Abstract
One-third of all congenital birth defects affect the head and face, and most craniofacial anomalies are considered to arise through defects in the development of cranial neural crest cells. Cranial neural crest cells give rise to the majority of craniofacial bones, cartilages and connective tissues. Therefore understanding the events that control normal cranial neural crest and subsequent craniofacial development is important for elucidating the pathogenetic mechanisms of craniofacial anomalies and for the exploring potential therapeutic avenues for their prevention. Treacher Collins syndrome (TCS) is a congenital disorder characterized by severe craniofacial anomalies. An animal model of TCS, generated through mutation of Tcof1, the mouse (Mus musculus) homologue of the gene primarily mutated in association with TCS in humans, has recently revealed significant insights into the pathogenesis of TCS. Apoptotic elimination of neuroepithelial cells including neural crest cells is the primary cause of craniofacial defects in Tcof1 mutant embryos. However our understanding of the mechanisms that induce tissue-specific apoptosis remains incomplete. In this review, we describe recent advances in our understanding of the pathogenesis TCS. Furthermore, we discuss the role of Tcof1 in normal embryonic development, the correlation between genetic and environmental factors on the severity of craniofacial abnormalities, and the prospect for prenatal prevention of craniofacial anomalies.
Keywords: craniofacial, DNA damage response/repair, neural crest cells, ROS, Tcof1
Introduction
Human faces exhibit an amazing diversity in size, shape and individual features. This diversity is derived from the complexity of craniofacial morphogenesis during early embryonic development. Central to proper craniofacial development is an important population of cells called cranial neural crest cells. Cranial neural crest cells comprise a multipotent, stem and progenitor cell population that forms most of the craniofacial bones, cartilages and connective tissues (Le Douarin & Kalcheim, 1999, Trainor, 2013). Consequently, defects in the formation, migration and/or differentiation of neural crest cells are thought to underlie many craniofacial malformations (Walker & Trainor, 2006, Crane & Trainor, 2006). Identifying the phase of neural crest cell development that is disrupted in association with a particular condition is very important for the understanding the pathogenesis of individual craniofacial abnormalities and for developing potential therapies. Conversely, analyzing craniofacial abnormalities can lead to the discovery of novel genes and/or mechanisms important for normal neural crest cell development, craniofacial morphogenesis and patterning.
Many congenital craniofacial abnormalities exhibit phenotypic variability without any correlation between genotype and phenotype. Environmental factors, such as pre-natal nutrition, and drug or chemical exposure are known to contribute to the severity of craniofacial abnormalities. However, how environmental and genetic factors interact and affect craniofacial development remains unclear. Animal models are a powerful tool for understanding the mechanisms that govern normal craniofacial development, for illustrating the pathogenesis of craniofacial abnormalities and uncovering the molecular mechanisms underlying the variability of phenotypic severity caused by environmental factors.
Here we initially summarize our knowledge of the multiple cellular functions of Tcof1, with an emphasis on the newly identified oxidative stress/DNA damage role during embryogenesis. Then we describe recent advances in our understanding of the pathogenesis of TCS, the effect of genetic and environmental factors on the severity of craniofacial anomalies and the prospect for prenatal prevention of TCS. Finally, we also discuss the role of endogenous reactive oxygen species on embryonic development.
What is Treacher Collins syndrome?
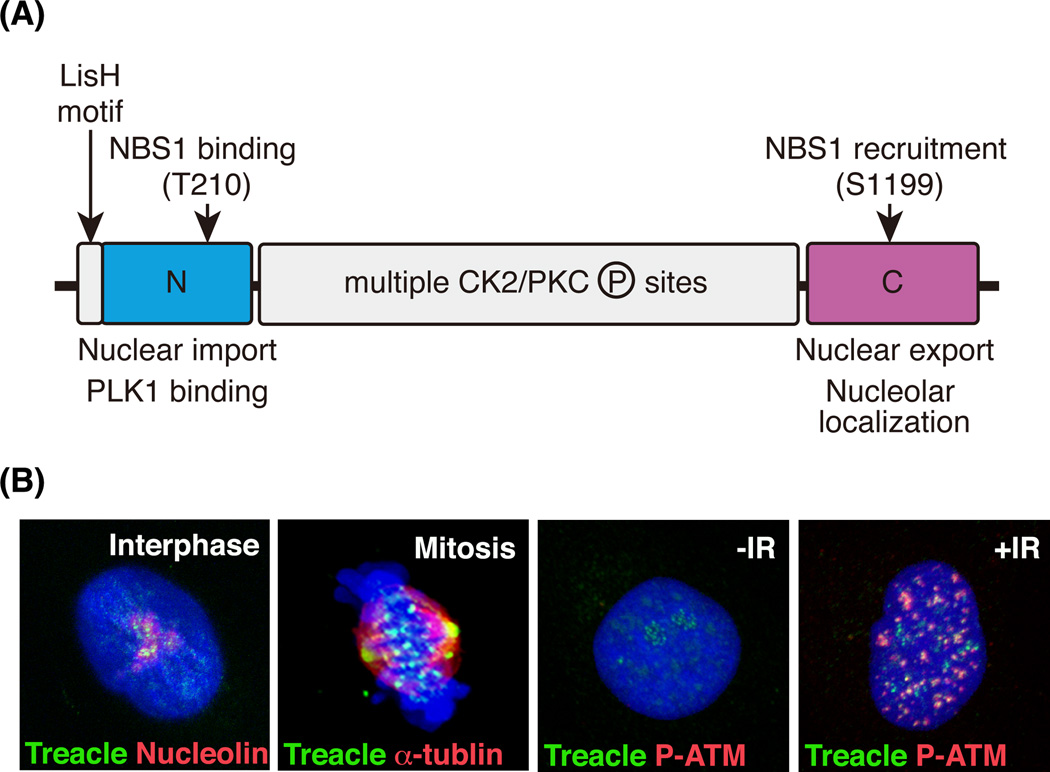
Craniofacial abnormalities account for approximately one-third of all congenital birth defects. Treacher Collins syndrome (TCS, OMIM number 1545000) is a severe congenital craniofacial disorder, and occurs with an incidence of 1 in 50,000 live births. Characteristic abnormalities associated with TCS include hypoplasia of jawbones and cheekbones, downward slanting of eyes, cleft palate and deformity of the external ears. Other clinical features of TCS include conductive hearing loss (Poswillo, 1975, Phelps et al., 1981, Trainor et al., 2009) and brain defects such as microcephaly and cognitive delay (Milligan et al., 1994, Cohen et al., 1995, Teber et al., 2004). The gene primarily mutated in association with TCS is TCOF1, which is located on chromosome 5 (Treacher Collins Syndrome Collaborative Group, 1996). Over 200 family specific mutations have been identified in TCOF1 gene and these include deletions, insertions, splicing, missense and nonsense mutations. The typical effect of these mutations is to introduce a premature termination codon which induces nonsense-mediated mRNA degradation and haploinsufficiency of TCOF1. TCOF1 encodes a 144kDa protein called Treacle (Fig. 1A). Treacle contains a putative nuclear export signal at its N-terminus, as well as nuclear and nucleolar import signals at its C-terminus, suggestive of dynamic localization (Winokur & Shiang, 1998). A LisH motif, which is typically associated with dimerization, microtubule binding, protein half-life and localization is also located at N-terminus (Wise et al., 1997, Dixon et al., 1997a). The most recognizable motifs in Treacle are the multiple casein kinase 2 (CK2)/protein kinase C (PKC) phosphorylation sites in the central region of the protein (Isaac et al., 2000). However, the importance of LisH motif and multiple CK2/PKC phosphorylation sites with respect to Treacle function remains unclear.
Fig.1.
Protein domain structure and dynamic cellular localization of Treacle. (A) Domain structure of Treacle is shown. Treacle consists of three regions, N-terminal, central and C-terminal region. A LisH motif is located at the N-terminus of Treacle. Threonine 210 is phosphorylated by CK2 and required for NBS1 binding. Serine 1199 is phosphorylated by ATM kinase and required for NBS1 recruitment into nucleolus. (B) Treacle localizes in the nucleolus during the interphase of the cell cycle (Interphase). Nucleoli are visualized by nucleolin immunostaining. During the mitotic phase, Treacle localizes to the centrosome and kinetochore (Mitosis). The mitotic spindle is marked by α-tubulin immunostaining. Nucleolar Treacle in non-irradiated cell (−IR) disperses in nucleus and forms DNA damage foci co-labeled with DNA damage response protein, phosphorylated-ATM (P-ATM, +IR).
Although penetrance of the TCS-causing mutations is very high, the severity of the TCS phenotype varies between families and within families (Dixon et al., 1994, Marres et al., 1995). The most severe cases can result in perinatal death due to a compromised airway (Edwards et al., 1996). However, some individuals are so mildly affected as to be indistinguishable from non-carriers of TCS-causing mutations. TCS associated mutations may therefore be carried more frequently than 1 in 50,000. Although over 200 family specific mutations have been identified, there is no clear evidence for a genotype-phenotype correlation (Gladwin et al., 1996, Edwards et al., 1997, Splendore et al., 2000). Genetic background, environmental factors and/or stochastic events could all contribute to the clinical variability of TCS.
Tcof1 is expressed broadly throughout the embryo during all stages of development with elevated levels in the neuroepithelium and neural crest between E8.5–10.5 (Dixon et al., 2006, Dixon et al., 1997b). To understand the mechanisms underlying the pathogenesis of TCS, a mouse model of TCS was generated (Dixon and Dixon 2004). Tcof1 homozygous mutants (Tcof1−/−) exhibit embryonic lethality around E5.0, while haploinsufficient mutant (Tcof1+/−) embryos mimic the characteristic features of TCS including hypoplasia of the maxillary and nasal bones (Dixon et al., 2000). Importantly, Tcof1+/− embryos also mimic the intra- and inter-familial variability in craniofacial abnormalities observed in human patients, particularly with respect to the frontonasal hypoplasia which varies within litters (Dixon & Dixon, 2004). Furthermore, while, Tcof1+/− mice on mixed DBA;C57BL/6 background exhibit severe craniofacial defects reminiscent of TCS, and die within 24 hours of birth due to respiratory failure, in contrast, the majority of Tcof1+/− mice on a pure DBA background look normal, having only subtle facial and brain defects. Moreover, these mice are postnatal viable and fertile. Thus, the Tcof1 knockout mouse is recognized as a good model for studying the pathogenesis of TCS.
Tcof1 is required for the maintenance of neural progenitor cells
Microcephaly is a congenital birth defect in which the cerebral cortex is considerably smaller than normal. Human genetic studies have identified links between mutations in genes associated with the process of mitosis and microcephaly. The majority of autosomal recessive primary microcephaly (MCHP) genes identified to date, including ASPM, CDK5RAP2, CENPJ and NDE1, encode centrosomal proteins. Mouse model studies have revealed that these genes are essential for the mitotic progression of neural progenitor cells and consequent proper neurogenesis (Fish et al., 2006, Bond et al., 2005, Feng & Walsh, 2004).
At the beginning of mitosis, the nucleolus disassembles in association with nuclear envelope breakdown, resulting in the release of proteins within the nucleolus and the nucleus into cytoplasm. Although, Treacle localizes to the nucleolus during interphase (Fig. 1B, Interphase)(Marsh et al., 1998), it disperses into the cytoplasm and re-localizes at the centrosomes and kinetochores during mitosis (Fig. 1B; Mitosis)(Sakai et al., 2012). This implies a mitotic function for Treacle, and consistent with this idea, TCOF1 knockdown delays mitotic progression in HeLa cells due to impairment of chromosome alignment along the metaphase plate. Biochemical analyses revealed direct binding between Treacle and Polo-like kinase 1 (Plk1), a known master regulator of mitosis. Knockdown of TCOF1 causes mis-localization of Plk1 and abnormal chromosome alignment in metaphase cells, illustrating the cooperation between these proteins during mitotic progression. Since Tcof1+/− mice on a pure DBA background exhibit smaller brains without craniofacial defects, the correlation between mitotic dysfunction and microcephaly was analyzed using this strain (Sakai et al., 2012). BrdU-IdU labeling and immunostaining for the mitotic cell marker, phosphorylated histoneH3 revealed delays in the mitotic progression of neural progenitor cells in Tcof1+/− embryos. Furthermore, loss of Tcof1 lead to randomization of the division plane of neural progenitor cells, which consequently perturbed the balance between neural progenitor cell expansion and differentiation in the forebrain of Tcof1+/− embryos. The depletion of neural progenitor cells specifically affected neurogenesis of upper layer neurons in association with reduced cerebral cortex size in Tcof1+/− pups (Sakai et al., 2012).
Tcof1 regulates ribosome biogenesis for neural crest cell survival
Treacle co-localizes with upstream binding factor (UBF) and RNA polymerase I in the nucleolus where ribosomal RNA transcription takes place (Valdez et al., 2004). Consistent with this intracellular localization, TCOF1 knockdown showed that Treacle is important for rRNA transcription and pre-RNA processing, implying a role of TCOF1/Treacle in ribosome biogenesis (Valdez et al., 2004, Gonzales et al., 2005). Recently, mutations in POLR1C and POLR1D which encode subunits of both RNA polymerase I and III have also been identified in association with Treacher Collins syndrome (Dauwerse et al., 2011). These findings imply a correlation between defects in ribosome biogenesis and the pathogenesis of TCS.
A role for Tcof1 in ribosome biogenesis has also been elucidated in the Tcof1+/− mouse model of TCS (Dixon et al., 2006, Jones et al., 2008). Tcof1+/− embryos exhibit a reduction of rRNA, which presages a deficiency in mature ribosome production. The perturbation of ribosome biogenesis appears to correlate with a decrease in cell proliferation in the neuroepithelium and premigratory cranial neural crest cells in Tcof1+/− embryos. Consequently, it has been hypothesized that deficient ribosome biogenesis is insufficient to meet the cellular needs of highly proliferative cell populations such as neuroepithelial cells, and it is directly responsible for the high levels of apoptosis in the neuroepithelium and premigratory cranial neural crest cells in Tcof1+/− embryos (Dixon et al., 2006, Jones et al., 2008). The majority of craniofacial cartilage and bone is derived from cranial neural crest cells. Therefore, neuroepithelial apoptosis and consequent reduction in the number of cranial neural crest cells underpins the craniofacial anomalies in Tcof1+/− embryos.
Deficient ribosome biogenesis is known to trigger nucleolar stress activation of p53, which then induces the expression of pro-apoptotic genes (Rubbi & Milner, 2003). In Tcof1+/− embryos, p53 is activated and stabilized, which induces expression of pro-apoptotic genes, such as Ccng1, Trp53inp1 and Wig1 specifically in neuroepithelium and premigratory cranial neural crest cells (Jones et al., 2008). These findings imply that haploinsufficiency of Tcof1 results in a deficiency in ribosome biogenesis which triggers nucleolar stress induced activation and stabilization of p53. Activated p53 leads to apoptosis through the induction of pro-apoptotic gene expression. The correlation between ribosome biogenesis defects and p53-dependent apoptosis of neuroepithelial cells in Tcof1+/− embryos raised the possibility that suppressing apoptosis via inhibition of p53 might prevent craniofacial anomalies. Indeed, in utero treatment of Tcof1+/− embryos with pifithrin-α, a specific inhibitor for p53, reduced neuroepithelial apoptosis and ameliorated the craniofacial anomalies in a dose-dependent manner. Similarly, genetic removal of p53 from the Tcof1+/− background (Tcof1+/−; p53+/−) also prevented the manifestation of craniofacial anomalies during embryogenesis. Furthermore, Tcof1+/−; p53+/− pups were viable and also fertile. Surprisingly, the craniofacial skeleton in Tcof1+/−; p53+/− embryos and newborn pups developed normally without restoration of ribosome biogenesis (Jones et al., 2008).
Recently, the ubiquitination of Treacle was shown to be required for the regulation of translation, and subsequent cell-fate determination of ES cells (Werner et al., 2015). The CUL3-KBTBD8 ubiquitin ligase complex monoubiquitinates Treacle, and this modification triggers a bridge between Treacle and NOLC1, another nucleolar protein involved in rRNA processing. The Treacle-NOLC1 complex connects RNA pol I with the H/ACA complex that catalyzes rRNA pseudouridylation and the SSU processome. This large complex appears to modify ribosomes and/or mRNA and induce distinct translational outputs, and as a result, ES cells differentiate towards a neural crest cell fate (Werner et al., 2015). Thus, in addition to the production of ribosomes, Treacle may be also be important for their modification.
Novel function of Tcof1: suppressing apoptosis induced by oxidative stress
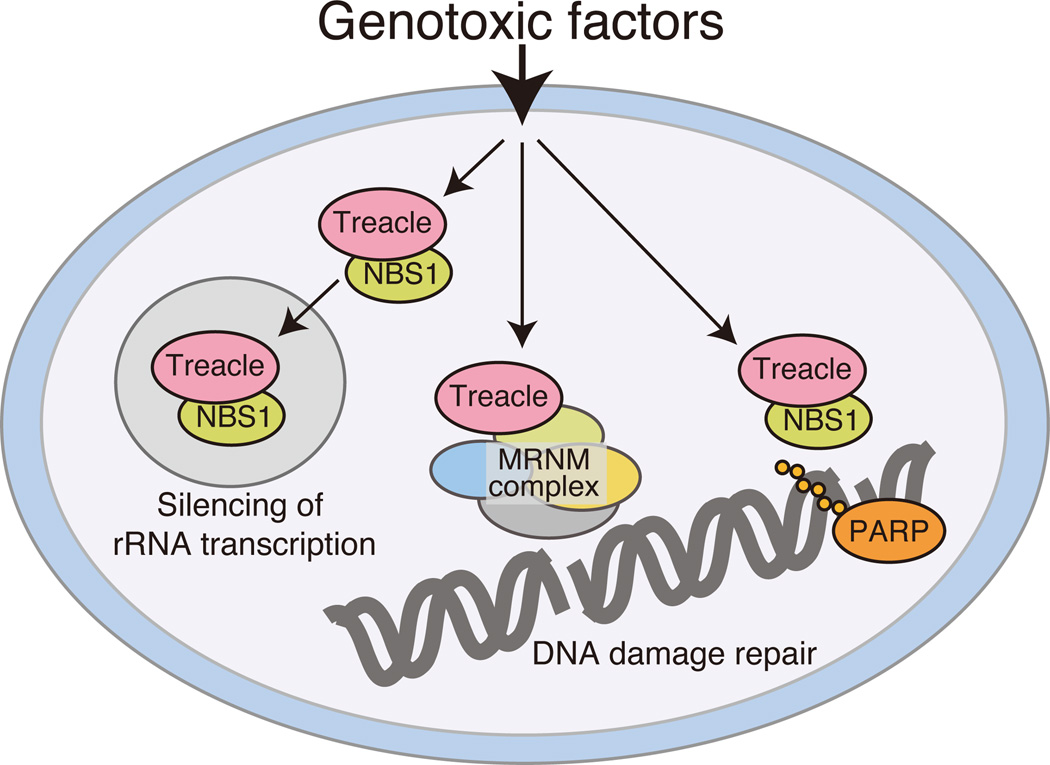
Rescue of the craniofacial skeleton in Tcof1+/−; p53+/− embryos and newborn pups occurred without restoration of ribosome biogenesis (Jones et al., 2008), suggesting that Tcof1/Treacle may also perform non-ribosome-associated functions. Consistent with this idea, three groups recently independently identified a role for Treacle in the DNA damage response/repair. In two studies, NBS1 as a bait, was shown to directly bind to Treacle in response to DNA damage induced by ionizing radiation in cultured cell lines (Ciccia et al., 2014, Larsen et al., 2014). In fact the translocation of NBS1 into nucleoli was dependent upon the phosphorylation of Treacle at threonine 210 by CK2 and at serine 1199 by ATM, a kinase which plays a central role in the DNA damage response (Fig. 1A). Treacle-dependent NBS1 translocation regulates silencing of RNA polymerase I-dependent rRNA transcription upon DNA damage (Fig. 2)(Ciccia et al., 2014, Larsen et al., 2014). It has also been reported that a small fraction of Treacle (isoform c) forms DNA damage foci dependent on Poly ADP ribose polymerase (PARP), which is involved in single-strand DNA damage response/repair (Fig. 2)(Larsen et al., 2014).
Fig.2.
Multiple functions of Treacle in response to DNA damage. DNA damage response protein NBS1 is recruited into the nucleolus in response to DNA damage. Nucleolar recruitment is dependent on Treacle, and an NBS1-Treacle complex silences rRNA transcription through the inhibition of RNA polymerase I function. Treacle also associates with DNA damage response and subsequent repair with an important DNA damage response complex MDC1-RAD50-NBS1-MRE11 complex. Treacle isoform C regulates PARP-dependent single-strand DNA damage repair.
In the third study, Multidimensional Protein Identification Technology (MudPIT) was used to identify novel functions for Treacle via the comprehensive detection of Treacle interacting proteins (Sakai et al., 2016). This approach resulted in identification of the MRNM (MDC1-RAD50-NBS1-MRE11) complex, which is an important mediator of double-strand DNA damage response/repair (Fig. 2). This study provided direct evidence that Tcof1 functioned in DNA damage response/repair in vivo, and elucidated a correlation between deficient DNA damage repair and the apoptotic elimination of cranial neural crest cells in Tcof1+/− embryos. In support of this finding, Treacle was shown to localize to sites of DNA damage and form DNA damage foci in response to X-ray irradiation (Fig. 1B; −IR and +IR). DNA damage foci localization of Treacle is diminished by knockdown of MDC1 in irradiated HeLa cells, validating the idea that Treacle functions as a component of MRNM complex. Moreover, Tcof1+/− embryo-derived mouse embryonic fibroblasts (MEFs) exhibit a delay in DNA damage repair together with multinucleation which is characteristic feature of DNA damage repair deficiency. Collectively this data confirms Treacle is a novel component of the MRNM complex and is essential for DNA damage response/repair (Fig. 2). Interestingly, the loss of TCOF1 affects the localization of BRCA1 to DNA damage foci without disruption of the BRCA1-A scaffold complex (Wu et al., 2012). Although direct binding between Treacle and BRCA1 has not been detected, ECT2 which is a BRCA1 C-terminus (BRCT) domain containing protein, interacts with BRCA1 as well as Treacle (Woods et al., 2012). Thus ECT2 may be a potential mediator of Treacle-BRCA1 binding. Mutations in BRCA1 are known to increase the risk of breast cancer (Miki et al., 1994), thus TCOF1/Treacle may play a role in the suppression of tumor formation and cancer formation through its interaction with BRCA1. It will be important in the future to determine how Treacle influences BRCA1 localization for a better understanding of the regulation of the DNA damage response/repair machinery during embryogenesis.
Under conditions whereby DNA damage is so severe as to not be repairable, damaged cells are eliminated by p53-dependent apoptosis in order to suppress tumor formation (Kaina, 2003). In Tcof1+/− embryos, phosphorylated-Chk2 positive and γH2AX-positive DNA damaged cells are detected specifically in the neuroepithelium, from which neural crest cells are derived. Phosphorylated-Chk2 can stabilize p53 and consequently induce apoptosis (Chehab et al., 2000, Shieh et al., 2000, Hirao et al., 2000) and consistent with these findings, p53 accumulates in the neuroepithelium of Tcof1+/− embryos. Furthermore, DNA damaged neuroepithelial cells are also labeled with apoptotic marker cleaved-Caspase3 (Sakai et al., 2016). Thus, Tcof1/Treacle links DNA damage response/repair to the apoptotic elimination of neuroepithelial cells and progenitor neural crest cells in Tcof1+/− embryos.
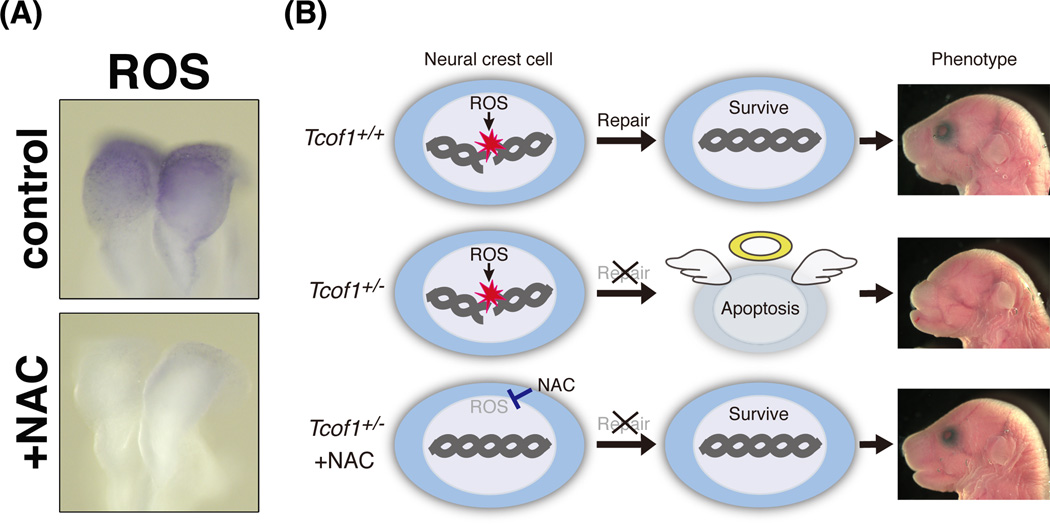
Interestingly, neuroepithelial cells including progenitor neural crest cells endogenously generate reactive oxygen species (ROS) at relatively high levels compared with other tissues (Fig. 3A; control)(Sakai et al., 2016). ROS are strong oxidants, suggesting that oxidative DNA damage occurs frequently in those cells. Indeed, in utero treatment of Tcof1+/− embryos with the strong antioxidant, N-acetyl-cysteine (NAC), suppresses DNA damage, preventing p53 accumulation and apoptosis (Fig. 3A; +NAC). Surprisingly, prenatal antioxidant supplementation with NAC reduced the incidence and severity of craniofacial anomalies. 30% of treated Tcof1+/− embryos were fully rescued and were morphologically indistinguishable from wild-type littermates (Sakai et al., 2016). The mechanisms underlying the craniofacial abnormalities observed in Tcof1+/− embryos, and prenatal restoration of craniofacial abnormalities based on its pathogenic mechanism are summarized in Fig.3B and 4B (non-NE, NE and NE +NAC). Briefly, perturbation of DNA damage repair leads to the apoptotic elimination of damaged neuroepithelial and neural crest cells in Tcof1+/− embryos, while apoptosis and subsequent craniofacial defects are ameliorated by quenching ROS with antioxidants in Tcof1+/− embryos. Taken together, Tcof1 is an essential gene for the protection of neuroepithelial and neural crest cells from endogenous and exogenous oxidative stress induced DNA damage during normal craniofacial development. Oxidative stress induced DNA damage appears to generally occur during embryonic development, thus other congenital craniofacial and brain disorders may be also be affected or caused by endogenous ROS. Therefore, these findings shed new light on potential therapeutic avenues for prevention of TCS as well as other congenital craniofacial and brain birth defects of similar origin.
Fig. 3.
Antioxidants reduce endogenous ROS and rescue craniofacial abnormalities. (A) Wild type embryos were treated with a strong antioxidant, NAC, via intraperitoneal injection of pregnant females at E8.5. After a 1hour incubation, embryos were subjected to NBT staining to visualize ROS. NAC effectively scavenged ROS. (B) Summary of the mechanism of preventing craniofacial abnormalities in Tcof1+/− embryos. Endogenous ROS frequently damages chromosomal DNA. Thus, the DNA damage response/repair machinery is dependent on Treacle which is required for prevention of oxidative DNA damage and subsequent apoptosis. In Tcof1+/− embryos, progenitor neural crest cells undergo apoptosis due to their perturbed ability to repair DNA. In contrast, scavenging of ROS via antioxidants such as NAC prevents DNA damage, reduces apoptosis and rescues the craniofacial abnormalities in Tcof1+/− embryos.
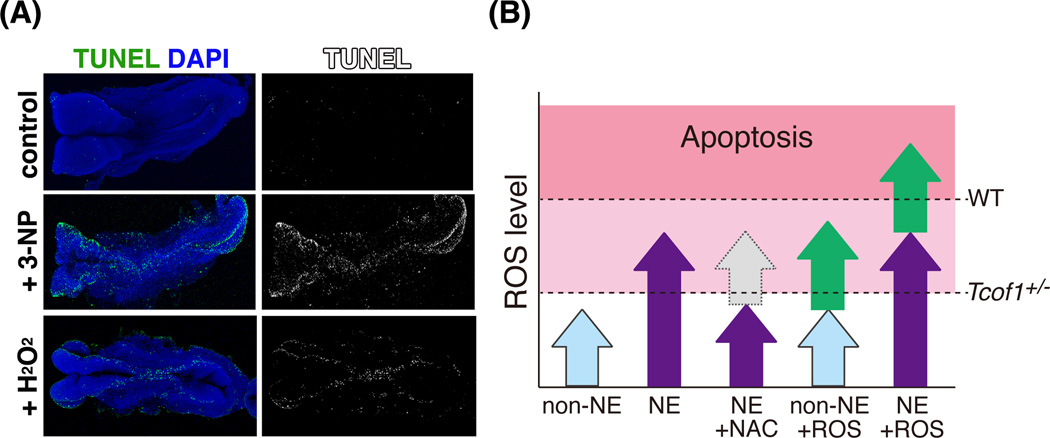
Fig. 4.
Induction of neuroepithelial apoptosis with excess ROS in wild type embryos. (A) Wild type E8.5 whole embryos were cultured in the presence of a strong oxidant H2O2 and ROS-generator, 3-NP. Apoptosis was detected by whole-mount TUNEL staining. Apoptosis was induced in neuroepithelium, especially at the dorsolateral edges where neural crest is formed. (B) Schematic representation of the relationship between ROS levels and induction of neuroepithelial apoptosis. Arrows indicate levels of ROS in non-neuroepithelium (light blue), neuroepithelium (purple), quenching by NAC (gray), addition or induction by 3-NP (green). Dotted lines indicate the threshold for oxidative stress-induced apoptosis in wild type (WT) and Tcof1 heterozygous mutant (Tcof1+/−) embryos, respectively.
Although craniofacial abnormalities can be ameliorated by antioxidant supplementation, Tcof1 mutant pups still exhibit nasal anomalies and die 2–3 days after the birth due to respiratory failure (Sakai et al., 2016). Further improvement in the degree of rescue may be attainable by varying the concentration of antioxidants and through combinatorial antioxidant supplementation. For future application in clinical medicine, antioxidants such as NAC, vitamin C, polyphenol and resveratrol which are popular, non-toxic, readily available and inexpensive dietary supplements, may provide avenues for exploration. Prenatal antioxidant supplementation may protect against craniofacial, brain and other congenital anomalies similar to prenatal folic acid supplementation in the prevention of anencephaly and spina bifida as recommended by the U.S. Public Health Service and CDC.
The neuroepithelium, which exists in an endogenously high oxidative state compare to non-neuroepithelium (Fig. 4B; non-NE and NE) is also more sensitive to increased ROS than its surrounding tissues. Exposing wild type embryos to ROS generator, 3-nitropropionic acid (3-NP) and the oxidant H2O2 induces massive apoptosis in the neuroepithelium and presumptive neural crest similar to the apoptotic pattern observed in Tcof1+/− embryos (Fig. 4A)(Sakai et al., 2016). High levels of endogenous ROS lower the threshold for oxidative stress-induced apoptosis, therefore neuroepithelial and neural crest cells are highly sensitive to exogenous ROS (Fig. 4B; non-NE +ROS andNE +ROS). Environmental factors such as chemicals, UV irradiation, alcohol, smoking and high-calorie diet (lipids, glucose) are strongly linked to increased risk and severity of congenital birth defects (Gilbert, 2003, Ornoy et al., 2015). Furthermore, such environmental factors are known to induce the generation of ROS (Wright et al., 1999, Ikehata & Ono, 2011, Zhao & Reece, 2005). Taken together, these findings suggest that the inter- and intra-familial variability in the TCS phenotype, may be influenced by exposure of embryos to environmental factor(s) during pregnancy. These studies of the pathogenesis of TCS illustrate the involvement of environmental factors in the variability of craniofacial abnormalities.
Perspectives
High levels of ROS in the neuroepithelium is a fundamental contributor to the pathogenesis of TCS. This raises the questions of what is the source of endogenous ROS, and why do neuroepithelial cells generate higher levels of ROS than their surrounding tissues. Experimental animals are kept in highly regulated and well controlled laboratory animal facilities, and are not exposed to radiation or high-calorie diets. However, there are many alternative mechanisms for the generation of ROS. One such source of ROS is the electron transport chain, which generates ATP during respiratory metabolism within mitochondria. Electrons derived from the oxidation of metabolic intermediates lead to the generation of ROS (Kirkinezos & Moraes, 2001).
Interestingly, the cranial region exhibits a relatively high uptake of glucose compared to trunk region (Hunter & Tugman, 1995). This further illustrates the contribution of respiratory metabolism to endogenous ROS generation. In addition, our recent findings suggest that some genes involved in respiratory metabolism exhibit neuroepithelium-specific expression in early stages of mouse embryo development (D. Sakai, unpublished data). This further supports the correlation between respiratory metabolism and endogenous ROS generation. Elucidating the precise sources of endogenous and exogenous ROS will be very important in the development of future therapeutic approaches for preventing or treating TCS.
Although, antioxidant treatment can ameliorate craniofacial abnormalities in Tcof1+/− embryos, high doses of antioxidants can lead to embryonic lethality (D. Sakai, unpublished data). This implies that specific levels of ROS might be required for normal craniofacial and brain development. In agreement with this idea, it has become evident that ROS can function as a signaling molecule to regulate many biological processes during embryonic development (Covarrubias et al., 2008, Le Belle et al., 2011). For example, the subventricular zone of the cortex in E14.5 mouse embryos exhibits elevated ROS and reducing endogenous ROS affects the proliferation of neural progenitor cells in vivo. Furthermore, the PI3 kinase-Akt-mTOR pathway is activated by endogenous ROS which also regulates neural stem and progenitor cell proliferation. Thus it will be very interesting to determine in the future whether endogenous ROS activates similar pathways and enhances proliferation in the neuroepithelium during early embryogenesis as a further step towards a better understanding of normal and abnormal craniofacial development.
Acknowledgments
The authors thank all members of our respective laboratories and numerous colleagues in the field for their constructive input over the many years, but in particular we would like to acknowledge Dr. Mike and Jill Dixon for their long term collaboration and interactions on our studies of Treacher Collins syndrome. Research in the Trainor laboratory is supported by the Stowers Institute for Medical Research and National Institute of Dental and Craniofacial Research (RO1 DE 016082). Research performed by D.S. was supported by Grants-in-Aid for Research Activity start-up Grant Number 24870028 and for Encouragement of Young Scientists (B) Grant Number 25840090.
References
- Bond J, Roberts E, Springell K, et al. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet. 2005;37:353–355. doi: 10.1038/ng1539. [DOI] [PubMed] [Google Scholar]
- Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- Ciccia A, Huang JW, Izhar L, Sowa ME, Harper JW, Elledge SJ. Treacher Collins syndrome TCOF1 protein cooperates with NBS1 in the DNA damage response. Proc Natl Acad Sci U S A. 2014;111:18631–18636. doi: 10.1073/pnas.1422488112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Ghezzi F, Goncalves L, Fuentes JD, Paulyson KJ, Sherer DM. Prenatal sonographic diagnosis of Treacher Collins syndrome: a case and review of the literature. Am J Perinatol. 1995;12:416–419. doi: 10.1055/s-2007-994511. [DOI] [PubMed] [Google Scholar]
- Covarrubias L, Hernandez-Garcia D, Schnabel D, Salas-Vidal E, Castro-Obregon S. Function of reactive oxygen species during animal development: passive or active? Dev Biol. 2008;320:1–11. doi: 10.1016/j.ydbio.2008.04.041. [DOI] [PubMed] [Google Scholar]
- Crane JF, Trainor PA. Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol. 2006;22:267–286. doi: 10.1146/annurev.cellbio.22.010305.103814. [DOI] [PubMed] [Google Scholar]
- Dauwerse JG, Dixon J, Seland S, et al. Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nat Genet. 2011;43:20–22. doi: 10.1038/ng.724. [DOI] [PubMed] [Google Scholar]
- Dixon J, Brakebusch C, Fassler R, Dixon MJ. Increased levels of apoptosis in the prefusion neural folds underlie the craniofacial disorder, Treacher Collins syndrome. Hum Mol Genet. 2000;9:1473–1480. doi: 10.1093/hmg/9.10.1473. [DOI] [PubMed] [Google Scholar]
- Dixon J, Dixon MJ. Genetic background has a major effect on the penetrance and severity of craniofacial defects in mice heterozygous for the gene encoding the nucleolar protein Treacle. Dev Dyn. 2004;229:907–914. doi: 10.1002/dvdy.20004. [DOI] [PubMed] [Google Scholar]
- Dixon J, Edwards SJ, Anderson I, Brass A, Scambler PJ, Dixon MJ. Identification of the complete coding sequence and genomic organization of the Treacher Collins syndrome gene. Genome Res. 1997a;7:223–234. doi: 10.1101/gr.7.3.223. [DOI] [PubMed] [Google Scholar]
- Dixon J, Hovanes K, Shiang R, Dixon MJ. Sequence analysis, identification of evolutionary conserved motifs and expression analysis of murine tcof1 provide further evidence for a potential function for the gene and its human homologue, TCOF1. Hum Mol Genet. 1997b;6:727–737. doi: 10.1093/hmg/6.5.727. [DOI] [PubMed] [Google Scholar]
- Dixon J, Jones NC, Sandell LL, et al. Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc Natl Acad Sci U S A. 2006;103:13403–13408. doi: 10.1073/pnas.0603730103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MJ, Marres HA, Edwards SJ, Dixon J, Cremers CW. Treacher Collins syndrome: correlation between clinical and genetic linkage studies. Clin Dysmorphol. 1994;3:96–103. [PubMed] [Google Scholar]
- Edwards SJ, Fowlie A, Cust MP, Liu DT, Young ID, Dixon MJ. Prenatal diagnosis in Treacher Collins syndrome using combined linkage analysis and ultrasound imaging. J Med Genet. 1996;33:603–606. doi: 10.1136/jmg.33.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SJ, Gladwin AJ, Dixon MJ. The mutational spectrum in Treacher Collins syndrome reveals a predominance of mutations that create a premature-termination codon. Am J Hum Genet. 1997;60:515–524. [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Walsh CA. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron. 2004;44:279–293. doi: 10.1016/j.neuron.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Fish JL, Kosodo Y, Enard W, Paabo S, Huttner WB. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc Natl Acad Sci U S A. 2006;103:10438–10443. doi: 10.1073/pnas.0604066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SF. Developmental Biology. 7th. Sinauer Associates; 2003. Environmental regulation of animal development. [Google Scholar]
- Gladwin AJ, Dixon J, Loftus SK, et al. Treacher Collins syndrome may result from insertions, deletions or splicing mutations, which introduce a termination codon into the gene. Hum Mol Genet. 1996;5:1533–1538. doi: 10.1093/hmg/5.10.1533. [DOI] [PubMed] [Google Scholar]
- Gonzales B, Henning D, So RB, Dixon J, Dixon MJ, Valdez BC. The Treacher Collins syndrome (TCOF1) gene product is involved in pre-rRNA methylation. Hum Mol Genet. 2005;14:2035–2043. doi: 10.1093/hmg/ddi208. [DOI] [PubMed] [Google Scholar]
- Group TCSC. Positional cloning of a gene involved in the pathogenesis of Treacher Collins syndrome. The Treacher Collins Syndrome Collaborative Group. Nat Genet. 1996;12:130–136. doi: 10.1038/ng0296-130. [DOI] [PubMed] [Google Scholar]
- Hirao A, Kong YY, Matsuoka S, et al. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- Hunter ES, 3rd, Tugman JA. Inhibitors of glycolytic metabolism affect neurulation-staged mouse conceptuses in vitro. Teratology. 1995;52:317–323. doi: 10.1002/tera.1420520602. [DOI] [PubMed] [Google Scholar]
- Ikehata H, Ono T. The mechanisms of UV mutagenesis. J Radiat Res. 2011;52:115–125. doi: 10.1269/jrr.10175. [DOI] [PubMed] [Google Scholar]
- Isaac C, Marsh KL, Paznekas WA, et al. Characterization of the nucleolar gene product, treacle, in Treacher Collins syndrome. Mol Biol Cell. 2000;11:3061–3071. doi: 10.1091/mbc.11.9.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Lynn ML, Gaudenz K, et al. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat Med. 2008;14:125–133. doi: 10.1038/nm1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaina B. DNA damage-triggered apoptosis: critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem Pharmacol. 2003;66:1547–1554. doi: 10.1016/s0006-2952(03)00510-0. [DOI] [PubMed] [Google Scholar]
- Kirkinezos IG, Moraes CT. Reactive oxygen species and mitochondrial diseases. Semin Cell Dev Biol. 2001;12:449–457. doi: 10.1006/scdb.2001.0282. [DOI] [PubMed] [Google Scholar]
- Larsen DH, Hari F, Clapperton JA, et al. The NBS1-Treacle complex controls ribosomal RNA transcription in response to DNA damage. Nat Cell Biol. 2014;16:792–803. doi: 10.1038/ncb3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Marres HA, Cremers CW, Dixon MJ, Huygen PL, Joosten FB. The Treacher Collins syndrome. A clinical, radiological, and genetic linkage study on two pedigrees. Arch Otolaryngol Head Neck Surg. 1995;121:509–514. doi: 10.1001/archotol.1995.01890050009002. [DOI] [PubMed] [Google Scholar]
- Marsh KL, Dixon J, Dixon MJ. Mutations in the Treacher Collins syndrome gene lead to mislocalization of the nucleolar protein treacle. Hum Mol Genet. 1998;7:1795–1800. doi: 10.1093/hmg/7.11.1795. [DOI] [PubMed] [Google Scholar]
- Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Milligan DA, Harlass FE, Duff P, Kopelman JN. Recurrence of Treacher Collins' syndrome with sonographic findings. Mil Med. 1994;159:250–252. [PubMed] [Google Scholar]
- Ornoy A, Reece EA, Pavlinkova G, Kappen C, Miller RK. Effect of maternal diabetes on the embryo, fetus, and children: congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Res C Embryo Today. 2015;105:53–72. doi: 10.1002/bdrc.21090. [DOI] [PubMed] [Google Scholar]
- Phelps PD, Poswillo D, Lloyd GA. The ear deformities in mandibulofacial dysostosis (Treacher Collins syndrome) Clin Otolaryngol Allied Sci. 1981;6:15–28. doi: 10.1111/j.1365-2273.1981.tb01782.x. [DOI] [PubMed] [Google Scholar]
- Poswillo D. The pathogenesis of the Treacher Collins syndrome (mandibulofacial dysostosis) Br J Oral Surg. 1975;13:1–26. doi: 10.1016/0007-117x(75)90019-0. [DOI] [PubMed] [Google Scholar]
- Rubbi CP, Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 2003;22:6068–6077. doi: 10.1093/emboj/cdg579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D, Dixon J, Achilleos A, Dixon M, Trainor PA. Prevention of Treacher Collins syndrome craniofacial anomalies in mouse models via maternal antioxidant supplementation. Nat Commun. 2016;7:10328. doi: 10.1038/ncomms10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D, Dixon J, Dixon MJ, Trainor PA. Mammalian neurogenesis requires Treacle-Plk1 for precise control of spindle orientation, mitotic progression, and maintenance of neural progenitor cells. PLoS Genet. 2012;8:e1002566. doi: 10.1371/journal.pgen.1002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- Splendore A, Silva EO, Alonso LG, et al. High mutation detection rate in TCOF1 among Treacher Collins syndrome patients reveals clustering of mutations and 16 novel pathogenic changes. Hum Mutat. 2000;16:315–322. doi: 10.1002/1098-1004(200010)16:4<315::AID-HUMU4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Teber OA, Gillessen-Kaesbach G, Fischer S, et al. Genotyping in 46 patients with tentative diagnosis of Treacher Collins syndrome revealed unexpected phenotypic variation. Eur J Hum Genet. 2004;12:879–890. doi: 10.1038/sj.ejhg.5201260. [DOI] [PubMed] [Google Scholar]
- Trainor PA. Neural Crest Cells. Elsevier; 2013. [Google Scholar]
- Trainor PA, Dixon J, Dixon MJ. Treacher Collins syndrome: etiology, pathogenesis and prevention. Eur J Hum Genet. 2009;17:275–283. doi: 10.1038/ejhg.2008.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez BC, Henning D, So RB, Dixon J, Dixon MJ. The Treacher Collins syndrome (TCOF1) gene product is involved in ribosomal DNA gene transcription by interacting with upstream binding factor. Proc Natl Acad Sci U S A. 2004;101:10709–10714. doi: 10.1073/pnas.0402492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MB, Trainor PA. Craniofacial malformations: intrinsic vs extrinsic neural crest cell defects in Treacher Collins and 22q11 deletion syndromes. Clin Genet. 2006;69:471–479. doi: 10.1111/j.0009-9163.2006.00615.x. [DOI] [PubMed] [Google Scholar]
- Werner A, Iwasaki S, Mcgourty CA, et al. Cell-fate determination by ubiquitin-dependent regulation of translation. Nature. 2015;525:523–527. doi: 10.1038/nature14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winokur ST, Shiang R. The Treacher Collins syndrome (TCOF1) gene product, treacle, is targeted to the nucleolus by signals in its C-terminus. Hum Mol Genet. 1998;7:1947–1952. doi: 10.1093/hmg/7.12.1947. [DOI] [PubMed] [Google Scholar]
- Wise CA, Chiang LC, Paznekas WA, et al. TCOF1 gene encodes a putative nucleolar phosphoprotein that exhibits mutations in Treacher Collins Syndrome throughout its coding region. Proc Natl Acad Sci U S A. 1997;94:3110–3115. doi: 10.1073/pnas.94.7.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NT, Mesquita RD, Sweet M, et al. Charting the landscape of tandem BRCT domain-mediated protein interactions. Sci Signal. 2012;5:rs6. doi: 10.1126/scisignal.2002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RM, Mcmanaman JL, Repine JE. Alcohol-induced breast cancer: a proposed mechanism. Free Radic Biol Med. 1999;26:348–354. doi: 10.1016/s0891-5849(98)00204-4. [DOI] [PubMed] [Google Scholar]
- Wu J, Liu C, Chen J, Yu X. RAP80 protein is important for genomic stability and is required for stabilizing BRCA1-A complex at DNA damage sites in vivo. J Biol Chem. 2012;287:22919–22926. doi: 10.1074/jbc.M112.351007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Reece EA. Nicotine-induced embryonic malformations mediated by apoptosis from increasing intracellular calcium and oxidative stress. Birth Defects Res B Dev Reprod Toxicol. 2005;74:383–391. doi: 10.1002/bdrb.20052. [DOI] [PubMed] [Google Scholar]