Abstract
Purpose
A prominent symptom of Myalgic Encephalomyelitis, Chronic Fatigue Syndrome, or Systemic Exertion Intolerance Disease (ME/CFS/SEID) is persistent fatigue that is worsened by physical exertion. Here the population effect of a single bout of exercise on fatigue symptoms in people with ME/CFS/SEID was estimated and effect moderators were identified.
Methods
Google Scholar was systematically searched for peer-reviewed articles published between February 1991 and May 2015. Studies were included where people diagnosed with ME/CFS/SEID and matched control participants completed a single bout of exercise and fatigue self-reports were obtained before and after exercise. Fatigue means, standard deviations, and sample sizes were extracted to calculate effect sizes and the 95% CI. Effects were pooled using a random-effects model and corrected for small-sample bias to generate mean Δ. Multi-level regression modeling adjusted for nesting of effects within studies. Moderators identified a priori were diagnostic criteria, fibromyalgia comorbidity, exercise factors (intensity, duration, type) and measurement factors.
Results
Seven studies examining 159 people with ME/CFS/SEID met inclusion criteria, and 47 fatigue effects were derived. The mean fatigue effect was Δ = 0.73 (95% CI = 0.24, 1.23). Fatigue increases were larger for people with ME/CFS/SEID when fatigue was measured four or more hours after exercise ended rather than during or immediately after exercise ceased.
Conclusions
This preliminary evidence indicates that acute exercise increases fatigue in people with ME/CFS/SEID more than in control groups, but effects were heterogeneous between studies. Future studies with no-exercise control groups of people with ME/CFS/SEID are needed to obtain a more precise estimate of the effect of exercise on fatigue in this population.
Keywords: acute, energy, mood, myalgic encephalomyelitis, exertion intolerance, malaise
Introduction
Myalgic encephalomyelitis, chronic fatigue syndrome, or Systemic Exertion Intolerance Disease (ME/CFS/SEID) is a medical condition in which the dominant symptom is unexplained fatigue that persists for at least 1 month (6, 11, 17, 56). Estimated prevalence of ME/CFS/SEID in the United States and England ranges from 0.007% to 2.8% (16, 27, 47, 55, 59). Diagnosis remains difficult because ME/CFS/SEID has no identified cause and requires that other illnesses and causes of fatigue be excluded (24). ME/CFS/SEID may be triggered by viral infections or environmental factors that cause immune dysfunction and inflammation (3) or autonomic and central nervous system pathologies, but the etiology remains uncertain (24). Although exercise-induced fatigue is a defining diagnostic feature, a full understanding of how much acute exertion or exercise might increase ME/CFS/SEID fatigue is unknown.
The lack of an apparent single cause of ME/CFS/SEID has led to a variety of treatments and to comparison studies of treatments (71). Exercise training increases feelings of energy and reduces feelings of fatigue in the general population (53) and has been used as a ME/CFS/SEID treatment. In 2004, a meta-analysis of five randomized controlled trials quantified the effect of exercise training on fatigue. Twelve weeks of exercise training resulted in a moderate-to-large reduction in fatigue when compared to control conditions. Exercise training combined with drug treatment (fluoxetine) was more effective than drug treatment alone, and exercise training combined with patient education was more effective than education alone (12). A 2011 meta-analysis reported that graded exercise training (5 trials) and cognitive behavioral therapy (16 trials) were equally effective for treating people with CFS/ME on a range of outcomes, including functional impairment, depression, anxiety, and fatigue (8). A 2015 quantitative review of eight randomized controlled trials including 1,518 CFS participants confirmed that exercise training more effectively reduced fatigue than passive control conditions such as usual care, relaxation, or flexibility training (32). This body of evidence suggests that exercise training could be efficacious for reducing fatigue states among many people with ME/CFS/SEID, although some individuals report that exercise training worsens their fatigue (63).
This literature has led to recommendations for designing exercise programs for people with ME/CFS/SEID (2, 64). But concern remains that acute physical exertion may result in adverse events for some people with ME/CFS/SEID (29, 30). Indeed, an exacerbation of symptoms after acute exertion is used to diagnose ME/CFS/SEID (11, 17). Fatigue is reduced after standardized (e.g., relative to each individual’s aerobic capacity) low-intensity acute exercise lasting at least 20 minutes in people without ME/CFS/SEID (38). No standardized acute exercise stimulus is used in diagnosis of ME/CFS/SEID, although it is noted that “minimal” activity may provoke symptoms in some people (7, 11). The treatment potential of exercise training and the risk of increased fatigue after acute exercise are paradoxical, as chronic exercise adaptations are often viewed as the cumulative effect of many acute bouts of exercise on both physiological (62) and psychological (52) outcomes.
Studies have measured the effect of acute exercise on fatigue states in people with ME/CFS/SEID, but the exercise features have varied (31, 34, 36, 42, 50, 58, 65). The purpose of this systematic review and meta-analysis was to estimate the size of the mean effect of completing a single bout of exercise on fatigue states reported by people with ME/CFS/SEID. A second objective was to determine what patient characteristics, exercise stimulus factors, or measurement features moderate the size of the effect.
Methods
This meta-analysis was completed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (33).
Data Searches
A search was conducted in Google Scholar for peer-reviewed studies written in English and published between February 1991 and May 2015. A single “Advanced Scholar Search” was conducted for articles with the exact phrase “Chronic Fatigue Syndrome” AND “exercise”. The reference list of each article that met the inclusion criteria was manually searched for relevant studies in addition to the reference lists of relevant review articles (8, 12, 14, 41, 48, 57).
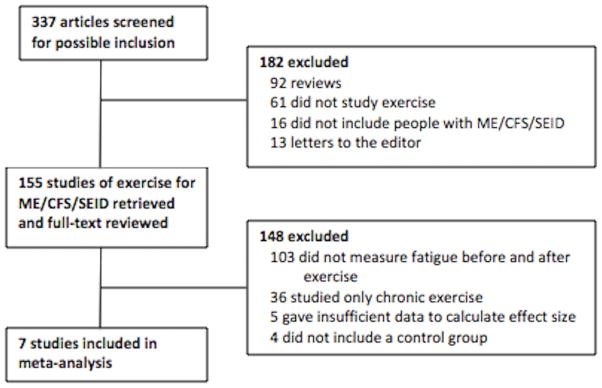
The inclusion criteria used to screen studies for the analysis was the following: (i) ME/CFS/SEID and healthy control participants completed a single bout of exercise; (ii) participants were diagnosed with CFS/ME using either the Centers for Disease Control (CDC)/Fukuda criteria (17), the Canadian Consensus Criteria (6), or the Myalgic Encephalomyelitis Consensus Criteria (7); (iii) self-reported fatigue was measured using a unipolar scale immediately before and after exercise; and (iv) data usable for the analysis appeared in a peer-reviewed publication written in the English language. A flow diagram (Figure 1) shows the process of study selection.
Figure 1.
Flow diagram of study selection. ME/CFS/SEID = Myalgic Encephalomyelitis/Chronic Fatigue Syndrome/Systemic Exertion Intolerance Disease.
Study Characteristics
A total of 7 studies contributing 47 total effects (median = 3 effects per study) met the inclusion criteria. The studies examined 159 people with ME/CFS/SEID, with a median of 13 people per study. Additional study features are shown in Table 1. Data were presented in text for 18 effects and in figures for 29 effects. Lead authors who presented data in figures were contacted by email with requests for precise data before the final analyses were completed. The lead author of one study (34) provided data and the final meta-analysis included 5 effects that were derived from figures. The first and second author independently derived all effects and a two-way (effects x raters) intraclass correlation for absolute agreement was calculated to test the reliability of effect size calculations. There was high initial agreement between authors (ICC [2, 46]=0.97, 95% CI=0.95, 0.98) and remaining discrepancies due to mathematical or data extraction errors were resolved.
Table 1.
Characteristics of Included Studies
| Authors | # of Effects | n (sex) | Fibromyalgia | Exercise Type (code) | Exercise Intensity (code) | Exercise Duration | Diagnostic Criteria | Fatigue Measure | Measurement Time (H) |
|---|---|---|---|---|---|---|---|---|---|
| LaManca et al. (1999) | 1 | 20 (f) | NR | treadmill exercise test (continuous-increasing) | maximal effort (vigorous) | NR (exhaustion) | CDC/Fkukuda | 0–3 Likert scale | 24 |
| Light et al. (2012) | 6 | 15 (m, f) | CFS only | arm-leg cycle ergometer (continuous-steady) | 70% age-predicted HR max (moderate) | 25 min | CDC/Fukuda | mental fatigue VAS | during exercise, 0, 0.5, 8, 24, 48 |
| “ | 6 | 15 (m, f) | CFS only | arm-leg cycle ergometer (continuous-steady) | 70% age-predicted HR max (moderate) | 25 min | CDC/Fukuda | physical fatigue VAS | during exercise, 0, 0.5, 8, 24, 48 |
| “ | 6 | 33 (m, f) | FM | arm-leg cycle ergometer (continuous-steady) | 70% age-predicted HR max (moderate) | 25 min | CDC/Fukuda | mental fatigue VAS | during exercise, 0, 0.5, 8, 24, 48 |
| “ | 6 | 33 (m, f) | FM | arm-leg cycle ergometer (continuous-steady) | 70% age-predicted HR max (moderate) | 25 min | CDC/Fukuda | physical fatigue VAS | during exercise, 0, 0.5, 8, 24, 48 |
| Lloyd et al. (1994) | 3 | 12 (m) | CFS only | hand grip dynamometer (discontinuous) | 40% MVC (light) | 30 min | CDC/Fukuda | POMS-fatigue | 0.1, 4, 24 |
| Meyer et al. (2013) | 3 | 13 (m, f) | CFS + FM | cycle ergometer exercise test (continuous-increasing) | maximal effort (vigorous) | NR (exhaustion) | CDC/Fukuda | POMS-fatigue | 0, 48, 72 |
| “ | 3 | 13 (m, f) | CFS + FM | cycle ergometer exercise test (continuous-increasing) | maximal effort (vigorous) | NR (exhaustion) | CDC/Fukuda | MFI-mental fatigue | 0, 48, 72 |
| “ | 3 | 13 (m, f) | CFS + FM | cycle ergometer exercise test (continuous-increasing) | maximal effort (vigorous) | NR (exhaustion) | CDC/Fukuda | 0–100 fatigue VAS | 0, 48, 72 |
| Peterson et al. (1994) | 2 | 10 (m, f) | NR | treadmill walk (continuous-steady) | 1 mph (light) | 30 min | CDC/Holmes et al. | 0–10 fatigue VAS | 0.1, 0.66 |
| Sisto et al. (1996) | 1 | 9 (f) | NR | treadmill exercise test (continuous-increasing) | maximal effort (vigorous) | NR (exhaustion) | CDC/Fukuda | POMS-fatigue | 96 |
| “ | 1 | 10 (f) | NR | treadmill exercise test (continuous-increasing) | sub-maximal effort (vigorous) | NR (exhaustion) | CDC/Fukuda | POMS-fatigue | 96 |
| Van Oosterwijck et al. (2010) | 2 | 22 (f) | NR | cycle ergometer exercise test (continuous-increasing) | incremental up to 75% HR max (moderate) | NR (to 75%) | CDC/Fukuda | 0–100 fatigue VAS | 0, 24 |
| “ | 2 | 22 (f) | NR | cycle ergometer (continuous-steady) | < 80% anaerobic threshold (moderate) | NR (self-selected) | CDC/Fukuda | 0–100 fatigue VAS | 0, 24 |
| “ | 1 | 22 (f) | NR | cycle ergometer exercise test (continuous-increasing) | incremental up to 75% HR max (moderate) | NR (to 75%) | CDC/Fukuda | CIS-fatigue subscale | 0 |
| “ | 1 | 22 (f) | NR | cycle ergometer (continuous-steady) | < 80% anaerobic threshold (moderate) | NR(self-selected) | CDC/Fukuda | CIS-fatigue subscale | 0 |
Note. n = number of ME/CFS patients per effect, FM=fibromyalgia comorbidity, NR = not reported, mixed = both HR = heart rate, MVC = maximum voluntary contraction, VAS = visual analog scale, POMS = Profile of Mood States, CIS = Checklist Individual Strength.
Effect size calculation
Hedges g effect sizes were calculated by subtracting the change in the control group (post-exercise fatigue mean minus pre-exercise fatigue mean) from the change in the ME/CFS/SEID group. This difference was divided by the pre-exercise pooled standard deviation (20). Positive effect sizes were calculated when larger post-exercise fatigue increases occurred in the CFS/ME/SEID group and negative effect sizes indicated larger post-exercise fatigue increases for the control group. Effect sizes were corrected for small sample bias to yield Hedges d (20, 35).
Moderator Selection and Coding
Several factors were hypothesized to influence the size and/or direction of the effect of acute exercise on fatigue. These factors, or moderators, were chosen and coded based on a priori rationale and the data consistently reported in the included studies. The seven moderators selected were categorized as diagnostic characteristics (ME/CFS diagnosis criteria, fibromyalgia comorbidity), exercise stimulus factors (intensity, type), or measurement features (construct, format, measurement time after exercise).
Diagnostic Characteristics
Although some organizations have worked to create international consensus criteria for diagnosing ME (7) and SEID (11), others suggest the varied diagnostic criteria reflect the continued debate and uncertainty over causes of ME/CFS (24). To reflect this debate, studies were coded based on the CDC (17), Oxford (56), or Canadian (6) criteria. Since fibromyalgia and ME/CFS are often comorbid (7), studies were also coded based on the inclusion of at least one person diagnosed with fibromyalgia using the American College of Rheumatology criteria (74).
Exercise Stimulus Factors
Light and moderate, but not vigorous, intensity exercise has previously been reported to reduce fatigue (38). Exercise intensity was coded as light, moderate, or vigorous according to a position stand from the American College of Sports Medicine (19). The exercise type used in each study was coded as continuous-increasing, continuous-steady, or discontinuous.
Measurement Features
The specific construct measured was coded (overall fatigue, mental fatigue, physical fatigue) since previous research has reported different effects of acute exercise on mental and physical fatigue (37). Visual analog scales (VAS) are more precise and potentially more sensitive to change than categorical questionnaires, therefore the measurement format was coded, as was measurement time (during to immediately after exercise, 5 minutes to ≤1 hour after, and ≥4 hours after exercise cessation).
Statistical Analysis
The mean effect size (Δ) and the 95% confidence interval (CI) were computed overall using a random effects model (35). The I2 statistic was used to test the heterogeneity of mean effects (22) and funnel plots with Egger’s test were used to examine risk of publication bias (13).
IBM SPSS version 20 was used with macros (73) to calculate the overall mean effect size and to conduct a moderator analysis using multiple linear regression analysis with maximum likelihood estimation. Use of more than one scale to measure fatigue or repeated time points yielded effects that were nested within studies (median of 3 effects per study) that might systematically differ from each other. Hence, a multi-level model with robust maximum likelihood estimation in Mplus 7.3.1 (46) was used to adjust for between-study variance and correlated effects within studies according to standard procedures (10, 25). Parameters and their errors were estimated with clustering on study using the Huber-White sandwich estimator to calculate standard errors that are robust to heteroscedasticity and correlated effects (15, 70, 72). Although simulations indicate that regression coefficients in multi-level models can be estimated without substantial bias in as few as 10 studies (39), maximum likelihood estimates of standard errors will be too small (39). Here, Monte Carlo integration was used provide robust maximum likelihood estimates (46). The conditional model (which included the intercept and the moderators) was compared with the unconditional intercept-only model using a likelihood ratio test and the adjusted Bayesian Information Criterion (BIC) (25). Two-way and three-way interactions of the exercise stimulus (i.e., intensity and type) and the time when fatigue was measured were also tested the same way in a single multiple linear regression model.
Results
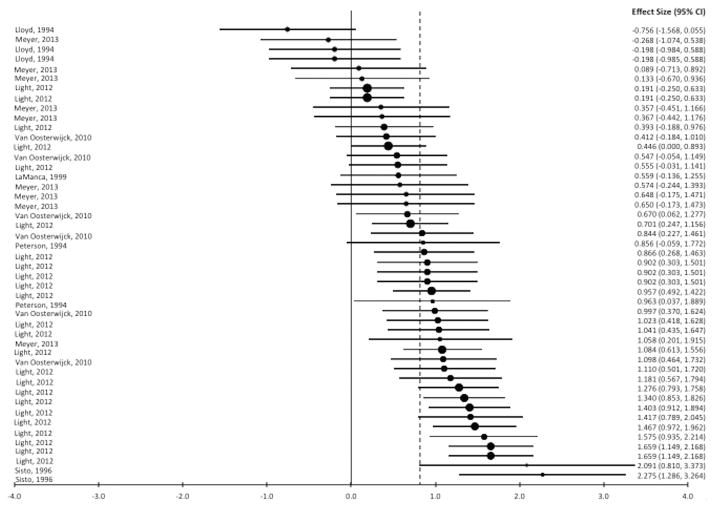
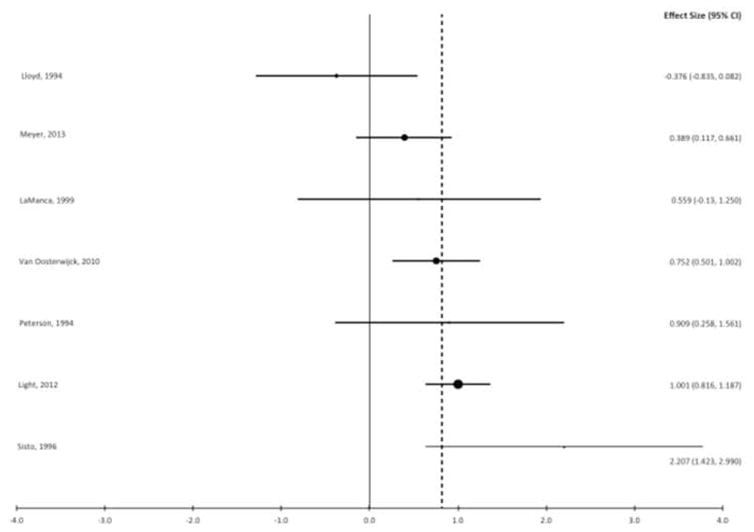
Fatigue increased after exercise in forty percent (19 of 47) of the effects for control groups and ninety-one percent (43 of 47) of the effects for ME/CFS/SEID. The distribution of effect sizes is shown in a forest plot (Figure 2, Panel A) and the weighted effect size of each study is shown in Figure 2, Panel B. Ninety-one percent (43 of 47) of the effects were positive which indicates that post-exercise fatigue increases were larger for people with ME/CFS/SEID than control participants. The mean effect size was Δ = 0.82 (95% CI = 0.66, 0.97) and heterogeneity was moderately high, I2 = 67% (95% CI = 61%, 72%). In the multi-level model (χ2 (2) = 190.1, BIC = 191.6) the mean delta was 0.73 (95% CI = 0.24, 1.23) with non-significant variance between studies (0.37, SE = 0.28, z = 1.29, p = .198). However, the between-study variance accounted for 55% of the total variance (ICC = .55). Because statistical power to detect variance that size was low here (.54 at an alpha of .05)(25), the planned moderator analysis was conducted using multilevel modeling to adjust standard errors for between-study variance.
Figure 2.
Panel A. Forest plot of fatigue effect sizes. Negative values represent larger increases in post-exercise fatigue from baseline for the control group, and positive values represent larger fatigue increases for the ME/CFS/SEID group. Each lead author name corresponds to a single effect (listed to the right of the name) derived from that study. The dashed line indicates the mean effect size.
Panel B. Forest plot of fatigue effect sizes for each individual study. Negative values represent larger increases in post-exercise fatigue from baseline for the control group, and positive values represent larger fatigue increases for the ME/CFS/SEID group. Each lead author name corresponds to an overall weighted mean effect size (listed to the right of the name) for the cited study. The dashed line indicates the mean effect size.
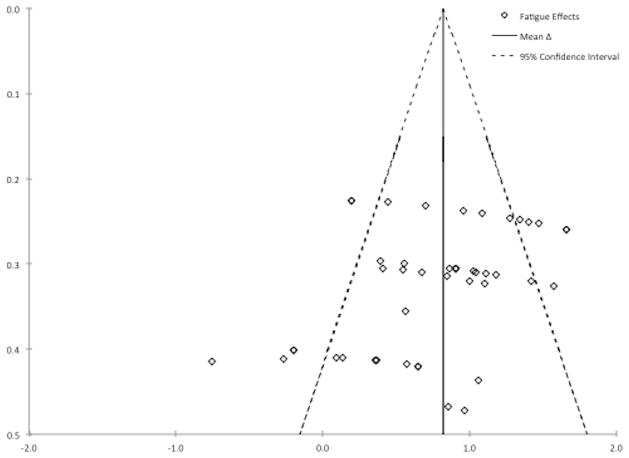
A funnel plot (Figure 3) of individual fatigue effects was asymmetrical. Visual examination of the funnel plot suggests that effects with larger standard errors were more likely to be zero or negative, showing a decrease or no change in fatigue for ME/CFS/SEID groups (outside the low 95% CI). Effects with smaller standard errors were more often positive (outside the high 95% CI), and should be interpreted as showing a larger increase in fatigue for ME/CFS/SEID than control groups. The estimate of the mean effect size also does not increase in precision as standard error decreases. Egger’s test for bias was statistically significant, F(1,46) =12.12, p = .001. However, funnel plot asymmetry and potential bias identified via Egger’s test can yield false-positive indication of bias when the true effect is actually larger in effects with small sample size because of unique characteristics of the sample population (60). A sensitivity analysis that excluded the four smallest and five largest outlying effects yielded a similar mean effect of exercise on fatigue, Δ = 0.81 (95% CI = 0.69, 0.94) and heterogeneity remained moderately high, I2 = 43% (95% CI = 31%, 53%). The multi-level (χ2 (2) = 132.1, BIC = 133.1) mean Δ was 0.76 (95% CI = 0.54, 0.97) with non-significant variance between studies (.013, SE = .015, z = 0.86, p = .392).
Figure 3.
Funnel plot of fatigue effect sizes. Effect size is on the x-axis and standard error is on the y-axis. Effects are expected to form a funnel shape in the absence of publication bias.
Univariate moderator results are shown in Table 2. Exercise intensity significantly moderated the effect, QB(2,44) = 13.29, p = 0.001 with only the 95% CI for light intensity exercise including zero. Exercise type also significantly moderated the effect, QB(2,44) = 21.48, p < 0.001. Discontinuous (handgrip) exercise resulted in a negative effect size estimate indicating larger increases in post-exercise fatigue for control than ME/CFS/SEID groups. Continuous-increasing and continuous-steady exercise both increased post-exercise fatigue and continuous-steady exercise led to the largest increases in fatigue for people with ME/CFS/SEID relative to control groups. The time that fatigue was measured also moderated the effect, QB(2,46) = 12.24, p = 0.002. The effect size was smaller when fatigue was measured during or ≤l hour after exercise compared to measurements made ≥4 hours after exercise. The construct measured (QB (2,44) = 10.85, p = 0.004) and the measurement format (QB (1,45) = 10.48, p = 0.001) also moderated the effect size (see Table 2). Fatigue was increased in ME/CFS/SEID regardless of the fatigue construct or scale format, but effects were significantly larger when measures specified fatigue as mental or physical and when visual analog scales were used.
Table 2.
Univariate Results for Fatigue Moderator Variables
| Effect Moderator | Number of Effects | Effect Size d (95% CI) | P Value |
|---|---|---|---|
| Patient Diagnostic Characteristics | |||
| Fibromyalgia Comorbidity | |||
| Yes | 21 | .808 (.580 to 1.036) | .73 |
| No | 15 | .755 (.481 to 1.029) | |
| Not reported | 11 | .931 (.590 to 1.271) | |
| Characteristics of the Exercise Stimulus | |||
| Intensity | |||
| Very Light to Light | 5 | .081 (-.402 to .565)a | .001 |
| Moderate | 30 | .955 (.795 to 1.115)b | |
| Vigorous/Incremental | 12 | .618 (.305 to .931)a,b | |
| Type | |||
| Continuous-increasing | 15 | .654 (.394 to .913)a | <.001 |
| Continuous-steady | 29 | .971 (.815 to 1.127)b | |
| Discontinuous | 3 | -.379 (-.961 to .203)c | |
| Measurement Features | |||
| Fatigue Measurement Time (hours) | |||
| During exercise - < 5 min post | 11 | .469 (.205 to .732)a | .002 |
| 5 min - 1 hr post | 10 | .732 (.448 to 1.016)a | |
| ≥4 hr post | 26 | 1.034 (.850 to 1.219)b | |
| Construct Measured | |||
| Overall Fatigue | 23 | .558 (.340 to .776)a | .004 |
| Mental Fatigue | 12 | .891 (.637 to 1.144)b | |
| Physical Fatigue | 12 | 1.112 (.855 to 1.368)b | |
| Measurement Format | |||
| Visual Analog Scale | 33 | .949 (.790 to 1.108)a | .001 |
| Categorical Questionnaire | 14 | .412 (.129 to .696)b | |
moderator levels with a different superscript differ significantly. The p value is for q-between.
Multiple linear regression analysis indicated that only intensity (B = .291, z = 2.23, p = .026) and the time when fatigue was measured (B = .657, z = 4.88, p < .001) were independently related to the increase in fatigue in ME/CFS/SEID relative to control groups. Effects for fatigue were greater after moderate intensity exercise and when fatigue was measured four hours or more after exercise ended. Type of exercise (B = -.007, z = 0.067, p = .95), fatigue construct (B = .153, z = 1.07, p = .29), and measurement format (B = .120, z = 0.598, p = .55) were not significantly related to effect size. After multi-level adjustment of standard errors, only the time when fatigue was measured was related to fatigue effect size (B = .683, z = 6.59, p < .001). Model fit (χ2 (7) = 151.9, BIC = 156.9) was improved (Δ χ2 (5) = −43.6, p < .001) compared to the intercept-only model (χ2 (2) = 190.1, BIC = 191.6). This effect remained in the sensitivity analysis that removed outlying effects (B = .580, z = 7.86, p < .001). Two-way and three-way interactions of the exercise stimulus (i.e., intensity and type) and the time when fatigue was measured were not significant (p-values ≥ .186).
Discussion
The primary finding of this meta-analysis is that a single session of exercise resulted in larger fatigue increases for people with ME/CFS/SEID than control groups. The overall effect size is consistent with previous reports of increased fatigue after exertion in ME/CFS/SEID (6, 7, 11, 17). Multiple regression analysis of moderators indicated that intensity of exercise and the time when fatigue was measured were independent influences on variation in fatigue effects. However, the heterogeneity of the fatigue effects was mainly explained by variation between studies. After multi-level adjustment of standard errors for between-study variation, only the time when fatigue was measured influenced effect size. The increase in fatigue among people with ME/CFS/SEID was greater when fatigue was measured more than four hours (and up to 96 hours) after exercise ended than when fatigue was measured during or within 40 minutes after the exercise session ceased. A previous meta-analysis of 16 studies, none of which included people diagnosed with ME/CFS/SEID, also reported that post-exercise fatigue effects were heterogeneous, but that fatigue was reduced in people with elevated fatigue when at least 20 minutes of exercise was performed at a light or moderate intensity (38). Thus, findings here and other meta-analytic reviews have found the fatigue effect size is influenced by exercise intensity, although fatigue is still generally increased post-exercise in people with ME/CFS/SEID.
Exercise intensity significantly moderated the size of the fatigue effect as moderate or vigorous intensity exercise increased post-exercise fatigue, but fatigue was not significantly increased in two studies of light intensity exercise (36, 50). Light intensity exercise has previously been shown to reduce fatigue in people with elevated fatigue, but not diagnosed with ME/CFS/SEID (38), although others have stated that any exertion may increase fatigue in ME/CFS/SEID (11). In addition, fatigue may not be caused by moderate-to-vigorous exercise itself, but could theoretically be triggered by related factors including post-exercise muscle injury, inflammation, or psychological stress caused by performing vigorous intensity exercise (41). Since only two studies contributing 5 effects (11% of the total) examined light intensity exercise, confidence in this result is minimal and future studies should examine the effect of light intensity on fatigue in people with ME/CFS/SEID.
The finding that fatigue is elevated from baseline for several days after exercise is not unexpected given that post-exertional malaise sometimes lasting greater than 24 hours is part of the diagnostic criteria for ME/CFS/SEID (6, 7, 11, 17). This result remained significant when a sensitivity analysis was conducted that included only studies that measured fatigue multiple times after exercise. Post-exertional malaise is considered a separate feature of ME/CFS/SEID that is related to fatigue, but also includes a cluster of other symptoms (e.g., pain, mood disturbance) that are worsened after exertion (26). Although one prior study suggested that symptoms categorizing post-exertional malaise do not peak until five days after exercise is performed (75), an unexpected result here was a larger fatigue effect size when measurements were conducted over 4 (and up to 96) hours post-exercise. There are several reasons this could have occurred. Delayed onset muscle soreness peaks ~48 hours after exercise is performed and is associated with pain and inflammation of the muscle (9). Although the exact mechanism of delayed onset muscle soreness is unknown, neutrophils may have a role (9). Neutrophils may also be regulated by the inflammatory cytokine IL-6 (28) and IL-6 has also been suggested as a fatigue mediator in both exercise and illness (66). In one study, the increase from baseline in several pro-inflammatory cytokines (IL-6, IL-1β) was larger at 8 hours post-exercise for ME/CFS/SEID than control participants and VAS ratings of mental and physical fatigue were also greater (69). Cessation of regular daily exercise also results in fatigue increases that peak 48 hours after the last exercise session (43), although there is no clear neurobiological explanation for this effect. Most (21 of 25, 84%) of the fatigue ratings made ≥4 hours after exercise were conducted in a laboratory setting along with other physiological measures, which might limit the influence of extraneous factors on fatigue ratings.
A potential limitation of the literature meta-analyzed here was the imprecise prescription of exercise intensity. One study used an absolute exercise prescription, i.e. 1 mph walk (50). Prescribing unstandardized exercise, rather than relativizing to fitness level, may lead to an over or underestimation of intensity depending on the fitness level of the participant. Another study assigned exercise intensity based on age-predicted heart rate max (34). Precise exercise prescription is important because intensity moderates fatigue (38). There is a possibility of substantial error when prescribing exercise intensity by age-predicted maximum heart rate because age-predicted maximum heart rate is only moderately correlated (r = 0.73) with actual maximal heart rate (61). Three other studies examined the effects of progressive, maximal exercise tests on fatigue (31, 42, 58). Such tests are used to assess cardiorespiratory fitness but do not represent the normal activity patterns of people with CFS/ME/SEID (67). This limitation may have influenced the precision of the exercise intensity moderator analysis. Since only two studies prescribed exercise relativized to the fitness level of each participant (36, 65), additional experiments are needed that document the effect of light and moderate intensity exercise in ME/CFS/SEID. In addition, trials that have reported fatigue reductions after chronic exercise training have prescribed exercise based on cardiorespiratory fitness tests (18, 68, 71). Future ME/CFS/SEID acute exercise studies should also conduct graded exercise tests to determine V̇O2peak (45), and then prescribe chosen exercise intensity as a percentage of the peak (19).
It remains uncertain why exercise training may effectively reduce fatigue in people with ME/CFS/SEID (8, 12, 32, 71), while the results of this meta-analysis support a worsening of fatigue after a single session of exercise. Differences between the respective bodies of literature include the study design, exercise stimulus chosen, and fatigue measures used. None of the studies in the meta-analysis included sedentary control conditions to account for confounding variables associated with the passage of time. For example, fatigue could change due to completion of experimental cognitive tasks (37) or circadian variation (54) and it is uncertain if changes in fatigue reported in the analysis here are due to exercise itself or extraneous factors. Conversely, exercise training trials have often compared exercise to a sedentary treatment, such as drug (68) or relaxation (32). Future acute exercise studies should use control groups or conditions, and it is recommended that repeated-measures designs be utilized because of the possibility that ME/CFS/SEID is not a homogeneous condition (1). No prior acute exercise study has used a within-subjects design with random assignment to exercise or quiet rest. It is unknown how the fatigue responses to exercise may differ as a function of time within the same person. Even though prior studies have shown that acute, high intensity exercise increases fatigue (51), it is possible these individuals would have reported even greater fatigue had they not exercised.
Second, when chronic exercise has reduced fatigue in people with ME/CFS/SEID (68, 71) graded exercise training is used where training begins at 40% of maximum heart rate (18), which is categorized as light intensity exercise (19). In the present meta-analysis, only 2 small studies (accounting for <15% of the patients) tested the effect of light intensity exercise on fatigue. Only one study included in the analysis examined a form of resistance exercise (36) and no studies were located that examined acute weight lifting exercise. Single sessions of weight lifting have previously been shown to reduce fatigue (21). In people who are not diagnosed with ME/CFS/SEID both acute aerobic and resistance exercise are equally efficacious for reducing fatigue (38). Acute weight lifting exercise could be more effective for people with ME/CFS/SEID because the only included study examining discontinuous exercise found statistically non-significant decreases in post-exercise fatigue. A single bout of weight lifting may also confer additional mental health benefits, including reductions in anxiety and depression symptoms, improved sleep-quality, enhanced cognition and increased self-esteem (49). It is recommended that future experiments determine if a single bout of weight lifting exercise is efficacious or harmful for people diagnosed with ME/CFS/SEID and that larger studies examine the effect of light intensity exercise (aerobic or resistance) on fatigue in this population. Future studies should also investigate the benefit of other forms of discontinuous exercise, as one study found larger fatigue reductions immediately after intermittent cycling sprints than during continuous exercise (44).
Third, the paradox (in the published literature) between acute and chronic exercise in ME/CFS/SEID may be related to fatigue measurement. In the moderator analysis, VAS resulted in larger fatigue effect sizes than categorical scales, although this did not remain significant after adjustment for correlated effects within studies. Previous exercise training trials in ME/CFS/SEID have used categorical scales (68, 71) rather than VAS, and some categorical scales may also measure multiple symptoms besides fatigue alone (23). In addition, baseline fatigue may also impact the effect of acute exercise on fatigue (38). This meta-analysis was not able to consider baseline fatigue as a moderator because some studies used measures without established normative values, which makes it difficult to directly compare fatigue measures. Future studies should use fatigue measures with normative values so such comparisons may be conducted.
Finally, related neurobiological precedent exists for an acute-chronic paradox. Initial treatment of anxiety disorders with selective serotonin reuptake inhibitors (SSRIs) can produce an increase in anxiety symptoms and the processing of anxiety-related stimuli (4), but over time the SSRIs are effective for the treatment of anxiety disorders (40). The available evidence from rodents supports a model to explain the paradox; specifically, that acute SSRI administration acts directly on the bed nucleus of the stria terminalis and central amygdala to augment the expression of cued fear conditioning while chronic SSRI treatment yields inhibition of fear learning by modulating glutamatergic actions in the amygdala (5). More human and animal data are needed before a plausible, defensible model can help to explain why acute exercise increases fatigue symptoms and chronic exercise reduces fatigue symptoms in people with ME/CFS/SEID.
Conclusion
The systematic review and meta-analysis limited to 7 studies including 159 people with ME/CFS/SEID found that a single bout of exercise moderately increased post-exercise feelings of fatigue in people diagnosed with ME/CFS/SEID more than control participants. Although more studies are needed to verify the accuracy of the effects estimated here, the effects size distributions observed here were each moderately heterogeneous and moderated by exercise intensity and the time after exercise that fatigue was measured. Based on the published acute exercise literature meta-analyzed here, it is too soon to conclude that all doses of exercise cause fatigue. Future experiments should be conducted to further elucidate the potential efficacy or harm of a range of doses (intensity, duration) and modalities of acute exercise for people with ME/CFS/SEID. Additional studies should also consider patient characteristics (e.g., symptom severity, illness length) that were not reported in studies analyzed here, but may influence the effect of exercise on fatigue. The current meta-analysis also could not consider diagnostic criteria as a moderator since all studies used the CDC criteria (17), and this should be a consideration in future research. Examining the full spectrum of exercise doses may also lead to additional mechanistic insight on the paradox between the harm of a single bout of exercise and the reported benefit of exercise training on fatigue in people with ME/CFS/SEID.
Acknowledgments
The authors wish to thank David Kupshik, Kaitlin Lang, Thomas Austin Nuckols, Daniel Schulz and Taylor Vaughn for assistance with article retrieval. Work supported by NIH-NCCIH T32 AT002688 (BDL).
Footnotes
Conflict of Interest
The authors declare no conflicts of interest. The results of the present study do not constitute endorsement by The American College of Sports Medicine (ACSM).
References
- 1.Afari N, Buchwald D. Chronic fatigue syndrome: A review. Am J Psychiat. 2003;160(2):221–36. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 2.Bailey SP. Tired of being tired: Exercise as a treatment for chronic fatigue syndrome. ACSM’s Health Fit J. 2010;15(1):20–5. [Google Scholar]
- 3.Bansal AS, Bradley AS, Bishop KN, Kiani-Alikhan S, Ford B. Chronic fatigue syndrome, the immune system and viral infection. Brain Behav Immun. 2012;26(1):24–31. doi: 10.1016/j.bbi.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer CJ. A single dose of citalopram increases fear recognition in healthy subjects. J Psychopharmacol. 2007;21(7):684–90. doi: 10.1177/0269881106074062. [DOI] [PubMed] [Google Scholar]
- 5.Burghardt NS, Bauer EP. Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: implications for underlying fear circuits. Neuroscience. 2013;247:253–72. doi: 10.1016/j.neuroscience.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 6.Carruthers BM, Jain AK, De Meirleir KL, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols. J Chronic Fatigue Syndr. 2003;11(1):7–36. [Google Scholar]
- 7.Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: international Consensus Criteria. J Intern Med. 2011;270(4):327–38. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castell BD, Kazantzis N, Moss-Morris R. Cognitive behavioral therapy and graded exercise for chronic fatigue syndrome: a meta-analysis. Clin Psychol: Sci Pr. 2011;18:311–24. [Google Scholar]
- 9.Cheung K, Hume PA, Maxwell L. Delayed onset muscle soreness: treatment strategies and performance factors. Sports Med. 2003;33(2):145–64. doi: 10.2165/00007256-200333020-00005. [DOI] [PubMed] [Google Scholar]
- 10.Cheung MA. A model for integrating fixed-, random-, and mixed-effects meta-analyses into structural equation modeling. Psychol Methods. 2008;13(3):182–202. doi: 10.1037/a0013163. [DOI] [PubMed] [Google Scholar]
- 11.Clayton EW, Alegria M, Bateman L, et al. Beyond myalgic encephalomyelitis/chronic fatigue syndrome-Redefining an illness. JAMA. 2015;313(11):1101–2. doi: 10.1001/jama.2015.1346. [DOI] [PubMed] [Google Scholar]
- 12.Edmonds M, McGuire H, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2004;(3) doi: 10.1002/14651858.CD003200.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Brit Med J. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evering RM, van Weering MG, Groothuis-Oudshoorn KC, Vollenbroek-Hutten MM. Daily physical activity of patients with the chronic fatigue syndrome: a systematic review. Clin Rehabil. 2011;25(2):112–33. doi: 10.1177/0269215510380831. [DOI] [PubMed] [Google Scholar]
- 15.Froot KA. Consistent covariance matrix estimation with cross-sectional dependence and heteroskedasticity in financial data. J Financ Quant Anal. 1989;24:333–55. [Google Scholar]
- 16.Fukuda K, Dobbins JG, Wilson LJ, Dunn RA, Wilcox K, Smallwood D. An epidemiologic study of fatigue with relevance for the chronic fatigue syndrome. J Psychiatric Res. 1997;31:19–29. doi: 10.1016/s0022-3956(96)00046-5. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its defintion and study. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 18.Fulcher KY, White PD. Chronic fatigue syndrome: a description of graded exercise treatment. Physiotherapy. 1998;84(5):223–6. [Google Scholar]
- 19.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 20.Hedges LV, Olkin I. Statistical methods for meta-analysis. New York: Academic Press; 1985. p. 369. [Google Scholar]
- 21.Herring MP, O’Connor PJ. The effect of acute resistance exercise on feelings of energy and fatigue. J Sports Sci Med. 2009;7:701–9. doi: 10.1080/02640410902777385. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. Brit Med J. 2003;327(6):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hjollund NH, Andersen JH, Bech P. Assessment of fatigue in chronic disease: a bibliographic study of fatigue measurement scales. Health Qual Life Outcomes. 2007;5:12. doi: 10.1186/1477-7525-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holgate ST, Komaroff AL, Mangan D, Wessely S. Chronic fatigue syndrome: understanding a complex illness. Nat Rev Neurosci. 2011;12(9):539–44. doi: 10.1038/nrn3087. [DOI] [PubMed] [Google Scholar]
- 25.Hox J. Multilevel Analysis: Techniques and Applications. New York: Routledge; 2010. p. 392. [Google Scholar]
- 26.Jason LA, Evans M, So S, Scott J, Brown A. Problems in defining post-exertional malaise. J Prev Interv Community. 2015;43(1):20–31. doi: 10.1080/10852352.2014.973239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jason LA, Richman JA, Rademaker AW, et al. A community-based study of chronic fatigue syndrome. Arch Intern Med. 1999;159:2129–37. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- 28.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regualtor of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24(1):25–9. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 29.Kindlon T. Reporting of harms associated with graded exercise therapy and cognitive behavioural therapy in myalgic encephalomyelitis/chronic fatigue syndrome. Bull IACFS/ME. 2011;19(2):59–111. [Google Scholar]
- 30.Kindlon T, Goudsmit EM. Graded exercise for chronic fatigue syndrome: too soon to dismiss reports of adverse reactions. J Rehabil Med. 2010;42(2):184. doi: 10.2340/16501977-0493. author reply -6. [DOI] [PubMed] [Google Scholar]
- 31.LaManca JJ, Sisto SA, Zhou X, et al. Immunological response in chronic fatigue syndrome following a graded exercise test to exhaustion. J Clin Immunol. 1999;19(2):135–42. doi: 10.1023/a:1020510718013. [DOI] [PubMed] [Google Scholar]
- 32.Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2015;(2) doi: 10.1002/14651858.CD003200.pub3. [DOI] [PubMed] [Google Scholar]
- 33.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W-65–W-94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 34.Light AR, Bateman L, Jo D, et al. Gene expression alterations at baseline and following moderate exercise in patients with Chronic Fatigue Syndrome and Fibromyalgia Syndrome. J Intern Med. 2012;271(1):64–81. doi: 10.1111/j.1365-2796.2011.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: SAGE Publications; 2001. p. 264. [Google Scholar]
- 36.Lloyd A, Gandevia S, Brockman A, Hales J, Wakefield D. Cytokine production and fatigue in patients with chronic fatigue syndrome and healthy control subjects in response to exercise. Clin Infect Dis. 1994;18(Suppl 1):S142–S6. doi: 10.1093/clinids/18.supplement_1.s142. [DOI] [PubMed] [Google Scholar]
- 37.Loy BD, O’Connor PJ. The effect of histamine on changes in mental energy and fatigue after a single bout of exercise. Physiol Behav. 2016;153:7–18. doi: 10.1016/j.physbeh.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Loy BD, O’Connor PJ, Dishman RK. The effect of a single bout of exercise on energy and fatigue states: a systematic review and meta-analysis. Fatigue. 2013:1–20. [Google Scholar]
- 39.Maas CJM, Hox JJ. Sufficient sample sizes for multilevel modeling. Methodology. 2005;1(3):86–92. [Google Scholar]
- 40.Mayo-Wilson E, Dias S, Mavranezouli I, et al. Psychological and pharmacological interventions for social anxiety disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2014;1(5):368–76. doi: 10.1016/S2215-0366(14)70329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCully KK, Sisto SA, Natelson BH. Use of exercise for treatment of chronic fatigue syndrome. Sports Med. 1996;21(1):35–48. doi: 10.2165/00007256-199621010-00004. [DOI] [PubMed] [Google Scholar]
- 42.Meyer JD, Light AR, Shukla SK, et al. Post-exertion malaise in chronic fatigue syndrome: symptoms and gene expression. Fatigue. 2013;1(4):190–209. [Google Scholar]
- 43.Mondin GW, Morgan WP, Piering PN, et al. Psychological consequences of exercise deprivation in habitual exercisers. Med Sci Sports Exerc. 1996;28(9):1199–203. doi: 10.1097/00005768-199609000-00018. [DOI] [PubMed] [Google Scholar]
- 44.Monroe DC, Gist NH, Freese EC, O’Connor PJ, McCully KK, Dishman RK. Effects of sprint interval cycling on fatigue, energy, and cerebral oxygenation. Med Sci Sports Exerc. 2016;48(4):615–24. doi: 10.1249/MSS.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 45.Mullis R, Campbell IT, Wearden AJ, Morriss RK, Pearson DJ. Prediction of peak oxygen uptake in chronic fatigue syndrome. Brit J Sports Med. 1999;33:352–6. doi: 10.1136/bjsm.33.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muthen LK, Muthen BO. Mplus Statistical Analysis with Latent Variables User’s Guide. Los Angeles: Muthen & Muthen; 1998–2012. p. 752. [Google Scholar]
- 47.Nacul LC, Lacerda EM, Pheby D, et al. Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: a repeated cross-sectional study in primary care. BMC Med. 2011:9. doi: 10.1186/1741-7015-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nijs J, Paul L, Wallman K. Chronic fatigue syndrome: an approach combining self-management with graded exercise to avoid exacerbations. J Rehabil Med. 2008;40(4):241–7. doi: 10.2340/16501977-0185. [DOI] [PubMed] [Google Scholar]
- 49.O’Connor PJ, Herring MP, Caravalho A. Mental health benefits of strength training in adults. Am J Lifestyle Med. 2010;4(5):377–96. [Google Scholar]
- 50.Peterson PK, Sirr SA, Grammith FC, et al. Effects of mild exercise on cytokines and cerebral blood flow in chronic fatigue syndrome patients. Clin Diagn Lab Immun. 1994;1(2):222–6. doi: 10.1128/cdli.1.2.222-226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pronk NP, Crouse SF, Rohack JJ. Maximal exercise and acute mood response in women. Physiol Behav. 1995;57(1):1–4. doi: 10.1016/0031-9384(94)00195-b. [DOI] [PubMed] [Google Scholar]
- 52.Puetz TW, Flowers SS, O’Connor PJ. A randomized controlled trial of the effect of aerobic exercise training on feelings of energy and fatigue in sedentary young adults with persistent fatigue. Psychother Psychosom. 2008;77(3):167–74. doi: 10.1159/000116610. [DOI] [PubMed] [Google Scholar]
- 53.Puetz TW, O’Connor PJ, Dishman RK. Effects of chronic exercise on feelings of energy and fatigue: a quantitative synthesis. Psychol Bull. 2006;132(6):866–76. doi: 10.1037/0033-2909.132.6.866. [DOI] [PubMed] [Google Scholar]
- 54.Rahman K, Burton A, Galbraith S, Lloyd A, Vollmer-Conna U. Sleep-wake behavior in chronic fatigue syndrome. Sleep. 2011;34(5):671–8. doi: 10.1093/sleep/34.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reyes M, Nisenbaum R, Hoaglin DC, et al. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Arch Intern Med. 2003;163(13):1530–6. doi: 10.1001/archinte.163.13.1530. [DOI] [PubMed] [Google Scholar]
- 56.Sharpe MC, Archard LC, Banatvala JE, et al. Chronic fatigue syndrome: guidelines for research. J Roy Soc Med. 1991;84:118–21. doi: 10.1177/014107689108400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shephard RJ. Chronic fatigue syndrome: an update. Sports Med. 2001;31(3):167–94. doi: 10.2165/00007256-200131030-00003. [DOI] [PubMed] [Google Scholar]
- 58.Sisto SA, LaManca J, Cordero DL, et al. Metabolic and cardiovascular effects of a progressive exercise test in patients with chronic fatigue syndrome. Am J Med. 1996;100:634–40. doi: 10.1016/s0002-9343(96)00041-1. [DOI] [PubMed] [Google Scholar]
- 59.Steele L, Dobbins JG, Fukuda K, et al. The epidemiology of chronic fatigue in San Francisco. Am J Med. 1998;105:83S–90S. doi: 10.1016/s0002-9343(98)00158-2. [DOI] [PubMed] [Google Scholar]
- 60.Sterne JAC, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–29. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–6. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 62.Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello LS. The acute versus the chronic response to exercise. Med Sci Sports Exerc. 2001;33(6):S438–S45. doi: 10.1097/00005768-200106001-00012. [DOI] [PubMed] [Google Scholar]
- 63.Twisk FN, Maes M. A review on cognitive behavioral therapy (CBT) and graded exercise therapy (GET) in myalgic encephalomyelitis (ME) / chronic fatigue syndrome (CFS): CBT/GET is not only ineffective and not evidence-based, but also potentially harmful for many patients with ME/CFS. Neuroendocrinol Lett. 2009;30(3):284–99. [PubMed] [Google Scholar]
- 64.Van Cauwenbergh D, De Kooning M, Ickmans K, Nijs J. How to exercise people with chronic fatigue syndrome: evidence-based practice guidelines. Eur J Clin Invest. 2012;42(10):1136–44. doi: 10.1111/j.1365-2362.2012.02701.x. [DOI] [PubMed] [Google Scholar]
- 65.Van Oosterwijck J, Nijs J, Meeus M, et al. Pain inhibition and postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: an experimental study. J Intern Med. 2010;268(3):265–78. doi: 10.1111/j.1365-2796.2010.02228.x. [DOI] [PubMed] [Google Scholar]
- 66.Vargas NT, Marino F. A neuroinflammatory model for acute fatigue during exercise. Sports Med. 2014;44(11):1479–87. doi: 10.1007/s40279-014-0232-4. [DOI] [PubMed] [Google Scholar]
- 67.Vercoulen JHMM, Bazelmans E, Swanink CMA, et al. Physical activity in chronic fatigue syndrome: assessment and its role in fatigue. J Psychiatr Res. 1997;31(6):661–73. doi: 10.1016/s0022-3956(97)00039-3. [DOI] [PubMed] [Google Scholar]
- 68.Wearden AJ, Morriss RK, Mullis R, et al. Randomised, double-blind, placebo-controlled treatment trial of fluoxetine and graded exercise for chronic fatigue syndrome. Brit J Psychiat. 1998;172:485–98. doi: 10.1192/bjp.172.6.485. [DOI] [PubMed] [Google Scholar]
- 69.White AT, Light AR, Hughen RW, et al. Severity of symptom flare after moderate exercise is linked to cytokine activity in chronic fatigue syndrome. Psychophysiology. 2010;47(4):615–24. doi: 10.1111/j.1469-8986.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–30. [Google Scholar]
- 71.White PD, Goldsmith KA, Johnson AL, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet. 2011;377:823–36. doi: 10.1016/S0140-6736(11)60096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–6. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 73.Wilson DB. Meta-analysis macros for SAS, SPSS, and Stata. [cited 2012 Feb 7]. Available from: http://mason.gmu.edu/~dwilsonb/ma.html2006.
- 74.Wolfe F, Smythe HA, Yunus MB, et al. The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33(2):160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 75.Yoshiuchi K, Cook DB, Ohashi K, et al. A real-time assessment of the effect of exercise in chronic fatigue syndrome. Physiol Behav. 2007;92(5):963–8. doi: 10.1016/j.physbeh.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]