Abstract
Sepsis is defined as life-threatening organ dysfunction caused by dysregulated host responses to infection (Third International Consensus definition for Sepsis and septic shock). Despite decades of research, sepsis remains the leading cause of death in intensive care units. More than 40 clinical trials, most of which have targeted the sepsis-associated pro-inflammatory response, have failed. Thus, antibiotics and fluid resuscitation remain the mainstays of supportive care and there is intense need to discover and develop novel, targeted therapies to treat sepsis. Both pre-clinical and clinical studies over the past decade demonstrate unequivocally that sepsis not only causes hyper-inflammation, but also leads to simultaneous adaptive immune system dysfunction and impaired antimicrobial immunity. Evidences for immunosuppression include immune cell depletion (T cells most affected), compromised T cell effector functions, T cell exhaustion, impaired antigen presentation, increased susceptibility to opportunistic nosocomial infections, dysregulated cytokine secretion, and reactivation of latent viruses. Therefore, targeting immunosuppression provides a logical approach to treat protracted sepsis. Numerous pre-clinical studies using immunomodulatory agents such as interleukin-7, anti-programmed cell death 1 antibody (anti-PD-1), anti-programmed cell death 1 ligand antibody (anti-PD-L1), and others have demonstrated reversal of T cell dysfunction and improved survival. Therefore, identifying immunosuppressed patients with the help of specific biomarkers and administering specific immunomodulators holds significant potential for sepsis therapy in the future. This review focusses on T cell dysfunction during sepsis and discusses the potential immunotherapeutic agents to boost T cell function during sepsis and improve host resistance to infection.
Keywords: Sepsis, Immunotherapy, T cell, immunosuppression, Interleukin-7, Anti-PD-1, Anti-PD-L1, Interleukin-15, IFN-γ, Flt3 Ligand, Biomarkers
Graphical Abstract
Introduction
Sepsis is associated with a high morbidity and mortality, and is the most common cause of death among critically ill patients in non-coronary intensive care units [1]. According to the earlier consensus criteria guidelines of 1992 and 2001, sepsis was defined as a systemic response to documented or suspected infection manifested by two or more of the systemic inflammatory response syndrome (SIRS) criteria as a result of infection: temperature >38 or <36; heart rate >90 beats per minute; respiratory rate >20 breaths per minute; and white blood cell count >12,000/cu mm or <4,000/cu mm, or >10% immature (band) forms [2]. Accordingly, severe sepsis was defined as sepsis associated with organ dysfunction, hypoperfusion, or hypotension and septic shock as sepsis induced hypotension which is not corrected by adequate resuscitation and pressor support along with presence of perfusion abnormalities [2]. Sepsis and severe sepsis account for an estimated 20.7 million and 10.7 million cases per year worldwide, respectively, and may contribute to up to 5.3 million deaths worldwide per annum [3]. The recently formulated guidelines by the third international consensus definition for sepsis and septic shock (Sepsis-3) define sepsis as life-threatening organ dysfunction caused by dysregulated host responses to infection [4]. Currently, there is no definitive therapy to treat sepsis and physicians must rely on supportive care alone in the form of antibiotics and fluid resuscitation. Care for septic patients costs approximately $17 billion in the United States alone [5] and undoubtedly much more worldwide. Moreover, the incidence of sepsis is increasing at an alarming rate in the ageing population, who have inadequate immune responses as a consequence of immunosenescence [6].
Numerous clinical trials have failed to yield any novel therapeutics for sepsis. A hyper-inflammatory response characterized by excessive release of pro-inflammatory mediators such as TNF-α and interleukin-1, was thought to be the hallmark of sepsis and the most viable therapeutic target based on numerous pre-clinical and clinical studies [7]. On the contrary, clinical trials that have targeted pro-inflammatory mediators using agents such as anti-endotoxin (LPS, Lipopolysaccharide) antibodies, proinflammatory cytokine (TNFα, IL-1β) blocking antibodies and inhibitors, and TLR (Toll like receptor) antagonists have been disappointing [8–10]. Thus, the foundation of current sepsis treatment is supportive and consists of timely antibiotic administration, fluid resuscitation and organ system support. That strategy has attenuated early deaths among septic patients and improved overall survival [11, 12]. Yet, despite these improvements, severe sepsis and septic shock still result in mortality rates of more than 30 % [13].
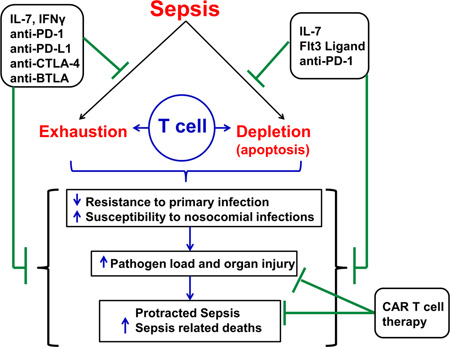
Extensive research and better understanding of the immunological alterations during sepsis during the past decade, has revealed a role for immunosuppression in the pathogenesis of sepsis [11, 14, 15]. Historically, sepsis was considered to be composed of an initial hyper-inflammatory phase (SIRS) followed by an anti-inflammatory or immunosuppressive phase (counter anti-inflammatory response syndrome, CARS) [7, 16]. This biphasic paradigm has been challenged by numerous recent reports, and it has now become evident that pro-inflammatory and anti-inflammatory phases can occur during variable time points during sepsis [17, 18]. Currently, immunosuppression during sepsis is a topic of intense research among numerous laboratories worldwide. Indeed, various studies show that patients surviving the initial inflammatory phase of sepsis are highly susceptible to nosocomial infections with opportunistic organisms and suffer late deaths among initial sepsis survivors [19, 20]. Although modern medicine strategies have resulted in improving the short term patient outcome in septic patients, it has equally resulted in a more protracted disease state with a shift towards immunosuppressive phenotype causing increased incidence of delayed deaths. In fact, greater than 70 % of deaths occur after the 3 days of sepsis initiation, many of which occur weeks after sepsis onset [20]. More recent studies indicate that patients discharged from the hospital after sepsis have a high one year mortality rate, often due to the development of secondary infections [3, 15]. With respect to the increasing findings of a shift in the time frame of mortality after sepsis, which can occur even years after initial septic insult, a trimodal distribution of deaths after sepsis has been recently postulated [15]. The three postulated phases include: early deaths due to inflammatory response, late deaths due to persistent organ injury and immunosuppression and delayed long term deaths (beyond 60–90 days post sepsis) due to persistent immune dysfunction and inflammation in the presence of other co-morbidities and advanced age [15]. It is important to note that, immune dysfunction or suppression is increasingly being recognized to play a critical role even in the pathology of delayed deaths after sepsis. Postmortem studies of patients who die of sepsis indeed have revealed marked immunosuppression [21] and pre-clinical studies equally support these findings [22–24]. Research by Hotchkiss et al. and others, have consistently shown that defects in effective adaptive immune system responses are a hallmark of immunosuppression during sepsis [11, 14, 16]. Immunotherapeutic strategies aimed at stimulating the immune system hold significant potential to reverse sepsis-induced immunosuppression and improve patient outcomes. The focus of this review is to highlight the major alterations in adaptive immune responses during sepsis, and the current and future potential for novel immunotherapeutic agents targeting reversal of T cell dysfunction.
Introduction to the adaptive immune system
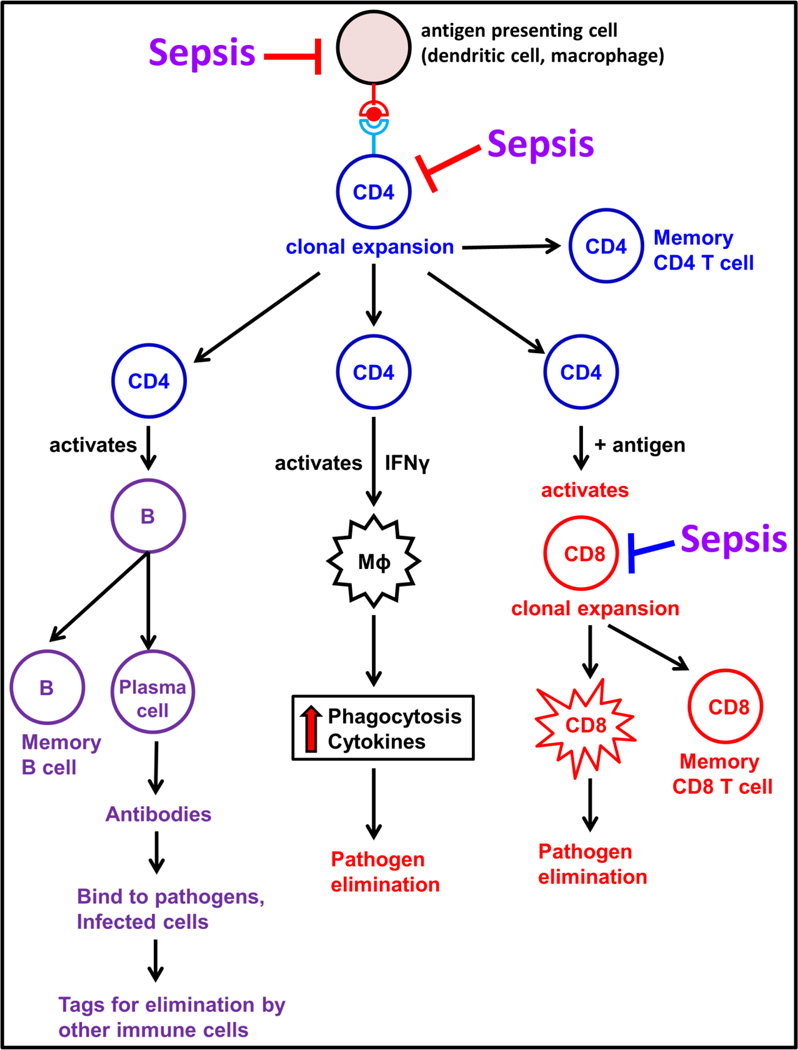
The adaptive immune system is composed of cells that respond in a highly specific manner to the particular antigen that induced them. It is composed of specialized cells known as lymphocytes, specifically T and B lymphocytes, which mediate the cell- and humoral immune responses respectively. Figure 1 shows a brief overview of various cells of the adaptive immune system. T cells play an important role in the elimination of infecting pathogens [25]. Innate immune cells such as dendritic cells, macrophages and monocytes prime naïve T cells by presenting specific pathogen-specific antigens in conjunction with major histocompatibility complex (MHC) class I and class II molecules [26]. Naïve T cells upon antigenic stimulation undergo clonal expansion, produce cytokines and generate antigen-specific effector cells, which help to clear the invading pathogen. Upon resolution of infection, the majority of effector T cells die (contraction phase) and the surviving T cells transform into the memory T cells, which are critical for generating recall responses to specific antigens upon reencounter with similar antigens [25]. CD4+ and CD8+ are the major T cell subsets. CD4+ T cells are also known as the helper T cells and play a critical role in orchestrating numerous responses of both the innate and adaptive immune systems [27]. CD8+ T cells, also known as cytotoxic T cells are important for targeted killing of tumor cells or virus-infected cells [25]. The antibody mediated responses are carried out by B cells, a process which is helped by CD4+ T cells [27]. The antibodies (immunoglobulins) produced by B cells are antigen specific. Interaction of immunoglobulin and antigen leads to myriad of effects including: inactivation of viruses or microbial toxins by blocking their interaction with host cells; as well as tagging of invading pathogens for destruction by phagocytes. Therefore, both T and B cells play a critical role in protecting host against life threatening infections. Impairment of such crucial defense mechanisms renders the host unable to eradicate primary infectious foci in the body, in addition to increasing susceptibility to secondary infections during sepsis [17]. The following sections will describe the current knowledge regarding impaired adaptive immune responses during sepsis and potential immunotherapeutic interventions.
Figure 1. Overview of the Adaptive Immune System.
The helper CD4+ T cells are activated upon antigen presentation by the antigen presenting cells. The activated CD4+ T cell undergo clonal expansion to form memory CD4+ T cells and effector CD4+ T cells. The effector CD4+ T cells serve to activate B cells, CD8 T cells and even macrophages to carry out pathogen elimination. Sepsis impairs the functions of both antigen presenting cells and the cells of adaptive immune system. (CD4 – CD4+ T lymphocytes, B – B lymphocytes, CD8 – CD8+ T lymphocytes and Mϕ – Macrophage).
Sepsis-induced T cell dysfunction
Patients respond to sepsis in a heterogeneous manner, with some patients producing both pro-inflammatory and anti-inflammatory cytokines early after sepsis onset, and some characterized by decreased cytokine secretion or anti-inflammatory response alone [14, 16]. Although some patients still succumb to death during the initial phase of sepsis, new treatment protocols rescue the majority of the patients from this phase, only to lead into an immunosuppressive phase [12]. Numerous pre-clinical and clinical studies show that multi-organ failure is a common consequence of sepsis which may lead to death [4, 28–30]. Growing evidence supports the hypothesis that immunosuppression is a major player in sepsis-induced mortality [15, 17]. Immunosuppression during sepsis is also evident from the fact that septic patients are highly susceptible to secondary infections caused by opportunistic pathogens such as Pseudomonas, Candida, Acinetobacter and Enterococcus [20], and a high incidence of reactivation of latent viruses such as cytomegalovirus and herpes simplex virus [31, 32]. Major mechanisms leading to sepsis induced impairment of adaptive immune system include: (i) apoptosis induced cellular depletion, and (ii) cellular exhaustion and impaired responsiveness to stimuli due to upregulation of inhibitory receptors or downregulation of essential co-stimulatory receptors, on the cell surface, and (iii) impaired cytokine responses [17, 21].
Sepsis-induced apoptosis of adaptive immune cells
Apoptosis is also known as programmed cell death. The term was first utilized in a historical paper by Kerr et al. in 1972, to describe a distinct form of cell death [33]. Apoptosis is a physiological process, known to occur during normal growth and development. But numerous pathological conditions including sepsis [34], exposure to irradiation and anti-cancer drugs [35], also cause cellular apoptosis, which can have deleterious consequences for the host survival.
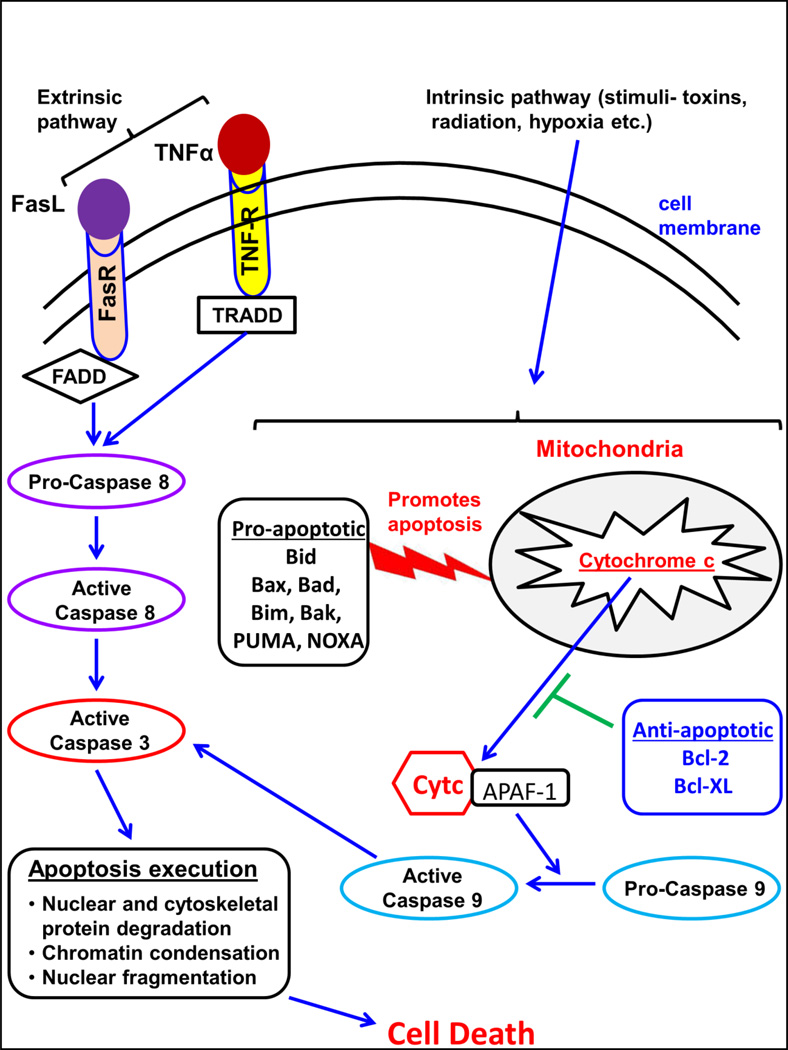
Classical mechanistic pathways of apoptosis include the extrinsic pathway and an intrinsic or the mitochondrial pathway [36]. Figure 2 describes the major pathways of apoptosis. The T cell-mediated perforin-granzyme-dependent killing of a cell also leads to apoptosis, which constitutes another known mechanism [36]. However, the ultimate executor of all the apoptotic pathways proceeds via cleavage of caspase-3, which causes DNA fragmentation and cleavage of cytoskeletal and nuclear proteins leading to cell death [36, 37]. The extrinsic pathway of apoptosis is mediated via interaction of the major death ligands such as Fas ligand (FasL or CD95L) and Tumor Necrosis Factor (TNFα) with a corresponding death receptor like Fas receptor (FasR or CD95R) and TNF receptor (TNFR), respectively, on the cell surface. Fas/FasR interaction leads to binding of the adapter protein FADD and the TNF/TNFR interaction leads to binding of the adapter protein TRADD followed by recruitment of FADD and receptor interacting protein (RIP) [38]. Ultimately, death- induced signaling complex (DISC) is formed via association with pro-caspase-8, leading to activation of caspse-8. Caspase-8 then triggers the execution phase of apoptosis through caspase-3 activation, leading to cell death. Alternatively, the intrinsic pathway can be triggered via various stimuli including radiation, hypoxia, free radicals, among others [36]. Such toxic stimuli disrupts mitochondrial membrane integrity leading to opening of the mitochondrial permeability transition pore (MPT), loss of membrane potential and release of mitochondrial cytochrome c into the cellular cytoplasm, which ultimately leads to activation of caspase-9, which cleaves caspase-3, leading to execution of apoptosis and cell death. One of the major controlling proteins for mitochondrial events of apoptosis is the Bcl-2 family of proteins, which govern cytochrome c release through regulation of mitochondrial membrane permeability [39]. Some of the anti-apoptotic proteins in Bcl-2 family include Bcl-2, Bcl-x, Bcl-XL, Bcl-XS, Bcl-w, BAG, while the pro-apoptotic proteins include Bcl-10, Bax, Bid, Bim, Bik, Bad, and Blk [36]. These proteins act through various mechanisms to induce apoptosis. The non-phosphorylated form of Bad can translocate to mitochondria and trigger cytochrome c release [40], and it can also bind to Bcl-Xl and Bcl-2, thereby inhibiting their protective effects [41]. Though the mechanisms are not well understood, Bcl-2 and Bcl-XL inhibit apoptosis by regulating caspase activation [42]. Another Bcl-2 family protein called Puma which can be induced by p53, acts with BAX protein as a pro-apoptotic molecule [43]. Mitochondrial damage is also known to occur via the extrinsic Fas pathway through caspase-8 mediated cleavage of Bid, which can translocate to mitochondria where it can bind to Bax/Bak of the intrinsic pathway leading to apoptosis [44, 45]. This mechanism is one of the known interaction points between the two classical pathways of apoptosis [46]. For both, the intrinsic and the extrinsic pathways, caspase-3 activation orchestrates the final apoptosis induced cell death [36].
Figure 2. Major pathways of Apoptosis.
Extrinsic pathways of apoptosis proceed through binding of Fas ligand to Fas receptor and/or TNFα to TNF receptor, leading to intracellular caspase 8 activation. Intrinsic pathway of apoptosis involves compromised mitochondrial membrane integrity due to various stimuli such as bacterial toxins, radiation and others. Various pro-apoptotic and anti-apoptotic mediators are shown. Mitochondrial membrane integrity is compromised leading to cytochrome c release, which ultimately leads to caspase-9 activation. Both activated caspase 8 and 9, further activate caspase 3, which carries out the final execution of the apoptosis cascade leading to cellular disintegration and death.
Apoptosis has been shown to be a significant pathological process during sepsis. In a remarkable study by Wang et al., injection of gram-negative bacteria into mice resulted in apoptosis of CD4+ and CD8+ cells in the thymus [47]. In the 1990’s, this study was followed by other reports which showed extensive apoptosis of lymphocytes in various organs including spleen, thymus, colon, ileum, lung, and skeletal muscle in a cecal ligation and puncture (CLP)-induced model of sepsis in mice [48] and one study which additionally showed apoptosis of B220+ cells in the bone marrow and immature T cells in the thymus [49, 50]. Following these studies, numerous pre-clinical studies provided definitive evidence that sepsis leads to significant apoptosis- induced loss of CD4+ and CD8+ lymphocytes [16, 22, 23, 51, 52]. A significant T cell depletion in spleen and wound draining lymph nodes has also been shown in systemic Pseudomonas infection during burn injury [53]. Importantly, inhibition of apoptosis using specific caspase inhibitors, caspase 3 knockout mice, or overexpression of anti-apoptotic Bcl-2 protein significantly decreased apoptosis of T cells and improved survival in a CLP model of sepsis [54, 55]. Another important finding was that T and B cell-deficient mice (Rag−/− knockout) showed increased mortality after sepsis as compared to wild type control mice [54] and caspase inhibitors did not afford any survival benefit after sepsis [24], implying that adaptive immune responses are essential for survival after sepsis. Indeed, these pre-clinical findings are also seen in clinical studies of septic patients. Numerous prospective studies, using blood samples collected from septic patients show that sepsis leads to severe lymphopenia early on with activation of pro-apoptotic caspase-8,9 and 3 in lymphocytes, reduced expression of anti-apoptotic Bcl-2 protein and Bcl-xl, and increased gene expression of pro-apoptotic proteins such as Bim, Bid and Bak. The degree of lymphocyte apoptosis correlated with the severity of sepsis in a subset of patients [56–58]. Moreover, postmortem analysis of sepsis patients also reveals an extensive loss of CD4+ T, CD8+ T and B lymphocyte populations in the spleen and lymph nodes [21, 34]. These studies also show that sepsis-induced apoptosis of immune cells can occur via multiple pathways including both extrinsic and mitochondrial- mediated mechanisms [59]. Tables 1 and 2 provide details of the major pre-clinical and clinical studies which show that immune cell apoptosis is an important factor in sepsis pathophysiology and provides an important target for therapeutic intervention in the future. However, this is challenging as such a therapy will need to be specifically targeted for lymphocyte cell populations which are undergoing accelerated apoptosis during sepsis. Moreover, the anti-apoptotic therapy should have a time limited effect until pathogen elimination and resolution of infection, to prevent any unnecessary prolonged blockade of physiological cell death.
Table 1.
Sepsis induced apoptosis (Pre-clinical studies)
| Experimental Model of Sepsis (Pre-clinical studies) |
Author | Major Findings |
|---|---|---|
| 1. Intraperitoneal injection of bacteria (Pseudomonas, Klebsiella, Streptococcus Pneumonia) |
Wang et al, 1994 [ref – 47] |
|
| 2. Cecal Ligation and Puncture (CLP) |
Ayala et al. 1995, 1996 [ref – 49, 50] |
|
| 3. CLP | Hotchkiss et al. 1997 [ref – 48] |
|
| 4. CLP | Hotchkiss et al. 1999, 2000 [ref – 24, 54, 55] |
|
| 5. CLP in Rag knockout mice | Hotchkiss et al. 1999 [ref – 54] |
Decreased 7 day survival in Rag KO mice (~5.3%) as compared to wild type mice (~45%), showing T cell are necessary for sepsis resolution |
| 6. CLP in FADD and pro- apoptotic Bid, PUMA and Noxa knockout mice |
Chang et al. 2007 [ref – 58] |
|
| 7. CLP in humanized mice model (functional human immune system in mice) |
Unsinger et al. 2009 [ref – 52] |
Increased T and B lymphocyte apoptosis in spleen (humanized cells undergo similar apoptosis as mice during sepsis) |
| 8. CLP | Unsinger et al. 2010 [ref – 23] Inoue et al. 2010 [ref – 51] |
|
| 9. Pseudomonas aeruginosa sepsis post burn injury |
Patil et al. 2016 [ref – 53] |
|
Table 2.
Sepsis induced apoptosis and T cell exhaustion (Clinical studies)
| Clinical Study overview |
Author | Major Findings |
|---|---|---|
| 1. Postmortem study of 20 sepsis patients |
Hotchkiss et al. 1997 [ref – 48] |
|
| 2. Postmortem study of 24 sepsis patients |
Hotchkiss et al. 2001 [ref – 34] |
|
| 3. Prospective study (blood samples, 71 sepsis patients) |
Hotchkiss et al.2005 [ref – 57] |
|
| 4. Prospective study (blood samples, 16 severe sepsis patients) |
Weber et al. 2008 [ref – 58] |
|
| 5. Postmortem study of 40 sepsis patients |
Boomer et al. 2011 [ref – 21] |
|
| 6. Prospective study (blood samples from 19 septic shock patients) |
Zhang et al. 2011 [ref – 75] |
|
| 7. Prospective study (blood samples from 64 sepsis patients) |
Guignant et al. 2011 [ref – 71] |
|
| 8. Prospective study (blood samples from 24 sepsis patients) |
Boomer et al. 2012 [ref – 56] |
|
| 9. Prospective study (blood samples, 24 sepsis patients) |
Shubin et al. 2013 [ref – 76] |
Increased BTLA expression on CD4+ T cells correlated with increased incidence of nosocomial infection |
| 10. Prospective study (blood samples, 27 Candida fungal sepsis patients) |
Spec et al. 2016 [ref – 72] |
|
T cell exhaustion and impaired response
T cell exhaustion represents an altered differentiation state, which is often characterized by features such as loss of effector functions, sustained upregulation of multiple cell surface inhibitory receptors, downregulation of co-stimulatory receptors, altered expression of key transcription factors and metabolic derangements [60]. It was first described in chronic viral infections in mice [61], and has even been shown in patients with HIV and hepatitis C virus infection, and cancer patients. Exhausted T cells have poor functional capacity with reduced cytokine production (interferon-gamma, interleukin-2, granzyme-B, TNF-α), impaired proliferation, and are prone to undergo apoptotic death. Major inhibitory cell surface receptors on T cells include: Programmed Death-1 (PD-1), B and T lymphocyte attenuator (BTLA or CD272), Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4 or CD152), T cell membrane protein-3 (TIM-3), and lymphocyte activation-gene-3 (LAG-3). The ligands for these inhibitory receptors are typically present on antigen presenting cells such as dendritic cells, macrophages and monocytes or on the target cells themselves. PD-1 interacts with Programmed Death-Ligand 1 and 2 (PD-L1, PD-L2), BTLA interacts with HVEM (herpes virus entry mediator), TIM-3 with CEACAM1 (carcinoembryonic antigen-related cell adhesion molecule 1) or Galectin 9, LAG3 interacts with antigen molecule presented in conjunction with major histocompatibility class II (MHC II) molecule and CTLA-4 interacts with CD80/CD86 on antigen presenting cells [60]. Co-stimulatory signals through cell surface CD28, along with T cell receptor (TCR), are required for optimal T cell functions including proliferation, cell survival and production of IFNγ and interleukin-2, which are critical to combat infections [62].
In addition to apoptosis, T cell exhaustion leading to impaired responsiveness is increasingly been recognized to play a key role in the pathophysiology of sepsis induced immunosuppression [16, 21]. [63]. T cell exhaustion often develops in the face of persistent antigen exposure and/or inflammation [60]. High antigen load and inflammation due to infection are characteristic features of sepsis [20]. Therefore, T cells have a prolonged exposure to persistent stimuli, which can lead to increased expression of cell surface inhibitory receptors. PD-1/PD-L1 is one of the major inhibitory receptor-ligand interactions studied during sepsis. PD-1 is usually upregulated on the surface of CD4+ and CD8+ T cells upon stimulation to prevent excessive T cell activation [60, 64]. The downstream signaling events of PD-1 that lead to T cell dysfunction include an intracellular immunoreceptor tyrosine -based switch (ITSM) and an immunoreceptor tyrosine-based inhibitory motif (ITIM), which recruit tyrosine-protein phosphatases SHP1 and/or SHP2 (SRC homology region 2 domain containing phosphatases) [60]. Numerous pre-clinical studies have shown a sustained increase in both PD-1 and PD-L1 expression during sepsis. In animal models, sepsis has been shown to cause increased expression of PD-1 on T cells and PD-L1 expression on monocytes which is associated with increased T cell apoptosis and mortality in a mouse model of CLP [65–67]. PD-L1 expression has also been shown to be increased on neutrophils in addition to monocytes in animal models of sepsis [68] and patients with active tuberculosis and HIV [69, 70]. Extensive clinical studies support these preclinical findings. In a postmortem study, T cells showed an increased expression of PD-1 and the activation marker CD69, and decreased expression of IL-7 receptor (IL-7R or CD127) and CD28 [21]. In the same study, ex vivo stimulation of splenocytes with LPS or anti-CD3/anti-CD28 showed a significant decrease in cytokine production (TNF-α, IFN-γ, IL-6, IL-10), implying impairment of lymphocyte function [21]. T cell proliferation is also impaired during sepsis [22, 23, 51, 71]. Recent reports show that PD-1 expression is significantly increased on peripheral blood CD4+ and CD8+ T cells during Candida bloodstream infection associated with CD8+ T cell activation (increased CD69 expression), a decrease in co-stimulatory CD28 expression and IL-7R expression [72]. Another prospective clinical study showed that as sepsis progressed, T lymphocytes in blood show increased expression of TIM-3, LAG-3, CTLA-4 and decreased IL-7R expression and IFN-γ secretion upon ex vivo simulation, along with an increased PD-L1 expression on dendritic cells [56]. This study showed some remarkable results because the inhibitory receptor expressions were comparatively low at the onset of sepsis but increased significantly over time as sepsis progressed [56, 73]. Another prospective clinical study also showed that severe sepsis caused a significant increase in PD-1 expression on CD4+ and CD8+ T lymphocytes and PD-L1 on monocytes, and furthermore monocyte PD-L1 expression on monocytes served as an independent predictor of 28-day mortality in septic shock patients [74, 75]. Shubin et al. showed that BTLA expression was significantly increased on circulating CD4+ T cells and B cells in septic mice, which was associated with loss of these cells [76]. Moreover, critically ill septic patients also show increased BTLA expression on circulating CD4+ T cells and higher BTLA expression (>80 %) correlated with increased susceptibility to acquiring nosocomial infections [76]. Ventilator associated pneumonia is known to be one of the most common nosocomial infections in ICU settings [77, 78] and interestingly, a postmortem study by Boomer et al. showed PD-L1 and HVEM expression (HVEM is ligand for BTLA) is detectable in the lungs of septic patients as compared to controls [21]. This leads to a presumptive hypothesis that septic patients have increased susceptibility to secondary lung infections in part due to increased expression of inhibitory ligands on lung parenchyma which downregulate T cell functions [17]. Table 2 describes some of the major clinical studies showing the evidence for T cell exhaustion markers and related functional alterations.
T cell exhaustion can also be facilitated by soluble mediators such as interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) [60]. A high ratio of IL-10 to TNF-α has been shown to be correlated with increased mortality in patients with sepsis and IL-10 is induced early on in the course of sepsis [79, 80]. IL-10 has been shown to suppress the production of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, IFN-γ, and GM-CSF (granulocyte monocyte colony stimulating factor) [81] and negatively affect immune cell responses. Numerous clinical studies have shown a global depression of cytokine production during sepsis, although the functional importance of that observation remains to be fully determined [82–84]. IL-10 can be produced by a variety of cell types, among which are regulatory T cells (Tregs). Tregs are characterized by flow cytometry as CD4+CD25+FoxP3+ and CD4+CD25+CD127+ and are elevated during the post-acute phase of sepsis. The emergence of Tregs during sepsis correlates with CD4+ T lymphocyte depletion [85]. Tregs can impair effector T cell functions, causing suppression of cellular proliferation and cytokine secretion, and induction of apoptosis [86]. Studies also demonstrate an interaction between Tregs and the PD-1 pathway, because a simultaneous depletion of Tregs and PD-1 blockade caused a synergistic augmentation of viral control and reversal of CD8+ T cell exhaustion [87]. IL-10 can also be induced by monocytes upon PD-1/PD-L1 ligation. TGF-β is another antiinflammatory cytokine that impairs T cell functions, such as IL-2 production and cell proliferation [88], and promotes the development of Tregs [89]. TGF-β levels have been shown to be increased in patients early during the course of sepsis [90, 91]. Similarly, pre-clinical studies also show an elevation in the TGF-β levels during experimental sepsis [92, 93]. TGF-β has been shown to negatively affect T cell proliferation and impair T cell mediated immune responses in the lung [94]. Ayala et al. showed that elevated TGF-β levels correlated with depressed splenocyte functions (interleukin-2 release) in a CLP model of sepsis and treatment with monoclonal antibody against TGF-β attenuated spleenocyte dysfunction [92]. Therefore, anti-inflammatory cytokines such as IL-10 and TGF-β might contribute significantly to sepsis induced impairment of T cell function.
Therefore, numerous mechanisms, which are potentially interconnected at different cellular signaling levels, have been implicated in T cell exhaustion during sepsis. As discussed above, increased expression of inhibitory receptors on T cells, increased Treg populations, and global cytokine depression with increased preponderance towards an anti-inflammatory phenotype may tilt the balance in favor of immunosuppression in the course of sepsis [17, 21, 56].
Is a need for immunotherapy justified?
Numerous clinical trials aimed at modulating the pro-inflammatory response during sepsis have failed [8]. As discussed in detail above, the traditional view of sepsis being a pre-dominantly inflammation-driven pathology is changing as a result of extensive research demonstrating early and concurrent immunosuppression [16]. Both pre-clinical and clinical studies clearly show that sepsis not only leads to T cell dysfunction, but also impairs the actions of antigen presenting cell functions such as monocytes, macrophages [95] and dendritic cells [96], resulting in an overall depression of normal immune responses. More than 70% of sepsis associated mortality occurs in the later course of disease, which is dominated by inability to control the primary infection and development of secondary nosocomial infections [20]. Sepsis still remains a serious threat with high mortality rates [4], the burden of which is compounded by a growing incidence of antibiotic resistant micro-organisms [97, 98]. Therefore, strategies to augment host immunity warrant detailed investigation and hold promise as a therapy in sepsis. The following sections of this review will focus on immunomodulatory agents targeted at boosting T cell functions during sepsis. The majority of knowledge about these immunomodulatory agents has been gained from animal studies and some clinical investigations.
Immunomodulatory agents in sepsis (summarized in Table 3)
Table 3.
Potential Immunotherapeutics for Sepsis
| Immunotherapy agent |
Mechanism of Action | Proposed benefit during sepsis |
Potential adverse effects |
|---|---|---|---|
| IL-7 |
|
|
|
| IL-15 |
|
|
|
|
Anti-PD-1 antibody |
|
|
|
|
Anti-PD-L1 antibody |
|
|
|
|
Anti-BTLA antibody |
|
|
|
|
Anti-CTLA4 antibody |
|
|
|
| IFNγ |
|
|
|
| Flt3 Ligand |
|
|
|
| CAR T cell therapy |
|
|
|
needs to be determined in future studies
1. Recombinant human Interleukin-7 (IL-7)
IL-7 (a 25 KDa glycoprotein) was discovered in 1988, a result of in vitro experiments demonstrating its growth stimulatory effect on B cell progenitors [99]. However, IL-7 also impacts T cell proliferation and differentiation and holds significant potential for improving T cell homeostasis during sepsis [23, 100, 101]. IL-7 is mainly produced by stromal cells in multiple organs including thymus, peripheral lymphoid organs, skin, intestine and liver [102]. The majority of immune cells do not produce IL-7 and only a small amount is produced by the dendritic cells [103]. The IL-7 receptor is a heterodimer composed of IL-7Rα (CD127) and the common receptor γ-chain (CD132) [104] and is expressed by most T cells (except for low levels on Treg cells). IL-7 is considered to be a pluripotent cytokine that is absolutely required for human T cell development, as evidenced by T cell absence in patients with severe combined immunodeficiency (SCID) as a result of mutations in the IL-7Rα or common γ chains [105, 106]. The major mechanisms of action of IL-7 relevant to sepsis therapy are to induce T cell proliferation, increased diversity of the T cell repertoire, decreased lymphocyte apoptosis by upregulating anti-apoptotic protein Bcl-2, increased expression of adhesion molecules causing better trafficking and recruitment and even helps neutrophil recruitment to sites of infection [22, 23, 104, 107, 108]. IL-7 has been shown to be well tolerated in clinical trials with no severe toxicities and more than doubling the levels of circulating CD4+ and CD8+ T cells in HIV and cancer patients [100, 109]. It does not induce a hyper-inflammatory cytokine storm and has been well tolerated in clinical trials [104]. Several pre-clinical studies indicate that IL-7 has significant beneficial effects during sepsis. In mouse models of sepsis, Hotchkiss and colleagues have shown that IL-7 treatment leads to restoration of depleted T cells in lymphoid organs, increased T cell proliferation, decreased lymphocyte apoptosis (via increased Bcl-2 protein expression), restoration of T cell IFNγ secretion, increased CD28 expression, and significant improvement in survival [22, 23, 107, 110]. IL-7 has also been shown to indirectly interact with the innate immune system by helping to recruit neutrophils to the site of infection via IL-17 secreted by γδT cells, which ultimately improved bacterial clearance and increased survival in a CLP model of sepsis [107]. Importantly, Venet et al. demonstrated that ex vivo treatment of T lymphocytes derived from septic patients with IL-7, significantly improved CD4+ and CD8+ T cell proliferation, IFN-γ production, STAT5 phosphorylation, and Bcl2 protein induction [108]. IL-7 mediates its proliferative and anti-apoptotic effects on T cells through activation of phosphoinositide 3-kinase (PI3K) and the Janus kinase-signal transducer and activator of transcription (JAK-STAT5 pathway) [104]. Therefore, STAT5 is one of the key signaling molecules which mediate the effects of IL-7. Low doses of IL-7 have been shown to preferentially activate effector T cells (Teff) as compared to Tregs in peripheral blood from septic patients [111]. Sepsis patients exhibit an extensive loss of T cell receptor (TCR) diversity, which is linked with an increased incidence of nosocomial infection and associated mortality [112]. IL-7 treatment has the potential to enhance TCR diversity and thereby help to mount an effective immune response against a variety of infecting pathogens [113]. In a clinical study in HIV pateints, IL-7 therapy also decreased PD-1 expression on lymphocytes [109]. Therefore, IL-7 has a highly versatile mechanism of action to attenuate T cell dysfunction. Though IL-7 therapy is a very tempting alternative to control sepsis, no actual clinical trial data exist to show its beneficial effects in septic patients. A clinical trial is in progress to study the effect of IL-7 to restore absolute lymphocyte counts in septic patients (registered on ClinicalTrials.gov # NCT02640807) and the results are much anticipated.
2. Interleukin-15 (IL-15)
IL-15 is a 14–15 KDa glycoprotein predominantly produced by dendritic cells, macrophages, monocytes, endothelial cells, stromal cells and renal epithelial cells [114–117]. IL-15 is transported to the surface of the cells complexed with IL-15 receptor-alpha (IL-15Rα) and presented to target cells (memory CD8+ T, NK and NKT cells) expressing IL-15 receptor β and common γ chains, through a unique mechanism called trans-presentation [118–120]. IL-15 signals through a heterodimeric receptor composed of the IL-2R/IL-15Rβ (CD122) beta and common gamma chains [121]. IL-15 is absolutely essential for the growth and survival of natural killer (NK) cells and NKT cells, and stimulates T cell proliferation (memory CD8+ T cells preferentially) and immunoglobulin synthesis by B cells and dendritic cells [122]. IL-15 has been shown to induce expansion of effector memory T cells and enhance their functions in HIV patients [123]. IL-15 therapy is being investigated as a cancer therapy and modified T cells that co-express IL-15 even showed a reduced expression of PD-1 [124]. IL-15 is also known to enhance the functions of multiple innate immune cells, including protection of neutrophils from apoptosis and modulation of neutrophil phagocytic functions [125]; increasing phagocytic action and cytokine secretion of macrophages [126]; and inducing maturation and inhibition of apoptosis among dendritic cells [127]. Although IL-15 has not been investigated in detail during sepsis, the potential beneficial effects of IL-15 on various immune cells make it an attractive tool in sepsis immunotherapy. In a mouse model of sepsis, IL-15 significantly decreased sepsis induced apoptosis of NK cells, dendritic cells, CD8+ T cells and gut epithelial cells, as result of increased Bcl-2 protein expression (anti-apoptotic) and decreased Bim and PUMA protein expression (anti-apoptotic) in immune cells [51]. Furthermore, IL-15 treatment also increased circulating IFN-γ levels and significantly improved survival in CLP sepsis and a Pseudomonas aeruginosa pneumonia model [51]. IL-15 has also been shown to be beneficial in reducing sepsis-induced muscle wasting in mice [128]. However, a recent study using burn wound infection induced Pseudomonas aeruginosa sepsis model showed that although IL-15 significantly increased CD8+, NK and NKT cells, but did not improve bacterial clearance or survival [53]. The contrasting findings might be the result of significant differences in the models used, as the latter study has a burn injury component as an additional insult prior to inducing infection and used BALB/c mice instead of the CD-1 mice strain used in the earlier CLP studies. IL-15 has also been shown to have potential toxic effects. It caused liver injury and cachexia in a mouse model [129] and caused fever, grade 3 hypotension and liver injury in a clinical trial for cancer patients [130]. Therefore, though IL15 possesses stimulating effects on numerous immune cells, its toxicity profile also needs to be taken into consideration. IL-15 does merit further investigation as an immunotherapeutic agent during sepsis. Based on the current knowledge as discussed above, future studies need to focus on testing various doses in different sepsis models to find an optimal dose with no overt toxicity. Moreover, rather than monotherapy, it might be worthwhile to explore the effects of IL-15 as combinatorial therapy with other immunotherapeutic agents. The combination of IL-15 with antibodies that block PD-1 or CTLA-4 has been shown to reduce IL-10 production and PD-1 expression on CD8+ T cells, which enhanced the anti-tumor activity of IL-15 [131].
3. Antibodies against inhibitory immune checkpoints
As discussed in detail in the T cell exhaustion section, expression of inhibitory receptors such as PD-1 on T cells and PD-L1 on antigen presenting cells and parenchymal cells downregulates T cell functions during sepsis [21, 65, 66, 68]. Blocking the PD-1/PD-L1 pathway has been successful in cancer therapy and FDA has recently approved PD-1 monoclonal antibodies to treat human cancers Bristol-Myers Squibb (Opdivo or nivolumab) and Merck (known as Keytruda or pembrolizumab) have developed and marketed antibodies to treat metastatic melanomas, non-small-cell lung carcinomas and metastatic renal cell carcinomas [132]. These therapies have induced successful regression of advanced stage cancers and improved survival rate. In pre-clinical mouse models of sepsis, blockade of either PD-1 or PD-L1 significantly improved survival [65, 66, 133]. Therefore, antibody therapies aimed at blocking PD-1 and/or PD-L1 are gaining significant attention as immunotherapeutic agents in sepsis. Studies show that increased PD-1 expression on circulating T cells in septic patients correlates with decreased T cell proliferation and increased secondary infections leading to higher mortality [71]. A current clinical trial is underway to assess the efficacy of anti-PDL1 in patients with severe sepsis (registered on ClinicalTrials.gov #NCT02576457).
BTLA is another immune checkpoint inhibitor that has been shown to be associated with increased morbidity and mortality in pre-clinical studies of sepsis [134]. Shubin et al. showed that BTLA expression is increased on circulating CD4+ T cells in sepsis patients and higher BTLA levels correlated with development of nosocomial infections [76]. BTLA knockout mice show reduced bacterial burden, decreased organ injury markers and improved survival in a CLP mouse model of sepsis [134]. CTLA is another member of the co-inhibitory receptor family. Pre-clinical studies show that bacterial sepsis causes increased expression of CTLA-4 on CD4+ and CD8+ T cells, and anti-CTLA-4 treatment dose dependently decreased CD4+ and CD8+ T lymphocyte apoptosis and improved survival [135]. Anti-CTLA-4 treatment also caused an increase in IFNγ production by splenocytes stimulated with anti-CD3/ani-CD28 (specific to T cell stimulation) and improved survival in a model of Candida fungal sepsis [133]. Therefore, blocking BTLA and CTLA-4 signaling might be another adjunct immunotherapy option during sepsis, which needs further exploration.
4. Interferon gamma (IFN-γ)
Activated CD4+ and CD8+ T cells, along with NK cells, are known to be the major source of IFNγ [136]. IFN-γ upregulates HLA-DR expression on monocytes and it is one of the key cytokines responsible for activation of monocytes and macrophages, which are essential for microbial elimination during sepsis [137, 138]. Both, pre-clinical and clinical studies show that T cell IFN-γ production is dramatically decreased during sepsis [21, 23, 56]. Therapies that restore T cell IFN-γ production have been shown to improve survival in animal models of sepsis [23, 133]. Infants with deficient production of IFN-γ exhibit decreased neutrophil mobility and NK cell activity and are more prone to recurrent infections, which further underscores the importance of IFN-γ in effective immunoregulation [139]. Recombinant IFN-γ treatment has been shown to increase HLA-DR expression on monocytes in septic patients, improve monocyte function and treatment was not associated with any adverse effects [140]. In another clinical report, treatment of a diabetic patient with severe staphylococcal sepsis which was refractory to antibiotic treatment, with IFN-γ resulted in elimination of the pathogen and resolution of sepsis within a week [141]. A large randomized, double-blind, placebo controlled clinical trial showed that IFN-γ treatment decreased the number of infection-related deaths in severely injured patients (like gunshot wound, motor vehicle accident and stab wound) [142]. Therefore, IFN-γ could be a potential immunomodulatory therapy during sepsis. But, it is very important to consider the fact that IFN-γ therapy might only be beneficial in sepsis patients with demonstrated immunosuppression such as those with downregulated HLA-DR on monocytes and it will not likely be beneficial in all the patients. Moreover, it may be harmful if administered during the pro-inflammatory phase of sepsis, by fueling further stimulation of monocytes and causing a vicious cycle of hyper inflammation. Therefore, IFN-γ therapy should be individualized to specific sepsis patients and can serve as an adjunctive therapy along with IL-7 and anti-PD1/anti-PD-L1, as IFN-γ alone is insufficient to correct the underlying T cell apoptosis and exhaustion during sepsis. A clinical trial is underway at the Radboud University in Netherlands, to assess the effects of adjunctive therapy with IFN-γ on immune functions in septic patients (Clinicaltrials.gov # NCT01649921), results of which are much anticipated.
5. Fms-like Tyrosine Kinase-3 ligand (Flt3L)
Flt3L is a cell surface transmembrane protein, which also exists as a soluble form owing to its proteolytic cleavage and both forms are biologically active [143]. Flt3L acts on a class III tyrosine kinase receptor, Flt3R, which is expressed predominantly on hematopoietic progenitor cells (including both myeloid and lymphoid) as well as on major steady state dendritic cell populations, and is essential for maintenance and expansion of early hematopoietic stem cells [143]. Flt3L affects the growth and mobilization of pluripotent hematopoietic stem and progenitor cells among both the lymphoid and myeloid pathways; thus it is considered to be a stem cell growth factor. Flt3L is usually considered to act in synergy with other endogenous cytokines such as IL-7, IL-3 and IL-11 to stimulate B cell lymphopoiesis in vitro, with IL-12 in thymus to promote T cell development, and with IL-15 to promote NK cell development [143]. Flt3L has been shown to an effective dendritic cell growth factor and a large expansion of dendritic cells has been observed in healthy human volunteers [144], as well as mice [145] receiving Flt3L. Flt3L treatment causes a potent expansion of splenic dendritic cells, which enhances neutrophil antimicrobial functions and improves survival during burn injury and Pseudomonas aeruginosa sepsis, and the protective effect was sustained even with adoptively transferring Flt3L-treated dendritic cells [145, 146]. Dendritic cells are a part of innate immune system and are the most effective antigen presenting cells for T cell activation [147]. Dendritic cell dysfunction is increasingly being recognized as an important factor in sepsis pathophysiology, which can indirectly impair T cell functions [96]. Importantly, recent studies from our laboratory using a mouse model of burn injury and Pseudomonas aeruginosa sepsis also show that Flt3L treatment attenuates T cell depletion, restores T lymphocyte CD28 and IFNγ secretion, attenuates increased PD-L1 expression on antigen presenting cells (dendritic cells, macrophages and monocytes), decreased organ injury markers and significantly improved survival (in press at SHOCK journal). Flt3L has also been shown to protect cardiomyocytes after infarction through reduction of oxidative stress and apoptosis [148]. Whether Flt3L has similar anti-apoptotic effects on T cells during sepsis, is yet to be determined. Moreover, Flt3L is already in clinical trials as a combination immunotherapy for lymphoma and has shown a good safety profile [149]. Therefore, Flt3L treatment certainly holds significant therapeutic potential during sepsis and merits further investigation as an immunotherapy alone or in combination with other therapies such as IL-7.
6. Chimeric Antigen Receptor (CAR) T cell therapy
CAR is composed of an extracellular antigen-recognition domain, which is usually an antibody single-chain variable fragment, but can also be a peptide or another protein, linked to an intracellular signaling domain – usually the CD3 zeta chain of the T cell receptor (TCR) [150]. CAR T cells are specially engineered in the laboratory and they specifically bind to only a particular antigen causing T cell activation, proliferation and cytokine secretion to eradicate the target cells, without the need for antigen presentation by the antigen presenting cells. CAR T cell therapy is being continuously improved upon and newer generation CAR T cells even have an additional integrated co-stimulatory signaling moiety such as CD28, to mount optimal T cell activation upon antigen encounter [151]. Therefore, CAR manipulation of T cells redirects them towards target cells to directly carry out critical effector functions. CAR T cells are being extensively investigated in cancer research to target T cells for destroying malignant cells and CD19 targeted CAR T cells have demonstrated marked anti-tumor responses in B cell acute lymphoblastic leukemia (B-ALL) [152–154]. In a remarkable study by Kumaresan et al., modified CAR T cells, which recognize Dectin-1 (mediates recognition of Aspergillus fumigatus fungus), inhibited the growth of fungal hyphae in vitro and diminished fungal infection in the mice as evidenced by decreased Aspergillus load in the lungs of treated mice as compared to untreated mice [155]. This study is the first to demonstrate that a pattern recognition receptor could be adapted to redirect cytotoxic CD8+ T cell specificity for pathogen elimination. Modified T cells were also able to secrete IFN-γ and might further potentiate the activation of granulocytes, especially macrophages, which remains to be determined. Therefore, such modified T cells could be developed if distinct antigen targets are identified among various infecting pathogens or a common antigen among groups of pathogens. For example, hypothetically the O antigen of gram negative bacterial lipopolysaccharide (LPS), could be a possible target. The O antigen of LPS is exposed on the extracellular surface of bacteria and could be adapted to produce specific cytotoxic CAR T cells against a group of gram negative bacteria. Such therapies could be beneficial for all sepsis patients and not just immunosuppressed patients, who are refractory to other therapies. Another important aspect of CAR T cell therapy is that the patient’s own cells could be genetically engineered for autologous transfusion, preventing any adverse effects of rejection. Development of CAR T cell therapy for infectious diseases has a long way to go and needs extensive pre-clinical research before any clinical study could be undertaken. CAR T cell therapy could have adverse effects which might be challenging to overcome. Retroviral and lentiviral vectors used to create CAR T cells could cause toxicity by insertional mutagenesis in the host, depletion of normal B cells (if CD19 CAR T cells used) leading to B cell aplasia, off target toxicities in other organs, and exaggeration of systemic inflammation or cytokine storm (due to excessive cytokine release upon antigen engagement by T cells) [156]. Numerous strategies to reduce such non-specific toxicities are being studied by scientists in the cancer field and the future might hold promise for CAR T cell therapies in sepsis as well, especially based on the encouraging results from the successful study of Aspergillus elimination by Dectin-1 modified cytotoxic T cells [155].
Biomarker guided patient stratification is essential is sepsis immunotherapy
Although, immunotherapy is a very promising approach in sepsis, it needs special consideration with respect to stratifying suitable patients. Immunotherapy needs to be directed towards patients who are actually immunosuppressed and developing specific biomarkers can aid in identifying specific patient populations. The following are few of the methods which could be used to detect immunosuppression and better direct the immunotherapeutics as discussed in previous sections:
Genetic profiling of individual patients to detect specific alterations that render an individual susceptible to infection [157–159]. A study by Wong et al. showed that pediatric septic shock is characterized by repression of genes corresponding to adaptive immunity as early as 1 to 3 days after septic shock [158]. Thus, the use of profiling could be used to identify immunosuppressed patients that would gain the greatest possible benefit of immunotherapy.
Sustained severe Lymphopenia as opposed to increased circulating Tregs [85]. Recent studies by Drewry and colleagues show that septic patients with sustained lymphopenia have increased in-hospital and long-term mortality [160]. Thus, measurement of blood absolute lymphocyte count provides an easily accessible and potentially valuable marker of ongoing immunosuppression.
Lymphocyte expression of inhibitory receptors such as PD-1, BTLA using flow cytometry technique on circulating T cells and PD-L1 expression on antigen presenting cells such as dendritic cells and monocytes, could serve as a marker to detect T cell exhaustion [21].
Ex vivo stimulation of peripheral blood mononuclear cells (with anti-CD3/anti-CD28) to detect altered, T cell cytokine secretion such as IFN-γ [21, 56].
Increased levels of anti-inflammatory cytokines (IL-10 and TGF-β) as compared to pro-inflammatory cytokines (TNF-α) [79, 80, 90, 91].
Measuring reactivation of latent viruses such as Cytomegalovirus, Epstein Barr virus and Herpes Simplex virus [32].
Development of secondary nosocomial infections such as opportunistic fungal infections (eg. Candida), Pseudomonas aeruginosa pneumonia and others [20].
Combination Immunotherapy for sepsis
As discussed above, numerous immunotherapeutic agents hold significant potential for sepsis therapy. Due to the wide variations in individual immune responses to a septic insult, combination of various immunomodulatory agents holds better chances of success rather than a standalone therapy by any individual agent. Moreover, individual therapies could be altered over the course of sepsis based on the temporal changes in immune responses [15].
The following represent some of the examples of combination strategies based on experimental evidence:
Combination of IL-7 with anti-PD-1 and/or anti-PD-L1 will be beneficial in patients with lymphopenia and T cell exhaustion [22, 23, 65, 66, 110, 133].
Flt3 ligand combined with IL-7 can lead to robust increase in T cell population in patients with severe lymphopenia. Flt3 ligand also leads to a potent expansion of dendritic cells, which are the most important antigen presenting cells to T cells [143, 145].
Anti-PD-1/anti-PD-L1 or IL-7 could be combined with IFN-γ in patients with decreased HLA-DR expression on monocytes and T cell dysfunction [161].
Anti-BTLA therapy in combination with anti-PD-L1 in patients with secondary ventilator associated pneumonia infections, as HVEM (ligand for BTLA) and PD-L1 expression were shown to be increased in lungs of patients who died of sepsis [21, 134].
IL-7 in combination with potential small molecule to inhibit lymphocyte apoptosis for increasing T lymphocyte counts and simultaneously decreasing sepsis induced ongoing lymphocyte depletion. Caspase inhibitors and HIV protease inhibitors have been tested in mice models of CLP sepsis and shown significant benefits with improved survival [24, 162]
Flt3 ligand in combination with Monophosphoryl Lipid A (MPLA) as a prophylactic therapy in critically ill burn patients to protect against sepsis [163–165]. MPLA is known to enhance innate immune cell functions and serves as a potent vaccine adjuvant [163–166].
Could Immunotherapy backfire in sepsis treatment?
The biggest fear with respect to immunotherapy is the development of an exaggerated inflammatory response, which can lead to fulminant hyper inflammation and rapid death during sepsis. Therefore, it is essential to stratify patients based on their immune status and administer personalized immunotherapy for individual patients as discussed above. However, such an adverse effect was not seen in clinical trials of immunomodulatory agents such as IFN-γ in critically ill trauma patients [161] or the staphylococcal sepsis patient [141], or IL-7 in HIV and cancer patients [100, 109]. Moreover, patients developing immunosuppression during protracted sepsis have significantly impaired production of inflammatory cytokines [21, 56]. Initial mouse studies after PD-1 discovery suggested that PD-1 deficiency also causes an increased incidence of autoimmune diseases leading to various pathologies such as lupus like syndromes, de novo type 1 diabetes, dilated cardiomyopathy and others [167, 168]. Therefore, the extension of PD-1/PD-L1 therapies for sepsis immunotherapy should be done with caution. Usually such side effects occur upon prolonged blockade of PD-1/PD-L1 pathway. During sepsis, such therapy that will be transient until resolution of infection and autoimmune side effects might not be a reality. However, only future clinical trials in sepsis can answer these questions. IL-15 has been shown to have potential toxic effects. It caused liver injury and cachexia in a mouse model [129] and caused fever, grade 3 hypotension and liver injury in a clinical trial for cancer patients [130]. Therefore, strict patient monitoring is needed with all immunotherapeutic agents. Ultimately, only clinical trials will be able to answer our concerns related to adverse effects of immunotherapy during sepsis.
Concluding Remarks
Stagnancy in development to any novel antibiotic therapies, growing emergence of antibiotic resistant pathogens and failed clinical trials focusing on modulating the pro-inflammatory phase of sepsis are major factors propelling the researchers in the sepsis field to develop and test immunomodulators aimed at attenuating immunosuppression during sepsis. Overall, immunotherapy could be successful in clinics for treating sepsis, if a personalized approach is taken for each patient. We believe that immunomodulatory agents such as IL-7 and antibodies to block T cell inhibitors such as anti-PD-1 and anti-PD-L1 hold significant future potential to end our decades of struggle to fight protracted sepsis.
Acknowledgments
N.K.P is supported by a Postdoctoral Grant from the American Heart Association (16POST29920007) and this work is also supported by the National Institute of Health grant NIH R01 GM66885 to E.R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s contributions:
NKP wrote the manuscript. ERS and JKB critically revised the manuscript for important intellectual content. Both the authors have read and approved the manuscript.
Disclosure of Conflicts of Interest
Authors declare no conflict of interest.
References
- 1.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5(1):4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101(6):1481–1483. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 3.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K. International Forum of Acute Care, T Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 4.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) Jama. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence outcome and associated costs of care. Critical care medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Critical care medicine. 2006;34(1):15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J, Opal S, Calandra T. Sepsis studies need new direction. Lancet Infect Dis. 2012;12(7):503–505. doi: 10.1016/S1473-3099(12)70136-6. [DOI] [PubMed] [Google Scholar]
- 9.Angus DC. The search for effective therapy for sepsis: back to the drawing board? Jama. 2011;306(23):2614–2615. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 10.Abraham E, Glauser MP, Butler T, Garbino J, Gelmont D, Laterre PF, Kudsk K, Bruining HA, Otto C, Tobin E, Zwingelstein C, Lesslauer W, Leighton A. p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. Ro 45-2081 Study Group. Jama. 1997;277(19):1531–1538. [PubMed] [Google Scholar]
- 11.Hotchkiss RS, Opal S. Immunotherapy for sepsis--a new approach against an ancient foe. N Engl J Med. 2010;363(1):87–89. doi: 10.1056/NEJMcibr1004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schorr CA, Dellinger RP. The Surviving Sepsis Campaign: past present and future. Trends Mol Med. 2014;20(4):192–194. doi: 10.1016/j.molmed.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Critical care medicine. 2013;41(5):1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nature medicine. 2009;15(5):496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? The Journal of clinical investigation. 2016;126(1):23–31. doi: 10.1172/JCI82224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13(3):260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boomer JS, Green JM, Hotchkiss RS. The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence. 2014;5(1):45–56. doi: 10.4161/viru.26516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang BM, Huang SJ, McLean AS. Genome-wide transcription profiling of human sepsis: a systematic review. Critical care. 2010;14(6):R237. doi: 10.1186/cc9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monneret G, Venet F, Kullberg BJ, Netea MG. ICU-acquired immunosuppression and the risk for secondary fungal infections. Med Mycol. 2011;49(Suppl 1):S17–S23. doi: 10.3109/13693786.2010.509744. [DOI] [PubMed] [Google Scholar]
- 20.Otto GP, Sossdorf M, Claus RA, Rodel J, Menge K, Reinhart K, Bauer M, Riedemann NC. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Critical care. 2011;15(4):R183. doi: 10.1186/cc10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. Jama. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unsinger J, Burnham CA, McDonough J, Morre M, Prakash PS, Caldwell CC, Dunne WM, Jr, Hotchkiss RS. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. The Journal of infectious diseases. 2012;206(4):606–616. doi: 10.1093/infdis/jis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, McDonough JS, Tschoep J, Ferguson TA, McDunn JE, Morre M, Hildeman DA, Caldwell CC, Hotchkiss RS. IL-7 promotes T cell viability trafficking and functionality and improves survival in sepsis. Journal of immunology. 2010;184(7):3768–3779. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nature immunology. 2000;1(6):496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 25.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annual review of immunology. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 26.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nature reviews. Immunology. 2007;7(7):543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 27.Zhu J, Paul WE. CD4 T cells: fates functions and faults. Blood. 2008;112(5):1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patil NK, Parajuli N, MacMillan-Crow LA, Mayeux PR. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol. 2014;306(7):F734–F743. doi: 10.1152/ajprenal.00643.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Critical care medicine. 2007;35(10):2408–2416. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 30.Holthoff JH, Wang Z, Patil NK, Gokden N, Mayeux PR. Rolipram improves renal perfusion and function during sepsis in the mouse. The Journal of pharmacology and experimental therapeutics. 2013;347(2):357–364. doi: 10.1124/jpet.113.208520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luyt CE, Kaiser L. Virus detection in patients with severe pneumonia: still more questions than answers? Am J Respir Crit Care Med. 2012;186(4):301–302. doi: 10.1164/rccm.201206-1119ED. [DOI] [PubMed] [Google Scholar]
- 32.Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, Pachot A, Brooks TL, Deych E, Shannon WD, Green JM, Storch GA, Hotchkiss RS. Reactivation of multiple viruses in patients with sepsis. PloS one. 2014;9(2):e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. Journal of immunology. 2001;166(11):6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 35.Norbury CJ, Hickson ID. Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol. 2001;41:367–401. doi: 10.1146/annurev.pharmtox.41.1.367. [DOI] [PubMed] [Google Scholar]
- 36.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. The EMBO journal. 2011;30(18):3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296(5573):1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 39.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 40.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87(4):619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 41.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad a heterodimeric partner for Bcl-XL and Bcl-2 displaces Bax and promotes cell death. Cell. 1995;80(2):285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 42.Newmeyer DD, Bossy-Wetzel E, Kluck RM, Wolf BB, Beere HM, Green DR. Bcl-xL does not inhibit the function of Apaf-1. Cell Death Differ. 2000;7(4):402–407. doi: 10.1038/sj.cdd.4400665. [DOI] [PubMed] [Google Scholar]
- 43.Liu FT, Newland AC, Jia L. Bax conformational change is a crucial step for PUMA-mediated apoptosis in human leukemia. Biochem Biophys Res Commun. 2003;310(3):956–962. doi: 10.1016/j.bbrc.2003.09.109. [DOI] [PubMed] [Google Scholar]
- 44.Esposti MD. The roles of Bid. Apoptosis. 2002;7(5):433–440. doi: 10.1023/a:1020035124855. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 46.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2(4):277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 47.Wang SD, Huang KJ, Lin YS, Lei HY. Sepsis-induced apoptosis of the thymocytes in mice. Journal of immunology. 1994;152(10):5014–5021. [PubMed] [Google Scholar]
- 48.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Critical care medicine. 1997;25(8):1298–1307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Ayala A, Herdon CD, Lehman DL, Ayala CA, Chaudry IH. Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset frequency and the nature of the mediators. Blood. 1996;87(10):4261–4275. [PubMed] [Google Scholar]
- 50.Ayala A, Herdon CD, Lehman DL, DeMaso CM, Ayala CA, Chaudry IH. The induction of accelerated thymic programmed cell death during polymicrobial sepsis: control by corticosteroids but not tumor necrosis factor. Shock. 1995;3(4):259–267. doi: 10.1097/00024382-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, Hotchkiss RS. IL-15 prevent apoptosis reverses innate and adaptive immune dysfunction and improves survival in sepsis. Journal of immunology. 2010;184(3):1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Unsinger J, McDonough JS, Shultz LD, Ferguson TA, Hotchkiss RS. Sepsis-induced human lymphocyte apoptosis and cytokine production in "humanized" mice. Journal of leukocyte biology. 2009;86(2):219–227. doi: 10.1189/jlb.1008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patil NK, Luan L, Bohannon JK, Guo Y, Hernandez A, Fensterheim B, Sherwood ER. IL-15 Superagonist Expands mCD8+ T NK and NKT Cells after Burn Injury but Fails to Improve Outcome during Burn Wound Infection. PloS one. 2016;11(2):e0148452. doi: 10.1371/journal.pone.0148452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. Journal of immunology. 1999;162(7):4148–4156. [PubMed] [Google Scholar]
- 55.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(25):14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, Green JM. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Critical care. 2012;16(3):R112. doi: 10.1186/cc11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. Journal of immunology. 2005;174(8):5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 58.Weber SU, Schewe JC, Lehmann LE, Muller S, Book M, Klaschik S, Hoeft A, Stuber F. Induction of Bim and Bid gene expression during accelerated apoptosis in severe sepsis. Critical care. 2008;12(5):R128. doi: 10.1186/cc7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, Hotchkiss RS. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21(3):708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 60.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nature reviews. Immunology. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. The Journal of experimental medicine. 1998;188(12):2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nature reviews. Immunology. 2003;3(12):939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 63.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends in immunology. 2014;35(2):51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Current opinion in immunology. 2009;21(2):179–186. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. Journal of leukocyte biology. 2010;88(2):233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, Wan X, Deng X, Cai Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Critical care. 2010;14(6):R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(15):6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang X, Chen Y, Chung CS, Yuan Z, Monaghan SF, Wang F, Ayala A. Identification of B7-H1 as a novel mediator of the innate immune/proinflammatory response as well as a possible myeloid cell prognostic biomarker in sepsis. Journal of immunology. 2014;192(3):1091–1099. doi: 10.4049/jimmunol.1302252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McNab FW, Berry MP, Graham CM, Bloch SA, Oni T, Wilkinson KA, Wilkinson RJ, Kon OM, Banchereau J, Chaussabel D, O'Garra A. Programmed death ligand 1 is over-expressed by neutrophils in the blood of patients with active tuberculosis. Eur J Immunol. 2011;41(7):1941–1947. doi: 10.1002/eji.201141421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bowers NL, Helton ES, Huijbregts RP, Goepfert PA, Heath SL, Hel Z. Immune suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway. PLoS Pathog. 2014;10(3):e1003993. doi: 10.1371/journal.ppat.1003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, Malcus C, Cheron A, Allaouchiche B, Gueyffier F, Ayala A, Monneret G, Venet F. Programmed death-1 levels correlate with increased mortality nosocomial infection and immune dysfunctions in septic shock patients. Critical care. 2011;15(2):R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spec A, Shindo Y, Burnham CA, Wilson S, Ablordeppey EA, Beiter ER, Chang K, Drewry AM, Hotchkiss RS. T cells from patients with Candida sepsis display a suppressive immunophenotype. Critical care. 2016;20:15. doi: 10.1186/s13054-016-1182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monneret G, Venet F. A rapidly progressing lymphocyte exhaustion after severe sepsis. Critical care. 2012;16(4):140. doi: 10.1186/cc11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shao R, Fang Y, Yu H, Zhao L, Jiang Z, Li CS. Monocyte programmed death ligand-1 expression after 3–4 days of sepsis is associated with risk stratification and mortality in septic patients: a prospective cohort study. Critical care. 2016;20(1):124. doi: 10.1186/s13054-016-1301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Li J, Lou J, Zhou Y, Bo L, Zhu J, Zhu K, Wan X, Cai Z, Deng X. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Critical care. 2011;15(1):R70. doi: 10.1186/cc10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shubin NJ, Monaghan SF, Heffernan DS, Chung CS, Ayala A. B and T lymphocyte attenuator expression on CD4+ T-cells associates with sepsis and subsequent infections in ICU patients. Critical care. 2013;17(6):R276. doi: 10.1186/cc13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonten MJ, Froon AH, Gaillard CA, Greve JW, de Leeuw PW, Drent M, Stobberingh EE, Buurman WA. The systemic inflammatory response in the development of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1997;156(4 Pt 1):1105–1113. doi: 10.1164/ajrccm.156.4.9610002. [DOI] [PubMed] [Google Scholar]
- 78.Pelekanou A, Tsangaris I, Kotsaki A, Karagianni V, Giamarellou H, Armaganidis A, Giamarellos-Bourboulis EJ. Decrease of CD4-lymphocytes and apoptosis of CD14-monocytes are characteristic alterations in sepsis caused by ventilator-associated pneumonia: results from an observational study. Critical care. 2009;13(6):R172. doi: 10.1186/cc8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Dissel JT, van Langevelde P, Westendorp RG, Kwappenberg K, Frolich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351(9107):950–953. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 80.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. The Journal of infectious diseases. 2000;181(1):176–180. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 81.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. The Journal of experimental medicine. 1991;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ertel W, Kremer JP, Kenney J, Steckholzer U, Jarrar D, Trentz O, Schildberg FW. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85(5):1341–1347. [PubMed] [Google Scholar]
- 83.Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. The Journal of clinical investigation. 1991;88(5):1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rigato O, Salomao R. Impaired production of interferon-gamma and tumor necrosis factor-alpha but not of interleukin 10 in whole blood of patients with sepsis. Shock. 2003;19(2):113–116. doi: 10.1097/00024382-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 85.Venet F, Chung CS, Kherouf H, Geeraert A, Malcus C, Poitevin F, Bohe J, Lepape A, Ayala A, Monneret G. Increased circulating regulatory T cells (CD4(+)CD25 (+)CD127 (−)) contribute to lymphocyte anergy in septic shock patients. Intensive care medicine. 2009;35(4):678–686. doi: 10.1007/s00134-008-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, O'Mara L, Yang S, Konieczny BT, Sharpe AH, Freeman GJ, Rudensky AY, Ahmed R. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. The Journal of experimental medicine. 2014;211(9):1905–1918. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gilbert KM, Thoman M, Bauche K, Pham T, Weigle WO. Transforming growth factor-beta 1 induces antigen-specific unresponsiveness in naive T cells. Immunol Invest. 1997;26(4):459–472. doi: 10.3109/08820139709022702. [DOI] [PubMed] [Google Scholar]
- 89.Wan YY, Flavell RA. TGF-beta and regulatory T cell in immunity and autoimmunity. Journal of clinical immunology. 2008;28(6):647–659. doi: 10.1007/s10875-008-9251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knapp S, Thalhammer F, Locker GJ, Laczika K, Hollenstein U, Frass M, Winkler S, Stoiser B, Wilfing A, Burgmann H. Prognostic value of MIP-1 alpha TGF-beta 2, sELAM-1and sVCAM-1 in patients with gram-positive sepsis. Clin Immunol Immunopathol. 1998;87(2):139–144. doi: 10.1006/clin.1998.4523. [DOI] [PubMed] [Google Scholar]
- 91.Marie C, Cavaillon JM, Losser MR. Elevated levels of circulating transforming growth factor-beta 1 in patients with the sepsis syndrome. Ann Intern Med. 1996;125(6):520–521. doi: 10.7326/0003-4819-125-6-199609150-00034. [DOI] [PubMed] [Google Scholar]
- 92.Ayala A, Knotts JB, Ertel W, Perrin MM, Morrison MH, Chaudry IH. Role of interleukin 6 and transforming growth factor-beta in the induction of depressed splenocyte responses following sepsis. Arch Surg. 1993;128(1):89–94. doi: 10.1001/archsurg.1993.01420130101015. discussion 94–5. [DOI] [PubMed] [Google Scholar]
- 93.Ahmad S, Choudhry MA, Shankar R, Sayeed MM. Transforming growth factor-beta negatively modulates T-cell responses in sepsis. FEBS letters. 1997;402(2–3):213–218. doi: 10.1016/s0014-5793(96)01535-9. [DOI] [PubMed] [Google Scholar]
- 94.Roth MD, Golub SH. Human pulmonary macrophages utilize prostaglandins and transforming growth factor beta 1 to suppress lymphocyte activation. Journal of leukocyte biology. 1993;53(4):366–371. doi: 10.1002/jlb.53.4.366. [DOI] [PubMed] [Google Scholar]
- 95.Williams MA, Withington S, Newland AC, Kelsey SM. Monocyte anergy in septic shock is associated with a predilection to apoptosis and is reversed by granulocyte-macrophage colony-stimulating factor ex vivo. The Journal of infectious diseases. 1998;178(5):1421–1433. doi: 10.1086/314447. [DOI] [PubMed] [Google Scholar]
- 96.Fan X, Liu Z, Jin H, Yan J, Liang HP. Alterations of dendritic cells in sepsis: featured role in immunoparalysis. Biomed Res Int. 2015;2015:903720. doi: 10.1155/2015/903720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pradipta IS, Sodik DC, Lestari K, Parwati I, Halimah E, Diantini A, Abdulah R. Antibiotic resistance in sepsis patients: evaluation and recommendation of antibiotic use. N Am J Med Sci. 2013;5(6):344–352. doi: 10.4103/1947-2714.114165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams FN, Herndon DN, Hawkins HK, Lee JO, Cox RA, Kulp GA, Finnerty CC, Chinkes DL, Jeschke MG. The leading causes of death after burn injury in a single pediatric burn center. Critical care. 2009;13(6):R183. doi: 10.1186/cc8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 100.Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, Buffet R, Mackall CL, Gress RE. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29(3):313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. The Journal of experimental medicine. 2008;205(7):1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. Journal of immunology. 2005;174(11):6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 103.Guimond M, Veenstra RG, Grindler DJ, Zhang H, Cui Y, Murphy RD, Kim SY, Na R, Hennighausen L, Kurtulus S, Erman B, Matzinger P, Merchant MS, Mackall CL. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nature immunology. 2009;10(2):149–157. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nature reviews. Immunology. 2011;11(5):330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20(4):394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 106.Cunningham-Rundles C, Ponda PP. Molecular defects in T- and B-cell primary immunodeficiency diseases. Nature reviews. Immunology. 2005;5(11):880–892. doi: 10.1038/nri1713. [DOI] [PubMed] [Google Scholar]
- 107.Kasten KR, Prakash PS, Unsinger J, Goetzman HS, England LG, Cave CM, Seitz AP, Mazuski CN, Zhou TT, Morre M, Hotchkiss RS, Hildeman DA, Caldwell CC. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infection and immunity. 2010;78(11):4714–4722. doi: 10.1128/IAI.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Venet F, Foray AP, Villars-Mechin A, Malcus C, Poitevin-Later F, Lepape A, Monneret G. IL-7 restores lymphocyte functions in septic patients. Journal of immunology. 2012;189(10):5073–5081. doi: 10.4049/jimmunol.1202062. [DOI] [PubMed] [Google Scholar]
- 109.Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, Molina JM, Fischl M, Goujard C, Rodriguez B, Rouzioux C, Avettand-Fenoel V, Croughs T, Beq S, Morre M, Poulin JF, Sekaly RP, Thiebaut R, Lederman MM. Effects of recombinant human interleukin 7 on T-cell recovery thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized placebo-controlled multicenter study. Clin Infect Dis. 2012;55(2):291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shindo Y, Unsinger J, Burnham CA, Green JM, Hotchkiss RS. Interleukin-7 and anti-programmed cell death 1 antibody have differing effects to reverse sepsis-induced immunosuppression. Shock. 2015;43(4):334–343. doi: 10.1097/SHK.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Demaret J, Dupont G, Venet F, Friggeri A, Lepape A, Rimmele T, Morel J, Monneret G. STAT5 phosphorylation in T cell subsets from septic patients in response to recombinant human interleukin-7: a pilot study. Journal of leukocyte biology. 2015;97(4):791–796. doi: 10.1189/jlb.5AB1114-545R. [DOI] [PubMed] [Google Scholar]
- 112.Venet F, Filipe-Santos O, Lepape A, Malcus C, Poitevin-Later F, Grives A, Plantier N, Pasqual N, Monneret G. Decreased T-cell repertoire diversity in sepsis: a preliminary study. Critical care medicine. 2013;41(1):111–119. doi: 10.1097/CCM.0b013e3182657948. [DOI] [PubMed] [Google Scholar]
- 113.Perales MA, Goldberg JD, Yuan J, Koehne G, Lechner L, Papadopoulos EB, Young JW, Jakubowski AA, Zaidi B, Gallardo H, Liu C, Rasalan T, Wolchok JD, Croughs T, Morre M, Devlin SM, van den Brink MR. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120(24):4882–4891. doi: 10.1182/blood-2012-06-437236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 115.Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. Journal of immunology. 1996;156(2):735–741. [PubMed] [Google Scholar]
- 116.Cui G, Hara T, Simmons S, Wagatsuma K, Abe A, Miyachi H, Kitano S, Ishii M, Tani-ichi S, Ikuta K. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(5):1915–1920. doi: 10.1073/pnas.1318281111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khawam K, Giron-Michel J, Gu Y, Perier A, Giuliani M, Caignard A, Devocelle A, Ferrini S, Fabbi M, Charpentier B, Ludwig A, Chouaib S, Azzarone B, Eid P. Human renal cancer cells express a novel membrane-bound interleukin-15 that induces in response to the soluble interleukin-15 receptor alpha chain epithelial-to-mesenchymal transition. Cancer research. 2009;69(4):1561–1569. doi: 10.1158/0008-5472.CAN-08-3198. [DOI] [PubMed] [Google Scholar]
- 118.Blaser BW, Schwind NR, Karol S, Chang D, Shin S, Roychowdhury S, Becknell B, Ferketich AK, Kusewitt DF, Blazar BR, Caligiuri MA. Trans-presentation of donor-derived interleukin 15 is necessary for the rapid onset of acute graft-versus-host disease but not for graft-versus-tumor activity. Blood. 2006;108(7):2463–2469. doi: 10.1182/blood-2006-04-019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Castillo EF, Stonier SW, Frasca L, Schluns KS. Dendritic cells support the in vivo development and maintenance of NK cells via IL-15 trans-presentation. Journal of immunology. 2009;183(8):4948–4956. doi: 10.4049/jimmunol.0900719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Han KP, Zhu X, Liu B, Jeng E, Kong L, Yovandich JL, Vyas VV, Marcus WD, Chavaillaz PA, Romero CA, Rhode PR, Wong HC. IL-15: IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells purification and characterization. Cytokine. 2011;56(3):804–810. doi: 10.1016/j.cyto.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends in pharmacological sciences. 2012;33(1):35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annual review of immunology. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 123.Mueller YM, Bojczuk PM, Halstead ES, Kim AH, Witek J, Altman JD, Katsikis PD. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood. 2003;101(3):1024–1029. doi: 10.1182/blood-2002-07-1957. [DOI] [PubMed] [Google Scholar]
- 124.Sim GC, Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine & growth factor reviews. 2014;25(4):377–390. doi: 10.1016/j.cytogfr.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 125.Pelletier M, Ratthe C, Girard D. Mechanisms involved in interleukin-15-induced suppression of human neutrophil apoptosis: role of the anti-apoptotic Mcl-1 protein several kinases including Janus kinase-2 p38 mitogen-activated protein kinase and extracellular signal-regulated kinases-1/2. FEBS letters. 2002;532(1–2):164–170. doi: 10.1016/s0014-5793(02)03668-2. [DOI] [PubMed] [Google Scholar]
- 126.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine & growth factor reviews. 2006;17(4):259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 127.Anguille S, Smits EL, Cools N, Goossens H, Berneman ZN, Van Tendeloo VF. Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties. Journal of translational medicine. 2009;7:109. doi: 10.1186/1479-5876-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim HC, Cho HY, Hah YS. Role of IL-15 in Sepsis-Induced Skeletal Muscle Atrophy and Proteolysis. Tuberc Respir Dis (Seoul) 2012;73(6):312–319. doi: 10.4046/trd.2012.73.6.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guo Y, Luan L, Rabacal W, Bohannon JK, Fensterheim BA, Hernandez A, Sherwood ER. IL-15 Superagonist-Mediated Immunotoxicity: Role of NK Cells and IFN-gamma. Journal of immunology. 2015;195(5):2353–2364. doi: 10.4049/jimmunol.1500300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM, Goldman CK, Bryant BR, Decker JM, Chen J, Worthy TA, Figg WD, Peer CJ, Sneller MC, Lane HC, Yovandich JL, Creekmore SP, Roederer M, Waldmann TA. Redistribution hyperproliferation activation of natural killer cells CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(1):74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010;16(24):6019–6028. doi: 10.1158/1078-0432.CCR-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past present and future. The Journal of clinical investigation. 2015;125(9):3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chang KC, Burnham CA, Compton SM, Rasche DP, Mazuski RJ, McDonough JS, Unsinger J, Korman AJ, Green JM, Hotchkiss RS. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Critical care. 2013;17(3):R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shubin NJ, Chung CS, Heffernan DS, Irwin LR, Monaghan SF, Ayala A. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. Journal of leukocyte biology. 2012;92(3):593–603. doi: 10.1189/jlb.1211641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Inoue S, Bo L, Bian J, Unsinger J, Chang K, Hotchkiss RS. Dose-dependent effect of anti-CTLA-4 on survival in sepsis. Shock. 2011;36(1):38–44. doi: 10.1097/SHK.0b013e3182168cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang L, Zhu J, Shan S, Qin Y, Kong Y, Liu J, Wang Y, Xie Y. Repression of interferon-gamma expression in T cells by Prospero-related homeobox protein. Cell research. 2008;18(9):911–920. doi: 10.1038/cr.2008.275. [DOI] [PubMed] [Google Scholar]
- 137.Murphey ED, Lin CY, McGuire RW, Toliver-Kinsky T, Herndon DN, Sherwood ER. Diminished bacterial clearance is associated with decreased IL-12 and interferon-gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock. 2004;21(5):415–425. doi: 10.1097/00024382-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 138.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annual review of immunology. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 139.Davies EG, Isaacs D, Levinsky RJ. Defective immune interferon production and natural killer activity associated with poor neutrophil mobility and delayed umbilical cord separation. Clinical and experimental immunology. 1982;50(2):454–460. [PMC free article] [PubMed] [Google Scholar]
- 140.Kox WJ, Bone RC, Krausch D, Docke WD, Kox SN, Wauer H, Egerer K, Querner S, Asadullah K, von Baehr R, Volk HD. Interferon gamma-1b in the treatment of compensatory anti-inflammatory response syndrome. A new approach: proof of principle. Arch Intern Med. 1997;157(4):389–393. [PubMed] [Google Scholar]
- 141.Nalos M, Santner-Nanan B, Parnell G, Tang B, McLean AS, Nanan R. Immune effects of interferon gamma in persistent staphylococcal sepsis. Am J Respir Crit Care Med. 2012;185(1):110–112. doi: 10.1164/ajrccm.185.1.110. [DOI] [PubMed] [Google Scholar]
- 142.Dries DJ, Jurkovich GJ, Maier RV, Clemmer TP, Struve SN, Weigelt JA, Stanford GG, Herr DL, Champion HR, Lewis FR, et al. Effect of interferon gamma on infection-related death in patients with severe A randomized injuries and double-blind placebo-controlled trial. Arch Surg. 1994;129(10):1031–1041. doi: 10.1001/archsurg.1994.01420340045008. discussion 1042. [DOI] [PubMed] [Google Scholar]
- 143.Wodnar-Filipowicz A. Flt3 ligand: role in control of hematopoietic and immune functions of the bone marrow. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2003;18:247–251. doi: 10.1152/nips.01452.2003. [DOI] [PubMed] [Google Scholar]
- 144.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, Caron D, Lebsack ME, McKenna HJ. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96(3):878–884. [PubMed] [Google Scholar]
- 145.Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T. Prophylactic treatment with fms-like tyrosine kinase-3 ligand after burn injury enhances global immune responses to infection. Journal of immunology. 2008;180(5):3038–3048. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- 146.Bohannon J, Cui W, Sherwood E, Toliver-Kinsky T. Dendritic cell modification of neutrophil responses to infection after burn injury. Journal of immunology. 2010;185(5):2847–2853. doi: 10.4049/jimmunol.0903619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nature reviews. Immunology. 2003;3(12):984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 148.Pfister O, Lorenz V, Oikonomopoulos A, Xu L, Hauselmann SP, Mbah C, Kaufmann BA, Liao R, Wodnar-Filipowicz A, Kuster GM. FLT3 activation improves post-myocardial infarction remodeling involving a cytoprotective effect on cardiomyocytes. Journal of the American College of Cardiology. 2014;63(10):1011–1019. doi: 10.1016/j.jacc.2013.08.1647. [DOI] [PubMed] [Google Scholar]
- 149.Marron NBTU, Hammerich L, George F, Kim-Schulze S, Keler T, Davis T, Crowley E, Salazar AM, Brody J. In Situ Vaccine for Low-Grade Lymphoma: Combination of Intratumoral Flt3L and Poly-ICLC With Low-Dose Radiotherapy (conference abstract) Journal of Clinical Oncology. 2016 [Google Scholar]
- 150.Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13(6):370–383. doi: 10.1038/nrclinonc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, Liu H, Grilley B, Rooney CM, Heslop HE, Brenner MK, Dotti G. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. The Journal of clinical investigation. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maude SL, Shpall EJ, Grupp SA. Chimeric antigen receptor T-cell therapy for ALL. Hematology Am Soc Hematol Educ Program. 2014;2014(1):559–564. doi: 10.1182/asheducation-2014.1.559. [DOI] [PubMed] [Google Scholar]
- 154.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, Jurcic J, Rosenblat T, Maslak P, Frattini M, Sadelain M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kumaresan PR, Manuri PR, Albert ND, Maiti S, Singh H, Mi T, Roszik J, Rabinovich B, Olivares S, Krishnamurthy J, Zhang L, Najjar AM, Huls MH, Lee DA, Champlin RE, Kontoyiannis DP, Cooper LJ. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(29):10660–10665. doi: 10.1073/pnas.1312789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunological reviews. 2014;257(1):107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG, Inflammation P. Host Response to Injury Large-Scale Collaborative Research, A genomic storm in critically injured humans. The Journal of experimental medicine. 2011;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wong HR, Cvijanovich N, Allen GL, Lin R, Anas N, Meyer K, Freishtat RJ, Monaco M, Odoms K, Sakthivel B, Shanley TP. Genomics of Pediatric SSSI Genomic expression profiling across the pediatric systemic inflammatory response syndrome sepsis and septic shock spectrum. Critical care medicine. 2009;37(5):1558–1566. doi: 10.1097/CCM.0b013e31819fcc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012;25(4):609–634. doi: 10.1128/CMR.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent Lymphopenia after Diagnosis of Sepsis Predicts Mortality. Shock. 2014 doi: 10.1097/SHK.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nature medicine. 1997;3(6):678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 162.Weaver JG, Rouse MS, Steckelberg JM, Badley AD. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. FASEB J. 2004;18(11):1185–1191. doi: 10.1096/fj.03-1230com. [DOI] [PubMed] [Google Scholar]
- 163.Bohannon JK, Luan L, Hernandez A, Afzal A, Guo Y, Patil NK, Fensterheim B, Sherwood ER. Role of G-CSF in monophosphoryl lipid A-mediated augmentation of neutrophil functions after burn injury. Journal of leukocyte biology. 2015 doi: 10.1189/jlb.4A0815-362R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hernandez A, Bohannon JK, Luan L, Fensterheim BA, Guo Y, Patil NK, McAdams C, Wang J, Sherwood ER. The role of MyD88- and TRIF-dependent signaling in monophosphoryl lipid A-induced expansion and recruitment of innate immunocytes. Journal of leukocyte biology. 2016 doi: 10.1189/jlb.1A0216-072R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Romero CD, Varma TK, Hobbs JB, Reyes A, Driver B, Sherwood ER. The Toll-like receptor 4 agonist monophosphoryl lipid a augments innate host resistance to systemic bacterial infection. Infection and immunity. 2011;79(9):3576–3587. doi: 10.1128/IAI.00022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 167.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 168.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]