academic Medicine Grant, The Margaret A. Baima Endowment Fund for Alcohol Research, and the Dr. Ralph and Marian C. Falk Medical Research Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank Melissa Hoo and Luis Ramirez for technical assistance.
Footnotes
Copyright form disclosures:
Dr. Kovacs received support for article research from the National Institutes of Health (NIH) and disclosed other support (The Dr. Ralph and Marian C. Falk Medical Research Trust). Her institution received funding from the NIH under award numbers (NIH AA012034, T32 AA013527, F30 AA022856, F31 AA022566), the Illinois Excellence in Academic Medicine Grant, and from The Margaret A. Baima Endowment Fund for Alcohol Research. Dr. Chen received support for article research from the NIH. His institution received grant support from the NIH (F30 AA022856). Dr. Ippolito received support for article research from the NIH. Her institution received grant support (F31 AA022566). Dr. O’Halloran disclosed that she does not have any potential conflicts of interest.
References
- 1.Barber RC, Maass DL, White DJ, et al. Increasing percent burn is correlated with increasing inflammation in an adult rodent model. Shock. 2008;30(4):388–393. doi: 10.1097/SHK.0b013e318164f1cd. [DOI] [PubMed] [Google Scholar]
- 2.Silver GM, Albright JM, Schermer CR, et al. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J Burn Care Res. 2008;29(5):784–789. doi: 10.1097/BCR.0b013e31818481bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelley D, Lynch JB. Burns in alcohol and drug users result in longer treatment times with more complications. J Burn Care Rehabil. 1992;13(2 Pt 1):218–220. doi: 10.1097/00004630-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Grobmyer SR, Maniscalco SP, Purdue GF, et al. Alcohol, drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. J Burn Care Rehabil. 1996;17(6 Pt 1):532–539. doi: 10.1097/00004630-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Davis CS, Esposito TJ, Palladino-Davis AG, et al. Implications of alcohol intoxication at the time of burn and smoke inhalation injury: an epidemiologic and clinical analysis. J Burn Care Res. 2013;34(1):120–126. doi: 10.1097/BCR.0b013e3182644c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brezel BS, Kassenbrock JM, Stein JM. Burns in substance abusers and in neurologically and mentally impaired patients. J Burn Care Rehabil. 1988;9(2):169–171. doi: 10.1097/00004630-198803000-00009. [DOI] [PubMed] [Google Scholar]
- 7.ABA. National Burn Repository: 2012 Report. Chicago, IL: American Burn Association; 2012. [Google Scholar]
- 8.Savola O, Niemela O, Hillbom M. Alcohol intake and the pattern of trauma in young adults and working aged people admitted after trauma. Alcohol and alcoholism. 2005;40(4):269–273. doi: 10.1093/alcalc/agh159. [DOI] [PubMed] [Google Scholar]
- 9.Naimi TS, Brewer RD, Mokdad A, et al. Binge drinking among US adults. JAMA: the journal of the American Medical Association. 2003;289(1):70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- 10.Chen MM, Zahs A, Brown MM, et al. An alteration of the gut-liver axis drives pulmonary inflammation after intoxication and burn injury in mice. American journal of physiology Gastrointestinal and liver physiology. 2014;307(7):G711–718. doi: 10.1152/ajpgi.00185.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen MM, Bird MD, Zahs A, et al. Pulmonary inflammation after ethanol exposure and burn injury is attenuated in the absence of IL-6. Alcohol. 2013;47(3):223–229. doi: 10.1016/j.alcohol.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. Journal of leukocyte biology. 1997;62(6):733–740. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- 13.Chen MM, Palmer JL, Ippolito JA, et al. Intoxication by intraperitoneal injection or oral gavage equally potentiates postburn organ damage and inflammation. Mediators of inflammation. 2013;2013:971481. doi: 10.1155/2013/971481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faunce DE, Llanas JN, Patel PJ, et al. Neutrophil chemokine production in the skin following scald injury. Burns: journal of the International Society for Burn Injuries. 1999;25(5):403–410. doi: 10.1016/s0305-4179(99)00014-5. [DOI] [PubMed] [Google Scholar]
- 15.Colantoni A, Duffner LA, De Maria N, et al. Dose-dependent effect of ethanol on hepatic oxidative stress and interleukin-6 production after burn injury in the mouse. Alcoholism, clinical and experimental research. 2000;24(9):1443–1448. [PubMed] [Google Scholar]
- 16.Chen MM, O’Halloran EB, Ippolito JA, et al. Alcohol potentiates postburn remote organ damage through shifts in fluid compartments mediated by bradykinin. Shock. 2015;43(1):80–84. doi: 10.1097/SHK.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emanuele NV, Emanuele MA, Morgan MO, et al. Ethanol potentiates the acute fatty infiltration of liver caused by burn injury: prevention by insulin treatment. Journal of burn care & research: official publication of the American Burn Association. 2009;30(3):482–488. doi: 10.1097/BCR.0b013e3181a28df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuriakose S, Muleme HM, Onyilagha C, et al. Diminazene aceturate (Berenil) modulates the host cellular and inflammatory responses to Trypanosoma congolense infection. PloS one. 2012;7(11):e48696. doi: 10.1371/journal.pone.0048696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies SP, Reddy H, Caivano M, et al. Specificity and mechanism of action of some commonly used protein kinase inhibitors. The Biochemical journal. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel PJ, Faunce DE, Gregory MS, et al. Elevation in pulmonary neutrophils and prolonged production of pulmonary macrophage inflammatory protein-2 after burn injury with prior alcohol exposure. American journal of respiratory cell and molecular biology. 1999;20(6):1229–1237. doi: 10.1165/ajrcmb.20.6.3491. [DOI] [PubMed] [Google Scholar]
- 21.van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol. 2010;605:189–203. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]
- 22.Chen XL, Xia ZF, Wei D, et al. Role of p38 mitogen-activated protein kinase in Kupffer cell secretion of the proinflammatory cytokines after burn trauma. Burns: journal of the International Society for Burn Injuries. 2003;29(6):533–539. doi: 10.1016/s0305-4179(03)00147-5. [DOI] [PubMed] [Google Scholar]
- 23.Izamis ML, Sharma NS, Uygun B, et al. In situ metabolic flux analysis to quantify the liver metabolic response to experimental burn injury. Biotechnology and bioengineering. 2011;108(4):839–852. doi: 10.1002/bit.22998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Akhtar S, Kovacs EJ, et al. Inflammatory response in multiple organs in a mouse model of acute alcohol intoxication and burn injury. J Burn Care Res. 2011;32(4):489–497. doi: 10.1097/BCR.0b013e3182223c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enomoto N, Ikejima K, Bradford B, et al. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology. 1998;115(2):443–451. doi: 10.1016/s0016-5085(98)70211-2. [DOI] [PubMed] [Google Scholar]
- 26.Enomoto N, Takei Y, Yamashina S, et al. Burn injury sensitizes rat Kupffer cells via mechanisms dependent on gut-derived endotoxin. J Gastroenterol. 2004;39(12):1175–1181. doi: 10.1007/s00535-004-1468-9. [DOI] [PubMed] [Google Scholar]
- 27.Drost AC, Burleson DG, Cioffi WG, Jr, et al. Plasma cytokines following thermal injury and their relationship with patient mortality, burn size, and time postburn. J Trauma. 1993;35(3):335–339. doi: 10.1097/00005373-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Zahs A, Bird MD, Ramirez L, et al. Anti-IL-6 antibody treatment but not IL-6 knockout improves intestinal barrier function and reduces inflammation after binge ethanol exposure and burn injury. Shock. 2013;39(4):373–379. doi: 10.1097/SHK.0b013e318289d6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costantini TW, Peterson CY, Kroll L, et al. Role of p38 MAPK in burn-induced intestinal barrier breakdown. The Journal of surgical research. 2009;156(1):64–69. doi: 10.1016/j.jss.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kita T, Ogawa M, Sato H, et al. Role of p38 mitogen-activated protein kinase pathway on heart failure in the infant rat after burn injury. International journal of experimental pathology. 2008;89(1):55–63. doi: 10.1111/j.1365-2613.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kita T, Yamaguchi H, Sato H, et al. Role of p38 mitogen-activated protein kinase pathway on renal failure in the infant rat after burn injury. Shock. 2004;21(6):535–542. doi: 10.1097/00024382-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Chen XL, Xia ZF, Ben DF, et al. Role of p38 mitogen-activated protein kinase in lung injury after burn trauma. Shock. 2003;19(5):475–479. doi: 10.1097/01.shk.0000055242.25446.84. [DOI] [PubMed] [Google Scholar]
- 33.Chen XL, Xia ZF, Yu YX, et al. p38 mitogen-activated protein kinase inhibition attenuates burn-induced liver injury in rats. Burns: journal of the International Society for Burn Injuries. 2005;31(3):320–330. doi: 10.1016/j.burns.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Chen LW, Chen PH, Chang WJ, et al. IKappaB-kinase/nuclear factor-kappaB signaling prevents thermal injury-induced gut damage by inhibiting c-Jun NH2-terminal kinase activation. Crit Care Med. 2007;35(5):1332–1340. doi: 10.1097/01.CCM.0000261891.30360.F0. [DOI] [PubMed] [Google Scholar]
- 35.Maung AA, Fujimi S, Miller ML, et al. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. Journal of leukocyte biology. 2005;78(2):565–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- 36.Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. American journal of physiology Gastrointestinal and liver physiology. 2001;281(5):G1135–1139. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- 37.Jeschke MG. The hepatic response to thermal injury: is the liver important for postburn outcomes? Molecular medicine. 2009;15(9–10):337–351. doi: 10.2119/molmed.2009.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin X, Zimmers TA, Perez EA, et al. Paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology. 2006;43(3):474–484. doi: 10.1002/hep.21087. [DOI] [PubMed] [Google Scholar]
- 39.Stienstra R, Saudale F, Duval C, et al. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51(2):511–522. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- 40.Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107(4):1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 41.Steinvall I, Bak Z, Sjoberg F. Acute respiratory distress syndrome is as important as inhalation injury for the development of respiratory dysfunction in major burns. Burns: journal of the International Society for Burn Injuries. 2008;34(4):441–451. doi: 10.1016/j.burns.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Liffner G, Bak Z, Reske A, et al. Inhalation injury assessed by score does not contribute to the development of acute respiratory distress syndrome in burn victims. Burns. 2005;31(3):263–268. doi: 10.1016/j.burns.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Bird MD, Zahs A, Deburghgraeve C, et al. Decreased pulmonary inflammation following ethanol and burn injury in mice deficient in TLR4 but not TLR2 signaling. Alcoholism, clinical and experimental research. 2010;34(10):1733–1741. doi: 10.1111/j.1530-0277.2010.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shults JA, Curtis BJ, Chen MM, et al. Impaired respiratory function and heightened pulmonary inflammation in episodic binge ethanol intoxication and burn injury. Alcohol. 2015;49(7):713–720. doi: 10.1016/j.alcohol.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carter D, Warsen A, Mandell K, et al. Delayed topical p38 MAPK inhibition attenuates full-thickness burn wound inflammatory signaling. J Burn Care Res. 2014;35(2):e83–92. doi: 10.1097/BCR.0b013e31828a8d6e. [DOI] [PMC free article] [PubMed] [Google Scholar]