Abstract
Objectives
To determine whether data obtained from the medical literature can be used to estimate the therapeutic index of 5 antiepileptic drugs (AEDs): carbamazepine, lamotrigine, phenobarbital, phenytoin, and valproate.
Methods
We performed a literature search using PubMed and Embase to collect published safety, efficacy, and therapeutic monitoring data for 5 AEDs, and extracted all relevant information into a drug- and study-specific drug database. For each AED, we summarized: 1) type, severity, and incidence of toxicity-related adverse events and toxicity-associated range of drug doses or concentrations; 2) effective versus toxic concentration and dose (therapeutic range); and 3) therapeutic drug monitoring practices. We defined therapeutic index as the ratio of the minimum toxic concentration to the minimum effective concentration.
Results
We reviewed a total of 810 full-text articles and extracted data from 163. The literature suggests that the therapeutic index of phenytoin is 2. The therapeutic indices of phenobarbital and valproate exceed 2. There was insufficient data to precisely quantify the therapeutic indices of carbamazepine and lamotrigine.
Conclusions
For some drugs, this approach offers a low-cost method of therapeutic index estimation. Our results can serve as preliminary data for future trials, and as guidance for FDA decision-making regarding narrow therapeutic index classification.
Keywords: Antiepileptic drugs, narrow therapeutic index, carbamazepine, lamotrigine, phenobarbital, phenytoin, valproate, valproic acid
INTRODUCTION
Over 150 generic antiepileptic drug (AED) formulations have been approved by the US Food and Drug Administration (FDA).1 In order to market a generic version of any drug, the sponsor must provide evidence of pharmaceutical equivalence and bioequivalence between the brand-name and the generic drug. In 2010, the FDA proposed that certain drugs classified as having a narrow therapeutic index (NTI) should achieve more stringent regulatory standards for approval.2 NTI drugs generally have the following characteristics: (a) there is little separation between therapeutic and toxic doses (or the associated blood/plasma concentrations); (b) sub-therapeutic concentrations may lead to serious therapeutic failure; (c) they are subject to therapeutic monitoring based on pharmacokinetic (PK) or pharmacodynamic (PD) measures; (d) they possess low-to-moderate (i.e., no more than 30%), within-subject variability; and (e) doses are often adjusted in very small increments (less than 20%) in clinical practice. For generic drugs classified as NTI, the proposed bioequivalence criteria require the use of reference-scaled testing and variability comparison, which makes these criteria tighter than the traditional average bioequivalence criteria.2–4
For these new standards to be implemented, it is critical to define which drugs can be classified as NTI. Conversion from brand-name to generic formulations of AEDs has been associated with increased frequency of seizures and adverse effects, which has led to a lack of confidence by some providers and patients in generic products.5–8 If certain AEDs could be identified as NTI drugs, application of the new criteria to the development of generic formulations could improve patient safety, enhance physician confidence in generic products, and result in overall cost savings due to increased generic drug prescribing. NTI classification requires therapeutic index estimation, which is not well-established for many generic AEDs. The purpose of our study was to determine whether data obtained from an exhaustive search of the medical literature could be used to estimate the therapeutic index of 5 off-patent AEDs.
MATERIALS AND METHODS
Through collaboration with the FDA, review of international regulatory agency websites (including Health Canada,9 the European Medicines Agency,10 and the National Institute of Health Sciences-Japan11), and consultation with neurology therapeutic experts, we identified the 5 AEDs as potential NTI drugs requiring closer evaluation to determine if they may benefit from tighter bioequivalence standards for generic drug development. carbamazepine, lamotrigine, phenobarbital, phenytoin, and valproate.
Literature search
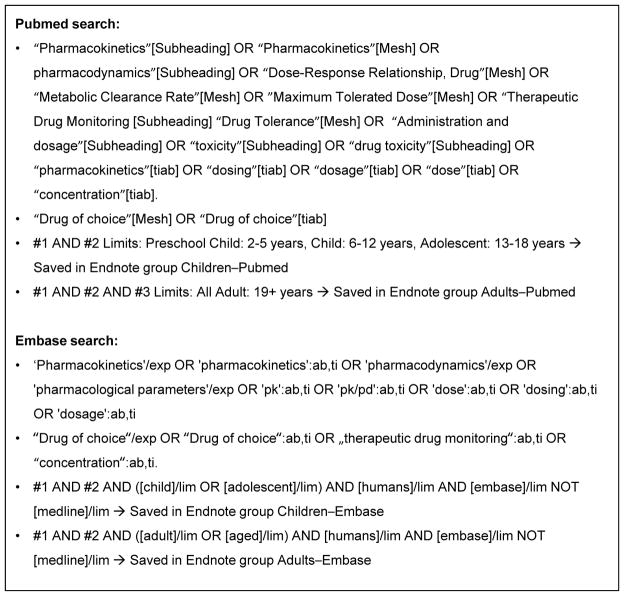
For each of the 5 AEDs, we performed an exhaustive literature search of all available indexed articles using Pubmed and Embase. We did not limit our search to a particular time period. The search was conducted with the aid of professional librarians from Duke University Medical Library (Figure 1). We reviewed abstracts for all articles identified by the search to determine whether they might contain relevant data focused on: 1) efficacy and/or safety data from prospective randomized controlled trials in the labeled indication for the drug; 2) therapeutic drug range and/or monitoring; or 3) adult PK data. Full text articles were reviewed for all abstracts of potential interest, and data was extracted into a drug- and study-specific drug database. Specific variables extracted included: study demographics, drug dosing and formulation, PK parameters (e.g., maximum concentration (Cmax), area under the curve (AUC), clearance, half-life (t1/2)), and efficacy data (study phase, primary outcome, and study result). Among safety data, we collected black box warnings and precautions indicated on the drug label, and all adverse events (AEs) with a placebo-adjusted frequency >10%. When it was uncertain whether data should be included, the manuscript was reviewed by a second study team member to reach consensus. As a quality assurance measure, 5% of the extracted data underwent an independent full review by 1 reviewer.
Figure 1.
Search strategies
Therapeutic index estimation
For each AED, we summarized by patient population: 1) type, severity, and incidence of toxicity-related AEs and toxicity-associated range of drug doses and concentrations; and 2) effective versus toxic concentration and dose (therapeutic range). To determine the therapeutic index of each drug, we aimed to determine the concentration of drug associated with selected AEs in approximately 50% of the population (TC50) and the concentration associated with efficacy in approximately 50% of the population (EC50). We defined the therapeutic index as TC50 divided by EC50. However, in the absence of available data in the literature to calculate TC50 and EC50, we defined therapeutic index as the ratio of the minimum toxic concentration to the minimum effective concentration.12,13 Even though the FDA does not specify a threshold value to determine whether a therapeutic index is narrow, in this study we considered a therapeutic index ≤2 as one criterion to support classification as an NTI drug.12,13
RESULTS
Carbamazepine
Safety
AEs associated with carbamazepine use in patients with epilepsy include somnolence, dizziness, gastrointestinal disturbance, and hematologic abnormalities (Table 1).14–22 There were conflicting results regarding the relationship between systemic exposure and toxicity. Several studies demonstrated that the prevalence of carbamazepine AEs was concentration-related, with toxicity occurring at concentrations >8. 7–11. 8 μg/mL.23–28 Conversely, other studies found no significant difference in mean carbamazepine serum concentrations between patients with and without AEs.18,19
Table 1.
Representative drug-related adverse events (AEs) and associated dose ranges
| Number of subjects | AE Type | AE Severity | Incidence | Range of AED doses |
|---|---|---|---|---|
| Carbamazepine | ||||
| 91–3011–3,5,6 | Somnolence Decreased appetite |
Mild/moderate | 8–36% 2% |
400–1200 mg/day |
| 91–37815, 16, 18–21 | Gastrointestinal | Mild/moderate | 4–29% | 400–2000 mg/day |
| 91–37815–20 | Rash | Mild/serious | 8–32% | 200–2000 mg/day |
| 20–23017, 21, 22 | Decreased white blood cells | Mild | Not reported | 200–1400 (5–24 mg/kg/day) |
| Lamotrigine | ||||
| 8 – 33435, 39, 40 | Rash | Moderate/serious | 1%, 3%, 8% | 100–500 mg (serious), 100 mg (moderate) |
| 156 – 33437, 40 | Stevens-Johnson syndrome | Serious | 0. 1%, 1% | 300–400 mg, 300 mg/day, and 250 mg bid |
| 14136 | Grand mal seizures | Serious | 1% | 300–400 mg/day |
| 12638 | Diplopia | Serious | 1% | 250 mg |
| 33440 | Dizziness | Serious | 0. 6% | 100–500 mg |
| 33440 | Blurry vision | Serious | 0. 6% | 100–500 mg |
| 33440 | Ataxia | Serious | 0. 3% | 100–500 mg |
| 33440 | Nausea | Serious | 0. 3% | 100–500 mg |
| Phenobarbital | ||||
| 11448 | Elevated GGT | Mild | 58% | NR |
| 18–124e-ref 82, e- ref 83 | Hypotension | NR | 11–49% | 10–30 mg/kg |
| 1854 | Intubation | Severe | 33% | 10–30 mg/kg |
| 10–143 e-ref 71, e- ref 78 | Somnolence | Mild | 30% | 8. 3–133. 3 mg/day |
| 14350 | Fatigue | Mild | 21% | 8. 3–133. 3 mg/day |
| Phenytoin | ||||
| 127–15159, 60 | Rash | Moderate/serious | 19% (mild), 8% (moderate), 2% (serious) | 300 mg (serious) |
| 114e-ref 61 | Stevens-Johnson syndrome | Serious | 1% | 300 mg |
| 14159 | Suicide attempt | Serious | 1% | 300 mg |
| 67e-ref 62 | Myalgia | Moderate | 2% | 200–450 mg |
| Valproate | ||||
| 112e-ref 76 | Somnolence | Mild | 10–15% | 1. 5–3. 0 mg/kg/min |
| 112e-ref 76 | Dizziness | Mild | 8–10% | 1. 5–3. 0 mg/kg/min |
| 112e-ref 76 | Nausea | Mild/moderate | 8–10% | 1. 5–3. 0 mg/kg/min |
| 112e-ref 76 | Paresthesia | Mild | 8% | 1. 5–3. 0 mg/kg/min |
GGT: gamma-glutamyl transpeptidase
Efficacy
Carbamazepine efficacy with mono- and combination therapy was assessed in studies using 3 different measures: 1) percentage of patients with a ≥50% reduction in seizures; 2) reduction in total seizures during the study period; and 3) percentage of patients who were seizure-free in specific time periods (Table 2).14, 17, 18, 20, 21 Only one study reported a positive correlation between carbamazepine concentration and seizure control.22
Table 2.
Representative range of antiepileptic drug (AED) doses in studies with successful monotherapy.
| Number of subjects | Outcome measure | Efficacy results | Dose range |
|---|---|---|---|
| Carbamazepine | |||
| 30014 | Seizure-free at 26 weeks | 75% | 400–1200 mg |
| 23017 | Withdrawn from treatment at <52 weeks | 42% | 200–1400 mg |
| 12918 | Seizure-free at 48 weeks | 38% | 300–1400 mg |
| 23620 | Seizure-free at 48–96 weeks | 34% | Mean: 722 mg |
| 10121 | Withdrawn from treatment at <36 months | 45% | 600 mg |
| Lamotrigine | |||
| 24942 | Seizure-free at 12 months | 61% | 25–600 mg |
| 22619 | Seizure-free at 7 weeks | 60% | 100–200 mg |
| 15637 | Remaining on monotherapy | 56% | 100–500 mg |
| 22243 | Seizure-free at 1 year | 89% | 50–150 mg |
| 13118 | Seizure-free at 40 weeks | 26% | 100–300 mg |
| Phenobarbital | |||
| 12453 | Resolution of all clinical and electrical evidence of seizure activity within 20 minutes of start of infusion | 58% | 15 mg/kg |
| 1854 | Resolution of status epilepticus | 61% | 5–23 mg/kg |
| 856 | Fewer seizures than clorazepate comparator | 50% | 148 ± 21. 8 mg/day |
| Phenytoin | |||
| 114e-ref 61 | >50% seizure reduction | 57% | 200–300 mg |
| 50e-ref 64 | Seizure-free at 6 months | 53% | 3–5 mg/kg |
| 26e-ref 65 | Seizure-free at 12–41 months | 76% | 200–300 mg |
| 37e-ref 66 | >50% reduction in seizure frequency at 14–24 months | 82% | 300 mg |
| 95e-ref 67 | Seizure-free at 10 months | 24% | 300 mg (mode) |
| Valproate | |||
| 238e-ref 75 | Time to treatment failure | Valproate significantly better than topiramate, but no significant difference between valproate and lamotrigine | 200–3000 mg |
| 16e-ref 77 | Seizure-free at 12 months | 25% | 1000–2000 mg |
| 6430 | >50% reduction in seizure frequency at >3 months | 80% | 600 mg (adults); 5–10 mg/kg (children) |
| 13e-ref 78 | Reduction in seizure frequency | A statistically significant difference was observed between VPA levels and seizure frequency. The relationship was curvilinear. | 300–4000 mg |
| 10e-ref 79 | >50% reduction in seizure frequency at 12 weeks | 50% | 900 mg |
Therapeutic range and drug monitoring
The conventionally accepted therapeutic range of plasma concentrations of carbamazepine in adults is 4–12 μg/mL.29 The dose range associated with efficacy and toxicity partially overlaps. Serum concentrations associated with efficacy range between 1.9 and 11.7 μg/mL, though concentrations less than 4 μg/mL result in less optimal seizure control in the overall epilepsy population.22,30,31 AEs have been reported in carbamazepine concentrations ranging from 4–19. 6 μg/mL, and higher concentrations are generally associated with more AEs.24,28,32–34
In clinical practice, therapeutic drug monitoring (TDM) is often not routinely performed for carbamazepine, though there is variability among clinicians. Drug levels may be checked when patients experience adverse effects or experience break-through seizures, and to monitor compliance with therapy. Patients taking interacting medications or patients converting from one drug formulation to another may require TDM.
Therapeutic index estimation
Information from the medical literature generally supports the accepted carbamazepine therapeutic range of 4–12 μg/mL. However, the response to carbamazepine therapy is reportedly variable, and patients may require lower or higher serum concentrations to achieve adequate seizure control. The available information suggests an approximate carbamazepine therapeutic index of 3. However, TDM may be required for patient safety in certain settings, such as when a patient experiences an adverse effect or break-through seizures. Further data, other than literature reviews, are needed to determine whether carbamazepine may require stricter bioequivalence criteria in these instances.
Lamotrigine
Safety
AEs associated with lamotrigine use in patients with refractory seizure occur in dose ranges of 100–500 mg/day (Table 1).35–40 The prevalence of AEs with lamotrigine use has been shown to be dose-related and generally independent of concomitantly administered medications.41 However, co-administration of valproate with lamotrigine has been associated with skin rash.42 Moreover, the fraction of subjects necessitating dose modification or discontinuation increased by 25% when plasma levels exceeded 20 μg/mL.41
Efficacy
Lamotrigine efficacy with mono- and combination therapy was assessed in studies using 3 different measures: 1) percentage of patients who had a >50% reduction in seizures; 2) reduction in total seizures during the study period; and 3) percentage of patients with seizure freedom in a specific time period (Table 2).18,19,37,43,44
Therapeutic range and drug monitoring
The dose range associated with efficacy and toxicity partially overlaps. In a large-scale observational study (N=811), overall toxicity of lamotrigine increased gradually with increasing serum concentrations, whereas efficacy of lamotrigine did not correlate with serum levels when the concentration of lamotrigine was below 15 μg/mL.41 In this study, about 50% of the population was seizure-free at 6 months at levels ranging from 1–15 μg/mL. The therapeutic index calculated based on the available data for lamotrigine was in the range of 1.3–20. In clinical practice, TDM is not routinely performed for lamotrigine. However, the American Academy of Neurology recommends that TDM of lamotrigine should be considered in pregnant women with epilepsy because pregnancy causes an increase in the clearance and a decrease in the level of lamotrigine.45,46 Due to the widely varying doses of lamotrigine, there is no well-defined one-size-fits-all target range in these patients. Individualized dosing or target concentrations are determined by attending physicians based on a range of other parameters including response, severity of symptoms, other drugs, and side effects.47
Therapeutic index estimation
Based on literature data, the therapeutic range of lamotrigine is not well established, nor is there a clear dose-to-response curve for safety or efficacy. The therapeutic index determined from the medical literature extends from a value on the border of supporting classification of the therapeutic index as narrow to a ten-fold higher value that would not be consistent with the therapeutic index being narrow (Table 3). Due to this uncertainty, our method of estimating the therapeutic index was unable to distinguish whether the therapeutic index of lamotrigine should be determined as narrow.
Table 3.
Narrow therapeutic index (NTI) classification. AED: antiepileptic drug.
| AED | Articles meeting search criteria (Pubmed / Embase) | Full text articles reviewed | Articles from which data extracted | Therapeutic index | NTI classification supported by literature? |
|---|---|---|---|---|---|
| Carbamazepine | 1022 / 344 | 364 | 34 | 3 | Possibly |
| Lamotrigine | 384 / 187 | 132 | 49 | 1. 3–20 | Possibly |
| Phenobarbital | 384 / 283 | 150 | 35 | ** | No |
| Phenytoin | 1007 / 284 | 111 | 34 | 2 | Yes |
| Valproate | 608 / 317 | 53 | 11 | ** | No |
Therapeutic index could not be estimated due to poor correlation of toxicity symptoms with drug concentration.
Phenobarbital
Safety
AEs associated with phenobarbital use occur at a wide range of doses (Table 1).48–51 In a single study evaluating the relationship between dose or plasma concentration and development of an AE, most AEs occurred in subjects with total serum phenobarbital levels at or below the standard accepted therapeutic range of 15–40 μg mL.51 The investigators did not find a relationship between phenobarbital levels and occurrence of AEs; however, there was a significant relationship between the number of concomitant antiepileptic agents (i.e., phenytoin, carbamazepine, and benzodiazepines) and occurrence of AEs.
Efficacy
Phenobarbital efficacy with mono- and combination therapy was assessed in studies using 3 different measures: 1) reduction in seizure frequency; 2) achievement of seizure remission for a predefined period of time; and 3) percentage of patients with treatment failure (Table 2).
Therapeutic range and drug monitoring
The conventional therapeutic range for phenobarbital is 15–40 μg/mL.52 In the vast majority of available studies, phenobarbital functioned as an active control or an adjunct drug in the study of other antiepileptic agents and was noted to be efficacious when serum concentrations were within the stated therapeutic range.31,53–57 In addition, it has been reported that some subjects have remained seizure-free at phenobarbital levels well below 10 μg/mL,52 while clearly other subjects can have seizures refractory to phenobarbital therapy despite levels in the therapeutic range.58
In clinical practice, TDM is routinely performed for phenobarbital.52 However, some clinicians choose to rely more on clinical response rather than targeting a range of concentrations. It has been reported that the majority of patients can achieve satisfactory results by adjusting dose based on clinical signs and symptoms.59
Therapeutic index estimation
The therapeutic range of phenobarbital has been well established and accepted. However, there are subjects who are successfully treated with phenobarbital levels below this range. Furthermore, there are numerous subjects who experience AEs despite having serum drug levels within this range, and there does not appear to be a significant relationship between drug level and frequency of AEs. Thus, we were unable to quantify the therapeutic index of phenobarbital. Studies evaluating the efficacy and safety of phenobarbital are frequently confounded by concomitant treatment with other antiepileptic medications. Despite these limitations, treatment strategies adjusting dose based on clinical signs and symptoms have been shown to be as successful as strategies relying on TDM.
Phenytoin
Safety
AEs associated with phenytoin use usually occur in dose ranges of 200–450 mg (Table 1).60–63 The prevalence of AEs with phenytoin use has been shown to be dose-related and independent of concomitantly administered medications.64 In one study, 86% of patients with toxicity had phenytoin-free levels >2 μg/mL.64
Efficacy
Phenytoin efficacy with mono- and combination therapy was assessed in studies using 3 different measures: 1) percentage of patients who had a >50% reduction; 2) reduction in total seizures during the study period; and 3) percentage of patients with seizure freedom in a specific time period (Table 2).62,65–68
Therapeutic range and drug monitoring
The conventionally accepted therapeutic range of phenytoin is 10–20 μg/mL. This therapeutic range was primarily established from small, often retrospective studies performed in the 1960s and 1970s combined with clinical experience and expert opinion.69 The dose range associated with efficacy and toxicity partially overlaps. The available studies evaluating serum phenytoin concentrations and seizure control suggest that seizure frequency is dose-related and seizure control is generally poor at concentrations <10 μg/mL.70–74 Serum concentrations between 15 and 20 are associated with improved seizure control. Although target total phenytoin concentrations of 10–20 μg/mL have been used in clinical trials, several studies have shown that total phenytoin values >20 μg/mL in certain patients may be optimal.70,75
In clinical practice, TDM is often not routinely performed for phenytoin. Levels may be checked when patients experience adverse effects or experience break-through seizures. There is a large inter-individual variability in the PK of phenytoin. For patients with ongoing physiological changes such as pregnancy, decreased renal clearance, decreased hepatic function, or microalbuminemia, TDM may be needed.64,71
Therapeutic index estimation
There is considerable overlap in the toxic and therapeutic ranges for phenytoin, and TDM is recommended in certain populations. Based on available literature, the therapeutic index for phenytoin is approximately 2. The relatively low therapeutic index is one criterion that supports NTI classification for phenytoin (Table 3).
Valproate
Safety
Valproate is generally well-tolerated at doses that produce concentrations that effectively reduce seizures (Table 1).76,77 One study evaluating the safety of rapidly infused valproate noted that adverse events often occurred around the time of maximal drug concentrations.77
Efficacy
Valproate efficacy was typically studied as a >50% reduction in seizure frequency or complete remission within a given time period (Table 2).30,76,78–80 Seizure control has been reported across a wide concentration range of 60–800 μmoles/L (8.7–115.4 ug/mL).30
Therapeutic range and drug monitoring
The therapeutic range of total valproate reported on the FDA label for epilepsy is 50–100 μg/mL (4–15 μg/mL unbound), which is consistent with published studies that have evaluated valproate efficacy and safety.81 Many clinicians perform TDM when adding drugs with potential interactions, when patients experience breakthrough seizures or toxicities, or to monitor compliance with therapy. However, clinicians do not consistently perform TDM.
Therapeutic index estimation
Valproate concentrations associated with adequate reductions in seizure frequency have been shown to vary widely, and there is little overlap between the toxic and therapeutic range. Thus, we were not able to estimate the therapeutic index. Clinicians do not consistently perform TDM.
DISCUSSION
There is considerable concern among physicians and patients regarding the safety and efficacy of generic AEDs.5 The American Academy of Neurology has issued a position statement opposing generic substitution of AEDs without attending physician’s approval, citing concerns for toxicity and break-through seizures.6 While much of the evidence for these concerns is anecdotal, there has been at least one survey study documenting cases in which patients who switched to generic AED formulations experienced lower drug levels and breakthrough seizures.82 In addition, a large Canadian claims database study compared the frequency of switchback rates for AEDs and other drugs in patients who had been compulsorily switched to generic formulations.83 In this study, the switchback rates to brand-name formulations were higher for both lamotrigine (13%) and valproate (20%) compared to non-AED drugs (1.5–2.9%).
If the FDA were to enact tighter bioequivalence standards for generic formulations of certain drugs, clinicians and patients could more confidently choose less expensive products, leading to health care cost savings. Tighter bioequivalence standards, however, are not necessary for drugs that have a wide therapeutic index, because larger variations in patient exposure can be well-tolerated. Identification of NTI drugs is therefore key to the implementation of new bioequivalence standards.
In our study, we completed a comprehensive literature search to estimate the therapeutic index of 5 AEDs, a crucial step towards determining whether these drugs could be classified as NTI. We found that the therapeutic index we estimated from the available literature supported NTI classification for 1 of the 5 AEDs studied (phenytoin), while 2 other AEDs (carbamazepine, lamotrigine) require the evaluation of multiple other factors to determine whether they may be classified as NTI. A recent study of generic-to-generic switches in epilepsy patients taking different forms of lamotrigine showed bioequivalence of disparate lamotrigine products, with no apparent difference in clinical effects.84 For the 2 drugs for which NTI status could not be adequately determined (carbamazepine and lamotrigine), TDM is advisable for at least some clinical situations, and there is some concentration-dependent efficacy and toxicity. Approval of generic formulations of these drugs without stringent testing of PK and pharmacodynamics properties could result in unacceptable risk of adverse effects or breakthrough seizures. In 2014 and 2015, the FDA updated the bioequivalence guidance for phenytoin and carbamazepine to request tighter bioequivalence standards for NTI drugs.85
The strengths of our study include a thorough review of all available toxicity and efficacy data for 5 AEDs. Based on the compiled information, we calculated therapeutic indices for use in classification of most of our drugs of interest. This study represents a proof-of-concept method that can be replicated to other drug classes to estimate therapeutic indices. During the period of brand-name drug exclusivity (5–7 years) when post-marketing data is generated, a data review can be performed to estimate the therapeutic index, and to potentially support its NTI classification.
Our study is limited by the heterogeneity of the data available in the existing literature; our results are based on a wide variety of trials that included different populations, study designs, indications, and drug dosages and regimens. In addition, for some drugs, very limited data were available from which we were able to draw conclusions. Our search methods, while comprehensive, may have led to incomplete retrieval of data. We also did not capture clinical practice data, which is an important component required for NTI classification. Finally, our estimation of therapeutic index was based on the population therapeutic range, which may be wider than that of an individual. Therefore, we may have overestimated the therapeutic index that can be applied to an individual. We have also included AE data from both titration and maintenance phases, and the therapeutic index may differ in different clinical situations. Given these limitations, combining the results of our review with other methods of NTI classification, including expert opinion and PK/PD modeling, may help determine which drugs should be subjected to more stringent bioequivalence criteria. The ideal source of data regarding safety and efficacy of generic formulations would be trials in which patients are randomized to continuing on brand-name formulations vs. switching to generics, with drug levels collected in patients experiencing toxicity. However, the cost of such trials to evaluate all possible NTI drugs is prohibitive. Our approach offers a low-cost method of therapeutic index determination. Our results can serve as preliminary data for future trials and as guidance for FDA decision-making regarding NTI drugs.
Acknowledgments
Sources of Funding:
This study was supported by a grant from the US Food and Drug Administration: NIH3U01FD00485801. Views expressed in written materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Dr. Greenberg received funding from NIH training grants (5T32HD043728-10 and 5T32HD043029-13).
Dr. Melloni received funding from industry for drug development in adults (https://dcri.org/about-us/conflict-of-interest), and from NICHD (HHSN275201000003I) and the FDA (3U01FD004858-01).
Dr. Gonzalez is funded by the nonprofit Thrasher Research Fund (www.thrasherresearch.org), and by the National Institute for Child Health and Human Development (NICHD) (K23HD083465).
Dr. Hill receives research support from industry for drug development in children (www.dcri.duke.edu/research/coi.jsp), the nonprofit Mend-A-Heart Foundation (www.medaheart.org), and from the National Center for Advancing Translational Sciences of the NIH (UL1TR001117).
Dr. Hornik receives salary support for research from the National Center for Advancing Translational Sciences of the NIH (UL1TR001117).
Dr. Cohen-Wolkowiez receives research support from industry for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp), the nonprofit Thrasher Research Fund (www.thrasherresearch.org), and from the NIH (1R01-HD076676-01A1), the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the NIAID (HHSN272201500006I and HHSN272201300017I), the NICHD (HHSN275201000003I), the FDA (1U01FD004858-01), and the Biomedical Advanced Research and Development Authority (HHSO100201300009C).
Dr. Guptill receives support from industry for drug development in adults (www.dcri.duke.edu/research/coi.jsp), from National Institute of Neurological Disorders and Stroke (K23NS085049-01A1), the Myasthenia Gravis Foundation of America, and the NICHD (HHSN275201000003I).
Footnotes
Conflicts of Interest
For the remaining authors none were declared.
References
- 1.Krauss GL, Caffo B, Chang YT, et al. Assessing bioequivalence of generic antiepilepsy drugs. Annals of neurology. 2011;70:221–228. doi: 10.1002/ana.22452. [DOI] [PubMed] [Google Scholar]
- 2.Yu LX, Jiang W, Zhang X, et al. Novel bioequivalence approach for narrow therapeutic index drugs. Clin Pharmacol Ther. 2015;97:286–291. doi: 10.1002/cpt.28. [DOI] [PubMed] [Google Scholar]
- 3.Jiang W, Makhlouf F, Schuirmann DJ, et al. A Bioequivalence Approach for Generic Narrow Therapeutic Index Drugs: Evaluation of the Reference-Scaled Approach and Variability Comparison Criterion. Aaps j. 2015;17:891–901. doi: 10.1208/s12248-015-9753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidance for Industry: Statistical Approaches to Establishing Bioequivalence. [08/25/2015]; Accessed at www.fda.gov/downloads/Drugs/Guidances/ucm070244.pdf on.
- 5.Berg MJ, Gross RA, Haskins LS, et al. Generic substitution in the treatment of epilepsy: patient and physician perceptions. Epilepsy & behavior : E&B. 2008;13:693–699. doi: 10.1016/j.yebeh.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Liow K, Barkley GL, Pollard JR, et al. Position statement on the coverage of anticonvulsant drugs for the treatment of epilepsy. Neurology. 2007;68:1249–1250. doi: 10.1212/01.wnl.0000259400.30539.cc. [DOI] [PubMed] [Google Scholar]
- 7.Wilner AN. Therapeutic equivalency of generic antiepileptic drugs: results of a survey. Epilepsy & behavior : E&B. 2004;5:995–998. doi: 10.1016/j.yebeh.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Haskins LS, Tomaszewski KJ, Crawford P. Patient and physician reactions to generic antiepileptic substitution in the treatment of epilepsy. Epilepsy & behavior: E&B. 2005;7:98–105. doi: 10.1016/j.yebeh.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 9. [9/23/2015];Health Canada [online] Accessed at: www.hc-sc.gc.ca/index-eng.php on.
- 10.European Medicines Agency [online] [9/23/15]; Accessed at: www.ema.europa.eu/ema/ on.
- 11.National Institute of Health Sciences-Japan [online] [9/23/15]; Accessed at: www.nihs.go.jp/ on.
- 12.Levy G. What are narrow therapeutic index drugs? Clin Pharmacol Ther. 1998;63:501–505. doi: 10.1016/S0009-9236(98)90100-X. [DOI] [PubMed] [Google Scholar]
- 13.Muller PY, Milton MN. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov. 2012;11:751–761. doi: 10.1038/nrd3801. [DOI] [PubMed] [Google Scholar]
- 14.Baulac M, Brodie MJ, Patten A, et al. Efficacy and tolerability of zonisamide versus controlled-release carbamazepine for newly diagnosed partial epilepsy: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Neurol. 2012;11:579–588. doi: 10.1016/S1474-4422(12)70105-9. [DOI] [PubMed] [Google Scholar]
- 15.Saetre E, Perucca E, Isojarvi J, et al. An international multicenter randomized double-blind controlled trial of lamotrigine and sustained-release carbamazepine in the treatment of newly diagnosed epilepsy in the elderly. Epilepsia. 2007;48:1292–1302. doi: 10.1111/j.1528-1167.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 16.Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000–1015. doi: 10.1016/S0140-6736(07)60460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chadwick D. Safety and efficacy of vigabatrin and carbamazepine in newly diagnosed epilepsy: a multicentre randomised double-blind study. Vigabatrin European Monotherapy Study Group. Lancet. 1999;354:13–19. doi: 10.1016/s0140-6736(98)10531-7. [DOI] [PubMed] [Google Scholar]
- 18.Brodie MJ, Richens A, Yuen AW. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK Lamotrigine/Carbamazepine Monotherapy Trial Group. Lancet. 1995;345:476–479. doi: 10.1016/s0140-6736(95)90581-2. [DOI] [PubMed] [Google Scholar]
- 19.Reunanen M, Dam M, Yuen AW. A randomised open multicentre comparative trial of lamotrigine and carbamazepine as monotherapy in patients with newly diagnosed or recurrent epilepsy. Epilepsy research. 1996;23:149–155. doi: 10.1016/0920-1211(95)00085-2. [DOI] [PubMed] [Google Scholar]
- 20.Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. The Department of Veterans Affairs Epilepsy Cooperative Study No. 264 Group. N Engl J Med. 1992;327:765–771. doi: 10.1056/NEJM199209103271104. [DOI] [PubMed] [Google Scholar]
- 21.Mattson RH, Cramer JA, Collins JF, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. N Engl J Med. 1985;313:145–151. doi: 10.1056/NEJM198507183130303. [DOI] [PubMed] [Google Scholar]
- 22.Monaco F, Riccio A, Benna P, et al. Further observations on carbamazepine plasma levels in epileptic patients. Relationships with therapeutic and side effects. Neurology. 1976;26:936–973. doi: 10.1212/wnl.26.10.936. [DOI] [PubMed] [Google Scholar]
- 23.Semah F, Gimenez F, Longer E, et al. Carbamazepine and its epoxide: an open study of efficacy and side effects after carbamazepine dose increment in refractory partial epilepsy. Ther Drug Monit. 1994;16:537–540. [PubMed] [Google Scholar]
- 24.Specht U, May TW, Rohde M, et al. Cerebellar atrophy decreases the threshold of carbamazepine toxicity in patients with chronic focal epilepsy. Arch Neurol. 1997;54:427–431. doi: 10.1001/archneur.1997.00550160063017. [DOI] [PubMed] [Google Scholar]
- 25.Bareggi SR, Tata MR, Guizzaro A, et al. Daily fluctuation of plasma levels with conventional and controlled-release carbamazepine: correlation with adverse effects. Int Clin Psychopharmacol. 1994;9:9–16. doi: 10.1097/00004850-199400910-00002. [DOI] [PubMed] [Google Scholar]
- 26.Riva R, Contin M, Albani F, et al. Lateral gaze nystagmus in carbamazepine-treated epileptic patients: correlation with total and free plasma concentrations of parent drug and its 10,11-epoxide metabolite. Ther Drug Monit. 1985;7:277–282. [PubMed] [Google Scholar]
- 27.Mayer M, Alternmuller, Sandmann, et al. Clinical Problems with Generic Antiepileptic Drugs. Comparison of Sustained-Release Formulations of Carbamazepine. Clinical Drug Investigation. 1999;18:17–26. [Google Scholar]
- 28.Chadwick D, Shaw MD, Foy P, et al. Serum anticonvulsant concentrations and the risk of drug induced skin eruptions. J Neurol Neurosurg Psychiatry. 1984;47:642–644. doi: 10.1136/jnnp.47.6.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomson T. Clinical pharmacokinetics of carbamazepine. Cephalalgia : an international journal of headache. 1987;7:219–223. doi: 10.1046/j.1468-2982.1987.0704219.x. [DOI] [PubMed] [Google Scholar]
- 30.Goggin T, Casey C, Callaghan N. Serum levels of sodium valproate, phenytoin and carbamazepine and seizure control in epilepsy. Ir Med J. 1986;79:150–156. [PubMed] [Google Scholar]
- 31.Schmidt D, Haenel F. Therapeutic plasma levels of phenytoin, phenobarbital, and carbamazepine: individual variation in relation to seizure frequency and type. Neurology. 1984;34:1252–1255. doi: 10.1212/wnl.34.9.1252. [DOI] [PubMed] [Google Scholar]
- 32.Hiremath GK, Kotagal P, Bingaman W, et al. Risk factors for carbamazepine elevation and toxicity following epilepsy surgery. Seizure. 2005;14:312–317. doi: 10.1016/j.seizure.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Hughes D-D. Chronic Leukopenia Associated with Carbamazepine and Other Antiepileptic Drugs. J Epilepsy. 1995:282–288. [Google Scholar]
- 34.Riva R, Contin M, Albani F, et al. Free and total plasma concentrations of carbamazepine and carbamazepine-10,11-epoxide in epileptic patients: diurnal fluctuations and relationship with side effects. Ther Drug Monit. 1984;6:408–413. doi: 10.1097/00007691-198412000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Matsuo F, Gay P, Madsen J, et al. Lamotrigine high-dose tolerability and safety in patients with epilepsy: a double-blind, placebo-controlled, eleven-week study. Epilepsia. 1996;37:857–862. doi: 10.1111/j.1528-1157.1996.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 36.Baulac M, Leon T, O’Brien TJ, et al. A comparison of pregabalin, lamotrigine, and placebo as adjunctive therapy in patients with refractory partial-onset seizures. Epilepsy research. 2010;91:10–19. doi: 10.1016/j.eplepsyres.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Gilliam F, Vazquez B, Sackellares JC, et al. An active-control trial of lamotrigine monotherapy for partial seizures. Neurology. 1998;51:1018–1025. doi: 10.1212/wnl.51.4.1018. [DOI] [PubMed] [Google Scholar]
- 38.Jozwiak S, Terczynski A. Open study evaluating lamotrigine efficacy and safety in add-on treatment and consecutive monotherapy in patients with carbamazepine- or valproate-resistant epilepsy. Seizure. 2000;9:486–492. doi: 10.1053/seiz.2000.0444. [DOI] [PubMed] [Google Scholar]
- 39.Binnie CD, Debets RM, Engelsman M, et al. Double-blind crossover trial of lamotrigine (Lamictal) as add-on therapy in intractable epilepsy. Epilepsy research. 1989;4:222–229. doi: 10.1016/0920-1211(89)90007-7. [DOI] [PubMed] [Google Scholar]
- 40.Schachter SC, Leppik E, Matsuo F, et al. Lamotrigine: A Six-Month, Placebo-Controlled, Safety and Tolerance Study. J Epilepsy. 1995;8:201–209. [Google Scholar]
- 41.Hirsch LJ, Weintraub D, Du Y, et al. Correlating lamotrigine serum concentrations with tolerability in patients with epilepsy. Neurology. 2004;63:1022–1026. doi: 10.1212/01.wnl.0000138424.33979.0c. [DOI] [PubMed] [Google Scholar]
- 42.Messenheimer J, Mullens EL, Giorgi L, et al. Safety review of adult clinical trial experience with lamotrigine. Drug Saf. 1998;18:281–296. doi: 10.2165/00002018-199818040-00004. [DOI] [PubMed] [Google Scholar]
- 43.Mohanraj R, Brodie MJ. Pharmacological outcomes in newly diagnosed epilepsy. Epilepsy & behavior: E&B. 2005;6:382–387. doi: 10.1016/j.yebeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Mauri Llerda JA, Tejero C, Mercade JM, et al. Lamotrigine and epilepsy in the elderly: observational study of low-dose monotherapy. International journal of clinical practice. 2005;59:651–654. doi: 10.1111/j.1742-1241.2005.00549.x. [DOI] [PubMed] [Google Scholar]
- 45.Deligiannidis KM. Therapeutic drug monitoring in pregnant and postpartum women: recommendations for SSRIs, lamotrigine, and lithium. The Journal of clinical psychiatry. 2010;71:649–650. doi: 10.4088/JCP.10ac06132gre. [DOI] [PubMed] [Google Scholar]
- 46.Pennell PB, Peng L, Newport DJ, et al. Lamotrigine in pregnancy: clearance, therapeutic drug monitoring, and seizure frequency. Neurology. 2008;70:2130–2136. doi: 10.1212/01.wnl.0000289511.20864.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris RG, Lee MY, Cleanthous X, et al. Long-term follow-up using a higher target range for lamotrigine monitoring. Ther Drug Monit. 2004;26:626–632. doi: 10.1097/00007691-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Wilder BJ, Willmore LJ, Bruni J, et al. Valproic acid: interaction with other anticonvulsant drugs. Neurology. 1978;28:892–896. doi: 10.1212/wnl.28.9.892. [DOI] [PubMed] [Google Scholar]
- 49.Sano J, Kawada H, Yamaguchi N, et al. Effects of phenytoin on serum gamma-glutamyl transpeptidase activity. Epilepsia. 1981;22:331–338. doi: 10.1111/j.1528-1157.1981.tb04117.x. [DOI] [PubMed] [Google Scholar]
- 50.Woo E, Chan YM, Yu YL, et al. If a well-stabilized epileptic patient has a subtherapeutic antiepileptic drug level, should the dose be increased? A randomized prospective study. Epilepsia. 1988;29:129–139. doi: 10.1111/j.1528-1157.1988.tb04408.x. [DOI] [PubMed] [Google Scholar]
- 51.Ieiri I, Hirata K, Higuchi S, et al. Pharmacoepidemiological study on adverse reactions of antiepileptic drugs. Chem Pharm Bull (Tokyo) 1992;40:1280–1288. doi: 10.1248/cpb.40.1280. [DOI] [PubMed] [Google Scholar]
- 52.Feldman RG, Pippenger CE. The relation of anticonvulsant drug levels to complete seizure control. Journal of clinical pharmacology. 1976;16:51–59. doi: 10.1002/j.1552-4604.1976.tb01491.x. [DOI] [PubMed] [Google Scholar]
- 53.Huber B, Hauser I, Horstmann V, et al. Long-term course of epilepsy in a large cohort of intellectually disabled patients. Seizure. 2007;16:35–42. doi: 10.1016/j.seizure.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 55.Shaner DM, McCurdy SA, Herring MO, et al. Treatment of status epilepticus: a prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology. 1988;38:202–207. doi: 10.1212/wnl.38.2.202. [DOI] [PubMed] [Google Scholar]
- 56.Wilensky AJ, Ojemann LM, Temkin NR, et al. Clorazepate and phenobarbital as antiepileptic drugs: a double-blind study. Neurology. 1981;31:1271–1276. doi: 10.1212/wnl.31.10.1271. [DOI] [PubMed] [Google Scholar]
- 57.Troupin AS, Friel P, Wilensky AJ, et al. Evaluation of clorazepate (Tranxene) as an anticonvulsant--a pilot study. Neurology. 1979;29:458–466. doi: 10.1212/wnl.29.4.458. [DOI] [PubMed] [Google Scholar]
- 58.Sironi VA, Cabrini G, Porro MG, et al. Antiepileptic drug distribution in cerebral cortex, ammon’s horn, and amygdala in man. Journal of neurosurgery. 1980;52:686–692. doi: 10.3171/jns.1980.52.5.0686. [DOI] [PubMed] [Google Scholar]
- 59.Jannuzzi G, Cian P, Fattore C, et al. A multicenter randomized controlled trial on the clinical impact of therapeutic drug monitoring in patients with newly diagnosed epilepsy. The Italian TDM Study Group in Epilepsy. Epilepsia. 2000;41:222–230. doi: 10.1111/j.1528-1157.2000.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 60.Ramsay E, Faught E, Krumholz A, et al. Efficacy, tolerability, and safety of rapid initiation of topiramate versus phenytoin in patients with new-onset epilepsy: a randomized double-blind clinical trial. Epilepsia. 2010;51:1970–1977. doi: 10.1111/j.1528-1167.2010.02670.x. [DOI] [PubMed] [Google Scholar]
- 61.Rapp RP, Norton JA, Young B, et al. Cutaneous reactions in head-injured patients receiving phenytoin for seizure prophylaxis. Neurosurgery. 1983;13:272–275. doi: 10.1227/00006123-198309000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Song C, Chen H, Wang X, et al. The efficacy and tolerability of lamotrigine adjunctive/monotherapy in patients with partial seizures refractory to poly-AEDs. Journal of Nanjing Medical University. 2009;23:322–327. [Google Scholar]
- 63.Craig I, Tallis R. Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single-blind randomized comparative study. Epilepsia. 1994;35:381–390. doi: 10.1111/j.1528-1157.1994.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 64.Peterson GM, Khoo BH, von Witt RJ. Clinical response in epilepsy in relation to total and free serum levels of phenytoin. Ther Drug Monit. 1991;13:415–419. doi: 10.1097/00007691-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Ramsay RE, Wilder BJ, Murphy JV, et al. Efficacy and safety of valproic acid versus phenytoin as sole therapy for newly diagnosed primary generalized tonic-clonic seizures. Journal of Epilepsy. 1992;5:55–60. [Google Scholar]
- 66.Shorvon SD, Chadwick D, Galbraith AW, Reynolds EH. One drug for epilepsy. Br Med J. 1978;1:474–476. doi: 10.1136/bmj.1.6111.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Callaghan N, Kenny RA, O’Neill B, et al. A prospective study between carbamazepine, phenytoin and sodium valproate as monotherapy in previously untreated and recently diagnosed patients with epilepsy. J Neurol Neurosurg Psychiatry. 1985;48:639–644. doi: 10.1136/jnnp.48.7.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steiner TJ, Dellaportas CI, Findley LJ, et al. Lamotrigine monotherapy in newly diagnosed untreated epilepsy: a double-blind comparison with phenytoin. Epilepsia. 1999;40:601–607. doi: 10.1111/j.1528-1157.1999.tb05562.x. [DOI] [PubMed] [Google Scholar]
- 69.Reynolds EH. Serum levels of anticonvulsant drugs. Interpretation and clinical value. Pharmacol Ther. 1980;8:217–235. doi: 10.1016/0163-7258(80)90047-9. [DOI] [PubMed] [Google Scholar]
- 70.Lund L. Anticonvulsant effect of diphenylhydantoin relative to plasma levels. A prospective three-year study in ambulant patients with generalized epileptic seizures. Arch Neurol. 1974;31:289–294. doi: 10.1001/archneur.1974.00490410037002. [DOI] [PubMed] [Google Scholar]
- 71.Turnbull DM, Rawlins MD, Weightman D, et al. “Therapeutic” serum concentration of phenytoin: the influence of seizure type. J Neurol Neurosurg Psychiatry. 1984;47:231–234. doi: 10.1136/jnnp.47.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buchthal F, Svensmark O, Schiller PJ. Clinical and electroencephalographic correlations with serum levels of diphenylhydanotin. Arch Neurol. 1960;2:624–630. doi: 10.1001/archneur.1960.03840120030004. [DOI] [PubMed] [Google Scholar]
- 73.Kutt H, Winters W, Kokenge R, McDowell F. Diphenylhydantoin metabolism, blood levels, and toxicity. Arch Neurol. 1964;11:642–648. doi: 10.1001/archneur.1964.00460240074010. [DOI] [PubMed] [Google Scholar]
- 74.Haerer AF, Grace JB. Studies of anticonvulsant levels in epileptics. I. Serum diphenylhydantoin concentrations in a group of medically indigent outpatients. Acta Neurol Scand. 1969;45:18–31. [PubMed] [Google Scholar]
- 75.Schmidt D. Single drug therapy for intractable epilepsy. J Neurol. 1983;229:221–226. doi: 10.1007/BF00313550. [DOI] [PubMed] [Google Scholar]
- 76.Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1016–1026. doi: 10.1016/S0140-6736(07)60461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramsay RE, Cantrell D, Collins SD, et al. Safety and tolerance of rapidly infused Depacon. A randomized trial in subjects with epilepsy. Epilepsy research. 2003;52:189–201. doi: 10.1016/s0920-1211(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 78.Sundqvist A, Tomson T, Lundkvist B. Valproate as monotherapy for juvenile myoclonic epilepsy: dose-effect study. Ther Drug Monit. 1998;20:149–157. doi: 10.1097/00007691-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 79.Gram L, Flachs H, Wurtz-Jorgensen A, et al. Sodium valproate, serum level and clinical effect in epilepsy: a controlled study. Epilepsia. 1979;20:303–311. doi: 10.1111/j.1528-1157.1979.tb04808.x. [DOI] [PubMed] [Google Scholar]
- 80.Adams DJ, Luders H, Pippenger C. Sodium valproate in the treatment of intractable seizure disorders: a clinical and electroencephalographic study. Neurology. 1978;28:152–157. doi: 10.1212/wnl.28.2.152. [DOI] [PubMed] [Google Scholar]
- 81.AbbVie. [5/21/15];Depakote package insert. 1983 online] Accessed at: www.rxabbvie.com/pdf/depakote.pdf on.
- 82.Berg MJ, Gross RA, Tomaszewski KJ, et al. Generic substitution in the treatment of epilepsy: case evidence of breakthrough seizures. Neurology. 2008;71:525–530. doi: 10.1212/01.wnl.0000319958.37502.8e. [DOI] [PubMed] [Google Scholar]
- 83.Andermann F, Duh MS, Gosselin A, et al. Compulsory generic switching of antiepileptic drugs: high switchback rates to branded compounds compared with other drug classes. Epilepsia. 2007;48:464–469. doi: 10.1111/j.1528-1167.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 84.Privitera MD, Welty TE, Gidal BE, et al. Generic-to-generic lamotrigine switches in people with epilepsy: the randomised controlled EQUIGEN trial. Lancet Neurol. 2016;15:365–372. doi: 10.1016/S1474-4422(16)00014-4. [DOI] [PubMed] [Google Scholar]
- 85.U.S. Food and Drug Administration. [3/29/16];Product-Specific Recommendations for Generic Drug Development [online] Accessed at: www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075207.htm on.