Abstract
Diastolic calcium (Ca) leak via cardiac ryanodine receptors (RyR2) can cause arrhythmias and heart failure (HF). Ca/calmodulin (CaM)-dependent kinase II (CaMKII) is upregulated and more active in HF, promoting RyR2-mediated Ca leak by RyR2-Ser2814 phosphorylation. Here, we tested a mechanistic hypothesis that RyR2 phosphorylation by CaMKII increases Ca leak by promoting a pathological RyR2 conformation with reduced CaM affinity. Acute CaMKII activation in wild-type RyR2, and phosphomimetic RyR2-S2814D (vs. non-phosphorylatable RyR2-S2814A) knock-in mouse myocytes increased SR Ca leak, reduced CaM-RyR2 affinity, and caused a pathological shift in RyR2 conformation (detected via increased access of the RyR2 structural peptide DPc10). This same trio of effects was seen in myocytes from rabbits with pressure/ volume-overload induced HF. Excess CaM quieted leak and restored control conformation, consistent with negative allosteric coupling between CaM affinity and DPc10 accessible conformation. Dantrolene (DAN) also restored CaM affinity, reduced DPc10 access, and suppressed RyR2-mediated Ca leak and ventricular tachycardia in RyR2-S2814D mice. We propose that a common pathological RyR2 conformational state (low CaM affinity, high DPc10 access, and elevated leak) may be caused by CaMKII-dependent phosphorylation, oxidation, and HF. Moreover, DAN (or excess CaM) can shift this pathological gating state back to the normal physiological conformation, a potentially important therapeutic approach.
Keywords: ryanodine receptor, calcium/calmodulin-dependent protein kinase II, calmodulin, dantrolene, fluorescence resonance energy transfer
1. Introduction
Heart failure (HF) and sudden cardiac death are leading causes of mortality worldwide. Almost half of HF patients die of sudden cardiac death due to lethal ventricular arrhythmias [1]. Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited stress-induced arrhythmogenic disease caused by mutations in cardiac ryanodine receptor (RyR2), calsequestrin 2 gene, or calmodulin (CaM) genes [2-4]. Recent research showed that diastolic Ca2+ (Ca) leak from the sarcoplasmic reticulum (SR), via dysfunctional RyR2, is important in the pathogenesis of HF and lethal arrhythmias such as CPVT [5-8]. This diastolic RyR2 leak in HF leads to systolic and diastolic dysfunction, and RyR2-mediated SR Ca leak activates inward Na/Ca exchange current to evoke delayed afterdepolarizations and arrhythmias [9].
In the heart, Ca/CaM-dependent protein kinase II (CaMKII) phosphorylates RyR2 at S2814, which increases RyR2-mediated SR Ca leak [10] and delayed afterdepolarizations [9]. CaMKII expression, activated state, and phosphorylation of RyR2 at S2814 are increased in HF [5]. CaMKII inhibition can also quiet SR Ca leak and arrhythmogenic events in HF [5, 11, 12]. Thus CaMKII may play a critical role in increased SR Ca leak in HF and lethal arrhythmias, but how S2814 phosphorylation alters RyR2 molecular conformation is unknown. The normal physiological role of CaMKII may be to fine-tune RyR Ca sensitivity during excitation-contraction coupling [13], and likewise for Ca-ATPase and Cav1.2 current. However, HF, reactive oxygen species (ROS), and hyperglycemia all promote chronic (autonomous) CaMKII activity that can mediate numerous adverse effects [14-16].
We have used the binding of fluorescent DPc10 peptide (F-DPc10; RyR2 residues 2460-2495) as a RyR2 conformational probe [17, 18]. DPc10 is in a RyR2 CPVT mutation hot-spot and may interfere with (or unzip) normal domain-domain interactions and promote pathological SR Ca leak like in CPVT and HF [7, 8]. Moreover, re-zipping of these domains by dantrolene (DAN) can stabilize both skeletal RyR (RyR1) in malignant hyperthermia and RyR2 in HF [19] and CPVT [8]. CaM binding to RyR2 is reduced in HF [5, 20, 21], CPVT [22], and by oxidation [18]. Oxidation can also directly promote RyR2 opening [18, 23] and CaMKII activation [15], and is involved in RyR2 dysfunction in HF and arrhythmias [24, 25].
CaM is a ubiquitous Ca-binding protein that modulates gating of RyR2, L-type Ca, Na+, and K+ channels, and activates CaMKII. CaM binds to RyR2 (involving RyR2 residues 3583-3603) with a Kd of 10-20 nM in myocytes [21] and is known to quiet RyR2 opening [21, 26, 27]. Ca/CaM also activates CaMKII by binding to the CaMKII regulatory domain with a Kd of 10-70 nM [28, 29]. Recent human CaM mutations are associated with long QT syndrome [30] and CPVT [3], and the CPVT CaM variants cause arrhythmogenic SR Ca leak by binding to RyR2 more tightly than wild-type (WT) CaM, and either activating or failing to quiet RyR2 gating [31]. We recently found that conformational unzipping within RyR2 reduces CaM-RyR2 affinity in canine and rat HF models [20, 21] and a CPVT mouse model (R2474S/+) [22]. Moreover, enhanced CaM-RyR2 binding can correct contractile dysfunction in disease models [32]. We also showed negative allosteric coupling between CaM affinity and binding access to DPc10 [17], where CaM binding reduces DPc10 access and SR Ca leak, and DPc10 reduces CaM affinity and promotes Ca leak. Thus, CaM can influence SR Ca leak in HF, independent of CaMKII.
Here we test whether CaMKII-dependent RyR2 phosphorylation and HF might both increase SR Ca leak by altering both CaM affinity and RyR2 conformation, as assessed by F-DPc10 access. We measure RyR2-bound CaM in saponin-permeabilized native cardiomyocytes using fluorescence resonance energy transfer (FRET) between fluorescent FK506-binding protein 12.6 (F-FKBP) and CaM (F-CaM) and assess RyR2 conformation by F-DPc10 access [17, 18, 21, 33]. We also use ventricular myocytes from knock-in mice expressing CaMKII phosphomimetic RyR2-S2814D (S2814D) [34] or non-phosphorylatable RyR2-S2814A (S2814A) [35], and also HF myocytes from a rabbit non-ischemic HF model, in which CaMKII activity is elevated, RyR2S2814 is phosphorylated, and is known to enhance diastolic SR Ca leak [5]. We conclude that CaMKII-dependent RyR2 phosphorylation and HF cause a common shift in RyR2 conformation that has increased leak and arrhythmogenesis and reduced CaM binding affinity, but that DAN can reverse these effects.
2. Materials and methods
An expanded Materials and Methods section can be found in the Online Data Supplement.
3. Results
3.1 Endogenous CaMKII activation decreases F-CaM binding to RyR2
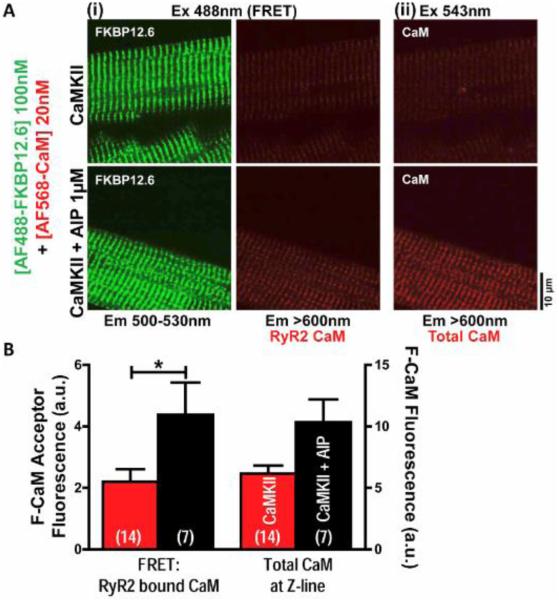
We previously showed that endogenous CaMKII can phosphorylate and activate RyR2 and promote Ca leak [26], and used that approach here to measure CaM-RyR2 binding after CaMKII activation in WT myocytes. Notably, under these conditions basal CaMKII-dependent RyR2-S2814 phosphorylation and spark activity was negligible (no different from negative controls that were pretreated with the selective CaMKII inhibitor, autocamtide-2 related inhibitory peptide [AIP]) [26]. Permeabilized myocytes were exposed for 1 min to 500 nM [Ca]i plus 1 μM exogenous CaM, to activate endogenous CaMKII and RyR2 phosphorylation [26]. FRET between donor-F-FKBP and acceptor-F-CaM was then measured to assess CaM-RyR2 binding after activation of endogenous CaMKII, with or without selective CaMKII block by 1 μM AIP (Fig. 1). After endogenous CaMKII activation, subsequent wash-off of exogenous CaM, and restoration of low [Ca]i (for 3 min), we washed in 100 nM F-FKBP (to saturate RyR2) and 20 nM F-CaM (near the Kd to optimize detection of binding changes). Fig. 1A-B show that when CaMKII was active, CaM binding to RyR2 was depressed. Thus CaMKII activation reduces CaM binding to RyR2.
Fig. 1.
CaMKII activation decreases CaM binding in permeabilized wild-type mouse myocytes. A, Confocal images of effect of AIP on fluorescent CaM (F-CaM) binding at Z-line (ii, Ex 543 nm) and to RyR2 detected by FRET between fluorescent donor FK506-binding protein12.6 (donor-F-FKBP) and acceptor-F-CaM (i, Ex 488 nm) after endogenous CaMKII activation. B, Analyzed data from A. N values on bars.
However, these F-CaM measurements are complicated by the washout of exogenous CaM used to activate CaMKII, and because CaMKII has multiple targets. Moreover, we measured exogenous CaM washout as only 30% complete in the 3 min period after the 500 nM [Ca]i exposure. Thus the apparent CaMKII-induced decrease in CaM binding in Fig. 1B probably underestimates the full effect. That is, phosphorylated RyR2 may lose non-fluorescent CaM faster, which would promote F-CaM binding in the next phase (due to more unoccupied CaM binding sites).
3.2 Reduced CaM-RyR2 affinity in permeabilized S2814D vs. S2814A myocytes
To simplify protocols and interpretations, and to more selectively test the effect of RyR2 S2814 phosphorylation, we used knock-in mouse myocytes that express only non-phosphorylatable S2814A or phosphomimetic S2814D. Notably, the Ca spark activity in S2814A myocytes is no different from that of basal WT or AIP-treated myocytes, whereas in phosphomimetic S2814D myocytes Ca spark frequency matched WT myocyte upon maximal activation of endogenous CaMKII, and was insensitive to AIP (see Fig 2 of [34]). After washout of endogenous CaM (25 min), we saturated RyR2 with 100 nM donor-F-FKBP, and varied acceptor-[F-CaM], to reach steady-state binding (20-120 min). FRET (as sensitized emission) between F-FKBP and F-CaM resolves RyR2-bound CaM from CaM bound to all other targets (Fig. 2Ai, right) [21]. Total Z-line associated F-CaM is detected by direct F-CaM excitation (Fig. 2Aii; λex=543 nm, λem=600 nm). Fig. 2A shows that S2814D myocytes exhibit reduced F-CaM binding both to RyR2 and overall at the Z-line in the Kd range of [F-CaM] (20 nM), which is similar to the endogenous free [CaM] level in cardiac myocytes (50-75 nM) [36]. Since most of the Z-line CaM is RyR2-bound [21], it is not surprising that the total CaM-Z-line binding has similar affinity as the RyR2-bound CaM. For phosphomimetic S2814D vs. S2814A myocytes, the CaM-RyR2 affinity was reduced 3-fold (Kd=72±9 vs. 20±2 nM), but FRETmax at saturating 500 nM CaM was unaltered. Thus CaMKII-dependent RyR2 phosphorylation reduces CaM-RyR2 affinity.
Fig. 2.
Steady-state CaM binding in permeabilized S2814D and S2814A myocytes. A, Confocal images of 20 nM F-CaM binding at Z-line (ii) and to RyR2 (i). B, Top, Steady-state F-CaM-RyR2 binding for S2814A (black) and S2814D (red) fit to a single-site binding isotherm (n=12-23). Bottom, Analysis for 20 and 40 (near Kd), and 500 nM (~FRETmax) [F-CaM]. C, same analysis as in B for Z-line-bound CaM (n=12-23). N values on bars.
3.3 FKBP12.6 binding at Z-line in permeabilized S2814A and S2814D myocytes
FKBP12.6 can bind to and stabilize RyR2 channel gating, although details are controversial [37]. We also measured FKBP12.6-RyR2 binding affinity in saponin-permeabilized S2814A and S2814D myocytes. Confocal image analysis of F-FKBP binding at 100 nM as in Figs. 1-2 indicates no difference in Bmax. Even at 3 nM F-FKBP (close to the Kd) [38] binding was unaltered in permeabilized S2814A vs. S2814D myocytes (Online Fig. 1). Thus, CaMKII-dependent RyR2 phosphorylation does not alter FKBP12.6-RyR2 affinity. DAN also did not alter FKBP12.6-RyR2 affinity in permeabilized S2814D myocytes (Online Fig. 1).
3.4 Faster access of F-DPc10 in permeabilized S2814D vs. S2814A myocytes
We previously showed negative allosteric coupling between CaM binding to RyR2 and accessibility of the unzipping peptide DPc10 to its RyR2 binding site [17]. That is, DPc10 reduces CaM binding and increases SR Ca leak, whereas CaM quiets SR Ca leak and inhibits DPc10 access. Fig. 3 assesses whether CaMKII-dependent RyR2 phosphorylation increases F-DPc10 access, using FRET between F-FKBP and F-DPc10. Fig. 3A shows kinetics of F-DPc10 binding in permeabilized myocytes from WT, S2814A, S2814D, and S2814D plus either saturating exogenous CaM (500 nM) or pretreatment with 1 μM DAN. Fig. 3B shows τwash-in of F-DPc10 as an index of F-DPc10 accessibility to its RyR2 binding site, and Fig. 3C shows Bmax of F-DPc10. S2814A myocytes had the same properties as WT myocytes for both F-DPc10 accessibility and Bmax. In S2814D myocytes (pseudo-phosphorylated RyR2) F-DPc10 binding was 2-fold faster than S2814A myocytes, while Bmax for F-DPc10 was unaltered in S2814D vs. S2814A myocytes. Both saturating CaM (500 nM) and 1 μM DAN (which stabilizes the normal zipped conformation), slowed DPc10 wash-in, and lowered Bmax in S2814D myocytes. Taken together, these results indicate that: (1) CaMKII-dependent phosphorylation of RyR2 enhances F-DPc10 access by inducing the pathological (unzipped) RyR2 conformation; (2) DAN and forced CaM binding can suppress the S2814D-induced F-DPc10 access by stabilizing the normal healthy (zipped) RyR2 conformation.
Fig. 3.
Kinetics of F-DPc10-RyR2 binding for permeabilized S2814A and S2814D myocytes, plus DAN and saturating [CaM] effects. A, Wash-in time course of F-DPc10-RyR2 binding for WT (gray), S2814A (black), S2814D (red), S2814D with 500 nM exogenous CaM (pink), and S2814D with 1 μM DAN (purple). B-C, Summary of τwash-in and Bmax values for data in A. N values on bars.
3.5 DAN restores high CaM-RyR2 affinity in permeabilized S2814D myocytes
Since DAN attenuated F-DPc10 access in S2814D myocytes, we tested whether DAN can also restore CaM-RyR2 affinity. Fig. 4 shows steady-state CaM binding using [F-CaM] at 20 nM CaM (near the Kd) in permeabilized S2814D myocytes, with or without 1 μM DAN. Representative confocal images show both RyR2-bound CaM measured via FRET (Fig. 4Ai, right), and total Z-line-bound CaM from fluorescence of directly excited F-CaM (Fig. 4A ii). 1 μM DAN enhances CaM-RyR2 affinity in permeabilized S2814D myocytes (Fig. 4A bottom vs. top and 4B; compare to Fig 2Bii).
Fig. 4.
DAN enhances CaM binding in permeabilized S2814D myocytes. 1 μM DAN was applied 1 hour before saponin-permeabilization and present throughout experiment. A, Confocal images of F-CaM-Z-line (ii) and F-CaM-RyR2 binding, (i) with or without 1 μM DAN. B, Analyzed data from A. N values on bars.
3.6 DAN or excess CaM reduces SR Ca2+ leak in S2814D myocytes
To test whether DAN or saturating CaM binding to RyR2 (which restored normal conformation) also reduce SR Ca leak, we measured Ca spark frequency (CaSpF) and SR Ca content (evaluated via rapid 10 mM caffeine application). We used two saponin-permeabilization protocols [21]. The first protocol used a longer saponin exposure (3 min) to allow F-CaM diffusion into the cell as in the F-CaM binding experiments above (e.g. Fig. 2). The second protocol used only brief saponin exposure (30 s) to allow [Ca]i control and Ca spark measurement, but keep endogenous CaM in the myocyte (verified by prevention of F-CaM entry) [21]. The latter protocol has the advantage of minimizing the loss of cellular contents such as CaM, better mimicking the intact myocyte effect of reduced CaM-RyR2 affinity. The normal 3-min permeabilization has the advantage of allowing us to control intracellular [F-CaM], while washing out endogenous CaM (and other factors). It was recently reported that CaM is essential for DAN inhibition of RyR1 and RyR2 [39]. We used the 30-s permeabilization protocol to assess DAN effects on CaSpF with endogenous [CaM] levels (except where we saturated RyR2 with CaM – which required the 3-min saponin exposure).
CaSpF was significantly higher in permeabilized S2814D vs. S2814A myocytes (Fig. 5A-B) as previously reported [34]. Since CaSpF is highly dependent on SR Ca content, we also normalized results for SR Ca load, assessed by rapid caffeine-induced SR Ca release (Fig. 5C). Both 1 μM DAN and 500 nM CaM (saturating) reversed the high CaSpF in S2814D myocytes (Fig. 5A-C). Ca spark characteristics are in Online Table 1.
Fig. 5.
Exogenous CaM and DAN quiet Ca sparks in S2814D permeabilized myocytes. Some myocytes were treated with 500 nM exogenous non-fluorescent CaM for 20 min after saponin-permeabilization. Some intact myocytes were pretreated for 1 hr with 1 μM DAN prior to and during experiment. A, Confocal line-scan images of Ca sparks. B, Summarized data of Ca spark frequency (CaSpF) and SR Ca content. C, CaSpF normalized to SR Ca load from panel B. N values on bars.
3.7 Reduced CaM-RyR2 affinity in myocytes of non-ischemic rabbit HF model
We previously reported reduced CaM-RyR2 affinity in both canine tachycardia-induced non-ischemic HF [20] and rat post-infarction ischemic HF [21]. We also recently showed that oxidative stress (50 μM H2O2) reduces CaM-RyR2 affinity in rat myocytes [18]. Furthermore, our non-ischemic HF rabbits exhibit elevated CaMKII activation and RyR2 phosphorylation at S2814, and increased SR Ca leak that is reversed by CaMKII inhibition [5]. Fig. 6A-B shows CaM-RyR2 binding in permeabilized HF rabbit myocytes vs. control (age-matched) (rabbit HF characteristics are in Online Table 2). In HF rabbits the CaM-RyR2 affinity was ~2 fold lower vs. control rabbits, but FRETmax was unaltered (Fig. 6B). Similar results were found for total Z-line-bound CaM (Fig. 6C).
Fig. 6.
CaM binding is reduced in permeabilized rabbit myocytes with HF, and restored by CaMKII inhibition and DAN. A, Confocal images of F-CaM-Z-line (ii) and F-CaM-RyR2 (i) for control and HF myocytes. B, Top, steady-state F-CaM-RyR2 binding for control (gray) and HF (black) fit to single-site binding isotherm (n=19-62). Bottom, data from top at 20, 40, and 500 nM [F-CaM]. C, same analysis as in B, but for Z-line-bound CaM (n=19-62). D, Analysis of data of DAN and AIP, on F-CaM-RyR2 binding, for control and HF myocytes. N values on bars.
We previously reported that FKBP12.6-RyR2 affinity is unaltered in a post-infarction rat ischemic HF model [21]. Here, we measured FKBP12.6-RyR2 binding in the near Kd range in control and HF rabbit myocytes. There was no significant change in FKBP12.6-RyR2 binding in HF vs. control myocytes (Online Fig. 2).
3.8 CaMKII inhibition and DAN restore CaM-RyR2 affinity in rabbit HF myocytes
Fig. 6D shows that in control rabbit myocytes, neither the CaMKII inhibitor AIP nor DAN (both at 1 μM) altered CaM-RyR2 binding, but in HF that both AIP and DAN restored normal CaM binding. These results are consistent with CaMKII being acutely responsible for the reduced CaM binding in HF, as we found for SR Ca leak and arrhythmogenic Ca waves [5, 12]. Furthermore, the ability of DAN to selectively reverse the pathological decrease in CaM-RyR2 binding (Fig. 6D) relates to the ability of DAN to inhibit SR Ca leak in this HF model [40], and parallel findings on RyR2 conformation and SR leak in a canine HF model [19].
3.9 Faster F-DPc10 access in rabbit HF vs. control myocytes
Our working hypothesis is that reduced CaM affinity and increased DPc10 accessibility are functionally linked as part of a pathological RyR2 resting conformation. Here, we tested whether the reduced CaM affinity in rabbit HF above is accompanied by increased DPc10 accessibility. To specifically detect F-DPc10 binding to RyR2, we monitored the F-DPc10 wash-in kinetics using FRET from donor-F-FKBP to acceptor-F-DPc10. Fig. 7A and 7C show that DPc10 accessibility is roughly twice as fast in HF vs. control (similar to the shift for S2814D phosphomimetic in Fig. 3B). Online Fig. 3A-B show that high [Ca]i (500 nM) only slightly increases accessibility of F-DPc10. The apparent reduction in Bmax (ns; Fig. 7D) could be partly due to lower RyR2 expression in HF rabbits [5].
Fig. 7.
F-DPc10-RyR2 binding kinetics in permeabilized control and heart failure (HF) rabbit myocytes, and effects of CaMKII inhibition and DAN. A, Wash-in time course of F-DPc10-RyR2 binding for control (gray) and HF (black) myocytes. B, Similar to A, but for HF myocytes without (black) or with pretreatment of 1 μM AIP (blue), 1 μM DAN (purple), and 500 nM exogenous CaM (pink). C-D, Mean of τwash-in and Bmax for the data in panel A and B. N values on bars.
These results indicate that in this rabbit non-ischemic HF model most RyR2s are in a structural state that has higher access to DPc10 (more in the pathological unzipped conformation). As above for CaM affinity, either raising [CaM] (500 nM) to force saturation of RyR2, adding 1 μM DAN, or inhibiting CaMKII with AIP, all partially restored RyR2 conformation to a lower DPc10 accessibility (Fig. 7B-D). For AIP this was only significant for the decrease of Bmax, while high [CaM] and DAN both significantly slowed τwash-in and reduced Bmax.
3.10 DAN quiets arrhythmogenic events in intact S2814D myocytes
We previously reported that constitutive CaMKII phosphomimetic S2814D mice exhibit higher propensity for lethal ventricular arrhythmias and sudden cardiac death [34]. Here we measured Ca waves or spontaneous Ca transients in the presence of 1 μM isoproterenol after 3 Hz field stimulation in intact S2814A and S2814D myocytes. Fig. 8A-B shows that with isoproterenol-induced stress, S2814D myocytes have an increased frequency of both Ca waves and spontaneous Ca transients, which can be suppressed by DAN. These results are consistent with the DAN-induced correction of pathologic conformational state and SR Ca leak (above) being able to also suppress arrhythmogenic events at the intact myocyte level.
Fig. 8.
DAN suppresses arrhythmias in S2814D-mouse intact myocyte and whole animal. A, Confocal line-scan images of spontaneous Ca waves (gray allows) and triggered activity where a wave triggers an action potential (black arrow) in 1 μM isoproterenol-treated intact S2814A and S2814D myocytes after 3 Hz pacing. Some intact S2814D myocytes were pretreated for 1 hr with 1 μM DAN before and during experiment. B, Percent of cells with spontaneous Ca2+ waves (left) and showing triggered activity. (events/total cells studied on bars). C, Representative recordings of surface ECG (lead I) and ventricular electrograms from saline-treated (top) and DAN-treated (bottom) S2814D mice reveal that DAN inhibits pacing-induced VT in S2814D mice. Black arrow indicates overdrive pacing. D, Percent incidence of pacing-induced VT after 0.5 mg/kg isoproterenol in each group. N values on bars. Fisher’s exact test.
3.11 DAN suppressed ventricular tachycardia inducibility in S2814D mice
Finally, we tested whether DAN treatment (20 mg/kg/day, i.p. for 10 days) could suppress ventricular tachycardia (VT) induction in intact S2814D mice. Programmed electrical stimulation was performed in anesthetized mice to induce VT after isoproterenol injection (0.5 mg/kg, i.p.). DAN did not alter baseline electrophysiological parameters in S2814D vs. saline treated S2814D mice (Online Table 3). Programmed ventricular pacing during isoproterenol was sufficient to induce VT in 100% of saline-treated S2814D mice, but in DAN-treated S2814D mice VT was only observed in 1 of 6 mice (Fig. 8C-D). DAN effectively suppressed VT induction in S2814D mice. Thus, restoration of CaM-RyR2 binding, and correction of conformation change and leak in S2814D mice by DAN may suffice to suppress VT inducibility caused by CaMKII-dependent RyR2 phosphorylation.
4. Discussion
CaMKII is known to increase RyR2 opening and leak [5, 10, 26, 34]. But it was unknown whether CaMKII-dependent RyR2 phosphorylation alters RyR2 conformation and sensitivity to CaM and DAN, as described in HF [19, 20], oxidation [18], or DPc10 (or CPVT) [8, 22]. We propose that CaMKII-dependent phosphorylation of RyR2 (at S2814) and HF promote a common pathological RyR2 conformation that exhibits reduced CaM affinity, elevated Ca leak, and increased DPc10 accessibility, and all these effects can be reversed by DAN (and high [CaM]).
4.1 Multiple pathways may converge to increase Ca2+ leak in pathological conditions
Four pathways proposed to promote pathological SR Ca leak in HF and arrhythmias are: (1) RyR2 structural unzipping between N-terminal and central domains [7, 8]; (2) reduced CaM binding to RyR2 [20, 22]; (3) oxidation [18]; and (4) increased CaMKII activity [5]. Endogenous CaMKII activation and the phosphomimetic S2814D mutant increase SR Ca leak and ventricular arrhythmias [26, 34], but the molecular mechanism was unclear. Our new working paradigm here is that all four of the above pathological RyR2 modifications converge on a common pathological conformational state in which CaM affinity is reduced and DPc10 access, SR Ca leakiness, and DAN sensitivity are all increased and functionally coupled. Our data here are consistent with this unifying working hypothesis, and this may provide the basis for a novel therapeutic approach.
In contrast, the FKBP12.6 affinity for RyR2 was remarkably unaltered in HF (here and [21]), in CPVT [8], upon RyR2 oxidation [18], upon phosphorylation by either CaMKII or Protein kinase A (PKA) [26, 38], or exposure to DPc10 [17]. We conclude that FKBP12.6 affinity changes in RyR2 are functionally unimportant in any of these pathological conditions. Caffeine and ryanodine may also work differently, strongly promoting RyR2 opening, but only slightly induce the conformational shift that is promoted by CaMKII, ROS, DPc10, and HF, and reversed by CaM and dantrolene [17, 41].
4.2 CaMKII phosphorylation promotes pathological conformational shift (reduces CaM-RyR2 binding and increased DPc10 access)
We found that both activation of endogenous CaMKII and phosphomimetic RyR2-S2814D reduced CaM-RyR2 affinity in situ (Figs. 1-2). This was accompanied by increased DPc10 accessibility that could be reversed by either DAN or excess CaM (Figs. 3-4). This is consistent with the negative allosteric coupling that we have shown between DPc10 access and CaM binding in the absence of phosphorylation [17]. Moreover, the roughly 3-fold reduction in CaM affinity and 2-fold acceleration of DPc10 binding with CaMKII is similar to that observed with oxidation and HF (and upon DPc10 addition alone). Indeed, three different HF models (volume/ pressure-overload induced in rabbit (here), tachycardia induced in dog [20], and post-myocardial infarction in rat [21]) all exhibit very similar RyR2-CaM binding changes associated with increased SR Ca leak and a more DPc10 accessible pathophysiological conformation.
A parsimonious conclusion is that these conditions can all cause a similar RyR2 conformational state, perhaps a different stable low energy closed state or mode, from which channel opening occurs more readily, thus causing higher diastolic SR Ca leak. Moreover, this increased diastolic leak can contribute to systolic and diastolic dysfunction, as well as arrhythmogenesis. The fact that the elevated leak in rabbit HF can be reversed by acute CaMKII inhibition [5, 12] is also consistent with CaMKII-dependent RyR2 phosphorylation being necessary and sufficient to drive RyR2 to this pathological state in HF, even if RyR2 oxidation, CPVT-linked mutations, or other modulators can promote the same altered structural state.
The explicit molecular mechanism for the CaMKII effect is unknown. We suspect that it increases RyR2 Ca-sensitivity (increased SR Ca release for the same SR Ca content and Ca current trigger [13]), but in our myocyte studies it is hard to distinguish cytosolic vs. luminal Ca sensitivity. It is unlikely that CaMKII significantly stabilizes the open state, because the Ca spark duration is only slightly prolonged [26].
Although we have shown that S2814 is mainly phosphorylated by CaMKII, and not PKA (e.g. [42]), PKA might phosphorylate S2814 under some conditions (in addition to its robust phosphorylation of S2808). However, we find very different functional effects of CaMKII (or phosphomimetic S2814D) vs. PKA on RyR2 in myocytes. CaMKII (or S2814D RyR2) strongly activates Ca sparks & waves, depresses CaM affinity, and enhances DPc10 access (present study, [26], and [34]). In contrast, even strong PKA activation has no detectable effect on Ca sparks & waves (when phospholamban is absent and SR Ca loading is prevented [43]).
4.3 DAN can shift the pathological conformation back to normal
In HF, DAN also drove RyR2 conformation back to the normal (zipped) conformation, restoring CaM affinity and low leak, and preventing triggered arrhythmias in S2814D mice (with unaltered RyR2 oxidation [18]). RyR2 unzipping and increased DPc10 binding was first suggested to explain CPVT linked to mutations in the N- and central-domain of RyR2 [8, 17]. As we show for CaMKII phoshomimetic S2814D mice here, DAN also restored CaM-RyR2 binding and inhibited DPc10 binding, SR Ca leak, and arrhythmias in R2474S knock-in CPVT mice [8, 22, 44]. Thus, some CPVT mutations may promote the same unzipped pathological RyR2 conformation as discussed above for CaMKII, HF, and oxidation. Furthermore, DAN-like drugs could be a tenable therapeutics for treating CaMKII-mediated arrhythmias in HF.
4.4 CaM plays an important role in RyR2 gating
Ca-free CaM (apo-CaM) and Ca-bound CaM (Ca-CaM) have different conformations, but both forms of CaM reduce RyR2 opening. Here, we clamped [Ca]i at 50 nM, favoring apo-CaM vs. Ca-CaM. We found reduced CaM-RyR2 affinity in canine [20], rat [21], and rabbit HF models (Fig. 6) and in a R2474S knock-in CPVT mouse model [22]. Enhanced CaM-RyR2 affinity could be therapeutic in these disease models [32, 45]. Moreover, human CPVT-associated CaM mutants bind to RyR2 with higher affinity than WT-CaM, but fail to inhibit RyR2 gating like WT-CaM, resulting in greater arrhythmogenic SR Ca leak [31]. Thus CaM-RyR2 binding importantly modulates RyR2 gating in the heart.
4.5 Pathological RyR2 conformation and high(er)-resolution RyR1 structures
Earlier work proposed an intrinsic structural interaction (zipping) between the N-terminal (aa 1-600) and central (aa 2000-2500) RyR2 domains, which could be disrupted (unzipped) by the DPc10 peptide (aa 2460-2495) to cause increased RyR2 opening and SR Ca leak in pathological conditions such as HF [7], CPVT [8], and oxidation [46]. This concept was consistent with a prior cryo-EM study suggesting that the N-terminal domain from one RyR protomer interacts with the central domain of an adjacent protomer (inter- vs. intra-subunit interaction) [41]. Dantrolene appears to bind to aa 601-620 of RyR2 [47] and corrects pathological RyR2 channel gating in HF [19, 40] and CPVT [44, 48] but has no effect on normal RyR2 gating in control healthy conditions [40]. Atomic models of the N-terminal module of each RyR subunit – consisting of three individual domains, A, B, and C – have been docked into cryo-EM maps of RyR1, identifying specific interactions between neighboring RyR subunits [49]. Domains A, B, and C form a central vestibule within the RyR tetrameric assembly, and dantrolene binding near this region may influence RyR functional stability [50]. Previous crystal structures of the RyR phosphorylation domain (aa 2700-2906, containing the sites of phosphorylation by CaMKII and PKA) and docking [51, 52] were updated based on newer cryo-EM maps. The probable location of the phosphorylation domain is in a density (turret) that is poorly resolved (indicating a dynamic underlying structure) at the top of the central region of the helical domain [53-56]. Phosphorylation could induce allosteric changes within the neighboring helical domain (aa 2110-2676 and 2981-3527, that includes the locus from which DPc10 is derived), which directly interacts with the N-terminal domain A [55]. Furthermore, the turret density is also near the density corresponding to Ca-free CaM previously resolved via cryo-EM [57]. Thus, CaMKII-dependent phosphorylation of RyR2 might induce RyR2 conformational changes that influence CaM binding and DPc10 accessibility. The new higher-resolution structural data published in 2015 [53-56] should help to further understand the structural basis for the normal and pathological RyR2 conformational states called zipped and unzipped and also the effects of CaMKII, CaM, and dantrolene.
4.6 Simple working model and therapeutic potential
We propose that the RyR2 has at least two stable baseline conformations or gating modes (Online Fig. 4). In this model, the “normal” healthy RyR2 conformation (or mode) has high CaM affinity and low DPc10 binding access, and the channel very rarely opens under resting physiological conditions. The “pathological” conformation (or mode) has lower CaM affinity, allows DPc10 to get to its binding site more easily, and exhibits more frequent RyR2 openings under otherwise normal intracellular conditions. Several factors promote shifts of RyR2 conformation from the normal to the less stable pathological mode, including CaMKII, oxidation, HF, CPVT, and DPc10. Conversely, CaM and DAN appear to shift the conformation and gating mode back toward the normal state. The pathological RyR conformation might exist in normal channels, under physiological conditions, but only becomes significantly populated when the promoting factors above are present. While this model is surely oversimplified, it provides a useful working framework for drug discovery and development, targeted at reducing pathological SR Ca leak and its consequent effects on systolic and diastolic function and arrhythmias. Notably, in our hands FKBP12.6 has no effect at all on this conformational equilibrium [17, 18]. So, to the extent that FKBP12.6 alters RyR2 gating, it would seem to do so by some other mechanism, not involving DPc10 or CaM binding.
There are attractive aspects of RyR2 conformation shift as a therapeutic target. First, neither DAN nor CaM, which both shift RyR2 from pathological toward the normal mode, inhibit normal SR Ca release during excitation-contraction coupling. This differs greatly from classical ion channel blockers. DAN is a clinically used drug that works as an effective mode-shifter. That is, DAN has no effect on normal healthy cardiac myocyte Ca transients, but it supresses pathological RyR2 leak and improves Ca transients [40]. Therefore, a second advantage is that DAN is already a known drug, used clinically for RyR1 dysfunction in malignant hyperthermia. A third advantage is that we have developed myocyte fluorescence assays that are quite sensitive to this conformational change, which can be used for drug discovery via high-throughput screening [17, 18, 21, 33]. Candidate small-molecule SR-leak inhibitors should enhance RyR2 F-CaM binding and suppress F-DPc10 access, but these effects should only be seen when RyR2 is pushed to the “pathological” state, and have no effect on “normal” RyR2 (like DAN). These aspects could help screening and development of novel candidate drugs.
During pathological states, CaMKII can be chronically and autonomously activated [5, 15, 16] strongly phosphorylating RyR2 at S2814, making the S2814D knock-in mouse a good mimic for this state. Under physiological changes in heart rate and Ca transient the amplitude the state of CaMKII activation can increase [29], and even at physiological heart rates one might expect RyR2 to have some S2814 phosphorylation. So while the extreme cases of S2814A- and S2814D-RyR2 have allowed us to identify apparent conformational and functional extremes, we cannot know how many RyR monomers within a tetramer need to be phosphorylated to produce the full functional phenotype. Moreover, these effects are likely graded physiologically, becoming increasingly trapped in the S2814D mode by progressive autonomous CaMKII activation in pathological states.
5. Conclusion
Novel findings of this study include: (1) CaMKII-dependent RyR2 phosphorylation (at S2814) reduces CaM-RyR2 affinity and (2) causes a pathological RyR2 conformation characterized by increased access of DPc10 (unzipping); (3) dantrolene prevents arrhythmogenic myocyte events by restoring normal RyR2 conformation and CaM-RyR2 affinity, thereby suppressing SR Ca leak; (4) dantrolene suppresses VT inducibility in S2814D mice; (5) elevated [CaM] (500 nM) can restore normal CaSpF and RyR2 conformation in S2814D mice; and (6) in rabbit non-ischemic HF myocytes, CaM-RyR2 affinity is reduced and RyR2 exhibits the pathological conformation as detected by DPc10 access (unzipping). Our working model is that CaMKII-dependent phosphorylation of RyR2 (at S2814) and HF promote a pathological RyR2 conformation which exhibits reduced CaM affinity, elevated Ca leak, and increased DPc10 accessibility, and all these effects can be reversed by dantrolene.
Supplementary Material
Highlights.
CaMKII-dependent RyR2 phosphorylation reduces CaM affinity and causes SR Leak.
RyR2 phosphorylation by CaMKII induces a pathological (unzipped) conformation.
Dantrolene restores CaM binding affinity and quiets diastolic SR Ca leak.
Dantrolene & CaM shift pathological conformation back to physiological (normal).
A common RyR2 pathological state may occur in heart failure, ROS, and CaMKII.
Acknowledgments
This work was supported by: (1) NIH P01-80101 (DMB), R01-HL092097 (DMB and RLC), R01-HL089598 (XHTW), R01-HL091947 (XHTW), R01-HL117641 (XHTW), and R41-HL129570 (XHTW); (2) the Uehara Memorial Foundation (HU); and (3) the American Heart Association 14SDG20080008 (NL), 15GRNT25610022 (RLC), and 13EIA14560061 (XHTW).
Abbreviations
- AIP
autocamtide-2 related inhibitory peptide
- Bmax
binding maximum
- CaM
calmodulin
- CaMKII
calcium/calmodulin-dependent protein kinase II
- CaSpF
calcium spark frequency
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- DAN
dantrolene
- F
fluorescent
- FKBP
FK506-binding protein
- FRET
fluorescence resonance energy transfer
- HF
heart failure
- PKA
protein kinase A
- ROS
reactive oxygen species
- RyR1
skeletal ryanodine receptor
- RyR2
cardiac ryanodine receptor
- SR
sarcoplasmic reticulum
- τwash-in
wash-in time constant
- VT
ventricular tachycardia
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- [1].Noseworthy PA, Newton-Cheh C. Genetic determinants of sudden cardiac death. Circulation. 2008;118:1854–63. doi: 10.1161/CIRCULATIONAHA.108.783654. [DOI] [PubMed] [Google Scholar]
- [2].Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69:1378–84. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nyegaard M, Overgaard MT, Sondergaard MT, Vranas M, Behr ER, Hildebrandt LL, et al. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am J Hum Genet. 2012;91:703–12. doi: 10.1016/j.ajhg.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- [5].Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–22. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- [6].Svensson B, Oda T, Nitu FR, Yang Y, Cornea I, Chen-Izu Y, et al. FRET-based trilateration of probes bound within functional ryanodine receptors. Biophys J. 2014;107:2037–48. doi: 10.1016/j.bpj.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oda T, Yano M, Yamamoto T, Tokuhisa T, Okuda S, Doi M, et al. Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure. Circulation. 2005;111:3400–10. doi: 10.1161/CIRCULATIONAHA.104.507921. [DOI] [PubMed] [Google Scholar]
- [8].Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, et al. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res. 2010;106:1413–24. doi: 10.1161/CIRCRESAHA.109.209312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–67. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- [10].Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–8. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- [11].Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, et al. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–93. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- [12].Curran J, Brown KH, Santiago DJ, Pogwizd S, Bers DM, Shannon TR. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca2+-calmodulin-dependent protein kinase II. J Mol Cell Cardiol. 2010;49:25–32. doi: 10.1016/j.yjmcc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca2+-calmodulin-dependent protein kinase II on cardiac excitation-contraction coupling in ferret ventricular myocytes. J Physiol. 1997;501:17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. ( Pt 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–73. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–74. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–6. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Oda T, Yang Y, Nitu FR, Svensson B, Lu X, Fruen BR, et al. In cardiomyocytes, binding of unzipping peptide activates ryanodine receptor 2 and reciprocally inhibits calmodulin binding. Circ Res. 2013;112:487–97. doi: 10.1161/CIRCRESAHA.111.300290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Oda T, Yang Y, Uchinoumi H, Thomas DD, Chen-Izu Y, Kato T, et al. Oxidation of ryanodine receptor (RyR) and calmodulin enhance Ca release and pathologically alter, RyR structure and calmodulin affinity. J Mol Cell Cardiol. 2015;85:240–8. doi: 10.1016/j.yjmcc.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, et al. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009;53:1993–2005. doi: 10.1016/j.jacc.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ono M, Yano M, Hino A, Suetomi T, Xu X, Susa T, et al. Dissociation of calmodulin from cardiac ryanodine receptor causes aberrant Ca2+ release in heart failure. Cardiovasc Res. 2010;87:609–17. doi: 10.1093/cvr/cvq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang Y, Guo T, Oda T, Chakraborty A, Chen L, Uchinoumi H, et al. Cardiac myocyte Z-line calmodulin is mainly RyR2-bound, and reduction is arrhythmogenic and occurs in heart failure. Circ Res. 2014;114:295–306. doi: 10.1161/CIRCRESAHA.114.302857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xu X, Yano M, Uchinoumi H, Hino A, Suetomi T, Ono M, et al. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem Biophys Res Commun. 2010;394:660–6. doi: 10.1016/j.bbrc.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–5. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- [24].Yano M, Okuda S, Oda T, Tokuhisa T, Tateishi H, Mochizuki M, et al. Correction of defective interdomain interaction within ryanodine receptor by antioxidant is a new therapeutic strategy against heart failure. Circulation. 2005;112:3633–43. doi: 10.1161/CIRCULATIONAHA.105.555623. [DOI] [PubMed] [Google Scholar]
- [25].Belevych AE, Terentyev D, Viatchenko-Karpinski S, Terentyeva R, Sridhar A, Nishijima Y, et al. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res. 2009;84:387–95. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- [27].Yamaguchi N, Takahashi N, Xu L, Smithies O, Meissner G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca release channel. J Clin Invest. 2007;117:1344–53. doi: 10.1172/JCI29515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gaertner TR, Kolodziej SJ, Wang D, Kobayashi R, Koomen JM, Stoops JK, et al. Comparative analyses of the three-dimensional structures and enzymatic properties of alpha, beta, gamma and delta isoforms of Ca2+-calmodulin-dependent protein kinase II. J Biol Chem. 2004;279:12484–94. doi: 10.1074/jbc.M313597200. [DOI] [PubMed] [Google Scholar]
- [29].Erickson JR, Patel R, Ferguson A, Bossuyt J, Bers DM. Fluorescence resonance energy transfer-based sensor Camui provides new insight into mechanisms of calcium/calmodulin-dependent protein kinase II activation in intact cardiomyocytes. Circ Res. 2011;109:729–38. doi: 10.1161/CIRCRESAHA.111.247148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Crotti L, Johnson CN, Graf E, De Ferrari GM, Cuneo BF, Ovadia M, et al. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation. 2013;127:1009–17. doi: 10.1161/CIRCULATIONAHA.112.001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hwang HS, Nitu FR, Yang Y, Walweel K, Pereira L, Johnson CN, et al. Divergent regulation of ryanodine receptor 2 calcium release channels by arrhythmogenic human calmodulin missense mutants. Circ Res. 2014;114:1114–24. doi: 10.1161/CIRCRESAHA.114.303391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hino A, Yano M, Kato T, Fukuda M, Suetomi T, Ono M, et al. Enhanced binding of calmodulin to the ryanodine receptor corrects contractile dysfunction in failing hearts. Cardiovasc Res. 2012;96:433–43. doi: 10.1093/cvr/cvs271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guo T, Fruen BR, Nitu FR, Nguyen TD, Yang Y, Cornea RL, et al. FRET detection of calmodulin binding to the cardiac RyR2 calcium release channel. Biophys J. 2011;101:2170–7. doi: 10.1016/j.bpj.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–79. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, deAlmeida A, et al. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110:1474–83. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu X, Bers DM. Free and bound intracellular calmodulin measurements in cardiac myocytes. Cell Calcium. 2007;41:353–64. doi: 10.1016/j.ceca.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bers DM. Ryanodine receptor S2808 phosphorylation in heart failure: smoking gun or red herring. Circ Res. 2012;110:796–9. doi: 10.1161/CIRCRESAHA.112.265579. [DOI] [PubMed] [Google Scholar]
- [38].Guo T, Cornea RL, Huke S, Camors E, Yang Y, Picht E, et al. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res. 2010;106:1743–52. doi: 10.1161/CIRCRESAHA.110.219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Oo YW, Gomez-Hurtado N, Walweel K, van Helden DF, Imtiaz MS, Knollmann BC, et al. Essential Role of Calmodulin in RyR Inhibition by Dantrolene. Mol Pharmacol. 2015;88:57–63. doi: 10.1124/mol.115.097691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maxwell JT, Domeier TL, Blatter LA. Dantrolene prevents arrhythmogenic Ca2+ release in heart failure. Am J Physiol Heart Circ Physiol. 2012;302:H953–63. doi: 10.1152/ajpheart.00936.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu Z, Wang R, Tian X, Zhong X, Gangopadhyay J, Cole R, et al. Dynamic, inter-subunit interactions between the N-terminal and central mutation regions of cardiac ryanodine receptor. J Cell Sci. 2010;123:1775–84. doi: 10.1242/jcs.064071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Huke S, Bers DM. Ryanodine receptor phosphorylation at Serine 2030, 2808 and 2814 in rat cardiomyocytes. Biochem Biophys Res Commun. 2008;376:80–5. doi: 10.1016/j.bbrc.2008.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–16. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- [44].Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, et al. Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2(R2474S/+) knock-in mouse model. Circ J. 2010;74:2579–84. doi: 10.1253/circj.cj-10-0680. [DOI] [PubMed] [Google Scholar]
- [45].Fukuda M, Yamamoto T, Nishimura S, Kato T, Murakami W, Hino A, et al. Enhanced binding of calmodulin to RyR2 corrects arrhythmogenic channel disorder in CPVT-associated myocytes. Biochem Biophys Res Commun. 2014;448:1–7. doi: 10.1016/j.bbrc.2014.03.152. [DOI] [PubMed] [Google Scholar]
- [46].Oda T, Yang Y, Uchinoumi H, Thomas DD, Chen-Izu Y, Kato T, et al. Oxidation of Ryanodine Receptor (RyR) and Calmodulin enhance Ca release and pathologically alter, RyR structure and Calmodulin affinity. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Paul-Pletzer K, Yamamoto T, Ikemoto N, Jimenez LS, Morimoto H, Williams PG, et al. Probing a putative dantrolene-binding site on the cardiac ryanodine receptor. Biochem J. 2005;387:905–9. doi: 10.1042/BJ20041336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jung CB, Moretti A, Mederos y Schnitzler M, Iop L, Storch U, Bellin M, et al. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol Med. 2012;4:180–91. doi: 10.1002/emmm.201100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tung CC, Lobo PA, Kimlicka L, Van Petegem F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature. 2010;468:585–8. doi: 10.1038/nature09471. [DOI] [PubMed] [Google Scholar]
- [50].Seidel M, Thomas NL, Williams AJ, Lai FA, Zissimopoulos S. Dantrolene rescues aberrant N-terminus intersubunit interactions in mutant pro-arrhythmic cardiac ryanodine receptors. Cardiovasc Res. 2015;105:118–28. doi: 10.1093/cvr/cvu240. [DOI] [PubMed] [Google Scholar]
- [51].Yuchi Z, Lau K, Van Petegem F. Disease mutations in the ryanodine receptor central region: crystal structures of a phosphorylation hot spot domain. Structure. 2012;20:1201–11. doi: 10.1016/j.str.2012.04.015. [DOI] [PubMed] [Google Scholar]
- [52].Sharma P, Ishiyama N, Nair U, Li W, Dong A, Miyake T, et al. Structural determination of the phosphorylation domain of the ryanodine receptor. FEBS J. 2012;279:3952–64. doi: 10.1111/j.1742-4658.2012.08755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yuchi Z, Yuen SM, Lau K, Underhill AQ, Cornea RL, Fessenden JD, et al. Crystal structures of ryanodine receptor SPRY1 and tandem-repeat domains reveal a critical FKBP12 binding determinant. Nat Commun. 2015;6:7947. doi: 10.1038/ncomms8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Efremov RG, Leitner A, Aebersold R, Raunser S. Architecture and conformational switch mechanism of the ryanodine receptor. Nature. 2015;517:39–43. doi: 10.1038/nature13916. [DOI] [PubMed] [Google Scholar]
- [55].Yan Z, Bai XC, Yan C, Wu J, Li Z, Xie T, et al. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature. 2015;517:50–5. doi: 10.1038/nature14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zalk R, Clarke OB, des Georges A, Grassucci RA, Reiken S, Mancia F, et al. Structure of a mammalian ryanodine receptor. Nature. 2015;517:44–9. doi: 10.1038/nature13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Samso M, Wagenknecht T. Apocalmodulin and Ca2+-calmodulin bind to neighboring locations on the ryanodine receptor. J Biol Chem. 2002;277:1349–53. doi: 10.1074/jbc.M109196200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.