Abstract
Objectives
Preoperative chemotherapy and radiation for localized esophageal cancer produces cure rates near 30% when combined with surgical resection. Vandetanib, a small molecule receptor tyrosine kinase inhibitor of VEGFR-2, VEGFR-3, RET and EGFR, demonstrated synergy with radiation and chemotherapy in preclinical models. We conducted a phase I study to assess the safety and tolerability of vandetanib when combined with preoperative chemoradiation in patients with localized esophageal carcinoma who were surgical candidates.
Methods
Patients with stage II–III esophageal and gastro-esophageal junction (GEJ) carcinoma without prior therapy were enrolled in a 3+3 phase 1 design. Patients received once daily vandetanib (planned dosing levels of 100, 200, and 300 mg) with concomitant daily radiotherapy (1.8 Gy/day, 45 Gy total) and chemotherapy, consisting of infusional 5-FU (225 mg/m2/day over 96 hours, weekly), paclitaxel (50 mg/m2, days 1, 8, 15, 22, 29) and carboplatin (AUC of 5, days 1, 29).
Results
A total 9 patients were enrolled with 8 having either distal esophageal or GEJ carcinomas. All patients completed the planned preoperative chemoradiation and underwent esophagectomy. Nausea (44%) and anorexia (44%) were the most common acute toxicities of any grade. One grade 4 non-hematologic toxicity was observed (gastro-bronchial fistula). One additional patient suffered a late complication, a fatal aorto-enteric hemorrhage, not definitively related to the investigational regimen. Five (56%) patients achieved a pathologic complete response (pCR). Three (33%) additional patients had only microscopic residual disease. Five (56%) patients remain alive and disease free with a median follow-up of 3.7 years and median overall survival of 3.2 years. The maximum tolerated dose was vandetanib 100 mg/day.
Conclusions
Vandetanib at 100 mg daily is tolerable in combination with preoperative chemotherapy (5-FU, paclitaxel, carboplatin) and radiation therapy with encouraging efficacy worthy of future study.
Keywords: Esophageal cancer, Radiotherapy, Vandetanib, Surgery, 5-fluorouracil, Paclitaxel, Carboplatin
Introduction
Annually, in the United States 17,040 cases of esophageal cancer are detected, a number staggeringly close to the estimated annual death rate of 15,070[1]. For those patients who are able to undergo surgical resection, cure rates remain at approximately 30% [2]. CALGB 9781, the CROSS trial, and a recent meta-analysis have demonstrated that the addition of neoadjuvant therapy to esophagectomy confers a survival advantage[3–5]. At present, combined chemoradiotherapy and surgery represents the standard of care for patients with locally advanced esophagogastric carcinoma. Two cycles of cisplatin and 5-fluorouracil (5-FU) on days 1 and 29, as used in CALGB 9781[3], or a regimen of weekly carboplatin and paclitaxel[4] have become acceptable radiosensitizing adjuncts to preoperative radiotherapy. Following multimodality therapy, up to 13% of patients develop at least local recurrence, though distant metastases remain the dominant mode of treatment failure, representing 77–84% of all recurrences[6, 7]. Thus, both improvements in systemic therapy and new radiosensitization strategies are urgently needed.
Additional novel chemotherapy regimens have been explored in hope of improving outcomes. A phase I/II trial demonstrated the feasibility of administering weekly paclitaxel, infusional 5-FU and carboplatin in conjunction with radiotherapy. One third of patients in this study achieved a pathologic complete response [8]. A further trial of neoadjuvant concurrent chemoradiation utilizing paclitaxel, infusional 5-FU, and carboplatin also produced encouraging results, with a pathologic complete response rates of 38% and a three year survival of 41% in a cohort of patients with potentially resectable esophageal carcinoma[9].
Vandetanib (ZD6474) is an oral tyrosine kinase inhibitor of VEGFR-2, VEGFR-3, RET and EGFR. VEGF expression has been linked to worse outcome in resectable esophageal cancer [10, 11]. EGFR over-expression is seen in 30–70% of esophageal cancers, and EGFR polysomy in approximately 25% [12–14]. EGFR expression is associated with worse prognosis in patients with locally advanced disease [12, 14, 15]. Pre-clinical work utilizing an esophageal squamous carcinoma cell line has supported the synergy of chemotherapy (both taxanes and platinum analogues) with EGFR inhibition, specifically vandetanib [16]. Xenograft studies evaluating multiple tumor types have demonstrated the potent radiosensitizing effects of vandetanib[17]. Vandetanib is slowly absorbed and eliminated with a median terminal half-life (t½) of approximately 5 days[18]. Common adverse events when utilized as a single agent include rash, diarrhea, hypertension and QTc prolongation[19]. Further work has demonstrated its tolerability when combined with carboplatin and paclitaxel [20, 21]. Based on this data, we conducted a phase I clinical trial to determine the feasibility of VEGFR, RET and EGFR targeting via vandetanib in combination with carboplatin, paclitaxel and 5FU chemoradiotherapy in localized stage II–III operable esophagogastric carcinoma.
Patients and Methods
Patients
Patients aged 18 years and older with previously untreated histologically documented carcinoma of the esophagus, GEJ, or stomach for whom preoperative chemoradiation was appropriate were eligible for enrollment. Adenocarcinomas and squamous cell carcinomas were both permissible. Patients were required to have potentially resectable disease, without evidence of distant metastases. All patients underwent pretreatment multidisciplinary evaluation, a contrast-enhanced CT of the chest and abdomen, esophagogastroscopy and endoscopic ultrasound (EUS) within 4 weeks of registration. Pre-treatment PET was recommended. Patients had to have an ECOG Performance Status of ≤ 2. Exclusion criteria included prior thoracic radiation, severe or uncontrolled systemic disease, uncontrolled hypertension (BP ≥ 160/100), active pregnancy or breast feeding, uncontrolled diarrhea, ANC < 1,500/mm3, platelet count ≤ 100 K/mm3, serum bilirubin > 1.5 times upper limit of normal, serum creatinine > 1.5 times upper limit of normal or creatinine clearance ≤ 50 mL/minute, AST or ALT > 2.5 times upper limit of normal, potassium < 4 mmol/L despite supplementation, and serum calcium or magnesium out of the normal range despite supplementation. Additional exclusion criteria included any of the following cardiac abnormalities: ≥ NYHA class 2 heart failure within 3 months of registration, LVEF < 45%, history of arrhythmia requiring treatment or producing symptoms, asymptomatic sustained ventricular tachycardia presence of a left bundle branch block, baseline QTc which was unmeasurable or ≥ 480 msec using Bazett’s correction, or use of concomitant medications that may cause QTc prolongation, Torsades de Pointes or induce CYP3A4 function. Patients provided written informed consent, and the protocol was approved by the Institutional Review Board of Fox Chase Cancer Center.
Study Design
This was a single-arm phase I dose escalation study. All patients received external beam radiotherapy (RT) with megavoltage linear accelerators (> 6 MV) to deliver multiple (> 2) fields. Patients were treated 5 days per week at 1.8 Gy/day for 25 fractions (total dose 45 Gy). The use of intensity modulated radiation therapy was not permitted. The superior and inferior borders of the field were 3 cm beyond the tumor. Lateral borders were 2 cm beyond the lateral edge of the tumor, as defined by EUS, esophagram or CT. Periesophageal nodes were included. For primary tumors above the carina (proximal esophagus), the supraclavicular nodes received treatment. Lower esophageal and GEJ patients received treatment to clinically negative celiac lymph nodes. All clinically positive lymph nodes received treatment. Dose was prescribed to the isocenter.
Chemotherapy with 5-FU, paclitaxel, and carboplatin was administered concurrently with radiation therapy. 5-FU, 225 mg/m2/day, was delivered via a 96 hour continuous infusion from Monday to Friday through the duration of radiotherapy. Paclitaxel, at a dose of 50 mg/m2, was administered via weekly infusion on days 1, 8, 15, 22, and 29 of radiotherapy. Carboplatin, AUC of 5, was administered via IV infusion on days 1 and 29 of radiotherapy. Vandetanib was administered as a daily tablet, continued from day 1 through the completion of radiation therapy. Dosing levels are summarized in Table 1. In the absence of dose-limiting toxicity, dose escalation would not proceed beyond dose level 3, as 300 mg per day had previously been shown to be the maximum tolerated single agent dose (MTD)[18, 20].
Table 1.
Dose Escalation Chart
| Dose Level | DOSE | ||||
|---|---|---|---|---|---|
| Vandetanib | Paclitaxel | Carboplatin | 5-FU | XRT | |
| Level 1 | 100 mg po qd | 50mg/m2/d qwk | AUC 5 q4wks | 225mg/m2/d PVI M-F | 1.8 Gy/d (total 45.0 Gy) |
| Level 2 | 200 mg po qd | 50mg/m2/d qwk | AUC 5 q4wks | 225mg/m2/d PVI M-F | 1.8 Gy/d (total 45.0 Gy) |
| Level 3 | 300 mg po qd | 50mg/m2/d qwk | AUC 5 q4wks | 225mg/m2/d PVI M-F | 1.8 Gy/d (total 45.0 Gy) |
Radiotherapy was temporarily suspended for ≥ grade 3 neutropenia or thrombocytopenia, vomiting for ≥ 3 days unresponsive to antiemetics, ≥ 5 watery stools per day unresponsive to anti-diarrheals or weight loss of > 10%. Interruption of therapy was to continue until the toxicity had resolved sufficiently to allow for resumption of therapy. For grade 3 toxicities attributable to vandetanib, the drug was held until resolution of toxicity to grade 1 or less. At that point, vandetanib was to be resumed with a dose reduction. If grade 3 or greater toxicity recurred or vandetanib was required to be held for > 3 weeks, the medication was permanently discontinued.
At 21 to 28 days following completion of induction chemoradiation, all patients underwent a repeat staging and pre-operative evaluation with physical examination, PFTs and a contrast-enhanced CT of the chest and abdomen. All patients underwent baseline and pre-operative PET-CT imaging. Esophagectomy was performed 6–8 weeks following completion of induction chemoradiation. The surgical approach was determined by the surgical investigator; transhiatal, Ivor-Lewis, or 3-hole approaches were acceptable. A baseline CT of the chest and abdomen was performed 6–8 weeks after surgery, with subsequent surveillance CT imaging of the chest and abdomen performed every 6 months through 36 months post-therapy, and then annually through 60 months.
Response Assessment
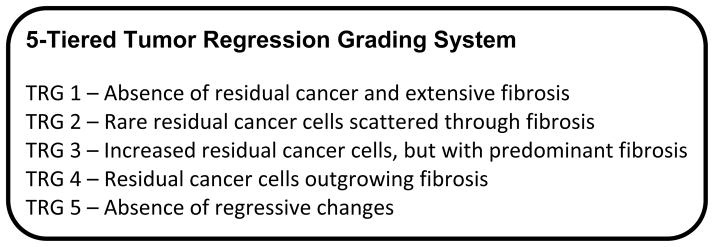
Pathologic response was assessed at the time of surgery. Tumor regression grading was additionally performed by independent pathologic review, utilizing the 5-tiered system previously described by Mandard, et al[22] (Figure 1).
Figure 1.
Dose Limiting Toxicity
Toxicity was scored according to the National Cancer Institute’s Common Toxicity Criteria (NCI CTCAE, version 3.0). Any patient who initiated the chemoradiation protocol and received at least one single dose of vandetanib was considered evaluable for toxicity. Evaluation for toxicity continued through the course of chemoradiation and for a period of 4 weeks post-surgery to evaluate for potential treatment effects on wound healing. DLT was defined as any of the following: ≥ grade 3 non-hematologic toxicity persisting for > 14 days, grade 4 non-hematologic toxicity, grade 4 hematologic toxicity lasting > 7 days, surgical complications of wound healing or hemorrhage grades 4–5 occurring ≤ 4 weeks from surgery, or any toxicity requiring > 14 day interruption of radiation therapy. Late toxicity (onset >90 days since initiation of RT or persisting beyond 90 days) monitoring was assessed using the RTOG Late Radiation Morbidity Scoring Schema.
Dose Escalation Schema
Three patients were entered into each dose level. If no patients experienced a dose-limiting toxicity (DLT), the next dose level was opened. If a DLT was observed in one of three patients, three additional patients (a total of six) were accrued at this level. If DLTs were observed in ≤ one of 6 patients, then escalation to the next level took place. If DLTs were observed in ≥ 2 of 3 or ≥ 2 of 6 patients, then the MTD had been exceeded and no further escalation took place. If not already accrued, three additional patients would be enrolled at the previous dose level. The MTD was predefined as the highest dose level where < 2 patients out of 6 experienced a DLT.
Statistical Methods
Progression-free survival (PFS) was measured from treatment start date until disease progression or death from any cause. Overall survival (OS) was measured from treatment start date until death from any cause. PFS and OS were determined using the Kaplan-Meier method, with times censored at the close-out date for patients still being followed without evidence of disease/death, or the date of last contact for those patients lost to follow-up.
Results
Patient characteristics
Nine patients were enrolled in this study between 3/2009 and 8/2010 at Fox Chase Cancer Center. The median patient age was 60 years (range, 33 – 77). The majority of patients had distal esophageal adenocarcinomas with involved lymph nodes. Further patient and tumor characteristics are listed in Table 2.
Table 2.
Patient and Tumor Characteristics (n = 9)
| Characteristic | Value | Percentage (%) |
|---|---|---|
|
| ||
| Age | ||
| Median | 60 | -- |
| Range | 35–77 | -- |
|
| ||
| Performance status | ||
| 0 | 5 | 56 |
| 1 | 4 | 44 |
|
| ||
| Tumor Location | ||
| Middle third | 1 | 11 |
| Lower third | 6 | 67 |
| GE junction | 2 | 22 |
|
| ||
| Histology | ||
| Squamous cell carcinoma | 2 | 22 |
| Adenocarcinoma | 7 | 78 |
|
| ||
| Stage Grouping (Clinical) | ||
| II | 3 | 33 |
| III | 6 | 67 |
|
| ||
| T Stage (Clinical) | ||
| T2 | 2 | 22 |
| T3 | 7 | 78 |
|
| ||
| N Stage (Clinical) | ||
| N0 | 2 | 22 |
| N1 | 6 | 67 |
| N2 | 0 | 0 |
| N3 | 1 | 11 |
|
| ||
| Grade | ||
| G1 | 2 | 22 |
| G2 | 2 | 22 |
| G3 | 4 | 44 |
| Unavailable | 1 | 11 |
Treatment Delivery
Locoregional therapy
All patients completed the planned course of radiation to the target radiation dose, and proceeded to definitive surgical resection without significant delay. There were no operative mortalities. Five patients did not require any treatment break from radiotherapy. For 2 patients, a treatment interruption of 2 consecutive days was necessary, and for 2 patients a treatment interruption of 5 consecutive days was required. Violation of the radiation treatment guidelines was noted in the second patient on protocol. As opposed to the protocol-defined conventional or 3D-conformal radiotherapy, he initiated therapy with an IMRT plan and received 540 cGy in this fashion. Once this was noted and corrected, he received the remainder of his therapy via either conventional AP/PA fields (900 cGy) or a 7-field 3D-conformal plan (3060 cGy). No further violations were noted through the duration of the protocol.
Chemotherapy
Nine patients were accrued across dose levels 1 and 2 as detailed in Table 1. Three patients were enrolled on dose level 1 without any dose limiting toxicity observed. Therefore, dose escalation proceeded to dose level 2, as planned. After 1 patient was enrolled onto dose level 2, a late sudden death occurred. The first patient enrolled on the trial suffered a fatal aorto-enteric hemorrhage at the gastro-esophageal anastomosis on day 99, more than 60 days since the last dose of vandetanib. Further enrollment to dose level 2 was temporarily halted. Upon review, the fatal event was deemed unlikely related to the study medication, and likely a complication of surgery. However, given that a relationship to the study drug could not be excluded, dose level 1 was then expanded. No DLTs were observed in the next three patients and enrollment thus proceeded to complete dose level 2.
The third patient enrolled at dose level 2 required a dose reduction of 5-FU during week 4 due to occurrence of grade 3 syncope. Chemotherapy was held altogether on day 29 due to grade 3 mucositis, with other therapy administered as planned. Following esophageal resection, his post-operative course was complicated by formation of a gastro-bronchial fistula, which was deemed to be a DLT (grade 4 wound healing) and required surgical repair. After discussion with the Data Safety Monitoring Committee, the decision was made to close the study, with the determination of 100 mg daily of vandetanib as the maximum tolerated dose.
In total, dose interruptions of vandetanib were necessary during chemoradiotherapy for 3 patients (33%). Grade 3 transaminitis necessitated a dose reduction of vandetanib in one patient. Chemotherapy was dose reduced in 2 patients (one in each dose level). In one of these patients it was held during the final 8 days of radiotherapy. One additional patient at each dose level had chemotherapy held for at least 7 days due to toxicity, allowing for the full delivery of radiotherapy in combination with vandetanib.
Toxicity
A summary of common toxicities is provided in Table 3. The most common non-hematologic toxicities were nausea and anorexia. Grade ≥2 nausea and ≥ grade 1 anorexia occurred in 4 patients (44%). Grade 3/4 adverse effects judged to be at least possibly related to vandetanib included anorexia (1), hyponatremia (1), abdominal pain (1), and depression (1). As previously noted, one patient at dose level 1, with a squamous carcinoma of the lower esophagus, suffered a fatal aorto-enteric hemorrhage at the anastomosis, deemed to be unlikely related to study treatment. A second patient, at dose level 2, developed a gastro-bronchial fistula, requiring surgical repair.
Table 3.
Toxicities at least possibly attributed to study treatment (n=9)
| Non-hematologic | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| ALT-SGPT | 0 | 0 | 1 | 0 | 0 |
| Anorexia | 1 | 2 | 1 | 0 | 0 |
| AST-SGOT | 0 | 0 | 1 | 0 | 0 |
| Nausea | 0 | 4 | 0 | 0 | 0 |
| Dehydration | 1 | 2 | 0 | 0 | 0 |
| Depression | 0 | 0 | 1 | 0 | 0 |
| Diarrhea | 0 | 1 | 0 | 0 | 0 |
| Dysphagia | 0 | 1 | 0 | 0 | 0 |
| Fatigue | 1 | 2 | 0 | 0 | 0 |
| Gastro-bronchial | 0 | 0 | 0 | 1 | 0 |
| Hemorrhage | 2 | 0 | 0 | 0 | 1* |
| Hypomagnesemia | 0 | 1 | 0 | 0 | 0 |
| Hyponatremia | 1 | 0 | 1 | 0 | 0 |
| Infection/sepsis | 0 | 1 | 0 | 0 | 0 |
| Mucositis | 0 | 1 | 0 | 0 | 0 |
| Pain, upper | 1 | 0 | 1 | 0 | 0 |
| Rash: dermatitis | 2 | 1 | 0 | 0 | 0 |
| Rash: acneiform | 1 | 0 | 0 | 0 | 0 |
| Syncope | 0 | 0 | 1 | 0 | 0 |
| Vomiting | 1 | 1 | 0 | 0 | 0 |
| Weight Loss | 1 | 0 | 0 | 0 | 0 |
| Hematologic | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Anemia | 4 | 0 | 0 | 0 | 0 |
| Leukopenia | 1 | 3 | 2 | 0 | 0 |
| Neutropenia | 3 | 0 | 1 | 0 | 0 |
Treatment Efficacy
All 9 patients underwent surgical resection (R0) of the primary lesion. Pathologic complete response (TRG 1) was noted in 5 patients (56%), including both of the patients with squamous histology. In 3 additional patients (33%), only rare residual cancer cells (TRG 2) were identifiable upon pathologic review. In sum, 8/9 (89%) patients demonstrated pathologic complete response or only microscopic residual disease at the time of resection.
At the time of this report, median patient follow-up stands at 3.7 years. 3 of the 8 evaluable patients (38%) have developed recurrent disease, all in the form of distant metastases. Five of 9 patients (56%) remain alive and disease free, with a median disease free survival of 2.6 years. Two of 4 evaluable patients (50%) who achieved a pCR remain disease free in follow-up. Median overall survival stands at 3.2 years. Response and outcomes are summarized in Table 4.
Table 4.
Treatment Response and Outcomes
| Patient | Histology | Dose Level | Initial Clinical Stage | Pathologic Response Grade | Pathologic Stage | Status in follow-up |
|---|---|---|---|---|---|---|
| 1001 | Squamous cell carcinoma | 1 | T3N1 | TRG 1 | T0N0 | Fatal GI bleed, day #99 |
| 1002 | Adenocarcinoma | 1 | T3N1 | TRG 2 | T2N0 | NED at 50 months |
| 1003 | Adenocarcinoma | 1 | T2N0 | TRG 2 | T1aN0 | NED at 50 months |
| 2004 | Squamous cell carcinoma | 2 | T3N1 | TRG 1 | T0N0 | Recurrent at 19 months |
| 1005 | Signet cell carcinoma | 1 | T3N1 | TRG 1 | T0N0 | NED at 45 months |
| 1006 | Adenocarcinoma | 1 | T2N1 | TRG 1 | T0N0 | Recurrent at 11 months |
| 1007 | Adenocarcinoma | 1 | T3N1 | TRG 1 | T0N0 | NED at 40 months |
| 2008 | Adenocarcinoma | 2 | T3N3 | TRG 3 | T1bN3 | Recurrent at 11 months |
| 2009 | Adenocarcinoma | 2 | T3N0 | TRG 2 | T2N0 | NED at 32 months |
Discussion
Our phase I study demonstrates that vandetanib can be safely combined with an aggressive chemoradiotherapy treatment program. A high pathologic complete response rate and impressive median survival were observed. Although acute toxicities were not severe, 2 of 9 patients had serious surgical complications and a relationship to vandetanib exposure could not be excluded. For future studies, we recommend a phase II dose of vandetanib of 100 mg daily. Consideration should be given to a longer interval between vandetanib and resection in future studies.
Fistulous tracts between the esophageal lumen and the bronchial airways or vasculature are uncommon complications in the setting of multimodality treatment of esophageal cancer, although minor anastomotic leakages occur at a rate of 22–30% following esophagectomy with or without neoadjuvant therapy[4]. It is established that inhibition of the VEGF pathway can result in wound healing complications[23]. In the treatment of non-small cell lung cancer, initial trials combining radiotherapy with the VEGF-targeted antibody bevacizumab produced several instances of tracheoesophageal fistula formation[24]. The majority of these cases were delayed, occurring many weeks after completion of chemoradiation. Most cases also involved instrumentation in the form of esophageal dilatation or esophageal stent placement. Thus, while the occurrence of two serious events related to complicating fistulas – an aorto-enteric hemorrhage and formation of a gastro-bronchial fistula – rightfully raise concern for the development of regimens combining VEGF inhibition with radiotherapy, the multiple factors at play prohibit firm conclusions of causality. In our trial, these events can be clearly attributed to the tri-modality treatment strategy, though a direct link to the addition of vandetanib cannot be made. In any case, this would necessitate close monitoring if this regimen were to be further investigated.
Although a small trial, our study produced an encouraging rate of pathologic complete responses (56%), substantially higher than the historic range of 21–29% [4, 25, 26]. Multiple studies have linked achievement of a pathologic complete response to superior long term outcomes, suggesting the potential to serve as a surrogate for treatment efficacy as PFS and OS data mature [27–29]. There is suggestion that pathologic assessment of the extent of residual disease may also be predictive of long term outcome; patients who achieve either a complete response or exhibit < 50% residual carcinoma may fare substantially better [30]. In our study, 8 of 9 patients (89%) demonstrated a pathologic CR or microscopic disease only. Thus far, responses have been durable, with a median disease free survival of 2.6 years and overall survival of 3.2 years at the time of reporting.
Our study does have several limitations to consider. While these efficacy data are encouraging, caution in their interpretation is necessary due to the small patient numbers. Our study was designed with a primary objective of evaluating toxicity and tolerability. An additional limitation is the generalizability of the 3-drug chemotherapy backbone we utilized to evaluate the addition of vandetanib. Since our study’s inception, the combination of carboplatin and paclitaxel to radiotherapy has demonstrated improved outcome as preoperative therapy compared to surgery alone. Given the better tolerance of the carboplatin/paclitaxel combination when compared to the older cisplatin/5-FU combination with radiotherapy, the former has gained wide acceptance in combined modality treatment of locally advanced esophageal cancer. As our regimen utilized a carboplatin and paclitaxel backbone, further study would be feasible with of this combination with vandetanib and radiotherapy. An improved toxicity profile may be observed with the administration of weekly carboplatin or elimination of 5-FU. Finally, given the complexity of our chemoradiotherapy backbone, it was quite challenging to definitively exclude additive toxicity or conclude additive efficacy from the addition of vandetanib. Of relevance, a recent trial of a neoadjuvant chemoradiation regimen incorporating infusional 5-FU, cisplatin and weekly docetaxel attained a pCR rate of 47%, which is similar to the pCR rate observed here [31]. However, heterogeneous populations and the histologic differences in response rate limit cross-trial inferences.
In summary, our phase I trial demonstrated that the administration of vandetanib with a chemotherapy regimen of infusional 5-FU, carboplatin and paclitaxel as well as concurrent radiation therapy is feasible in the neoadjuvant setting for treatment of locally advanced esophageal cancer. The MTD and recommended phase II dose of vandetanib is 100 mg daily for future studies of this combination. As toxicities are manageable with encouraging initial efficacy data, further investigation is warranted.
Summary.
Over-expression of VEGF and EGFR is linked to worse outcomes in esophageal cancer. The multi-targeted tyrosine kinase inhibitor, vandetanib, demonstrates potent inhibition of VEGF and EGFR and synergizes with chemotherapy and radiotherapy. This phase I trial evaluated the addition of vandetanib to neoadjuvant concurrent chemoradiotherapy (5-FU, carboplatin, paclitaxel) in the treatment of Stage II-III esophagogastric cancers. This strategy proved to be tolerable with encouraging efficacy. The maximum tolerated dose of vandetanib was 100 mg daily.
Acknowledgments
Source of funding: Supported by AstraZeneca, IIRIS NUMBER: IRUSZACT0064. IA received support of the Russian Government Program of competitive growth of Kazan Federal University. This publication was supported by grant number P30 CA006927 from the National Cancer Institute, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflict of interest
The authors declared no conflicts of interest.
Presented in part at ASCO GI, Jan 19–21, 2012, San Fransisco, CA, USA.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Wijnhoven BP, et al. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg. 2007;245(5):717–25. doi: 10.1097/01.sla.0000251703.35919.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tepper J, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–92. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Hagen P, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 5.Sjoquist KM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–92. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 6.van Hagen P, et al. Recurrence pattern in patients with a pathologically complete response after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. Br J Surg. 2013;100(2):267–73. doi: 10.1002/bjs.8968. [DOI] [PubMed] [Google Scholar]
- 7.Meguid RA, et al. Recurrence after neoadjuvant chemoradiation and surgery for esophageal cancer: does the pattern of recurrence differ for patients with complete response and those with partial or no response? J Thorac Cardiovasc Surg. 2009;138(6):1309–17. doi: 10.1016/j.jtcvs.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anne P, AR, Rosato F, et al. A phase I trial of preoperative paclitaxel, carboplatin, 5-FU, and radiation in patients with resectable esophageal or gastric cancer. J Clin Oncol. 2004;22:4031. [Google Scholar]
- 9.Meluch AA, et al. Preoperative therapy with concurrent paclitaxel/carboplatin/infusional 5-FU and radiation therapy in locoregional esophageal cancer: final results of a Minnie Pearl Cancer Research Network phase II trial. Cancer J. 2003;9(4):251–60. doi: 10.1097/00130404-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Choi JY, et al. Prognostic significance of vascular endothelial growth factor expression and microvessel density in esophageal squamous cell carcinoma: comparison with positron emission tomography. Ann Surg Oncol. 2006;13(8):1054–62. doi: 10.1245/ASO.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, et al. Prognostic value of vascular endothelial growth factor expression in patients with esophageal cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1126–34. doi: 10.1158/1055-9965.EPI-12-0020. [DOI] [PubMed] [Google Scholar]
- 12.Gibault L, et al. Diffuse EGFR staining is associated with reduced overall survival in locally advanced oesophageal squamous cell cancer. Br J Cancer. 2005;93(1):107–15. doi: 10.1038/sj.bjc.6602625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandla S, et al. Comparative genomics of esophageal adenocarcinoma and squamous cell carcinoma. Ann Thorac Surg. 2012;93(4):1101–6. doi: 10.1016/j.athoracsur.2012.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang KL, et al. Expression of epidermal growth factor receptor in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Cancer. 2007;109(4):658–67. doi: 10.1002/cncr.22445. [DOI] [PubMed] [Google Scholar]
- 15.Gibson MK, et al. Epidermal growth factor receptor, p53 mutation, and pathological response predict survival in patients with locally advanced esophageal cancer treated with preoperative chemoradiotherapy. Clin Cancer Res. 2003;9(17):6461–8. [PubMed] [Google Scholar]
- 16.Morelli MP, et al. Sequence-dependent antiproliferative effects of cytotoxic drugs and epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16(Suppl 4):iv61–68. doi: 10.1093/annonc/mdi910. [DOI] [PubMed] [Google Scholar]
- 17.Frederick B, et al. ZD6474, an inhibitor of VEGFR and EGFR tyrosine kinase activity in combination with radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64(1):33–7. doi: 10.1016/j.ijrobp.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 18.Holden SN, et al. Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol. 2005;16(8):1391–7. doi: 10.1093/annonc/mdi247. [DOI] [PubMed] [Google Scholar]
- 19.Kiura K, et al. A randomized, double-blind, phase IIa dose-finding study of Vandetanib (ZD6474) in Japanese patients with non-small cell lung cancer. J Thorac Oncol. 2008;3(4):386–93. doi: 10.1097/JTO.0b013e318168d228. [DOI] [PubMed] [Google Scholar]
- 20.Tamura T, et al. A phase I dose-escalation study of ZD6474 in Japanese patients with solid, malignant tumors. J Thorac Oncol. 2006;1(9):1002–9. [PubMed] [Google Scholar]
- 21.Heymach JV, et al. Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(33):5407–15. doi: 10.1200/JCO.2008.17.3138. [DOI] [PubMed] [Google Scholar]
- 22.Mandard AM, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–6. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Hompes D, Ruers T. Review: incidence and clinical significance of Bevacizumab-related non-surgical and surgical serious adverse events in metastatic colorectal cancer. Eur J Surg Oncol. 2011;37(9):737–46. doi: 10.1016/j.ejso.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Spigel DR, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2010;28(1):43–8. doi: 10.1200/JCO.2009.24.7353. [DOI] [PubMed] [Google Scholar]
- 25.Walsh TN, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335(7):462–7. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 26.Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185(6):538–43. doi: 10.1016/s0002-9610(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 27.Defoe SG, et al. Retrospective review of patients with locally advanced esophageal cancer treated at the University of Pittsburgh. Am J Clin Oncol. 2011;34(6):587–92. doi: 10.1097/COC.0b013e3181f942af. [DOI] [PubMed] [Google Scholar]
- 28.Chirieac LR, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103(7):1347–55. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 29.Berger AC, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23(19):4330–7. doi: 10.1200/JCO.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Wu TT, et al. Excellent interobserver agreement on grading the extent of residual carcinoma after preoperative chemoradiation in esophageal and esophagogastric junction carcinoma: a reliable predictor for patient outcome. Am J Surg Pathol. 2007;31(1):58–64. doi: 10.1097/01.pas.0000213312.36306.cc. [DOI] [PubMed] [Google Scholar]
- 31.Pasini F, et al. Neoadjuvant therapy with weekly docetaxel and cisplatin, 5-fluorouracil continuous infusion, and concurrent radiotherapy in patients with locally advanced esophageal cancer produced a high percentage of long-lasting pathological complete response: a phase 2 study. Cancer. 2013;119(5):939–45. doi: 10.1002/cncr.27822. [DOI] [PubMed] [Google Scholar]