Abstract
Disparities in outcomes for African-American (AA) kidney transplant recipients have persisted for 40 years without a comprehensive analysis of evolving trends in risks associated with this disparity. Here we analyzed United States transplant registry data, which included adult Caucasian or AA solitary kidney recipients transplanted between 1990 and 2009 encompassing 202,085 transplants. Over this 20 year period, the estimated rate of 5 year graft loss decreased from 27.6% to 12.8%. Notable trends in baseline characteristics that significantly differed by race over time included: increased prevalence of diabetes from 2001–09 in AAs (5 year slope difference: 3.4%), longer time on the waiting list (76.5 more days per 5 years in AAs), fewer living donors in AAs from 2003–09 (5 year slope difference: −3.36%), more circulatory death donors in AAs from 2000–09 (5 year slope difference: 1.78%), and a slower decline in delayed graft function in AAs (5 year slope difference: 0.85%). The absolute risk difference between AAs and Caucasians for 5 year graft loss significantly declined over time (−0.92% decrease per 5 years), while the relative risk difference actually significantly increased (3.4% increase per 5 years). These results provide a mixed picture of both promising and concerning trends in disparities for AA kidney transplant recipients. Thus, although the disparity for graft loss has significantly improved, equity is still far off and other disparities, including living donation rates and delayed graft function rates have widened during this time.
Keywords: Kidney transplant, African American, Racial disparities, Graft loss
INTRODUCTION
It is well established that African-Americans (AAs), as compared to Caucasians, are at a significantly increased risk of developing hypertension and diabetes, major risk factors for developing end-stage renal disease.1–3 As such, the lifetime prevalence of ESRD in AAs is nearly three times higher as compared to non-Hispanic Whites (AAs: 8.5% for men and 7.8% for women vs. non-Hispanic Whites [NHWs]: 3.3% in men and 2.2% in women).4,5 For those that develop ESRD, kidney transplantation offers the optimal treatment option, as it has demonstrated substantial advantages to dialysis, both in terms of life longevity and quality.6–9 Over the past 40 years, there have been remarkable enhancements in kidney allograft survival rates. In 1973, the average kidney transplant lasted approximately 1.5 to 2 years,10 whereas in 2012, the kidney allograft half-life was 11.3 years.11
During this same timeframe, it is unclear if racial disparities in allograft survival have significantly changed, both in scope and magnitude. As AAs are dramatically over-represented on the U.S. dialysis and transplant waiting lists, this disparity has enormous public health implications.5 In 1977, Opelz and colleagues demonstrated that at 3 years post-transplant, AAs had a 10% absolute lower rate of graft survival, as compared to Caucasians (25% in AA, 35% in Caucasians).12 The most recent Scientific Registry of Transplant Recipients (SRTR) annual report that details racial differences in outcomes, demonstrates a 12% difference in absolute five year graft survival rates.11 An analysis recently published, using SRTR data, demonstrated a 20–30% reduction in the adjusted risk for 5-year graft loss for both deceased and living donor AA recipients, but did not fully assess change in baseline risk over time and determine the trends in factors associated with this racial disparity, beyond delayed graft function (DGF) and acute rejection.13
Racial disparities in transplant have primarily been attributed to immunologic risk factors in AAs which lead to higher acute rejection rates,14–16 lower socioeconomic status,17,18 medication non-adherence,19,20 reduced access to care21 and more frequent comorbid conditions.22–25 Recent studies demonstrate gene variants may also account for this disparity, both in the higher prevalence of ESRD in AAs and the increased risk of graft loss after transplant. Polymorphisms within Apolipoprotein L1 (APOL1), which are only present in those of African ancestry, are significantly associated with the risk of developing ESRD in AAs and graft loss in those that receive AA donor organs.26 To date, there are limited studies that seek to determine if the prevailing etiologies attributed to racial disparities in kidney transplantation have evolved over time.27 Such an analysis may provide insightful information regarding which factors to focus interventional studies within the contemporary era of kidney transplantation in the hopes of removing racial disparities for AA recipients.
The objective of this study was to utilize national registry data from all adult kidney transplant recipients accumulated over a 20 year time period to determine which baseline and follow up variables that are implicated in racial disparities have significantly evolved over time.
RESULTS
Between Oct 1, 1987 and Sept 30, 2014, there were 394,359 kidney transplants identified in the UNOS dataset. Of these, 19,313 were excluded for age <18 years, 37,810 were excluded for receiving non-renal transplants, 62,813 were excluded for non-AA or Caucasian race/ethnicity and 72,338 were excluded for being outside the study time period (Jan 1, 1990 to Dec 31, 2009); leaving 202,085 transplant events included in the final analysis. Supplemental Figure 1 displays the study flowchart and number of transplants per year, stratified by race. Of the 202,085 transplants, 144,081 (71%) were Caucasian, while 58,004 (29%) were AA recipients. The proportion of kidney transplants performed in AAs substantially increased over the 20 year time period, starting at 24% in 1990 and increasing to 33% in 2009 (2.11% increase per five years, see top left chart in Supplemental Figure 2 and top of Table 2). The mean follow-up for the entire cohort was 7.0±4.9 years.
Table 2.
Five-year trajectories of baseline characteristics for adult kidney transplant recipients transplanted between 1990 and 2009, stratified by recipient race
| Baseline Characteristic | Slope in Caucasians⇂ | Slope in African Americans⇂ | Difference in Slopes by Race⇂ | p-value difference in slopes |
|---|---|---|---|---|
| Population Proportion | −2.11% | 2.11% | 4.22% | <0.0001 |
|
| ||||
| Median Age (years) | 3.57 | 2.71 | −0.87 | 0.0002 |
|
| ||||
| Female Gender | −0.59% | 0.44% | 1.02% | 0.0007 |
|
| ||||
| Median BMI (kg/m2) | 1.05 | 1.00 | −0.05 | 0.4198 |
|
| ||||
| Median Functional Status | −1.98% | −3.30% | −1.48% | 0.7405 |
|
| ||||
| Some College or College Graduate | 2.97% | 2.16% | −0.81% | 0.0841 |
|
| ||||
| Medicare or Medicaid 1990–1998 | −16.3% | −13.4% | 3.0% | 0.3531 |
| Medicare or Medicaid 1999–2009 | 2.7% | 2.6% | −0.07% | 0.9717 |
|
| ||||
| Primary Diagnosis for ESRD | ||||
| Hypertension | 2.45% | 1.56% | −0.90% | 0.0541 |
| Diabetes | −0.17% | 2.16% | 2.33% | <0.0001 |
| Other | −2.28% | −3.72% | −1.44% | 0.0144 |
|
| ||||
| Comorbidities | ||||
| Angina | 0.21% | −0.34% | −0.55% | 0.5237 |
| Diabetes 1990–2000 | −3.8 % | −8.6% | −4.8% | 0.0108 |
| Diabetes 2001–2009 | 2.3% | 5.7% | 3.4% | 0.0004 |
| Cerebrovascular Accident | 0.21% | 0.49% | 0.29% | 0.3405 |
| Hypertension 1990–2002 | −5.1% | −7.5% | −2.4% | <0.0001 |
| Hypertension 2003–2009 | 7.3% | 5.6% | −1.7% | 0.2144 |
| Peripheral Vascular Disease | 0.33% | −0.01% | −0.37% | 0.1906 |
|
| ||||
| Receiving Dialysis at Time of Transplant | −5.18% | −2.45% | 2.73% | <0.0001 |
|
| ||||
| Median Days on Wait List (days) | 38.9 | 115.5 | 76.5 | <0.0001 |
|
| ||||
| Median Donor Age (yrs) | 2.73 | 3.15 | 0.43 | 0.3083 |
|
| ||||
| Median Donor BMI (kg/m2) | 1.01 | 1.02 | 0.01 | 0.8609 |
|
| ||||
| Donor Female | 2.23% | 1.62% | −0.61% | 0.2278 |
|
| ||||
| Donor Race | ||||
| Caucasian | −0.76% | −2.70% | −1.95% | 0.0052 |
| African-American | 0.10% | 1.22% | 1.12% | 0.0668 |
| Other | 0.65% | 1.46% | 0.81% | <0.0001 |
|
| ||||
| Living Donor 1990–2002 | 14.3% | 12.9% | −1.33% | 0.3244 |
| Living Donor 2003–2009 | −3.67% | −7.00% | −3.36% | 0.0030 |
|
| ||||
| Expanded Criteria Donor 1990–1995 | 3.9% | 6.00% | 2.1% | 0.0959 |
| Expanded Criteria Donor 1996–2009 | 0.83% | 1.10% | 0.27% | 0.5082 |
|
| ||||
| Donor after Cardiac Death 1990–1999 | −3.02% | −4.43% | −1.42% | 0.0175 |
| Donor after Cardiac Death 2000–2009 | 3.51% | 5.30% | 1.78% | <0.0001 |
|
| ||||
| Median HLA Mismatches | 0.28 | 0.00 | −0.28 | 0.2077 |
| A Mismatches | 0.00 | >0.00 | >0.00 | 0.0017 |
| B Mismatches | 0.00 | 0.36 | 0.36 | <0.0001 |
| DR Mismatches | 0.00 | 0.00 | 0.00 | N/A |
|
| ||||
| Median Current PRA | <0.00% | 0.00% | <0.00 % | 0.0023 |
| Median Peak PRA | −1.07% | −1.23% | −0.15% | 0.6265 |
| Current PRA >20% 1990–1997 | −7.5% | −7.20% | 0.32% | 0.3159 |
| Current PRA >20% 1998–2009 | 4.7% | 5.60% | 0.95% | 0.1267 |
| Current PRA >80% 1990–1997 | −2.8% | −2.90% | −0.08% | 0.8674 |
| Current PRA >80% 1998–2009 | 2.0% | 2.60% | 0.65% | 0.0056 |
|
| ||||
| Median Cold Time Deceased Donor (hrs) | −1.67 | −1.55 | 0.11 | 0.6875 |
| Median Cold Time Living Donor (hrs) | 0.00 | 0.00 | 0.00 | N/A |
|
| ||||
| Previous Kidney Transplant 1990–1994 | −5.00% | −8.60% | −3.70% | 0.0007 |
| Previous Kidney Transplant 1995–2009 | −0.13% | 0.44% | 0.57% | 0.0344 |
|
| ||||
| Induction Therapy | ||||
| IL-2 Receptor Antagonist 1990–1997 | 9.70% | 10.1% | 0.44% | 0.9323 |
| IL-2 Receptor Antagonist 1998–2000 | 39.3% | 38.1% | −1.19% | 0.9404 |
| IL-2 Receptor Antagonist 2001–2009 | 60.0% | −64.7% | −4.67% | 0.7396 |
| Cytolytic Therapy 1990–1993 | 20.4% | 23.4% | 3.0% | 0.8291 |
| Cytolytic Therapy 1994–1995 | −24.1% | −18.6% | 5.6% | 0.8726 |
| Cytolytic Therapy 1996–1999 | −20.8% | −36.6% | −15.8% | 0.6301 |
| Cytolytic Therapy 2000–2009 | 52.6% | 62.0% | 9.40% | 0.5133 |
|
| ||||
| Immunosuppression at Discharge | ||||
| Tacrolimus 1990–1995 | −16.8% | −21.1% | 6.30% | 0.3674 |
| Tacrolimus 1996–2009 | 31.8% | 31.6% | −0.19% | 0.9341 |
| Cyclosporine 1990–1995 | 19.4% | 14.6% | −3.3% | 0.5629 |
| Cyclosporine 1996–2009 | −32.3% | −32.4% | −0.09% | 0.9724 |
| Mycophenolate 1990–1998 | 43.9% | 45.0% | 1.11% | 0.9231 |
| Mycophenolate 1999–2009 | 8.42% | 7.64% | −0.78% | 0.8990 |
| Azathioprine 1990–1994* | 41.3% | 41.4% | 0.08% | 0.9977 |
| Azathioprine 1995–2009* | −77.9% | −87.5 | −9.7% | 0.1994 |
| mTOR Inhibitor 1990–1995 | −0.29% | 0.12% | 0.41% | 0.8545 |
| mTOR Inhibitor 1996–2001 | 14.4% | 14.6% | 0.15% | 0.9684 |
| mTOR Inhibitor 2002–2009 | −24.2% | −26.7% | −2.54% | 0.4527 |
| Corticosteroids 1990–2001 | −1.89% | −1.61% | 0.29% | 0.8133 |
| Corticosteroids 2002–2005 | −35.4% | −27.1% | 8.29% | 0.0853 |
| Corticosteroids 2006–2009 | 38.6% | 31.2% | −7.44% | 0.4489 |
slope is estimated in 5 year increments
log transformed slope difference
Table 1 displays the baseline sociodemographics, comorbidities, donor characteristics, immunologic risks and immunosuppression for the entire cohort, stratified by race. During the 20 year period, AAs were significantly younger, had a higher BMI, were less likely to have graduated college, were more likely to be receiving public insurance and more likely to have hypertension or diabetes. For donor characteristics, AAs were less likely to receive a living donor, but more likely to receive an expanded criteria donor (ECD), donor after circulatory death (DCD) and substantially more likely to receive an AA donor organ. AAs also had significantly higher immunologic risks, including more HLA mismatches, higher PRA levels above 20% and 80% and longer cold ischemic times. Finally, AAs were more likely to receive potent immunosuppression regimens, as compared to Caucasians, including cytolytic induction therapy, tacrolimus, mycophenolate and corticosteroids at discharge.
Table 1.
Baseline recipient sociodemographics, donor information and transplant characteristics for adult kidney transplant recipients transplanted between 1990 and 2009, stratified by recipient race
| Variable | Caucasian | African-American |
|---|---|---|
| Number of Patients | 144,081 (71%) | 58,004 (29%) |
|
| ||
| Median Age (IQR) | 48 (37, 58) | 47 (37, 56) |
|
| ||
| Female Gender | 39.1% | 40.9% |
|
| ||
| Median BMI (IQR) | 25.7 (22.6, 29.7) | 26.7 (23.2, 30.9) |
|
| ||
| Median Karnofsky Functional Status (IQR) | 100% (80, 100%) | 100% (80, 100%) |
|
| ||
| Some College or College Graduate | 52.3% | 43.1% |
|
| ||
| Primary Insurance - Medicare or Medicaid | 52.0% | 73.4% |
|
| ||
| Primary Diagnosis for ESRD | ||
| Hypertension | 12.3% | 40.0% |
| Diabetes | 21.8% | 20.0% |
| Other | 65.9% | 40.0% |
|
| ||
| Comorbidities | ||
| Hypertension | 82.5% | 91.3% |
| Diabetes | 31.7% | 32.6% |
| Angina | 11.9% | 8.1% |
| Cerebrovascular Accident | 2.5% | 2.5% |
| Peripheral Vascular Disease | 4.5% | 2.8% |
|
| ||
| Receiving Dialysis at Time of Transplant | 81.8% | 93.2% |
|
| ||
| Median Days on Wait List (IQR) | 295 (119, 647) | 553 (221, 1093) |
|
| ||
| Median Donor Age (IQR) | 40 (27, 50) | 37 (24, 49) |
|
| ||
| Donor Female Gender | 48.0% | 43.4% |
|
| ||
| Donor Race | ||
| Caucasian | 88.8% | 55.0% |
| African-American | 4.5% | 35.3% |
| Other | 6.7% | 9.7% |
|
| ||
| Living Donor | 41.3% | 21.6% |
|
| ||
| Expanded Criteria Donor | 10.3% | 12.2% |
|
| ||
| Donor after Cardiac Death | 2.6% | 4.3% |
|
| ||
| Median HLA Mismatches (IQR) | 3 (2–4) | 4 (3–5) |
| A Mismatches (IQR) | 1 (0–2) | 1 (1–2) |
| B Mismatches (IQR) | 1 (1–2) | 2 (1–2) |
| DR Mismatches (IQR) | 1 (0–1) | 1 (1–2) |
|
| ||
| Median Peak PRA (IQR) | 0% (0, 11%) | 0% (0, 21%) |
| Median Current PRA (IQR) | 0% (0, 4%) | 0% (0, 7%) |
| Current PRA >20% | 13.3% | 15.9% |
| Current PRA >80% | 4.1% | 4.9% |
|
| ||
| Median Cold Ischemic Time (hrs±SD) | 15.0 (2.0, 23.0) | 17.0 (10.0, 24.0) |
|
| ||
| Previous Kidney Transplant | 13.4% | 9.9% |
|
| ||
| Induction Therapy | ||
| IL-2 Receptor Antagonist | 20.1% | 18.8% |
| Cytolytic Therapy | 36.8% | 42.2% |
|
| ||
| Immunosuppression at Discharge | ||
| Tacrolimus | 44.3% | 51.1% |
| Cyclosporine | 48.5% | 41.7% |
| Mycophenolate | 60.7% | 64.9% |
| Azathioprine | 26.3% | 22.2% |
| mTOR Inhibitor | 6.5% | 6.7% |
| Corticosteroids | 82.7% | 84.1% |
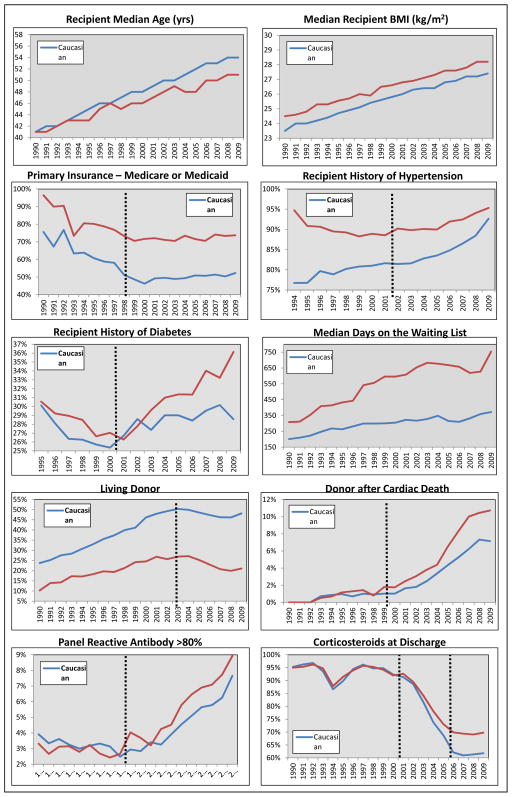
Over the 20 year study, there were significant evolutions in these variables, a number of which differed substantially by race. Recipient age in Caucasians significantly increased at a faster rate over time, as compared to AAs (3.54 vs. 2.69 years of age per 5 year study period, p<0.0001). Other trajectories that differed by race include female gender (faster increase in AAs), hypertension (increased faster in Caucasians from 1990–2002), diabetes (increased faster in AAs from 2001–2009), receiving dialysis at the time of transplant (decreased faster in Caucasians), waiting list time (increased faster in AAs), receiving an organ from a Caucasian donor (decreased faster in AAs), living donors (decreased more in AAs from 2003 to 2009), circulatory death donors (increased faster in AAs from 2000 to 2009), HLA Type A and B mismatches (increased faster in AAs), PRA >80% (increased faster in AAs from 1998 to 2009) and previous transplant (decreased faster in AAs from 1990–94, then increased in AAs from 1995–2009); see Table 2 for slope estimates, Figure 1 and Supplemental Figure 2 to visualize temporal trends and knots in slopes (dotted vertical lines).
Figure 1.
Annual trends in baseline variables for adult kidney transplant recipients transplanted between 1990 and 2009, stratified and compared based on recipient race. Dotted vertical lines represent knots in the trends over time.
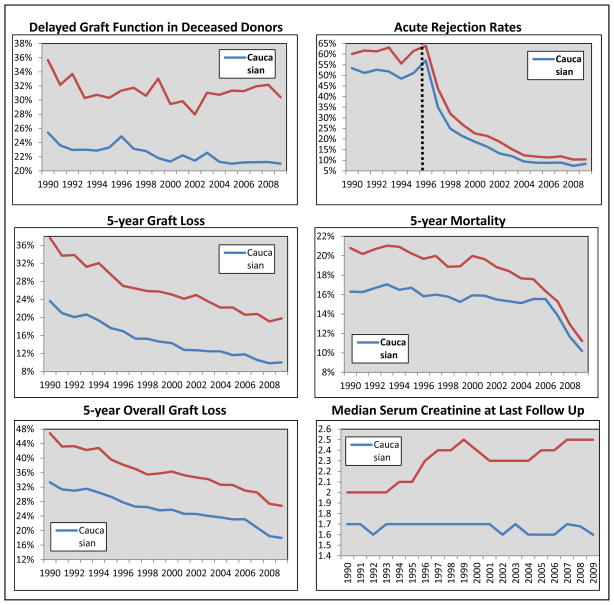
Post-transplant clinical outcomes compared by race are displayed in Table 3; the prevalence of all events were significantly higher in AA recipients (p<0.001). The trajectories of these events over time and compared by race are displayed in Table 4 and Figure 2. The rate of decline in delayed graft function was significantly faster in Caucasians; while graft loss, death and overall graft loss rates all declined at a faster rate in AAs. The rate of decline in 5-year graft loss rates was 0.92% faster in AAs, as compared to Caucasians (p=0.0066), while the rate in decline in 5-year mortality rates was 0.87% faster in AAs (p=0.0172). Post-transplant outcomes, stratified by donor type (living and deceased) and compared by race are presented in Supplementary Table 2. The estimated decrease in disparities for graft loss and death were all significantly higher in magnitude within deceased donor recipients, although all estimates were statistically significant in both living and deceased donor recipients.
Table 3.
Graft and patient outcomes for adult kidney transplant recipients transplanted between 1990 and 2009, stratified by race
| Outcome | Caucasian | African American |
|---|---|---|
|
| ||
| Delayed Graft Function | ||
| Overall | 15.0% | 25.8% |
| Deceased Donor | 22.4% | 31.2% |
| Living Donor | 4.4% | 6.4% |
|
| ||
| Acute Rejection | ||
| 6 month | 15.0% | 16.2% |
| 1 year | 15.9% | 17.4% |
| Overall | 25.5% | 28.8% |
|
| ||
| Graft Loss | ||
| 1 year | 5.60% | 9.96% |
| 3 year | 10.10% | 17.61% |
| 5 year | 14.68% | 25.07% |
|
| ||
| Death | ||
| 1 year | 3.96% | 4.84% |
| 3 year | 8.93% | 10.85% |
| 5 year | 15.17% | 18.28% |
|
| ||
| Overall Graft Loss | ||
| 1 year | 8.84% | 12.72% |
| 3 year | 16.85% | 23.77% |
| 5 year | 25.52% | 35.16% |
|
| ||
| Median SrCr at Last Follow Up (mg/dL [IQR]) | 1.7 (1.2–2.7) | 2.3 (1.5–4.5) |
Table 4.
Five-year trajectories of clinical outcomes for adult kidney transplant recipients transplanted between 1990 and 2009, stratified and compared by recipient race
| Post-Transplant Outcome | Slope in Caucasians⇂ | Slope in African Americans⇂ | Difference in Slopes by Race⇂ | p-value for difference in slopes |
|---|---|---|---|---|
| Delayed Graft Function | −2.06% | −1.22% | 0.85% | 0.0433 |
|
| ||||
| Acute Rejection 1990–1996* | 20.5% | 22.7% | 2.19% | 0.9215 |
| Acute Rejection 1997–2009* | −71.16% | −67.6% | 3.61% | 0.6813 |
|
| ||||
| Graft Loss | ||||
| 1 year | −2.09% | −3.16% | −1.07% | 0.0035 |
| 3 year | −2.80% | −3.72% | −0.93% | 0.0401 |
| 5 year | −3.34% | −4.26% | −0.92% | 0.0066 |
|
| ||||
| Death | ||||
| 1 year | −0.39% | −0.67% | −0.28% | 0.0014 |
| 3 year | −0.52% | −1.05% | −0.54% | 0.0002 |
| 5 year | −1.08% | −1.95% | −0.87% | 0.0172 |
|
| ||||
| Overall Graft Loss | ||||
| 1 year | −2.23% | −3.16% | −0.93% | 0.0053 |
| 3 year | −2.84% | −3.82% | −0.98% | 0.0046 |
| 5 year | −3.56% | −4.52% | −0.96% | 0.0018 |
|
| ||||
| Median Last SrCr (mg/dL) | −0.01 | 0.14 | 0.14 | <0.0001 |
slope is estimated in 5 year increments
log transformed slope difference
Figure 2.
Annual trends in post-transplant clinical outcomes for adult kidney transplant recipients transplanted between 1990 and 2009, stratified and compared based on recipient race. Dotted vertical lines represent knots in the trends over time.
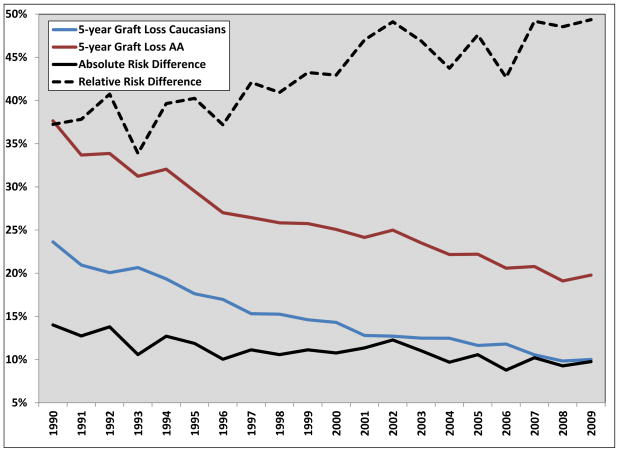
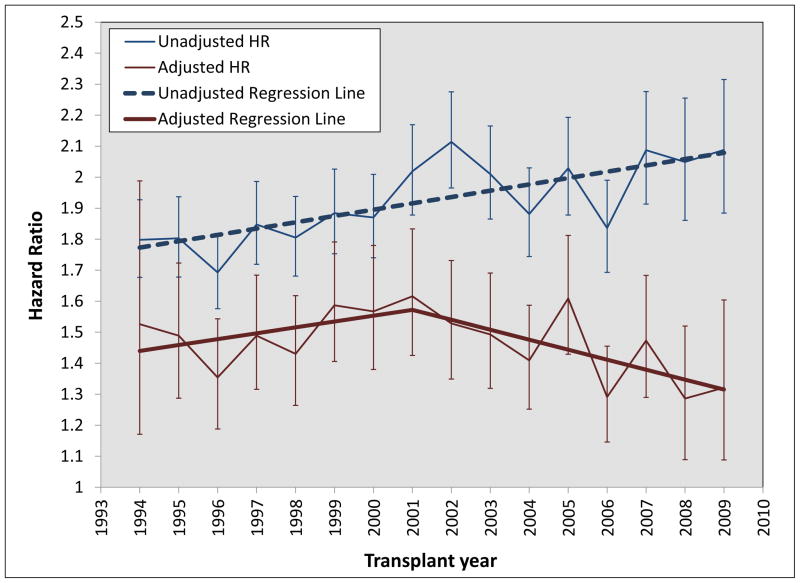
The absolute risk difference between AAs and Caucasians for 5 year graft loss significantly declined over time (−0.92% decrease per 5 years, p<0.001, see Figure 3), while the relative risk difference increased (3.4% increase per 5 years, p<0.001, see Figure 3). Figure 4 displays the unadjusted and adjusted hazard-ratios for AA race (Caucasians are the referent group) for graft loss between 1994 and 2009. The unadjusted hazard-ratios for AAs significantly increased during the entire time period (0.10 increase in hazard-ratio per 5 years, p=0.0008), while the adjusted hazard-ratios increased from 1994–2001 (0.26 increase per 5 years, p=0.0165) then reversed and decreased from 2002–2009 (0.16 decrease per 5 years, p=0.0052).
Figure 3.
Annual five-year death-censored graft loss rates and absolute and relative risk differences for adult kidney transplant recipients transplanted between 1990 and 2009, compared based on recipient race
Figure 4.
Annual trends in unadjusted and adjusted hazard ratios in African-Americans for the outcome of graft loss (death as a competing risk event) in adult kidney transplant recipients transplanted between 1940 and 2009
DISCUSSION
This study provides a detailed assessment of 20 years of evolving trends in racial disparities in kidney transplantation, demonstrating a number of interesting findings. Most importantly, although there is still a long way to go to achieve equity, the disparity in AAs for graft loss has significantly improved over the past 20 years, with an absolute risk difference decrease of 0.9% per 5 years, when compared to Caucasians. Yet, the relative risk difference between AAs and Caucasians has actually increased (3.4% increase per 5 year time period). Thus, depending on your viewpoint, the conclusions with regards to trends in racial disparities over time in kidney transplantation are paradoxical. Further, when the risk of graft loss in AAs is fully adjusted using explanatory variables, the hazard-ratios after 2001 significantly decrease (−0.16 per 5 years), suggesting more of the inherent risk in AA recipients is captured in measured covariates during recent transplant years. These results, in terms of graft loss disparities, are similar to those reported in a recently published analysis.13
With regards to other post-transplant outcomes, these results present a mixed message of both significant improvements and areas of concern. Five year graft loss and mortality rates have decreased significantly faster in AAs, as compared to Caucasians (~0.9% difference per 5 years, p<0.02). Additionally, acute rejection rates have dramatically decreased since 1996 (~70% per 5 years), which was similar in magnitude in AAs and Caucasians. However, the difference in delayed graft function rates between AA and Caucasians has widened over time, which is a strong risk factor for acute rejection and graft loss.28,29 This is a concerning trend, and one that may be a function of donor type.28,30 Between 2003 and 2009, the disparity between AAs and Caucasians in living donor rates has also significantly widened, and this likely represents an important disparities driving outcome differences.31 Further, AA recipients had an increased utilization of circulatory death donors between 2000 and 2009, likely a compensatory effect of the decrease in living donor rates. While this probably contributed to the increase in transplant rates within AAs, it may also be influencing post-transplant outcomes, including delayed graft function and graft function.28,30 These results demonstrate that living donation rates have decreased recently in the U.S.; while other international data indicates continued increased rates across the EU and Scandinavia. However, living donation rates per million are still very high in the U.S, only surpassed by the Netherlands, U.K., Turkey, Iceland and Macedonia.32 It is clear from this data that future interventions in racial disparities need to focus on improving living donation rates, particularly within the AA community.
At the time of transplant, there were a number of interesting trends in baseline variables that significantly differed by race over time. Between 2001 and 2009, the proportion of AAs that were females and those that had pre-existing diabetes significantly increased, as did time on the waiting list; conversely, the number of preemptive transplants decreased more so in AAs. Some of these issues are likely to be related, as it is well known that AAs are referred for transplant evaluation later in their course, usually after chronic renal replacement therapy has already been initiated. Thus, earlier referral and listing, coupled with more living donors in AAs will likely improve these growing disparities.33
It is interesting that the proportion of AA donors increased within AA recipients, as compared to Caucasians (with a statistically significant compensatory decrease in Caucasian donors in AAs). This is likely an unintentional effect of significant changes in U.S. organ allocation policies that occurred during this time period. AAs have markedly different HLA polymorphisms, and thus, are less likely to have unacceptable HLA antibodies (negative virtual cross-match) with AA donors. This trend is further exaggerated by the increasing rates of HLA mismatches and PRA levels that are seen in AAs over this time period.34,35 There may be significant clinical implications of this, as it relates to racial disparities. Recent data demonstrates that APOL1 gene variants, which are only present in those of African ancestry, are strongly associated with increased risk of graft loss.26 Thus, as the proportion of AA donors allocated to AA recipients has grown, so has the potential impact of the APOL1 gene variant on graft outcomes and disparity gaps. There were major changes made to the U.S. organ allocation policy for kidneys in December of 2014. It will be interesting to determine what impact these have on donor race trends. Without widespread donor genotyping for APOL1, we can only speculate the impact these trends have on graft outcomes and racial disparities.36
The use of potent immunosuppressive regimens, which includes cytolytic induction therapy, tacrolimus and mycophenolate, dramatically increased during this study period for the entire cohort, particularly since the mid to late 1990s. Concurrently, there was a compensatory drop in acute rejection rates, starting in 1996. Overall, this occurred at a similar rate in both AAs and Caucasians; but in the most recent years (2006–2009), the absolute difference in rejection rate was only 1.7% between AAs and Caucasians (vs. 4.7% in years prior). This decrease in disparities for acute rejection is likely due to the known immunologic risk factors common in AA recipients, including HLA mismatches, higher PRAs, immune hyper-responsiveness and genetic polymorphisms in cytokine production and immunosuppressant metabolism.37, 38 Steroid utilization at discharge substantially decreased between 2002 and 2005, with a levelling off starting in 2006. Yet, steroids continue to be used more often in AA recipients. This is perhaps because there continues to be controversy regarding the safety of steroid withdrawal in AA recipients.39–41
Globally, outside of the U.S., there is controversy whether disparities in outcomes for those of African descent are of significant magnitude. Large studies from both France and Canada failed to demonstrate a significant difference in outcomes between those of African descent, as compared to those of European ancestry.42,43 Both countries have universal healthcare access and there is conjecture that this is a major factor driving U.S. disparities. However, there are significant disparities in those of African descent within both the U.K. and Brazil, which have universal healthcare access.44,45 There are likely other more complex issues driving these differences in outcomes as it relates to racial disparities across global regions. Migratory patterns and etiologies for migration are vastly different based on country of destination. Thus, the constitutions of those of African descent substantially differ across the U.S., Canada, Brazil, France and the U.K. Comorbidities, such as hypertension and diabetes are significantly more common in AAs and those of African descent in Brazil. Gene variants associated with outcomes, including APOL1, cytochrome P450, and MDR1 also likely differ across these heterogeneous populations. Healthcare access and socio, cultural and economic disparities also differ substantially across these global regions. Thus, the reasons for differences in racial disparities across the Americas and Europe are likely a complex convergence of different populations, socioeconomics and culture.46 The results from this analysis demonstrate that the risk factors likely driving these disparities do evolve over time, with improvements juxtaposed with increasing challenges. However, there is reason to believe that achieving equity in outcomes across racial groups can be achieved by intensely focusing on these evolving trends.
Although the results of this analysis provide considerable insights into the trends in racial disparities, this study does have several significant limitations. This was a retrospective analysis that relied on registry data input by transplant programs without detailed validation. Additionally, the level of missingness and definitions of a number of variables evolved over time.
Although data were available back to 1987, we chose to start the analysis in 1990 because of missingness issues (with Cox regression adjusted models starting in 1994 for this issue). The number of patients with missing data decreased over time and the definitions of important variables, included diabetes, panel reactive antibody, functional status and expanded criteria donor changed as well. Also, using regressed slopes to define and compare trends assumes linearity, and clearly, a number of these trends were not linear across the entire 20 year period. To account for this, we utilized spline and knot analysis, which improved model fit (see Supplemental Table 1). Because of these issues and the inherent flaws embedded within retrospective analyses, including being prone to residual confounding and misclassification of variables that have evolved over time, these results should not be misconstrued as cause and effect. Rather, they provide associations that can be utilized to guide future prospective observational and interventional studies and allow the transplant community to focus on the pertinent mutable variables that are likely driving racial disparities in the contemporary era of kidney transplantation.
In conclusion, although equity is still far off, over the past 20 years, there has been a significant improvement in racial disparities in graft loss rates for AA kidney transplant recipients. However, there are a number of concerning trends for AA recipients, as compared to Caucasians, including a decrease in living donation rates, coupled with higher rates of delayed graft function, diabetes, longer waiting times, and less preemptive transplants. Disparity research endeavors should focus on reducing these growing differences or mitigating their influence on outcomes, as a mechanism to move closer to equitable outcomes.
METHODS
Study design
This was an analysis of the United Network of Organ Sharing (UNOS) Transplant Registry database, which was implemented in 1987 to track baseline and follow up data for all patients awaiting and undergoing solid organ transplants within the United States. These data were merged with the Social Security Death Master File (SSDMF) to obtain accurate patient death dates. After local IRB approval and a data use agreement (DUA) with UNOS, we obtained national Standard Transplant Analysis and Research (STAR) de-identified datasets, which were pre-linked to the SSDMF data. This study focused on patients transplanted between January 1, 1990 and December 31, 2009 (20 years), with follow up through December 31, 2014. This time period was chosen because of the large amount of missing data prior to 1990 and to ensure all patients had at least 5 years of potential follow up. Inclusion criteria were adult (≥18 years of age at the time of transplant) recipients of solitary kidney transplants. Those that were not either AA or Caucasian were excluded for ease of comparison and reporting of results.
Primary Outcome
The primary outcome for this study was graft loss, with death analyzed as a competing risk event, which was defined as a composite of returning to chronic dialysis or undergoing preemptive retransplant. We also assessed mortality and overall graft loss, which was defined as a composite of either graft loss or death.
Risk Factors
We assessed for a large number of risk factors that have been previously identified as potential explanatory variables for racial disparities in kidney transplantation. These included recipient sociodemographics (age, gender, body mass index [BMI], functional status, education and insurance), recipient comorbidities (reason for ESRD, cardiovascular disease [CVD] comorbid conditions and time on waitlist), donor characteristics (age, gender, race, and donor type), transplant characteristics/immunologic risks (HLA mismatches, PRA, cold ischemic time, previous kidney transplant) and finally, baseline immunosuppression (induction and maintenance therapy). The definitions of these risk factors can be found in the Supplemental Methodology.
Statistical Analysis
First, we assessed baseline variables aggregated for the 20-year cohort and compared by recipient race (Caucasian vs. AA). This was done using standard descriptive and univariate statistics. Continuous variables are reported as medians with interquartile ranges (IQR), with comparisons made using the Mann Whitney U test. Categorical variables are presented as percentages with comparisons made using the Chi square test. One, three, and five year event rates for graft loss, death and overall graft loss were analyzed using Cox regression to estimate survival and cumulative incidence functions, with death events accounted for as a competing risk in the graft loss models (Fine and Gray method).47 Repeated patients were accounted for within a given year through the use of a marginal model (COVS(aggregate) option).
Next, we assessed the temporal trends in these variables by transplant year and whether these differed by race. This was done by plotting the frequency or median of the variable on the y-axis and the transplant year on the x-axis, stratified by race. We utilized linear regression for frequencies and quantile regression for medians (PROC QUANTREG) to estimate the slope change per year; visualization of the data was used in conjunction with formal spline and knot analysis to determine if significant changes in the direction or magnitude of slopes were present (PROC TRANSREG and PBSSPLINE). If so, dummy terms were created from the time variable to account for the knots; goodness of fit (R2) was used to assess optimal knots and data transformations through comparison of iterative models. Interaction terms (race*time) were utilized to assess if the variable slope significant differed by race over time. The number of knots and transformations utilized for each variable can be found in Supplementary Table 1. For non-categorical variables, we conducted analyses using both means and medians and linear and quantile regression; based on model fit and variable distribution, chose to report medians with quantile regression in the final results. Results using means were comparable, with differences noted for functional status, BMI, PRA, HLA subtypes and cold time.
Finally, we determined if the absolute and relative risk of 5-year graft loss by recipient race (Caucasian set as referent group) changed over time. We calculated the graft loss relative risk difference (AA − Caucasian / AA), absolute risk difference (AA − Caucasian), unadjusted and adjusted hazard-ratios for each transplant year and assessed the temporal trends in these using linear regression with spline analysis (same methodology detailed above). To estimate the hazard-ratios for each year, we utilized Cox regression, with death treated as a competing risk (Fine and Gray method).47 Repeated patients were accounted for within a given year through the use of a marginal model; with retransplant entered as a covariate to adjust for between years retransplant status. The following were included in the model for adjustment: delayed graft function, acute rejection, re-transplant, primary insurance, recipient gender, recipient BMI, education, diabetes, hypertension, pre-emptive transplant, cold ischemic time, HLA mismatches, PRA, living donor, ECD, DCD, induction therapy, tacrolimus, cyclosporine, mycophenolate, azathioprine, mTOR inhibitors and steroids. Statistical significance was based on a two-sided p-value of less than 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Supplementary Material
Supplementary Table 1 – List of variables included in the analysis with the time periods used for calculation of the regressed slope and whether the data was log-transformed
Supplemental Table 2 - Five-year trajectories of clinical outcomes for adult kidney transplant recipients transplanted between 1990 and 2009, stratified by recipient race and donor type
Supplemental Figure 1 – Study cohort flowchart displaying the method in which the study population was created, with specifics regarding the number and reasons for exclusions and annual transplant numbers stratified by recipient race
Supplemental Figure 2 – Additional charts displaying annual trends in baseline variables not in Figure 1 in adult kidney transplant recipients transplanted between 1990 and 2009. Dotted vertical lines represents knots in slopes.
Acknowledgments
Source of Support: Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K23DK099440. Running Headline: Trends in racial disparities in kidney transplantation
Footnotes
Disclosure
The authors have no conflicts of interest to disclose as it relates to the content of this manuscript.
References
- 1.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The third national health and nutrition examination survey, 1988–1994. Diabetes Care. 1998;21(4):518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 2.Kato N. Ethnic differences in genetic predisposition to hypertension. Hypertension Research. 2012;35(6):574–581. doi: 10.1038/hr.2012.44. [DOI] [PubMed] [Google Scholar]
- 3.Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: The multi-ethnic study of atherosclerosis. Hypertension. 2011;57(6):1101–1107. doi: 10.1161/HYPERTENSIONAHA.110.168005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. J Am Med Assoc. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 5.U S Renal Data System. USRDS 2011 annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2011. [Google Scholar]
- 6.Griva K, Davenport A, Harrison M, Newman SP. The impact of treatment transitions between dialysis and transplantation on illness cognitions and quality of life–A prospective study. British Journal of Health Psychology. 2012;17(4):812–27. doi: 10.1111/j.2044-8287.2012.02076.x. [DOI] [PubMed] [Google Scholar]
- 7.Landreneau K, Lee K, Landreneau MD. Quality of life in patients undergoing hemodialysis and renal transplantation--a meta-analytic review. Nephrol Nurs J. 2010;37(1):37–44. [PubMed] [Google Scholar]
- 8.Rana A, Gruessner A, Agopian VG, et al. Survival benefit of solid-organ transplant in the united states. JAMA Surgery. 2015;150(3):252–259. doi: 10.1001/jamasurg.2014.2038. [DOI] [PubMed] [Google Scholar]
- 9.Tonelli M, Wiebe N, Knoll G, et al. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 10.Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973;5(1):253–259. [PubMed] [Google Scholar]
- 11.Matas A, Smith J, Skeans M, et al. OPTN/SRTR 2012 annual data report: Kidney. Am J Transplant. 2014;14(S1):11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 12.Opelz G, Mickey MR, Terasaki PI. Influence of race on kidney transplant survival. Transplant Proc. 1977;9(1):137–142. [PubMed] [Google Scholar]
- 13.Purnell TS, Luo X, Kucirka LM, Cooper LA, Crews DC, et al. Reduced racial disparity in kidney transplant outcomes in the United States from 1990 to 2012. J Am Soc Nephrol. 2016 doi: 10.1681/ASN.2015030293. epub ahead of print Feb 4, 2016. [DOI] [PMC free article] [PubMed]
- 14.Ciancio G, Burke GW, Suzart K, et al. The use of daclizumab, tacrolimus and mycophenolate mofetil in African-American and hispanic first renal transplant recipients. Am J Transplant. 2003;3(8):1010–1016. doi: 10.1034/j.1600-6143.2003.00181.x. [DOI] [PubMed] [Google Scholar]
- 15.Neylan JF. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. FK506 kidney transplant study group. Transplantation. 1998;65(4):515–523. doi: 10.1097/00007890-199802270-00011. [DOI] [PubMed] [Google Scholar]
- 16.Podder H, Podbielski J, Hussein I, Katz S, Buren C, Kahan BD. Sirolimus improves the two-year outcome of renal allografts in African-American patients. Transplant Int. 2001;14(3):135–142. doi: 10.1007/s001470100315. [DOI] [PubMed] [Google Scholar]
- 17.Butkus DE, Meydrech EF, Raju SS. Racial differences in the survival of cadaveric renal allografts. overriding effects of HLA matching and socioeconomic factors. N Engl J Med. 1992;327(12):840–845. doi: 10.1056/NEJM199209173271203. [DOI] [PubMed] [Google Scholar]
- 18.Curtis JJ. Kidney transplantation: Racial or socioeconomic disparities? Am J Kidney Dis. 1999;34(4):756–758. doi: 10.1016/S0272-6386(99)70404-X. [DOI] [PubMed] [Google Scholar]
- 19.Kalil R, Heim-Duthoy K, Kasiske B. Patients with a low income have reduced renal allograft survival. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1992;20(1):63. doi: 10.1016/s0272-6386(12)80318-0. [DOI] [PubMed] [Google Scholar]
- 20.Schweizer RT, Rovelli M, Palmeri D, Vossler E, Hull D, Bartus S. Noncompliance in organ transplant recipients. Transplantation. 1990;49(2):374–377. doi: 10.1097/00007890-199002000-00029. [DOI] [PubMed] [Google Scholar]
- 21.Young CJ, Kew C. Health disparities in transplantation: Focus on the complexity and challenge of renal transplantation in African Americans. Med Clin N Am. 2005;89:1003–1031. doi: 10.1016/j.mcna.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Cosio FG, Dillon JJ, Falkenhain ME, et al. Racial differences in renal allograft survival: The role of systemic hypertension. Kidney Int. 1995;47(4):1136–1141. doi: 10.1038/ki.1995.162. [DOI] [PubMed] [Google Scholar]
- 23.Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation: IV. impact of post-transplant diabetes. Kidney Int. 2002;62(4):1440–1446. doi: 10.1111/j.1523-1755.2002.kid582.x. [DOI] [PubMed] [Google Scholar]
- 24.Cosio FG, Hickson LJ, Griffin MD, Stegall MD, Kudva Y. Patient survival and cardiovascular risk after kidney transplantation: The challenge of diabetes. Am J Transplant. 2008;8(3):593–599. doi: 10.1111/j.1600-6143.2007.02101.x. [DOI] [PubMed] [Google Scholar]
- 25.Taber DJ, Meadows HB, Pilch NA, Egede LE. The impact of diabetes on ethnic disparities in kidney transplantation. Ethnicity and Disease. 2013;23:238–44. [PubMed] [Google Scholar]
- 26.Freedman BI, Julian BA, Pastan SO, Israni AK, Schladt D, et al. Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. Am J Transplant. 2015;15:1615–22. doi: 10.1111/ajt.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young CJ, Gaston RS. African Americans and renal transplantation: Disproportionate need, limited access, and impaired outcomes. Am J Med Sci. 2002;323(2):94–9. doi: 10.1097/00000441-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Tansplant. 2011;11(11):2279–2296. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yarlagadda SG, Coca SG, Formica RN, Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24(3):1039–1047. doi: 10.1093/ndt/gfn667. [DOI] [PubMed] [Google Scholar]
- 30.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Tansplant. 2011;11(11):2279–2296. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gore JL, Danovitch G, Litwin M, Pham P, Singer JS. Disparities in the utilization of live donor renal transplantation. Am J Transplant. 2009;9(5):1124–1133. doi: 10.1111/j.1600-6143.2009.02620.x. [DOI] [PubMed] [Google Scholar]
- 32.Anonymous. [Accessed May 14, 2016];2014 Nov; http://ec.europa.eu/health/blood_tissues_organs/docs/ev_20141126_factsfigures_en.pdf.
- 33.Patzer RE, Plantinga LC, Paul S, et al. Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. J Am Med Assoc. 2015;314(6):582–594. doi: 10.1001/jama.2015.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts JP, Wolfe RA, Bragg-Gresham JL, Rush SH, Wynn JJ, et al. Effect of changing the priority of HLA matching on the rates and outcomes of kidney transplantation in minority groups. New Eng J Med. 2004;350:545–51. doi: 10.1056/NEJMoa025056. [DOI] [PubMed] [Google Scholar]
- 35.Baxter-Lowe LA. [Accessed May 16, 2016];2014 Oct; http://2014.ashi-hla.org/sites/default/files/docs/2014/Baxter-Lowe2.pdf.
- 36.Stewart DE, Kucheryavaya AY, Klassen KD, Turgeon NA, Formica RN, Aeder MI. Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant. 2016 doi: 10.1111/ajt.13770. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Padiyar A, Hricik DE. Immune factors influencing ethnic disparities in kidney transplantation outcomes. Expert Review of Clinical Immunology. 2011;7(6):769–778. doi: 10.1586/eci.11.32. [DOI] [PubMed] [Google Scholar]
- 38.Malat GE, Culkin C, Palya A, Ranganna K, Kumar MS. African American kidney transplantation survival: The ability of immunosuppression to balance the inherent pre- and post-transplant risk factors. Drugs. 2009;69(15):2045–2062. doi: 10.2165/11318570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Hricik DE, Knauss TC, Bodziak KA, et al. Withdrawal of steroid therapy in African American kidney transplant recipients receiving sirolimus and tacrolimus. Transplantation. 2003;76(6):938–942. doi: 10.1097/01.TP.0000089440.47239.3F. [DOI] [PubMed] [Google Scholar]
- 40.Hricik DE, Augustine JJ, Knauss TC, et al. Long-term graft outcomes after steroid withdrawal in African American kidney transplant recipients receiving sirolimus and tacrolimus. Transplantation. 2007;83(3):277–281. doi: 10.1097/01.tp.0000251652.42434.57. [DOI] [PubMed] [Google Scholar]
- 41.Kumar M, Khan S, Ranganna K, Malat G, Sustento-Reodica N, Meyers W. Long-Term outcome of early steroid withdrawal after kidney transplantation in African American recipients monitored by surveillance biopsy. Am J Transplant. 2008;8(3):574–585. doi: 10.1111/j.1600-6143.2007.02099.x. [DOI] [PubMed] [Google Scholar]
- 42.Pallet N, Thervet E, Alberti C, Emal-Algae V, Bedrossian J, et al. Kidney transplant in black recipients: are African Europeans different from African Americans? Am J Transplant. 2005;5:2682–7. doi: 10.1111/j.1600-6143.2005.01057.x. [DOI] [PubMed] [Google Scholar]
- 43.Yeates K, Wiebe N, Gill J, Sima C, Schaubel D, et al. Similar outcomes among Black and White renal allograft recipients. J Am Soc Nephrol. 2009;20:172–9. doi: 10.1681/ASN.2007070820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medina-Pestana JO. Clinical Transplants 2010. Chapter 9: Renal transplant – Sao Paulo, Brazil. Terasaki Foundation Laboratory; Los Angeles, CA: pp. 107–26. [PubMed] [Google Scholar]
- 45.Medcalf JF, Andrews PA, Bankart J, Bradley C, Carr S, et al. Poorer graft survival in ethnic minorities: results from a multi-centre UK study of kidney transplant outcomes. Clin Nephrol. 2044;75:294–301. doi: 10.5414/cn106675. [DOI] [PubMed] [Google Scholar]
- 46.Malek SK, Keys BJ, Kumar S, Milford E, Tullius SG. Racial and ethnic disparities in kidney transplantation. Transplant Int. 2011;24:419–24. doi: 10.1111/j.1432-2277.2010.01205.x. [DOI] [PubMed] [Google Scholar]
- 47.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 – List of variables included in the analysis with the time periods used for calculation of the regressed slope and whether the data was log-transformed
Supplemental Table 2 - Five-year trajectories of clinical outcomes for adult kidney transplant recipients transplanted between 1990 and 2009, stratified by recipient race and donor type
Supplemental Figure 1 – Study cohort flowchart displaying the method in which the study population was created, with specifics regarding the number and reasons for exclusions and annual transplant numbers stratified by recipient race
Supplemental Figure 2 – Additional charts displaying annual trends in baseline variables not in Figure 1 in adult kidney transplant recipients transplanted between 1990 and 2009. Dotted vertical lines represents knots in slopes.