Abstract
US28 transcripts have been detected in primary monocytes and in THP-1 monocytes infected with HCMV but US28 protein expression has not yet been demonstrated in these cell types. Moreover, the mechanism(s) by which US28 signals and contributes to viral pathogenesis in monocytes remains unclear. Here, we show that US28 protein is robustly expressed in HCMV infected THP-1 monocytes and that US28 can trigger Gαq dependent signaling in THP-1 cells infected with HCMV and in THP-1 cells stably expressing US28. US28 signaling in these cells is dependent on G-protein coupling, but independent of chemokine binding. Importantly, we demonstrate that this US28 signaling is functionally important as it stimulates the adhesion of monocytes to an endothelial monolayer. Our studies, which demonstrate that US28-driven Gαq signaling has profound effects on monocyte biology, suggest that US28 driven phenotypic changes in HCMV infected monocytes may play important roles in HCMV dissemination and/or pathogenesis.
Keywords: HCMV, cytomegalovirus, latency, lytic replication, US28, vGPCR, Gαq, endothelial adhesion, monocytes, macrophages
Introduction
US28 is a HCMV encoded GPCR, which is structurally similar to human CC chemokine receptors, most notably, CCR-1 (Gao and Murphy, 1994). The HCMV genome encodes four such GPCRs (US27, US28, UL33 and UL78), and of these four, US28 appears to be the most potent signaler and have the most significant phenotypic effects on infected cells (Vischer et al., 2014). Although not essential for replication in vitro (Vieira et al., 1998), previous studies have shown that US28 can promote migration of vascular smooth muscle cells and rat macrophages (Streblow et al., 1999; Vomaske et al., 2009a), function as a chemokine sink to reduce chemokine availability in the milieu surrounding infected cells (Randolph-Habecker et al., 2002; Vieira et al., 1998), facilitate cell to cell viral transmission in epithelial cells (Noriega et al., 2014), and support latent infection of hematopoietic progenitor cells (Humby and O’Connor, 2015). In human foreskin fibroblasts and smooth muscle cells, the US28 protein is expressed with early to late phase kinetics (Miller et al., 2012; Stropes and Miller, 2008). In monocytes, which initially typically support a “dormant” or “abortive” HCMV phase, US28 transcripts have been demonstrated to be expressed either transiently or persistently after infection depending on the cell type used for the experiment (Beisser et al., 2001; Hargett and Shenk, 2010). However, since the presence of US28 transcripts may not necessarily reflect US28 protein expression, whether or not the US28 protein is expressed and present in monocyte and/or macrophages cells after de novo HCMV infection remains an interesting and important open question. Moreover, although US28 protein is thought to be produced in HCMV infected monocytes and macrophages, whether or not US28 plays an important functional role in this cell type during infection remains unclear.
Previous results from our lab and others have shown that US28 triggers constitutive signaling by coupling to Gαq in HCMV infected human foreskin fibroblasts, endothelial cells, vascular smooth muscle cells, and glioblastoma derived tumor cells (Casarosa et al., 2001; Casarosa et al., 2005; Miller et al., 2012; Minisini et al., 2003; Stropes and Miller, 2008). In the canonical Gαq signaling pathway, Gαq can activate phospholipase C-β to induce inositol triphosphate (IP3) accumulation, which leads to the release of calcium from the endoplasmic reticulum (ER) and the activation of protein kinases such as Protein Kinase C (PKC) (Rozengurt, 2007). In addition to Gαq, other Gα subunits including Gα12, Gα13, Gα16, and Gαi have been shown to be involved in US28-dependent constitutive and/or ligand-dependent signaling (Billstrom et al., 1998; Joshi et al., 2015; Melnychuk et al., 2004; Moepps et al., 2008). However, whether or not US28 triggers a similar or distinct set of signaling pathways in monocytes remains unexplored. Therefore, in this research we sought to examine whether US28 can trigger constitutive signals in a monocytic cell line, and if so, determine what Gα subunit is used by US28 to activate signaling. Pharmacological inhibitors have been widely used to assess G-protein signaling activity and many such inhibitors are available including Pertussis toxin (Gαi inhibitor) (Karimian et al., 2012), YM-254890 (Gαq inhibitor) (Takasaki et al., 2004), U-73122 (phospholipase C inhibitor) (Smith et al., 1990), and Ro-32-0432 (PKC inhibitor) (Wilkinson et al., 1993) all of which could be used to tease out the signaling mechanism(s) used by US28 in monocytes.
US28 is a seven-membrane spanning protein with an extracellular amino terminus and an intracellular carboxy terminal tail (Chee et al., 1990a; Chee et al., 1990b; Gao and Murphy, 1994; Vomaske et al., 2009b). US28, like most members of the GPCR superfamily contains a “DRY box” motif (aspartate-arginine-tyrosine) located in second intracellular loop at residues 128–130 that is essential for G protein coupling (Gether, 2000), and replacement of arginine 129 with alanine (R129A) abolishes G protein coupling (Waldhoer et al., 2003). In addition, amino acids between residues 11 and 16 in the amino terminus of US28 are required for ligand binding (Casarosa et al., 2005), and deletion of residues 2 through 16 (ΔN) eliminates all known chemokine binding to US28 (Stropes and Miller, 2008). US28 mutants such as US28-R129A and US28-ΔN are useful tools that can be used to dissect and analyze the signals, functions, and mechanisms of US28 action within cells.
The monocyte is not only a cell type in which HCMV may establish a latent or abortive type of infection but it also a cell type believed to play an important role in viral dissemination. Cell migration and extravasation across the blood vessel endothelial layer are crucial factors involved in viral dissemination, and previous studies have shown that the binding of MCP-1 or RANTES to US28 promotes vascular smooth muscle cell migration (Streblow et al., 1999), while binding of Fractalkine to US28 enhances macrophage migration in a rat macrophage model (Vomaske et al., 2009a). Thus, it appears that chemokine binding to US28 can have distinct effects on cell migration in a cell type dependent fashion. In addition to cellular migration and chemotaxis, monocyte adhesion to the endothelium is another essential element believed to be important for HCMV dissemination (Bentz et al., 2006). It is known that HCMV infected monocytes display increased monocyte/endothelial cell adhesion (Smith et al., 2007), but whether US28 can influence monocyte adhesion to the endothelium remains unknown.
In this study, we found that the US28 protein is expressed in HCMV infected THP-1 cells, a “monocytic-like” myeloid cell line that has proven to be a useful model for monocyte/macrophage development and biology and has been utilized for studies aimed at understanding HCMV latent/lytic switching in infected myeloid cells. We also find that US28 can constitutively couple to Gαq leading to activation of phospholipase C-β (PLC-β). In addition, we demonstrate that the US28 triggered Gαq signal promotes monocyte adhesion to endothelial cells via a Gαq➔PLC-β➔PKC signaling axis. The results from these studies suggest that US28 protein expression and signaling may influence the biology of HCMV infected monocytes thereby facilitating hematogenous dissemination of virus.
Material and methods
General reagents
Anti-human CD36 antibodies were purchased from eBioscience. Anti-FLAG M2 agarose beads and anti-FLAG M2 antibody conjugated to biotin were obtained from Sigma-Aldrich. Anti-IE1/IE2 mAb810 was purchased from Millipore, Inc. Anti-UL44 antibody is a gift from John D. Shanley; University of Connecticut. Streptavidin conjugated to HRP was purchased from BD Pharmagen.
Cell culture
Human endothelial cells (HECV) were obtained from the Interlab Cell Line Collection in Naples, Italy and cultured in DMEM (Dulbecco Modified Eagle’s Medium) supplemented with 10% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin at 37°C in 5% CO2. HS68 human foreskin fibroblasts (HFFs) were purchased from ATCC and maintained in Dulbecco’s modified Eagle’s medium (Mediatech) supplemented with 10% Fetal Clone III serum (Hyclone) and 1% penicillin/streptomycin (Mediatech). HECVs and HFFs were passaged every 3 days to maintain a sub-confluent state. The THP-1 monocyte-like cell line was maintained in RPMI-1640 (Roswell Park Memorial Institute-1640) supplemented with 10% FBS, 100 IU/ml penicillin and 100g/ml streptomycin at 37°C in 5% CO2. Cells were passaged every 3 days to keep the cell density below 106 cells /ml.
Propagation and purification of HCMV virus
The HCMV TB40E-US28FL (containing a mCherry cassette and a 3XFLAG added to US28) and TB40E-ΔUS28 viruses were generously provided by Dr. Christine O’ Connor (Miller et al., 2012; O’Connor and Shenk, 2011). These viruses were characterized and demonstrated to grow with similar kinetics and to similar titers to HCMV TB40E in HFFs. To propagate virus, HFFs were infected with TB40E viruses at MOI of 0.04. Viral supernatant was harvested at days 9, 11, and 13 post-infection. Cell culture supernatant containing virus was centrifuged at 1800 × G for 3 minutes at 21°C to remove cellular debris. The clarified supernatant was overlaid on a 20% D-sorbitol/1mM MgCl2 cushion and subjected to ultracentrifugation at 24,000 rpm for 1 hr at 21°C. Supernatant was decanted, and the viral pellet was resuspended in RPMI-1640 culture media. Viral supernatant was aliquoted and stored at −80°C.
Construction of US28 lentiviral mutant constructs
pcDNA3-US28FLAG, and pcDNA3-FLAG US28 R129A derived from the VHL/E strain of HCMV (Miller et al., 2003) were used as templates for construction of US28 lentivirus expression constructs. Primers for amplification of wildtype US28 (US28-WT): Forward primer: GTTCGGACTAGTGCCACCATGACACCGACGACGCGACCGCG; Reverse primer: GTTCGGTCTAGATTACTTGTCATCATCGTCCTTGTAGTC. Primers for amplification of amino terminal truncated US28 (US28-ΔN): Forward primer: GTTCGGACTAGTGCCACCATGGACGATGAAGCGACTCCCTGTGTC; Reverse primer is the same as reverse primer for wildtype PCR reaction. Primers for amplification of US28 containing an Arg to Ala mutation at amino acids 129 (US28- R129A) were the same as for wildtype US28. PCR reactions were performed for 30 cycles with denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 1 minute using Phusion High – Fidelity DNA polymerase (New England BioLabs Inc.). The fragments were gel purified using GeneJET Gel Extraction Kit (Thermo Scientific), and were digested with SpeI and XbaI. Digested fragments were inserted into the SpeI and XbaI sites of the pEN-TmiRC3 entry vector (provided by Iain Fraser via Addgene). US28 inserts in pEN-TmiRC3 entry vector were transferred to pSLIK-Venus (provided by Iain Fraser via Addgene) using the Gateway LR Clonase II Enzyme (Invitrogen). pSLIK-Venus encodes a DOX-regulatable US28 expression construct and the Venus fluorescent protein to identify transduced cells. All constructs were verified by DNA sequencing.
Reverse transcription PCR
Infected THP-1 cells were harvested, and RNA was isolated using RNA-Bee (Tel-Test, Inc.). 1μg of total RNA was converted to complementary DNA using an iScript cDNA Synthesis Kit (Bio-Rad). PCR reactions were performed for 30 cycles with Taq DNA polymerase using similar conditions to those described above. Primers for US28 PCR are WEM-116 (forward primer) GCTATGTTTGCCAGTTTGTGTTTTATCACGG and WEM-117 (reverse primer) AGGCTCTAGACTAGCCCACGAAGACGTACAGCAGCGG.
Production of lentivirus particles and transduction of THP-1 cells
2×106 293T cells were transfected with 3 μg of pSLIK-Venus lentiviral backbone containing US28-FLAG or US28-FLAG mutants, 1.5 μg of psPAX2 packing construct, and 0.5 μg of pVSVg envelope construct using a 4:1 ratio of Mirus LT-1 transfection reagent according to the manufacturer’s and cultured in DMEM supplemented with 10% FBS, penicillin and streptomycin. 24 hours after transfection, media were removed and cells were fed with fresh media. Culture media containing lentiviruses were then harvested at 48 and 72 hours post transfection and pooled. Viruses were concentrated by ultracentrifugation at 25,000 RPM for 90 minutes at 4°C. Supernatants were aspirated, and viral pellets were resuspended in 2 ml RPMI-1640 with 10% FBS and penicillin/streptomycin. 2ml of viral supernatant was used to infect 4×105 THP-1 cells via spinfection (i.e. centrifugation at 1000 × G for 90 minutes at 37°C). After spinfection, THP-1 cells were cultured overnight, and media were changed with fresh media in the morning. At day 2 and day 4 after transduction, THP-1 cells were analyzed by flow cytometry to examine the transduction efficiency.
HCMV infection of THP-1 cells
THP-1 cells were infected with TB40E-US28FL or TB40E-ΔUS28 at MOIs as indicated in the figure legends. After the viral supernatant was added, cells were spinfected at 21°C and 1000 × G for 30 minutes to enhance infectivity. After overnight culture, cells were spun down and the inocula were removed. Cells were incubated in 1X trypsin for 5 minutes to remove attached but un-internalized virions (O’Connor and Murphy, 2012). The trypsin reactions were neutralized by adding equal volumes of fresh culture media. The supernatant was aspirated, and cells were resuspended in fresh culture media. UV-inactivated virus was processed by exposing viral supernatant to UV in the cell culture hood for 15–20 minutes.
IP3 accumulation assays
2×105 cells were plated into each well in 12-well plates and cultured in inositol free RPMI-1640 (Roswell Park Memorial Institute Institute-1640) supplemented with 10% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin and 2 μCi of 3H-myoinositol for 24 hours at 37°C in 5% CO2. 1 μg of doxycycline or vehicle was the added to each well to induce gene expression for another 24 hours. Cells were harvested and washed with serum free RPMI once. THP-1 cells were resuspended in 1 ml serum free RPMI supplemented with 20 mM LiCl and incubated at 37°C in 5% CO2 for 2 hours to allow constitutive US28 driven IP3 accumulation to occur. Cells were pelleted by centrifugation at 500 × G for 5 minutes. Supernatant was removed. Cells were resuspended in 1 ml percholic acid and incubated at 4°C for 15 minutes. Cells were pelleted and 800 μl of supernatant was added to fresh Eppendorf containing 400 μl of neutralizing buffer (0.72M KOH and 0.6M KHCO3). The mixture was mixed by vortex and centrifuged at 13,000 rpm for 2 minutes. 50 μl of the supernatant was then mixed with 10 ml of scintillation fluid and used as a reference for “total labeling” for each sample. 1 ml of the supernatant was added to 3 ml of distilled water and passed through freshly prepared Dowex columns (AG1-8X; Bio-Rad). The columns were washed 2X with distilled water. Bound IP3 was eluted with 4 ml elution buffer (0.1 M formic acid and 1M ammonium formate). After elution, 10 ml scintillation fluid was added into each sample and counted in a liquid scintillation counter (Beckman Coulter). The eluted samples containing IP3 were divided by the reference “total labelling” sample to obtain the percent conversion of myo-inositol into IP3 for each sample. For infected cell IP3 experiments, THP-1 and PMA-differentiated THP-1 cells were infected at MOI of 10. Infected cells were fed with fresh media one day after infection. At day 2 post-infection, 2 μCi of 3H-myoinositol was added to each well containing 5×105 cells to label cells for 48 hours. Analyses of IP3 accumulation was performed as mentioned above.
Immunoprecipitation and western blotting
THP1 cells infected with HCMV TB40E-US28FL were harvested at multiple time points post infection as indicated in the figure legends and lysed in RIPA buffer. THP-1 control cells and THP-1 cells stably expressing US28, US28-R129, and US28-ΔN mutants were treated with 1 μg/ml doxycycline for 24 hours to induce US28 expression and similarly harvested in RIPA buffer. Cell lysates were passed through a 22G × 1 ½ needle ten times to shear chromosomal DNA, and centrifuged at 12000 × G to remove cellular debris. 50 μl of cell lysate was saved as whole cell lysate. 20 μl of M2-agarose beads were added to the remaining cell lysate, and samples were incubated at 4°C overnight. M2-agarose beads were pelleted by centrifugation and supernatant were aspirated. Agarose beads were washed 3 times with RIPA buffer. 50 μl of 3X sample buffer was added to each sample and the samples were incubated at room temperature for 30 minutes to elute the US28 protein. Immunoprecipitates or whole cell lysate were used for 10% SDS-PAGE electrophoresis. After electrophoresis, proteins were transferred from SDS-PAGE to nitrocellulose using a semi-dry transfer apparatus. Nitrocellulose membranes were blocked in 5% skim milk for 1 hour. For detection of FLAG-tagged US28 in immunoprecipitates, nitrocellulose membranes were incubated in rabbit polyclonal anti-flag antibody (diluted in TBST) at 4°C overnight. This combination immunoprecipitation/western blot approach is typically used for detection of US28 as its low level of expression combined with its strong membrane association makes it difficult to detect by straight western blot. For detection of IE, UL44, and actin proteins in whole cell extracts, nitrocellulose membranes were incubated in the appropriate antibody (diluted in TBST) at 4°C overnight. All membrane were washed 3 times with TBST after incubation with the primary antibody and incubated with goat anti-rabbit or goat anti-mouse HRP conjugated secondary antibody (diluted in TBST containing 5% milk) for 3 hours. The membranes were washed 3 times with TBST, and subjected to antibody detection using the SuperSignal West Pico or Femto chemiluminescent substrates (Thermo Scientific). Luminescence emitted from the membranes was detected by blue autoradiography film BX. Films were developed by Kodak min-R mammography processor.
Monocyte-endothelial cell adhesion assays
1×105 HECV endothelial cells were plated into each well in a 12 well plate and cultured overnight. 500,000 THP-1 cells were harvested and plated into each well to co-culture for 1 hour. The suspended cells were removed and each well was washed twice with culture media. Culture wells were then trypsinized to detach endothelial cells and attached THP-monocytes from the plate. Cells were collected and stained with anti-human CD36 antibody for one hour. Cells were washed once with DPBS. Cells were resuspended in 200 μl DPBS. Cells from each well were counted by flow cytometry, and the number of total cells and CD36 positive cells (THP-1) are measured by flow cytometry. The number of CD36 positive THP-1 cells adhered to the endothelial monolayer was divided by the number of input THP-1 cells put into each well and the ratio was converted to percentage.
Results
Examination of US28 RNA and protein expression patterns in the THP-1 monocytic cell line
While previous studies have indicated that US28 transcripts can be detected in HCMV infected primary CD14+ monocytes and in the THP-1 monocytic cell line (Beisser et al., 2001; Cheung et al., 2006; Hargett and Shenk, 2010), it remained unclear if the US28 protein is indeed expressed in any of these settings. We sought to examine this question in THP-1 cells as this cell line could serve as a useful model to readily probe mechanistic and functional effects of US28 expression in monocytes and other related myeloid cell types. We initially repeated the experiments of Beisser and colleagues to confirm the expression of US28 mRNAs. THP-1 cells were infected with TB40E-US28FL (an otherwise wildtype HCMV strain) and TB40E-ΔUS28 (a US28 null HCMV strain). Infected cells were harvested at various time points post-infection and RNA was isolated for use in RT-PCR experiments. US28 transcripts were detected from 1–4 days post-infection in TB40E-US28FL infected cells (Figure 1A), consistent with the earlier observation of Beisser et. al. Infection with TB40E-ΔUS28 virus failed to produce detectable US28 mRNAs as would be expected considering this virus is deleted for the US28 ORF.
Figure 1. US28 RNA and protein expression in HCMV infected THP-1 cells.
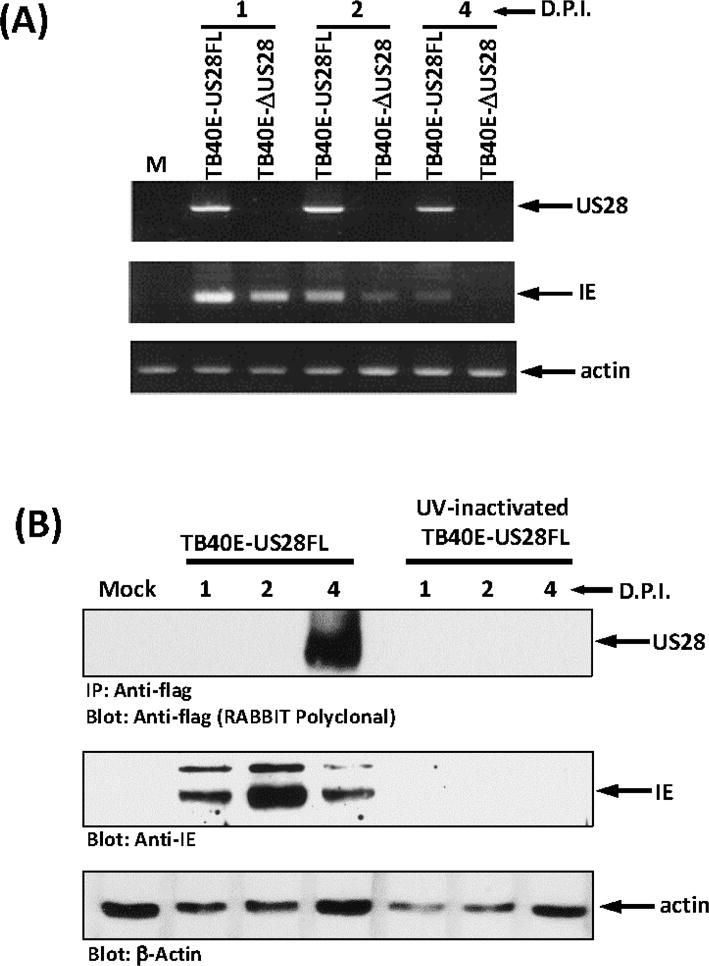
(A) THP-1 cells were infected with TB40E-US28FL virus or TB40E-ΔUS28 viruses at MOI of 10. Cells were harvested at days 1, 2, and 4 post-infection. RNA samples were converted into cDNA by reverse transcription. PCR was performed to analyze the expression of HCMV US28, immediate-early genes, and cellular actin (internal control). (B) THP-1 cells were infected with TB40E-US28FL virus or UV-inactivated virus at MOI of 10. Cells were harvested at days 1, 2, and 4 post-infection. Whole cell lysates were analyzed by western blot to detect HCMV immediate early gene (IE) protein, UL44, and actin (internal control). Immunoprecipitation and western blot with anti-flag antibody were performed to analyze US28 protein expression. d.p.i: days post infection.
We next examined US28 protein expression in HCMV TB40E infected THP-1 cells. THP-1 cells were infected with wildtype or UV-inactivated HCMV TB40E-US28FL. Since UV-inactivated virus infected cells do not initiate viral gene expression, this serves as an appropriate control to tell us whether US28 protein expression in the monocytes would be the result of de novo protein synthesis or delivered into the cell by the infecting viral particle. This latter part of the experiment was included in our studies as several groups have proposed that US28 present in the viral particles could be inserted into the plasma membrane of the infected cells and recent studies by Humby and O’Connor did find virion delivered US28 in latently infected Kasumi-3 cells (Fraile-Ramos et al., 2001; Humby and O’Connor, 2015; Penfold et al., 2003). THP-1 monocytes infected with a MOI of 10 were harvested at days 1, 2 and 4 post-infection, FLAG-tagged US28 protein was immunoprecipitated with anti-FLAG M2 affinity gel, and analyzed by western blotting using rabbit polyclonal anti-FLAG antibodies (Figure 1B, upper panel). Our results demonstrate that US28 protein is expressed in HCMV infected THP-1 cells accumulating to a maximal level at day 4 post-infection (Figure 1B and data not shown). US28 protein expression is delayed somewhat relative to immediate early IE1/IE2 proteins, which peaked at day 2 post-infection (Figure 1B, middle panel). US28 protein expression was also delayed somewhat relative to US28 mRNA expression (compare Figure 1A and 1B). While US28 mRNA is detectable at day 1 post-infection, we are unable to detect US28 protein until day 4 post-infection. This could be a reflection of the fact that two mRNAs encode for US28, one containing both US27/US28 ORFs and one containing the US28 ORF. We have not further explored the exact structure of the mRNAs present in the infected THPs, but it seems likely that transcripts giving rise to US28 protein accumulate with somewhat delayed kinetics such that the protein is made maximally by day 4 post-infection. Surprisingly, we were unable to detect US28 in cells infected with UV-inactivated virus. We cannot, however, rule out the possibility that a small amount of virion delivered US28 could be present, though undetectable, at early times post-infection. These results indicate that US28 protein is expressed in HCMV-infected THP-1 monocytes and that the presence of US28 protein in these cells is a result of de novo protein synthesis rather than being delivered by the infecting virus particle. We performed similar experiments in HCMV-infected CD14+ primary human monocytes and we were indeed able to detect US28 expression at days 2 and 3 post-infection (data not shown). Taken together, our results indicate that US28 protein is expressed in HCMV infected monocytes.
Infection of THP-1 monocytes with HCMV induces US28-dependent signaling to PLC-β
While previous studies have shown that US28 can constitutively activate Gαq and induce PLC-β dependent IP3 accumulation in HCMV infected fibroblasts, smooth muscle cells, and endothelial cells (Miller et al., 2012; Minisini et al., 2003; Stropes and Miller, 2008), whether US28 can trigger constitutive signaling in HCMV infected myeloid cells remains unknown. Given that US28 is predominantly a Gαq coupled GPCR, we chose to examine US28 signaling initially by assessing US28-promoted phospholipase C-β (PLC-β) activity. THP-1 cells were infected with TB40E-US28FL or TB40E-ΔUS28 at an MOI of 10 and PLC-β signaling was assessed at 96 hpi, by measuring the accumulation of inositol phosphates (InsP). Under these infection conditions, we were unable to detect any US28 dependent PLC-β signaling (Figure 2A, left panel). We surmised that the lack of detectable US28 signaling under these conditions might be reflective of the fact that only ~8% of infected THP-1 express viral proteins and the assay is simply not sensitive enough to detect US28-dependent signaling (Wu and Miller, 2015). Treatment with phorbol esters like PMA drives THP-1 cells into more of a macrophage-like phenotype and drives the lytic phase such that ~50% of the infected cells begin to express significant levels of immediate early, early, and late proteins (Wu and Miller, 2015). Therefore, we treated THP-1 cells with PMA for 72 hours before infecting with HCMV. Using these conditions, we observed a strong increase in the expression of US28 as examined by immunoprecipitation/western blot (Figure 2B). We then repeated the PLC-β signaling experiments following infection with TB40E-US28FL and TB40E-ΔUS28 viruses (Figure 2A, right panel). Interestingly, TB40E-US28FL induced PLC-β signaling 5.9-fold over mock infected cells, while the US28 deletion mutant failed to induce PLC-β dependent signaling. Taken together, these results indicate that US28 does in fact function as an active signaling molecule capable of activating PLC-β in HCMV infected myeloid cells.
Figure 2. US28 constitutively induces PLC-β signaling in HCMV infected THP-1 cells.
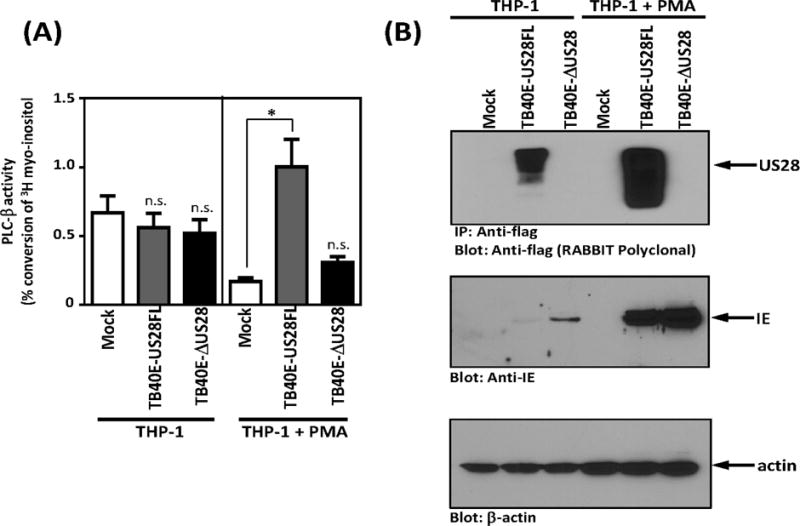
(A) THP-1 and PMA differentiated THP-1 cells were mock infected or infected with TB40E-US28FL or TB40E-ΔUS28 viruses. At day 4 post-infection, cells were harvested and PLC-β activity was assessed. (B) THP-1 and PMA-differentiated THP-1 cells were mock infected or infected with the indicated TB40E viruses. US28 protein expression was analyzed by immunoprecipitation and western blot at day 4 post-infection. Data are representative of 3–4 independent experiments. Statistical significance was determined by performing unpaired two-tailed Student’s t tests with GraphPad Prism® software. **: p<0.01, *: p<0.05.
Mechanistic analyses of US28 stimulated PLC-β signaling in THP-1 monocytes
To explore the mechanism by which US28 induces constitutive PLC-β signaling in infected monocytes, we developed stable and doxycycline inducible THP-1 cell lines expressing wildtype and mutant versions of US28. THP-1 cells were transduced with lentiviruses (pSLIK-Venus) carrying doxycycline/tetracycline inducible US28 expression cassettes. We established stable THP-1 cell lines carrying wildtype US28 (US28-WT), chemokine binding deficient US28 (US28-ΔN), and signaling deficient US28 (US28-R129A). Transduction efficiency was monitored using the co-expressed Venus fluorescent protein (data not shown). Immunoprecipitation and western blot techniques were then used to confirm US28 expression. Using this system, we find that US28 protein is tightly repressed in the absence of DOX, but robustly induced within 24 hours after addition of DOX to the media (Figure 3A). Subcellular localization of US28 was also assessed in the THP-1 cells following induction (Figure 3B). Interestingly, while previous studies with US28 expression in fibroblasts and epithelial cells has demonstrated significant endosomal associated US28, indicative of constitutive US28 internalization (Fraile-Ramos et al., 2003), in the THP monocytes we observe mostly cell surface US28 that predominantly clusters to one region of the cell. It remains unclear as to the nature of these clusters and if they are localized to cholesterol-rich lipid rafts. Similar clusters containing the signaling receptor SIRPα have recently been observed in monocytes/macrophages and in this case the clusters serve to bring signaling molecules into microdomains with SIRPα (Ha et al., 2013). Further studies will be necessary to determine if similar mechanisms are exploited by US28 to facilitate signaling. This newly developed, tightly regulatable myeloid-specific US28 expression system provides a robust model to explore US28 signaling as it affords us with the ability to direct US28 expression only when desirable and alleviates any problems that could be attributed to chronic long-term expression of this highly active viral protein.
Figure 3. Ectopic US28 expression induces PLC-β signaling in THP-1 cells.
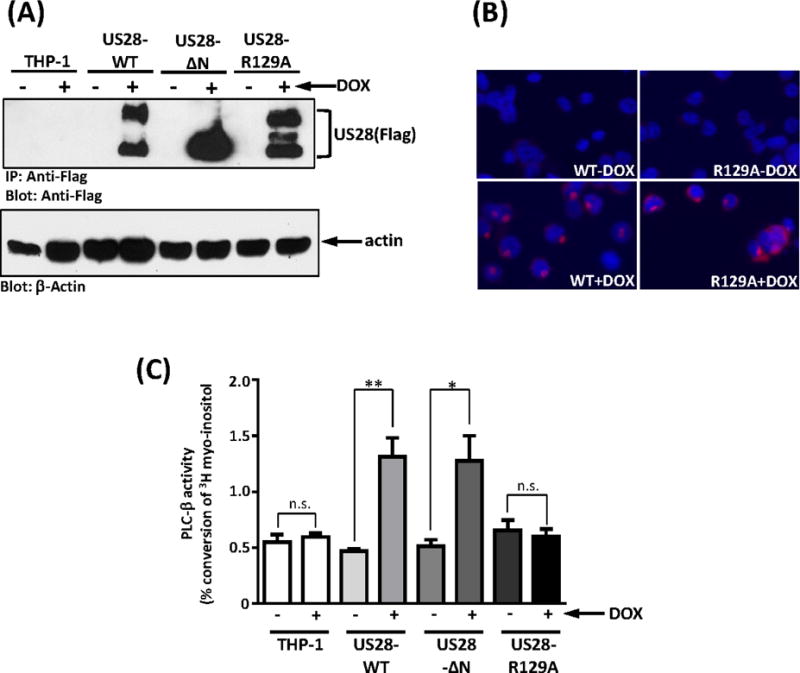
(A) Transduced THP-1 cells were treated with 1 μg/ml of doxycycline for 24 hours. Immunoprecipitation and western blot with anti-flag antibody were used to detect US28 and mutant protein expression. Whole cell lysates were analyzed by western blot to detect actin expression. (B) The subcellular localization of US28 and US28-R129A were analyzed by immunostaining with anti-flag antibody and fluorescence microscopy. (C) Transduced THP-1 cells were labeled with 3H-myoinositol 1 day before induction, and treated with 1 μg/ml of doxycycline or vehicle (H2O) for 24 hours. Percentage of PLC-β induced IP conversion was calculated using the reading from column elution divided by the measure of total labelling. The graph represented four independent experiments. Statistical significance was determined by performing unpaired two-tailed Student’s t tests with GraphPad Prism® software. ΔN: N-terminal deletion US28, R129A: US28 with DRY box mutation. **:p<0.01, *: p<0.05, n.s.: not statistically significant.
To examine the mechanism underlying the ability of US28 to promote PLC-β driven IP3 accumulation (Figure 2A), THP-1 cells expressing wildtype, ΔN, and R129A US28 proteins were labeled with 3H-myoinisitol for 48 hours, and US28 expression was induced with 1μg/ml DOX for the final 24 hours of the labeling protocol. The results of IP3 accumulation assays demonstrated that wildtype US28 causes a 3-fold increase in IP3 accumulation compared to control THP-1 cells or US28 cells without DOX (Figure 3C). Since IP3 accumulation is an indicator of the activated of phospholipase C-β (Kawakami and Xiao, 2013), a downstream effector of Gαq, these results suggested that US28 can constitutively activate Gαq in THP-1 cells. To verify that the IP3 signaling is triggered by US28 coupling to G proteins, we used the US28 DRY box mutant US28-R129A, which cannot couple to heterotrimeric G proteins (Waldhoer et al., 2003). Expression of US28-R129A was confirmed by immunoprecipitation/western blot and immunofluorescence and its expression and subcellular localization shown to be similar to that of wildtype US28 (Figures 3A and 3B). While the protein expression levels of US28-R129A are similar to that of wildtype US28 after doxycycline treatment, IP3 signaling is not triggered by US28-R129A mutant (Figure 3C), indicating that the US28-driven IP3 accumulation is indeed G protein-dependent.
Since it is known that US28 can bind to multiple chemokines (Casarosa et al., 2005; Vomaske et al., 2009b), it is possible that chemokines secreted by monocytes such as MCP-1 (Cignetti et al., 2003; Steube et al., 1999) could be binding to US28 and activating signaling in an autocrine fashion. Therefore the US28 N-terminal truncated mutant (US28-ΔN), which lacks amino acids 2 to 16 and cannot bind to chemokines (Stropes and Miller, 2008) was used to determine whether the IP3 accumulation induced by US28 is occurring via a constitutive or ligand dependent signaling process. The expression of US28-ΔN was verified by immunoprecipitation and western blot (Figure 3A), and the expression levels were found to be similar to both US28 wildtype and US28-R129A. THP-1 cells expressing US28-ΔN showed a significant induction of IP3 accumulation when compared to control cells or US28-ΔN cells without DOX and the level of signaling was comparable to that of US28-WT (Figure 3C). These results indicate that the US28 signaling we observe is due to constitutive signaling activity and unlikely to involve autocrine signaling mediated by secreted chemokines. Taken together, the data obtained from these experiments indicated that US28 can constitutively couple to heterotrimeric G proteins to kindle signal transduction in monocytes.
While the cellular chemokine receptors most highly similar to US28 are classically coupled to Gαi (Neptune and Bourne, 1997; White et al., 2013), much of the previous work with US28 in non-myeloid cells has demonstrated that it is constitutively coupled to Gαq. Therefore, to determine which Gα subunit is utilized by US28 for this constitutive signaling in monocytes, we re-examined US28 induced IP3 accumulation using pharmacological inhibitors of the relevant G-proteins (Figure 4). In particular, Gαi and Gαq inhibitors were used along with a PLC-specific inhibitor to examine this question. YM-254890, a Gαq specific inhibitor (Takasaki et al., 2004), suppressed the IP3 signaling induced by US28, as did U-73122, a PLC inhibitor (Smith et al., 1990). However, pertussis toxin, a Gαi inhibitor (Karimian et al., 2012), had no effect on IP3 accumulation activated by US28 indicating that in the case of US28 signaling in THP-1 monocytes, Gαq and not Gαi is the specific G-protein responsible for activating this signaling (Figure 4). In summary, using a combination of US28 mutants and pharmacological inhibitors, we demonstrate that US28 can constitutively trigger Gαq-dependent signaling in monocytes leading to PLC-β activation and accumulation of the second messenger IP3.
Figure 4. US28 induced PLC-β signaling in stable THP-1 lines is blocked by Gαq and PLC-β inhibitors but not Gαi inhibitors.
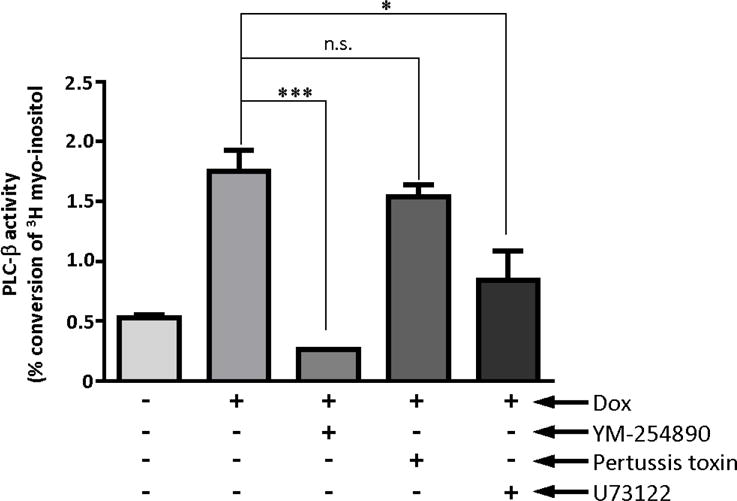
Transduced THP-1 cells were labeled with 3H-myoinositol 1 day before induction, and treated with 1 μg/ml of doxycycline or vehicle (H2O) for 24 hours. THP-1 cells were pretreated with inhibitors of Gαq (YM-254890, 100 nM), PLC-β (U73122, 2 μM), or Gαi (pertussis toxin, 200nM) for 30 minutes before IP3 assay. Percentage of IP3 conversion was calculated using the reading from column elution divided by the measure of total labelling. The graph represented three independent experiments. Statistical significance was determined by performing unpaired two-tailed Student’s t tests with GraphPad Prism® software. YM: YM-254890, PTX: pertussis toxin, ***: p=0.001,**:p<0.01, *: p<0.05, n.s.: not statistically significant.
US28 promotes THP-1 monocyte adhesion to endothelial cells
Monocytes, a cell type in which HCMV is prevalent in vivo, typically circulate throughout the blood system, and when stimulated by inflammatory signals, extravasate across the endothelial lining into surrounding tissues where they differentiate into macrophages (Gerna et al., 2004; Stevenson et al., 2014). In the case of HCMV infected monocytes, as monocyte➔macrophage differentiation proceeds during and after adhesion to the endothelial cell layer (Chan et al., 2008; Chan et al., 2012; Smith et al., 2007), HCMV is thought to transition from a non-permissive stage into a lytic one. Since US28 induces significant signaling in the monocytes, we hypothesized that US28 might also affect the ability of THP-1 monocytes to adhere to endothelial cells. THP-1 cells induced to express US28 via DOX addition were added to HECV endothelial cell monolayers and allowed to adhere for a time course between 5 and 60 minutes (Figure 5). We chose this time course based on published studies by other groups using THP-1 cells as a model for endothelial adhesion (Kawakami et al., 2007; Manna and Jain, 2014; Ruan et al., 2015). At each time point, non-adhered THP-1 cells were removed from the HECV endothelial monolayer by washing twice with culture media. Monolayers containing endothelial cells and adhered THP-1 cells were detached from the plates and stained with the CD36, which is highly expressed on THP-1 cells but not on the underlying endothelial monolayer. US28 expressing cells showed a significant increase in endothelium adhesion at all time points tested (Figure 5).
Figure 5. US28 expression in monocytes drives increased monocyte to endothelial cell adhesion.
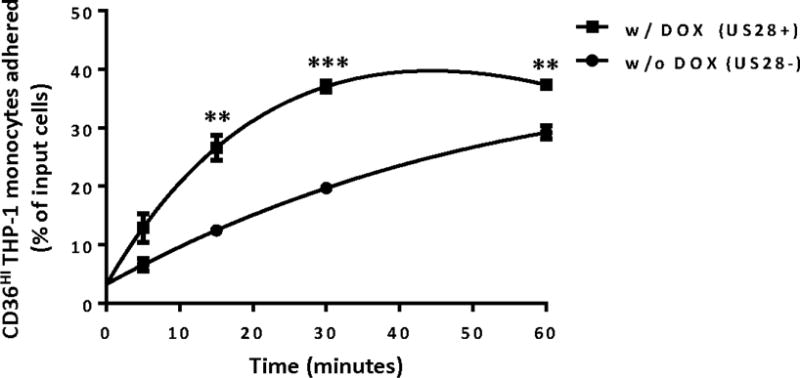
Transduced THP-1 monocyte cells were treated with doxycycline (1 μg/ml) for 24 hours to induce US28 expression. Cells were then co-cultured with an endothelial cell monolayer (HECV) for indicated times. Unattached cells were removed by vigorous washing with media alone. The number of attached cells were counted by flow cytometry and calculated as a percentage of input cells used in the assay. The data represent 3 independent experiments. Statistical significance was determined by performing unpaired two-tailed Student’s t tests with GraphPad Prism® software. ** : p<0.01, *** : p<0.001
We then examined the chemokine binding domain mutant US28-ΔN and the G-protein signaling deficient mutant US28-R129A for their abilities to promote monocyte adhesion. Cells expressing either wildtype US28 or the US28-ΔN mutant exhibited a significant increase in cell adhesion at 60 minutes, while cells expressing the US28-R129A mutant did not demonstrate any increase in THP-1 monocyte adhesion (Figure 6). Based on the data with US28 mutants, our data indicates that US28 derived signaling is playing an active role in facilitating cell adhesion to endothelial cells (Figure 6). It is intriguing that the US28-ΔN mutant induced monocyte adhesion similarly to that of US28 WT. US28 has been shown to be able to bind to Fractalkine (Kledal et al., 1998), an adhesion molecule which can be expressed by endothelial cells (Imaizumi et al., 2004; Umehara et al., 2001). Therefore, it was possible that US28-mediated monocyte adhesion to endothelial cells could be occurring through a mechanism involving binding of US28 to Fractalkine present on the endothelial cell surface. However, our results show that this is not the mechanism that US28 uses to promote monocyte-endothelial cell adhesion because US28-WT and US28-ΔN both promote monocyte adherence to the endothelial cells while the signaling dead US28-R129A mutant does not. Therefore, based on these data we conclude that ligand binding of endothelial cell surface proteins to the amino terminus of US28 is not involved in the US28-promoted monocyte/endothelium adhesion observed in our studies. Rather, the data support the notion that constitutive US28 signaling is an important factor in this process.
Figure 6. US28 induced monocyte-endothelial cell adhesion is dependent on constitutive G-protein based signaling but not the extracellular ligand binding domain.
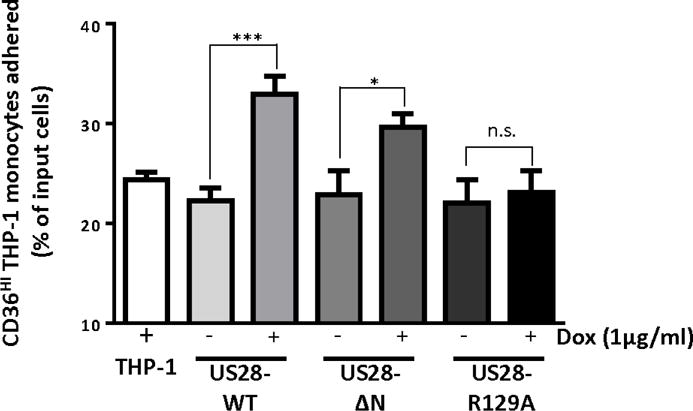
Transduced THP-1 cells were treated with 1 μg/ml of doxycycline or vehicle (H2O) for 24 hours to induce US28 expression. 5×105 THP-1 cells were then co-cultured with HECV cells for one hour, and unattached cells were washed away with media. Numbers of input cells and cells attaching to HECV cells were determined by flow cytometry. The ratios between cells attaching to HECV cells and input cells were converted into a percentage. The graph represented eight independent experiments. Statistical significance was determined by performing unpaired two-tailed Student’s t tests with GraphPad Prism® software. ***: p<0.0001, *: p<0.05, n.s.: not statistically significant.
To explore the mechanistic nature of the constitutive US28-based signaling involved in regulation of monocyte-endothelial adhesion, we investigated the effects of the Gαq YM-254890 inhibitor that we previously used in PLC-β signaling assays as well as the PKC inhibitor Ro32-0432 (Figure 7). We chose to include the PKC inhibitor in our investigations because PKC is a downstream mediator of PLC-β signaling/IP3 accumulation and because PKC activity has been previously shown to promote monocyte adhesion (Chang et al., 2012). Cells were treated with DOX overnight to induce US28 expression and then treated with YM-254890 or Ro32-0432 inhibitors for 60 minutes before being overlaid onto the HECV endothelial cell monolayer. Both inhibitors significantly inhibited the US28 induced increase in cell adhesion, demonstrating that US28 induced monocyte adhesion involves a Gαq➔PLC-β➔IP3➔ PKC signaling pathway (Figure 7). Interestingly, while the Gαq inhibitor YM-254890 completely abolished US28-promoted adhesion, the PKC inhibitor Ro-32-0432 only inhibited about 50% of the US28-promoted adhesion (Figure 7). This difference could be due to variable potency of the two inhibitors or could suggest that an additional PKC-independent Gαq signal is also involved in adhesion. Further experimentation will be necessary to fully explore this point. Although in the monocyte-endothelium adhesion assays the media containing inhibitors was replaced with fresh media without inhibitors, it is possible that trace levels of the inhibitors could be carried over thereby influencing monocyte-endothelial adhesion by modulating the phenotype of the endothelial cell and not the monocyte. To address this issue, we examined THP-1 monocyte adhesion to plastic culture dishes that were coated with collagen. Interestingly, US28 also modestly promoted the ability of THP-1 monocytes to adhere to the collagen coated plastic (data not shown). This adhesion was similarly blocked by both the Gαq and PKC inhibitors. Therefore, we conclude that the inhibition of US28 induced adherence of THP-1 monocytes by the Gαq and PKC inhibitors is due to a direct inhibition of US28 signaling in the THP-1 monocytes and not due to an indirect effect of the inhibitors on the underlying endothelial monolayer. In summary, we demonstrate that US28 protein is expressed in monocytes, actively induces signaling through Gαq, PLC-β and PKC, ultimately leading to the induction of monocyte-endothelial adhesion.
Figure 7. US28 induced monocyte-endothelial cell adhesion requires the downstream signaling molecules Gαq and PKC.
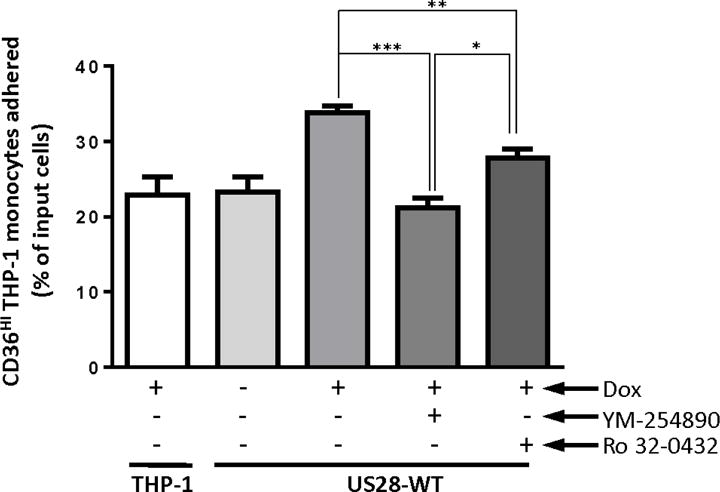
Transduced THP-1 cells were treated with 1 μg/ml of doxycycline or vehicle (H2O) for 24 hours to induce US28 expression. Before co-culture, cells were pretreated with inhibitors of Gαq (YM-254890, 100 nM) and PKC (Ro-32-0432, 5 μM), or vehicle for an hour. 5×105 THP-1 cells were then co-cultured with HECV cells for one hour, and unattached cells were washed away with media. Numbers of input cells and cells attaching to HECV cells were determined by flow cytometry. The ratios between cells attaching to HECV cells and input cells were converted into a percentage. The graph represented four independent experiments. Statistical significance was determined by performing unpaired two-tailed Student’s t tests with GraphPad Prism® software. *: p<0.05, **: p<0.01, ***: p<0.0001.
Discussion
In this research, we demonstrate that the HCMV US28 vGPCR protein is expressed in latently infected THP-1 cells and show that US28 can productively activate signaling pathways including one leading to PLC-β. Moreover, using an inducible system for US28 expression in THP-1 cells, we provide mechanistic information indicating that US28 specifically activates the Gαq family of G-proteins in an agonist-independent manner leading to PLC-β and PKC activation and induction of monocyte➔endothelial cell adhesion. From a signaling perspective, the proximal events downstream of US28 elucidated in this study remain consistent with findings in other cell types such as fibroblasts and smooth muscle cells (Miller et al., 2012; Minisini et al., 2003; Stropes and Miller, 2008), but our results for the first time reveal that US28 signaling through Gαq/PKC can promote the adhesive properties of the cell. These results provide novel insights into the function and signaling of US28 in a cell type that can serve as a reservoir for hematogenous dissemination of HCMV. Since monocyte migration and adhesion to endothelial cells are cellular properties important for viral dissemination (Stevenson et al., 2014), our results raise the possibility that US28 may play an essential role in HCMV dissemination. In murine cytomegalovirus (MCMV) studies, it has been shown MCMV M33-null virus has a defect in viral dissemination as it fails to grow in the salivary gland and that a US28 rescued virus showed partially restoration in salivary gland growth (Farrell et al., 2011), supporting the hypothesis that US28 can influence viral dissemination.
Our studies primarily use inducible US28 expressing THP-1 cells to explore the functional activities and signaling pathways induced by US28 expression because only 5–8% of monocytes or THP-1 cells express HCMV genes after infection (Wu and Miller, 2015) and it is difficult to examine signaling and function when the uninfected cells outnumber the infected cells by such a large margin. In addition, as HCMV encodes over 200 gene products, many of which influence cell signaling and could thereby mask the effects of US28, it is important to first examine US28 function in isolation in specific cell types and then to revisit interesting questions at a later point in the context of infection. Due to these limitations, we chose to use an inducible ectopic expression system to investigate the signaling and function of US28 in THP-1 cells. Having established that US28 signaling in the THP-1 monocytes leads to the induction of cellular adhesion, we are now poised to further explore these functions in CD14+ monocytes from peripheral blood. Our data does indicate that US28 protein is expressed in the primary cells, an important step in providing the necessary data in a natural environment. To pursue the US28 signaling and adhesion experiments in primary monocytes it will be necessary to establish pure populations of infected monocytes by flow cytometry due to the low percentage of infected cells and the short-lived nature of these cells. Moreover, it will be interesting to determine whether one of the MCMV GPCR homologs similarly affects monocyte adhesion and develop an appropriate system in the mouse model in which to explore this avenue of research.
We also provide evidence that US28 promotes monocyte/endothelial adhesion via the activation of a Gαq➔PLC-β➔PKC pathway. This mechanism of US28 promoted adhesion is somewhat surprising given that US28 can bind cell surface ligands such as Fractalkine (Kledal et al., 1998), but it is clear from our mutational studies that the amino-terminus of US28 is not required for this activity and that pharmacological inhibition of monocyte signaling pathways abolishes this activity. The target(s) downstream of PKC in this process have not yet been identified. One possible candidate PKC target is α4β1 integrin. Previous studies have demonstrated that PLC-β activation leads to an inside-out signal for α4β1 integrin activation (Ashida et al., 2003) which causes increased adhesion to VCAM-1 and fibronectin found on endothelial cells (Kawakami et al., 2007). Future studies aimed at identifying the target of PKC signaling downstream of US28 and involved in the promotion of monocyte adhesion are needed and could be explored by RNA-seq analyses of transcriptional changes in US28 expressing cells followed by siRNA knockdown of identified targets. It is interesting to speculate that inhibition of US28 signaling could have profound effects on HCMV dissemination and provide an attractive therapeutic target.
HCMV infection has frequently been reported as a potential co-factor in the promotion of atherosclerosis (Caposio et al., 2011; Popovic et al., 2012; Streblow et al., 2001a). Several groups have speculated that US28 could impact the progression of atherosclerosis via the stimulation of smooth muscle cell migration (Streblow et al., 1999; Vomaske et al., 2010; Zhou et al., 1999). However, many studies have implied that monocyte-endothelium adhesion is a crucial factor for atherosclerosis (Aoki et al., 2007; Shagdarsuren et al., 2009; Tatara et al., 2009) and our results suggest that US28 could have an additional function in the development or promotion of atherosclerosis (Streblow et al., 2001b) by promoting monocyte adhesion to the endothelium adjacent to the developing plaque. Future research and the development of appropriate animal models will be needed to verify whether US28 can indeed affect atherosclerosis.
The ability of US28 to activate Protein kinase C may have broad implications beyond the scope of the current study. PKC is known to affect the differentiation of myeloid cells (Barbosa et al., 2014; Lin et al., 2011), a process clearly central to the HCMV latent➔lytic switch (Sinclair, 2010). We have not observed any significant effect of US28 on myeloid maturation and differentiation in the THP-1 model (data not shown). However, it is possible that US28 signaling through PKC may have profound effects on the differentiation of myeloid progenitor cells and influence progression of HCMV into the lytic phase. Humby and O’Connor have recently published that US28 is important for the establishment of latency (Humby and O’Connor, 2015), so it will be interesting to determine if US28 regulates PKC signaling in hematopoietic and myeloid progenitors. It is formally possible that US28 may function early during infection to regulate the establishment of latency and at later time points to regulate the reactivation of virus replication. Under such a scenario, US28 may exhibit differential patterning of signaling such that regulation of PKC signaling may not occur until later time points post-infection when US28 protein levels are high and when US28 induced signaling may regulate reactivation through PKC signaling.
It is interesting that in the before mentioned studies by Humby and O’Connor (Humby and O’Connor, 2015), they could detect virion derived US28 that is brought into the Kasumi-3 cells during viral entry. In our studies, the US28 protein that is expressed in the monocytes is due to de novo production of US28 protein that is not apparent until 3–4 days post-infection. It is intriguing to speculate that regulation of US28 stability and protein degradation could be different depending on the cell type being studied. US28 has been demonstrated to be have diverse functions in regulating signal transmission and it is feasible that US28 turnover may more readily occur in the monocyte/macrophages than in the Kasumi-3 myeloid progenitors leading to our inability to detect virion delivered US28 in the THP-1 monocytes. Lysosomal protein degradation would be expected to be more efficient in phagocytic cells like dendritic cells and macrophages, and this could have a profound effect on HCMV biology as turnover of critical viral proteins could be altered relative to that in non-phagocytic cells. Detailed analyses of the US28 half-life in different cells could provide important information regarding the interpretation of these results.
In summary, in these studies we demonstrate that US28 protein is expressed in non-permissively infected monocyte cells and has the potential to rewire monocyte signal transduction and promote cell adhesion. Therefore, we speculate that US28 not only has important functions in the permissive phase in fibroblasts and smooth muscle cells, but also plays a crucial role in cells such as monocytes that typically harbor virus in a non-productive or semi-permissive state. Monocytes are central to HCMV dissemination and the establishment of US28 as a viral regulator of monocyte biology provides the basis for studies aimed at developing novel therapeutics to target US28 that could eventually be used to combat HCMV pathogenesis and disease.
Highlights.
The HCMV US28 vGPCR protein is present in HCMV infected monocytes
US28 induces potent signaling through the Gaq family of G-proteins
This Gaq signaling is constitutive and requires the DRY box but not the amino terminus
US28 signaling via this pathway promotes monocyte adhesion to endothelial cells
Acknowledgments
We would like to thank Christine O’Connor for providing the recombinant TB40E viruses, J. Shanley for UL44 antibody, and Astellas Pharmaceuticals for providing the YM-254890. We thank the Cell Processing and Manipulation Core in the Translational Cores, and physicians and nurses at CCHMC for obtaining and processing peripheral blood samples for monocyte purification. We also thank the CCHMC Translational Research Trials Office for providing the regulatory and administrative support for this endeavor. S.E. Wu was supported by a Teaching Assistantship at the University of Cincinnati. This work was supported by National Institutes of Health Grants R01-AI058159 and R56-AI095442 awarded to W.E.M and also by R21-AI119415 awarded to Christine O’Connor and W.E.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki S, Yatomi Y, Shimosawa T, Yamashita H, Kitayama J, Tsuno NH, Takahashi K, Ozaki Y. The suppressive effect of sphingosine 1-phosphate on monocyte-endothelium adhesion may be mediated by the rearrangement of the endothelial integrins alpha(5)beta(1) and alpha(v)beta(3) J Thromb Haemost. 2007;5:1292–1301. doi: 10.1111/j.1538-7836.2007.02559.x. [DOI] [PubMed] [Google Scholar]
- Ashida N, Takechi H, Kita T, Arai H. Vortex-mediated mechanical stress induces integrin-dependent cell adhesion mediated by inositol 1,4,5-trisphosphate-sensitive Ca2+ release in THP-1 cells. J Biol Chem. 2003;278:9327–9331. doi: 10.1074/jbc.M212316200. [DOI] [PubMed] [Google Scholar]
- Barbosa CM, Bincoletto C, Barros CC, Ferreira AT, Paredes-Gamero EJ. PLCgamma2 and PKC are important to myeloid lineage commitment triggered by M-SCF and G-CSF. J Cell Biochem. 2014;115:42–51. doi: 10.1002/jcb.24653. [DOI] [PubMed] [Google Scholar]
- Beisser PS, Laurent L, Virelizier JL, Michelson S. Human cytomegalovirus chemokine receptor gene US28 is transcribed in latently infected THP-1 monocytes. J Virol. 2001;75:5949–5957. doi: 10.1128/JVI.75.13.5949-5957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz GL, Jarquin-Pardo M, Chan G, Smith MS, Sinzger C, Yurochko AD. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J Virol. 2006;80:11539–11555. doi: 10.1128/JVI.01016-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billstrom MA, Johnson GL, Avdi NJ, Worthen GS. Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J Virol. 1998;72:5535–5544. doi: 10.1128/jvi.72.7.5535-5544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caposio P, Orloff SL, Streblow DN. The role of cytomegalovirus in angiogenesis. Virus Res. 2011;157:204–211. doi: 10.1016/j.virusres.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarosa P, Bakker RA, Verzijl D, Navis M, Timmerman H, Leurs R, Smit MJ. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J Biol Chem. 2001;276:1133–1137. doi: 10.1074/jbc.M008965200. [DOI] [PubMed] [Google Scholar]
- Casarosa P, Waldhoer M, LiWang PJ, Vischer HF, Kledal T, Timmerman H, Schwartz TW, Smit MJ, Leurs R. CC and CX3C chemokines differentially interact with the N terminus of the human cytomegalovirus-encoded US28 receptor. J Biol Chem. 2005;280:3275–3285. doi: 10.1074/jbc.M407536200. [DOI] [PubMed] [Google Scholar]
- Chan G, Bivins-Smith ER, Smith MS, Smith PM, Yurochko AD. Transcriptome analysis reveals human cytomegalovirus reprograms monocyte differentiation toward an M1 macrophage. J Immunol. 2008;181:698–711. doi: 10.4049/jimmunol.181.1.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Nogalski MT, Yurochko AD. Human cytomegalovirus stimulates monocyte-to-macrophage differentiation via the temporal regulation of caspase 3. J Virol. 2012;86:10714–10723. doi: 10.1128/JVI.07129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MY, Huang DY, Ho FM, Huang KC, Lin WW. PKC-dependent human monocyte adhesion requires AMPK and Syk activation. PLoS One. 2012;7:e40999. doi: 10.1371/journal.pone.0040999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MS, Bankier AT, Beck S, Bohni R, Brown CM, Cerny R, Horsnell T, Hutchison CA, 3rd, Kouzarides T, Martignetti JA, et al. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990a;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Chee MS, Satchwell SC, Preddie E, Weston KM, Barrell BG. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990b;344:774–777. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- Cheung AK, Abendroth A, Cunningham AL, Slobedman B. Viral gene expression during the establishment of human cytomegalovirus latent infection in myeloid progenitor cells. Blood. 2006;108:3691–3699. doi: 10.1182/blood-2005-12-026682. [DOI] [PubMed] [Google Scholar]
- Cignetti A, Vallario A, Roato I, Circosta P, Strola G, Scielzo C, Allione B, Garetto L, Caligaris-Cappio F, Ghia P. The characterization of chemokine production and chemokine receptor expression reveals possible functional cross-talks in AML blasts with monocytic differentiation. Exp Hematol. 2003;31:495–503. doi: 10.1016/s0301-472x(03)00066-3. [DOI] [PubMed] [Google Scholar]
- Farrell HE, Abraham AM, Cardin RD, Sparre-Ulrich AH, Rosenkilde MM, Spiess K, Jensen TH, Kledal TN, Davis-Poynter N. Partial functional complementation between human and mouse cytomegalovirus chemokine receptor homologues. J Virol. 2011;85:6091–6095. doi: 10.1128/JVI.02113-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraile-Ramos A, Kledal TN, Pelchen-Matthews A, Bowers K, Schwartz TW, Marsh M. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol Biol Cell. 2001;12:1737–1749. doi: 10.1091/mbc.12.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraile-Ramos A, Kohout TA, Waldhoer M, Marsh M. Endocytosis of the viral chemokine receptor US28 does not require beta-arrestins but is dependent on the clathrin-mediated pathway. Traffic. 2003;4:243–253. doi: 10.1034/j.1600-0854.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- Gao JL, Murphy PM. Human cytomegalovirus open reading frame US28 encodes a functional beta chemokine receptor. J Biol Chem. 1994;269:28539–28542. [PubMed] [Google Scholar]
- Gerna G, Baldanti F, Revello MG. Pathogenesis of human cytomegalovirus infection and cellular targets. Hum Immunol. 2004;65:381–386. doi: 10.1016/j.humimm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- Ha B, Lv Z, Bian Z, Zhang X, Mishra A, Liu Y. ‘Clustering’ SIRPalpha into the plasma membrane lipid microdomains is required for activated monocytes and macrophages to mediate effective cell surface interactions with CD47. PLoS One. 2013;8:e77615. doi: 10.1371/journal.pone.0077615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargett D, Shenk TE. Experimental human cytomegalovirus latency in CD14+ monocytes. Proc Natl Acad Sci U S A. 2010;107:20039–20044. doi: 10.1073/pnas.1014509107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humby MS, O’Connor CM. Human Cytomegalovirus US28 Is Important for Latent Infection of Hematopoietic Progenitor Cells. J Virol. 2015;90:2959–2970. doi: 10.1128/JVI.02507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Yoshida H, Satoh K. Regulation of CX3CL1/fractalkine expression in endothelial cells. J Atheroscler Thromb. 2004;11:15–21. doi: 10.5551/jat.11.15. [DOI] [PubMed] [Google Scholar]
- Joshi S, Wels C, Beham-Schmid C, Fukunaga-Kalabis M, Holmen SL, Otte M, Herlyn M, Waldhoer M, Schaider H. Galpha13 mediates human cytomegalovirus-encoded chemokine receptor US28-induced cell death in melanoma. Int J Cancer. 2015 doi: 10.1002/ijc.29506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimian G, Buist-Homan M, Faber KN, Moshage H. Pertussis toxin, an inhibitor of G(alphai) PCR, inhibits bile acid- and cytokine-induced apoptosis in primary rat hepatocytes. PLoS One. 2012;7:e43156. doi: 10.1371/journal.pone.0043156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A, Aikawa M, Nitta N, Yoshida M, Libby P, Sacks FM. Apolipoprotein CIII-induced THP-1 cell adhesion to endothelial cells involves pertussis toxin-sensitive G protein- and protein kinase C alpha-mediated nuclear factor-kappaB activation. Arterioscler Thromb Vasc Biol. 2007;27:219–225. doi: 10.1161/01.ATV.0000249620.68705.0d. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Xiao W. Phospholipase C-beta in immune cells. Adv Biol Regul. 2013;53:249–257. doi: 10.1016/j.jbior.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kledal TN, Rosenkilde MM, Schwartz TW. Selective recognition of the membrane-bound CX3C chemokine, fractalkine, by the human cytomegalovirus-encoded broad-spectrum receptor US28. FEBS Lett. 1998;441:209–214. doi: 10.1016/s0014-5793(98)01551-8. [DOI] [PubMed] [Google Scholar]
- Lin YF, Leu SJ, Huang HM, Tsai YH. Selective activation of specific PKC isoforms dictating the fate of CD14(+) monocytes towards differentiation or apoptosis. J Cell Physiol. 2011;226:122–131. doi: 10.1002/jcp.22312. [DOI] [PubMed] [Google Scholar]
- Manna P, Jain SK. Effect of PIP3 on adhesion molecules and adhesion of THP-1 monocytes to HUVEC treated with high glucose. Cell Physiol Biochem. 2014;33:1197–1204. doi: 10.1159/000358688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnychuk RM, Streblow DN, Smith PP, Hirsch AJ, Pancheva D, Nelson JA. Human cytomegalovirus-encoded G protein-coupled receptor US28 mediates smooth muscle cell migration through Galpha12. J Virol. 2004;78:8382–8391. doi: 10.1128/JVI.78.15.8382-8391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WE, Houtz DA, Nelson CD, Kolattukudy PE, Lefkowitz RJ. G-protein-coupled receptor (GPCR) kinase phosphorylation and beta-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. J Biol Chem. 2003;278:21663–21671. doi: 10.1074/jbc.M303219200. [DOI] [PubMed] [Google Scholar]
- Miller WE, Zagorski WA, Brenneman JD, Avery D, Miller JL, O’Connor CM. US28 is a potent activator of phospholipase C during HCMV infection of clinically relevant target cells. PLoS One. 2012;7:e50524. doi: 10.1371/journal.pone.0050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minisini R, Tulone C, Luske A, Michel D, Mertens T, Gierschik P, Moepps B. Constitutive inositol phosphate formation in cytomegalovirus-infected human fibroblasts is due to expression of the chemokine receptor homologue pUS28. J Virol. 2003;77:4489–4501. doi: 10.1128/JVI.77.8.4489-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moepps B, Tulone C, Kern C, Minisini R, Michels G, Vatter P, Wieland T, Gierschik P. Constitutive serum response factor activation by the viral chemokine receptor homologue pUS28 is differentially regulated by Galpha(q/11) and Galpha(16) Cell Signal. 2008;20:1528–1537. doi: 10.1016/j.cellsig.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Bourne HR. Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs. Proc Natl Acad Sci U S A. 1997;94:14489–14494. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega VM, Gardner TJ, Redmann V, Bongers G, Lira SA, Tortorella D. Human cytomegalovirus US28 facilitates cell-to-cell viral dissemination. Viruses. 2014;6:1202–1218. doi: 10.3390/v6031202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor CM, Murphy EA. A myeloid progenitor cell line capable of supporting human cytomegalovirus latency and reactivation, resulting in infectious progeny. J Virol. 2012;86:9854–9865. doi: 10.1128/JVI.01278-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor CM, Shenk T. Human cytomegalovirus pUS27 G protein-coupled receptor homologue is required for efficient spread by the extracellular route but not for direct cell-to-cell spread. J Virol. 2011;85:3700–3707. doi: 10.1128/JVI.02442-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold ME, Schmidt TL, Dairaghi DJ, Barry PA, Schall TJ. Characterization of the rhesus cytomegalovirus US28 locus. J Virol. 2003;77:10404–10413. doi: 10.1128/JVI.77.19.10404-10413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M, Smiljanic K, Dobutovic B, Syrovets T, Simmet T, Isenovic ER. Human cytomegalovirus infection and atherothrombosis. J Thromb Thrombolysis. 2012;33:160–172. doi: 10.1007/s11239-011-0662-x. [DOI] [PubMed] [Google Scholar]
- Randolph-Habecker JR, Rahill B, Torok-Storb B, Vieira J, Kolattukudy PE, Rovin BH, Sedmak DD. The expression of the cytomegalovirus chemokine receptor homolog US28 sequesters biologically active CC chemokines and alters IL-8 production. Cytokine. 2002;19:37–46. doi: 10.1006/cyto.2002.0874. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- Ruan Q, Zhao C, Ye Z, Ruan J, Xie Q, Xie W. Effect and possible mechanism of monocyte-derived VEGF on monocyte-endothelial cellular adhesion after electrical burns. Burns. 2015;41:825–832. doi: 10.1016/j.burns.2014.10.030. [DOI] [PubMed] [Google Scholar]
- Shagdarsuren E, Djalali-Talab Y, Aurrand-Lions M, Bidzhekov K, Liehn EA, Imhof BA, Weber C, Zernecke A. Importance of junctional adhesion molecule-C for neointimal hyperplasia and monocyte recruitment in atherosclerosis-prone mice-brief report. Arterioscler Thromb Vasc Biol. 2009;29:1161–1163. doi: 10.1161/ATVBAHA.109.187898. [DOI] [PubMed] [Google Scholar]
- Sinclair J. Chromatin structure regulates human cytomegalovirus gene expression during latency, reactivation and lytic infection. Biochim Biophys Acta. 2010;1799:286–295. doi: 10.1016/j.bbagrm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Smith MS, Bivins-Smith ER, Tilley AM, Bentz GL, Chan G, Minard J, Yurochko AD. Roles of phosphatidylinositol 3-kinase and NF-kappaB in human cytomegalovirus-mediated monocyte diapedesis and adhesion: strategy for viral persistence. J Virol. 2007;81:7683–7694. doi: 10.1128/JVI.02839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- Steube KG, Meyer C, Drexler HG. Constitutive protein expression of monocyte chemotactic protein-1 (MCP-1) by myelomonocytic cell lines and regulation of the secretion by anti- and proinflammatory stimuli. Leuk Res. 1999;23:843–849. doi: 10.1016/s0145-2126(99)00107-1. [DOI] [PubMed] [Google Scholar]
- Stevenson EV, Collins-McMillen D, Kim JH, Cieply SJ, Bentz GL, Yurochko AD. HCMV reprogramming of infected monocyte survival and differentiation: a Goldilocks phenomenon. Viruses. 2014;6:782–807. doi: 10.3390/v6020782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streblow DN, Orloff SL, Nelson JA. Do pathogens accelerate atherosclerosis? J Nutr. 2001a;131:2798S–2804S. doi: 10.1093/jn/131.10.2798S. [DOI] [PubMed] [Google Scholar]
- Streblow DN, Orloff SL, Nelson JA. The HCMV chemokine receptor US28 is a potential target in vascular disease. Curr Drug Targets Infect Disord. 2001b;1:151–158. doi: 10.2174/1568005014606080. [DOI] [PubMed] [Google Scholar]
- Streblow DN, Soderberg-Naucler C, Vieira J, Smith P, Wakabayashi E, Ruchti F, Mattison K, Altschuler Y, Nelson JA. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell. 1999;99:511–520. doi: 10.1016/s0092-8674(00)81539-1. [DOI] [PubMed] [Google Scholar]
- Stropes MP, Miller WE. Functional analysis of human cytomegalovirus pUS28 mutants in infected cells. J Gen Virol. 2008;89:97–105. doi: 10.1099/vir.0.83226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki J, Saito T, Taniguchi M, Kawasaki T, Moritani Y, Hayashi K, Kobori M. A novel Galphaq/11-selective inhibitor. J Biol Chem. 2004;279:47438–47445. doi: 10.1074/jbc.M408846200. [DOI] [PubMed] [Google Scholar]
- Tatara Y, Ohishi M, Yamamoto K, Shiota A, Hayashi N, Iwamoto Y, Takeda M, Takagi T, Katsuya T, Ogihara T, Rakugi H. Macrophage inflammatory protein-1beta induced cell adhesion with increased intracellular reactive oxygen species. J Mol Cell Cardiol. 2009;47:104–111. doi: 10.1016/j.yjmcc.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Umehara H, Goda S, Imai T, Nagano Y, Minami Y, Tanaka Y, Okazaki T, Bloom ET, Domae N. Fractalkine, a CX3C-chemokine, functions predominantly as an adhesion molecule in monocytic cell line THP-1. Immunol Cell Biol. 2001;79:298–302. doi: 10.1046/j.1440-1711.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- Vieira J, Schall TJ, Corey L, Geballe AP. Functional analysis of the human cytomegalovirus US28 gene by insertion mutagenesis with the green fluorescent protein gene. J Virol. 1998;72:8158–8165. doi: 10.1128/jvi.72.10.8158-8165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer HF, Siderius M, Leurs R, Smit MJ. Herpesvirus-encoded GPCRs: neglected players in inflammatory and proliferative diseases? Nat Rev Drug Discov. 2014;13:123–139. doi: 10.1038/nrd4189. [DOI] [PubMed] [Google Scholar]
- Vomaske J, Melnychuk RM, Smith PP, Powell J, Hall L, DeFilippis V, Fruh K, Smit M, Schlaepfer DD, Nelson JA, Streblow DN. Differential ligand binding to a human cytomegalovirus chemokine receptor determines cell type-specific motility. PLoS Pathog. 2009a;5:e1000304. doi: 10.1371/journal.ppat.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vomaske J, Nelson JA, Streblow DN. Human Cytomegalovirus US28: a functionally selective chemokine binding receptor. Infect Disord Drug Targets. 2009b;9:548–556. doi: 10.2174/187152609789105696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vomaske J, Varnum S, Melnychuk R, Smith P, Pasa-Tolic L, Shutthanandan JI, Streblow DN. HCMV pUS28 initiates pro-migratory signaling via activation of Pyk2 kinase. Herpesviridae. 2010;1:2. doi: 10.1186/2042-4280-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M, Casarosa P, Rosenkilde MM, Smit MJ, Leurs R, Whistler JL, Schwartz TW. The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. J Biol Chem. 2003;278:19473–19482. doi: 10.1074/jbc.M213179200. [DOI] [PubMed] [Google Scholar]
- White GE, Iqbal AJ, Greaves DR. CC chemokine receptors and chronic inflammation–therapeutic opportunities and pharmacological challenges. Pharmacol Rev. 2013;65:47–89. doi: 10.1124/pr.111.005074. [DOI] [PubMed] [Google Scholar]
- Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294(Pt 2):335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SE, Miller WE. The human cytomegalovirus lytic cycle is induced by 1,25-dihydroxyvitamin D3 in peripheral blood monocytes and in the THP-1 monocytic cell line. Virology. 2015;483:83–95. doi: 10.1016/j.virol.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YF, Yu ZX, Wanishsawad C, Shou M, Epstein SE. The immediate early gene products of human cytomegalovirus increase vascular smooth muscle cell migration, proliferation, and expression of PDGF beta-receptor. Biochem Biophys Res Commun. 1999;256:608–613. doi: 10.1006/bbrc.1999.0387. [DOI] [PubMed] [Google Scholar]


