Abstract
The mechanisms behind the low viral loads and lower mortality rates of HIV-2+ individuals remain unknown. We hypothesized that reduced interaction of HIV-2 with CD169, the primary HIV-1 attachment factor on monocyte-derived dendritic cells (DCs) that targets captured virus particles to the trans infection pathway, contributes to its diminished pathogenic phenotype in vivo. We observed a significant decrease in capture of HIV-2 Gag-eGFP virus-like particles (VLPs) and infectious GFP-containing HIV-2 particles compared to corresponding HIV-1 particles by CD169+ mature DCs. Interestingly, there was decreased co-localization of HIV-2 with HIV-1 Gag at plasma membrane microdomains in virus producer cells which correlated with reduced incorporation of GM3, the CD169 ligand, in HIV-2 virions, and reduction in mature DC-mediated HIV-2 trans infection compared to HIV-1. We conclude that limited interaction of HIV-2 with CD169 diminishes virus access to the mature DC-mediated trans infection pathway and might result in attenuated HIV-2 dissemination in vivo.
Keywords: HIV-2, dendritic cells, virus capture, trans infection, CD169, GM3, cell-associated virus transfer
Introduction
There are two viruses that cause Acquired Immune Deficiency Syndrome (AIDS) in humans, HIV-1 and HIV-2. Zoonotic transmissions of both viruses to the human populations have occurred presumably due to repeated human exposure to infected non-human primates (Sharp et al., 2001). HIV-2, is believed to have made a zoonotic jump from SIVsmm-infected sooty mangabeys (Cercocebus atys) in 1940 ± sixteen years while the major pandemic strain of HIV-1 (Group M), has been hypothesized to have made the jump from SIVcpz-infected chimpanzees (Pan troglodytes) in 1930 ± fifteen years (Lemey et al., 2003). Less is known about HIV-2 than HIV-1, although it appears to have the same mode of transmission and is associated with similar immune deficiency in end stage disease as HIV-1. In contrast to the rapid disease course of HIV-1 in the absence of highly active antiretroviral therapy, progression to end-stage disease in HIV-2-infected individuals is characterized as heterogeneous, with a minority of infected individuals progressing to AIDS, while a majority of HIV-2 infected individuals display a longer asymptomatic stage with relative viral control and high CD4+ T cell counts (de Silva et al., 2008; MacNeil et al., 2007). Interestingly, the survival rate of HIV-2+ individuals is 100% five years post-seroconversion compared to 67% in HIV-1+ individuals (de Silva et al., 2008). Though the mechanistic explanation behind these differences is not fully understood, these observed differences in the course of disease in HIV-1 and HIV-2 infections has lead to the hypothesis that HIV-2 is an attenuated form of HIV-1.
CD4+ T cells are the primary targets of both HIV-1 and HIV-2. As the main target cell for HIV pathogenesis, dissemination to and establishment of virus replication in CD4+ T cells is critical for viral spread. While most studies are in agreement that HIV-2 is capable of infecting CD4+ T cells in vitro at an equivalent level as HIV-1, there have been reported disparities in infections of dendritic cells (Chauveau et al., 2015; Duvall et al., 2007). Dendritic cells (DCs) are sentinel cells that bridge innate and adaptive immune responses, which have been postulated to play a role in HIV pathogenesis (Wu and KewalRamani, 2006). The ability of HIV-1 to establish productive infection in DCs is attenuated primarily due to the existence of numerous post-entry restrictions to virus replication, such as SAMHD1 (Hrecka et al., 2011; Laguette et al., 2011), that block virus replication at the reverse transcription step. In contrast, HIV-2 encoded Vpx can counteract SAMHD1 activity by targeting it for proteasomal degradation and facilitate productive infection of DCs by HIV-2 (Hrecka et al., 2011; Laguette et al., 2011). It should be noted, that overcoming SAMHD1 restriction alone in DCs is not sufficient, since infections with a select subset of Vpx-encoding primary or lab-adapted HIV-2 isolates does not result in robust replication in DCs (Chauveau et al., 2015; Duvall et al., 2007). In addition, productive infection of DCs with HIV-2 can elicit robust antiviral responses (Manel et al., 2010). Therefore, it remains unclear whether the ability of HIV to productively infect DCs is correlated to its pathogenesis.
In addition to CD4 and chemokine co-receptors, DCs express a number of virus attachment factors, such as DC-SIGN and CD169 that can bind HIV-1 particles and mediate trans infection of CD4+ T cells (Geijtenbeek et al., 2000; Izquierdo-Useros et al., 2012b; Jameson et al., 2002; Puryear et al., 2013). Unlike all previously identified HIV-1 attachment factors that recognize the virus envelope glycoprotein, CD169, also called sialoadhesin or Siglec1 (sialic acid binding Ig-like lectin 1), a member of the immunologlobin-like lectin superfamily (Hartnell, 2001), binds α2,3-sialylated glycospingolipids (GSLs), such as GM3, incorporated in the virus particle membrane (Izquierdo-Useros et al., 2012a; Puryear et al., 2012). GM3-dependent capture of HIV-1 particles by CD169 results in virus particle localization and preservation of virus particle infectivity within CD81+ CD169+ plasma membrane invaginations in mature DCs (Akiyama et al., 2015), and upon establishment of mature DC – CD4+ T cell conjugates, transfer of captured particles to CD4+ T cells across virological synapses (Izquierdo-Useros et al., 2012b; Puryear et al., 2013).
In our study, we have investigated whether HIV-2 is capable of utilizing the CD169/GM3 dependent mature DC-mediated trans infection pathway for efficient dissemination to CD4+ T cells. We observe significantly less capture of HIV-2 by mature DCs and hence, subsequently less mature DC-mediated transfer of HIV-2 to autologous CD4+ T cells. We demonstrate that incorporation of GM3 is reduced in HIV-2 virions, presumably due to HIV-2 assembly and exit from GSL-deficient membrane sites in virus-producer cells. These findings suggest that attenuated access of HIV-2 to the CD169-dependent trans infection pathway might contribute to the diminished spread and reduced pathogenicity of HIV-2 in vivo.
Methods
Ethics Statement
This research has been determined to be exempt by the Institutional Review Board of the Boston University Medical Center since it does not meet the definition of human subjects research, since all human samples were collected in an anonymous fashion and no identifiable private information was collected.
Cells
Human cell lines, HEK293T (a human kidney epithelial cell line) and TZM-bl, have been described before (Puryear et al., 2013). THP1 and CD169 expressing THP1 (THP1/CD169) cells have been described previously (Akiyama et al., 2015). Immature DCs were derived from CD14+ peripheral blood monocytes from healthy donors, as previously described (Puryear et al., 2012). DCs were matured for two days with ultrapure Escherichia coli K-12 lipopolysaccharide (100ng/ml; Invivogen). Autologous CD4+ T cells were isolated by positive selection from the CD14-depleted PBMCs using CD4-conjugated magnetic beads and LS columns (Miltenyi Biotech). CD4+ T cells were then stimulated with 2% phytohemagglutinin (PHA) (Invitrogen) for two days, washed and cultured in interleukin-2 (NIH AIDS Reagent Program, contributed by Dr. Maurice Gately) (50U/ml) containing- RPMI-10% heat inactivated fetal bovine serum (FBS) (Invitrogen).
Plasmids
The proviral plasmid, HIV-1/Lai-YU2env (CCR5-tropic) and the CCR5-tropic Lai-Bal envelope expression plasmid have has been described previously (Akiyama et al., 2015; Puryear et al., 2013). pROD9 was a generous gift from Dr. Geoffrey Gottlieb, (Smith et al., 2008). HIV-2 ST envelope expression plasmid was a gracious gift of Dr. Paula Cannon (Hauser et al., 2010). HIV-1Δenv and HIV-2Δenv provirus plasmids encoding gfp in place of nef, have been described previously (Pertel et al., 2011; Yamashita and Emerman, 2004). HIV-2iGFP, an infectious molecular clone encoding GFP between MA and CA flanked by protease cleavage sites, was constructed using a cloning strategy similar to the one previously described for construction of HIV-1iGFP proviral plasmid (Hubner et al., 2007). Briefly, sequences encoding HIV-2/ROD9 matrix (MA) and capsid (CA) were cloned into a cloning vector, pSL1180, using KasI and ApaI restriction enzymes, and was used for subsequent cloning steps. HIV-2 ROD9 MA sequence containing the protease cleavage site was amplified using the following primers: ROD9 matrix-Kas-F (5′-CGGTGTGAAATACCGCACAGATGCGTAA-3′), ROD9 matrix-R (TTTTTTACGCGTGTGCTGTACAGGATAGTTACCACCCTTCTCGCTAGATGGTGCTG TTGG). HIV-2 ROD9 CA sequence including the protease cleavage site was amplified with the following primers: ROD9 CAC_F (TTTTTTTCTAGAGGAGGAAA TTACCCAGTG CAACATGTAG G) and M13-R-long (5′-GGATAACAATTTCACACAGGAAACAGCTATGAC-3′). The PCR-amplified HIV-2 ROD9 MA and CA fragments and an AflIII-XbaI fragment from the HIV-iGFP proviral clone containing only the GFP sequence were assembled into pROD9 to generate HIV-2iGFP. The plasmids, HIV-1 Gag-eGFP and HIV-1 Gag-mCherry express a Gag-eGFP (NIH AIDS Reagent Program; contributed by Marilyn D. Resh and George Pavlakis) or Gag-mCherry fusion proteins (Izquierdo-Useros et al., 2009). HIV-2 Gag-eGFP was constructed by PCR amplifying HIV-2 ROD Gag orf with the following primers: ROD_GAG_BSSH2_F (TTT GCG CGC ATT GTG GGA GAT GGG) and ROD_GAG_BGLII_R (TTT AGA TCT CCG TTT CTG TTC TGG TCT TTT CCA AAG AGA G), and cloning the PCR product into the HIV Gag-eGFP plasmid using the restriction enzymes BsshII and BamHI. Additionally, HIV-1 RRE (rev responsive element) was inserted after Gag-eGFP orf using XbaI and HpaI restriction sites. All plasmids were verified by sequencing.
Viruses
Infectious viral stocks and Gag-eGFP VLPs were produced by calcium phosphate mediated transfections of HEK293T cells. To limit GSL incorporation in virions budding from transfected HEK293Ts, transfections were performed with media containing phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP; 10uM; Calbiochem) as described previously (Hatch et al., 2009). Two days post transfection cell free virus particle containing supernatants were harvested. HIV-2 CDC77618 was originally isolated from diagnostic culture of Ivorian AIDS patient (NIH AIDS Reagent Program, contributed by Dr. Stefan Wiktor and Dr. Mark Rayfield) (Owen et al., 1998) and HIV-1/Lai-YU2 (CCR5-tropic) proviral clone has been described previously (Puryear et al., 2013). These viruses were propagated in PHA-stimulated PBMCs for twelve days. Cell-free virus containing supernatants were collected every three days, filtered through a 0.45 μM syringe filter, pooled and concentrated via ultracentrifugation. Both transfection supernatants and PBMC derived viruses were concentrated through a 20% sucrose (Fisher) cushion using ultracentrifugation (100,000xg, two hours, 4°C). Virus pellets were resuspended in HeNa (140 mM NaCl, 10 mM HEPES) buffer and aliquoted prior to storage at −80°C. Infectivity of virus stocks was tittered on TZM-bl cells (NIH AIDS Reagent Program, contributed by Dr. John C. Kappes and Dr. Xiaoyun Wu).
Western blots
GFP content of Gag-eGFP VLPs and iGFP infectious viruses were titered using quantitative western blot analysis, as described previously (Puryear et al., 2013). Briefly, virus particle lysates were run on 10% SDS-PAGE gel, transferred to nitrocellulose membrane and blocked with Odyssey Blocking Buffer (Licor). Membranes were probed for GFP (goat anti-GFP polyclonal antibody, Novus #NB600-313) followed by a donkey anti-goat-IgG-IRDye 800CW (Licor). Membranes were scanned with an Odyssey Scanner (Licor) and the GFP content in VLPs or infectious viruses was determined by comparison to recombinant GFP standard (Roche). To determine if HIV-2iGFP viruses incorporated Env, virus particle lysates were probed for Gag and Env expression using anti-Gag mouse mAb (AG3.0) (Sanders-Beer et al., 2012) and anti-HIV-2 Env antisera (obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: Antiserum to HIV-2ST gp120 from Dr. Raymond Sweet, SmithKline Beecham Pharmaceuticals).
Immunofluorescence Microscopy
To visualize virus assembly sites in HEK293T cells, cells were co-transfected with Gag-eGFP (HIV-1 or HIV-2) and HIV-1 Gag-mCherry expression plasmids. Cells were fixed with 4% paraformaldehyde (PFA; Boston Bioproducts) at 24 h post transfection, stained with DAPI (4=,6-diamidino-2-phenylindole; Sigma) to visualize nuclei and processed for confocal microscopy. Images were acquired on a Leica SP5 confocal microscope and analyzed with Image J and pseudo-colored for data presentation. Extent of co-localization between eGFP and mCherry fusion proteins was analyzed using Pearson’s correlation of coefficient analysis in Image J from at least 7 fields of view and 95–155 cells per transfection. Alternatively, HEK293T cells were transfected with either HIV-1iGFP or HIV-2iGFP proviral plasmids, fixed with 4% paraformaldehyde and stained with AlexaFluor 594-conjugated Wheat Germ Aggluttinin (WGA-AF594) and counterstained with DAPI at 24 hr post-transfection. To determine extent of co-localization between Gag and GM3, mock, HIV-1iGFP or HIV-2iGFP transfected cells were fixed with 4% paraformaldehyde at 24 h post transfection, and incubated with anti-GM3 monoclonal antibody (Clone M2590, Cosmo Bio) and stained with AlexaFluor 594-conjugated goat anti-mouse IgM (Invitrogen) and counterstained with DAPI. Co-localization between green (GFP) and red (WGA or GM3) signals is reported as mean (±SEM) Pearson’s coefficient and was analyzed using Image J of at least 3 fields of view and 30–45 cells per condition.
Virus Capture & Transfer Assays
Mature DCs or THP1/CD169 cells (1×105 cells) were challenged with indicated doses of GFP-containing VLPs or infectious viruses for 2 hours at 37°C. In some experiments, cells were pre-incubated with anti-CD169 blocking antibody (10 μg/ml, Clone 7D2, Novus Biologicals) for 20 min at room temperature prior to virus exposure. Cells were thoroughly washed, fixed with 4% PFA and analyzed via flow cytometry (BD Calibur). To determine the extent of virus transfer to CD4+ T cells, mature DCs (2×105) were incubated with GFP-expressing single cycle of replication viruses (MOI=5) for 2 hours at 37°C, washed thoroughly and then autologous CD4+ T cells (4×105) were added and co-cultured for six days. Alternatively, activated CD4+ T cells were infected with GFP-expressing single-cycle of replication competent viruses (MOI =5) by spinoculation (cell-free infection). Infected cultures were stained with PE-conjugated α-CD3 monoclonal antibody (BD), and the number of GFP+ CD3+ T cells was determined by FACS performed on LSRII (BD) and data analyzed with FlowJo software (FlowJo).
Lipid measurements
The GM3 ELISA was adapted from previous lipid extraction (Bligh and Dyer, 1959) and GM3 detection protocols (Daino et al., 1999; Tai et al., 1984). Briefly, lipids from equal amounts (ng GFP) of Gag-eGFP VLPs (total volume 40ul) were extracted with chloroform: methanol (1:2) mixture (150ul). Subsequently, chloroform (50ul) and water (50ul) were sequentially added with vortexing in a glass tube (Pyrex), and were centrifuged for five minutes at 1500rpm. The non-aqueous (bottom) phase containing chloroform was discarded. The aqueous phase containing lipids was mixed with additional 100ul of methanol and added to an ELISA plate (Thermo-Scientific #442404) and allowed to dry overnight. Soluble GM3 (Avanti) standards were added in parallel. The wells were blocked with PBS/5% nonfat milk for 2 hours at room temperature followed by addition of α-GM3 monoclonal antibody (DH2 hybridoma supernatant, gracious gift of Dr. Sen-Itiroh Hakomori (Dohi et al., 1988) for one hour at 37°C. The plates were washed four times with PBS followed by addition of HRP-conjugated goat anti-mouse IgG antibody (Sigma). Finally, the plates were readout using TMB (KPL) as a substrate and a micro plate reader.
GM1 and PS Quantification by Plasmon Coupling
GM1 and PS quantification by plasmon coupling followed a recently described procedure (Feizpour et al., 2015). Briefly, biotinylated gold nanoparticles (NPs) were targeted to the lipid of interest on HIV-1 and HIV-2 VLPs functionalized with either biotinylated Annexin V (in case of PS, Abcam) or cholera toxin B (in case of GM1, Life Technologies) using Neutravidin (Thermo Fisher Scientific) as a linker. The spectral response of the VLP-bound gold NPs depends on the label density. After calibrating the spectral response with liposomes of known target lipid concentration, the measured spectral response provides a quantification of target lipids in individual VLPs. 50–300 VLPs were evaluated per condition.
Statistical Analysis
Statistical significance was determined by Student’s two-tailed t test or non-parametric Friedman test, and significant P values (≤0.05) are indicated on the figures.
Results
Attenuated Capture of HIV-2 Gag-eGFP VLPs by CD169
CD169 or Siglec 1, a myeloid cell-specific protein, captures HIV-1 and facilitates mature DC-mediated HIV-1 trans infection of CD4+ T cells (Izquierdo-Useros et al., 2012b; Puryear et al., 2013). We hypothesized that lower HIV-2 viral loads in vivo (Arien et al., 2005; Blaak et al., 2006; MacNeil et al., 2007) might be attributed to inefficient access to the CD169-dependent mature DC-mediated trans infection pathway. We have previously described a Gag-eGFP VLP based virus capture assay (Hatch et al., 2009; Izquierdo-Useros et al., 2009) to determine that CD169 recognizes sialylated-GSLs, such as GM1 and GM3, in the virus particle membrane (Izquierdo-Useros et al., 2012a; Izquierdo-Useros et al., 2012b; Puryear et al., 2013; Puryear et al., 2012). To determine if HIV-2 particles are also captured by CD169, and if HIV-2 capture by CD169 is also dependent on sialylated GSLs, mature DCs were challenged with fluorescent unmodified or GSL-depleted HIV-2 Gag-eGFP VLPs. Virus particle inputs for the different VLP preparations were normalized using a quantitative western blot analysis for GFP content (Figure 1A). We observed a significant decrease in the capture of HIV-2 VLPs by mature DCs compared to capture of HIV-1 Gag-eGFP VLPs (Figure 1B). Depletion of GSLs, the ligand for CD169, from both HIV-1 and HIV-2 Gag-eGFP VLPs (PDMP treatment of virus producer cells) resulted in a further decrease in VLP capture suggesting that capture of both HIV-1 and HIV-2 VLPs by mature DCs is dependent on GSLs (Figure 1B). Furthermore, capture of HIV-2 Gag-eGFP VLPs by THP/CD169 cells was significantly lower than that observed with HIV-1 Gag-eGFP VLPs (Figure 1C), suggesting a diminished ability of CD169 to recognize HIV-2 Gag-eGFP VLPs.
Figure 1.
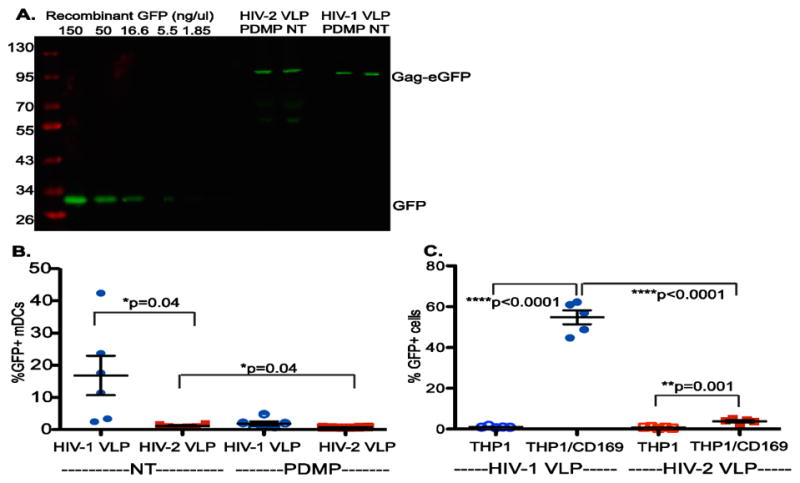
Attenuated Capture of HIV-2 VLPs by CD169. (A) Representative quantitative western blot analysis of VLP lysates for measuring GFP content of virions. Standard curve of recombinant GFP (included in each western blot) was used to quantify the GFP content in HIV-2 Gag-eGFP and HIV-1 Gag-eGFP VLPs. PDMP treatment of HEK293T cells was used to deplete GSLs in VLPs. NT=non-treated (B) Quantitative FACS analysis of VLP capture by mature DCs. Mature DCs were challenged with 10ng GFP containing HIV-1 or HIV-2 VLPs (NT = non-treated or PDMP = GSL depleted) at 37°C for 2 hr. The data from six independent experiments with cells derived from six independent donors (each symbol) is shown as means ± SEM (horizontal lines). *p=0.04 (C) Attenuated capture of HIV-2 VLPs by THP-1/CD169. THP-1 cells or THP1 cells constitutively expressing CD169 (THP1/CD169) were challenged with 10ng GFP containing HIV-1 or HIV-2 VLPs at 37°C for 2 hr, washed, fixed and quantified via flow cytometry. The data from 5 independent experiments (each symbol) is shown as means ±SEM (horizontal lines). ****p<0.0001; **p=0.001.
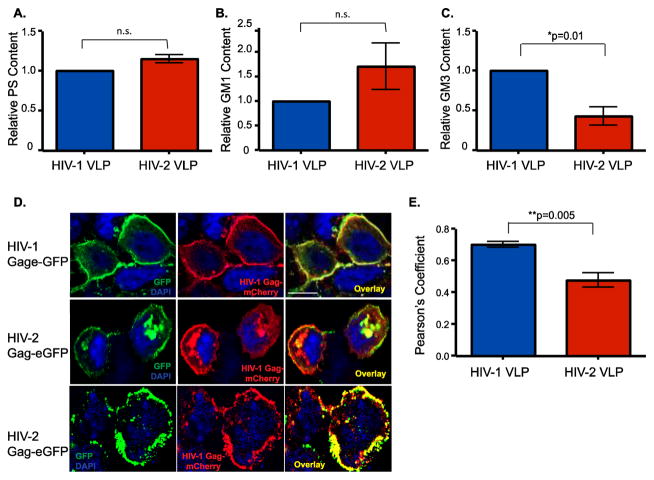
To determine if the significantly reduced capture of HIV-2 VLPs by mature DCs and THP1/CD169 cells is attributed to reduced levels of GSL incorporation by HIV-2 virions, we quantified the lipidomic contents of HIV-1 and HIV-2 Gag-eGFP VLPs. We employed both a multi-spectral plasmonic nanocoupling analysis to measure phosphatidylserine (PS) and GM1 levels (Feizpour et al., 2015) and ELISA to measure GM3 incorporation in VLP membranes. While both HIV-1 and HIV-2 Gag-eGFP VLPs contained similar levels of PS (Figure 2A) and GM1 (Figure 2B), there was a 2–3-fold decrease in GM3 content in HIV-2 Gag-eGFP VLPs (Figure 2C).
Figure 2.
Decreased Incorporation of GM3 in HIV-2 Gag-eGFP VLPs. Phosphatidylserine (PS) (A) and GM1 (B) content of HIV-1 and HIV-2 Gag-eGFP VLPs was determined by multi-spectral plasmonic nanocoupling analysis; n.s. = not significant (C) GM3 content in HIV-1 and HIV-2 Gag-eGFP VLPs was determined by an ELISA. Values for HIV-2 Gag-eGFP VLPs were normalized to those observed with HIV-1 Gag-eGFP VLPs (set as 1) and is the mean of 3 (for PS and GM1) and 4 (GM3) independent measurements ± SEM. *p=0.01. (D) HEK293T cells were co-transfected on glass cover slips with HIV-1 Gag-eGFP or HIV-2 Gag-eGFP expression plasmids with HIV-1 Gag-mCherry expression plasmid. Cells were counterstained with DAPI to visualize nuclei. The left panel shows expression of the HIV-1 or HIV-2 Gag-eGFP, middle panel shows the HIV-1 Gag-mCherry expression and the right panel is the overlay. (E) Co-localization between green (Gag-eGFP) and red (Gag-mCherry) is reported as mean Pearson’s coefficient of co-localization ± SEM. **p=0.005.
HIV-1 Gag defines the virus particle assembly site and hence determines specificity of incorporation of host-determinants such as GSLs (Akiyama et al., 2014). To determine if HIV-1 and HIV-2 assemble at divergent membrane locations, HEK293T cells were co-transfected with HIV-1 Gag-mCherry and HIV-1 or HIV-2 Gag-eGFP expression plasmids and processed for confocal microscopy. As expected, there was extensive co-localization between HIV-1 Gag-eGFP and HIV-1 Gag-mCherry fusion proteins at the plasma membrane (Figure 2D and E). Interestingly, HIV-2 Gag-eGFP was localized at both the plasma membrane and intracellular sites (Figure 2D, middle and bottom rows), and co-localization between HIV-2 Gag-eGFP and HIV-1 Gag-mCherry at both these sites was infrequently observed (Figure 2D and E), suggesting that altered choice of HIV-2 Gag-directed virus assembly site might account for the reduced GM3 incorporation in HIV-2 VLPs.
Interactions of HIV-2iGFP Virions with Mature DCs and THP1/CD169 Cells are Attenuated
To determine if infectious HIV-2 particles are also deficient for capture by CD169, we constructed a proviral plasmid that encodes GFP between MA and CA and flanked by protease cleavage sites (HIV-2-iGFP), such that upon virus maturation GFP is cleaved out and remains associated as a fluid phase fluorescent marker of virions, similar to the previously described HIV-1-iGFP virus (Hubner et al., 2007) (Figure 3A). GFP was incorporated in both HIV-1 and HIV-2 virions and was cleaved by the viral protease (Figure 3B). While HIV-2-iGFP virions incorporated Env to similar levels as HIV-1iGFP (Figure 3B), infectivity of HIV-2-iGFP was attenuated compared to HIV-2/ROD (Figure 3C), similar to what has been previously reported with HIV-iGFP (Hubner et al., 2007) (Figure 3C). To determine the site of Gag localization, HIV-1iGFP or HIV-2iGFP transfected HEK293T cells were stained with wheat germ agglutinin (WGA-AF594), which binds sialic acid and N-acetylglucosamine residues in the plasma membrane, or GM3 and processed for laser scanning confocal microscopy. Interestingly, while GFP expression in HIV-1iGFP transfected cells was primarily co-localized at the plasma membrane with WGA (Figure 3D and E), and GM3 (Figure 3F and G), intracytoplasmic GFP puncta were clearly visible in HIV-2iGFP transfected cells and co-localized poorly with WGA (Figure 3D and E) and GM3 (Figure 3F and G) at the plasma membrane.
Figure 3.
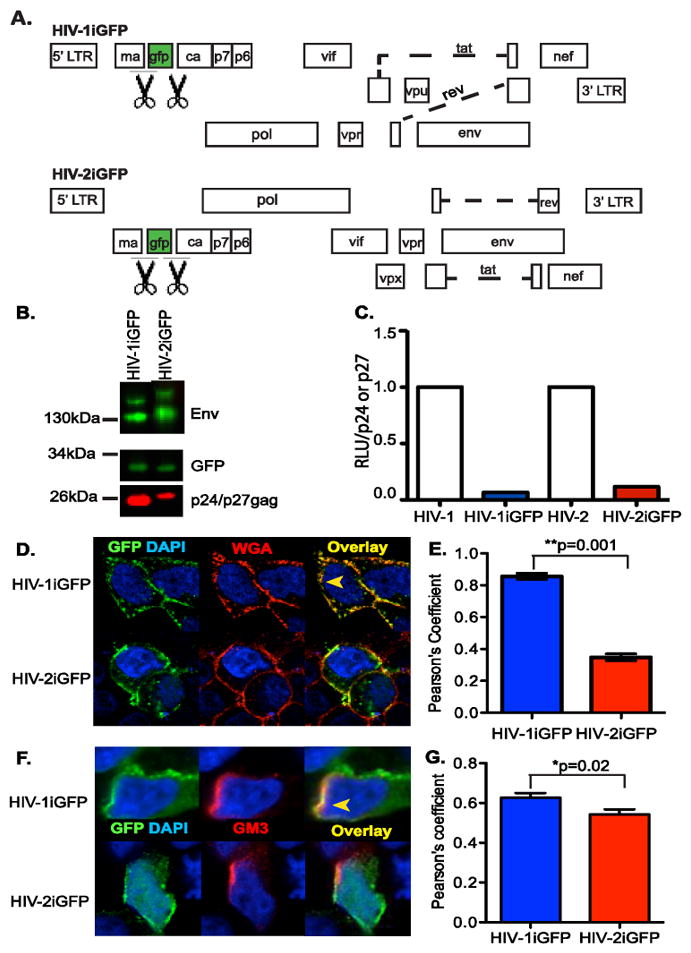
Characterization of HIV-2iGFP. (A) Genome organization of the HIV-1 and HIV-2 proviral plasmids used in this study, which encode GFP between MA and CA, flanked by protease cleavage sites (indicated by the scissors symbol), such that infectious virus particles contain GFP. (B) Representative western blot analysis for Env, GFP, and p24gag or p27gag in virus particle lysates. (C) TZM-bl cells were infected with virus particles (HIV-1/Lai-Bal, HIV-1iGFP, HIV-2/ROD9 or HIV-2iGFP), washed, and cultured for 2 days. Cells were lysed 2 days post infection and the luciferase activity (RLU =relative light units) per p24gag or p27gag measured via TZM-bl infections and AG3.0 ELISA were determined and the values for the iGFP proviruses normalized to that observed with isogenic viruses lacking GFP. The data shown is from a representative experiment (out of 2 independent experiments) performed in triplicate (D) HEK293T cells were transfected with either HIV-1iGFP or HIV-2iGFP plasmid and stained with AlexaFluor 594-conjugated Wheat Germ Agluttinin (WGA-AF594) and counterstained with DAPI, one day post-transfection. (F) HEK293T cells were transfected with either HIV-1iGFP or HIV-2iGFP plasmid and stained with anti-GM3 and AlexaFluor 594-conjugated goat anti-mouse antibodies and counterstained with DAPI, one day post-transfection. The yellow arrowhead in panels D and F indicates an area of co-localization while a white arrowhead indicates lack of co-localization between GFP and WGA (D) or GM3 (F) staining. (E and G) Co-localization between green (GFP) and red (WGA or GM3) is reported as mean (±SEM) Pearson’s coefficient of co-localization. **p=0.001; *p=0.02.
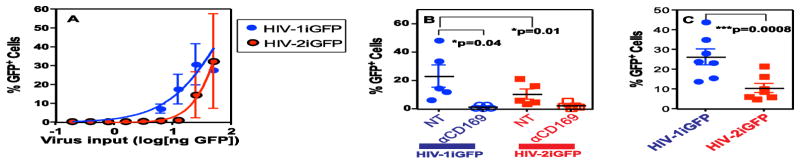
GFP content of HIV-1iGFP and HIV-2iGFP virions derived from transient transfections of HEK293T cells was determined using a quantitative western blot analysis. Virus particles containing equal amounts of GFP were used in virus capture experiments with mature DCs. These viruses allowed us to employ a FACS-dependent measure of capture of fluorescent virus particles by cells. Although capture of both HIV-1iGFP and HIV-2iGFP by mature DCs increased in a dose-dependent manner (Figure 4A), capture of HIV-2iGFP by mature DCs was consistently lower than that observed with HIV-1iGFP especially, at lower viral inputs (Figure 4A). We performed virus capture studies with both mature DCs and THP1/CD169 cells with a single virus input (10 ng or 8.3 ng of GFP equivalent, respectively). Though the extent of virus capture varied amongst mature DCs derived from multiple independent donors, the differences in virus capture (2-fold) between HIV-1iGFP and HIV-2iGFP were statistically significant (p=0.01; Figure 4B). Additionally, there was a 2.5-fold decrease (p=0.0008) in capture of HIV-2iGFP relative to HIV-1iGFP by THP1/CD169 cells (Figure 4C). Furthermore, capture of both HIV-1 iGFP and HIV-2 iGFP virus particles was inhibited upon blocking CD169 by anti-CD169 blocking antibodies, though inhibition of HIV-2 iGFP capture by anti-CD169 antibody was not statistically significant (Figure 3B). These findings suggest that CD169-dependent capture of HIV-2 by mature DCs is attenuated.
Figure 4.
Capture of HIV-2iGFP Virions by Mature DCs is Attenuated. (A) Mature DCs were challenged with increasing amounts (ng of GFP) of HIV-1iGFP (blue) or HIV-2iGFP (red) virions at 37°C for 2 hours, washed, fixed and the percentage of GFP+ cells quantified using flow cytometry. The data shown is the mean ± SEM from 3 independent donors. Nonlinear regression was used to estimate a fitted curve in GraphPad Prism 5 (B) Mature DCs were challenged with 10 ng of GFP containing HIV-1iGFP or HIV-2iGFP virus particles in the absence (NT) or presence of anti-CD169 monoclonal antibody (10 μg/ml) at 37°C for 2 hours, washed, fixed and the percentage of GFP+ cells quantified using flow cytometry. (C) THP1/CD169 cells were challenged with 8.3 ng of GFP containing HIV-1iGFP or HIV-2iGFP virions at 37°C for 2 hours, washed, fixed and the percentage of GFP+ cells quantified using flow cytometry. The data shown in panels B and C is the mean percentage of GFP positive cells ± SEM of 5 or 7 independent experiments, respectively; *p=0.04. ***p=0.0008.
Reduced Access of HIV-2 to the Mature-DC Mediated Trans Infection Pathway
We next asked whether reduced capture of HIV-2 by CD169 results in decreased access to the mature DC-mediated trans infection pathway of CD4+ T cells. Mature DCs were challenged with replication competent HIV-1 (Lai-YU2) or HIV-2 (CDC77618) virus particles, washed and cultured alone or co-cultured with autologous CD4+ T cells. Cell-free supernatants on day six post-infection were added to TZM-bl cells to measure the amount of virus produced by the co-cultures compared to virus produced by mature DCs or CD4+ T cells alone. Note that CD4+ T cells were infected to a similar extent by HIV-1 and HIV-2 (Figure 5B). While infecting mature DCs with either HIV-1/Lai-YU2 or HIV-2/CDC77618 and culturing alone in the absence of CD4+ T cells did not result in production of infectious virus (data not shown), infections of mature DC – T cell co-cultures resulted in robust production of infectious HIV-1 virions (Figure 5A). Surprisingly, production of infectious HIV-2 virions was dramatically attenuated in mature DC-T cell co-cultures (~100-fold reduction compared to HIV-1) (Figure 5A). These findings suggest that HIV-2 is less efficiently transferred by mature DCs to autologous CD4+ T cells.
Figure 5.
Access of HIV-2 to Mature DC-Mediated Trans Infection Pathway is Diminished. Mature DCs (A) or activated CD4+ T cells (B) were pulsed with either HIV-1 (Lai-YU2) or HIV-2 (CDC77618) at an MOI=0.1. Virus-exposed mature DCs were washed to remove unbound virus and co-cultured with autologous CD4+ T cells. Mature DC-T cell co-culture supernatants or CD4+ T cell supernatants were harvested on day 6 post-infection and added to TZM-bl cells. The data shown is luciferase activity (RLU) in TZM-Bl cell lysates harvested 48 hr post initiation of infection and is the mean ±SEM from 4 experiments derived from cells from 4 independent donors. (C) Mature DCs were infected with GFP expressing single cycle replication competent viruses (pseudotyped with HIV-1/BaL Env or HIV-2 ST Env) at MOI=5. Virus-exposed mDCs were washed and cultured with autologous CD4+ T cells. Additionally, CD4+ T cells were infected directly (cell-free infection) in parallel. The percentage of GFP positive CD4+ T cells was quantified via flow cytometry. The data from 6 independent infections with cells from 6 donors is shown (mean ±SEM). *p=0.01
Next we used GFP expressing single-cycle viruses to determine if the differences in HIV-1 and HIV-2 virus replication in mature DC – CD4+ T cell co-cultures can be attributed to the inability of HIV-2 to access the mature DC-mediated trans infection pathway. Mature DCs were challenged with GFP-expressing (upon establishment of infection) single-cycle HIV-1 or HIV-2, pseudotyped with either HIV-1 Bal Env or HIV-2 ST Env. There was no difference in the level of cell-free infection of CD4+ T cells by Bal-Env pseudotyped HIV-1/GFP or ST-Env pseudotyped HIV-2/GFP (Figure 5C). However, transfer of ST-Env pseudotyped HIV-2/GFP by mature DCs was 4.5-fold less efficient than transfer of Bal-Env pseudotyped HIV-1/GFP (Figure 5C). These findings suggest that inefficient access of HIV-2 to the CD169-dependent mature DC mediated trans infection pathway results in reduced virus transmission to autologous CD4+ T cells and consequently less virus spread.
Discussion
In this study, we investigated the efficiency of mature DCs to transmit captured HIV-1 and HIV-2 particles to autologous CD4+ T cells. Previous studies by others and us have demonstrated that virion incorporation of host-derived ganglioside GM3 is crucial for Env-independent capture and dissemination of HIV-1 by mature DCs (Izquierdo-Useros et al., 2012a; Puryear et al., 2012). However, very little is known about HIV-2 capture and transfer by mature DCs. We constructed a HIV-2 Gag-eGFP expression plasmid and an HIV-2 proviral plasmid that encodes GFP between MA and CA and flanked by protease cleavage sites (HIV-2-iGFP), such that VLPs and infectious virions contain GFP. We were thus able to compare HIV-1 and HIV-2 Gag-eGFP VLP and HIV-iGFP virus capture by a quantitative FACS based assay, and demonstrated that HIV-2 capture by CD169-expressing mature DCs and THP1 cells was decreased compared to HIV-1 (Figures 1 and 4). Interestingly, decreased HIV-2 capture by CD169 could primarily be attributed to reduced incorporation of GM3, but not GM1, in HIV-2 virions (Figure 2). While infectious virus particles derived from PBMCs might have differing amounts of GM3 than those derived from transiently transfected HEK293T cells, HIV-2 replication in mature DC-T cell co-cultures and mature DC-mediated HIV-2 trans infection was substantially attenuated compared to HIV-1 (Figure 5). These findings provide additional confirmation that GM3 is the critical virus particle determinant necessary for optimal interactions of HIV with CD169.
In the lentiviral replication cycle, newly synthesized Gag proteins are targeted to the plasma membrane for assembly and budding of new virions. Our previous studies have suggested that GM3 incorporation in HIV-1 virions is dependent on HIV-1 MA-defined virus assembly sites (Akiyama et al., 2014). MA proteins of HIV-1 and HIV-2 Gag are both co-translationally myristoylated at the N-terminal end, which plays a critical role in virus assembly at the plasma membrane. HIV-1 MA binding to phosphatidylinositol 4,5-biphosphate (PI(4,5)P2), which is enriched at the inner leaflet of plasma membrane microdomains, induces exposure of the myristyl group. This myristyl group conformation change from hidden to exposed is critical for stable plasma membrane association of HIV-1 Gag (Saad et al., 2008). In contrast, the myristyl group in HIV-2 MA is more tightly sequestered and less sensitive to PI(4,5)P2 binding resulting in a lower rate of association of HIV-2 Gag with the plasma membrane (Saad et al., 2008). Additionally, decreased affinity of HIV-2 Gag to model yeast spheroplasts (membranes) has been hypothesized to result in HIV-2 assembly and budding defects from these model membranes (Morikawa et al., 2007). Our confocal microscopy studies of transfected HEK293T cells revealed reduced co-localization of HIV-2 Gag with HIV-1 Gag at plasma membrane microdomains (Figures 2), as well as increased presence of HIV-2 Gag at GM3-deficient intracellular membranes (Figure 3). Since GSLs, such as GM1 and GM3 are enriched at the plasma membrane in distinct microdomains (Fujita et al., 2007), and intracellular membrane composition of lipids is distinct from that of plasma membranes (Klaas et al., 2012), reduced incorporation of GM3, but not GM1, in HIV-2 virions might reflect the heterogeneity in HIV-2 assembly site as observed with HIV-1 MA mutants (Akiyama et al., 2014). Studies are in progress to determine if mutations in HIV-2 MA that alter virus assembly site result in enhanced virion incorporation of GM3 and rescue capture of HIV-2 virions by CD169.
In contrast to reduced capture by CD169, HIV-1 and HIV-2 are equally captured by the DC attachment factors, galactosylceramide (Hammache et al., 1998) and DC-SIGN (Pohlmann et al., 2001). As opposed to CD169 whose expression is induced upon maturation of DCs (Izquierdo-Useros et al., 2012b; Puryear et al., 2013), expression of DC-SIGN is decreased in DCs upon maturation (Geijtenbeek et al., 2000), and neutralization of DC-SIGN function has negligible impact on mature DC-mediated HIV-1 trans infection (Puryear et al., 2013). Thus, we hypothesize that selective deficiency in the ability of HIV-2 to interact with CD169 compromises its ability to access the mature DC trans infection pathway. As opposed to trans infection, HIV-2 virus particle fusion (cis infection) is significantly more promiscuous than HIV-1 (Morner et al., 1999). HIV-2 can utilize multiple co-receptors, such as CCR1, CCR2b, CCR3, orphan receptor BONZO or BOB in addition to CD4, CCR5 and CXCR4, for fusing with target cells (Morner et al., 1999; Owen et al., 1998). Though HIV-2 Vpx can overcome restriction by SAMHD1 (Hrecka et al., 2011; Laguette et al., 2011), LPS-matured DCs present a hostile cellular environment for HIV-2 replication (Pertel et al., 2011) that places additional roadblocks to establishment of productive infection. Whether engagement of CD169-independent attachment factors and chemokine co-receptors by HIV-2 results in fusion and entry within mature DCs remains to be determined, though recent studies have questioned the ability of HIV-2 to successfully fuse and replicate in immature DCs (Chauveau et al., 2015).
We have previously postulated that mature DCs in an inflammatory microenvironment are critical cellular vectors for mediating HIV-1 dissemination by capturing virus, trafficking to lymph nodes and efficiently transferring captured virus to CD4+ T cells (Izquierdo-Useros et al., 2014; Kijewski and Gummuluru, 2015). CD169 is a myeloid cell specific antigen (Crocker et al., 2007) whose expression is positively correlated with viral loads in HIV-1 infected individuals (Rempel et al., 2008; van der Kuyl et al., 2007). For instance, increased expression of CD169 on circulating monocytes has been observed in acutely infected and untreated HIV-1 patients (Pino et al., 2015; Rempel et al., 2008; van der Kuyl et al., 2007) and in pathogenic lentiviral infections of non-human primates (Bosinger et al., 2009; Jaroenpool et al., 2007; Kim et al., 2015). Additionally, CD169 is also constitutively expressed on tissue resident myeloid cells, particularly macrophages in the subcapsular sinus and the marginal zone of secondary lymph nodes (Hartnell, 2001; Kim et al., 2015). Since CD169 is an interferon stimulated gene (Puryear et al., 2013; Rempel et al., 2008), early type I interferon responses to acute lentivirus infection that induce expression of CD169 on peripheral blood monocytes and tissue-resident macrophages, can paradoxically result in enhanced dissemination of HIV-1 (Rempel et al., 2008). Additionally, CD169 expression on DCs can also be induced by TGFβ, the most abundant cytokine found in semen, and hence CD169 may also play a role in in sexual transmission of HIV-1 (De Saint Jean et al., 2014). Thus, the relative inability of HIV-2 to exploit the CD169-dependent trans infection pathway might contribute to the decreased transmission rates and reduced viral loads in HIV-2 infected individuals. These studies highlight the need for the development of therapeutics, which specifically inhibit CD169-dependent HIV-1 dissemination, as additional approaches to curtail the virus pandemic.
Highlights.
Inefficient capture of HIV-2 by CD169
Reduced incorporation of GM3 in HIV-2 virions
HIV-2 has diminished access to the mature DC-mediated trans infection pathway
Acknowledgments
We thank, the BUMC flow cytometry and imaging core facility for technical assistance. We thank the NIH AIDS Research and Reference Reagent Program for providing us with the following reagents: TZM-bl (Catalog # 8129 contributed by Dr. John C. Kappes, Dr. Xiaoyun Wu and Tranzyme Inc), Antiserum to HIV-2ST gp120 (Catalog # 1410 contributed by Dr. Raymond Sweet, SmithKline Beecham Pharmaceuticals), pGag-EGFP (Catalog # 11468 contributed by Marilyn D. Resh and George Pavlakis), HIV-2CDC77618 (Catalog #3932 contributed by Dr. Stefan Wiktor and Dr. Mark Rayfield), HIV-IG (Catalog # 3957 contributed by NABI and NHLBI, Dr. Luiz Barbosa), and recombinant human IL-2 (Catalog # 136 contributed by Dr. Maurice Gately). We thank Dr. Benjamin Chen for the HIV-iGFP proviral clone, Dr. Geoffery Gottlieb for the pROD9 proviral clone, Dr. Paula Cannon for the HIV-2 ST envelope expression plasmid, Dr. Nancy Haigwood for the α-gp120 polyclonal antibody, Dr. Sen-Itiroh Hakamori for the gracious gift of the α-GM3 hybridoma, and Dr. Sanders-Beer for the generous gift of the α-p24gag monoclonal antibody (Clone AG3.0).
Funding
This study was supported by NIH grants R01AI064099 (S.G.) and R01CA138509 (B.M.R.), and Research Training in Immunology T32 AI007309-23 (S.D.G.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Suzanne D. G. Kijewski, Email: sgeer@bu.edu.
Hisashi Akiyama, Email: hakiyama@bu.edu.
Amin Feizpour, Email: feizpour@bu.edu.
Caitlin Miller, Email: cm12@bu.edu.
Nora-Guadalupe P. Ramirez, Email: Nora-guadalupe.Ramirez@utsouthwestern.edu.
Björn M. Reinhard, Email: bmr@bu.edu.
Suryaram Gummuluru, Email: rgummulu@bu.edu.
References
- Akiyama H, Miller C, Patel HV, Hatch SC, Archer J, Ramirez NG, Gummuluru S. Virus particle release from glycosphingolipid-enriched microdomains is essential for dendritic cell-mediated capture and transfer of HIV-1 and henipavirus. J Virol. 2014;88:8813–8825. doi: 10.1128/JVI.00992-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Ramirez NG, Gudheti MV, Gummuluru S. CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies. PLoS Pathog. 2015;11:e1004751. doi: 10.1371/journal.ppat.1004751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arien KK, Abraha A, Quinones-Mateu ME, Kestens L, Vanham G, Arts EJ. The replicative fitness of primary human immunodeficiency virus type 1 (HIV-1) group M, HIV-1 group O, and HIV-2 isolates. J Virol. 2005;79:8979–8990. doi: 10.1128/JVI.79.14.8979-8990.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaak H, van der Ende ME, Boers PH, Schuitemaker H, Osterhaus AD. In vitro replication capacity of HIV-2 variants from long-term aviremic individuals. Virology. 2006;353:144–154. doi: 10.1016/j.virol.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol. 1959;37:7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, Zeng M, Masopust D, Carlis JV, Ran L, Vanderford TH, Paiardini M, Isett RB, Baldwin DA, Else JG, Staprans SI, Silvestri G, Haase AT, Kelvin DJ. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauveau L, Puigdomenech I, Ayinde D, Roesch F, Porrot F, Bruni D, Visseaux B, Descamps D, Schwartz O. HIV-2 infects resting CD4+ T cells but not monocyte-derived dendritic cells. Retrovirology. 2015;12:2. doi: 10.1186/s12977-014-0131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker PR, Paulson JC, Varki A. Siglecs and their role in the immune system. Nat Rev Immunol. 2007;7:12. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- Daino T, Tsuchihashi K, Kashiwagi M, Yachida Y, Akino T, Gasa S. Antigenicity of the carbohydrate moiety of gangliosdie GM3 having 3-O-acetyl ceramid. Glycoconj J. 1999;16:5. doi: 10.1023/a:1006949719565. [DOI] [PubMed] [Google Scholar]
- De Saint Jean A, Lucht F, Bourlet T, Delezay O. Transforming growth factor beta 1 up-regulates CD169 (sialoadhesin) expression on monocyte-derived dendritic cells: role in HIV sexual transmission. Aids. 2014;28:2375–2380. doi: 10.1097/QAD.0000000000000431. [DOI] [PubMed] [Google Scholar]
- de Silva TI, Cotten M, Rowland-Jones SL. HIV-2: the forgotten AIDS virus. Trends Microbiol. 2008;16:588–595. doi: 10.1016/j.tim.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Dohi T, GN, Hakomori S. An IgG3 Monoclonal Antibody Established after Immunization with GM3 Lactone: Immunochemical Specificity and Inhibition of Melanoma Cell Growth in Virtro and in Vivo. Cancer Res. 1988;48:6. [PubMed] [Google Scholar]
- Duvall MG, Lore K, Blaak H, Ambrozak DA, Adams WC, Santos K, Geldmacher C, Mascola JR, McMichael AJ, Jaye A, Whittle HC, Rowland-Jones SL, Koup RA. Dendritic cells are less susceptible to human immunodeficiency virus type 2 (HIV-2) infection than to HIV-1 infection. J Virol. 2007;81:13486–13498. doi: 10.1128/JVI.00976-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizpour A, Yu X, Akiyama H, Miller CM, Edmans E, Gummuluru S, Reinhard BM. Quantifying lipid contents in enveloped virus particles with plasmonic nanoparticles. Small. 2015;11:1592–1602. doi: 10.1002/smll.201402184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Cheng J, Hirakawa M, Furukawa K, Kusunoki S, Fujimoto T. Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Molecular biology of the cell. 2007;18:2112–2122. doi: 10.1091/mbc.E07-01-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek T, Kwon D, Torensma R, van Vliet S, van Duijnhoven G, Middel J, Cornelissen I, Nottet H, KewalRamani V, Littman D, Figdor C, van Kooyk Y. DC-SIGN, a Dendritic Cell-Specific HIV-1-Binding Protein that Enhances trans-Infection of T cells. Cell. 2000;100:11. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Hammache D, Pieroni G, Yahi N, Delezay O, Koch N, Lafont H, Tamalet C, Fantini J. Specific Interaction of HIV-1 and HIV-2 Surface Envelope Glycoproteins with Monolayers of Galactosylceramid and Ganglioside GM3. J Biol Chem. 1998;273:5. doi: 10.1074/jbc.273.14.7967. [DOI] [PubMed] [Google Scholar]
- Hartnell A. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. 2001;97:288–296. doi: 10.1182/blood.v97.1.288. [DOI] [PubMed] [Google Scholar]
- Hatch S, Archer J, Gummuluru S. Glycosphingolipid composition of human immunodeficiency virus type 1 (HIV-1) particles is a crucial determinant for dendritic cell-mediated HIV-1 trans-infection. J Virol. 2009;83:3496–3506. doi: 10.1128/JVI.02249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H, Lopez LA, Yang SJ, Oldenburg JE, Exline CM, Guatelli JC, Cannon PM. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology. 2010;7:51. doi: 10.1186/1742-4690-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner W, Chen P, Del Portillo A, Liu Y, Gordon RE, Chen BK. Sequence of human immunodeficiency virus type 1 (HIV-1) Gag localization and oligomerization monitored with live confocal imaging of a replication-competent, fluorescently tagged HIV-1. J Virol. 2007;81:12596–12607. doi: 10.1128/JVI.01088-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Useros N, Lorizate M, Contreras FX, Rodriguez-Plata MT, Glass B, Erkizia I, Prado JG, Casas J, Fabrias G, Krausslich HG, Martinez-Picado J. Sialyllactose in viral membrane gangliosides is a novel molecular recognition pattern for mature dendritic cell capture of HIV-1. PLoS Biol. 2012a;10:e1001315. doi: 10.1371/journal.pbio.1001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Useros N, Lorizate M, McLaren PJ, Telenti A, Krausslich HG, Martinez-Picado J. HIV-1 capture and transmission by dendritic cells: the role of viral glycolipids and the cellular receptor Siglec-1. PLoS Pathog. 2014;10:e1004146. doi: 10.1371/journal.ppat.1004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, Pino M, Erkizia I, Glass B, Clotet B, Keppler OT, Telenti A, Krausslich HG, Martinez-Picado J. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012b;10:e1001448. doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Useros N, Naranjo-Gomez M, Archer J, Hatch S, Erkizia I, Blanco J, Borras F, Puertas M, Connor J, Fernandez-Figueras M, Moore L, Clotet B, Gummuluru S, Martinez-Picado J. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113:10. doi: 10.1182/blood-2008-05-158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, Iwasaki A. Expression of DC-SIGN by Dendritic Cells of Intestinal and Genital Mucosae in Humans and Rhesus Macaques. J Virol. 2002;76:1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaroenpool J, Rogers KA, Pattanapanyasat K, Villinger F, Onlamoon N, Crocker PR, Ansari AA. Differences in the constitutive and SIV infection induced expression of Siglecs by hematopoietic cells from non-human primates. Cellular immunology. 2007;250:91–104. doi: 10.1016/j.cellimm.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijewski SDG, Gummuluru S. A mechanistic overview of dendritic cell-mediated HIV-1 trans infection: the story so far. Future Virology. 2015;10:14. doi: 10.2217/fvl.15.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WK, McGary CM, Holder GE, Filipowicz AR, Kim MM, Beydoun HA, Cai Y, Liu X, Sugimoto C, Kuroda MJ. Increased Expression of CD169 on Blood Monocytes and Its Regulation by Virus and CD8 T Cells in Macaque Models of HIV Infection and AIDS. AIDS Res Hum Retroviruses. 2015 doi: 10.1089/aid.2015.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaas M, Oetke C, Lewis LE, Erwig LP, Heikema AP, Easton A, Willison HJ, Crocker PR. Sialoadhesin promotes rapid proinflammatory and type I IFN responses to a sialylated pathogen, Campylobacter jejuni. J Immunol. 2012;189:2414–2422. doi: 10.4049/jimmunol.1200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemey P, Pybus OG, Wang B, Saksena NK, Salemi M, Vandamme AM. Tracing the origin and history of the HIV-2 epidemic. Proc Natl Acad Sci U S A. 2003;100:6588–6592. doi: 10.1073/pnas.0936469100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil A, Sarr AD, Sankale JL, Meloni ST, Mboup S, Kanki P. Direct evidence of lower viral replication rates in vivo in human immunodeficiency virus type 2 (HIV-2) infection than in HIV-1 infection. J Virol. 2007;81:5325–5330. doi: 10.1128/JVI.02625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y, Goto T, Yasuoka D, Momose F, Matano T. Defect of human immunodeficiency virus type 2 Gag assembly in Saccharomyces cerevisiae. J Virol. 2007;81:9911–9921. doi: 10.1128/JVI.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morner A, Bjorndal A, Albert J, Kewelramani V, Littman D, Inoue R, Thorstensson R, Fenyo E, Bjorling E. Primary Human Immunodeficiency Virus Type 2 (HIV-2) Isolates, Like HIV-1 Isolates, Frequently Use CCR5 but Show Promiscuity in Coreceptor Usage. J Virol. 1999;73:7. doi: 10.1128/jvi.73.3.2343-2349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SM, Ellenberger D, Rayfield M, MP, Hahn BH, Lal RB. Genetically divergent strains of human immunodeficiency type 2 use multiple corecptors for viral entry. J Virol. 1998;72:8. doi: 10.1128/jvi.72.7.5425-5432.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertel T, Reinhard C, Luban J. Vpx rescues HIV-1 transduction of dendritic cells from the antiviral state established by type 1 interferon. Retrovirology. 2011;8:49. doi: 10.1186/1742-4690-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino M, Erkizia I, Benet S, Erikson E, Fernandez-Figueras MT, Guerrero D, Dalmau J, Ouchi D, Rausell A, Ciuffi A, Keppler OT, Telenti A, Krausslich HG, Martinez-Picado J, Izquierdo-Useros N. HIV-1 immune activation induces Siglec-1 expression and enhances viral trans-infection in blood and tissue myeloid cells. Retrovirology. 2015;12:37. doi: 10.1186/s12977-015-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann S, Baribaud F, Lee B, Leslie GJ, Sanchez MD, Hiebenthal-Millow K, Munch J, Kirchhoff F, Doms RW. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J Virol. 2001;75:4664–4672. doi: 10.1128/JVI.75.10.4664-4672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puryear W, Akiyama H, Geer S, Ramirez N, Yu X, Reinhard B, Gummuluru S. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog. 2013;9:e1003291. doi: 10.1371/journal.ppat.1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puryear W, Yu X, Ramirez N, Reinhard B, Gummuluru S. HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells. Proc Natl Acad Sci U S A. 2012;109:7475–7480. doi: 10.1073/pnas.1201104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel H, Calosing C, Sun B, Pulliam L. Sialoadhesin Expressed on IFN-Induced Monocytes Binds HIV-1 and Enhances Infectivity. PloS one. 2008;3:9. doi: 10.1371/journal.pone.0001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad JS, Ablan SD, Ghanam RH, Kim A, Andrews K, Nagashima K, Soheilian F, Freed EO, Summers MF. Structure of the myristylated human immunodeficiency virus type 2 matrix protein and the role of phosphatidylinositol-(4,5)-bisphosphate in membrane targeting. Journal of molecular biology. 2008;382:434–447. doi: 10.1016/j.jmb.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders-Beer BE, Eschricht M, Seifried J, Hirsch VM, Allan JS, Norley S. Characterization of a monoclonal anti-capsid antibody that cross-reacts with three major primate lentivirus lineages. Virology. 2012;422:402–412. doi: 10.1016/j.virol.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P, Bailes E, Chaudhuri R, Rodenburg C, Santiago M, Hahn B. The origins of acquired immune deficiency syndrome viruses: where and when? Phil Trans R Soc Lond. 2001;356:10. doi: 10.1098/rstb.2001.0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Gottlieb GS, Anderson DJ, Pyrak CL, Preston BD. Human immunodeficiency virus types 1 and 2 exhibit comparable sensitivities to Zidovudine and other nucleoside analog inhibitors in vitro. Antimicrob Agents Chemother. 2008;52:329–332. doi: 10.1128/AAC.01004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T, Cahan LD, Paulson JC, Saxton RE, Irie RF. Human Monoclonal Antibody Against Ganglioside GD2: Use in Development of Enzyme-Linked Immunosorbent Assay for the Monitoring of Anti-GD2 in Cancer Patients. J Natl Cancer Inst. 1984;73:7. [PubMed] [Google Scholar]
- van der Kuyl AC, van den Burg R, Zorgdrager F, Groot F, Berkhout B, Cornelissen M. Sialoadhesin (CD169) expression in CD14+ cells is upregulated early after HIV-1 infection and increases during disease progression. PloS one. 2007;2:e257. doi: 10.1371/journal.pone.0000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, KewalRamani V. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:10. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Emerman M. Capsid is a dominant determinant of retrovirus infectivity in nondividing cells. J Virol. 2004;78:5670–5678. doi: 10.1128/JVI.78.11.5670-5678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]