Abstract
Rationale
Catecholamines increase cardiac contractility, but exposure to high concentrations or prolonged exposures can cause cardiac injury. A recent study demonstrated that a single subcutaneous injection of isoproterenol (ISO; 200 mg/kg) in mice causes acute myocyte death (8-10%) with complete cardiac repair within a month. Cardiac regeneration was via endogenous cKit+ cardiac stem cell (CSC)-mediated new myocyte formation.
Objective
Our goal was to validate this simple injury/regeneration system and use it to study the biology of newly forming adult cardiac myocytes.
Methods and Results
C57BL/6 mice (n=173) were treated with single injections of vehicle, 200mg/kg or 300mg/kg ISO, or with two daily doses of 200mg/kg ISO for 6 days. Echocardiography revealed transiently increased systolic function and unaltered diastolic function 1 day after single ISO injection. Single ISO injections also caused membrane injury in about 10% of myocytes but few of these myocytes appeared to be necrotic. Circulating troponin I levels after ISO were elevated, further documenting myocyte damage. However, myocyte apoptosis was not increased after ISO injury. Heart weight to body weight ratio and fibrosis were also not altered 28 days after ISO injection. Single or multiple dose ISO injury was not associated with an increase in the percentage of 5-ethynyl-2’-deoxyuridine (EdU)-labeled myocytes. Furthermore, ISO injections did not increase new myocytes in cKit+/Cre × R-GFP transgenic mice.
Conclusions
A single dose of ISO causes injury in about 10% of the cardiomyocytes. However, most of these myocytes appear to recover and do not elicit cKit+ cardiac stem cell (CSC)-derived myocyte regeneration.
Keywords: Myocardium, heart failure, catecholamine, drug, remodeling
INTRODUCTION
Many forms of cardiovascular disease can lead to death of cardiac myocytes. Effective repair requires the generation of new cardiac muscle cells, however the adult myocyte has lost all or most of its ability to reenter the cell cycle and divide1. Therefore, stem cells with the capacity to differentiate into new cardiac myocytes could be useful for cardiac regeneration. Endogenous cKit+ cardiac stem cells (CSCs) were first described over a decade ago 2. There has been great debate over their ability to form new myocytes in the normal adult heart or to regenerate new cardiomyocytes after injury. Some studies suggest that these cells can robustly form new myocytes in the aging heart 3 or in the adult heart after injury 2-5, while others find very small numbers of new myocytes during aging or after injury 6. A minority of studies suggest that these cells cannot differentiate into new myocytes 7.
The rate of new myocyte formation (myocyte turnover) in the normal adult mammalian heart is also not well established, with recent studies reporting annual rates of myocyte turnover at 1% 8, or 4-10% 9 in humans, and as low as 0.003% 5 or as high as 8-10% 10 in rodent studies using a variety of cell fate-mapping strategies.
The ability of the adult mammalian heart to regenerate in response to multiple forms of injury has been studied and again the results have been highly variable 11-13. A recent study by Ellison et al. 10 utilized a single dose of subcutaneous isoproterenol (ISO) to induce what was described as a “takotsubo-like” cardiomyopathy. This study showed that a single injection of ISO causes death of about 10% of the cardiac myocytes in the mouse heart, and over the next month there is complete cardiac regeneration. The newly formed cardiac myocytes in this study were exclusively derived from cKit+ CSCs.
Our laboratories have studied myocyte regeneration in multiple small and large animal cardiac injury models, including continuous ISO infusion via osmotic minipumps 5, myocardial infarction 14, myocardial infarction in the presence of cardiotoxic agents 15, and myocardial infarction with exogenous implantation of CSCs 14. All of these studies have shown low rates (< 1%) of new myocyte formation. The recent study by Ellison et al. 10 describes a simple system that should be easily reproduced and would be a useful system to study the properties of newly forming cardiac myocytes in the adult heart. The goal of the present study was to validate the data suggesting that a single ISO injection in mice kills ~10% of the cardiac myocytes and that over the next month these are replaced by new myocytes derived from endogenous CSCs.
Our study establishes that a single ISO dose does indeed injure about 10% of C57BL/6 mouse ventricular myocytes. However, the majority of these myocytes appear to recover normal function over the next few weeks. We also show that in spite of the ISO injury, there is a transient increase in ventricular function, rather than the decrease in cardiac function with widespread myocyte death described previously 10. Additionally, we show that ISO injury did not increase the percentage of EdU+ myocytes (<0.008%) or the percentage of myocytes derived from CSCs. We also show that inducing more severe cardiac injury with multiple injections of ISO did not elicit a robust regenerative response.
METHODS
Please refer to the Materials and Methods section in the Online Data Supplement for a detailed description of experimental methods.
Experimental animals
All animal procedures were approved by the Temple University School of Medicine Institutional Animal Care and Use Committee. For most mouse experiments, wild-type 10-12-week-old male C57BL/6 mice (n=173) were used (The Jackson Laboratory; Bar Harbor, ME). For fate mapping of endogenous c-kit+ cardiac myocytes, constitutive and inducible Kit-Cre-IRES-GFPnls (n=9) or Kit-MerCreMer knock-in mice (n=5) were generated 6. In short, the Kit locus was targeted in mice to express Cre recombinase and eGFP with a nuclear localization sequence (eGFPnls). These mice were crossed with Cre-responsive Rosa26 mice (R-GFP) for lineage tracing.
Isoproterenol dosing
Single-dose isoproterenol (ISO) was administered by subcutaneous injections. We used 200mg/kg ISO and a higher dose of 300 mg/kg (as this was the highest tolerable non-lethal dose). For the chronic ISO experiments 200mg/kg ISO was injected twice daily for 6 consecutive days. For all experiments, a stock solution of 50mg/mL isoproterenol (Sigma-Aldrich #I6504; St. Louis, MO) was prepared in sterile 0.9% saline.
Statistical analysis
All data analyses were performed using SAS version 9.3. Data are shown as mean ± SEM. A mixed-effects model approach was employed for variables that were measured repeatedly. For variables that were measured only once, overall group comparisons were tested using the Kruskal-Wallis test or ANOVA F-test, when appropriate, while the pairwise group comparisons were performed using the exact Wilcoxon rank-sum test or unequal variance two-sample t-test.
RESULTS
Isoproterenol dosing
An ISO dose titration study was performed in mice to define a non-lethal ISO dose that injures the heart. Mice were injected with 5 (n=5), 200 (n=5), 300 (n=1), 400 (n=4), 500 (n=1), 600 (n=1), 700 (n=1), 800 (n=1), or 1000mg/kg (n=5) ISO. Doses greater than or equal to 400mg/kg caused death of every animal within a few hours. Therefore, vehicle (saline), 200mg/kg (dose used by Ellison et al. 10) and 300mg/kg (highest non-lethal dose) were used for all experiments.
To document the lethal arrhythmias induced by high-dose ISO, continuous ECGs were recorded while animals were injected with 200, 300, 400 or 1000mg/kg ISO. Injection of ISO induced many different types of arrhythmias. After injection of 400 and 1000mg/kg, ECG tracings initially revealed a slight increase in heart rate (data not shown) followed by sinus arrest and heart block with a ventricular escape rhythm within a few minutes that ended either in lethal ventricular tachycardia or asystole (Online Figure I).
ISO transiently increases myocardial contractility and does not depress diastolic function
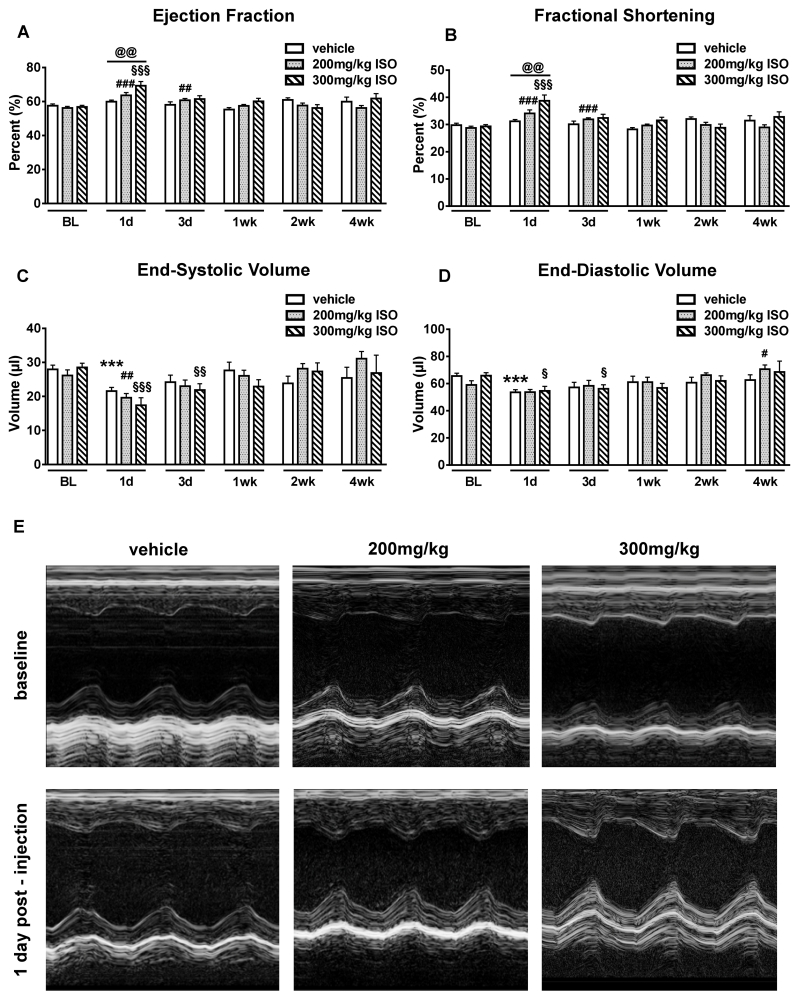
Mice were injected subcutaneously with vehicle (n=14), 200mg/kg (n=15) or 300mg/kg (n=14) ISO. All animals underwent baseline echocardiography (ECHO) prior to dosing and then at 1, 3, 7, 14, and 28 days after ISO/vehicle injection. Conventional ECHO revealed that 200 and 300mg/kg ISO significantly increased left ventricular ejection fraction (EF) and fractional shortening (FS) after 1 day compared to baseline (BL). Injection with 300mg/kg ISO significantly increased EF (59.9±0.9% vs 69.3±2.5%; p<0.01) and FS (FS: 31.2±0.6% vs 38.8±2.1%; p<0.01) after 1 day compared to the vehicle injected group (Figures 1A and 1B; Online Videos I-IV). Cardiac function returned to baseline levels at one week and was unchanged for the remainder of the study. End-systolic and end-diastolic volumes did not differ between groups at any time point (Figures 1C and 1D). Figure 1E shows representative M-mode images of all 3 treatment groups at BL and 1 day post-ISO injection. Heart rates during ECHO did not significantly differ between groups at any time point (Online Figure I).
Figure 1. Cardiac function post-ISO injection.
ECHO measurements were performed at baseline (BL), 1, 3, 7, 14, 28 days post-injection (vehicle (n=14), 200mg/kg isoproterenol (ISO) (n=15) and 300mg/kg ISO (n=14). A-D, ECHO-derived cardiac function and volumes. ***p<0.001 vs. BL vehicle, #p<0.05, ##p<0.01 and ###p<0.001 vs. BL 200mg/kg, §p<0.05, §§p<0.01 and §§§p<0.001 vs. BL 300mg/kg; @@p<0.01 between groups. Data are shown as mean ± SEM. E, Representative M-mode echocardiograms of mice at baseline (upper panel) and 1 day post vehicle, 200mg/kg, or 300mg/kg ISO injection (lower panel). ISO increased cardiac function. See also Online Figure II.
Diastolic function was determined using ECHO coupled with pulsed Doppler and tissue Doppler imaging (TDI) techniques. No differences in diastolic function (E, E/A, E′, EAT) (Online Figure II) between groups at any time point were found.
Subendocardial and apical myocardial contractility are not impaired after ISO injury
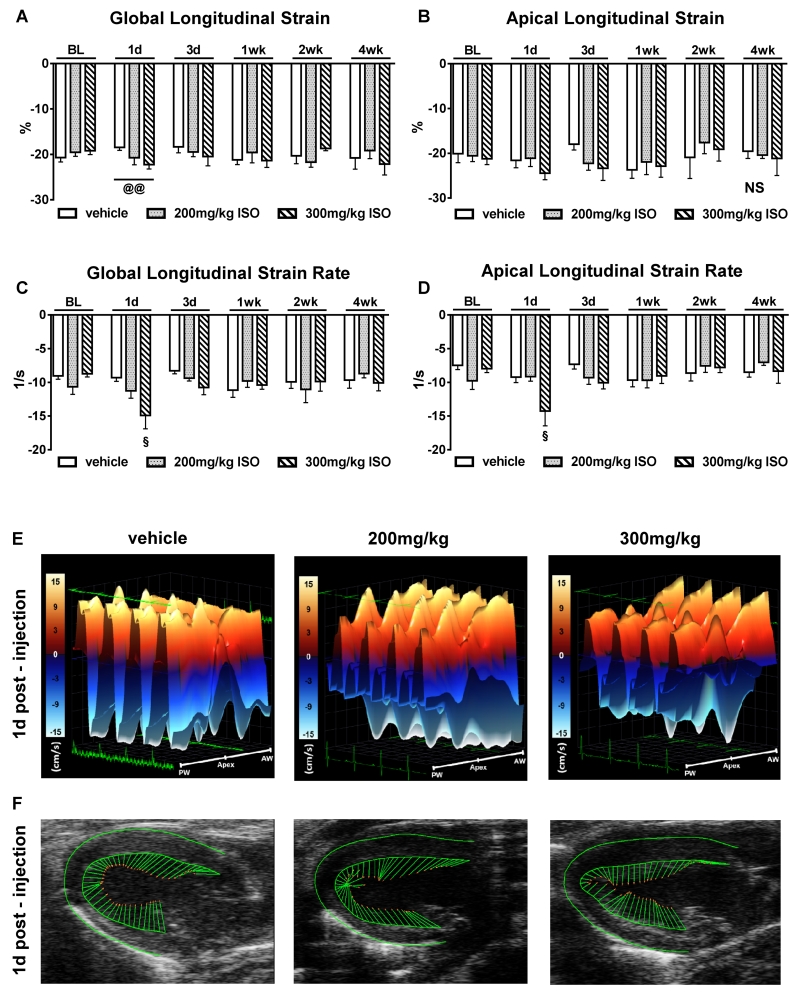
To determine the effects of ISO on regional myocardial function, speckle-tracking based strain analyses on long axis B-mode loops were performed. Due to myocardial fiber orientation at varying levels of the LV wall, longitudinal strain is most representative of myocardial shortening at the level of the endocardium 16. Furthermore, the software allows analysis of regional wall segments. Consistent with the results from conventional ECHO, 300mg/kg ISO significantly increased the magnitude of global longitudinal strain (Figure 2A) compared with vehicle controls after 1 day (−22.26±0.98% vs −18.48%±0.64%; p<0.01) and returned to baseline after 3 days. Apical longitudinal strain (Figure 2B) was not different between the groups. Strain rate reflects the rate of changes in strain (strain/time). Both global and apical strain rate (Figures 2C and 2D) were significantly improved by 300mg/kg ISO after 1 day compared to baseline. In line with our previous ECHO findings, this effect was transient. After 3 days, global and apical longitudinal strain, as well as strain rate, returned to baseline values and remained unchanged throughout the study. Figures 2E and 2F show normal three-dimensional regional wall velocity diagrams and vector diagrams of all 3 groups, respectively, 1 day after injection. These results demonstrate that a single subcutaneous ISO injection (200 or 300mg/kg) does not impair subendocardial or apical contractility and therefore, from a functional point of view, does not produce a “takotsubo-like” cardiomyopathy in these mice.
Figure 2. Speckle-tracking based strain imaging.
A and B, Global and apical longitudinal strain after vehicle (n=14), 200mg/kg (n=15), and 300mg/kg (n=14) ISO injection. C and D, Global and apical longitudinal strain rate after vehicle (n=14), 200mg/kg (n=15), and 300mg/kg (n=14) ISO injection. §p<0.05 vs. BL 300mg/kg; @@p<0.01 between groups. NS=not significant (p>0.05). Data are shown as mean ± SEM. E, Three-dimensional regional wall velocity diagrams show contraction (orange/positive values) or relaxation (blue/negative values) of 4 consecutive cardiac cycles 1 day post-injection. F, Vector diagrams show the direction and magnitude of endocardial contraction at mid-systole 1 day post-injection. Anterior wall (AW), Posterior wall (PW).
A single ISO injection alters myocyte membrane permeability and induces necrosis but not apoptosis in vivo
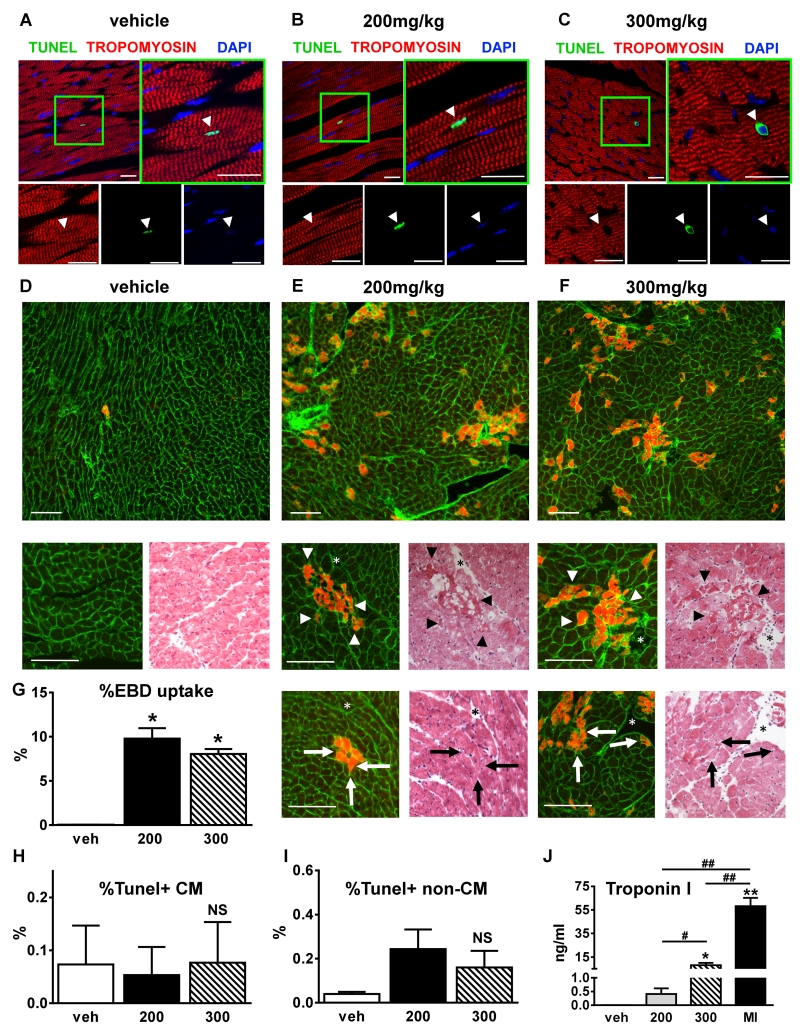
Comprehensive functional assessment of myocardial function revealed no functional deterioration after ISO injection at any time point. Injection with 300mg/kg ISO even increased systolic function transiently. We next explored the idea that ISO induces cardiac myocyte injury. To investigate the effects of ISO on myocyte and non-myocyte apoptosis, we performed terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining on myocardial tissue fixed at 1 day after ISO injection. Figures 3A-3C display representative confocal micrographs of all three groups 1 day after injections with the magnified panels showing TUNEL+ nuclei. There were no significant increases in the percentage of TUNEL+ myocytes after ISO treatment compared to vehicle (Figure 3H). The percentage of TUNEL+ non-myocytes was slightly increased after ISO but this did not reach statistical significance (Figure 3I). Confirming these results, Western blot analysis revealed no increase in activated caspase-3 after ISO injection (Online Figure III).
Figure 3. ISO induced myocyte membrane damage and does not induce apoptosis.
A-C, Tissue sections from hearts fixed at 1 day post-injection were immunostained for terminal deoxynucleotidyl transferase mediated deoxyuridine triphosphate nick-endlabeling (TUNEL; green), tropomyosin (red), and nuclei were labeled with 4’,6-diamidino-2-phenylindole (DAPI, blue). Vehicle (A), 200mg/kg ISO (B) and 300mg/kg ISO (C). White arrowheads=myocyte nuclei. Scale bars=25μm. D-F, H&E (right small panels) and Evans Blue Dye (EBD) uptake (upper and left small panels) analysis of consecutive 7-micron thick histological sections from hearts of mice 24 hours after one-time injection of vehicle or ISO (vehicle, n=4 (D), 200mg/kg ISO, n=5 (E), and 300 mg/kg ISO, n=5 (F)). Membranes are stained green, EBD+ myocytes are in red. Scale bars=100μm. Star=reference point. Arrowheads=potential necrotic areas in agreement with EBD uptake. Arrows=EBD uptake without histological signs of necrosis. G-I, The percentages of EBD positive myocytes (G), TUNEL+ myocyte (H) (n=9, 3 each group) and non-myocyte nuclei (I) (n=9, 3 each group) were quantified. J, Tn-I levels were measured at day 1 post-injection. Veh=vehicle (n=5); 200=200mg/kg ISO (n=5), 300=300mg/kg ISO (n=4); MI=myocardial infarction (n=4). *p<0.05 vs. veh, **p<0.01 vs. veh, #p<0.05 between groups, ##p<0.01 between groups, NS=not significant (p>0.05). G-J, Data are shown as mean ± SEM.
To further investigate ISO-induced myocyte injury, myocyte membrane permeability and necrosis were evaluated by measuring Evans Blue Dye (EBD) uptake 24 hours after ISO or vehicle injection. Figures 3D-3F show representative images of EBD uptake and H&E staining of the matched histological sections from all 3 groups. Quantification of myocyte EBD uptake (Figure 3G) revealed a significant increase after 200mg/kg (9.79±0.58%) and 300mg/kg ISO (8.04±0.58%) compared to vehicle (0.04±0.02%; p<0.05). EBD uptake certainly defines ISO-induced membrane damage but does not necessarily document myocyte necrosis 17, 18. We found areas of EBD+ myocytes where the matching H&E stained section revealed intact, non-necrotic myocytes (Figures 3E and 3F, lower small images). We also identified EBD+ myocytes with histological features of necrosis (Figures 3E and 3F, upper small images). These results suggest that ISO can alter myocyte membrane permeability without necessarily causing necrosis. EBD+ myocytes were found throughout the ventricle and were not localized exclusively to the endocardium or the apical regions of the LV.
To further characterize injury induced by ISO, troponin I (Tn-I) levels were measured 24 hours after ISO injection in vehicle, 200mg/kg and 300mg/kg ISO injected mice and after myocardial infarction (MI) induced by permanent occlusion of the left anterior descending (LAD) artery, as a positive control (Figure 3J). Tn-I levels were significantly increased after 300mg/kg ISO (8.2±1.9ng/ml, p<0.05) and after MI (58.1±7.1ng/ml, p<0.01) compared to vehicle, whereas 200mg/kg ISO caused only a slight increase in Tn-I, which did not reach statistical significance (0.4±0.2ng/ml, p=NS). Injury with 300mg/kg ISO caused a significantly higher increase in Tn-I levels compared to 200mg/kg (p<0.05). Notably, compared to Tn-I levels after MI, 200 and 300mg/kg ISO caused significantly smaller increases (p<0.01). In the vehicle group, the Tn-I levels were too low for quantification (<0.01ng/ml). These results suggest that a single injection of either 200 or 300mg/kg ISO caused transient changes in myocyte membrane permeability but that not all EBD+ myocytes were necrotic.
A single ISO injection does not induce cardiac fibrosis or change heart size in C57BL/6 mice
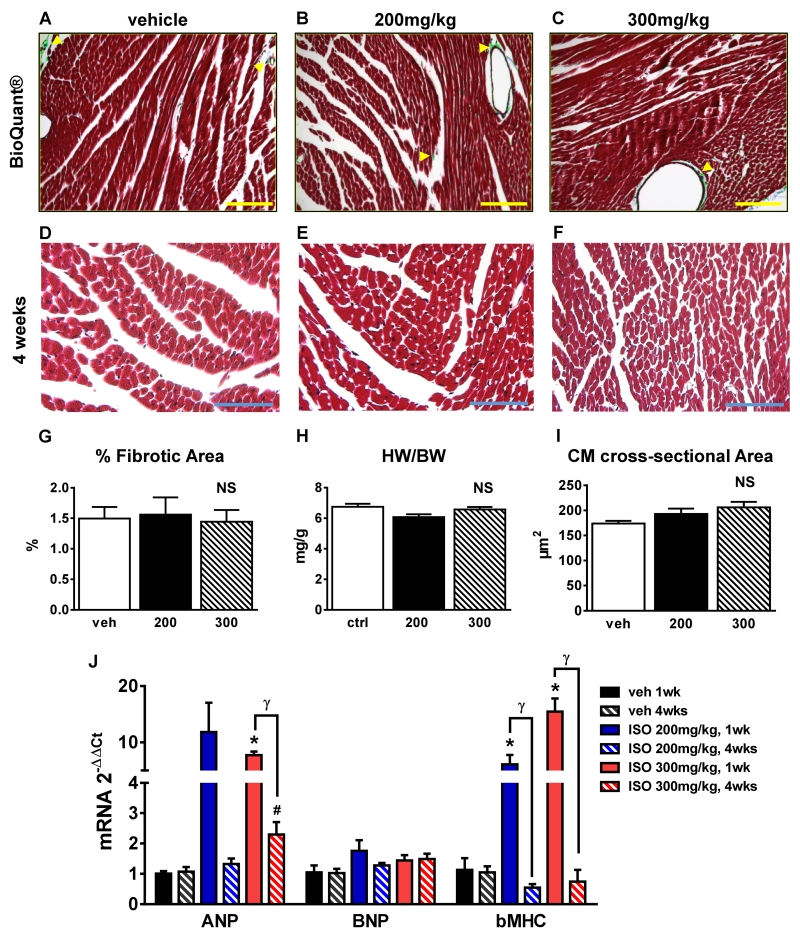
Myocardial injury from MI 15, pressure overload, or prolonged ISO infusion 5 elicits a fibrotic response that is associated with changes in myocardial diastolic compliance. If ISO caused significant myocardial necrosis, an increase in fibrotic area would be expected. Since we observed a significant increase in EBD uptake after ISO treatment, which is associated with myocyte injury 19, we measured cardiac fibrosis. Masson’s trichrome-stained tissue sections from hearts fixed at 4 weeks post-ISO or vehicle injections were studied (Figures 4A-4C show representative images). Bioquantification analysis did not show significant differences in the percentage of fibrotic tissue between groups (Figure 4G). Furthermore, no changes in heart weight to body weight ratio (HW/BW) at 4 weeks post-ISO were observed (Figure 4H), documenting the lack of organ hypertrophy.
Figure 4. Myocardial fibrosis and hypertrophy.
A-C, Tissue sections from hearts fixed at 4 weeks post-ISO injection (vehicle (A and D); 200mg/kg ISO (B and E), and 300mg/kg ISO (C and F)) were stained with Masson’s trichrome. Bright-field micrographs with bioquantification indicating Masson’s trichrome blue-stained fibrotic areas (green). Scale bars=150μm. D-F, Representative bright-field micrographs of vehicle (D), 200mg/kg ISO (E), and 300mg/kg ISO (F) hearts studied 4 weeks after injection. Scale bars=50μm. G, Percentage of fibrotic area determined by bioquantification (n=9, 3 each group). H, Heart weight/body weight ratio (HW/BW) (n=14). I, Average myocyte cross-sectional area (n=9, 3 each group). J, Atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and β-myosin heavy chain (βMHC).
mRNA levels at 1 week (n=11) and 4 weeks (n=12) after vehicle, 200mg/kg ISO, and 300mg/kg ISO. ANP and βMHC expression levels were upregulated at 1 week post-ISO (300mg/kg). *p<0.05 vs. veh 1wk, #p<0.05 vs. veh 4wks, γ p<0.05 1wk vs. 4wks, NS=not significant (p>0.05). G-I, Data are shown as mean ± SEM.
Diseases that induce cardiac myocyte death are usually associated with hypertrophy of surviving myocytes. To explore this possibility, myocyte cross-sectional area was measured in Masson’s trichrome-stained tissue sections (Figures 4D-4F and 4I) and cell surface area was measured in myocytes isolated from vehicle and ISO-injected animals (Figure 6B). A trend towards an increase in myocyte size after ISO injury was observed, but this effect did not reach statistical significance. To further explore the idea that ISO injury induces reactive pathological hypertrophy, mRNA expression levels of the hypertrophic markers atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and β-myosin heavy chain (βMHC) were measured at 1 and 4 weeks post-ISO. In the 300mg/kg ISO-treated animals compared to vehicle-treated controls (Figure 4J), significant increases in ANP and βMHC expression patterns were observed at 1 week. After 200mg/kg ISO injection, βMHC was also upregulated at 1 week. βMHC expression in both groups (200 and 300mg/kg ISO) had returned to normal by 4 weeks post-ISO injection. Collectively, our results suggest that a single ISO injection causes a small amount of cardiac myocyte necrosis and a small amount of reactive cardiac hypertrophy, with no detectable fibrosis.
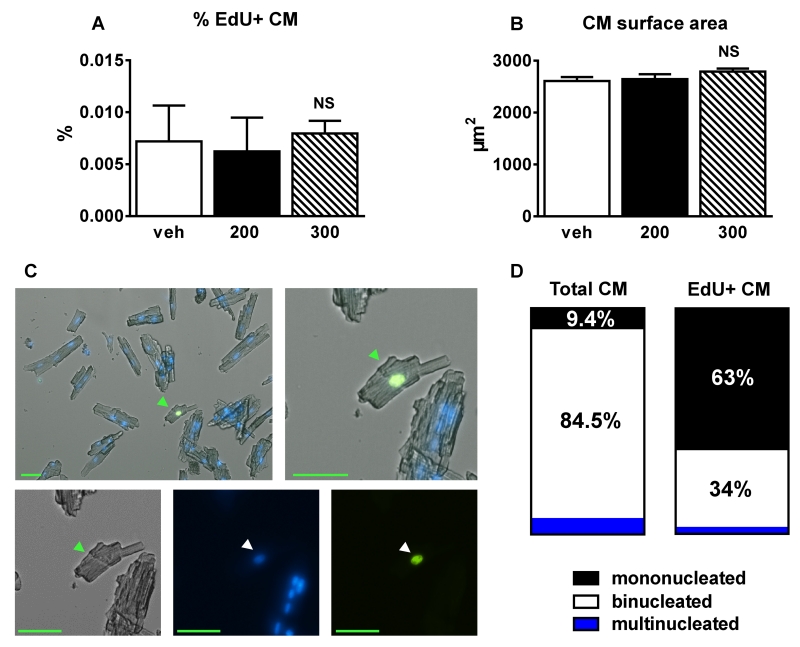
Figure 6. ISO did not increase the % of EdU+ myocyte nuclei in isolated cardiomyocytes.
A, The percentage of EdU+ cardiomyocytes (CM) was less than 0.008% and did not differ between groups (veh (n=3), 200mg/kg ISO (n=3) and 300mg/kg ISO (n=4)) at 4 weeks post-ISO. B, Cardiomyocyte surface area at 4 weeks post-ISO. C, Representative fluorescent micrograph of a small EdU+ mononucleated myocyte. Scale bars=50μm. Green arrowheads=cardiomyocyte. White arrowheads=myocyte nuclei. D, The percentage of mono-, bi-, and multinucleated cells in all CMs (left) and EdU+ CMs (right) at 4 weeks post-ISO. NS=not significant (p>0.05). Data are shown as mean ± SEM.
ISO does not increase EdU incorporation in myocyte nuclei in vivo
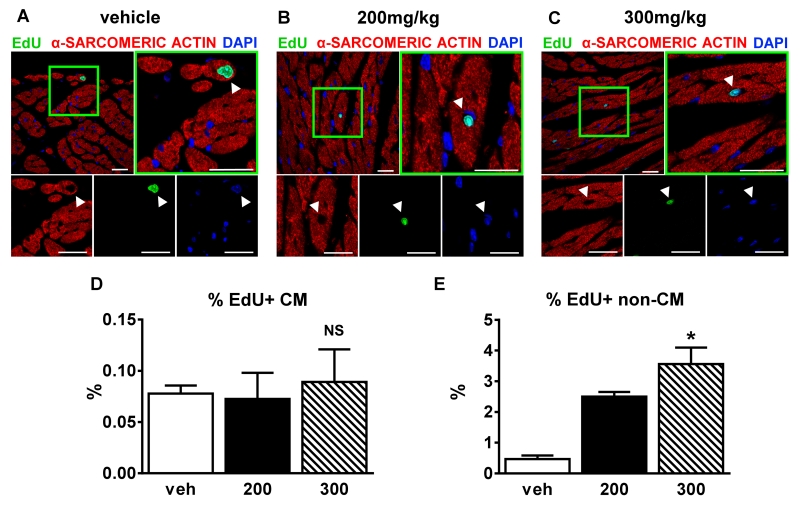
Our experiments show that ISO caused myocyte injury (EBD uptake in 10% of ventricular myocytes) but was not associated with an increase in fibrosis. These findings could be due to necrotic myocytes being replaced by CSC-derived new myocytes as suggested in the previous study 10 or because most of the damaged and EBD-labeled myocytes recover normal function. To assess whether ISO injury induces increased myocyte and non-myocyte proliferation and DNA replication in vivo, a 5-ethynyl-2’-deoxyuridine (EdU) pulse-chase labeling strategy was used. A similar approach with 5-bromo-2’-deoxyuridine (BrdU) has been used previously by our group to identify newly generated feline myocytes after cardiac injury caused by 10 days of continuous ISO infusion 5. Figures 5A-5C display representative confocal micrograph images of hearts treated with a single injection of vehicle or ISO (200 and 300mg/kg), one week after injection and EdU labeling (via minipump). Quantitative histology (Figure 5D) revealed no differences in the percentage of EdU+ myocytes between ISO and vehicle-treated animals at 4 weeks (veh 0.08±0.01%, 200mg/kg ISO 0.07±0.03%, 300mg/kg ISO 0.09±0.03%; p=NS). ISO caused a significant dose-dependent increase in the percentage of EdU+ non-myocytes (veh 0.47±0.12%, 200mg/kg ISO 2.5±0.15%, 300mg/kg ISO 3.57±0.53%; p<0.05) (Figure 5E). After myocardial infarction, a much larger number of EdU+ non-myocyte nuclei were observed in the injured border zone and infarct area, with fewer in the viable tissue of the remote zone (Online Figure IV). These results suggest that ISO causes a dose-dependent injury that elicits a proportional post-injury inflammatory response.
Figure 5. 200mg/kg ISO does not increase the % of EdU+ myocyte nuclei.
A-C, Confocal micrographs of fixed hearts at 1 week post-injection: veh (A); 200mg/kg ISO (B), and 300mg/kg ISO (C) that were immunostained against α-sarcomeric actin(red), 5-ethynyl-2′-deoxyuridine (EdU; green), and nuclei labeled with 4’,6-diamidino-2-phenylindole (DAPI, blue). White arrowheads=myocyte nuclei. Scale bars=25μm. D and E, Percentage of EdU+ myocyte (D) (n=9, 3 each group) or non-myocyte (E) (n=9, 3 each group) nuclei 4 weeks post-injection. Veh=vehicle; 200=200mg/kg ISO, 300=300mg/kg ISO. *p<0.05 vs. veh, NS=not significant (p>0.05). D and E, Data are shown as mean ± SEM. See also Online Figure IV.
To more conclusively document the percentage of EdU+ myocytes, single cells were isolated from vehicle, 200mg/kg or 300mg/kg ISO-treated mice 4 weeks post-ISO injection (Figure 6). The percentage of EdU+ cardiomyocytes was very low and did not differ between groups (veh 0.007±0.003%, 200mg/kg 0.006±0.003%, 300mg/kg 0.008±0.001; p=NS). Many of the EdU+ myocytes were small and mononucleated (Figure 6C). 63% of EdU+-isolated cardiomyocytes were mononucleated, 34% binucleated and 3% multinucleated, whereas among all myocytes 9.4% were mononucleated and 84.5% were binucleated (Figure 6D). These results suggest that there is a low rate of new myocyte formation in the adult C57BL/6 mouse and this rate is not significantly increased after cardiac injury via a single injection of ISO.
Isoproterenol does not increase CSC-derived new myocytes
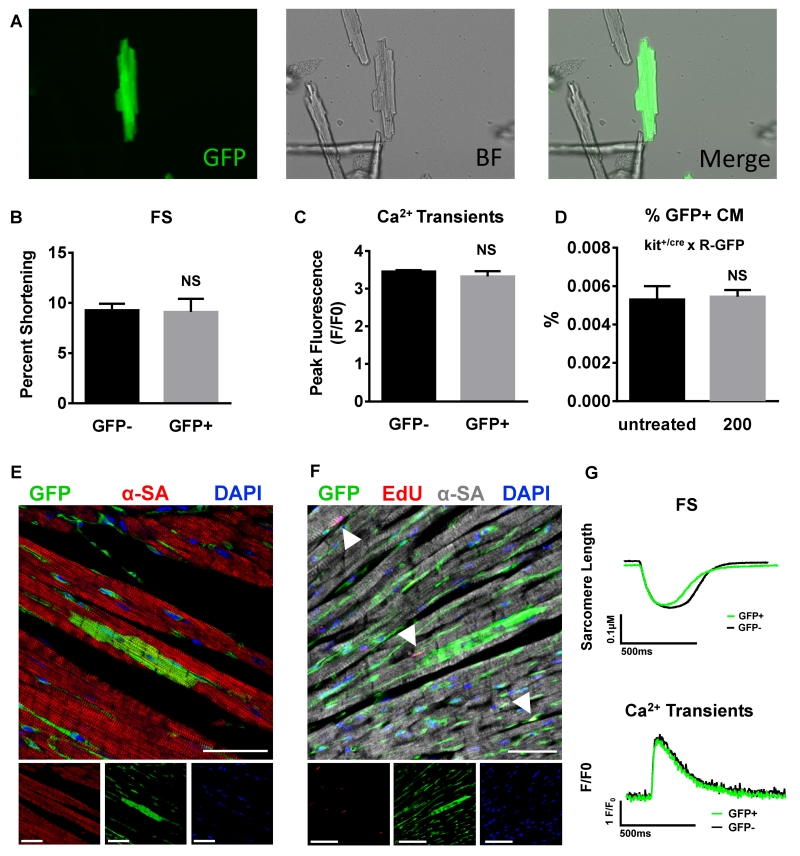
To determine if the new myocytes seen in these studies were derived from CSCs, transgenic mice that utilize the Kit locus for lineage tracing were used 6. The constitutive Kit+/Cre × R-GFP mice (Kit+/Cre × R-GFP) irreversibly mark any cell that previously or currently expresses cKit with green fluorescent protein (GFP) 6. These mice were injected with 200mg/kg ISO and after 4 weeks, myocytes were isolated or hearts were fixed for immunostaining. Figure 7A displays a representative fluorescent micrograph of an eGFP+ myocyte. Quantification of eGFP+ fluorescent myocytes (Figure 7D) from the hearts of ISO injected Kit+/Cre × R-GFP mice revealed a similar percentage of eGFP+ myocytes compared to untreated Kit+/Cre × R-GFP mice (0.0055±0.0004% vs 0.0053±0.0007 %; p=NS). The low percentage of eGFP+ myocytes is similar to that reported previously by van Berlo et al. 6.
Figure 7. ISO injury did not increase the % of ckit+ CSC-derived new myocytes or alter their physiology.
A, Confocal micrographs of an eGFP+ myocyte isolated from a transgenic kit+/cre × R-GFP mouse. Average fractional shortening (FS) data (B), peak Ca2+-transient measurements (C), Fractional Shortening (FS) traces and Ca2+ transients (G) in eGFP+ myocytes vs. normal non-GFP+ (GFP−) myocytes from the same heart. D, 200mg/kg ISO injection did not alter the percentage of eGFP+ myocyte compared to untreated kit+/cre × R-GFP mouse. E and F, Representative confocal micrographs of fixed hearts from transgenic kit+/cre × R-GFP mice at 4 weeks after ISO (200mg/kg) injection that were immunostained against GFP (green), α-sarcomeric actin (α-SA)(red in E; gray in F), 5-ethynyl-2′-deoxyuridine (EdU; red), and nuclei labeled with 4’,6-diamidino-2-phenylindole (DAPI, blue). White arrowheads=Edu+ nuclei. Scale bars=50μm. NS= not significant (p<0.05). B-D, Data are shown as mean ± SEM. See also Online Figure V.
Tissue sections from hearts fixed at 4 weeks were stained for GFP (green) and α-sarcomeric actin (red) (Figure 7E). 16 sections from each heart were analyzed for GFP+ myocytes. One to two GFP+ myocytes were identified per entire heart section, which is consistent with findings from van Berlo et al. 6. The majority of GFP+ cells in every section appeared to be endothelial cells. Furthermore, none of the GFP+ myocytes were positive for EdU (Figure 7F). The ejection fraction in Kit+/Cre × R-GFP mice and non-transgenic littermates was not impaired after 200mg/kg ISO injection at any time point (Online Figure V). The functional properties of eGFP+ myocytes were also studied. Fractional shortening (Figures 7B and 7G) and Ca2+-transients (Figures 7C and 7G) did not differ between GFP+ and GFP− myocytes isolated from the same hearts. Thus, a single injection of 200mg/kg ISO does not increase the percentage of cKit+-derived new myocytes in the Kit+/Cre × R-GFP mice or alter the physiology of myocytes isolated from them.
Chronic ISO causes cardiac injury without relevant new cardiomyocyte formation
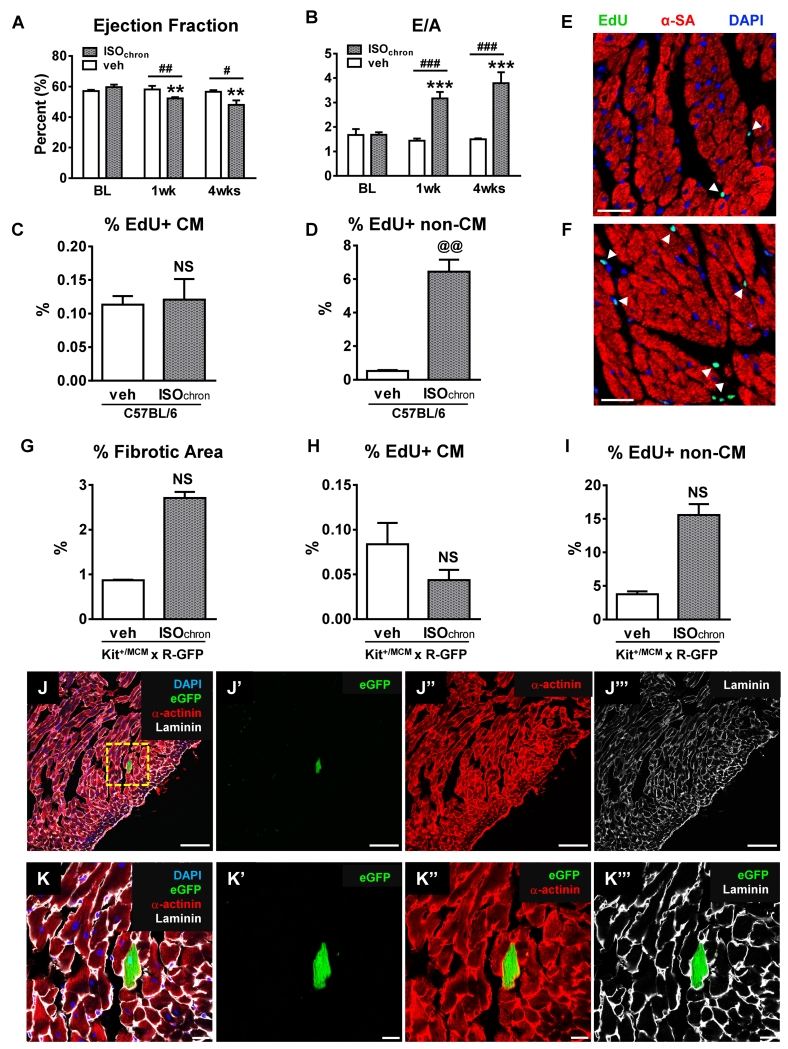
We were concerned that our failure to see myocyte regeneration after a single ISO injection might have resulted from insufficient myocyte injury. Therefore, C57BL/6 mice were injected with 200mg/kg ISO twice daily for 6 consecutive days. In contrast to a one-time ISO injection, multiple ISO injections caused a significant decrease in EF at 7 and 28 days post injection compared to vehicle-injected animals (28 days: ISO 48.0±2.9% vs veh 56.6±1.1%; p<0.05) (Figure 8A, Online Figure VI). Furthermore, diastolic function, represented by an increased E/A ratio, was also significantly impaired at 7 and 28 days after ISO injection (28 days: ISO 3.8±0.5 vs veh 1.5±0.04; p<0.001) (Figure 8B, Online Figure VI). However, the percentage of fibrotic area (data not shown), myocyte cross-sectional area (Online Figure VI), and heart weight to body weight ratio (Online Figure VI) were comparable to vehicle-injected mice, consistent with our findings after single ISO injection. The EdU pulse-chase labeling strategy revealed no differences in the percentage of EdU+ myocyte nuclei between ISO and vehicle-treated animals at 28 days after injections (ISO 0.12±0.03% vs veh 0.11±0.01%; p=NS) (Figure 8C). The percentage of EdU+ non-myocyte nuclei was significantly increased after multiple ISO injections (ISO 6.44±0.71% vs veh 0.52±0.06%; p<0.01) (Figure 8D). This rise significantly exceeded the percentage of EdU+ non-myocyte nuclei after both 200 and 300mg/kg single ISO injections (fold-change: 200mg/kg 5±0.31, 300mg/kg 7.1±1.07 and chronic ISO 12.88±1.43, p<0.05) (Online Figure VI).
Figure 8. Chronic ISO administration caused cardiac injury without new myocyte formation.
A-F, C57BL/6 mice were injected with 200mg/kg ISO (n=9) or vehicle (n=5) twice daily for 6 days. A and B, ISO caused significant systolic (Ejection Fraction) and diastolic (E/A) impairment in the ISO group (n=9) compared to vehicle group (n=5). C and D, The percentage of EdU+ myocyte (C) or non-myocyte (D) nuclei (veh (n=4), ISOchron (n=5)). E and F, Confocal micrographs of fixed hearts at 4 weeks post-injection: veh (E) and ISOchro (F) that were immunostained against α-sarcomeric actin(α-SA, red), 5-ethynyl-2′-deoxyuridine (EdU; green), and nuclei labeled with 4’,6-diamidino-2-phenylindole (DAPI, blue). White arrowheads=EdU+ nuclei. Scale bars=25μm. **p<0.01 and ***p<0.001 vs. BL ISOchron, #p<0.05, ##p<0.01 and ###p<0.001 between groups, @@p<0.01 vs. veh, NS=not significant (p>0.05). A-D, Data are shown as mean ± SEM. G-I, Kit+/MCM × R-GFP mice were injected with 200mg/kg ISO (n=4) or vehicle (n=2) twice daily for 6 days. G, Percentage of fibrotic area determined by bioquantification (veh (n=2), ISOchron (n=3)). H and I, Percentage of EdU+ myocyte (CM) (H) or non-myocyte (non-CM) (I) nuclei (veh (n=2), ISOchron (n=3)) at 4 weeks post-injections. J and K, Representative low (J) and high magnification (K) immunofluorescent images of a fixed heart from a transgenic Kit+/MCM × R-GFP mouse at 4 weeks after 6 days of 2×/daily ISO (200mg/kg) injections, that was immunostained against laminin (white) and α-sarcomeric actinin (red). Endogenous eGFP fluorescence from the R-GFP reporter is shown in green. Nuclei were labeled with 4’,6-diamidino-2-phenylindole (DAPI, blue). Individual fluorescent channels are shown alongside each image. Scale bars=100μm (J) and 10μm (K). NS=not significant (p<0.05). A-D and G-I, Data are shown as mean ± SEM.
To further explore if a more pronounced cardiac injury elicited CSC-mediated new cardiomyocytes, Kit+/MCM × R-GFP mice were injected with 200mg/kg ISO twice daily for 6 consecutive days. Prior to ISO dosing, Kit+/MCM × R-GFP mice were induced with tamoxifen for 2 weeks to activate the lineage tracing system 6. In line with our findings in C57BL/6 mice, myocyte cross-sectional area (Online Figure VI), heart weight to body weight ratio (Online Figure VI), and the percentage of EdU+ myocyte nuclei (ISO 0.04±0.01% vs veh 0.08±0.02%; p=NS) (Figure 8H) were unaltered after ISO. Although there was a 4-fold increase in the percentage of EdU+ non-myocyte nuclei (ISO 15.6±1.64% vs veh 3.8±0.45%; p=NS) (Figure 8I), as well as an increase in the percentage of fibrotic area (ISO 2.7±0.13% vs veh 0.9±0.01%; p=NS) (Figure 8G, Online Figure VI) in the ISO group compared to vehicle, both did not reach statistical significance, which is most likely explained by a small sample size. However, we did not observe any increase in eGFP+ myocytes. Thus, chronic ISO exposure caused more robust cardiac injury and depressed systolic and diastolic function, but did not elicit ckit+-CSC derived myocyte regeneration.
DISCUSSION
The objective of this study was to validate published work 10 demonstrating that a single injection of ISO in mice induces myocyte necrosis localized to the ventricular endocardium and apex, with transient depression of cardiac pump function, followed by a regenerative response in which CSCs are activated to proliferate and differentiate into new cardiac myocytes that fully regenerate the myocardium. Our goal was to use this system to study the properties of newly forming adult cardiac myocytes. We conducted a dose titration study in C57BL/6 mice and demonstrated that ISO doses ≥ 400mg/kg were lethal. We then explored the effects of single injections of 200mg/kg (the concentration used in the previous study 10), the highest non-lethal dose of 300mg/kg, and chronic ISO administration for 6 days (to increase injury). We defined the effects of these ISO doses on cardiac systolic and diastolic function, myocyte injury, myocyte size, cardiac fibrosis and new myocyte generation.
A single injection of high dose ISO causes transient positive inotropic effects
ISO is a potent β-adrenergic agonist that increases systolic [Ca2+] to enhance myocyte contractility 20. Toxic levels of catecholamines or prolonged exposure can induce cardiac injury in part by inducing Ca2+ overload-mediated myocyte necrosis 21. The previous study 10 showed that a single injection of 200mg/kg ISO caused endocardial and apical myocyte death and reduced cardiac function. To validate these observations, we first performed comprehensive ECHO evaluation for 4 weeks after ISO injection and we further performed speckle-tracking based strain imaging, a high-sensitivity method for phenotyping cardiac function. These techniques are known to detect small global and regional changes in contractility 16, 22. Neither conventional nor strain analyses revealed any functional deterioration at any time point after ISO injection. In fact, we observed a transient enhancement of myocardial contractility (EF, FS, global longitudinal strain; Figures 1A and 1B and Figure 2A) 1 day after ISO injection compared to vehicle, consistent with the well-known positive inotropic action of ISO. Ellison et al. 10 did not present ECHO data in mice, only hemodynamic measurements, where they demonstrated a significant decrease in left ventricular developed pressure (LVDevP) and +dP/dt 1 day post-ISO injection, that returned to normal baseline values after 28 days. The reasons why we were unable to confirm transient depression of global cardiac function is unclear.
The effects of single-dose ISO administration has been studied in other animal models. In an earlier 2007 study in rats, Ellison et al. 23 reported that a single subcutaneous ISO (5mg/kg) injection induced acute LV failure at 1 day with full recovery by 6 days. Sachdeva et al.24 described a “takotsubo-like” cardiomyopathy in rats after a single subcutaneous injection that used a 20-fold higher concentration than used by Ellison et al. 23. In mice, Shao et al. 25 demonstrated LV systolic dysfunction 1 day after intraperitoneal injection of 400 mg/kg ISO. After 1 week, cardiac function was normalized and no significant fibrosis was detected 25. Our findings indicate that a single ISO dose (200 or 300mg/kg) causes diffuse cardiac myocyte injury (Evans Blue Dye uptake) without reducing global cardiac function (Figures 1A and 1B and Figure 2A). Given the time course of the functional effects reported in these studies (either no change in global function or full recovery within a week), we conclude that a single injection of ISO injures the heart but does not cause widespread myocyte death. This is consistent with the findings that cardiac fibrosis was not observed and heart function recovered quickly; if it was indeed reduced in these studies.
The previous study 10 showed that myocytes in the ventricular apex and subendocardium are killed after ISO injection and referred to their own study in rats 23. However, they did not provide functional evidence of ISO induced “takotsubo-like” cardiomyopathies in mice. Speckle-tracking based longitudinal strain analyses, which can define myocardial performance in individual cardiac segments and layers 16, did not demonstrate regional apical or subendocardial impairments after ISO administration (Figures 2A-2D). Therefore, from a functional point of view, a single ISO injection did not induce a “takotsubo-like” cardiomyopathy in C57BL/6 WT mice.
There have been reports of cardiac dysfunction after ISO injections in mice, but these studies have usually used multiple, serial, ISO injections 26, 27. It seems clear that chronic ISO administration can induce myocardial injury with subsequent functional impairment, myocardial necrosis, fibrosis, and hypertrophy in rats and cats 5, 24, 28-30. Studies in mice have shown that serial subcutaneous ISO administration induces hypertrophy, diastolic dysfunction and increased myocardial fibrosis, but does not affect systolic function 26, 27. Kudej et al. demonstrated that chronic ISO administration via minipumps induced cardiac hypertrophy without cellular necrosis 31. Hohimer et al. highlighted that different routes of ISO administration have different hemodynamic sequelae and could potentially evoke different cardiac phenotypes in mice 32. In our view, the variability in ISO effects on cardiac function reported in the literature likely results from different ISO dosing strategies.
ISO causes myocyte injury without inducing apoptosis or widespread necrosis
We did not observe a significant increase in the percentage of TUNEL+ myocytes (Figure 3H) or non-myocytes (Figure 3I) or an increase in activated caspase-3 (Online Figure III) after ISO exposure. These data suggest that apoptosis plays a minor role in ISO-induced myocardial injury, which is consistent with Ellison’s et al. 10 observations in mice and rats. We found that 200mg/kg and 300mg/kg ISO caused about 10% of the ventricular myocytes to take up EBD, documenting membrane permeability abnormalities 19. EBD uptake is further associated with loss of membrane integrity and loss of mitochondrial membrane potential 33, 34. Myocardial necrosis after ISO administration has been reported in several animal models 5, 10, 23, 24, 35, 36, but we did not find global functional defects or cardiac fibrosis. Therefore, we questioned if EBD+ myocytes were indeed all destined for necrosis. H&E stained slides of the matched EBD+ sections showed some areas in which EBD+ myocytes showed features of necrotic tissue (Figures 3E and 3F, upper small images). However, many areas with EBD+ myocytes showed no other histological features of necrosis (in the matched H&E sections) (Figures 3E and 3F, lower small images). Others have shown that ISO can induce a transient alteration in sarcolemmal membrane permeability without necrosis 17. A transient membrane instability could cause EBD uptake in some myocytes, with subsequent recovery after elimination of the stressor. While Ellison et al. 10 reported that 200mg/kg ISO caused the same type of myocardial damage in mice and rats, they did not document how they measured myocardial necrosis in mice. Interestingly, they reported that 200mg/kg ISO caused necrosis in 10% of myocytes, which is very similar to our findings with EBD uptake. In our study, Tn-I levels measured 1 day after ISO injection were significantly elevated after 300mg/kg ISO, but not after 200mg/kg ISO. In addition, we showed that ISO-injured animals had significantly lower levels of serum Tn-I levels compared to mouse hearts with MI (which clearly induces myocyte death, inflammation and reactive fibrosis 15), (Figure 3J). In humans, Tn-I levels correlate with myocardial necrosis after radiofrequency ablation 37 and infarct volume after acute myocardial infarction 38. A close correlation between cardiac troponin T and histological infarct size has been observed in mice 39. Interestingly, the increase in Tn-I after 300mg/kg ISO was significantly higher compared to 200mg/kg ISO, which suggests increased injury. The percentage of EBD+ myocytes was similar between 200 and 300mg/kg ISO suggesting that EBD uptake may identify both damaged (but able to recover) and necrotic cells. Collectively, our results suggest that a single sub-lethal ISO injection in C57BL/6 mice causes transient membrane damage in about 10% of the myocytes, and some of these myocytes appear to undergo necrosis with a small, ISO dose-dependent increase in the infiltration of proliferative inflammatory cells, but no reactive fibrosis.
ISO causes dose-dependent injury without inducing cardiac regeneration
We used an EdU pulse-chase labeling strategy to identify newly forming myocytes and non-myocytes at 4 weeks after ISO injury. Newly formed myocytes (from any source) that entered the cell cycle would be detected by EdU nuclear incorporation. The study by Ellison et al. 10 showed that 8-10% of the ventricular myocytes were labeled with BrdU 6 and 28 days after 200mg/kg ISO. We did not find any increase in EdU+ myocytes at 4 weeks after injection with 200 or 300mg/kg ISO (Figure 5D). We used EdU rather than BrdU in our study because, in our hands, detection of EdU is much more reliable. The smaller molecular size of the EdU reagents, higher diffusion rates, and more effective tissue penetration without denaturation allows for better structural preservation 40. EdU could also label damaged DNA, thus it is possible that we detected false-positive proliferating cells. Given the fact that only a few cardiomyocytes were EdU+ (<0.10%) and the fact that most of these myocytes were small and mononucleated makes the idea that they represent damaged DNA rather than new DNA highly unlikely. Our findings suggest that there is a very low rate of new myocyte formation in the adult mouse heart and the rate of new myocyte formation is not increased significantly after ISO injury. The idea that the EdU+ myocytes represent newly formed myocytes was strongly supported by our finding that these cells were largely mononucleated while the majority of EdU− myocytes were binucleated (Figure 6D).
We also explored whether ISO injury induces cardiac myocyte regeneration and whether new myocytes are derived from CSCs. We utilized a cKit lineage fate-mapping strategy in which Kit+/cre × R-GFP transgenic mice 6 were given a single 200mg/kg ISO injection. The results of these experiments were consistent with our EdU findings. 200mg/kg ISO did not increase the percentage of eGFP+ myocytes compared to control (untreated Kit+/cre × R-GFP). Interestingly, the percentage of EdU+-isolated myocytes observed after ISO injury or in controls was similar to the percentage of GFP+ myocytes derived from CSCs (about 0.005%). The somewhat higher percentage of EdU+ myocytes found in tissue sections could simply reflect differences in experimental approaches such as difficulties with reliable identification of myocyte nuclei in tissue sections. Interestingly, none of the GFP+ myocytes we identified after ISO injury had an EdU+ nucleus (Figure 7F). These findings suggest that the new EdU+ myocytes we observed may not have been derived from a cKit-expressing precursor cell.
Chronic ISO causes cardiac injury without activating CSC-mediated regeneration
Multiple high dose ISO injections were used to determine if a more pronounced cardiac injury could induce cardiomyocyte regeneration. In C57BL/6 mice, multiple high dose ISO injections caused significant impairments in systolic and diastolic function (Figure 8A and 8B; Online Figure VI) and increased the percentage of EdU+ non-myocyte nuclei (Figure 8D, Online Figure VI), but not of EdU+ myocytes (Figure 8C). Interestingly, we could not find evidence for significant reactive hypertrophy (normal HW/BW and myocyte cross-sectional area) after multiple ISO injections in C57BL/6 mice. In line with the findings in C57BL/6 mice, multiple high dose ISO injections increased the percentage of EdU+ non-myocyte nuclei (Figure 8I) in Kit+/MCM × R-GFP mice. Myocyte cross-sectional area (Online Figure VI), HW/BW ratio (Online Figure VI), and the percentage of EdU+ myocyte nuclei (Figure 8H) were also not altered after multiple high dose ISO injections in Kit+/MCM × R-GFP mice. Interestingly, ISO caused LV fibrosis in Kit+/MCM × R-GFP (Figure 8G, Online Figure VI) but not in C57BL/6 mice (data not shown). The difference might be related to tamoxifen induction prior to ISO dosing in Kit+/MCM × R-GFP mice. Furthermore, we cannot rule out strain- and/or sex-related differences in the response to isoproterenol. Faulx et al. reported that ISO causes a more pronounced cardiac injury in A/J than C57BL/6J, due to ß-adrenergic receptor (ß-AR) downregulation in C57BL/6J mice 41. However, we do not believe that sex and/or strain differences can account for the differences between Ellison’s and our findings.
The percentage of EdU+ myocytes did not increase after multiple high dose ISO administration, both in C57BL/6 and in Kit+/MCM × R-GFP mice, suggesting that the rate of new myocyte formation is not significantly increased after this form of ISO injury. Notably, we did not observe an increase in eGFP+ myocytes either after multiple ISO injections (Kit+/MCM × R-GFP mice), which is in line with our findings in Kit+/cre × R-GFP mice after a single 200mg/kg ISO injection. Our findings suggest that ISO induces dose-dependent cardiac injury (Tn-I levels, EdU+ non-myocyte nuclei), but despite induction of several pathological alterations, neither single nor multiple ISO injections induced cardiomyocyte regeneration. In a previous study in felines 5, continuous ISO infusion for 10 days via osmotic minipumps was shown to induce myocyte death, cardiac fibrosis, and depressed cardiac contractile function. This strategy for ISO injury was shown to activate cKit+ CSC (BrdU labelling). However 28 days after ISO injury, BrdU labelling of ventricular myocytes increased by only 0.04%, suggesting that larger amounts of ISO injury than caused in the present study induces a small regenerative response. The source of these new myocytes in the feline study could not be determined conclusively.
In summary, the current study shows that a single injection of 200 or 300mg/kg ISO caused myocyte membrane permeability alterations sufficient to allow EBD uptake and Tn-I leak from about 10% of the ventricular myocytes. These injured myocytes were observed diffusely throughout the heart and were not localized to the endocardium or apical regions of the heart. No reductions in global or regional myocardial performance were observed. Therefore, a single dose of 200mg/kg ISO in mice does not produce a “takotsubo-like” cardiomyopathy in C57BL/6 mice. A small amount of myocyte necrosis was induced by ISO injury, and we found a small, ISO dose-dependent increase in the infiltration of proliferative inflammatory cells but no significant myocardial fibrosis that is normally associated with widespread necrotic injury. Multiple ISO injections caused a more pronounced cardiac injury with systolic and diastolic cardiac dysfunction. Although ISO induced a dose-dependent cardiac injury, we did not find any evidence for cardiac regeneration either after a single or after multiple injections of high dose ISO, using two independent techniques and two ckit lineage tracing mouse models. Collectively, our results in mice do not support the idea that ISO injury elicits cardiomyocyte regeneration.
Supplementary Material
Novelty and Significance.
What Is Known?
Endogenous cKit+ cardiac stem cells (CSCs) have the ability to form new myocytes in the normal adult heart and to regenerate new cardiomyocytes after cardiac injury. However, the extent to which CSCs form new myocytes after cardiac injury is highly variable in different studies.
High levels of circulating catecholamines can induce reversible cardiac stunning and/or myocyte death.
A previous report showed that a single injection of Isoproterenol (ISO: 200 mg/Kg) kills 8-10% of the myocytes in the mouse heart and over the 28 days, these lost myocytes are regenerated from cKit+ cardiac stem cells.
What New Information Does This Article Contribute?
A single ISO injection (200 or 300 mg/Kg) in C57BL/6 mice causes dose-dependent injury in 8-10% of the ventricular myocytes, but hearts recover without significant fibrosis or abnormal systolic and diastolic cardiac function.
Single or multiple injections of ISO cause injury but do not induce an increase in the number of newly generated myocytes, as determined by Ethynyl DeoxyUridine (EdU) labeling.
ISO injury in a cKit+-lineage tracing mouse model does not result in new myocyte generation from cKit+ precursor cells.
The adult mammalian heart appears to have a very limited ability to regenerate myocytes that die with disease or aging. Developing novel approaches to replace lost myocytes and their supportive vasculature would be a major medical advance. Endogenous cKit+ cardiac stem cells are thought to have the capacity to differentiate into new myocytes and vascular tissue. We addressed the question of whether or not cardiac stem cells can significantly regenerate the adult heart after ISO-induced injury. ISO caused significant myocyte injury and infiltration of proliferative inflammatory cells. However, after recovery, no significant late myocardial fibrosis or impairment of systolic and diastolic cardiac function was found. While ISO induced a dose-dependent increase in cardiac injury, the percentage of EdU+ cardiomyocytes did not increase. Experiments in a cKit+-lineage-tracing mouse model did not reveal new myocytes derived from cKit+ cardiac precursors. This study does not support the concept that transient ISO-induced cardiac injury activates cardiomyocyte regeneration.
ACKNOWLEDGEMENTS
We would like to thank Sarah Anne Tupchong for her assistance in editing the manuscript.
SOURCES OF FUNDIG
Supported by NIH grants R01HL089312, T32HL091804, P01HL091799, and R01HL033921 to SRH. S.M. received a SDG grant from AHA
Non-standard Abbreviations and Acronyms
- ANP
atrial natriuretic peptide
- BL
baseline
- BNP
brain natriuretic peptide
- BrdU
5-bromo-2’-deoxyuridine
- BW
body weight
- CSC
cardiac stem cell
- dP/dt
maximal rate of rise of pressure
- EBD
Evans Blue Dye
- EdU
5-ethynyl-2’-deoxyuridine
- EF
ejection fraction
- FS
fractional shortening
- GFP
green fluorescent protein
- HW
heart weight
- ISO
isoproterenol
- LAD
left anterior descending
- LV
left ventricle
- LVDevP
left ventricular developed pressure
- MI
myocardial infarction
- ßMHC
ß-myosin heavy chain
- TDI
tissue doppler imaging
- Tn-I
troponin I
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling
- veh
vehicle
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–35. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nature medicine. 2007;13:970–4. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angert D, Berretta RM, Kubo H, Zhang H, Chen X, Wang W, Ogorek B, Barbe M, Houser SR. Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells. Circulation research. 2011;108:1226–37. doi: 10.1161/CIRCRESAHA.110.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–41. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science (New York, NY) 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–6. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfo M, Agosti V, Viglietto G, Condorelli G, Indolfi C, Ottolenghi S, Torella D, Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–42. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 11.Nigro P, Perrucci GL, Gowran A, Zanobini M, Capogrossi MC, Pompilio G. c-kit(+) cells: the tell-tale heart of cardiac regeneration? Cell Mol Life Sci. 2015;72:1725–40. doi: 10.1007/s00018-014-1832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hesse M, Fleischmann BK, Kotlikoff MI. Concise review: The role of C-kit expressing cells in heart repair at the neonatal and adult stage. Stem Cells. 2014;32:1701–12. doi: 10.1002/stem.1696. [DOI] [PubMed] [Google Scholar]
- 13.Madonna R, Ferdinandy P, De Caterina R, Willerson JT, Marian AJ. Recent developments in cardiovascular stem cells. Circulation research. 2014;115:e71–8. doi: 10.1161/CIRCRESAHA.114.305567. [DOI] [PubMed] [Google Scholar]
- 14.Duran JM, Makarewich CA, Sharp TE, Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo H, Houser SR. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circulation research. 2013;113:539–52. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duran JM, Makarewich CA, Trappanese D, Gross P, Husain S, Dunn J, Lal H, Sharp TE, Starosta T, Vagnozzi RJ, Berretta RM, Barbe M, Yu D, Gao E, Kubo H, Force T, Houser SR. Sorafenib cardiotoxicity increases mortality after myocardial infarction. Circulation research. 2014;114:1700–12. doi: 10.1161/CIRCRESAHA.114.303200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer M, Cheng S, Jain M, Ngoy S, Theodoropoulos C, Trujillo A, Lin FC, Liao R. Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circulation research. 2011;108:908–16. doi: 10.1161/CIRCRESAHA.110.239574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. The Journal of cell biology. 1997;139:375–85. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Togo T, Alderton JM, Bi GQ, Steinhardt RA. The mechanism of facilitated cell membrane resealing. J Cell Sci. 1999;112(Pt 5):719–31. doi: 10.1242/jcs.112.5.719. [DOI] [PubMed] [Google Scholar]
- 19.Han R, Bansal D, Miyake K, Muniz VP, Weiss RM, McNeil PL, Campbell KP. Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury. The Journal of clinical investigation. 2007;117:1805–13. doi: 10.1172/JCI30848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou Y, Komuro I, Yamazaki T, Kudoh S, Uozumi H, Kadowaki T, Yazaki Y. Both Gs and Gi proteins are critically involved in isoproterenol-induced cardiomyocyte hypertrophy. The Journal of biological chemistry. 1999;274:9760–70. doi: 10.1074/jbc.274.14.9760. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. The Journal of clinical investigation. 2007;117:2431–44. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer M, Cheng S, Unno K, Lin FC, Liao R. Regional cardiac dysfunction and dyssynchrony in a murine model of afterload stress. PloS one. 2013;8:e59915. doi: 10.1371/journal.pone.0059915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellison GM, Torella D, Karakikes I, Purushothaman S, Curcio A, Gasparri C, Indolfi C, Cable NT, Goldspink DF, Nadal-Ginard B. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. The Journal of biological chemistry. 2007;282:11397–409. doi: 10.1074/jbc.M607391200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachdeva J, Dai W, Kloner RA. Functional and histological assessment of an experimental model of Takotsubo’s cardiomyopathy. J Am Heart Assoc. 2014;3:e000921. doi: 10.1161/JAHA.114.000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao Y, Redfors B, Stahlman M, Tang MS, Miljanovic A, Mollmann H, Troidl C, Szardien S, Hamm C, Nef H, Boren J, Omerovic E. A mouse model reveals an important role for catecholamine-induced lipotoxicity in the pathogenesis of stress-induced cardiomyopathy. European journal of heart failure. 2013;15:9–22. doi: 10.1093/eurjhf/hfs161. [DOI] [PubMed] [Google Scholar]
- 26.Schumacher A, Khojeini E, Larson D. ECHO parameters of diastolic dysfunction. Perfusion. 2008;23:291–6. doi: 10.1177/0267659109102485. [DOI] [PubMed] [Google Scholar]
- 27.Brooks WW, Conrad CH. Isoproterenol-induced myocardial injury and diastolic dysfunction in mice: structural and functional correlates. Comparative medicine. 2009;59:339–43. [PMC free article] [PubMed] [Google Scholar]
- 28.Korkmaz S, Radovits T, Barnucz E, Hirschberg K, Neugebauer P, Loganathan S, Veres G, Pali S, Seidel B, Zollner S, Karck M, Szabo G. Pharmacological activation of soluble guanylate cyclase protects the heart against ischemic injury. Circulation. 2009;120:677–86. doi: 10.1161/CIRCULATIONAHA.109.870774. [DOI] [PubMed] [Google Scholar]
- 29.Teerlink JR, Pfeffer JM, Pfeffer MA. Progressive ventricular remodeling in response to diffuse isoproterenol-induced myocardial necrosis in rats. Circulation research. 1994;75:105–13. doi: 10.1161/01.res.75.1.105. [DOI] [PubMed] [Google Scholar]
- 30.Rona G, Chappel CI, Balazs T, Gaudry R. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch Pathol. 1959;67:443–55. [PubMed] [Google Scholar]
- 31.Kudej RK, Iwase M, Uechi M, Vatner DE, Oka N, Ishikawa Y, Shannon RP, Bishop SP, Vatner SF. Effects of chronic beta-adrenergic receptor stimulation in mice. Journal of molecular and cellular cardiology. 1997;29:2735–46. doi: 10.1006/jmcc.1997.0508. [DOI] [PubMed] [Google Scholar]
- 32.Hohimer AR, Davis LE, Hatton DC. Repeated daily injections and osmotic pump infusion of isoproterenol cause similar increases in cardiac mass but have different effects on blood pressure. Can J Physiol Pharmacol. 2005;83:191–7. doi: 10.1139/y04-137. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Xie W, Zhang Y, Lin P, Han L, Han P, Wang Y, Chen Z, Ji G, Zheng M, Weisleder N, Xiao RP, Takeshima H, Ma J, Cheng H. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circulation research. 2010;107:76–83. doi: 10.1161/CIRCRESAHA.109.215822. [DOI] [PubMed] [Google Scholar]
- 34.Yue Y, Li Z, Harper SQ, Davisson RL, Chamberlain JS, Duan D. Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation. 2003;108:1626–32. doi: 10.1161/01.CIR.0000089371.11664.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L, Cui J, Yang Q, Jia C, Xiong M, Ning B, Du X, Wang P, Yu X, Li L, Wang W, Chen Y, Zhang T. Apocynin attenuates isoproterenol-induced myocardial injury and fibrogenesis. Biochemical and biophysical research communications. 2014;449:55–61. doi: 10.1016/j.bbrc.2014.04.157. [DOI] [PubMed] [Google Scholar]
- 36.Grimm D, Elsner D, Schunkert H, Pfeifer M, Griese D, Bruckschlegel G, Muders F, Riegger GA, Kromer EP. Development of heart failure following isoproterenol administration in the rat: role of the renin-angiotensin system. Cardiovascular research. 1998;37:91–100. doi: 10.1016/s0008-6363(97)00212-5. [DOI] [PubMed] [Google Scholar]
- 37.Madrid AH, del Rey JM, Rubi J, Ortega J, Gonzalez Rebollo JM, Seara JG, Ripoll E, Moro C. Biochemical markers and cardiac troponin I release after radiofrequency catheter ablation: approach to size of necrosis. American heart journal. 1998;136:948–55. doi: 10.1016/s0002-8703(98)70148-6. [DOI] [PubMed] [Google Scholar]
- 38.Di Chiara A, Dall’Armellina E, Badano LP, Meduri S, Pezzutto N, Fioretti PM. Predictive value of cardiac troponin-I compared to creatine kinase-myocardial band for the assessment of infarct size as measured by cardiac magnetic resonance. J Cardiovasc Med (Hagerstown) 2010;11:587–92. doi: 10.2459/JCM.0b013e3283383153. [DOI] [PubMed] [Google Scholar]
- 39.Metzler B, Hammerer-Lercher A, Jehle J, Dietrich H, Pachinger O, Xu Q, Mair J. Plasma cardiac troponin T closely correlates with infarct size in a mouse model of acute myocardial infarction. Clin Chim Acta. 2002;325:87–90. doi: 10.1016/s0009-8981(02)00296-6. [DOI] [PubMed] [Google Scholar]
- 40.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2415–20. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faulx MD, Ernsberger P, Vatner D, Hoffman RD, Lewis W, Strachan R, Hoit BD. Strain-dependent beta-adrenergic receptor function influences myocardial responses to isoproterenol stimulation in mice. American journal of physiology Heart and circulatory physiology. 2005;289:H30–6. doi: 10.1152/ajpheart.00636.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.