Abstract
Oxygen is vital for all aerobic life forms. Oxygen-dependent hydroxylation of hypoxia-inducible factor (HIF)-1α by prolyl hydroxylase domain enzymes (PHDs) is an important step for controlling the expression of oxygen-regulated genes in metazoan species, thereby constituting a molecular mechanism for oxygen sensing and response. Herein, we report a genetically encoded dual-emission ratiometric fluorescent sensor, Pro-CY, which responds to PHD activities in vitro and in live cells. We demonstrated that ProCY could monitor hypoxia in mammalian cells. By targeting this novel genetically encoded biosensor to the cell nucleus and cytosol, we determined that, under normoxic conditions, the HIF-prolyl hydroxylase activity was mainly confined to the cytosol of HEK 293T cells. The results collectively suggest broad applications of ProCY on evaluation of hypoxia and PHD activities and understanding of pathways for the control of hypoxic responses.
Graphical Abstract
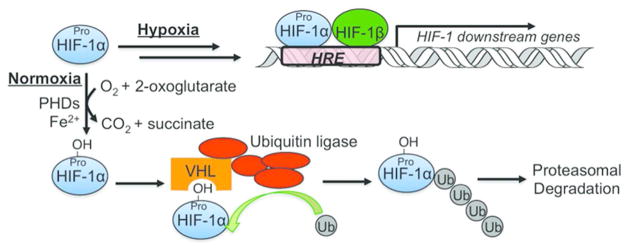
Oxygen is required for all aerobic organisms on Earth for metabolism and energy production. In the meanwhile, oxygen overexposure may increase the production of reactive oxygen species (ROS), leading to oxidative damage.1 Therefore, biological systems have evolved complex mechanisms to sense changes in oxygen concentrations and respond accordingly to maintain homeostasis. Hypoxia-inducible factor (HIF)-1 is an important component of a cellular signaling cascade responsible for oxygen-dependent responses in various animals.2 HIF-1 is a transcription factor, which regulates the expression of a number of downstream genes, whereas the stability of the α-subunit of HIF-1 (HIF-1α) is controlled by oxygen-dependent hydroxylation of conserved proline residues by prolyl hydroxylase domain enzymes (PHDs), thereby leading to subsequent ubiquitination and proteasomal degradation of HIF-1α (Figure 1).2–5 Consequently, HIF-1α and its prolyl hydroxylation serve as a mechanism to transduce the cellular oxygen level into changes in gene expression.6, 7
Figure 1.
Schematic illustration of the regulation of HIF-1α. Under normoxia, PHDs use molecular oxygen (O2) as a substrate to generate proline-hydroxylated HIF-1α, which next binds to von Hippel-Lindau (VHL) to trigger polyubiquitination and proteasomal degradation of HIF-1α. Under hypoxia, the PHD activity is reduced because of low oxygen levels, so HIF-1α is stabilized and enters the nucleus to bind hypoxia responsive elements (HREs) to induce the expression of downstream genes.
Hypoxia, a condition defined by low oxygen levels, has been linked to diverse pathophysiological processes and diseases, such as cancer, anemia, ischemia, and stroke.8–11 Because of the high sensitivity and versatility of fluorescence measurements, hypoxia-responsive fluorescent probes are considered important research tools.12, 13 Previous studies have reported several synthetic fluorescent probes sensitive to bioreduction reactions under hypoxia, such as synthetic molecules containing a nitro group,14–16 a quinone group,17 an azo group,18, 19 or a xanthine ring20 as their sensory moieties. Despite the progress, none of these aforementioned fluorescent probes directly senses PHD-induced hydroxylation, and their fluorescence change is often dependent of the activities of cellular reductases under hypoxia. Furthermore, it is not trivial to deliver these synthetic dyes to specific subcellular locations. In other studies, fluorescent proteins (FPs) have been fused downstream to tandem repeats of the hypoxia transcriptional response element (HRE) to afford genetic tools for monitoring the transcriptional activity of HIF-1.21 Because prolyl hydroxylation is not the only regulatory mechanism for HRE-dependent transcription,2 the fluorescence of the FP reporters does not necessarily reflect oxygen abundance and the activities of PHDs. Herein, we describe a novel genetically encoded single-chain fluorescent biosensor, which can ratiometrically respond to PHD activities in vitro and in live cells. We also show that the Förster resonance energy transfer (FRET) ratios of the biosensor are indicative of hypoxic and normoxic conditions in living systems.
RESULTS AND DISCUSSION
Design and in vitro Characterization
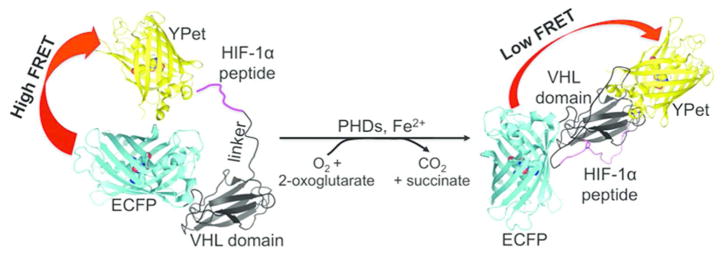
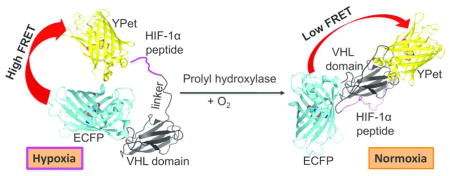
We designed the biosensor by sandwiching a proline-containing substrate peptide derived from HIF-1α and a small 10-kDa protein domain derived from the von Hippel-Lindau tumor suppressor (VHL) between an enhanced cyan FP (ECFP) and a yellow FP YPet (Figure 2). We selected a 22-aa peptide from HIF-1α (residues 556 to 577 with a hydroxylation site at residue 564), because of its reasonable length and excellent substrate activity for various PHDs.22, 23 The hydroxylation of Pro564 can greatly enhance the interaction of the peptide with VHL, whereas residues 60–154 of VHL form a distinct domain responsible for the interaction (Supporting Information Figure S1).24 ECFP and YPet were selected, because they form a highly optimized FRET pair due to their weak dimerization propensity, which typically leads to relatively large dynamic ranges in FP-based biosensors.25–28 We reasoned that the oxygen-dependent hydroxylation of the HIF-1α derived peptide by PHDs might induce the interaction of the peptide with the VHL domain, leading to a conformational change that alters the distance and/or relative orientation between ECFP and YPet to further trigger a measurable change in the FRET efficiency.28
Figure 2.
Illustration of the FRET change of the genetically encoded ratiometric fluorescent sensor, ProCY, upon the actions of PHDs.
We constructed five genetic variants, which differ from each other in terms of the length and composition of the linkers (Figures S2–S4). We expressed all fusion proteins in Escherichia coli, by co-expressing GroEL and GroES chaperones to assist protein folding.29 We next purified the proteins in two steps using Ni-NTA affinity chromatography and size-exclusion columns, and determined their fluorescence responses to a catalytically active fragment of PHD2 (a.k.a. HIF-P4H-2, or EglN1), which is the most abundant form of HIF-prolyl hydroxylases, using an in vitro enzyme assay.30, 31 We named the variant showing the largest dynamic range (defined as the ratio of the acceptor-to-donor emission ratios before and after treatment with PHD2) ProCY. It contains a 21-amino acid Gly- and Ser- rich linker between the VHL domain and the HIF-1α derived peptide; all other elements were fused with short dipeptide sequences (Figure 3A). The FRET ratio (YPet/ECFP) of this variant changed from 2.95 to 1.76 after addition of PHD2, corresponding to a dynamic range of 168% (Figure 3B). We further constructed a negative-control fluorescent probe, ProCY-N, which is identical to ProCY except for that both proline residues (Pro564 and Pro567) in the HIF-1α derived peptide were mutated to alanine (Figure 3A). The fluorescence of ProCY-N is irresponsive to the PHD2 activity (Figure 3C).
Figure 3.
(A) Domain arrangements of ProCY and the negative-control probe, ProCY-N. (B) Fluorescence emission spectra for ProCY before (cyan) and after (orange) treatment with a catalytically active PHD2 fragment. (C) Fluorescence emission spectra for ProCY-N, showing no response to PHD2 treatment. ECFP and YPet are an enhanced cyan fluorescent protein and a yellow fluorescent protein, respectively.
Imaging of Hypoxia and Prolyl Hydroxylases in Mammalian Cells
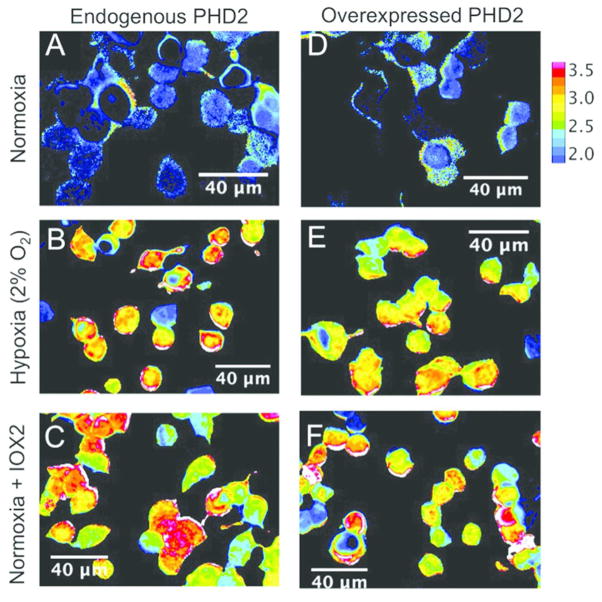
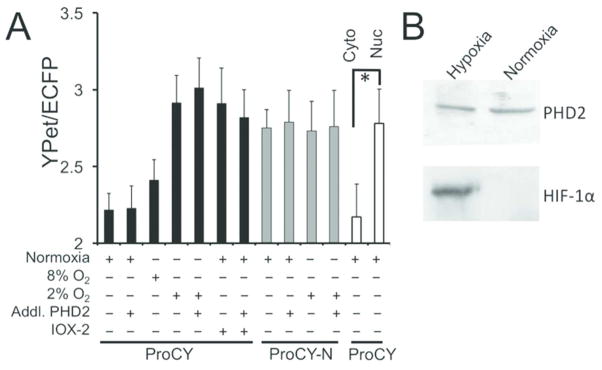
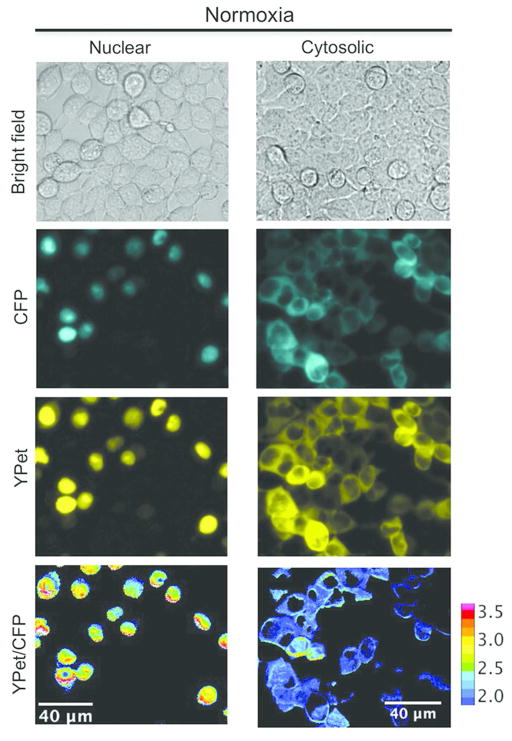
We next expressed ProCY in human embryonic kidney (HEK) 293T cells, and examined its responses to intracellular PHD activities. We cultured cells in a typical cell culture incubator (~ 20% O2), or in a hypoxic chamber supplied with 2% O2. A lower FRET ratio was observed for the cells under normoxia (Figure 4A) compared to the cells under hypoxia (Figure 4B), corroborating our in vitro characterization with purified proteins. Under normoxia, addition of 10 μM IOX2,32 a commercially available PHD inhibitor, resulted in a FRET ratio close to that of cells under hypoxia (Figure 4C). We further co-expressed ProCY with the catalytic fragment of PHD2 in HEK 293T cells. Under all conditions, the FRET ratios of the cells overexpressing the PHD2 fragment were essentially indistinguishable from the ratios of the cells without PHD2 overexpression (Figures 4D–F and 5A), indicating that the endogenous PHD level in HEK 293T cells is high and it is not the limiting factor for the intracellular prolyl hydroxylation reaction. Previous studies suggest that oxygen is a key regulator for PHD activities.33 We examined the FRET ratios of ProCY at three different oxygen concentrations (~ 20%, 8%, and 2%). Indeed, there was an inverse relationship between FRET ratios and oxygen concentrations (Figures 5A). To further confirm that the FRET changes of ProCY were caused by intracellular PHD activities, we expressed the negative-control probe, ProCY-N, in HEK 293T cells under normoxia and hypoxia, and no FRET ratio change was observed (Figures 5A and S5A). All data collectively support that ProCY is an effective sensor for monitoring the PHD activities and hypoxia in mammalian cells. We also utilized Western blot to probe the expression levels of PHD2 and HIF-1α in HEK 293T (Figure 5B). PHD2 was observed for cells under both normoxia and hypoxia, whereas HIF-1α was only observed for cells under hypoxia. The result is aligned with previous reports,2 indicating that our experimental conditions were well controlled.
Figure 4.
Pseudocolored ratio images of representative ProCY-expressing HEK 293T cells without (A–C) or with (D–F) overexpression of PHD2. The cells were cultured under either normoxia (A and D) or hypoxia (B and E), or treated with a PHD2 inhibitor, IOX2, under normoxia (C and F). The color bar represents the ratio of sensitized YPet fluorescence emission to direct ECFP donor emission (YPet/ECFP).
Figure 5.
(A) The FRET ratios of ProCY or ProCY-N in HEK 293T cells under the corresponding conditions. PHD2 overexpression had little effect, indicating the endogenous PHD level was high. Compartmental expression of ProCY suggests a higher PHD activity in the cytosols than the nuclei of HEK 293T cells (*p < 0.05). Plotted are average values of three replicates with error bars representing standard deviations. (B) Western blot of PHD2 and HIF-1α for HEK 293T cells under hypoxia or normoxia.
Prolyl hydroxylation is generally considered as an irreversible post-translational modification.34 This limits the capability of ProCY for real-time monitoring of cellular dynamics of PHD activities. We observed slow responses of ProCY after switching cells from normoxia to hypoxia. This is not surprising, because hydroxylated ProCY sensor proteins have to be largely degraded before FRET changes become obvious. Our ProCY sensor took ~ 48 h to reach steady states, corroborating previous reports that FPs have long half-lives.35 In order to gain faster responses, one may choose to fuse ProCY with a destabilizing sequence (degron) to enhance the turnover rate of hydroxylated ProCY.35 On the other hand, one could express ProCY under hypoxia to monitor re-oxygenation (Figures S5B). Although PHD-induced FRET changes usually completed in 30–40 min in our in vitro assays, most FRET changes of ProCY-expressing cells were in the first 2–3 hours during re-oxygenation under normoxic conditions. This difference was likely caused by the slow dissolution and diffusion of oxygen in the culture medium. Biological systems often use oxygen transporter proteins, such as hemoglobin, to increase the solubility and transport of oxygen.36
Subcellular Imaging of the Prolyl Hydroxylase Activity
It is generally accepted that the localization of PHDs in distinct subcellular compartments is regulated.37, 38 Their localization and translocation between the nucleus and the cytosol are important mechanisms for the control of hypoxic responses.38, 39 Recently, the subcellular localization of PHDs has even been linked to tumor development and resistance to radiotherapy.40–42 Despite that a number of studies, which mainly relied on nuclear/cytoplasmic fractionation followed by Western blotting of PHDs or direct fluorescence imaging of labeled PHDs, have sought to elucidate the distribution of PHD enzymes between the nucleus and the cytosol, conflicting results have been reported.37, 43–45 This discrepancy may be attributed to different cell types used in these studies, or may be caused by the inaccuracy of their detection methods.43 Since ProCY is a genetically encoded indicator for the HIF-prolyl hydroxylase activity, we simply fused ProCY with a nuclear localization sequence (NLS) or a nuclear export signal (NES) to achieve the complete localization of ProCY in either the nucleus or the cytosol. When HEK 293T cells were cultured in normoxia, reduced FRET ratios were observed for cytosolic ProCY, but not for nuclear ProCY (Figures 5A and 6). The result suggests that the endogenous PHD activity is mainly in the cytosols of HEK 293T cells. This experiment demonstrates the usefulness of ProCY to directly image subcellular HIF-prolyl hydroxylase activities. This method may be further applied to other cell lines to investigate cell-type specific impacts and to elucidate molecular mechanisms that control the shuttling of PHDs between subcellular domains.
Figure 6.
Microscopic images of representative HEK 293T cells expressing nuclear or cytosolic ProCY. The ratio images are pseudocolored and the color bar represents the ratio of sensitized YPet fluorescence emission to direct ECFP donor emission (YPet/ECFP).
Conclusions
We have developed a novel genetically encoded dual-emission ratiometric fluorescent biosensor for HIF-prolyl hydroxylases, by sandwiching a proline-containing HIF-1α derived peptide and a 4-hydroxylproline interacting domain between an FP-based FERT pair. We demonstrated that the probe could be utilized to monitor hypoxia and PHD activities in live mammalian cells. We targeted this biosensor to the nucleus and cytosol, and determined that, under normoxia, the HIF-prolyl hydroxylase activity was mainly confined to the cytosols of HEK 293T cells. Our data collectively suggest broad applications of ProCY on evaluation of hypoxia and prolyl hydroxylase activities and understanding of relevant molecular mechanisms. Moreover, as HIF-prolyl hydroxylases have emerged as promising drug targets for a variety of diseases,46 such as myocardial infarction, stroke, cancer, diabetes, and severe anemia, ProCY may be utilized in high-throughput assays to identify inhibitors or activators of PHDs.
METHODS
Materials, reagents, and general methodology
Ascorbic acid was purchased from Spectrum Chemical. Disodium 2-oxoglutarate was purchased from TCI America. Iron (II) sulfate heptahydrate was purchased from Thermo Fisher Scientific. Catalase from Bovine Liver was purchased from Sigma-Aldrich. IOX2 was purchased from Cayman Chemical. Gases for cell culture were purchased from Airgas USA. SuperSignal West Pico Chemiluminescent substrate and HRP-conjugated HIF-1α antibody were from Thermo Fisher Scientific. HRP-conjugated antibody to c-Myc was from Abcam. Colorimetric One-Component TMB Membrane Peroxidase Substrate (Prod # 50–77–18) was purchased from Kierkegaard & Perry Laboratories. Synthetic DNA oligonucleotides were purchased from Integrated DNA Technologies. HA-EglN1-pcDNA3 (Plasmid # 18963) was purchased from Addgene. Restriction endonucleases were purchased from New England Biolabs or Thermo Scientific Fermentas. PCR products and products of restriction digestion were purified by gel electrophoresis and extracted using Syd Laboratories Gel Extraction columns. Plasmid DNA was purified using Syd Laboratories Miniprep columns. DNA sequence was analyzed by Retrogen.
Construction of E. coli Expression Plasmids
To construct ProCY, polymerase chain reactions (PCR) were utilized to amplify the pVHL fragment from a synthetic gene block (Integrated DNA Technologies), and YPet and ECFP from a previously reported Src sensor.27 In particular, YPet was amplified with oligos YPet-For and YPet-Rev (see Supporting Information Table S1 for sequences). The PCR product was digested with Kpn I and Hind III and ligated into a compatible pre-digested pBAD/His B plasmid to afford pBAD-YPet. In the next step, two PCR reactions were performed to amplify ECFP and pVHL and add sequences for linkers and a HIF-1α derived peptide. Oligos ECFP-For and pVHL-Rev were used in the first reaction, and oligos Pst1-For, Kpn1-Rev1, Kpn-Rev2 and Kpn-Rev3 were used in the second PCR reaction, respectively. Products of these two reactions were then assembled using an overlap PCR to produce the full-length gene containing ECFP, pVHL, the linkers, and a HIF-1α derived peptide. The PCR product was digested with Xho I and Kpn I, and ligated into a pre-digested compatible pBAD-YPet plasmid to derive pBAD-ProCY. To construct ProCY-B, two separate PCR reactions with oligos ECFP-For and GGSG-Rev, and GGSG-For and Kpn-Rev2, were utilized to amplify gene fragments from pBAD-ProCY. The products of the two reactions were assembled using overlap PCR, followed by digestion with Xho I and Kpn I, and ligation into a predigested pBAD-YPet to generate pBAD-ProCY-B. To create ProCY-C, two separate PCR reactions were performed using pBAD-ProCY-B as the template. In the first PCR reaction, oligos ECFP-For and MidFloppy-R were used, while in the second reaction, MidFloppy-F1, MidFloppy-F2, and pBAD-Rev were used. The two PCR reactions were assembled using overlap PCR, digested with Xho I and Hind III, and ligated into a compatible pre-digested pBAD/His B vector. To construct ProCY-D, we performed a PCR reaction on ProCY-B using oligos HIF-Rigid-F and pBAD-Rev. The PCR product was digested with Kpn I and Hind III and ligated into a pre-digested pBAD-ProCY-B plasmid to afford pBAD-ProCY-D. To build ProCY-E, a PCR reaction was performed on pBAD-ProCY using oligos ECFP-For and SacI-Rev. The resulting PCR product was digested with Xho I and Sac I and ligated into a pre-digested compatible pBAD-ProCY plasmid to afford pBAD-ProCY-E. The control construct ProCY-N was generated by a PCR reaction on pBAD-ProCY using ECFP-For and PAPA-Rev. The resulting product was digested with Xho I and Kpn I and ligated to a pre-digested pBAD-YPet. Similarly, PCR was used to amplify the catalytically active fragment of PHD2 from HA-EglN1-pcDNA3 using oligos PHD2-Kpn1-F and PHD2-HindIII-R. The PCR product was digested with Kpn I and Hind III restriction enzymes and ligated into a pre-digested compatible pCDF-1b plasmid to afford pCDF-PHD2. All resultant ligation products were used to transform E. coli DH10B competent cells, which were next plated on LB agar plates supplemented with appropriate antibiotics. Correct assembly was confirmed by sequencing.
Protein Expression and Purification
To express all FRET constructs, DH10B cells were co-transformed with the corresponding pBAD plasmid and a pGro7 chaperone plasmid (Takara), which conditionally overexpresses GroEL/ES. Cells were grown on LB agar containing 100 μg/mL ampicillin and 50 μg/mL chloramphenicol at 37°C overnight. A single colony was grown in a starter culture of 5 mL LB broth with appropriate antibiotics at 37°C and 220 rpm overnight. A saturated starter culture was next diluted 100-fold into 2YT medium containing the appropriate antibiotics and grown under the same condition. When OD600 reached 0.8, the expression culture was induced with 0.2% L-arabinose. Culture flasks were then moved to room temperature where growth continued with vigorous shaking for 48 hours. Cells were then harvested, resuspended in 1× PBS supplied with cOmplete™ EDTA-free Protease Inhibitor (Roche), and then lysed with sonication. His6-tagged proteins were affinity-purified with nickel-nitrilotriacetic acid (Ni-NTA) agarose beads (Qiagen) under native conditions according to the manufacturer’s instruction and then buffer-exchanged into Tris-HCl (30 mM, pH 7.4) using Thermo Scientific Snakeskin dialysis tubing (7 k molecular cutoff). Proteins were further purified by size exclusion column chromatography (HiPrep Sephacryl S-200 HR, GE Healthcare) and concentrated using a 3K molecular weight cutoff Amicon Ultra centrifugal filter (Millipore). The concentration was determined using Beer’s Law and the reported extinction coefficients of ECFP and YPet.47
To express the catalytically active fragment of PHD2, BL21(DE3) cells were transformed with pCDF-PHD2 and grown on LB agar containing 50 μg/mL spectinomycin at 37 °C overnight. A single colony was used to inoculate 5 mL LB supplied with 50 μg/mL spectinomycin at 37 °C overnight. Saturated cell culture was then diluted 100-fold with fresh 2YT containing 50 μg/mL spectinomycin, and grown at 37 °C and 220 rpm. When OD600 reached 0.8, protein expression was induced with IPTG (1 mM). Growth continued at room temperature for 24 hours. Cells were then harvested and lysed. His6-tagged PHD2 was affinity purified with Ni-NTA agarose beads (Qiagen) and dialyzed into Tris-HCl (30 mM, pH 7.4) as mentioned above. Glycerol was added to a final concentration of 10% (v/v), and the mixture was then stored immediately at −80 °C.
Spectroscopic Characterization
A monochromator-based Synergy Mx Microplate Reader (BioTek) was used to record all absorbance and fluorescence spectra. To record the emission spectra, the excitation wavelength was set at 434 nm, and the emission scanned from 455 nm to 600 nm. The instrumental bandwidth was 9 nm. FRET ratios were calculated by dividing the fluorescence intensity of YPet at 525 nm over the fluorescence intensity of ECFP at 480 nm. For single point measurements, excitation at 434 nm and emission at 480 nm and 525 nm were used to calculate FRET ratios. Absorbance spectra were recorded by scanning from 250 nm to 600 nm, and a blank solution was used to subtract the background.
Assays of the PHD2 Activity
Freshly made stock solutions were all prepared in Tris-HCl (30 mM, pH 7.4). The PHD2 activity assay was carried out by mixing 2 mg/mL BSA, 1 mM DTT, 2 mM ascorbic acid, 0.6 mg/mL catalase, 25 μM Fe (II) solution (initially prepared as a 500 mM stock in 20 mM HCl) with 1 μM FRET biosensor in the wells of a black 96-well plate.30, 48 The resultant mixture was first incubated at room temperature for 20 min. Next, the catalytic fragment of PHD2 (2 μM) was added to the reaction mixture. The reaction was then initiated by addition of 200 μM 2-oxoglutarate. The 96-well plate was incubated at 37 °C for another 40 min. Excitation at 434 nm was used to measure emission spectra from 455 nm to 600 nm. FRET ratios were calculated by dividing the emission intensity at 525 nm over the emission intensity at 480 nm. Control experiments were performed with the same procedure except for that no PHD2 was added.
Construction of mammalian reporter plasmids
To insert the ProCY and ProCY-N genes into the mammalian expression vector pcDNA3, oligos HydpcDNA3-F and HydpcDNA3-R were used to amplify both genes from the corresponding pBAD plasmids and add Hind III and Xba I restriction sites. HydpcDNA3-F also includes a Kozak sequence and a start codon for efficient translation initiation. Each PCR product was digested with Hind III and Xba I, and inserted into a compatible pre-digested pcDNA3 plasmid. Similarly, the PHD2 active fragment was amplified using PHD2-pcDNA3-F and PHD2-pcDNA3-R, digested with Hind III and Xba I restriction enzymes, and ligated into a compatible pre-digested pcDNA3 vector. To add a myc-tag at the C-terminus of PHD2 for Western blot, pcDNA3-F and mycPHD2-R were used. The PCR product was digested with Hind III and Xba I and ligated into a pre-digested pcDNA3 plasmid. To create a plasmid for nuclear expression of ProCY, we used oligonucleotides PLJMI-NheI-F and pHP-Nuc-R to amplify the ProCY gene fragment from pBAD-ProCY. The PCR product was digested with Nhe I and Xho I and ligated into a predigested pEYFP-Nuc plasmid (Clontech) containing three copies of a nuclear localization sequence (DPKKKRKV). To construct a plasmid for cytosolic expression of ProCY, we used oligos NES-HP-F1, NES-HP-F2 and NES-HP-XbaI-R to amplify the ProCY gene fragment from pBAD-ProCY and further extend it to include an N-terminal nucleus export signal (LQLPPLERLTL). The product was digested with Hind III and Xba I, and then ligated into a predigested pNES-rxRFP1 plasmid49, which contains two C-terminal repeats of the nuclear export signal. The resultant pNES-ProCY has three NES repeats, one at the N-terminus and two at the C-terminus.
Design of hypoxia chambers
Hypoxia chambers were constructed as previously described.50 150 x 15 mm petri dishes were tightly sealed with playdough. Two holes were drilled in the lid of each petri dish. A valve was placed in each hole, one allowing gas in and one allowing gas out. Transfected HEK293 cells were placed into the sealed chamber. A small 35-mm dish containing distilled water was also placed in the chamber to humidify the incoming gas. Unless otherwise specified, the chamber was flushed with a gas mixture containing 93% N2, 5% CO2 and 2% O2 for 5 min. Next, the two valves were closed. In every 12 hours, new gas containing 93% N2, 5% CO2 and 2% O2 was used to reflush the chamber. Cells in the hypoxia chamber were incubated at 37 °C for 48 hours before imaging. For the purpose of comparison, a gas mixture containing 87% N2, 5% CO2 and 8% O2 was also utilized to maintain a hypoxic condition with 8% O2.
Mammalian Cell Culture and Imaging
Human Embryonic Kidney (HEK) 293T cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cells were incubated at 37°C with 5% CO2 in humidified air for 24 hours. HEK 293T cells were then co-transfected with 2 μg pcDNA3-ProCY and 2 μg pcDNA3-PHD2 in the presence of 12 μg PEI (polyethyleneimine, linear, M.W. 25 kDs). Transfected cells were then divided in two groups. The first group was cultured in complete media under normoxic conditions for 48 hours, and the second group of transfected cells was cultured in complete media in hypoxic chambers for 48 hours. Another group of Transfected HEK 293T cells were treated with 10 μM IOX2 inhibitor for 48 hours. Cells were washed with 2 mL Dulbecco’s Phosphate Buffered Saline (DPBS), and left in 1 mL DPBS for immediate imaging on a Motic AE31 inverted epifluorescence microscope equipped with a 20× objective lens. Excitation was achieved using a 100-W Short-Arc Mercury lamp and a 436/20 nm excitation filter. ECFP and YPet emission were collected with a 480/40 nm and a 535/40 emission filter, respectively. A 455 nm longpass dichroic mirror was used in all experiments. Resultant images were processed with ImageJ. To compare FRET ratios under various conditions, results from at least three replicate samples were used to derive means and standard deviations.
Western blotting
HEK293T cells seeded on a 100-mm dishes were exposed to hypoxia conditions for 24 hours. Cells were then harvested and nuclear proteins were extracted using a well-established extraction method.51 Proteins were then analyzed on a sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. Membranes were then blocked with 5% low fat milk for 1 hour followed by incubation with HRP-conjugated anti-HIF-1α antibody. Signals were detected using SuperSignal West Pico Chemiluminescent substrate (Thermo Fisher). Western blot analysis of PHD2 was carried out by transfecting HEK293T cells with pcDNA3-mycPHD2 and exposing cells to either normoxic or hypoxic conditions. Cell lysates were then prepared and analyzed on SDS-PAGE, followed by blotting on nitrocellulose membranes. Membranes were blocked with 5% low fat milk for 1 hour followed by overnight incubation with HRP-conjugated anti-c-Myc antibody at 4°C. Signals were then detected using colorimetric One-Component TMB Membrane Peroxidase Substrate (Kirkegaard & Perry Laboratories).
Supplementary Material
Acknowledgments
This work was supported in part by the National Science Foundation (CHE-1351933), the National Institutes of Health (R03EB020211 and R01GM118675), and the University of California, Riverside. We also acknowledge Z. Chen for intellectual input. HA-EglN1-pcDNA3 was a gift from W. Kaelin (Addgene plasmid # 18963).
Footnotes
Notes
The authors declare no competing financial interest.
Additional notes on the mechanism, sequence information of biosensors, additional characterization, FRET images of cells expressing the ProCY-N control probe, and sequences of oligonucleotides used in this study. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Berkelhamer SK, Kim GA, Radder JE, Wedgwood S, Czech L, Steinhorn RH, Schumacker PT. Developmental differences in hyperoxia-induced oxidative stress and cellular responses in the murine lung. Free Radic Biol Med. 2013;61:51–60. doi: 10.1016/j.freeradbiomed.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiol. 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 3.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Molle I, Thomann A, Buckley DL, So EC, Lang S, Crews CM, Ciulli A. Dissecting fragment-based lead discovery at the von Hippel-Lindau protein:hypoxia inducible factor 1alpha protein-protein interface. Chem Biol. 2012;19:1300–1312. doi: 10.1016/j.chembiol.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 6.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 7.Giaccia AJ, Simon MC, Johnson R. The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004;18:2183–2194. doi: 10.1101/gad.1243304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhao S, Wu J. Hypoxia inducible factor stabilization as a novel strategy to treat anemia. Curr Med Chem. 2013;20:2697–2711. doi: 10.2174/0929867311320210006. [DOI] [PubMed] [Google Scholar]
- 10.Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol. 2004;207:3149–3154. doi: 10.1242/jeb.01064. [DOI] [PubMed] [Google Scholar]
- 11.Shi H. Hypoxia inducible factor 1 as a therapeutic target in ischemic stroke. Curr Med Chem. 2009;16:4593–4600. doi: 10.2174/092986709789760779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Zhang W, Li J, Zhang Y. Optical imaging of tumor microenvironment. Am J Nucl Med Mol Imaging. 2013;3:1–15. [PMC free article] [PubMed] [Google Scholar]
- 13.Papkovsky DB, Dmitriev RI. Biological detection by optical oxygen sensing. Chem Soc Rev. 2013;42:8700–8732. doi: 10.1039/c3cs60131e. [DOI] [PubMed] [Google Scholar]
- 14.Cui L, Zhong Y, Zhu W, Xu Y, Du Q, Wang X, Qian X, Xiao Y. A new prodrug-derived ratiometric fluorescent probe for hypoxia: high selectivity of nitroreductase and imaging in tumor cell. Org Lett. 2011;13:928–931. doi: 10.1021/ol102975t. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Liu H, Mack J, Tian J, Zou B, Lu H, Li Z, Jiang J, Shen Z. A BODIPY-based ‘turn-on’ fluorescent probe for hypoxic cell imaging. Chem Commun. 2015;51:13389–13392. doi: 10.1039/c5cc05139h. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Li X, Gao X, Zhang Y, Shi W, Ma H. Nitroreductase detection and hypoxic tumor cell imaging by a designed sensitive and selective fluorescent probe, 7-[(5-nitrofuran-2-yl)methoxy]-3H-phenoxazin-3-one. Anal Chem. 2013;85:3926–3932. doi: 10.1021/ac400750r. [DOI] [PubMed] [Google Scholar]
- 17.Komatsu H, Harada H, Tanabe K, Hiraoka M, Nishimoto S-i. Indolequinone-rhodol conjugate as a fluorescent probe for hypoxic cells: enzymatic activation and fluorescence properties. MedChemComm. 2010;1:50–53. [Google Scholar]
- 18.Kiyose K, Hanaoka K, Oushiki D, Nakamura T, Kajimura M, Suematsu M, Nishimatsu H, Yamane T, Terai T, Hirata Y, Nagano T. Hypoxia-sensitive fluorescent probes for in vivo real-time fluorescence imaging of acute ischemia. J Am Chem Soc. 2010;132:15846–15848. doi: 10.1021/ja105937q. [DOI] [PubMed] [Google Scholar]
- 19.Cai Q, Yu T, Zhu W, Xu Y, Qian X. A turn-on fluorescent probe for tumor hypoxia imaging in living cells. Chem Commun. 2015;51:14739–14741. doi: 10.1039/c5cc05518k. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi S, Piao W, Matsumura Y, Komatsu T, Ueno T, Terai T, Kamachi T, Kohno M, Nagano T, Hanaoka K. Reversible off-on fluorescence probe for hypoxia and imaging of hypoxia-normoxia cycles in live cells. J Am Chem Soc. 2012;134:19588–19591. doi: 10.1021/ja310049d. [DOI] [PubMed] [Google Scholar]
- 21.Vordermark D, Shibata T, Brown JM. Green fluorescent protein is a suitable reporter of tumor hypoxia despite an oxygen requirement for chromophore formation. Neoplasia. 2001;3:527–534. doi: 10.1038/sj.neo.7900192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsila M, Koivunen P, Gunzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- 23.Koivunen P, Hirsila M, Kivirikko KI, Myllyharju J. The length of peptide substrates has a marked effect on hydroxylation by the hypoxia-inducible factor prolyl 4-hydroxylases. J Biol Chem. 2006;281:28712–28720. doi: 10.1074/jbc.M604628200. [DOI] [PubMed] [Google Scholar]
- 24.Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, Pugh CW, Maxwell PH, Ratcliffe PJ, Stuart DI, Jones EY. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature. 2002;417:975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 25.Ohashi T, Galiacy SD, Briscoe G, Erickson HP. An experimental study of GFP-based FRET, with application to intrinsically unstructured proteins. Protein Sci. 2007;16:1429–1438. doi: 10.1110/ps.072845607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vinkenborg JL, Evers TH, Reulen SW, Meijer EW, Merkx M. Enhanced sensitivity of FRET-based protease sensors by redesign of the GFP dimerization interface. ChemBioChem. 2007;8:1119–1121. doi: 10.1002/cbic.200700109. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang M, Sun J, Chien S, Wang Y. Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors. Proc Natl Acad Sci U S A. 2008;105:14353–14358. doi: 10.1073/pnas.0807537105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ai HW. Fluorescent-protein-based probes: general principles and practices. Anal Bioanal Chem. 2015;407:9–15. doi: 10.1007/s00216-014-8236-3. [DOI] [PubMed] [Google Scholar]
- 29.Nishihara K, Kanemori M, Kitagawa M, Yanagi H, Yura T. Chaperone coexpression plasmids: differential and synergistic roles of DnaK-DnaJ-GrpE and GroEL-GroES in assisting folding of an allergen of Japanese cedar pollen, Cryj2, in Escherichia coli. Appl Environ Microbiol. 1998;64:1694–1699. doi: 10.1128/aem.64.5.1694-1699.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hewitson KS, Schofield CJ, Ratcliffe PJ. Hypoxia-inducible factor prolyl-hydroxylase: purification and assays of PHD2. Methods Enzymol. 2007;435:25–42. doi: 10.1016/S0076-6879(07)35002-7. [DOI] [PubMed] [Google Scholar]
- 31.Ivan M, Haberberger T, Gervasi DC, Michelson KS, Gunzler V, Kondo K, Yang H, Sorokina I, Conaway RC, Conaway JW, Kaelin WG., Jr Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S A. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhury R, Candela-Lena JI, Chan MC, Greenald DJ, Yeoh KK, Tian YM, McDonough MA, Tumber A, Rose NR, Conejo-Garcia A, Demetriades M, Mathavan S, Kawamura A, Lee MK, van Eeden F, Pugh CW, Ratcliffe PJ, Schofield CJ. Selective small molecule probes for the hypoxia inducible factor (HIF) prolyl hydroxylases. ACS Chem Biol. 2013;8:1488–1496. doi: 10.1021/cb400088q. [DOI] [PubMed] [Google Scholar]
- 33.Dao JH, Kurzeja RJ, Morachis JM, Veith H, Lewis J, Yu V, Tegley CM, Tagari P. Kinetic characterization and identification of a novel inhibitor of hypoxia-inducible factor prolyl hydroxylase 2 using a time-resolved fluorescence resonance energy transfer-based assay technology. Anal Biochem. 2009;384:213–223. doi: 10.1016/j.ab.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 34.Gorres KL, Raines RT. Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol. 2010;45:106–124. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Zhao X, Fang Y, Jiang X, Duong T, Fan C, Huang CC, Kain SR. Generation of destabilized green fluorescent protein as a transcription reporter. J Biol Chem. 1998;273:34970–34975. doi: 10.1074/jbc.273.52.34970. [DOI] [PubMed] [Google Scholar]
- 36.Mairbaurl H, Weber RE. Oxygen transport by hemoglobin. Comprehensive Physiology. 2012;2:1463–1489. doi: 10.1002/cphy.c080113. [DOI] [PubMed] [Google Scholar]
- 37.Metzen E, Berchner-Pfannschmidt U, Stengel P, Marxsen JH, Stolze I, Klinger M, Huang WQ, Wotzlaw C, Hellwig-Burgel T, Jelkmann W, Acker H, Fandrey J. Intracellular localisation of human HIF-1 alpha hydroxylases: implications for oxygen sensing. J Cell Sci. 2003;116:1319–1326. doi: 10.1242/jcs.00318. [DOI] [PubMed] [Google Scholar]
- 38.Pientka FK, Hu J, Schindler SG, Brix B, Thiel A, Johren O, Fandrey J, Berchner-Pfannschmidt U, Depping R. Oxygen sensing by the prolyl-4-hydroxylase PHD2 within the nuclear compartment and the influence of compartmentalisation on HIF-1 signalling. J Cell Sci. 2012;125:5168–5176. doi: 10.1242/jcs.109041. [DOI] [PubMed] [Google Scholar]
- 39.Steinhoff A, Pientka FK, Mockel S, Kettelhake A, Hartmann E, Kohler M, Depping R. Cellular oxygen sensing: Importins and exportins are mediators of intracellular localisation of prolyl-4-hydroxylases PHD1 and PHD2. Biochem Biophys Res Commun. 2009;387:705–711. doi: 10.1016/j.bbrc.2009.07.090. [DOI] [PubMed] [Google Scholar]
- 40.Jokilehto T, Rantanen K, Luukkaa M, Heikkinen P, Grenman R, Minn H, Kronqvist P, Jaakkola PM. Overexpression and nuclear translocation of hypoxia-inducible factor prolyl hydroxylase PHD2 in head and neck squamous cell carcinoma is associated with tumor aggressiveness. Clin Cancer Res. 2006;12:1080–1087. doi: 10.1158/1078-0432.CCR-05-2022. [DOI] [PubMed] [Google Scholar]
- 41.Jokilehto T, Jaakkola PM. The role of HIF prolyl hydroxylases in tumour growth. J Cell Mol Med. 2010;14:758–770. doi: 10.1111/j.1582-4934.2010.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luukkaa M, Jokilehto T, Kronqvist P, Vahlberg T, Grenman R, Jaakkola P, Minn H. Expression of the cellular oxygen sensor PHD2 (EGLN-1) predicts radiation sensitivity in squamous cell cancer of the head and neck. Int J Radiat Biol. 2009;85:900–908. doi: 10.1080/09553000903074104. [DOI] [PubMed] [Google Scholar]
- 43.Yasumoto K, Kowata Y, Yoshida A, Torii S, Sogawa K. Role of the intracellular localization of HIF-prolyl hydroxylases. Biochim Biophys Acta. 2009;1793:792–797. doi: 10.1016/j.bbamcr.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Berchner-Pfannschmidt U, Tug S, Trinidad B, Oehme F, Yamac H, Wotzlaw C, Flamme I, Fandrey J. Nuclear oxygen sensing: induction of endogenous prolyl-hydroxylase 2 activity by hypoxia and nitric oxide. J Biol Chem. 2008;283:31745–31753. doi: 10.1074/jbc.M804390200. [DOI] [PubMed] [Google Scholar]
- 45.Barth S, Nesper J, Hasgall PA, Wirthner R, Nytko KJ, Edlich F, Katschinski DM, Stiehl DP, Wenger RH, Camenisch G. The peptidyl prolyl cis/trans isomerase FKBP38 determines hypoxia-inducible transcription factor prolyl-4-hydroxylase PHD2 protein stability. Mol Cell Biol. 2007;27:3758–3768. doi: 10.1128/MCB.01324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myllyharju J. HIF prolyl 4-hydroxylases and their potential as drug targets. Curr Pharm Des. 2009;15:3878–3885. doi: 10.2174/138161209789649457. [DOI] [PubMed] [Google Scholar]
- 47.Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2:905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 48.Ma X, Wang X, Cao J, Geng Z, Wang Z. Effect of proline analogues on activity of human prolyl hydroxylase and the regulation of HIF signal transduction pathway. PLoS One. 2014;9:e95692. doi: 10.1371/journal.pone.0095692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan Y, Ai HW. Development of redox-sensitive red fluorescent proteins for imaging redox dynamics in cellular compartments. Anal Bioanal Chem. 2016;408:2901–2911. doi: 10.1007/s00216-015-9280-3. [DOI] [PubMed] [Google Scholar]
- 50.Signorelli S, Jennings P, Leonard MO, Pfaller W. Differential effects of hypoxic stress in alveolar epithelial cells and microvascular endothelial cells. Cell Physiol Biochem. 2010;25:135–144. doi: 10.1159/000272066. [DOI] [PubMed] [Google Scholar]
- 51.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.