Abstract
Vitamin D deficiency in human subjects is associated with hypertension, metabolic syndrome and related risk factors of cardiovascular diseases. Serum 25-hydroxyvitamin D levels correlate inversely with adiposity in obese and lean individuals. Bioactive vitamin D, or calcitriol, exerts anti-inflammatory effects on adipocytes, preadipocytes and macrophages in vitro. We tested the hypothesis that vitamin D deficiency alters the phenotype of perivascular adipose tissue (PVAT) leading to impaired function in resistance artery. To examine the effects of vitamin D and PVAT on vascular reactivity, myograph experiments were performed on arteries, with or without intact PVAT, from mice maintained on vitamin D-deficient, vitamin D-sufficient or vitamin D-supplemented diet. Systolic blood pressure was significantly increased in mice on vitamin D-deficient diet. Importantly, vitamin D deficiency enhanced angiotensin II-induced vasoconstriction and impaired the normal ability of PVAT to suppress contractile responses of the underlying mesenteric resistance artery to angiotensin II and serotonin. Furthermore, vitamin D deficiency caused upregulation of the mRNA expression of tumor necrosis factor-α, hypoxia-inducible factor-1α and its downstream target lysyl oxidase in mesenteric PVAT. Incubation of mesenteric arteries under hypoxic conditions impaired the anti-contractile effects of intact PVAT on those arteries from mice on vitamin D-sufficient diet. Vitamin D supplementation protected arteries against hypoxia-induced impairment of PVAT function. The protective effects of vitamin D against vascular dysfunction, hypertension and cardiovascular diseases may be mediated, at least in part, through regulation of inflammatory and hypoxia signaling pathways in PVAT.
Keywords: Perivascular adipose tissue, resistance artery, vitamin D, hypoxia, inflammation
1. Introduction
The prevalence of metabolic syndrome and cardiovascular disease poses serious health problems. In obese individuals, adipose tissue displays increased macrophage accumulation and increased production of pro-inflammatory molecules compared to that of lean individuals (1). Most blood vessels are surrounded by PVAT and it produces factors that affect atherogenesis and smooth muscle cell proliferation. In addition, PVAT produces transferable vasoactive substances including adipocyte-derived relaxing factors and adipocyte-derived contractile factors (2). The effect of PVAT on arterial reactivity has been examined in human small arteries obtained from subcutaneous adipose tissue biopsies using wire myography (3, 4). PVAT-containing arteries displayed a blunted response to vasoconstrictor agonist stimulation compared to arteries without PVAT from healthy lean subjects. Importantly, the anti-contractile effect of PVAT was lost in arteries taken from subjects with metabolic syndrome (3, 4). Obese subjects tend to have lower levels of serum 25-hydroxyvitamin D (25(OH)D) (5). Vitamin D is a fat-soluble vitamin stored in adipose tissue. It is hypothesized that the lower status of circulating 25(OH)D in obese subjects is a consequence of volumetric dilution within adipose tissue mass (5).
Bioactive vitamin D, or 1,25(OH)2D3 (1,25-dihydroxycholecalciferol or calcitriol), is a steroid hormone with classical roles in calcium and phosphorus homeostasis and bone metabolism (6). The 1,25(OH)2D3 functions as a ligand of the vitamin D receptor (VDR), which forms a heterodimer with retinoid X receptor (RXR) that binds vitamin D response elements to regulate target gene transcription. Vitamin D3 (cholecalciferol) is generated in the skin of animals through ultraviolet B radiation from sun exposure (6). There is a high prevalence of vitamin D insufficiency/deficiency, or hypovitaminosis D, in the general population (7). An analysis from the Intermountain Healthcare System (>41,000 patients) showed that vitamin D insufficiency (≤30 ng/ml serum 25(OH)D) was associated with higher incidence of coronary artery disease, myocardial infarction, heart failure, stroke, hypertension and type II diabetes (7).
In cell culture studies, 1,25(OH)2D3 has been shown to suppress the induction of pro-inflammatory mediators in adipocytes, preadipocytes and monocytes/macrophages (8). Our group discovered a crucial role for dietary vitamin D in determining the phenotype of epicardial adipose tissue. The epicardial adipose tissue from Yucatan miniswine maintained on vitamin D-deficient diet exhibited an increase in immune cell infiltration, increased expression of pro-inflammatory cytokines (e.g. tumor necrosis factor-α (TNFα), interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1)), as well as decreased adiponectin compared to control miniswine on vitamin D-sufficient diet (9). These results led us to the hypothesis that vitamin D deficiency causes impaired resistance artery function through direct effects on PVAT inflammation and adipokine production.
2. Methods
2.1 Animals and diets
The animal procedures in this study were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of Creighton University. BALB/c mice were purchased from Harlan Laboratories. Mice were maintained in a pathogen-free environment at the Animal Resource Facility of Creighton University. Three different diets were used in this study (Teklad Diets, Harlan Laboratories). Mice were fed either a vitamin D-deficient diet (TD110272), a standard vitamin D-sufficient diet containing 1.5 IU/g of vitamin D3 (TD2018), or a vitamin D-supplemented diet containing 10 IU/g of vitamin D3 (TD110742), as in previous studies by our group (10). The vitamin D-deficient diet (TD110272) was fortified with vitamins A, E and K in addition to 1.2% calcium to prevent hypocalcemia. For each of the three different diet groups, male and female breeding pairs were maintained on the same diet as their offspring. Food and water were provided ad libitum. Mice were euthanized between 4–6 months of age by intraperitoneal injection of sodium pentobarbital (150 mg/kg) followed by exsanguination.
2.2 Measurement of vascular reactivity
Isometric tension was measured in rings of second order mesenteric artery (~2.5 mm length, ~100 µm diameter) using the Radnoti M4 series wire myograph system (Radnoti, Monrovia, CA). Isometric tension was measured in rings of thoracic aorta (~4 mm length) using a Graz organ bath myograph system (Hugo Sachs Elektronik, Harvard Apparatus, Germany). Arteries were removed and placed in cold oxygenated Krebs physiological buffer (in mM: NaCl 118.3, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11 and CaCl2 2.5). Arterial rings were either dissected free of adventitial PVAT or remained with the PVAT intact. The study was designed to compare an arterial ring plus PVAT and an arterial ring minus PVAT from within the same animal. The n-values represent a single replicate for each arterial ring type (plus or minus PVAT) used from each mouse. Arterial rings were connected to the force transducer via two steel wires (aorta: 125 µm; mesenteric artery: 25 µm) and suspended in organ baths containing 5 ml Krebs buffer (maintained at 37°C and 95% O2 / 5% CO2). Resting tension was set to ~0.5 g for aortic rings and ~0.25 g for mesenteric artery rings, and the vessels were equilibrated for 45 minutes. Dose-dependent contraction was recorded for cumulative doses of KCl, serotonin (5-hydroxytryptamine; 5-HT), angiotensin II (Ang II), prostaglandin F2α (PGF2α), endothelin 1 (ET-1) or phenylephrine (PE). Dose-dependent relaxation was recorded for aortic rings in response to Ach or SNP after initial submaximal pre-contraction (~50% of max) with PGF2α. Following application of each agonist, vessels were dilated to baseline tension with SNP and washed three times with fresh Krebs buffer over a thirty-minute recovery period. Data were collected with PowerLab/8SP and analyzed with LabChart 7 software (AD Instruments, Colorado Springs, CO).
2.3 Blood pressure recordings
Systolic blood pressure was recorded in conscious restrained mice (4–6 months of age) using the CODA non-invasive blood pressure system (Kent Scientific, Torrington, CT) by the automated tail-cuff method. Recordings (20 cycles) were taken each day for a period of three days. Mice were trained and acclimated the first two days. The mean of 20 cycles of recordings on the third day was calculated for the determination of baseline systolic blood pressure.
2.4 Histology of arteries and adipose depots
Histological analysis was performed on perigenital adipose tissue, mesenteric adipose tissue, inter-scapular brown adipose tissue, and aorta with intact PVAT. Tissues were fixed in 10% neutral buffered formalin, processed and embedded in paraffin blocks. Sections (5 µm thickness) were cut from paraffin blocks and mounted on microscope slides. Slides were stained with hematoxylin and eosin. Images were taken at 10× and 20× magnification using a Nikon Eclipse Ci microscope (Nikon Instruments, Melville, NY). Adipocyte cross-sectional area was quantified from perigenital and mesenteric adipose tissues according to a protocol adapted from previously described methods (11). Using ImageJ program, the images were converted to 8-bit image type. The threshold of the image was adjusted manually using the Threshold command. Cross-sectional areas (µm2) of adipocytes were calculated using the Measure command. Three sections were evaluated from each mouse for each adipose depot examined. Values less than 100 µm2 were excluded from the analysis.
2.5 Gene expression analysis
Samples of perigenital adipose tissue were removed from the mice and snap frozen on dry ice before storage at −80°C. The mesentery and aorta were removed and placed in cold Krebs buffer. Mesenteric PVAT and aortic PVAT were dissected away from the arteries, and the samples were snap frozen on dry ice before storage at −80°C. Total RNA was isolated using the Trizol reagent method. RNA concentration was quantified using a Nanodrop (Thermo-Scientific, Rockford, IL). cDNA was synthesized from 400 ng of total RNA using oligo(dT) primers and ImProm-II reverse transcription system reagents (Promega, Madison, WI) according to the manufacturer's protocol. Quantitative real-time PCR reactions were performed using iQ SYBR Green Supermix and CFX96 Real-Time PCR System (Bio-Rad Laboratories, Hercules, CA). The thermocycler conditions were as follows: 2 minutes at 95°C for initial denaturation, and 45 cycles of 30 seconds at 95°C, 30 seconds at 62°C, and 30 seconds at 72°C. Relative mRNA expression was determined from ΔΔCT values calculated with GAPDH as the reference gene. Primers were obtained from Integrated DNA Technologies (Coralville, IA). Primer pairs were designed for the following mouse genes:
Adiponectin (forward): 5’-AAGGACAAGGCCGTTCTCT-3’
Adiponectin (reverse): 5’-TATGGGTAGTTGCAGTCAGTTGG-3’
Leptin (forward): 5’-TGACACCAAAACCCTCATCA-3’
Leptin (reverse): 5’-TCATTGGCTATCTGCAGCAC-3’
UCP1 (forward): 5’-CGACTCAGTCCAAGAGTACTTCTCTTC-3’
UCP1 (reverse): 5’-GCCGGCTGAGATCTTGTTTC-3’
HIF1α (forward): 5’-TCAAGTCAGCAACGTGGAAG-3’
HIF1α (reverse): 5’-TATCGAGGCTGTGTCGACTG-3’
LOX (forward): 5’-CATTCTTCTGCTGCGTGACAAC-3’
LOX (reverse): 5’-CCCGACGGCGAGAAACCAG-3’
PTGIS (forward): 5’-CAAACATGGAGGAATCGTCTTCG-3’
PTGIS (reverse): 5’-GGGGGACCAGATTAAATAGTGTG-3’
TNFα (forward): 5’-CGGAGTCCGGGCAGGT-3’
TNFα (reverse): 5’-GCTGGGTAGAGAATGGATGAACA-3’
IL1β (forward): 5’-AGGCAGGCAGTATCACTCATTGT-3’
IL1β (reverse): 5’-GGAAGGTCCACGGGAAAGA-3’
GAPDH (forward): 5’-CATGGCCTTCCGTGTTCCTA-3’
GAPDH (reverse): 5’-CCTGCTTCACCACCTTCTTGAT-3’
2.6 Western blotting
Protein lysates were prepared by homogenizing samples of mesenteric PVAT in RIPA buffer containing protease inhibitors and phosphatase inhibitors. Homogenates were centrifuged at 4°C, and the protein concentration of the lysates was quantified by Bradford assay. Protein lysate samples (10 µg) were separated by SDS-PAGE and transferred onto a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). The membrane was immunoblotted with primary antibody followed by HRP-conjugated secondary antibody. Protein bands were detected by chemiluminescence using a ChemiDoc MP system (Bio-Rad Laboratories, Hercules, CA) and quantified using ImageJ software. Hypoxia-inducible factor-1α (HIF-1α) protein was probed using anti-HIF-1α antibody (ab179483, Abcam, San Francisco, CA). As a loading control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was probed using anti-GAPDH antibody (NB300-221, NOVUS Biological, Littleton, CO).
2.7 Hypoxic chamber incubation
Arterial rings and adipose tissue samples were cultured in DMEM/F12 medium supplemented with 1% penicillin/streptomycin in 12-well plates. Hypoxia was induced in the tissues using a modular incubator chamber (Billups-Rothenberg, Del Mar, California). Rings of thoracic aorta (~4 mm length) and first order mesenteric artery (~2.5 mm length, ~150 µm diameter) with or without intact PVAT were used to test the effects of hypoxia on vascular reactivity. Samples of perigenital adipose tissue, aortic PVAT, mesenteric PVAT, aorta and mesenteric artery tissues were used to test the effects of hypoxia on gene expression. The plate for hypoxic incubation was placed inside the modular incubator chamber, which was then flushed with a gas mixture of 2% O2, 5% CO2, and 93% N2 for 10 minutes. The chamber was then sealed and maintained at 37°C with 5% CO2 in a standard cell culture incubator for 24 hours (2% O2). Control tissues were maintained under normoxic conditions in the same incubator for 24 hours (20% O2). O2 levels were monitored using a MaxO2+ analyzer (Maxtec, Salt Lake City, Utah). The arteries were then used for isometric tension experiments via wire myograph. The vascular reactivity study was designed to compare an arterial ring plus PVAT and an arterial ring minus PVAT under hypoxic condition or normoxic condition that was taken from within the same animal. The n-values represent a single replicate for each arterial ring type (plus or minus PVAT) and treatment group (hypoxic or normoxic) used from each mouse. RNA was isolated from the other tissues for gene expression analysis. The gene expression study was designed to compare tissue samples incubated under hypoxic condition or normoxic condition that were taken from within the same animal. The n-values represent a single replicate for each tissue sample type (perigenital adipose tissue, aortic PVAT, mesenteric PVAT or aortic tissue) and treatment group (hypoxic or normoxic) taken from each mouse. Each mesenteric artery tissue sample used for RNA isolation was taken from an individual mouse due to the small quantity of tissue.
2.8 Statistical analysis
Data are expressed as means ± SE. Myograph data were analyzed with two-way repeated-measures analysis of variance. Gene expression fold changes were calculated according to the Livak method. One-way analysis of variance or Student’s t-test were used to analyze statistically significant differences between groups where required. P value <0.05 was considered statistically significant.
3. Results
3.1 Vitamin D diet and PVAT control resistance artery reactivity
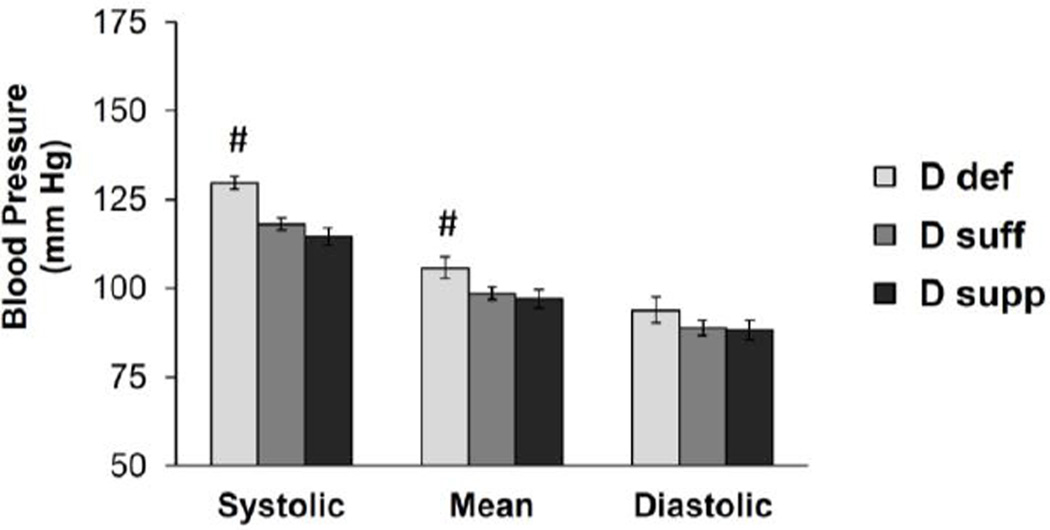
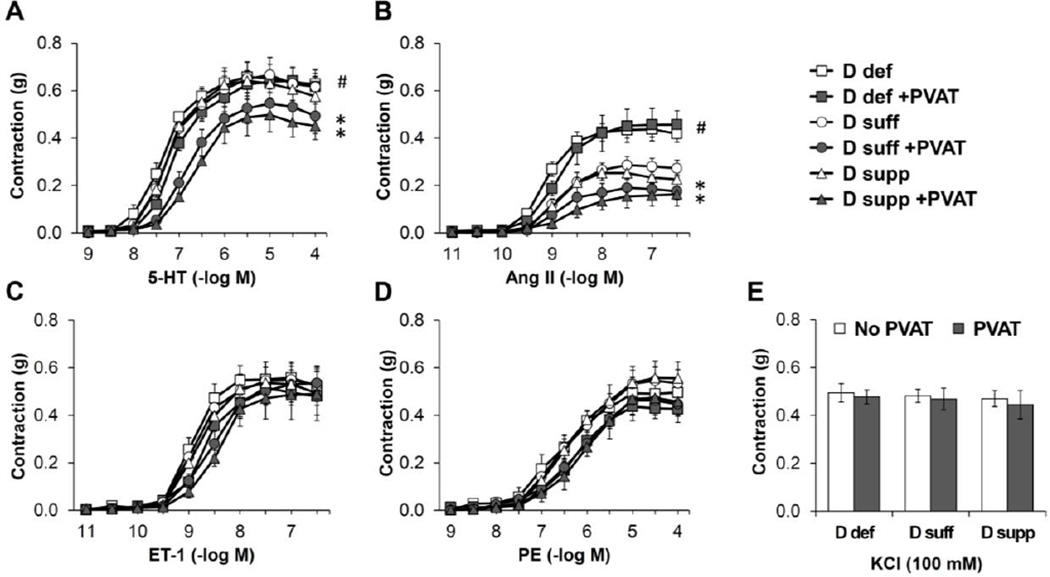
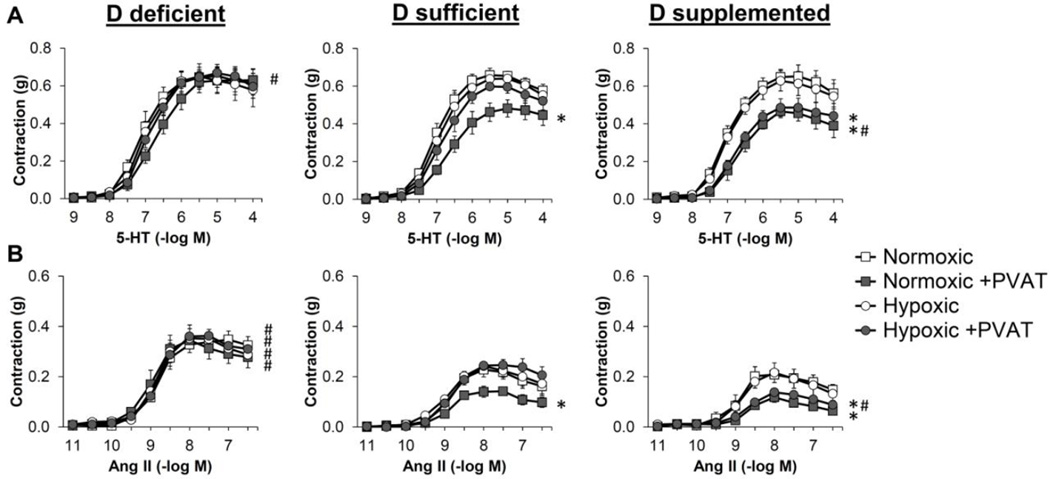
The goal of this study was to evaluate the influence that dietary vitamin D has on PVAT phenotype and vascular function. To achieve this, we utilized mice maintained on vitamin D-deficient, vitamin D-sufficient (1.5 IU/g) or vitamin D-supplemented (10 IU/g) diet. Mice maintained on vitamin D-deficient diet displayed a significant increase in systolic and mean blood pressure compared to mice on vitamin D-sufficient or vitamin D-supplemented diet (Figure 1). To evaluate the effects of vitamin D and PVAT on resistance vessel tone, we performed myograph experiments on second order mesenteric artery rings with or without PVAT attached (Figure 2). The presence of intact PVAT prohibits the use of methods that rely on imaging detection of inner lumen and outer edges of the vessel. Hence, isometric tension was measured via wire myography. Vasoconstriction was selectively increased in response to angiotensin II (Ang II) in mesenteric arteries without PVAT from mice maintained on vitamin D-deficient diet (Figure 2B). Vitamin D diet did not alter responses to other agonists including KCl, serotonin (5-hydroxytryptamine; 5-HT), endothelin 1 (ET-1) or phenylephrine (PE) in the absence of PVAT. The presence of intact PVAT caused a significant decrease in contractile responses to 5-HT and Ang II in mesenteric arteries from mice maintained on vitamin D-sufficient or vitamin D-supplemented diet but not those from mice on vitamin D-deficient diet (Figure 2A–B). Intact PVAT did not significantly alter the maximal contractile responses to KCl, ET-1 or PE, suggesting that PVAT did not simply act as a barrier to agonist stimulation. Following the myograph experiments, the mesenteric artery rings with intact PVAT were blotted dry and their mass was recorded. This confirmed that the mass of mesenteric artery rings was not different between the vitamin D diet groups (Table S1).
Figure 1. Vitamin D-deficient mice display increased blood pressure.
Blood pressure was measured using the CODA non-invasive blood pressure system on mice maintained on the different vitamin D diets. Data represent means ± SE (n=8–10 mice). #p<0.05 D def or D supp vs. D suff.
Figure 2. Vitamin D deficiency eliminates anti-contractile effects of PVAT in mesenteric resistance arteries.
Isometric tension was measured on second order mesenteric arteries from mice maintained on vitamin D-deficient diet (D def; squares), vitamin D-sufficient diet (D suff; circles) or vitamin D-supplemented diet (D supp; triangles). The mesenteric artery rings were either dissected free of PVAT (open symbols) or had the PVAT remaining intact (filled symbols). Dose-dependent contraction was assessed in response to 5-HT (A), angiotensin II (Ang II) (B), endothelin-1 (ET-1) (C), PE (D) or KCl (E). Data represent means ± SE (n=5 mice). *p<0.05 PVAT vs. no PVAT. #p<0.05 D def or D supp vs. D suff.
3.2 Vitamin D diet alters the anti-contractile properties of aortic PVAT
Different vascular beds exhibit unique properties in terms of PVAT phenotype and vascular reactivity. We examined thoracic aortic rings, with or without the PVAT (e.g. periaortic adipose tissue) attached, via wire myography (Figure S1). Vitamin D diet did not alter contractile responses to agonists including KCl, 5-HT, prostaglandin F2α (PGF2α) or PE in aortic rings without PVAT when comparing aorta from mice maintained on vitamin D-deficient or vitamin D-supplemented diet to those from mice on vitamin D-sufficient diet. Aortic rings exhibited very minimal contraction in response to Ang II or ET-1 regardless of vitamin D diet (data not shown). The presence of intact PVAT caused a significant decrease in contractile responses to 5-HT and PGF2α in aorta from mice maintained on vitamin D-sufficient or vitamin D-supplemented diet but not those from mice on vitamin D-deficient diet. Therefore, the normal anti-contractile effects of aortic PVAT are diminished by vitamin D deficiency.
We also examined the influence of aortic PVAT on vasodilation (Figure S1). Endothelium-dependent relaxation in response to acetylcholine was not largely affected by vitamin D diet or the presence of intact PVAT. Smooth muscle-dependent relaxation in response to the nitric oxide donor sodium nitroprusside was not affected by vitamin D diet or the presence of PVAT. Following the myograph experiments, the mass of the aortic rings with or without intact PVAT was recorded to confirm that it was similar between the vitamin D diet groups (Table S1).
3.3 Effects of vitamin D diet on the phenotype of different adipose tissue depots
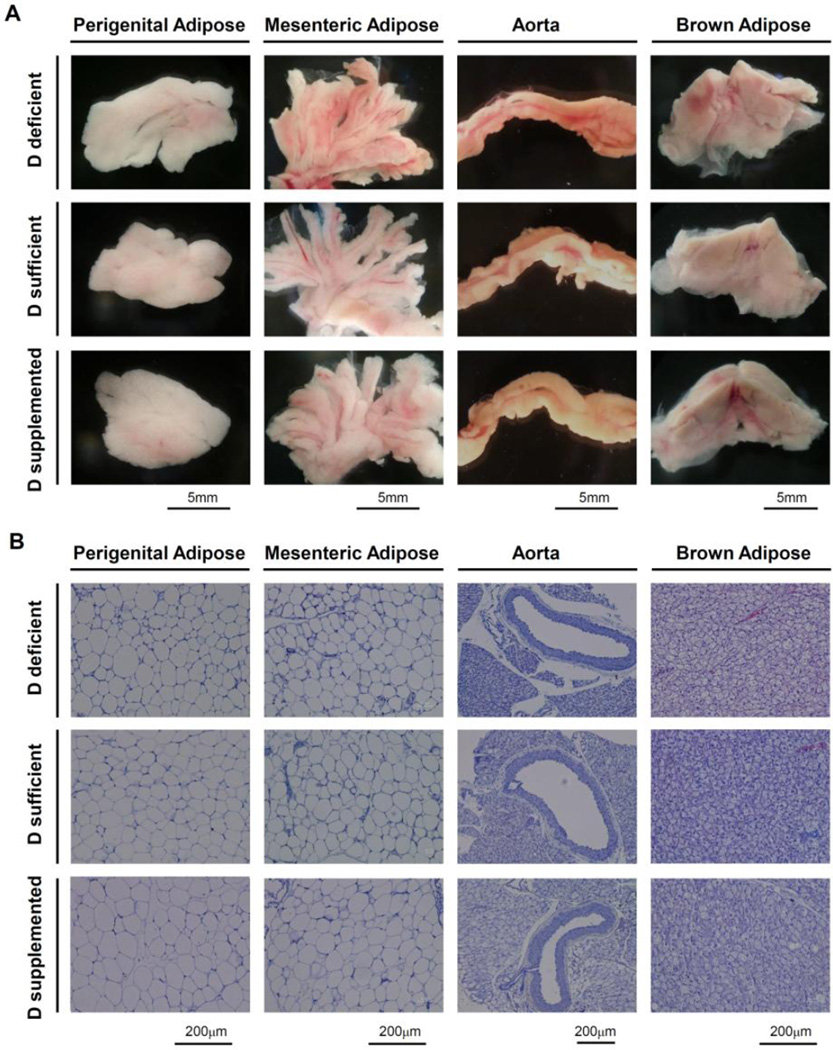
To investigate the mechanisms by which dietary vitamin D modifies the phenotype of adipose tissue and PVAT, we performed a series of histological and gene expression analyses. We began by examining the gross anatomy and the histology of perigenital white adipose tissue, mesenteric adipose tissue, interscapular brown adipose tissue and aorta with periaortic adipose tissue intact (Figure 3). We observed that the mesenteric bed from mice on vitamin D-sufficient or vitamin D-supplemented diet displayed the typical lighter appearance of visceral white adipose tissue (Figure 3A). In contrast, the mesenteric bed from vitamin D-deficient mice often displayed areas with a darker appearance than normal visceral white adipose tissue. However, the perigenital adipose tissue displayed the normal appearance of visceral white adipose tissue in each of the three vitamin D diet groups. By histology, hematoxylin and eosin staining revealed no obvious effects of vitamin D diet on the phenotype of the different adipose tissue depots or the aorta (Figure 3B). Hematoxylin and eosin stained sections of mesenteric adipose tissue exhibited a phenotype similar to perigenital white adipose tissue, whereas periaortic adipose tissue exhibited a phenotype similar to interscapular brown adipose tissue. Vitamin D diet had no significant effect on body mass, organ mass or adipose tissue depot mass (Figure S3). A shift toward enlargement of adipocyte cross-sectional area was observed within perigenital adipose tissue from vitamin D-supplemented mice, compared to vitamin D-deficient and vitamin D-sufficient mice.
Figure 3. Effects of dietary vitamin D on morphology of different adipose depots.
Representative images are shown for perigenital adipose tissue, mesenteric adipose tissue, aorta with intact PVAT and interscapular brown adipose tissue from mice maintained on the different vitamin D diets. (A) Images of the gross anatomy of tissue samples placed in a dish in Krebs buffer taken using a dissecting microscope (n=5–6 mice). (B) Images of sections from paraffin-embedded tissues stained with hematoxylin and eosin taken using a Nikon Eclipse Ci microscope (n=6–8 mice).
3.4 Vitamin D deficiency activates hypoxia signaling pathways in mesenteric PVAT
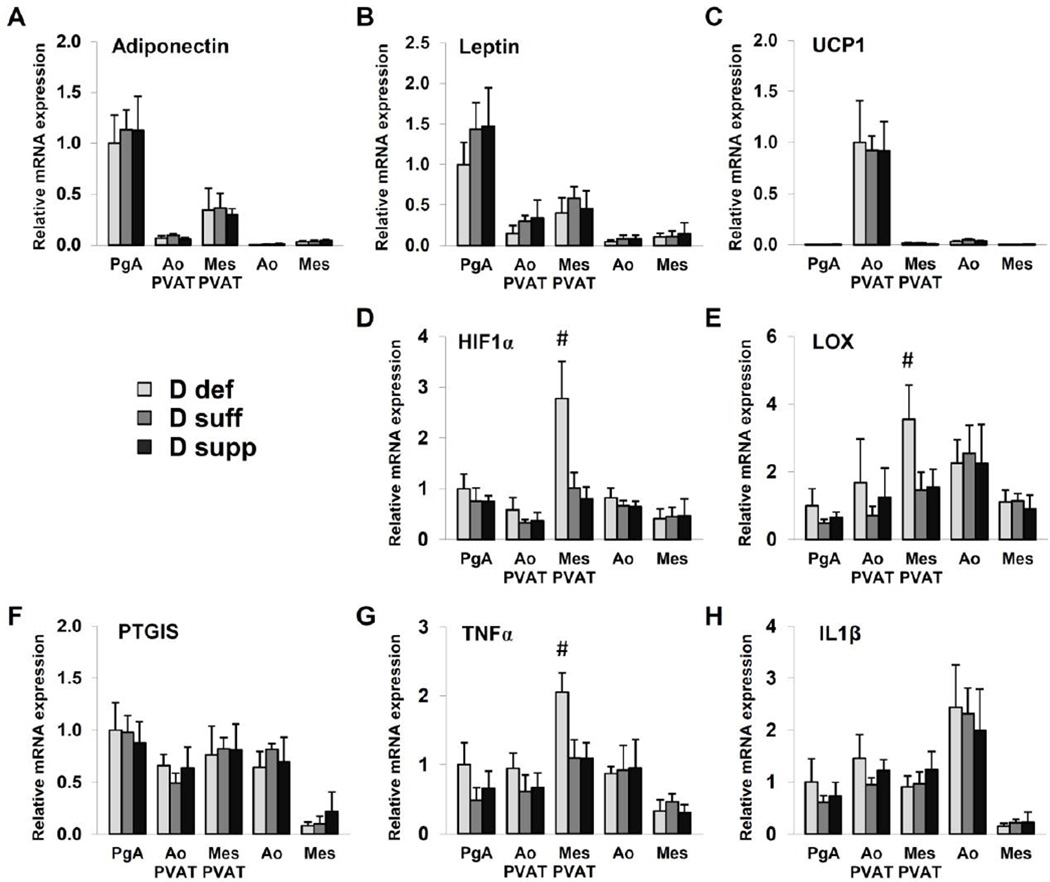
The vitamin D metabolite 1,25(OH)2D3 mediates its effects through ligand activation of the nuclear receptor transcription factor VDR. The effects of dietary vitamin D diet on PVAT and arterial function may be mediated through direct or indirect transcriptional regulation of several factors within pathways that converge to regulate the contractile apparatus of smooth muscle. We analyzed the gene expression of potential mediators using samples of aortic tissue, mesenteric artery tissue, perigenital adipose tissue, aortic PVAT and mesenteric PVAT (Figure 4). Leptin and adiponectin are important adipokines that exert direct vasodilatory effects. We observed higher expression of leptin and adiponectin mRNA in perigenital adipose tissue than other adipose tissues or vascular tissues. However, vitamin D diet did not affect leptin or adiponectin mRNA expression. Prostacyclin (prostaglandin I2) is a vasodilator synthesized by prostaglandin I2 synthase (PTGIS) and released by PVAT. PTGIS mRNA expression was not altered by dietary vitamin D in the tissues tested. Uncoupling protein-1 (UCP1) is a mitochondrial protein that contributes to the characteristic thermogenic properties of brown adipose tissue. Thoracic aortic PVAT displayed abundant expression of UCP1 mRNA, consistent with its similar appearance to interscapular brown adipose tissue (12). Again, vitamin D diet did not affect expression of UCP1 mRNA.
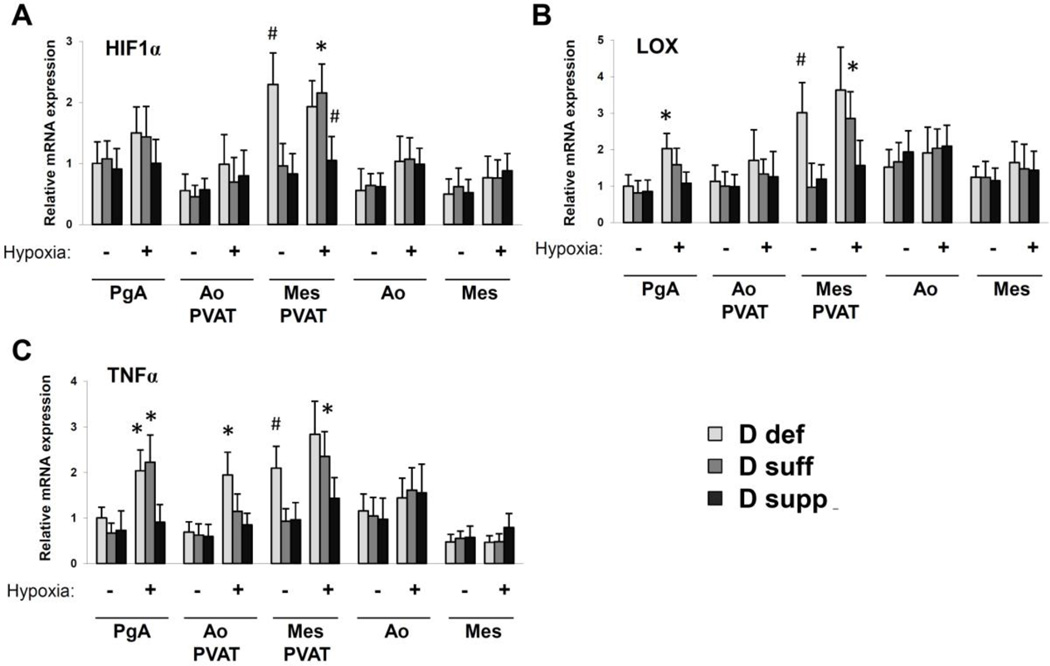
Figure 4. Vitamin D deficiency upregulates mediators of hypoxia and inflammation in mesenteric PVAT.
Gene expression analysis was performed on samples of perigenital adipose tissue (PgA), aortic PVAT (Ao PVAT), mesenteric PVAT (Mes PVAT), aortic tissue (Ao) and mesenteric artery tissue (Mes) by quantitative real-time PCR. Relative expression of mRNA transcripts was determined after normalization to GAPDH. Data for adiponectin (A), leptin (B), HIF-1α (D), LOX (E), PTGIS (F), TNFα (G) and IL1β (H) are fold-change of vitamin D-deficient perigenital adipose tissue. UCP1 data (C) are fold change of vitamin D-deficient aortic PVAT. Data represent means ± SE (n=6 mice). #p<0.05 D def or D supp vs. D suff.
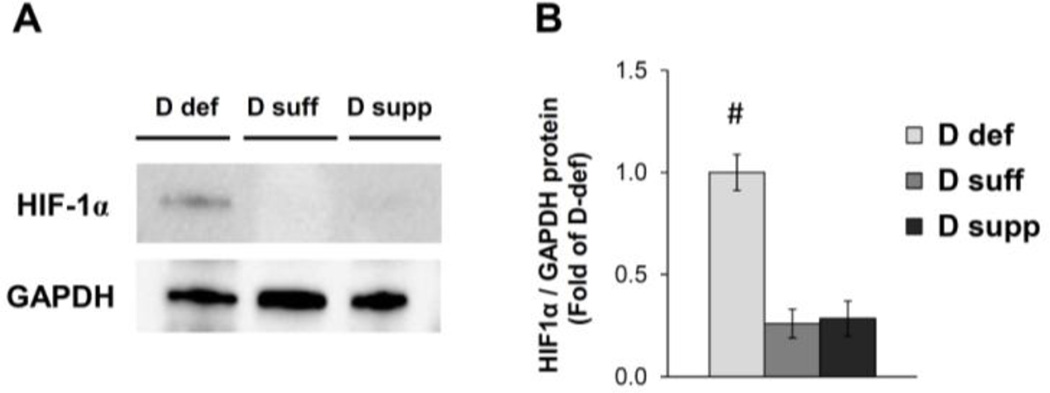
Vitamin D is implicated in the regulation of inflammatory and hypoxia signaling pathways. Vitamin D deficiency significantly increased the expression of TNFα mRNA in mesenteric PVAT (Figure 4). We also detected a significant increase in the expression of hypoxia-inducible factor 1α (HIF-1α) and lysyl oxidase (LOX) mRNA in mesenteric PVAT from vitamin D-deficient mice (Figure 4). HIF-1α is a subunit of the oxygen-sensing transcription factor HIF-1, and LOX is a target gene positively regulated by HIF-1 (13). We found that HIF-1α protein expression was induced in mesenteric PVAT from mice on vitamin D-deficient diet compared to that of mice on vitamin D-sufficient or vitamin D-supplemented diet (Figure 5). Together, these results indicate that vitamin D deficiency may cause activation of hypoxia signaling pathways in mesenteric PVAT.
Figure 5. Vitamin D deficiency induces HIF-1α protein expression in mesenteric PVAT.
Western blot analysis was performed on samples of mesenteric PVAT. A representative image of a blot is shown (A). Relative expression of HIF-1α protein was determined after normalization to GAPDH (B). Data are fold-change of vitamin D-deficient mesenteric PVAT. Data represent means ± SE (n=5 mice). #p<0.05 D def or D supp vs. D suff.
3.5 Vitamin D supplementation protects against hypoxia-induced PVAT dysfunction
Next, we tested the effects of hypoxic incubation of arteries from mice in the three vitamin D diet groups. Hypoxia was induced in the tissues in vitro using a modular incubator chamber. To test the effects of hypoxia on vascular reactivity, arterial rings with or without intact PVAT were incubated under hypoxic (2% O2) or normoxic (20% O2) conditions for 24 hours then used for isometric tension experiments via wire myograph (Figure 6; Figure S2). First order mesenteric arteries were used in this experiment due to the decrease in viability observed with second order mesenteric arteries following 24 hours of incubation (data not shown). Following normoxic incubation, the presence of intact PVAT blunted contraction in response to 5-HT and Ang II in mesenteric arteries from mice in vitamin D-sufficient and vitamin D-supplemented diet groups (Figure 6; Figure S2). For the case of vitamin D-sufficient mice, hypoxic incubation enhanced contractile responses to 5-HT and Ang II in arteries with intact PVAT when compared to normoxic incubation (Figure 6). For vitamin D-supplemented mice, however, hypoxic incubation of arteries with intact PVAT did not display a similar reversal of the anti-contractile effect on 5-HT or Ang II-induced contraction. For vitamin D-deficient mice, mesenteric arteries with or without PVAT incubated under normoxic and hypoxic conditions exhibited similar contractile responses to 5-HT and Ang II (Figure 6; Figure S2). Responses to Ang II were augmented by vitamin D deficiency compared to the other groups tested (Figure 6). These results demonstrate that hypoxic incubation of mesenteric arteries from vitamin D-sufficient mice can recapitulate features of PVAT dysfunction caused by vitamin D deficiency.
Figure 6. Hypoxic incubation inhibits anti-contractile effects of PVAT in normal mesenteric arteries.
Isometric tension was measured on first order mesenteric arteries from mice maintained on the three vitamin D diets. The mesenteric artery rings were either dissected free of PVAT (open symbols) or had the PVAT remaining intact (filled symbols) and incubated for 24 hours under normoxic (squares) or hypoxic conditions (circles). Dose-dependent contraction was assessed in response to 5-HT (A) and Ang II (B) (see also Figure S2). Data represent means ± SE (n=4–5 mice). *p<0.05 PVAT vs. no PVAT. #p<0.05 D def or D supp vs. D suff.
The same incubation and myograph experiments were performed using aortic rings (Figure S4). The presence of intact PVAT caused blunting of receptor-independent contraction to KCl for aortic rings incubated under either normoxic or hypoxic conditions independent of vitamin D diet. Intact PVAT also suppressed contractile responses to 5-HT or PGF2α in aortic rings from mice maintained on vitamin D-sufficient or vitamin D-supplemented diet, but not those on vitamin D-deficient diet. Unlike mesenteric arteries, hypoxic incubation did not enhance contractile responses in aortic rings with intact PVAT from vitamin D-sufficient mice.
3.6 Vitamin D supplementation attenuates hypoxia and inflammation in adipose tissues
Next, we tested the effects of hypoxic incubation on the expression of HIF-1α, LOX and TNFα (Figure 7). Artery and adipose tissue samples were incubated under hypoxic (2% O2) or normoxic (20% O2) conditions for 24 hours and RNA was harvested for gene expression analyses. Under normoxic conditions, the levels of HIF-1α, LOX and TNFα mRNA were significantly increased in mesenteric PVAT from vitamin D-deficient mice compared to that of vitamin D-sufficient or vitamin D-supplemented mice. Hypoxic incubation induced significant upregulation of HIF-1α, LOX and TNFα mRNA in mesenteric PVAT from vitamin D-sufficient mice but not that of vitamin D-supplemented mice. Hypoxia also induced significant upregulation of TNFα mRNA in perigenital adipose tissue from vitamin D-sufficient and vitamin D-deficient mice as well as in aortic PVAT from vitamin D-deficient mice. Altogether these results support the concept that vitamin D signaling pathways may counteract the effects of hypoxia and inflammation on adipose tissues.
Figure 7. Vitamin D supplementation negatively regulates expression of mediators of hypoxia and inflammation in adipose tissues.
Gene expression analysis was performed on samples of perigenital adipose tissue (PgA), aortic PVAT (Ao PVAT), mesenteric PVAT (Mes PVAT), aortic tissue (Ao) and mesenteric artery tissue (Mes) by quantitative real-time PCR. Tissues were incubated for 24 hours under normoxic or hypoxic conditions Relative expression of mRNA transcripts was determined after normalization to GAPDH. Data for HIF-1α (A), LOX (B), and TNFα (C) are fold-change of vitamin D-deficient perigenital adipose tissue. Data represent means ± SE (n=3–4 mice). *p<0.05 hypoxic vs. normoxic. #p<0.05 D def or D supp vs. D suff.
4. Discussion
Studies on genetic mouse models have greatly enhanced our current understanding of the physiological functions of the vitamin D system. In terms of the cardiovascular system, VDR−/− mice develop hypertension, vascular calcification and ventricular hypertrophy (14, 15). Rats fed a vitamin D-deficient diet likewise developed hypertension related to increased myogenic tone and impaired acetylcholine-mediated relaxation in mesenteric artery (16). The role of VDR in vascular function was recently examined in endothelium-specific VDR knockout mice (17). Deletion of VDR in endothelial cells caused impaired Ach-mediated relaxation in aortic rings and decreased expression of endothelial nitric oxide synthase in aortic tissue. However, endothelium-specific VDR knockout mice displayed normal baseline blood pressure (17). These results suggest that hypertension in VDR−/− mice may be caused by additional deficits in other tissues such as vascular smooth muscle and PVAT.
In the current study, we examined the effects of dietary vitamin D on arterial reactivity and PVAT phenotype. Systolic and mean blood pressure was significantly increased in vitamin D-deficient mice. Our results indicate that vitamin D-deficient treatment in mice enhanced Ang II-stimulated vasoconstriction in mesenteric artery. It is established that vitamin D signaling pathways can antagonize components of the renin-angiotensin system. VDR−/− mice display increased renal expression of renin mRNA, high plasma Ang II levels, hypertension and cardiac hypertrophy (18). Vascular smooth muscle cells cultured from VDR−/− mice displayed increased expression of Ang II type 1 receptor (AT1R) and generated a higher level of Ang II compared to control cells from wild-type mice (19). Therefore, vitamin D deficiency may enhance Ang II-mediated vasoconstriction through changes in expression or activity of AT1R and downstream signaling mediators.
Through this study, we also found that vitamin D deficiency impaired the normal ability of intact PVAT to suppress contraction of the underlying aorta or mesenteric artery in response to certain vasoconstrictor agonists. PVAT is a complex mixture of adipocytes, small vessels, fibroblasts and immune cells including macrophages and T lymphocytes (20). PVAT modulates reactivity of the underlying vessel by releasing products including adipokines, cytokines and vasoactive substances. Human and animal studies have shown that various PVAT depots increase production of cytokines and chemokines (i.e. Ang II, TNFα, IL-1β, IL-6, MCP-1) during disease states including atherosclerosis, hypertension and obesity (2, 21–23). Vitamin D deficiency is associated with numerous inflammatory diseases. A recent microarray study revealed that plasma 25(OH)D levels display an inverse correlation with mRNA expression levels of pro-inflammatory cytokines (e.g. TNFα, IL-6, MCP-1 and pentraxin 3) in epicardial adipose tissue biopsy samples from human patients with coronary artery disease (24). Mechanistically, vitamin D controls inflammatory signaling pathways through regulation of key molecules including importin-α3 (karyopherin-α4), NF-κβ, and suppressor of cytokine signaling 3 (SOCS3) (9, 25, 26).
The role of vitamin D in adipose tissue or PVAT is not completely understood. VDR is expressed in adipocytes, and siRNA-mediated knockdown of VDR was shown to inhibit adipogenesis (27). There are conflicting reports, however, that 1,25(OH)2D3 has both inhibitory and stimulatory effects on adipogenesis in cell culture (28). VDR−/− mice and Cyp27b1−/− mice exhibit a similar lean phenotype, characterized by decreased adiposity and increased energy expenditure compared to wild-type control mice (29). Cyp27b1 is the 1-hydroxylase enzyme that generates the VDR ligand 1,25(OH)2D3. VDR−/− mice and Cyp27b1−/− mice were also resistant to high fat diet-induced weight gain (29). In contrast, transgenic mice with targeted overexpression of human VDR in adipose tissue exhibited an obese phenotype along with decreased energy expenditure compared to non-transgenic control mice (30).
Factors including hypoxia, pro-inflammatory cytokines, reactive oxygen species and cyclooxygenase products (i.e. thromboxane) have the ability to potentiate the contractile effects of PVAT (2). For instance, in aortic rings from high fat diet-induced obese mice, the presence of intact PVAT potentiated contractile responses to PE or 5-HT (31, 32). The anti-contractile function of PVAT seen in aortic rings from lean mice could be restored in rings from obese mice by pre-incubation with a cyclooxygenase inhibitor (e.g. meclofenamate) or scavenger of superoxide (e.g. tiron) (31, 32). Studies on small arteries obtained from subcutaneous adipose tissue of healthy lean subjects revealed that PVAT naturally has an anti-contractile effect on these arteries. This effect is not witnessed in the corresponding arteries from obese subjects with metabolic syndrome (3, 4). The deleterious effects of metabolic syndrome on PVAT function can be recapitulated by initiating hypoxia or inflammation in vitro. Hypoxic incubation with 95% N2 and 5% CO2 gas impaired the normal anti-contractile effects of PVAT in response to norepinephrine in human and mouse arteries (3, 33). Likewise, induction of inflammation by incubation with TNFα or IL-6 impaired the anti-contractile effects of PVAT in human subcutaneous small arteries (3, 4). Intriguingly, incubation with an anti-TNFα antibody or anti-IL-6 antibody was able to suppress hypoxia-induced PVAT dysfunction in the human small arteries (3). These examples illustrate how PVAT has a central role in mediating vascular dysfunction induced by hypoxic and inflammatory stimuli.
Adiponectin, leptin, and prostacyclin are potential factors that mediate the direct vasodilatory or anti-contractile effects of PVAT in a paracrine manner (2). In the current study, we observed no effect of vitamin D diet on expression of adiponectin, leptin or PTGIS mRNA in mouse aortic PVAT or mesenteric PVAT. We did find, however, that vitamin D deficiency appeared to cause activation of hypoxia signaling pathways in mesenteric PVAT. Both HIF-1α and LOX mRNA were upregulated in mesenteric PVAT from vitamin D-deficient mice. HIF-1 is an oxygen-regulated transcription factor that mediates cellular responses to hypoxia. HIF-1 is a heterodimer consisting of HIF-1α and HIF-1β subunits. HIF-1α is expressed at basal levels in most cell types and becomes targeted for ubiquitination and proteasomal degradation by von Hippel–Lindau protein (pVHL) through an oxygen-dependent prolyl hydroxylation mechanism during normoxic conditions (1). Under hypoxic conditions, HIF-1α protein is stabilized and the HIF-1 heterodimer then regulates gene expression by binding to hypoxia response elements. HIF-1α transcription is also often upregulated during hypoxia (1). Upregulation of the HIF-1 target LOX may indicate a functional increase in HIF-1 transcriptional activity. Vitamin D has been implicated in the regulation of HIF-1. The VDR ligand 1,25(OH)2D3 inhibits the proliferation of diverse human cancer cell lines in part by decreasing HIF-1α expression and HIF-1 transcriptional activity (34). The mechanisms by which vitamin D regulates HIF-1 are unclear. One mechanism could be related to vitamin D3 up-regulated protein 1 (VDUP1), which interacts with the pVHL-HIF-1α complex, stimulating the nuclear export and degradation of HIF-1α (35).
LOX is a copper-dependent amine oxidase that catalyzes the crosslinking of collagens and elastins in the extracellular matrix. The physiological relevance of HIF-1α and LOX in adipose tissues has been examined in different mouse models. Treatment of mice with a high-fat diet has been shown to induce fibrosis in white adipose tissue, which is associated with increased expression of HIF-1α and LOX. Concurrent treatment with the selective HIF-1α inhibitor PX-478 mitigated these effects of diet-induced obesity (36). Transgenic mice that overexpress a constitutively active form of HIF-1α under the control of an adipocyte-specific promoter developed fibrosis in white adipose tissue that could be reversed by treatment with the LOX inhibitor β-aminoproprionitrile (BAPN) (37). The therapeutic usefulness of such treatments is tempered by the consequences of modulating LOX activity within the cardiovascular system. LOX has crucial functions to maintain the integrity of vascular mechanical properties. LOX deficiency in LOX−/− mice causes perinatal lethality due to defects in vascular development including a high incidence of aortic aneurysms (38). Chronic administration with the LOX inhibitor BAPN has also been shown to reduce both the strength and stiffness of the aorta in rats (39). Altogether these studies suggest that modulation of HIF-1α and LOX could have important ramifications on adipose and vascular function and remodeling.
Vitamin D therapy may be an effective strategy to improve cardiovascular health. Currently, the clinical relevance of vitamin D supplementation is in question. Meta-analyses of human clinical studies contend that vitamin D supplementation fails to reverse obesity, diabetes and hypertension (40–43). Nonetheless, trial designs with more sophisticated endpoints are warranted. Assaying of VDR target gene transcripts as biomarkers has utility in telling how a patient respond to vitamin D supplementation. For instance, studies on pre-diabetic individuals demonstrated that 5 month-term vitamin D3 supplementation did not cause a uniform induction of VDR target gene mRNA expression (i.e. CD14, thrombomodulin) in primary peripheral blood mononuclear cells or adipose tissue biopsy samples (44, 45). Thus, individuals can be stratified into populations those who respond strongly or weakly to vitamin D supplementation (44, 45). Factors that influence the potential efficacy of vitamin D intervention include polymorphisms in genes related to 25(OH)D metabolism, alterations in vitamin D binding protein levels or binding affinity, and post-translational modification of VDR or VDR coactivators (i.e. inhibitory phosphorylation). Elucidation of the mechanisms that regulate VDR activation could lead to the development of novel and improved treatments to combat human metabolic and cardiovascular diseases.
Supplementary Material
Highlights.
Perivascular fat elicits anti-contractile effects in mouse mesenteric artery
Vitamin D deficiency activates hypoxia signaling in mesenteric perivascular fat
Hypoxia induces inflammation and impairs perivascular adipose function
Vitamin D supplementation protects against hypoxia-induced vascular dysfunction
Acknowledgments
This work was supported by research grants R01 HL104516, R01HL116042, and R01 HL120659 and the Office of Dietary Supplements to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this original research article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial and competing interests’ disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
- 1.Trayhurn P. Hypoxia and adipocyte physiology: implications for adipose tissue dysfunction in obesity. Annu Rev Nutr. 2014;34:207–236. doi: 10.1146/annurev-nutr-071812-161156. [DOI] [PubMed] [Google Scholar]
- 2.Szasz T, Bomfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag. 2013;9:105–116. doi: 10.2147/VHRM.S33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009 Mar 31;119(12):1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 4.Aghamohammadzadeh R, Greenstein AS, Yadav R, Jeziorska M, Hama S, Soltani F, et al. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol. 2013 Jul 9;62(2):128–135. doi: 10.1016/j.jacc.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012 Jul;20(7):1444–1448. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 6.Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. 2014 May 29;7:69–87. doi: 10.2147/JIR.S63898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010 Oct 1;106(7):963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Ding C, Gao D, Wilding J, Trayhurn P, Bing C. Vitamin D signalling in adipose tissue. Br J Nutr. 2012 Dec 14;108(11):1915–1923. doi: 10.1017/S0007114512003285. [DOI] [PubMed] [Google Scholar]
- 9.Gupta GK, Agrawal T, DelCore MG, Mohiuddin SM, Agrawal DK. Vitamin D deficiency induces cardiac hypertrophy and inflammation in epicardial adipose tissue in hypercholesterolemic swine. Exp Mol Pathol. 2012 Aug;93(1):82–90. doi: 10.1016/j.yexmp.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal T, Gupta GK, Agrawal DK. Vitamin D supplementation reduces airway hyperresponsiveness and allergic airway inflammation in a murine model. Clin Exp Allergy. 2013 Jun;43(6):672–683. doi: 10.1111/cea.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen HC, Farese RV., Jr Determination of adipocyte size by computer image analysis. J Lipid Res. 2002 Jun;43(6):986–989. [PubMed] [Google Scholar]
- 12.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011 Oct;301(4):H1425–H1437. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006 Apr 27;440(7088):1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, et al. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011 Oct 25;124(17):1838–1847. doi: 10.1161/CIRCULATIONAHA.111.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt N, Brandsch C, Kuhne H, Thiele A, Hirche F, Stangl GI. Vitamin D receptor deficiency and low vitamin D diet stimulate aortic calcification and osteogenic key factor expression in mice. PLoS One. 2012;7(4):e35316. doi: 10.1371/journal.pone.0035316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tare M, Emmett SJ, Coleman HA, Skordilis C, Eyles DW, Morley R, et al. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J Physiol. 2011 Oct 1;589(Pt 19):4777–4786. doi: 10.1113/jphysiol.2011.214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni W, Watts SW, Ng M, Chen S, Glenn DJ, Gardner DG. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension. 2014 Dec;64(6):1290–1298. doi: 10.1161/HYPERTENSIONAHA.114.03971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002 Jul;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valcheva P, Cardus A, Panizo S, Parisi E, Bozic M, Lopez Novoa JM, et al. Lack of vitamin D receptor causes stress-induced premature senescence in vascular smooth muscle cells through enhanced local angiotensin-II signals. Atherosclerosis. 2014 Aug;235(2):247–255. doi: 10.1016/j.atherosclerosis.2014.05.911. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Agrawal DK. Dysregulation of T cell subsets in the pathogenesis of hypertension. Curr Hypertens Rep. 2015 Feb;17(2) doi: 10.1007/s11906-014-0521-1. 8,014-0521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003 Nov 18;108(20):2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 22.Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, et al. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation. 2011 Sep 6;124(10):1160–1171. doi: 10.1161/CIRCULATIONAHA.111.027375. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Wang X, Mai J, Zhao X, Liang Y, Gu M, et al. C-reactive protein promotes vascular endothelial dysfunction partly via activating adipose tissue inflammation in hyperlipidemic rabbits. Int J Cardiol. 2013 Oct 3;168(3):2397–2403. doi: 10.1016/j.ijcard.2013.01.158. [DOI] [PubMed] [Google Scholar]
- 24.Dozio E, Briganti S, Vianello E, Dogliotti G, Barassi A, Malavazos AE, et al. Epicardial adipose tissue inflammation is related to vitamin D deficiency in patients affected by coronary artery disease. Nutr Metab Cardiovasc Dis. 2015 Mar;25(3):267–273. doi: 10.1016/j.numecd.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal T, Gupta GK, Agrawal DK. Calcitriol decreases expression of importin alpha3 and attenuates RelA translocation in human bronchial smooth muscle cells. J Clin Immunol. 2012 Oct;32(5):1093–1103. doi: 10.1007/s10875-012-9696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S, Swier VJ, Boosani CS, Radwan MM, Agrawal DK. Vitamin D deficiency accelerates coronary artery disease progression in swine. Arterioscler Thromb Vasc Biol. 2016 Jul;36(7):1–9. doi: 10.1161/ATVBAHA.116.307586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blumberg JM, Tzameli I, Astapova I, Lam FS, Flier JS, Hollenberg AN. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J Biol Chem. 2006 Apr 21;281(16):11205–11213. doi: 10.1074/jbc.M510343200. [DOI] [PubMed] [Google Scholar]
- 28.Mutt SJ, Hypponen E, Saarnio J, Jarvelin MR, Herzig KH. Vitamin D and adipose tissue-more than storage. Front Physiol. 2014 Jun 24;5:228. doi: 10.3389/fphys.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology. 2009 Feb;150(2):651–661. doi: 10.1210/en.2008-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong KE, Kong J, Zhang W, Szeto FL, Ye H, Deb DK, et al. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J Biol Chem. 2011 Sep 30;286(39):33804–33810. doi: 10.1074/jbc.M111.257568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer MR, Fredette NC, Barton M, Prossnitz ER. Regulation of vascular smooth muscle tone by adipose-derived contracting factor. PLoS One. 2013 Nov 11;8(11):e79245. doi: 10.1371/journal.pone.0079245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J. 2010 Jul;74(7):1479–1487. doi: 10.1253/circj.cj-09-0661. [DOI] [PubMed] [Google Scholar]
- 33.Withers SB, Simpson L, Fattah S, Werner ME, Heagerty AM. cGMP-dependent protein kinase (PKG) mediates the anticontractile capacity of perivascular adipose tissue. Cardiovasc Res. 2014 Jan 1;101(1):130–137. doi: 10.1093/cvr/cvt229. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. 2007 Apr;6(4):1433–1439. doi: 10.1158/1535-7163.MCT-06-0677. [DOI] [PubMed] [Google Scholar]
- 35.Shin D, Jeon JH, Jeong M, Suh HW, Kim S, Kim HC, et al. VDUP1 mediates nuclear export of HIF1alpha via CRM1-dependent pathway. Biochim Biophys Acta. 2008 May;1783(5):838–848. doi: 10.1016/j.bbamcr.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Mol Cell Biol. 2013 Mar;33(5):904–917. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009 Aug;29(16):4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez C, Martinez-Gonzalez J, Raposo B, Alcudia JF, Guadall A, Badimon L. Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res. 2008 Jul 1;79(1):7–13. doi: 10.1093/cvr/cvn102. [DOI] [PubMed] [Google Scholar]
- 39.Bruel A, Ortoft G, Oxlund H. Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis. 1998 Sep;140(1):135–145. doi: 10.1016/s0021-9150(98)00130-0. [DOI] [PubMed] [Google Scholar]
- 40.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10(2):e1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vimaleswaran KS, Cavadino A, Berry DJ, LifeLines Cohort Study investigators. Jorde R, Dieffenbach AK, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014 Sep;2(9):719–729. doi: 10.1016/S2213-8587(14)70113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014 Apr;2(4):307–320. doi: 10.1016/S2213-8587(13)70212-2. [DOI] [PubMed] [Google Scholar]
- 43.Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, et al. Clinical review: Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014 Oct;99(10):3551–3560. doi: 10.1210/jc.2014-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlberg C, Seuter S, de Mello VD, Schwab U, Voutilainen S, Pulkki K, et al. Primary vitamin D target genes allow a categorization of possible benefits of vitamin D(3) supplementation. PLoS One. 2013 Jul 29;8(7):e71042. doi: 10.1371/journal.pone.0071042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vukic M, Neme A, Seuter S, Saksa N, de Mello VD, Nurmi T, et al. Relevance of vitamin d receptor target genes for monitoring the vitamin d responsiveness of primary human cells. PLoS One. 2015 Apr 13;10(4):e0124339. doi: 10.1371/journal.pone.0124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.