Abstract
Scope
Tannin-rich fruits have been evaluated as alternative prevention strategies for colorectal cancer based on their anti-inflammatory properties. This study compared tannin-rich preparations from mango (rich in gallotannins) and pomegranate (rich in ellagitannins) in the dextran sodium sulfate-induced colitis model.
Methods and results
In rats, mango and pomegranate beverages decreased intestinal inflammation and the levels of pro-inflammatory cytokines in mucosa and serum. The mango beverage suppressed the ratio of phosphorylated/total protein expression of the IGF-1R-AKT/mTOR axis and down-regulated mRNA expression of Igf1, Insr, and pik3cv. Pomegranate decreased p70S6K and RPS6, as well as Rps6ka2, Map2k2, and Mapk1 mRNA. In silico modeling indicated a high binding-docked of gallic acid to the catalytic domain of IGF-1R, which may suppress the activity of the enzyme. Ellagic acid docked effectively into the catalytic domains of both IGF-1R and EGFR. In vitro assays with lipopolysaccharide-treated CCD-18Co cells using polyphenolic extracts from each beverage, as well as pure compounds, corroborated the predictions made in silico.
Conclusion
Mango polyphenols inhibited the IGF-1R- AKT/mTOR axis, and pomegranate polyphenols downregulate the mTOR downstream pathway through reductions in ERK1/2. These results suggest that extracts rich in gallo- and ellagitannins act on different molecular targets in the protection against ulcerative colitis.
Keywords: Mango, pomegranate, rat, colitis, mTOR pathway
1 Introduction
Ulcerative colitis (UC) is a chronic inflammatory condition that affects 1.4 million people in the United States [1]. Although UC is rarely fatal on its own, it is associated with high levels of inflammation that increase the risk of colorectal cancer [2]. More than 18% of inflammatory bowel disease (IBD) patients develop colon cancer within 30 years of disease onset [3] and have a higher mortality risk than patients diagnosed with sporadic colon cancer [4]. Anti-inflammatory drugs play a significant role in the prevention and treatment of UC [5]; however, standard clinical care drug regimens are often associated with severe side effects [6]. Several studies have focused on alternative approaches to managing UC, based on natural anti-inflammatory products in the diet or in dietary supplements [7].
Polyphenolics derived from fruits and vegetables have demonstrated anti-inflammatory effects via the inhibition of nuclear factor kappa-B (NF-κB) and induction of antioxidant defense systems [8]. However, polyphenolics can exert distinct anti-inflammatory activities based on their specific structural features and affinities for different cellular targets [9]. Numerous polyphenols have shown anti-inflammatory, anti-cancer, and anti-obesity effects by binding to their target receptors as antagonists. Examples include trans-resveratrol and curcumin that bind to the human CB1 cannabinoid receptors [10]; as well as andepigallocatechin-3-gallate, which interacts with the 67-kDa laminin receptor [11], CD4 receptor [12], and hepatocyte growth factor receptor (Met) [13–16]. The affinity of polyphenols to receptors on the cell membrane is based on their chemical structures, and binding can modulate a host of downstream signaling pathways inside the cell.
Mango is rich in polyphenols, including gallic acid and gallotannins [17]. It was reported that mango extract exerted anti-inflammatory properties via reduction of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and tumor necrosis factor (TNF)-α in a dextran sodium sulfate (DSS)-induced model of colitis [18]. Unlike mango, pomegranate is rich in ellagic acid, ellagitannins, certain flavonoids, and anthocyanins. Pomegranate exhibited anti-inflammatory properties through suppression of COX-2 and iNOS expression, mitogen-activated protein kinase pathway (MAPK; p38MAPK, JNK, and Extracellular signal-regulated kinases (ERK)1/2), and NF-κB activities in chemically-induced colitis [19, 20]. Both mango and pomegranate polyphenolics showed anti-inflammatory effects in rats with chemically-induced colitis. Therefore, the mechanisms underlying anti-inflammatory activities of polyphenolics appear to be dependent on the predominant active constituents in mango and pomegranate.
The Mammalian Target of Rapamycin (mTOR) pathway has been implicated in UC [21–24]. The mTOR pathway can be triggered via signals acting on the insulin receptor (IR)/insulin like growth factor-1 receptor (IGF-1R), which increase cell growth and cell proliferation via downstream effectors, such as eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) and p70 ribosomal protein S6 kinase (p70S6K1) [25]. mTOR can also be regulated through the ERK1/2 pathway [26]. mTOR inhibitors have shown to be effective as anti-inflammatory drugs in colitis, attenuating NF-κB activation and T-cell function [21, 23, 27]. Targeted suppression of these activated pathways during colitis by natural compounds may provide an important strategy in the prevention of colitis.
Proteomic and gene expression analysis enables the identification of potential therapeutic targets in the prevention of inflammatory disease and underlying mechanisms [28]. In addition, molecular docking studies add mechanistic understanding of receptor tyrosine kinases (RTKs) inhibition by polyphenols in cancer prevention and other therapeutic benefits [15, 29]. These analytical tools aid in the comparison of gene expression and binding affinities of polyphenols to different targets.
The objective of this study was to determine the potential anti-inflammatory properties of mango and pomegranate polyphenolics, and to identify the underlying mechanisms in DSS-treated rats. We hypothesized that mango and pomegranate polyphenolics would decrease chemically induced colitis in rats through suppression of the mTOR and associated pathways. We further postulated that the underlying mechanisms would differ according to the structures of predominant polyphenolics in each treatment regimen.
2 Materials and methods
2.1 Beverage preparation
Mango (Keitt, Mexico) was peeled, homogenized, and heated with cellulase and pectinase to break down fiber. The puree was centrifuged and the supernatant was stored at −20°C to prevent oxidation. Pomegranate was received from Stiebs LLC (Kirkland, WA). The phenolic concentration in the experimental beverages was measured spectrophotometrically by the Foline-Ciocalteu assay against an external standard of gallic acid. Individual polyphenolics in the beverages (Table 1) were analyzed with a Waters Alliance 2690 HPLC-MS system (Milford, MA). In addition, identification by mass spectrometric analyses was performed with a Thermo Finnigan LCQ Deca XP Max mass spectrometer equipped with an ESI ion source (Thermo Fisher, San Jose, CA) [30]. The concentrations of total polyphenolics were as follows: mango beverage (475.90mg/L Gallic acid equivalent (GAE)) and pomegranate beverage (2504.74mg/L GAE) (Table 1). The caloric content and pH of the control beverage was adjusted by adding 15.7g sugar and 0.05g citric acid in 100ml distilled water (Brix 15.7 and pH 3.4) (Table 1) [31].
Table 1.
The content of experimental beverages and body weight changes, food intake, beverage intake, and caloric intake.
| Control | Mango | Pomegranate | |
|---|---|---|---|
| Brix | 15.7 | 15.6 | 15.8 |
| pH | 3.4 | 3.3 | 3.5 |
| Vitamin C (mg/L) | 6.13 | 10.17 | |
| Total polyphenolics (mg GAE/L) | 475.90 | 2504.74 | |
| Monogalloyl glucoside | Punicalins | ||
| Gallic acid | Punicalagins | ||
| OH-Benzoic acid hexoside | Ellagic acid | ||
| Dihydrophaseic acid | Chlorogenic acid | ||
| Gallotannins | Quercetin-3-rutinoside | ||
| Quercetin | |||
| Flavonols | |||
| Body weight changes (g) | 84.36 ± 7.70 | 42.75 ± 3.02* | 51.51 ± 4.26* |
| Food intake (g/day)a | 8.73 ± 0.28 | 125% | 130% |
| Beverage intake (ml/day)a | 91.24 ± 0.99 | 92% | 89% |
| Intake of polyphenols/rat (mg GAE) | 39.98 | 204.06 |
Values are the mean ± SEM (n=10 per group). Food intake measured as the mean (± SEM) weight (g) of food intake per 48 hour period at 8 weeks. Beverage intake was calculated as the mean (± SEM) volume (ml) of beverage consumed for the whole study. Caloric intake was calculated on the basis of 3 kcal/g of pellet and 0.612 kcal/ml of juice.
Reproduced with slight modification from reference 31.
The values are statistically significant compared to the control at p<0.05.
2.2 Animal treatment and tissue sampling
DSS-induced colitis is an excellent preclinical model of colitis that exhibits many phenotypic characteristics (such as loss of epithelial homeostasis, regeneration, and wound healing) relevant to the human disease [32]. This model is a valuable tool for investigating the involvement of dietary factors in the pathogenesis of IBD and therapeutic options [33]. Ten-week old male Sprague-Dawley rats obtained from Harlan Teklad (Houston, TX) were acclimated to the cages for 1 week and randomly assigned into control, mango, and pomegranate beverage groups (n=10/group). After 3 weeks of consuming rat food pellets (Cat # 8604, Harlan Teklad, Houston, TX) and experimental beverages ad libitum, all rats were administered 3% (w/v) DSS (Molecular weight, 36 000–50 000; Cat # 02160110; MP Biomedicals, Solon, OH) (3 cycles, 14 day separation) for 48 h to induce chronic inflammation [34–36]. Rats were killed at 10 weeks, and the entire colon was removed (Figure 1A). A one-centimeter section was cut from the distal end of each rat colon and fixed in 4% paraformaldehyde (PFA) for embedding in paraffin. The remaining colon was gently scraped and frozen at −80°C for protein and RNA samples. Preclinical experiments in rats were approved by the Texas A&M University Animal Care and Use Committee (TAMU AUP 2011-73).
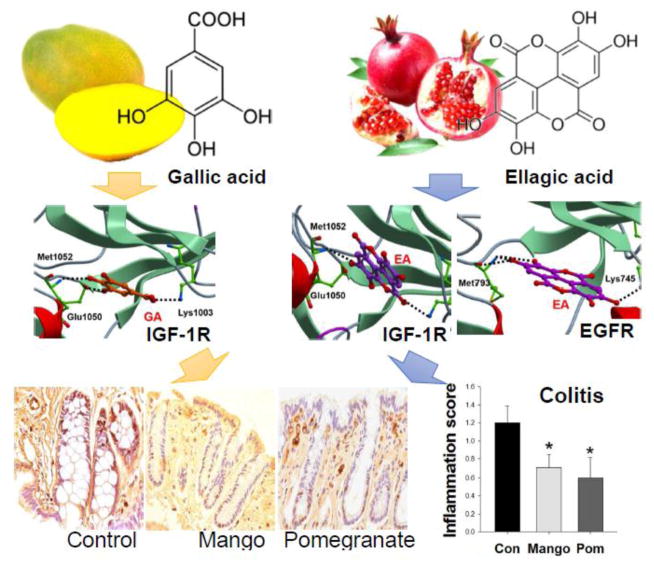
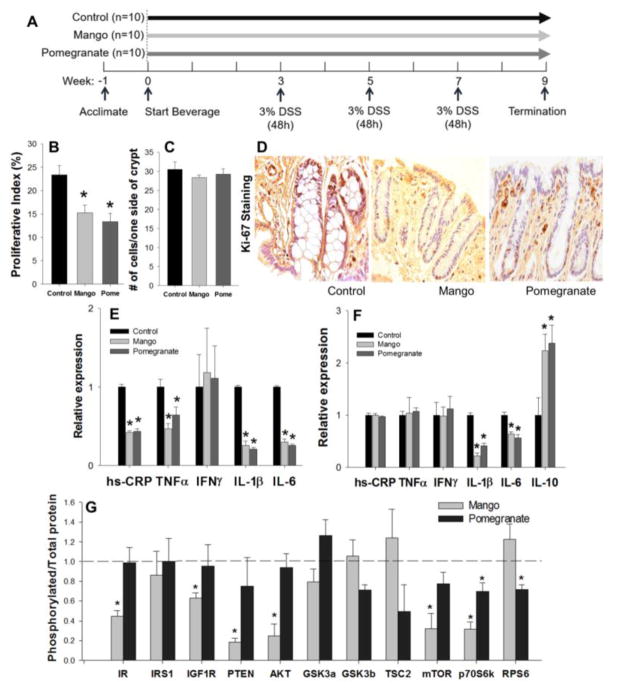
Figure 1. Effects of mango and pomegranate beverages on colonocyte proliferation, pro-inflammatory cytokines, and AKT/mTOR pathway in DSS-treated rats.
Values are the mean ± SEM (n=10 per group). Asterisk indicates significance compared to the control at p<0.05. (A) Experimental design of the in vivo study. (B) Mango and pomegranate beverages decreased the colonocyte proliferative index compared to control beverage in DSS-treated rats (n=10 per group, 25 crypts/rat). (C) There was no difference in the total number of cells on one side of a crypt. (D) Representative images of Ki-67 immunohistochemistry. (E) Mango and pomegranate beverages decreased the expression of hs-CRP and pro-inflammatory cytokines TNFα, IL-1β, and IL-6 levels in the intestinal mucosa of DSS-treated rats. (F) Mango and pomegranate beverages decreased the levels of IL-1β and IL-6 and increased the level of IL-10 in the DSS-treated rat serum. (G) The phosphorylated/total protein ratios of IR, IGF1R, PTEN, AKT, mTOR, and p70S6K1 were decreased by mango beverage, whereas p70S6K1 and RPS6 ratios were reduced by pomegranate. The dashed bar represents the control group, and the phosphorylation to protein ratio was normalized to the control.
2.3 Quantifying inflammation and cell proliferation index
DSS treatment causes histopathological changes in the colon (inflammation and mucosal ulceration) [37]. After DSS removal, re-epithelization of the damaged lining occurs via the squamous epithelium [38]. Severity of ulcerative colitis is correlated with the histological changes [39]. For histopathological analysis, hematoxylin and eosin stained slides were blindly scored (0 = none; 1 = mild; 2 = moderate; 3 = severe) by a veterinary pathologist (C.P.) to assess the degree of inflammation, ulceration, and squamous metaplasia. Immunohistochemical staining was performed using primary antibody against Ki-67 (Dilution 1:50, BD Pharmingen, San Jose, CA). Tissue sections were incubated with biotinylated anti-mouse IgG from the Vectastain ABC Elite kit (Vector Lab, Burlingame, CA) and 3,3′-Diaminobenzidine tetrahydrochloride as the chromagen. Hematoxylin was used as a counterstain. Ki-67 is indicative of proliferating cells, which showed up as brown spots within colonic crypt columns [40]. Twenty-five crypt columns per rat were selected for quantitative analysis (n=10/group). The total number of cells in each crypt (side) was counted, and the number and position of the Ki-67 positive cells were recorded from the base of the crypt along one side. The proliferative index was calculated as the percentage of positive cells divided by the total number of cells on the one side of the crypt column.
2.4 Inflammatory biomarkers
Colonic mucosa tissues (100μg) and serum samples were assayed for inflammatory markers, C-reactive protein (CRP) and cytokines (TNFα, IFNγ, IL-1β, IL-6, and IL-10) using multiplex kits (Millipore, Billerica, MA). Phosphorylation of the activation loop is required for activating signal kinases, including AKT, mTOR, and p70S6K [41, 42]. Protein extracts (50μg) from mucosal tissue were used to determine the levels of phosphorylated protein and total protein with mTOR signaling kits (Akt, GSK3β, GSK3α, IGF1R, IR, IRS1, mTOR, p70 S6 kinase, PTEN, RPS6, and TSC2, Millipore, Billerica, MA), according to the manufacturer’s protocol. Multiplex analysis was performed on a Luminex L200 instrument (Luminex, Austin, TX), and data were analyzed by Luminex xPONENT software. The levels of phosphorylated protein were normalized against total protein levels; the relative activation level of the signal pathway was expressed as the ratio of phosphorylated protein/total protein.
2.5 mTOR and MAPK gene expression analysis
mRNA was extracted from rat mucosal scrapings. 400ng of mRNA was converted to cDNA using a RT2 First-strand cDNA synthesis kit (Qiagen, Valencia, CA). The mTOR and MAPK pathways were examined using RT2-Profiler PCR arrays (n=6/group; Qiagen, Valencia, CA) and the ABI 7900HT PCR system (Applied biosystems, Foster City, CA). Each plate contains gene-specific primer sets for 84 known targets in the corresponding pathways of interest. Analysis of the results was performed by the zRMicroArray [43]. Genes with non-adjusted p<0.05 were considered as significant. Additionally, the false discovery rate (FDR) adjusted p-values were calculated.
2.6 Molecular modeling and docking
Molecular Docking was run with Molsoft ICM software (MolSoft LLC, San Diego, CA) as reported by [44, 45]. In the ICM-VLS screening procedure, each compound is assigned a score according to its fit within the receptor. The ICM score accounts for continuum and discrete electrostatics, hydrophobicity, and entropy parameters [46]. The protocols were validated by docking the co-crystallized inhibitors into the binding site of interest. The 3D-coordinates of the human IGF-1R and epidermal growth factor receptor (EGFR) catalytic domain in complex with inhibitors (1,3-disubstituted-8-amino-imidazopyrazine derivative and 4-Amino-6-arylamino-pyrimidine-5-carbaldehyde hydrazones) were retrieved from the Protein Data Bank (Structure ID= 3D94 [47] and Structure ID= 2RGP [48]) and energetically minimized in the internal coordinate space [44]. The inhibitors docked with the high score of −58.13 (1,3-disubstituted-8-amino-imidazopyrazine derivative to IGF-1R) and −54.75 (4-Amino-6-arylamino-pyrimidine-5-carbaldehyde hydrazones to EGFR), reproducing the non-covalent binding pattern observed in the crystal structures [47, 48].
2.7 Cell-based assays
Human CCD-18Co colon myofibroblast cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA). Intestinal myofibroblasts are located subjacent to the epithelium, and play crucial roles in intestinal inflammation through the production of pro-inflammatory cytokines [49, 50]. The LPS-treated CCD-18Co myofibroblast cells model is used to investigate mechanisms of inflammation [51]. Cells were seeded onto a 12-well plate (100, 000 cells/well) for mRNA analysis, and onto a 6-well plate (200,000 cells/well) for the protein analysis. After 24 hours, cells were incubated with mango or pomegranate extract (2.5–10 mg/L GAE), gallic acid (1–4 mg/L GAE), or ellagic acid (1–4 mg/L GAE) without lipopolysaccharide (LPS). The extracts for the in vitro study were prepared as described in our previous literature [30, 52]. The treatment was repeated again after 1 hour, but with media containing 2μg/mL LPS to determine the preventive activities of polyphenols. Simultaneous treatment of polyphenols and LPS would only show the therapeutic effects of polyphenols instead of both the preventative and therapeutic effects. In this study, cells were treated with polyphenols for 1 hour before the combined polyphenol and LPS treatments to study the preventative effects of polyphenols. mRNA was extracted for gene expression analysis after 3h, and protein was extracted after 23h. The extracted mRNA were converted to cDNA using a reverse transcription kit (Invitrogen, Grand Island, NY). The SYBR Green PCR master Mix (Applied Biosystems, Foster City, CA) was used for the qPCR analyses on an Applied Biosystems 7900HT Fast Real-Time PCR instrument. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. In addition, 50μg of lysed protein were loaded onto acrylamide gel, followed by transfer onto PVDF membranes. The membranes were incubated with primary antibodies against IGF-1R, EGFR (Cell Signaling Technology, Beverly, MA), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA).
2.8 Statistical analyses
Data were expressed as means with standard errors. All the data were analyzed by ANOVA and post-hoc Dunnett test (p<0.05) using JMP 10 (SAS Institute Inc., Cary, NC). Results from the gene expression arrays were analyzed by ANOVA R-package embedded in zRMicroArray [43]. Non adjusted p values <0.05 were accepted as significant.
3 Results
3.1 Mango and pomegranate beverages reduced the inflammatory response, and modified AKT/mTOR signaling pathway in DSS-treated rats
Histological scores were performed to assess the extent of colitis. Mango beverage reduced the inflammation score by 41% (p=0.05). Pomegranate beverage decreased the inflammation and ulceration scores by 50% and 63%, respectively (p=0.049 and 0.042) compared to the control group (Table 2). Compared to controls, mango and pomegranate beverages reduced Ki-67 labeling by 37% and 42%, respectively (p=0.0245 and 0.0259, Figure 1B and D), indicating significant inhibition of the colonic cell proliferation index. There was no difference in the total number of cells per side of a crypt (Figure 1C). The level of hs-CRP and pro-inflammatory cytokines (such as TNFα, IL1β, and IL-6) in the mucosal samples also were lowered by the mango and pomegranate treatments (Figure 1E). In addition, the beverages decreased the level of IL-1β and IL-6, and induced the level of IL-10 in serum samples (Figure 1F). In multiplex assays for mTOR pathway intermediates, mango beverage decreased the ratio of phosphorylated/total protein for IR, IGF1R, PTEN, AKT, mTOR, and p70S6K. The pomegranate beverage decreased the ratio of phosphorylated/total protein of p70S6K and RPS6 (Figure 1G).
Table 2.
Histological scores for inflammation-associated changes in DSS treated rats.
| Inflammationa | Ulcerationa | Squamous Metaplasia | |
|---|---|---|---|
| Control | 1.2 ± 0.19 | 0.9 ± 0.23 | 1.3 ± 0.44 |
| Mango | 0.7 ± 0.15* | 0.7 ± 0.21 | 0.7 ± 0.36 |
| Pomegranate | 0.6 ± 0.22* | 0.3 ± 0.15* | 0.9 ± 0.37 |
Values are the mean ± SEM (n=10 per group).
Reproduced with slight modification from reference 31.
The values are statistically significant compared to the control at p<0.05.
3.2 Mango and pomegranate beverages modulate mucosal mTOR and MAPK gene expression
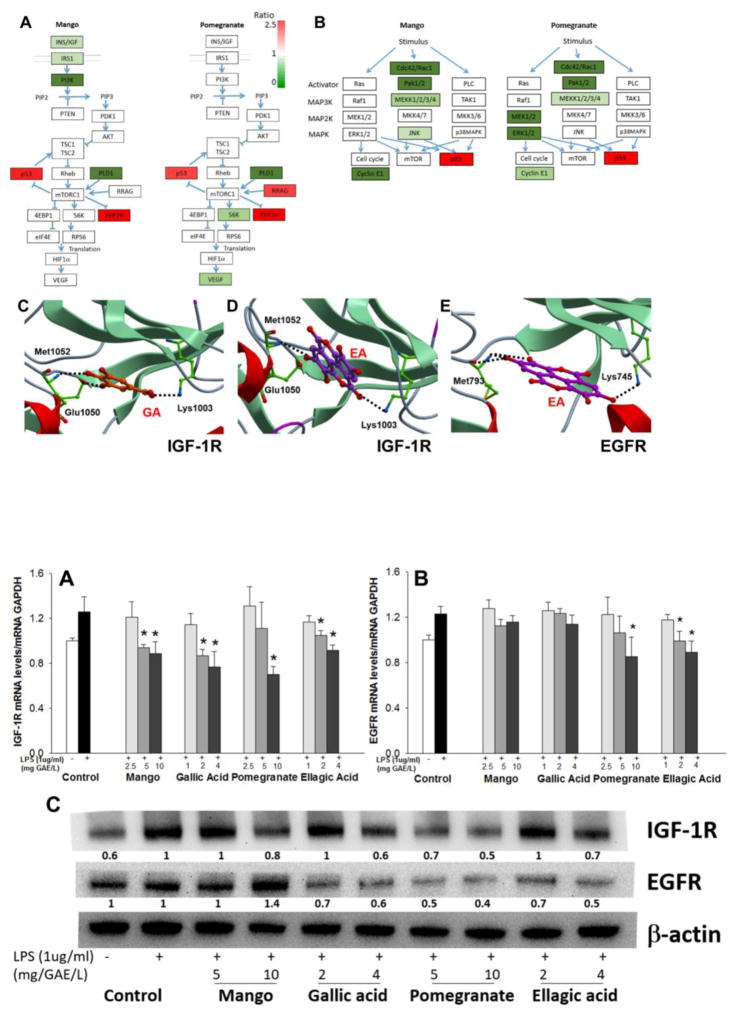
PCR arrays were performed with mRNA isolated from rat mucosal scrapings (n=6/group). Among the 84 genes profiled in the mTOR pathway, six genes had a significant differential expression after mango or pomegranate treatment, including downregulation of Pld1, and upregulation of Ppp2r2b and Tp53. After FDR adjustment for multiple testing, there was no significant difference. Genes Igf1, Insr, and Pik3cv were downregulated by mango beverage, whereas pomegranate beverage upregulated Rragd and attenuated Rps6ka2 and Vegfc (Figure 2A and Table 3). Among the 84 genes profiled in the MAPK pathway, several targets had a significant differential expression after mango and pomegranate beverages, including consistent upregulation of Tp53 and downregulation of Map3k2, Pak1, and Ccne1. Genes Mapk9, Mapk10, and Rac1 were downregulated by mango beverage, while pomegranate beverage downregulated Cdc42, Map2k2, Mapk1, and sfn mRNA (Figure 2B and Table 3).
Figure 2. Differentially expressed genes altered by mango or pomegranate beverages and docking of gallic acid and ellagic acid with IGF-1R and EGFR catalytic domains.
(A) mTOR signaling and (B) MAPK pathway. RNA was isolated from rat mucosal scrapings (n=6/group). Relative expression was presented as a comparison of the experimental group to the control group. Red boxes, significantly upregulated genes; green boxes, significantly downregulated genes (P<0.05, respectively); white boxes, no significant change detected. (C) Docking of gallic acid (GA) into the inhibited conformation of human IGF-1R catalytic domains, (D) ellagic acid (EA) into the IGF-1R, (E) EA into the EGFR. Ligands are displayed as sticks and colored by atom type, with carbon atoms in orange (GA) and magenta (EA). Protein residues are shown as a stick with the carbon atoms colored in green. Secondary structure is displayed as ribbon. Protein-ligand hydrogen bond interactions are displayed as dashed black lines (Molsoft ICM v3.7-2d).
Table 3.
Differentially expressed genes in colonic mucosa following mango and pomegranate treatment.
| Symbol | Gene name | GenebankID | Relative expressiona | p-valueb | Relative expressiona | p-valueb |
|---|---|---|---|---|---|---|
|
| ||||||
| Mango | Pomegranate | |||||
| mTOR pathway | ||||||
| Ppp2r2b | Protein phosphatase 2 (formerly 2A), regulatory subunit B, beta isoform | NM_022209 | 2.993 | 0.026 | 2.792 | 0.037 |
| Tp53 | Tumor protein p53 | NM_030989 | 2.585 | 0.045 | 2.367 | 0.049 |
| Pld1 | Phospholipase D1 | NM_030992 | 0.342 | 0.049 | 0.350 | 0.044 |
| Igf1 | Insulin-like growth factor 1 | NM_178866 | 0.573 | 0.038 | ||
| Pik3cv | Phosphoinositide-3-kinase, catalytic, beta polypeptide | NM_053481 | 0.351 | 0.045 | ||
| Insr | Insulin receptor | NM_017071 | 0.608 | 0.047 | ||
| Vegfc | Vascular endothelial growth factor C | NM_053653 | 0.428 | 0.007 | ||
| Rps6ka2 | Ribosomal protein S6 kinase polypeptide 2 | NM_057128 | 0.455 | 0.035 | ||
| Rragd | Ras-related GTP binding D | NM_001106641 | 2.241 | 0.040 | ||
|
| ||||||
| MAPK pathway | ||||||
| Ccne1 | Cyclin E1 | M_00110082 | 0.460 | 0.022 | 0.532 | 0.023 |
| Pak1 | P21 protein (Cdc42/Rac)-activated kinase 1 | NM_017198 | 0.456 | 0.042 | 0.480 | 0.044 |
| Tp53 | Tumor protein p53 | NM_030989 | 3.991 | 0.045 | 3.093 | 0.026 |
| Map3k2 | Mitogen activated protein kinase kinase kinase 2 | NM_138503 | 0.570 | 0.047 | 0.639 | 0.032 |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 | NM_134366 | 0.489 | 0.017 | ||
| Mapk10 | Mitogen activated protein kinase 10 | NM_012806 | 0.445 | 0.036 | ||
| Mapk9 | Mitogen-activated protein kinase 9 | NM_017322 | 0.564 | 0.049 | ||
| Map2k2 | Mitogen activated protein kinase kinase 2 | NM_133283 | 0.495 | 0.027 | ||
| Cdc42 | Cell division cycle 42 (GTP binding protein) | NM_171994 | 0.499 | 0.030 | ||
| Mapk1 | Mitogen activated protein kinase 1 | NM_053842 | 0.432 | 0.036 | ||
| Sfn | Stratifin | XM_232745 | 0.443 | 0.037 | ||
Relative expression was presented as a comparison of the experimental group to the control group.
Non-adjusted p values <0.05. After FDR adjustment for multiple testing, the p-values were non-significant.
3.3 Molecular modeling and docking
All kinase enzymes have a catalytic domain that binds ATP. Inhibited conformation refers to a conformational change of the enzyme upon the binding of inhibitors into the ATP binding pocket, inactivating the kinase [53]. Human IGF-1R and EGFR catalytic domains in the inhibited conformation were retrieved from the Protein Data Bank (IGF-1R)(Structure ID=3D94) [47] and EGFR (Structure ID=2RGP) [48]). Gallic acid and ellagic acid docked favorably into the IGF-1R ATP pocket, with scores of −27.5 and −34, respectively (Table 4, Figure 2C and D). Phenol hydroxyls of gallic acid and ellagic acid interacted with hinge residues of Met 1052 (N-H) and Glu 1050 (C=O), whereas the ligand carboxylate group associated with the side chain of Lys 1003, as experimentally observed with the inhibitor 1,3-disubstituted-8-amino-imidazopyrazine derivative (Score: −58.13) [47]. Ellagic acid also docked favorably into the EGFR ATP pocket, with a score of −21.87 (Table 4, Figure 2E). Similar to 4-amino-6-arylamino-pyrimidine-5-carbaldehyde hydrazones, ellagic acid established two hydrogen bonds between the phenol hydroxyls and the carbonyl C=O and N-H backbone of hinge residue Met 793 [48]. A non-covalent interaction between the ligand and the side chain of Lys 745 also was detected (Figure 2E). Gallic acid did not dock favorably into the EGFR ATP pocket (Table 4).
Table 4.
Docking into human IGF-1R and EGFR catalytic domains in the inhibited conformation.
| Score IGF-1R | Score EGFR | |
|---|---|---|
| 1,3-disubstituted-8-amino-imidazopyrazine derivative | − 58.13 | - |
| 4-Amino-6-arylamino-pyrimidine-5-carbaldehyde hydrazones | - | − 54.75 |
| Gallic acid (GA) | − 27.50 | nd* |
| Ellagic acid (EA) | − 34.00 | − 21.87 |
nd- not docked favorably
3.4 Effects of mango, gallic acid, pomegranate, and ellagic acid on IGF-1R and EGFR in cells
Based on the predictions from molecular docking in silico, we hypothesized that mango and pomegranate beverages, and their major polyphenolics, would exert anti-inflammatory activities, at least in part, by inhibiting IGF-1R and EGFR associated pathways. In LPS-treated CCD-18Co cells, mango extract and gallic acid suppressed the expression of IGF-1R mRNA by 29% at 10mg/L GAE of mango extract and by 39% at 4mg/L of gallic acid (Figure 3A). Mango and gallic acid also attenuated the corresponding protein expression by 20% at 10mg/L GAE of mango extract and by 40% at 4mg/L of gallic acid (Figure 3C). Pomegranate extract suppressed the expression of IGF-1R mRNA by 46% at 10mg/L GAE, compared to a 27% reduction by 4mg/L ellagic acid (Figure 3A). The expression of EGFR mRNA was attenuated by 31% at 10mg/L GAE of pomegranate extract, compared to 27% at 4mg/L of ellagic acid (Figure 3B). In LPS-treated CCD-18Co cells, protein expression levels of IGF-1R were reduced at 5–10mg/L GAE of pomegranate extract, whereas EGFR protein expression was diminished by gallic acid (2–4 mg/L GAE), ellagic acid (2–4 mg/L GAE), and pomegranate (5–10 mg/L GAE) (Figure 3C).
Figure 3. Effects of mango, gallic acid, pomegranate, and ellagic acid on IGF-1R and EGFR mRNA and protein expression in LPS-treated CCD-18Co cells.
(A) Mango, gallic acid, pomegranate, and ellagic acid lowered the expression of IGF-1R mRNA, whereas (B) pomegranate and ellagic acid decreased the expression of EGFR mRNA, 4 h after treatment. Values are the mean ± SEM (n=3 per group). Asterisk indicate significance compared to the control at p<0.05. (C) Western blot of IGF-1R and EGFR protein, 24 h after treatment with test agents. The number under each band represents protein expression (relative densitometry) normalized to β-actin.
4 Discussion
This is the first time that mango and pomegranate beverages as well as their effects on mTOR and related signaling pathways have been compared in a preclinical model of colitis. Moreover, mechanistic insights were derived from molecular docking predictions in silico and cell-based assays with lipopolysaccharide (LPS)-treated CCD-18Co cell lines, which corroborated findings from the studies in vivo. In the rat/DSS model, mango and pomegranate beverages decreased the inflammatory response and attenuated the levels of pro-inflammatory cytokines in mucosal and serum samples. These findings are clearly consistent with an overall protective effect of mango and pomegranate beverages under the conditions reported here.
In this study, mango and pomegranate beverages decreased the colonocyte proliferation index in DSS treated rats. Cell proliferation is advantageous for healing and regeneration of damaged epithelium in acute cases [54]. In this study, however, chronic colitis was induced by 3 cycles of 3% DSS treatment for 2 days followed by 12 days of water [34, 35]. Histological assessment showed complete re-epithelialization of distal colon with squamous metaplasia in rats at the end of this study. Re-epithelialization is the last stage of the wound healing process, and is frequently observed in mice 1–4 weeks after DSS removal (this study was terminated 2 weeks after the third DSS administration) [55]. In addition, it was reported that cell proliferation in chronic phases of DSS was induced 40–60 fold compared to no DSS [56]. Cell proliferation is advantageous for acute damage repair, but not in long-term progression of DSS-induced damage. It detrimental to have higher levels of proliferation in the long-term, as it is related to the induction of cell inflammation and dysplasia [55]. Therefore, decreased proliferation in the experimental beverage group would likely relate to lower inflammation and cellular damage.
Sequential activation of the AKT/mTOR pathway plays critical roles in regulation of cell proliferation and inflammation [57, 58]. The binding of IGF-1, insulin, and other growth signals to their receptors (IGF-1R) phosphorylates several intracellular kinases including AKT [59]. Phosphorylated AKT is catalytically active and contributes to the phosphorylation (activation) of the downstream kinases, including mTOR and p70S6K [41, 60]. The activation of p70S6K and RPS6 by phosphorylation induced HIF-1α protein by increasing its translation, resulting in cell proliferation and inflammation [61–63]. In this study, levels of phosphorylated protein and total protein were measured to determine the relative activities of the components involved in the mTOR signaling pathway. Mango beverage decreased the activities of upstream components of the mTOR pathway in the colonic mucosa, such as IR, IGF-1R, and AKT, whereas pomegranate beverage decreased the activities of the downstream components of the mTOR pathway, such as p70S6K and RPS6. Suppression of the mTOR pathway activity by our experimental beverages may result in decreased cell proliferation and inflammation.
Previously, it was reported that 3% DSS treatment altered 1609 genes (among 39,000 genes) related with inflammation, cell proliferation, and cell signaling responses including upregulation of igf1 gene expression in the intestinal mucosa during 6 days compared to the control sacrificed prior to DSS treatment [64]. We hypothesized that mango and pomegranate beverages would suppress inflammation via downregulation of the mTOR pathway, which is activated in colitis and impacts cell growth, proliferation, and inflammatory responses in the gut [21, 23, 27]. Dietary pomegranate was previously reported to exert anti-inflammatory activities through inhibition of the MAPK pathway in chronic TNBS-induced colitis [19, 20]. Supporting evidence was provided by the current investigation using the DSS/colitis model, with evidence for decreased expression of several targets by the pomegranate treatment in the MAPK pathway, including Cdc42, Pak1, Mapk1, Map2k2, Map3k2, Sfn, and Ccne1. These findings are consistent with the protective outcomes of mango and pomegranate beverages, although the constituent flavonoids might differentially impact the same molecular targets involved in chronic inflammation [9], such as Ccne1, Map3k2, Pak1, Pld1, Ppp2r2b, and Tp53.
In silico docking provided insights into the possible interactions of mango and pomegranate constituents with IGF-1R and EGFR, which may influence EGFR-mediated activation of the MAPK pathway and cell proliferation [65, 66]. Gallic acid and ellagic acid bind to not only to IGF-1R and EGFR, but other receptors as well. However, this study aimed to determine the interaction of the major active components (gallic and ellagic acid from tannins) of each fruit preparation on the receptors involved in the mTOR and MAPK pathways. Predictions obtained through computational modeling were corroborated in cell-based assays. For example, IGF-1R was confirmed at the mRNA and protein level as a target for downregulation in cells treated with gallic acid, ellagic acid, mango, and pomegranate. Interestingly, gallic acid was not predicted to interact favorably with the EGFR catalytic domain, but showed evidence for downregulation of EGFR protein expression in cell-based assays. Further studies might be warranted on alternative binding sites for gallic acid on EGFR. Unlike gallic acid, ellagic acid docked favorably with EGFR in the inhibited conformation and significantly lowered the corresponding mRNA and protein expression levels of EGFR in cell-based assays. It is likely that metabolism also plays an important role in determining the relative efficacy of targeting IGF-1R and EGFR in vivo, via conversion of ellagic acid to urolithins in the gut or feedback signaling mechanisms [67–69].
In this study, even though mango and pomegranate beverages contained representative amounts of the nutrients naturally found in fruits, such as sugar, vitamin C, and other polyphenolics, only the effects of gallic acid and ellagic acid were tested in a cell-based assay,. Gallic and ellagic acid are the most prevalent of the polyphenolic compounds found in mango and pomegranate. The effects of the other mango polyphenols were tested in previous studies, and their effects in inflammation were not significant (as yet unpublished). Even though it was reported that vitamin C intake (100mg/kg body weight) decreased inflammation in DSS-treated mice [70], the amount of vitamin C in the beverages is insignificant compared to the polyphenolic content. The intake of vitamin C in each animal is negligible compared to studies performed with Vitamin C that report any efficacy. Overall, a more elaborate research design is needed to determine the anti-inflammatory activities of each component and their respective combinations that are represented in a whole fruit preparation. This study aimed to test only the major active compounds (Gallic acid and ellagic acid) of each fruit preparation.
In conclusion, the present investigation demonstrated that mango and pomegranate beverages exert beneficial protective outcomes in a preclinical model of colitis. Mango beverage appears to target multiple points along the IGF-1R-AKT/mTOR axis, whereas pomegranate beverage regulates downstream components of the mTOR pathway through the reduction of ERK1/2. Constituents of the respective beverages were predicted in silico to target specific members of the signaling cascade, such as IGF-1R and EGRF; this was corroborated in cell-based assays. The current work does not exclude the possibility that other constituents in mango and pomegranate beverages, or their metabolites, might be effective towards the mTOR pathway, or indeed might act cooperatively to protect against the chronic inflammation that underlies UC in humans.
Acknowledgments
The study was supported by the National Mango Board. RHD is supported by P01 grant CA090890 from the National Cancer Institute, by P30 grant ES023512 from the National Institute of Environmental Health Sciences, by the John S. Dunn Foundation, and by a Chancellor’s Research Initiative from Texas A&M University.
Abbreviations
- 4E-BP1
Eukaryotic translation initiation factor 4E-binding protein 1
- COX-2
Cyclooxygenase-2
- DSS
dextran sodium sulfate
- EA
ellagic acid
- EGFR
epidermal growth factor receptor
- ERK
Extracellular signal-regulated kinases
- FDR
false discovery rate
- GA
gallic acid
- GAE
gallic acid equivalent
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- IBD
inflammatory bowel disease
- IGF-1R
insulin like growth factor-1 receptor
- iNOS
inducible nitric oxide synthase
- IR
insulin receptor
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase pathway
- Met
hepatocyte growth factor receptor
- mTOR
mammalian target of rapamycin
- NF-κB
Nuclear factor-kappa B
- p70S6K
p70 ribosomal protein S6 kinase
- RPS6
Ribosomal protein s6
- RTKs
receptor tyrosine kinases
- TNF-α
Tumor necrosis factor alpha
- UC
ulcerative colitis
Footnotes
Study conception and design: HK, STT, SUMT; Acquisition of data: HK, NB; Analysis and interpretation of data: HK, II, KRP, WHB, RHD, SUMT; Drafting of manuscript: HK, SUMT; Critical Revision: WHB, RHD, SUMT
The authors have declared no conflict of interest.
References
- 1.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence prevalence and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. American journal of physiology Gastrointestinal and liver physiology. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 3.Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World journal of gastroenterology: WJG. 2008;14:3937–3947. doi: 10.3748/wjg.14.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Alimentary pharmacology & therapeutics. 2003;18:1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, DuBois RN. The role of anti-inflammatory drugs in colorectal cancer. Annual review of medicine. 2013;64:131–144. doi: 10.1146/annurev-med-112211-154330. [DOI] [PubMed] [Google Scholar]
- 6.Sachedina B, Saibil F, Cohen LB, Whittey J. Acute pancreatitis due to 5-aminosalicylate. Annals of internal medicine. 1989;110:490–492. doi: 10.7326/0003-4819-110-6-490. [DOI] [PubMed] [Google Scholar]
- 7.Yuan G, Wahlqvist ML, He G, Yang M, Li D. Natural products and anti-inflammatory activity. Asia Pacific journal of clinical nutrition. 2006;15:143–152. [PubMed] [Google Scholar]
- 8.Shapiro H, Singer P, Halpern Z, Bruck R. Polyphenols in the treatment of inflammatory bowel disease and acute pancreatitis. Gut. 2007;56:426–435. doi: 10.1136/gut.2006.094599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez R, Ballester I, Lopez-Posadas R, Suarez MD, et al. Effects of flavonoids and other polyphenols on inflammation. Critical reviews in food science and nutrition. 2011;51:331–362. doi: 10.1080/10408390903584094. [DOI] [PubMed] [Google Scholar]
- 10.Seely KA, Levi MS, Prather PL. Retraction: The Dietary Polyphenols trans-Resveratrol and Curcumin Selectively Bind Human CB1 Cannabinoid Receptors with Nanomolar Affinities and Function as Antagonists/Inverse Agonists. Journal of Pharmacology and Experimental Therapeutics. 2009;330:31–39. doi: 10.1124/jpet.109.151654. [DOI] [PubMed] [Google Scholar]
- 11.Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nature structural & molecular biology. 2004;11:380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 12.Williamson MP, McCormick TG, Nance CL, Shearer WT. Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: Potential for HIV-1 therapy. Journal of allergy and clinical immunology. 2006;118:1369–1374. doi: 10.1016/j.jaci.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Larsen CA, Dashwood RH. (−)-Epigallocatechin-3-gallate inhibits Met signaling proliferation and invasiveness in human colon cancer cells. Archives of biochemistry and biophysics. 2010;501:52–57. doi: 10.1016/j.abb.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen CA, Dashwood RH. Suppression of Met activation in human colon cancer cells treated with (−)-epigallocatechin-3-gallate: minor role of hydrogen peroxide. Biochemical and biophysical research communications. 2009;389:527–530. doi: 10.1016/j.bbrc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen CA, Dashwood RH, Bisson WH. Tea catechins as inhibitors of receptor tyrosine kinases: mechanistic insights and human relevance. Pharmacological Research. 2010;62:457–464. doi: 10.1016/j.phrs.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen CA, Bisson WH, Dashwood RH. Tea catechins inhibit hepatocyte growth factor receptor (MET kinase) activity in human colon cancer cells: kinetic and molecular docking studies. Journal of medicinal chemistry. 2009;52:6543–6545. doi: 10.1021/jm901330e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y, Brecht JK, Talcott ST. Antioxidant phytochemical and fruit quality changes in mango (Mangifera indica L) following hot water immersion and controlled atmosphere storage. Food Chemistry. 2007;105:1327–1334. [Google Scholar]
- 18.Marquez L, Perez-Nievas BG, Garate I, Garcia-Bueno B, et al. Anti-inflammatory effects of Mangifera indica L. extract in a model of colitis. World journal of gastroenterology: WJG. 2010;16:4922–4931. doi: 10.3748/wjg.v16.i39.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boussetta T, Raad H, Letteron P, Gougerot-Pocidalo MA, et al. Punicic acid a conjugated linolenic acid inhibits TNFalpha-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats. PloS one. 2009;4:e6458. doi: 10.1371/journal.pone.0006458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosillo MA, Sanchez-Hidalgo M, Cardeno A, Aparicio-Soto M, et al. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacological research: the official journal of the Italian Pharmacological Society. 2012;66:235–242. doi: 10.1016/j.phrs.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Bhonde MR, Gupte RD, Dadarkar SD, Jadhav MG, et al. A novel mTOR inhibitor is efficacious in a murine model of colitis. American journal of physiology Gastrointestinal and liver physiology. 2008;295:G1237–1245. doi: 10.1152/ajpgi.90537.2008. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda C, Ito T, Song J, Mizushima T, et al. Therapeutic effect of a new immunosuppressive agent, everolimus, on interleukin-10 gene-deficient mice with colitis. Clin Exp Immunol. 2007;148:348–359. doi: 10.1111/j.1365-2249.2007.03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farkas S, Hornung M, Sattler C, Guba M, et al. Rapamycin decreases leukocyte migration in vivo and effectively reduces experimentally induced chronic colitis. Int J Colorectal Dis. 2006;21:747–753. doi: 10.1007/s00384-005-0793-7. [DOI] [PubMed] [Google Scholar]
- 24.Sun X, Threadgill D, Jobin C. Campylobacter jejuni induces colitis through activation of mammalian target of rapamycin signaling. Gastroenterology. 2012;142:86–95. e85. doi: 10.1053/j.gastro.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang YJ, Dai Q, Sun DF, Xiong H, et al. mTOR signaling pathway is a target for the treatment of colorectal cancer. Ann Surg Oncol. 2009;16:2617–2628. doi: 10.1245/s10434-009-0555-9. [DOI] [PubMed] [Google Scholar]
- 26.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends in biochemical sciences. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deore V, Yewalkar N, Bhatia D, Desai N, et al. Synthesis and therapeutic evaluation of pyridyl based novel mTOR inhibitors. Bioorg Med Chem Lett. 2009;19:2949–2952. doi: 10.1016/j.bmcl.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 28.Dolara P, Luceri C, De Filippo C, Femia AP, et al. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat Res. 2005;591:237–246. doi: 10.1016/j.mrfmmm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Smith DM, Daniel KG, Wang Z, Guida WC, et al. Docking studies and model development of tea polyphenol proteasome inhibitors: applications to rational drug design. Proteins: Structure, Function, and Bioinformatics. 2004;54:58–70. doi: 10.1002/prot.10504. [DOI] [PubMed] [Google Scholar]
- 30.Noratto GD, Bertoldi MC, Krenek K, Talcott ST, et al. Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. J Agric Food Chem. 2010;58:4104–4112. doi: 10.1021/jf903161g. [DOI] [PubMed] [Google Scholar]
- 31.Kim H, Banerjee N, Barnes R, Pfent CM, et al. Mango polyphenolics reduce inflammation in intestinal colitis - involvement of the miR-126/PI3K/AKT/mTOR axis in vitro and in vivo. Molecular carcinogenesis. 2016 doi: 10.1002/mc.22484. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, et al. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240–248. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 33.Melgar S, Karlsson L, Rehnström E, Karlsson A, et al. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. International immunopharmacology. 2008;8:836–844. doi: 10.1016/j.intimp.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 34.Hong MY, Bancroft LK, Turner ND, Davidson LA, et al. Fish oil decreases oxidative DNA damage by enhancing apoptosis in rat colon. Nutrition and cancer. 2005;52:166–175. doi: 10.1207/s15327914nc5202_7. [DOI] [PubMed] [Google Scholar]
- 35.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 36.Ritchie LE, Sturino JM, Carroll RJ, Rooney LW, et al. Polyphenol-rich sorghum brans alter colon microbiota and impact species diversity and species richness after multiple bouts of dextran sodium sulfate-induced colitis. FEMS microbiology ecology. 2015;91:fiv008. doi: 10.1093/femsec/fiv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Y, Kolachala V, Dalmasso G, Nguyen H, et al. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PloS one. 2009;4:e6073. doi: 10.1371/journal.pone.0006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta RB, Harpaz N, Itzkowitz S, Hossain S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. Journal of cellular physiology. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annual review of biochemistry. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 42.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nature cell biology. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 43.Zlatev Z. Sofia University; Bulgaria: 2009. [Google Scholar]
- 44.Perkins A, Phillips JL, Kerkvliet NI, Tanguay RL, et al. A Structural Switch between Agonist and Antagonist Bound Conformations for a Ligand-Optimized Model of the Human Aryl Hydrocarbon Receptor Ligand Binding Domain. Biology. 2014;3:645–669. doi: 10.3390/biology3040645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abagyan R, Totrov M, Kuznetsov D. ICM—a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. Journal of computational chemistry. 1994;15:488–506. [Google Scholar]
- 46.Totrov M, Abagyan R. Proceedings of the third annual international conference on Computational molecular biology; ACM; 1999. pp. 312–320. [Google Scholar]
- 47.Wu J, Li W, Craddock BP, Foreman KW, et al. Small-molecule inhibition and activation-loop trans-phosphorylation of the IGF1 receptor. The EMBO journal. 2008;27:1985–1994. doi: 10.1038/emboj.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu G, Abad MC, Connolly PJ, Neeper MP, et al. 4-Amino-6-arylamino-pyrimidine-5-carbaldehyde hydrazones as potent ErbB-2/EGFR dual kinase inhibitors. Bioorganic & medicinal chemistry letters. 2008;18:4615–4619. doi: 10.1016/j.bmcl.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 49.Kawasaki H, Ohama T, Hori M, Sato K. Establishment of mouse intestinal myofibroblast cell lines. World journal of gastroenterology: WJG. 2013;19:2629–2637. doi: 10.3748/wjg.v19.i17.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De LKT, Whiting CV, Bland PW. Proinflammatory cytokine synthesis by mucosal fibroblasts from mouse colitis is enhanced by interferon-gamma-mediated up-regulation of CD40 signalling. Clin Exp Immunol. 2007;147:313–323. doi: 10.1111/j.1365-2249.2006.03267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogler G, Gelbmann C, Vogl D, Brunner M, et al. Differential activation of cytokine secretion in primary human colonic fibroblast/myofibroblast cultures. Scandinavian journal of gastroenterology. 2001;36:389–398. doi: 10.1080/003655201300051216. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee N, Talcott S, Safe S, Mertens-Talcott SU. Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and vivo: potential role of miRNA-27a and miRNA-155 in cell survival and inflammation. Breast cancer research and treatment. 2012;136:21–34. doi: 10.1007/s10549-012-2224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao JJL. Molecular recognition of protein kinase binding pockets for design of potent and selective kinase inhibitors. Journal of medicinal chemistry. 2007;50:409–424. doi: 10.1021/jm0608107. [DOI] [PubMed] [Google Scholar]
- 54.Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World journal of gastroenterology. 2008;14:348. doi: 10.3748/wjg.14.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. BioMed Research International. 2012;2012 doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vetuschi A, Latella G, Sferra R, Caprilli R, Gaudio E. Increased proliferation and apoptosis of colonic epithelial cells in dextran sulfate sodium-induced colitis in rats. Digestive diseases and sciences. 2002;47:1447–1457. doi: 10.1023/a:1015931128583. [DOI] [PubMed] [Google Scholar]
- 57.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Lim HK, Choi YA, Park W, Lee T, et al. Phosphatidic acid regulates systemic inflammatory responses by modulating the Akt-mammalian target of rapamycin-p70 S6 kinase 1 pathway. Journal of Biological Chemistry. 2003;278:45117–45127. doi: 10.1074/jbc.M303789200. [DOI] [PubMed] [Google Scholar]
- 59.Stitt TN, Drujan D, Clarke BA, Panaro F, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Molecular cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 60.Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. Journal of Biological Chemistry. 2005;280:26089–26093. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- 61.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 62.Weichhart T, Costantino G, Poglitsch M, Rosner M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 63.Land SC, Tee AR. Hypoxia-inducible factor 1α is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. Journal of Biological Chemistry. 2007;282:20534–20543. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 64.Fang K, Bruce M, Pattillo CB, Zhang S, et al. Temporal genome wide expression profiling of DSS colitis reveals novel inflammatory and angiogenesis genes similar to ulcerative colitis. Physiological genomics. 2010 doi: 10.1152/physiolgenomics.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hewish M, Chau I, Cunningham D. Insulin-like growth factor 1 receptor targeted therapeutics: novel compounds and novel treatment strategies for cancer medicine. Recent patents on anti-cancer drug discovery. 2009;4:54–72. doi: 10.2174/157489209787002515. [DOI] [PubMed] [Google Scholar]
- 66.Fischer O, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochemical Society Transactions. 2003;31:1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- 67.Landete J. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Research International. 2011;44:1150–1160. [Google Scholar]
- 68.Giorgio C, Mena P, Del Rio D, Brighenti F, et al. The ellagitannin colonic metabolite urolithin D selectively inhibits EphA2 phosphorylation in prostate cancer cells. Molecular nutrition & food research. 2015;59:2155–2167. doi: 10.1002/mnfr.201500470. [DOI] [PubMed] [Google Scholar]
- 69.Nuñez-Sánchez MA, García-Villalba R, Monedero-Saiz T, García-Talavera NV, et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Molecular nutrition & food research. 2014;58:1199–1211. doi: 10.1002/mnfr.201300931. [DOI] [PubMed] [Google Scholar]
- 70.Yan H, Wang H, Zhang X, Li X, Yu J. Ascorbic acid ameliorates oxidative stress and inflammation in dextran sulfate sodium-induced ulcerative colitis in mice. Int J Clin Exp Med. 2015;8:20245–20253. [PMC free article] [PubMed] [Google Scholar]