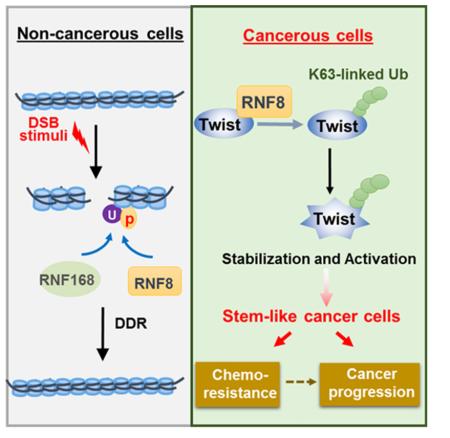
Abstract
Twist has been shown to cause treatment failure, cancer progression and cancer-related death. However, strategies that directly target Twist are not yet conceivable. Here we revealed K63-linked ubiquitination is a crucial regulatory mechanism for Twist activation. Through an E3 ligase screen and biochemical studies, we unexpectedly identified that RNF8 functions as a direct Twist activator by triggering K63-linked ubiquitination of Twist. RNF8-promoted Twist ubiquitination is required for Twist localization to the nucleus for subsequent EMT and CSC functions, thereby conferring chemoresistance. Our histological analyses showed that RNF8 expression is upregulated and correlated with disease progression, EMT features and poor patient survival in breast cancer. Moreover, RNF8 regulates cancer cell migration and invasion and cancer metastasis, recapitulating the effect of Twist. Together, our findings reveal a previously unrecognized tumor-promoting function of RNF8 and provide the first evidence that targeting RNF8 is an appealing strategy to tackle tumor aggressiveness and treatment resistance.
Graphical abstract
Introduction
The emergence of adjuvant and neoadjuvant chemotherapy has substantially improved short-term cancer outcomes, including reduced tumor burden and a better five-year survival rate. In contrast, progress toward dramatic improvements in long-term survival and tumor eradication, particularly for metastatic patients, remains disappointing (Holohan et al., 2013). The epithelial-to-mesenchymal transition (EMT) process is central to the reprogramming of differentiated epithelial cells to a mesenchymal-like phenotype (Thiery et al., 2009). This process and the underlying transcription control factors, so-called EMT-TFs, that mediate it have also been linked to the acquisition of cancer stem cell properties (Mani et al., 2008; Puisieux et al., 2014; Ye and Weinberg, 2015). Cancer stem cells (CSCs) are intrinsically quiescent and highly resistant to chemotherapy (Singh and Settleman, 2010). CSCs are pluripotent with self-renewing capacity that allows a few tumor cells, including those residual after treatment, to give rise to numerous, differentiated progeny. This cellular plasticity is thought to explain, in large part, the short durability of current treatments in advanced cancer patients and cause of death (Ye and Weinberg, 2015). In light of the significance of EMT and CSCs in cancer progression and treatment failure, there is a clear need to better understand the biological processes that give rise to and maintain treatment-resistant tumor cells.
Triple negative breast cancer (TNBC) is the most aggressive subtype of breast cancer that exhibits overall poor outcome to current dose-dense chemotherapy regimens (Andre and Zielinski, 2012; Dent et al., 2007). Studies of TNBC cells and tissues have shown that TNBCs commonly exhibit an activated EMT program and an enriched CSC population (Lehmann et al., 2011; Perou, 2010). Furthermore, a recent single-cell analysis of patient-derived tumor xenografts identified that the CSCs accounting for TNBC metastasis harbor an EMT gene signature including Twist upregulation (Lawson et al., 2015). Expression of Twist is repressed in normal adult tissues but is upregulated in TNBC and high-grade breast cancer (Lawson et al., 2015; Yang et al., 2004). Twist, as a key EMT-TF, triggers EMT, CSC self-renewal and metastasis in breast cancer (Mani et al., 2008; Yang et al., 2004). Twist overexpression was demonstrated to drive EMT and CSC features and the development of TNBC in xenograft and transgenic mouse models (Morel et al., 2012; Shi et al., 2014). These studies unequivocally emphasize the pathological importance of Twist in TNBC, suggesting that blocking EMT by inhibiting Twist is a compelling approach to target TNBC. However, development of pharmacological agents to specifically target Twist and other EMT-TFs are lagging because of lack of ligand binding domain in them (Surade and Blundell, 2012), creating a need for identification of more druggable targets for EMT-based therapy.
Ubiquitination is a reversible post-translational modification that is essential for regulation of protein stability, function and subcellular localization (Chan et al., 2014; Mattiroli and Sixma, 2014). The versatility of ubiquitination stems from its ability to form topologically different ubiquitin chains. The best characterized and most abundant form of ubiquitination in cells is the ubiquitin chain linked via its lysine (K) residue at amino acid 48 (Phu et al., 2011): a modification that targets protein substrates for proteasomal degradation (Mattiroli and Sixma, 2014). In contrast, the second most abundant K63-linked ubiquitination, has recently been demonstrated to selectively activate protein functions and signaling pathways by creating a molecular platform for protein/protein interactions (Chan et al., 2012; Hoeller and Dikic, 2009). As a consequence, increased K63-linked ubiquitination hyper-activates oncogenic signaling and tumor progression (Chan et al., 2012; Hoeller and Dikic, 2009). Among the ubiquitination machinery, E3 ligases recognize and select the protein substrates for ubiquitination and thus impart specificity to this process (Berndsen and Wolberger, 2014; Cohen and Tcherpakov, 2010). The fact that these ubiquitination-controlling enzymes are deregulated in diseases, including cancer, has made them attractive as potential drug targets (Chan et al., 2013; Hoeller and Dikic, 2009; Skaar et al., 2014).
RNF8 is a RING-finger E3 ligase that has been best characterized for its involvement in DNA damage repair and telomere end protection (Bennett and Harper, 2008; Huen et al., 2007). RNF8 has been shown to recruit to sites of DNA double strand breaks (DSBs), where it promotes K63-linked ubiquitination of histones H2A and H2AX. This in turn creates an environment to recruit RNF168 as well as downstream repair factors (e.g., 53BP1 and BRCA1) to the damage (Bennett and Harper, 2008; Huen et al., 2007). Aside from histone substrates, RNF8 stabilizes Tpp1 to increase the formation of the Tpp1–Pot1 heterodimer, a protein complex critical for telomere maintenance and end protection (Rai et al., 2011). Intriguingly, a recent publication discovered that during mitosis, RNF8 is released from the DSB sites for halting DNA repair by CDK-mediated phosphorylation (Orthwein et al., 2014). This report implies that RNF8 exhibits a chromosome-independent function. In the current study, we identified that Twist is an unrevealed, chromosome-independent substrate of RNF8. We found that the DSB-dissociated RNF8 directly activates Twist via K63-linked ubiquitination and consequently promotes EMT, CSCs, chemoresistance and cancer progression. Elucidation of the mechanistic insight into and clinical significance of RNF8 in cancer progression advances current understanding of how cancer cells resist chemotherapy, survive and ultimately metastasize. Such knowledge offers strategies to conquer treatment-resistant and metastatic tumors.
Results
RNF8 identified by an E3 ligase screen is a Twist activator and a commonly upregulated gene in TNBC
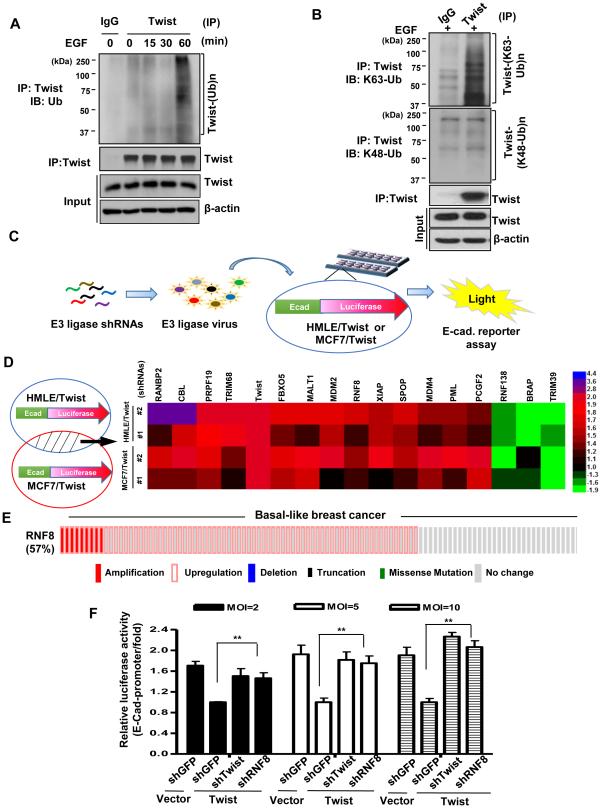
While much has emerged regarding the regulation of Twist gene expression, including induction by a number of pro-oncogenic cues such as growth factors and hypoxia (Thiery et al., 2009; Yang et al., 2008; Zheng and Kang, 2014), less is known about the post-translational regulation of Twist. To determine whether K63-linked ubiquitation controls Twist activity posttranslationally, we first tested if Twist undergoes ubiquitination in BT549 TNBC cells. Indeed, endogenous Twist ubiquitination was drastically induced upon stimulation with epidermal growth factor (EGF) in the absence of proteasome inhibitor MG132 (Figure 1A). Of note, this ubiquitination event did not lead to Twist degradation (Figures 1A and 1B). Using ubiquitin linkage-specific antibodies, we found that EGF induced K63-but not K48-linked ubiquitination of Twist (Figure 1B), suggesting that the non-proteolytic K63-linked ubiquitination is a regulatory mode for orchestrating Twist functions.
Figure 1. Identification of RNF8 as a Twist activator through an E3 ligase screening.
(A) BT549 TNBC cells were serum-starved and treated with or without EGF for various time periods; whole cell extracts were harvested for immunoprecipitation (IP) with Twist, followed by immunoblotting (IB) analysis. (B) BT549 cells were serum-starved, treated with EGF for 60 min. and harvested for IP with Twist, followed by IB analysis. (C) Schematic illustration of the strategy screening for E3 ligases that activate Twist. (D) Overlapping Candidate E3 ligases that regulate Twist activity in both HMLE and MCF7 cells are shown in the heat map. The experiment was independently performed for two times (n=3). (E) cBioPortal was used to access and to visualize the cancer genome atlas (TCGA) data for 107 cases of invasive carcinoma of basal-like breast cancer. Each column represents an individual sample. (F) Twist-overexpressing HMLE cells were infected with various doses of viruses containing shRNAs as indicated and were harvested for an E-cadherin luciferase reporter assay. Quantified results are presented as means ± SEM (n=3); **, p < 0.01. See also Figure S1 and Table S1-S3.
To identify E3 ligases responsible for Twist activation in an unbiased manner, we designed a luciferase-based short hairpin RNA (shRNA) screening strategy. Human mammary epithelial (HMLE) and MCF7 cancer cells harbor very low levels of Twist hence overexpression of Twist is known to robustly induce EMT in these cells (Mani et al., 2008; Vesuna et al., 2012). Transcriptional repression of E-cadherin gene is a well-characterized readout for Twist activity as well as EMT (Yang et al., 2004). Therefore, we integrated a luciferase reporter construct fused to the E-cadherin promoter into Twist-overexpressing HMLE and MCF7 cells. E-cadherin luciferase assay illustrated that Twist overexpression effectively suppressed E-cadherin transcription as expected, whereas genetic silencing of Twist compromised this effect in both cell models (Figure S1A), validating our screening approach. We then used the established cell platforms to screen an shRNA library consisting of 150 different E3 ligases for their effects on E-cadherin gene expression (Figure. 1C and 1D; Table S1). The E3 ligase candidates that consistently altered over 50% of Twist transcriptional activity in both cell platforms were plotted in the heat map (Figure 1D) and verified with an additional shRNA (Table S2 and S3). Gene silencing of FBXO5, MALT1, MDM2, RNF8, XIAP, SPOP, MDM4, PML and PCGF2 reduced Twist activity more than 50%, while downregulation of TRIM39 induced 50% of Twist activity (Fig. 1D, right ; Table S2 and S3), indicating their roles in EMT regulation. Downregulation of RNF138 and BRAP were found to inactivate Twist as well, albeit to a lesser extent (Fig. 1D, right; Table S3). Deficiency of RanBp2, CBL, PRPF19 and TRIM68 exhibited stronger effect than Twist knockdown in E-cadherin derepression (Fig. 1D), suggesting Twist-independent mechanisms underlying their actions on EMT. To explore the clinical relevance of candidate E3 ligases specific for Twist in TNBC, we analyzed TCGA data with cBioPortal (Cerami et al., 2012; Ciriello et al., 2015) for gene alterations in invasive ductal carcinomas of basal-like breast cancer (BLBC), a subtype largely overlaps with TNBC (Kreike et al., 2007). A majority of BLBCs were found to display gene amplification and/or upregulation of RNF8 (57% of TCGA data as shown in Figure 1E; Figure S1B and S1C), uncovering that RNF8 is a common risk factor for basal-like TNBC. We further silenced RNF8 expression in Twist-overexpressing cells to determine its relationship with Twist. E-cadherin reporter assay showed that RNF8 deficiency attenuated Twist-driven E-cadherin repression to an extent similar to Twist knockdown in both cell models (Figure 1F; Figure S1D). Together, these results uncover a previously unrecognized function of RNF8 in cancer and suggest that RNF8-mediated cancer development is at least in part through Twist activation.
RNF8 directly promotes K63-linked ubiquitination and influences Twist nuclear localization and protein stability
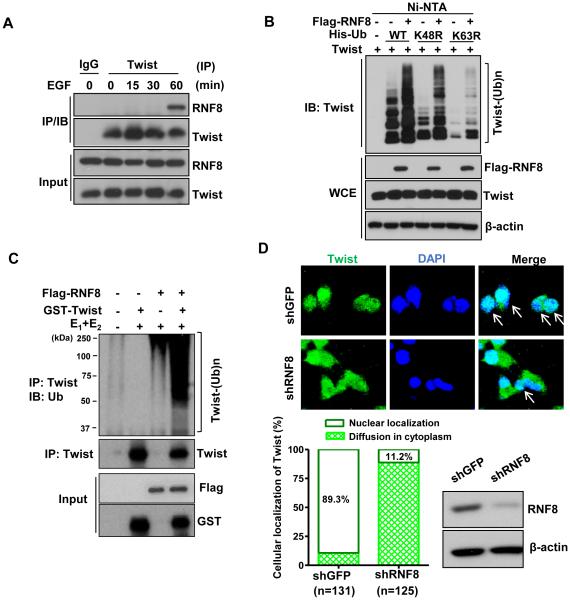
To determine the mechanism by which RNF8 activates Twist, we examined the interaction between RNF8 and Twist. RNF8 was shown to associate with Twist endogenously upon EGF stimulation for 60 minutes (Figure 2A), at which point endogenous Twist underwent K63-linked ubiquitination (Figure 1A and 1B). Moreover, in vivo ubiquitination assay revealed that RNF8 induces K63- but not K48-linked ubiquitination of Twist (Figure 2B). RNF8 overexpression promoted Twist ubiquitination, whereas a RNF8 RING domain deletion mutant (ΔR) devoid of the E3 ligase activity failed to trigger this ubiquitination event, despite its intact binding affinity to Twist (Figure S2A and S2B). These data demonstrate that RNF8-mediated K63-linked ubiquitination of Twist is dependent on its E3 ligase activity. RNF8 triggers K48- and K63-linked ubiquitination by interacting with different E2 ubiquitin-conjugating enzymes, UBCH8 and UBC13, respectively (Lok et al., 2012). A point mutation (I405A) on RNF8, which disrupts its binding to UBCH8 and subsequent formation of K48-based ubiquitination (Lok et al., 2012), retains the ability to induce Twist ubiquitination via the K63-linkage (Figure S2C). Importantly, RNF8 in collaboration with UBC13 readily induced Twist ubiquitination in vitro (Figure 2C), indicating that RNF8 is a direct E3 ligase for promoting Twist K63-linked ubiquitination.
Figure 2. RNF8 regulates K63-linked ubiquitination and nuclear localization of Twist.
(A) BT549 cells were serum-starved, treated with or without EGF for various time periods and harvested for co-IP assay followed by IB analysis. (B) In vivo ubiquitination assay in 293T cells transfected with Twist, Flag-tagged-RNF8 (Flag-RNF8) along with Histidine-tagged ubiquitin (His-Ub), His–Ub K48R, or His–Ub K63R constructs. Ni-nitrilotriacetic acid (NTA) indicates nickel bead precipitate; WCE indicates whole-cell extracts. (C) GST-Twist proteins were incubated with adenosine triphosphate, E1 and E2 (Ubc13/Uev1) along with or without purified Flag-RNF8 for in vitro Twist ubiquitination assay. (D) Confocal image analysis of Twist subcellular localization in BT549 cells with GFP or RNF8 knockdown. The arrow indicates the nuclear localization of Twist (upper). IB analysis of RNF8 expression and the quantification results are shown in the bottom of the graph. See also Figure S2.
Twist is a nuclear protein that regulates a variety of cellular functions controlled by gene transcription events. K63-linked ubiquitination has emerged as a post-translational modification for fine-tuning signal transduction pathways (Chan et al., 2014; Mattiroli and Sixma, 2014), and has been reported to do so through regulating nuclear translocation of protein targets (Geetha et al., 2005; Xue et al., 2012). We therefore reasoned that RNF8-mediated K63-linked ubiquitination of Twist may be crucial for Twist’s localization to the nucleus, where it executes its function as a transcription factor. Our immunofluorescent study showed that Twist is primarily present in the nucleus of BT549 TNBC cells as expected (Figure 2D). In contrast, RNF8 deficiency results in defective nuclear import of Twist (Figure 2D; Figure S2D), revealing RNF8-mediated Twist nuclear localization as a critical mechanism controlling Twist regulation. Of note, silencing RNF8 significantly reduced Twist protein expression with no impact on gene expression (Figure S2E and S2F). Moreover, inhibition of proteasome-mediated degradation rescued this defect (Figure S2G), dictating that RNF8-controlled Twist proteolysis is mediated by the proteasome. In line with this observation, RNF8 overexpression increases Twist protein level and such effect depends on the E3 ligases activity of RNF8 (Figure S2H). These findings implicate that RNF8 orchestrates Twist nuclear localization and protein stability, thereby activating Twist transcriptional ability. Consistent with our findings, an earlier study reported that cytosolic Twist was prone to K48-linked ubiquitination and consequent proteasome-dependent proteolysis due to increased Twist access to its proteolytic E3 ligase, β-TrCP (Zhong et al., 2013). This report along with our data thus establish that subcellular localization is a regulatory event for Twist protein stabilization.
K63-linked ubiquitination regulates Twist nuclear localization and protein stability for subsequent EMT
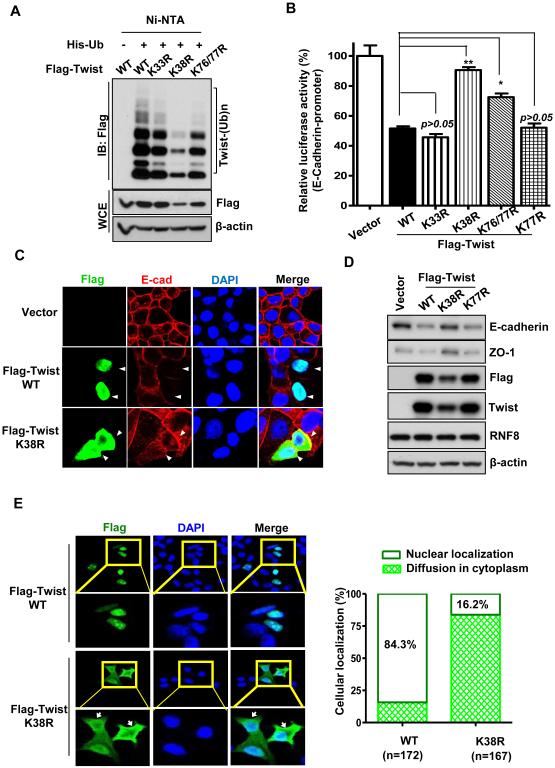
To characterize the function of the K63-linked ubiquitination, we next sought to identify the K residue(s) on Twist where K63-linked ubiquitination takes place. We individually mutated each of the evolutionarily conserved K residues to arginine (R) in Twist and examined whether any of the Twist mutants failed to be ubiquitinated. Of the 10 conserved K residues, mutation on K38 to R most profoundly impaired Twist ubiquitination (Figure 3A; Figure S3A and S3B). We then compared the transcriptional activity of Twist wild-type and various mutants by assessing their effects on suppressing E-cadherin gene expression. We found that Twist K38R mutant greatly impaired Twist’s activity in repressing E-cadherin transcription (Figure 3B and Figure S3C). Immunofluorescent staining and immunoblotting analysis also showed that compared to wild-type, Twist K38R mutant up regulated the expression of epithelial markers including E-cadherin and ZO-1 (Figure 3C and 3D; Figure S3D), suggesting that K63-linked ubiquitination is crucial for regulating Twist function in EMT. Consistent with the phenotype in RNF8-knockdown cells, the majority of Twist K38R mutant was mislocalized to the cytoplasm (Figure 3C and 3E; Figure S3D and S3E). Moreover, protein expression of cytosolic Twist K38R was reduced compared to wild type and inhibition of proteosomal activity rescued such defect (Figure 3A; Figure S3F and S3G); supporting an essential role of K63-linked ubiquitination in regulating nuclear localization and stability of Twist. We reasoned that the phenotypes associated with K38R mutant was not resulted from an altered protein structure since such point mutation did not affect other Twist actions including acetylation and its interaction with its regulator, BRD4 (Shi et al., 2014). Instead, we found that cytosolic Twist was preferentially degraded (Figure S3H), recapitulating the Twist K38R mutant phenotype. Our results therefore identify that RNF8-mediated K63-linked ubiquitination is an unrevealed posttranslational control of Twist protein expression and activity by enforcing its proper nuclear localization.
Figure 3. K63-linked ubiquitination is essential for Twist nuclear transport and EMT.
(A) In vivo ubiquitination assay in 293T cells transfected with His-Ub and Flag-Twist or various Flag-Twist mutants. (B) MCF7 cells were transfected with the indicated plasmids and harvested for an E-cadherin luciferase reporter assay. Quantified results are presented as means ± SEM (n=3); *, p < 0.05 and **, p < 0.01. (C) MCF7 cells transfected with mock, wild-type and the K38R mutant of Flag-Twist were fixed and stained with indicated antibodies, and subjected to confocal microscopy analysis. The arrow indicates the cells harboring ectopic expression of Flag-Twist wild-type or K38R mutant. (D) MCF7 cells stably expressing mock, Flag-Twist or various Flag-Twist mutants were harvested for IB analysis. (E) MCF7 cells transfected with mock, Flag-Twist wild-type or Flag-Twist K38R mutant were fixed and stained with indicated antibodies, and subjected to confocal microscopy analysis. Representative confocal images of nuclear and cytosolic Twist were shown in the left panel. The arrow indicates the cytosolic localization of Twist. Quantification results of nuclear and cytosolic Twist were shown in the right panel. See also Figure S3.
RNF8 regulates EMT and CSC self-renewal through K63-linked ubiquitination of Twist
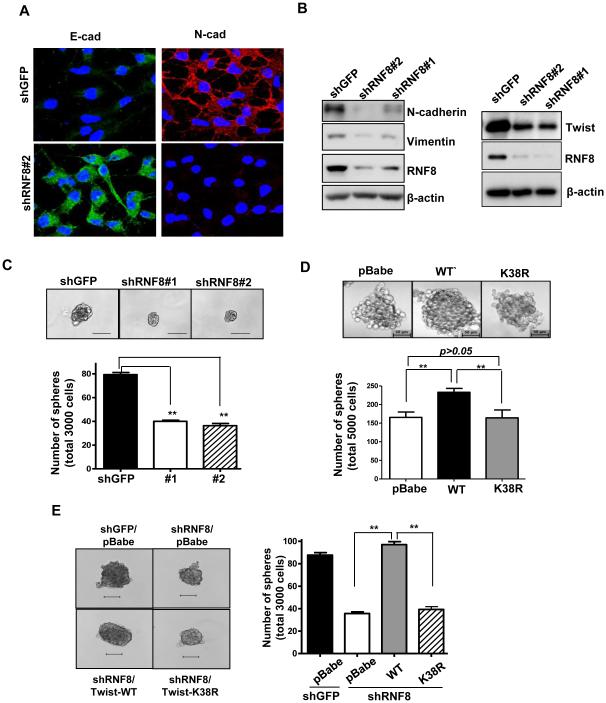
EMT is a reversible process that is activated during tumor progression/metastasis in response to a deregulated genome and altered microenvironment (Kalluri and Weinberg, 2009). Twist, by controlling EMT, has been shown to drive self-renewal of CSC to increase the CSC population, which in turn confers treatment resistance and tumor recurrence (Mani et al., 2008). Having shown the mechanistic link between RNF8 and Twist, we next determined if RNF8 is required for Twist functions in EMT and CSC regulation. RNF8 knockdown in both BT549 and MDA-MB-231 TNBC cells decreased expression of Twist and mesenchymal markers (e.g., N-cadherin and Vimentin), and increased expression of epithelial markers (e.g., E-cadherin and ZO-1), indicating an impairment of EMT process (Figure 4A and 4B; Figure S4A and S4B). We next determined if RNF8 regulates CSCs and if RNF8’s action on CSCs depends on Twist K63-linked ubiquitation. Mammosphere formation assay is a well-established assay for demonstrating the self-renewal ability of stem cells (Dontu et al., 2003). We found that RNF8 silencing mitigated the size and number of mammosphere in both BT549 and MDA-MB-231 cells (Figure 4C; Figure S4C), demonstrating a critical function of RNF8 in CSC regulation. Likewise, the ubiquitination-deficient Twist K38R mutant failed to enhance CSC self-renewal as wild-type did (Figure 4D). The inability of CSCs to self-renew induced by RNF8 deficiency was rescued in full by wild-type, but not the K38R mutant of Twist (Figure 4E; Figure S4D). These findings dictate that RNF8 regulates CSC function through promoting Twist K63-linked ubiquitination.
Figure 4. RNF8 regulates EMT and CSC self-renewal via promoting K63-linked ubiquitination of Twist.
(A) BT549 cells with GFP or RNF8 knockdown were fixed and stained with indicated antibodies, and subjected to confocal microscopy analysis for expression and subcellular localization of E-cadherin and N-cadherin. (B) IB analysis for expression of Twist, N-cadherin and Vimentin in BT549 cells with GFP or RNF8 knockdown. (C) Mammosphere formation assay in BT549 cells with GFP or RNF8 knockdown. (D) Mammosphere formation assay in MDA-MB-231 cells with stable expression of vector alone, Flag-Twist wild-type or K38R mutant. (E) Mammosphere formation assay in BT549 cells with GFP, RNF8 knockdown or with RNF8 knockdown plus overexpression of Twist wild-type or K38R mutant. The quantified results are presented as means ± SEM (n=3); **, p < 0.01. Scale bar, 100 µm in (C, E) and 50 µm in (D). See also Figure S4.
RNF8 deficiency synergistically sensitizes CSCs and cancer cells to chemotherapy
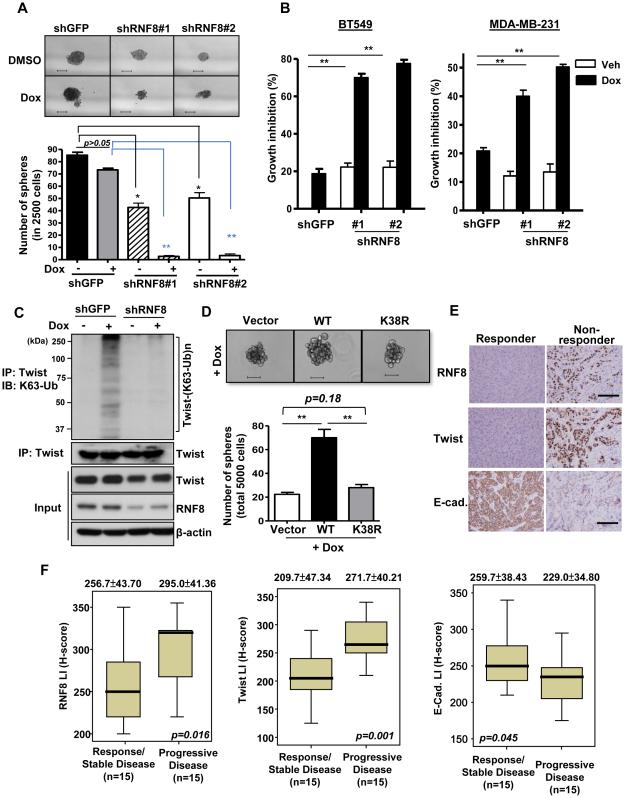
Similar to stem cells, CSCs reside in a slow-cycling state that makes them more resistant to cytotoxic agents – a feature that poses major challenges for tumor eradication (Cojoc et al., 2015; Sampieri and Fodde, 2012). We tested the role of RNF8 in CSC sensitivity to currently used breast cancer chemotherapies, such as doxorubicin and paclitaxel (Taxol). We found that these chemotherapeutic agents modestly or partially reduced the CSC population, but were able to almost eradicate CSCs when combined with RNF8 deficiency (Figure 5A; Figure S5A). As a result, RNF8 knockdown greatly augmented the chemosensitivity of BT549 and MDA-MB-231 TNBC cells (Figure 5B; Figure S5B). RNF8 is known as a DNA damage response transducer recruited to the DNA double strand break (DSB) sites to repair DNA lesions. While doxorubicin and etoposide effectively induced DSBs, evident by the formation of γ-H2A foci (Figure S5C), we surprisingly found that a large amount of RNF8 was dissociated from DSB loci in BT549 cancer cells (Figure S5D and S5E). Moreover, these DSB-inducing agents greatly promoted Twist K63-linked ubiquitination (Figure 5C; Figure S5F) and doxorubicin-induced Twist K63-linked ubiquitination relied on RNF8 expression (Figure 5C). Consistent to the doxorubicin effect, RNF8 deficiency combined with etoposide also eliminates most CSCs (Figure S5G). In support of this notion, we found that Twist K63-linked ubiquitination is required for conferring chemoresistance of CSCs (Figure 5D; Figure S5H). Our results highlight the critical role of RNF8 and Twist K63-linked ubiquitination in regulating tumor response to chemotherapy and suggest that RNF8 expression in cancer may exacerbate patient outcomes of DSB-inducing therapy.
Figure 5. RNF8 confers cancer chemoresistance through K63-linked ubiquitination of Twist.
(A) Mammosphere formation assay in BT549 cells with GFP or RNF8 knockdown in response to doxorubicin (Dox). (B) Cell growth inhibition assay in BT549 and MDA-MB-231 cells with GFP or RNF8 knockdown in response to Dox. (C) GFP and RNF8-knockdown BT549 cells treated with vehicle or doxorubicin for 2 hrs were harvested for IP with Twist, followed by IB analysis to determine the level of K63-linked ubiquitination of Twist. (D) Mammosphere formation assay in MDA-MB-231 cells with stable expression of vector alone (pBabe), Twist wild-type and Twist K38R mutant in the presence of doxorubicin treatment. The quantified results are presented as means ± SEM (n=3); *, p < 0.05 and **, p < 0.01. Scale bar, 100 µm in (A) and 50 µm in (D). (E and F) Histological (E) and quantification (F) analyses of RNF8, Twist and E-cadherin in breast cancer patients with progressive or stable disease following receiving Dox. as a primary treatment (n=15 in each group). Scale bar represents 200 µm. The quantified results are presented as means ± SEM. See also Figure S5.
To further examine the role of RNF8 in chemoresistance in vivo, we explore whether RNF8 levels were related to patient responses to chemotherapy. We conducted immunohistochemistry studies of RNF8 in breast tumors from patients who had unresectable tumors and received doxorubicin (Adriamycin) as primary therapy. Compared to doxorubicin-responsive cases, patients who eventually developed recurrent or metastatic breast tumors following doxorubicin therapy possessed higher RNF8 expression in their primary tumors (Figure 5E and 5F). This clinical phenotype is also correlated with Twist upregulation and E-cadherin downregulation (Figure 5E and 5F). These findings reveal RNF8-Twist axis as a pathological-relevant mechanism in chemoresistance.
RNF8 expression is upregulated in advanced breast cancer and is positively correlated with disease progression and poor patient outcomes
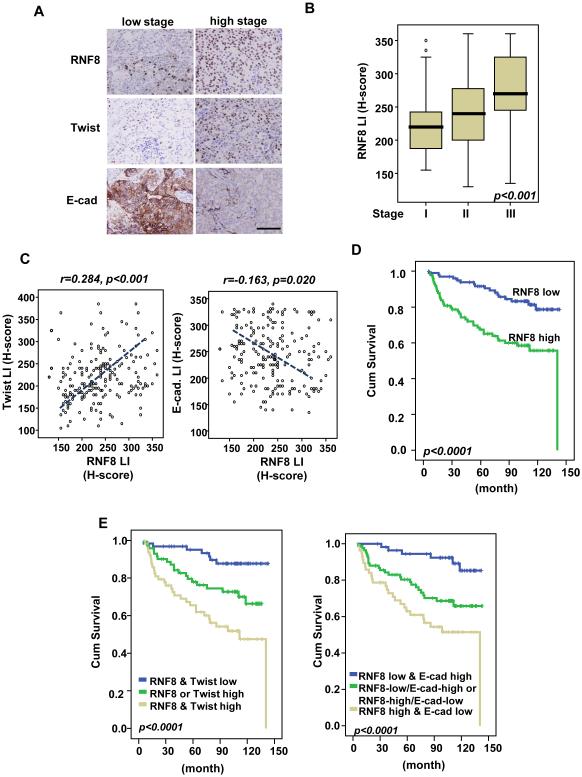
A recent single-cell analysis showed that CSCs can initiate metastasis of TNBC (Lawson et al., 2015). This finding and the observed amplification/overexpression of RNF8 in invasive basal-like breast cancer in TCGA samples led us to elucidate a cancer-promoting role for RNF8. We analyzed the protein expression of RNF8 and its relationship to disease progression in 202 breast tumor cases (all stages). We found that RNF8 is overexpressed in breast cancer and its overexpression is significantly correlated with numerous adverse clinicopathological factors, including increments of nodal metastasis (pN, p < 0.001), and tumor stage (p < 0.001; Figure 6A and 6B; Table S4). RNF8 expression also significantly correlates with Twist upregulation (p < 0.001) and E-cadherin downregulation (p = 0.02) (Figure 6C; Figure S6A and S6B; Table S4). Consistent with the previous report (Grzegrzolka et al., 2015), we also observed variable Twist expression in the myofibrobalsts of the tumor-associated stroma in some tumor cases (data not shown). In the univariate survival analysis, RNF8 overexpression (p < 0.0001 by continuous scoring), Twist overexpression (p = 0.0002) and E-cadherin downregulation (p = 0.0042) together with pT status (p < 0.0001), pN status (p < 0.0001), and stage (p < 0.0001) effectively predicted inferior metastasis-free survival (p < 0.0001) (Figure 6D; Figure S6C and S6D; Table S5). In the multivariate analysis, RNF8 and Twist overexpression remained prognostically significant for metastasis-free survival in breast cancer patients (p = 0.019 and p = 0.034, respectively in Table S6). To determine if RNF8 alone or in combination with Twist or E-cadherin performed as a more accurate prognostic marker, patient survival outcomes in relation to varied expressions of RNF8, Twist and E-cadherin were analyzed. Kaplan-Meir analysis showed that RNF8 overexpression in combination with Twist overexpression or E-cadherin downregulation is a stronger predictor of metastasis-free survival than RNF8 alone (Figure 6E).
Figure 6. RNF8 is overexpressed in breast cancer and is positively correlated with poor disease outcomes.
(A) Representative images of histological analyses of RNF8, Twist and E- cadherin expressions in breast cancer patients in low (stage I) and high (stage III) stages of tumor. Scale bar represents 200 µm. (B) Quantification analysis of RNF8 expressions in 202 cases of resected breast tumors. (C) Scatter plot analysis for the correlation between RNF8 expression versus Twist and E-cadherin expression levels was determined using the Pearson correlation coefficient analysis (n=202). LI represents labeling index. (D) Kaplan-Meier plot analysis of metastasis-free survival of 202 cases of breast cancer patients with low or high expression of RNF8. (E) Kaplan-Meier plot analysis of metastasis-free survival of 202 cases of breast cancer patients with low or high expression of RNF8, Twist and E-cadherin individually and concurrently. See also Figure S6, and Table S4-S6.
RNF8 regulates cancer cell migration, invasion and metastasis
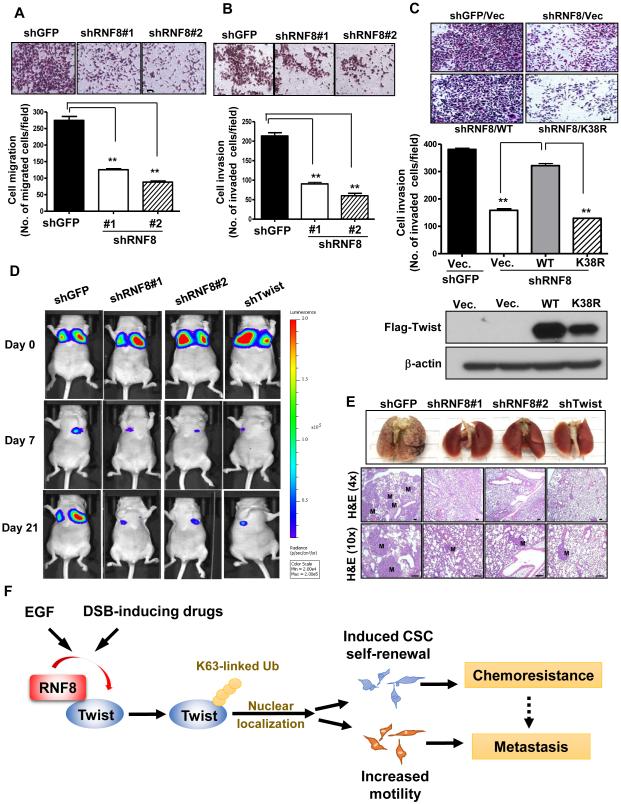
To examine the role of RNF8 in cancer cell migration, invasion and metastasis, transwell cell migration and Matrigel-coated invasion assays were performed and showed that RNF8 is required for migration and invasion of TNBC cells (Figure 7A and 7B; Figure S7A and S7B). Conversely, overexpression of RNF8 promoted cell migration (Figure S7C and S7D). Compared to wild-type, RNF8-ΔR mutant failed to enhance migration ability of cancer cells (Figure S7C and S7D), suggesting that the E3-ligases activity of RNF8 is required for cancer cell’s ability to migrate. The defect in cell invasion observed in RNF8-deficient cancer cells was restored by Twist wild-type but not the K38R mutant (Figure 7C), illustrating RNF8-regulated cell invasiveness through promoting Twist K63-linked ubiquitination. Of note, RNF8-mediated Twist activation, EMT and cancer cell migration is independent of RNF168 (Figure S7E-S7H), indicating that RNF8 exhibits a non-canonical role in regulating cancer progression.
Figure 7. RNF8 regulates cancer cell migration, invasion and cancer metastasis as Twist does.
(A and B) Transwell cell migration (A) and Matrigel cell invasion (B) assays in BT549 cells with GFP and RNF8 knockdown. (C) Matrigel cell invasion assay and IB analysis in MDA- MB-231 cells with GFP, RNF8 knockdown or with RNF8 knockdown plus expression of Flag- Twist wild-type and K38R mutant. The quantified results are presented as means ± SEM (n=3); *, p < 0.05 and **, p < 0.01. Scale bar, 100 µm. (D) MDA-MB-231-Luciferase cells with GFP, RNF8 or Twist knockdown were injected into nude mice, and the kinetics of breast cancer metastasis to the lung were measured and quantified using the IVIS bioluminescence system (n = 6 per group). Representative bioluminescent images are shown for days 0, 7 and 21. (E) Histological analysis of lung metastases derived from MDA-MB-231-Luciferase cells with GFP, RNF8 or Twist knockdown. Lung tissues were isolated 28 days after injection. H&E indicates haematoxylin and eosin stain; M indicates metastatic breast tumors. Scale bar, 100 µm. (F) A working model depicting the mechanism by which cancer exploits overexpressed RNF8 to drive chemoresistance and cancer metastasis via Twist activation. Ub indicates ubiquitination. See also Figure S7.
There is considerable evidence that upregulation of Twist in cancer cells leads to cancer metastasis including TNBC (Yang et al., 2004; Yang et al., 2008). Having shown that RNF8 is overexpressed in invasive TNBC and that RNF8 regulates cancer cell migration and invasion (Figure 1E and 7A-7C), we next determine the requirement of RNF8 in TNBC metastasis. We employed a bioluminescence imaging approach to examine the kinetics of cancer metastasis. Our data showed that circulation of MDA-MB-231 with control, RNF8 or Twist silencing to the lung was comparable 2h (day 0) after injection (Figure 7D; Figure S7I), indicating that neither RNF8 nor Twist affects survival of breast cancer cells during circulation to the lung. The bioluminescence signal in the lung was greatly reduced upon RNF8 or Twist deficiency within the first week, when the cancer cells began to grow in the lungs (Figure 7D; Figure S7I). Such a defect continued to inhibit metastasis outgrowth at later time points (day 21 in Figure 7D; Figure S7I, and day 28 in Figure 7E), establishing a previous uncharacterized function of RNF8 in cancer metastasis.
Discussion
Our results reveal several unexpected findings with important clinical implications. We identify that K63-linked ubiquitination is a critical post-translational control of Twist. We discover the link between RNF8 and Twist by an unbiased approach and further reveal an essential role of RNF8-mediated Twist K63-linked ubiquitination in CSC regulation, cancer cell migration and invasion (Figure 7F). This study describes the unknown oncogenic activities of RNF8 and demonstrates its clinical relevance as a poor prognostic marker in breast cancer. Our findings imply that current chemotherapy using DSB-inducing agents such as doxorubicin and etoposide may exacerbate therapeutic outcomes in patients with RNF8 overexpression. Our results therefore call for clinical assessment of RNF8 for optimal use of chemotherapy.
Our study provides preclinical evidence demonstrating that targeting RNF8-Twist axis is an effective approach to inhibit cancer metastasis, supporting the previously established functional role of Twist during metastatic process in basal-like TNBC. Although a recent study reported that EMT is dispensable for tumor dissemination in MMTV-Neu and MMTV-PyMT transgenic mice, breast tumors developed in these models are primarily luminal-type (Fischer et al., 2015). These studies along with ours imply that EMT pathway selectively regulates cancer spread in certain cancer types or subtypes. In the current study, we noticed that E-cadherin downregulation does not have the significant prediction power for breast cancer progression as RNF8 or Twist does (Figure S6A and S6B), albeit E-cadherin downregulation remains a significant predictor of metastasis-free survival in breast cancer patients (Figure 6D; Figure S6C and S6D). We thus cannot rule out the possibility that additional mechanism is engaged in RNF8/Twist-regulated metastasis. Twist has been shown to promote cancer cell migration and metastasis via Rac1 activation in head and neck cancer (Yang et al., 2012). In TNBC model, we found RNF8 deficiency reduced the level of GTP-bound Rac1, a readout for Rac1 activity (Figure S7J). This result demonstrates that RNF8 regulates multiple Twist downstream substrates and thus is a bona fide regulator of Twist.
Numerous studies have reported RNF8’s vital roles in DSB-induced DNA damage repair and telomere end protection in non-cancerous cells (Rai et al., 2011; Thorslund et al., 2015). During cellular mitosis, RNF8 was found to be released from DSBs by CDK1-mediated phosphorylation (Orthwein et al., 2014). However, the precise biological function of DSB-dissociated RNF8 remains a mystery. Here, we found that a majority of RNF8 is dissociated from DNA damage foci following doxorubicin and etoposide stimulation in breast cancer cells. This is plausibly attributed to the frequent alterations in the expression and activity of CDK1 in breast cancer (Horiuchi et al., 2012; Kim et al., 2008). The DSB-dissociated RNF8 can thus interact with Twist for promoting Twist-mediated chemoresistance and cancer progression. This study along with previous reports indicate that RNF8 exhibits distinct functions in cancer and normal cells via crosstalk with distinct downstream substrates. Our findings therefore provide a perspective on the cancer regulatory functions of DNA damage response regulator.
Previous publications have reported that several kinases including MAPK, AKT, GSK3β and IKKβ can phosphorylate Twist (Hong et al., 2011; Lander et al., 2013; Li et al., 2016; Zhong et al., 2013). Twist phosphorylation by MAPK, AKT, GSK3 and IKKβ reduces Twist protein expression via recruiting FBXL14/Ppa and/or β-TRCP E3 ligases, which target Twist for K48- linked ubiquitination and subsequent proteasomal degradation (Lander et al., 2011; Zhong et al., 2013). In our research, we identified that K63-linked ubiquitination governs Twist localization to the nucleus where Twist triggers EMT and resultant CSC phenotypes, revealing that K63-linked ubiquitination is an active signal for Twist regulation. Additionally, K63-linked ubiquitination can prevent proteasome-mediated degradation of Twist. These results suggest that K63-linked ubiquitination acts to stabilize Twist by preventing Twist access to its proteolytic E3 ligases, β- TrCP and FBXL14 in the cytoplasm. Detailed mechanisms underlying the crosstalk between K63- and the canonical K48-linked ubiquitination of Twist require further investigation.
Aside from ubiquitination, multiple post-translational modifications, such as acetylation and methylation, occur at lysine residues. Methylation of lysine residues on non-histone proteins has emerged as a prevalent post-translational modification and as an important regulator of signal transduction in cells (Biggar and Li, 2015). To date, there is no evidence demonstrating the occurrence of lysine methylation on Twist. Shi and colleagues have shown that Twist is regulated by acetylation at K73 and K76, but not at K38 residues (Shi et al., 2014). Acetylation of Twist recruits the BRD4/P-TEFb/RNA-Pol II transcription complex to activate Wnt5A gene expression and subsequent Wnt5a-mediated EMT process, yet it does not affect nuclear transport of Twist (Shi et al., 2014). This study along with ours elucidate that different types of lysine modifications regulate Twist functions via diverse mechanisms.
E3 ligases confer specificity to ubiquitination by selecting substrates for this process. Proteomics approach was widely used to identify binding partners of protein of interest including Twist (Shi et al., 2014), yet this approach may miss important E3 ligase targets since the interaction between E3 ligases and protein substrates is often transient. In this study, a lentiviral shRNA-based system followed by clinical assessment of gene alternation in TCGA database was employed to functionally screen for candidate E3 ligases as Twist and EMT activators. Characterization and functional studies of RNF8 identified by this strategy established an uncharacterized pathological function of RNF8 in breast cancer. These results also indicate that our screening platform is a powerful tool for effective identification of druggable targets that block EMT. Besides breast cancer, such screening and validation strategy can be adopted for other types of tumors where Twist activation is implicated such as ovarian, prostate and lung cancers (Khan et al., 2013). It is noteworthy that our research uncovers several E3 ligases, such as PML FBXO5, MALT1 and MDM4, as potential EMT regulators despite their TNBC- associated gene mutations are not as common as RNF8 (Figure S1C). FBXO5 (also known as FBX5 and Emi1), MALT1 and MDM4 have been reported as therapeutic targets for breast cancer (Haupt et al., 2015; Pan et al., 2016; Wang et al., 2014). Even though PML is a legitimate tumor suppressor in leukemia, it has been shown to be upregulated in breast cancer in which PML facilitates fatty acid metabolism to support breast cancer survival (Carracedo et al., 2012). Theses E3 ligases appear to express in TNBC tissues as independent and joint events due to the inter-individual heterogeneity in cancer (Figure S1B). Further efforts are needed to explore their actions on Twist and interactions in regulating EMT. Nevertheless, data generated in this study inform the expression pattern and clinical relevance of E3 ligases as biomarkers for TNBC. Our work thus provides a proof-of-principle that targeting E3 ligases for K63-linked ubiquitination of EMT-TFs represents new opportunities for effective cancer therapy.
Experimental Procedures
E3 ligase screening
Twist-overexpressed HLME and MCF7 were integrated with the E-cadherin promoter reporter constructs via viral infection. The resulting stable cells were infected with various viral particles for 24h, then refreshed with regular medium. 48h later, luciferase activity was measured by using the dual luciferase system (Promega). Each sample was analyzed in triplicate and experiments were performed at least three times. The relative luciferase activity was calculated as a ratio between firefly luciferase activity and Renilla luciferase activity.
Immunohistochemistry and Scoring
All of the immunohistochemistry experiments dealing with human subjects were performed under protocol approved by the institutional review board of Chi-Mei Medical Center (IRB# 10501-012). Detailed information please refer to the Supplemental Experimental Procedures.
Statistical Analysis
All data are shown as means ± SEM for at least three independent experiments, unless otherwise indicated. All statistical significance was determined by unpaired two-tailed Student’s t tests, and p values less than 0.05 were considered statistically significant. For in vivo mouse experiment, the mouse number is determined by power analysis based on the fact that with a sample size of 6 with a two-sided type I error rate of 0.05 the study will have 90% power to detect a 30% difference in cancer metastasis.
Metastasis assay
MDA-MB-231 cells stably overexpressed with luciferase gene (MDA-MB-231-Luc) silenced with GFP, RNF8 or Twist lentiviral shRNAs were injected into the lateral tail vein (2 × 106 cells) of athymic female nude mice (Harlen Laboratories). For bioluminescence imaging, mice were anaesthetized and given 150 mg/mice of D-luciferin via intraperitoneal injection. 5 min after injection, bioluminescence was detected using an IVIS imaging system (PerkinElmer) and analyzed by the Living Image software (PerkinElmer). 6-7 weeks after, mice were killed and lung tissues were obtained for analysis of cancer metastasis. All animal experiments were performed under the protocol (IACUC# 623918) approved by the Institutional Animal Care and Use Committee (IACUC) of Stony Brook University.
Supplementary Material
Highlights.
K63-linked ubiquitination activates Twist and EMT
RNF8 activates EMT and CSC self-renewal via Twist K63-linked ubiquitination
RNF8 overexpression in breast cancer predicts poor disease outcomes
RNF8 deficiency inhibits CSC self-renewal and cancer metastasis
eTOC.
RNF8 is previously known as a genome guardian. Lee et al. employed an E3 ligase screen revealing RNF8 as an activator of Twist and cancer. RNF8 regulates cancer metastasis and its overexpression is correlated with poor disease outcomes, opening up a new perspective on cancer-promoting actions of DNA damage regulators.
RNF8 is known as a genome guardian. Lee et al. employed an E3 ligase screen revealing RNF8 as an activator of Twist and cancer. RNF8 regulates cancer metastasis and its overexpression is correlated with poor disease outcomes, opening up a new perspective on cancer-promoting actions of DNA damage regulators.
Acknowledgments
We thank Dr. M.C. Hung for cell lines and reagents. We also thank the members of Chan’s laboratory for their valuable comments and suggestions. This work was supported by the Stony Brook School of Medicine and Cancer Center Fund, the TRO Carol M. Baldwin Award, the Feldstein Medical Foundation Award, the Peter Rowley Breast Cancer Research Project grant and the NIH grant (5 K22 CA181412) to C.H.C., as well as the grants from the Department of Health in Taiwan (MOHW103-TD-B-111-05, MOHW103-TDU-M-221-123017 and DOH102- TD-M-111-102001) to C.F.L
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Supplemental Information includes Supplemental Experimental Procedures, seven figures and six tables can be found with this article online.
Author contributions
H. J. L. conceived the project, designed the experiments and wrote the manuscript. C. F. L. performed the histological analyses and analyzed the clinical data. H. J. L. and D. R. performed the experiments; R. S. P. contributed to the bioinformatics analysis. P. A. T and M. A. F contributed to manuscript completion. C. H. C. conceived the project, provided overall guidance and contributed to manuscript completion.
References
- Andre F, Zielinski CC. Optimal strategies for the treatment of metastatic triple- negative breast cancer with currently approved agents. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(Suppl 6):vi46–51. doi: 10.1093/annonc/mds195. [DOI] [PubMed] [Google Scholar]
- Bennett EJ, Harper JW. DNA damage: ubiquitin marks the spot. Nature structural & molecular biology. 2008;15:20–22. doi: 10.1038/nsmb0108-20. [DOI] [PubMed] [Google Scholar]
- Berndsen CE, Wolberger C. New insights into ubiquitin E3 ligase mechanism. Nature structural & molecular biology. 2014;21:301–307. doi: 10.1038/nsmb.2780. [DOI] [PubMed] [Google Scholar]
- Biggar KK, Li SS. Non-histone protein methylation as a regulator of cellular signalling and function. Nature reviews. Molecular cell biology. 2015;16:5–17. doi: 10.1038/nrm3915. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Weiss D, Leliaert AK, Bhasin M, de Boer VC, Laurent G, Adams AC, Sundvall M, Song SJ, Ito K, et al. A metabolic prosurvival role for PML in breast cancer. The Journal of clinical investigation. 2012;122:3088–3100. doi: 10.1172/JCI62129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer discovery. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CH, Jo U, Kohrman A, Rezaeian AH, Chou PC, Logothetis C, Lin HK. Posttranslational regulation of Akt in human cancer. Cell & bioscience. 2014;4:59. doi: 10.1186/2045-3701-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng Z, Huang HY, Tsai KK, Flores LG, Shao Y, et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell. 2012;149:1098–1111. doi: 10.1016/j.cell.2012.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CH, Morrow JK, Li CF, Gao Y, Jin G, Moten A, Stagg LJ, Ladbury JE, Cai Z, Xu D, et al. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell. 2013;154:556–568. doi: 10.1016/j.cell.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell. 2015;163:506–519. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Tcherpakov M. Will the ubiquitin system furnish as many drug targets as protein kinases? Cell. 2010;143:686–693. doi: 10.1016/j.cell.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Cojoc M, Mabert K, Muders MH, Dubrovska A. A role for cancer stem cells in therapy resistance: cellular and molecular mechanisms. Seminars in cancer biology. 2015;31:16–27. doi: 10.1016/j.semcancer.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & development. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha T, Kenchappa RS, Wooten MW, Carter BD. TRAF6-mediated ubiquitination regulates nuclear translocation of NRIF, the p75 receptor interactor. The EMBO journal. 2005;24:3859–3868. doi: 10.1038/sj.emboj.7600845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzegrzolka J, Biala M, Wojtyra P, Kobierzycki C, Olbromski M, Gomulkiewicz A, Piotrowska A, Rys J, Podhorska-Okolow M, Dziegiel P. Expression of EMT Markers SLUG and TWIST in Breast Cancer. Anticancer research. 2015;35:3961–3968. [PubMed] [Google Scholar]
- Haupt S, Buckley D, Pang JM, Panimaya J, Paul PJ, Gamell C, Takano EA, Lee YY, Hiddingh S, Rogers TM, et al. Targeting Mdmx to treat breast cancers with wild-type p53. Cell death & disease. 2015;6:e1821. doi: 10.1038/cddis.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. 2009;458:438–444. doi: 10.1038/nature07960. [DOI] [PubMed] [Google Scholar]
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nature reviews. Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- Hong J, Zhou J, Fu J, He T, Qin J, Wang L, Liao L, Xu J. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer research. 2011;71:3980–3990. doi: 10.1158/0008-5472.CAN-10-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi D, Kusdra L, Huskey NE, Chandriani S, Lenburg ME, Gonzalez-Angulo AM, Creasman KJ, Bazarov AV, Smyth JW, Davis SE, et al. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. The Journal of experimental medicine. 2012;209:679–696. doi: 10.1084/jem.20111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen MS, Grant R, Manke I, Minn K, Yu X, Yaffe MB, Chen J. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–914. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Chen HC, Zhang D, Fu J. Twist: a molecular target in cancer therapeutics. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:2497–2506. doi: 10.1007/s13277-013-1002-x. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Nakayama S, Miyoshi Y, Taguchi T, Tamaki Y, Matsushima T, Torikoshi Y, Tanaka S, Yoshida T, Ishihara H, et al. Determination of the specific activity of CDK1 and CDK2 as a novel prognostic indicator for early breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008;19:68–72. doi: 10.1093/annonc/mdm358. [DOI] [PubMed] [Google Scholar]
- Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast cancer research : BCR 9. 2007:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander R, Nasr T, Ochoa SD, Nordin K, Prasad MS, Labonne C. Interactions between Twist and other core epithelial-mesenchymal transition factors are controlled by GSK3-mediated phosphorylation. Nature communications. 2013;4:1542. doi: 10.1038/ncomms2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander R, Nordin K, LaBonne C. The F-box protein Ppa is a common regulator of core EMT factors Twist, Snail, Slug, and Sip1. The Journal of cell biology. 2011;194:17–25. doi: 10.1083/jcb.201012085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, Zhou A, Eyob H, Balakrishnan S, Wang CY, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. The Journal of clinical investigation. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CW, Xia W, Lim SO, Hsu JL, Huo L, Wu Y, Li LY, Lai CC, Chang SS, Hsu YH, et al. AKT1 inhibits epithelial-to-mesenchymal transition in breast cancer through phosphorylation-dependent Twist1 degradation. Cancer research. 2016 doi: 10.1158/0008-5472.CAN-15-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok GT, Sy SM, Dong SS, Ching YP, Tsao SW, Thomson TM, Huen MS. Differential regulation of RNF8-mediated Lys48- and Lys63-based poly-ubiquitylation. Nucleic acids research. 2012;40:196–205. doi: 10.1093/nar/gkr655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiroli F, Sixma TK. Lysine-targeting specificity in ubiquitin and ubiquitin-like modification pathways. Nature structural & molecular biology. 2014;21:308–316. doi: 10.1038/nsmb.2792. [DOI] [PubMed] [Google Scholar]
- Morel AP, Hinkal GW, Thomas C, Fauvet F, Courtois-Cox S, Wierinckx A, Devouassoux-Shisheboran M, Treilleux I, Tissier A, Gras B, et al. EMT inducers catalyze malignant transformation of mammary epithelial cells and drive tumorigenesis towards claudin-low tumors in transgenic mice. PLoS genetics. 2012;8:e1002723. doi: 10.1371/journal.pgen.1002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthwein A, Fradet-Turcotte A, Noordermeer SM, Canny MD, Brun CM, Strecker J, Escribano-Diaz C, Durocher D. Mitosis inhibits DNA double-strand break repair to guard against telomere fusions. Science. 2014;344:189–193. doi: 10.1126/science.1248024. [DOI] [PubMed] [Google Scholar]
- Pan D, Zhu Y, Zhou Z, Wang T, You H, Jiang C, Lin X. The CBM Complex Underwrites NF-kappaB Activation to Promote HER2-Associated Tumor Malignancy. Molecular cancer research : MCR. 2016;14:93–102. doi: 10.1158/1541-7786.MCR-15-0229-T. [DOI] [PubMed] [Google Scholar]
- Perou CM. Molecular stratification of triple-negative breast cancers. The oncologist. 2010;15(Suppl 5):39–48. doi: 10.1634/theoncologist.2010-S5-39. [DOI] [PubMed] [Google Scholar]
- Phu L, Izrael-Tomasevic A, Matsumoto ML, Bustos D, Dynek JN, Fedorova AV, Bakalarski CE, Arnott D, Deshayes K, Dixit VM, et al. Improved quantitative mass spectrometry methods for characterizing complex ubiquitin signals. Molecular & cellular proteomics : MCP 10. 2011;M110:003756. doi: 10.1074/mcp.M110.003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nature cell biology. 2014;16:488–494. doi: 10.1038/ncb2976. [DOI] [PubMed] [Google Scholar]
- Rai R, Li JM, Zheng H, Lok GT, Deng Y, Huen MS, Chen J, Jin J, Chang S. The E3 ubiquitin ligase Rnf8 stabilizes Tpp1 to promote telomere end protection. Nature structural & molecular biology. 2011;18:1400–1407. doi: 10.1038/nsmb.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampieri K, Fodde R. Cancer stem cells and metastasis. Seminars in cancer biology. 2012;22:187–193. doi: 10.1016/j.semcancer.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang Q, Lin Y, Li J, Kang T, Tao M, et al. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer cell. 2014;25:210–225. doi: 10.1016/j.ccr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar JR, Pagan JK, Pagano M. SCF ubiquitin ligase-targeted therapies. Nature reviews. Drug discovery. 2014;13:889–903. doi: 10.1038/nrd4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surade S, Blundell TL. Structural biology and drug discovery of difficult targets: the limits of ligandability. Chemistry & biology. 2012;19:42–50. doi: 10.1016/j.chembiol.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Thorslund T, Ripplinger A, Hoffmann S, Wild T, Uckelmann M, Villumsen B, Narita T, Sixma TK, Choudhary C, Bekker-Jensen S, et al. Histone H1 couples initiation and amplification of ubiquitin signalling after DNA damage. Nature. 2015;527:389–393. doi: 10.1038/nature15401. [DOI] [PubMed] [Google Scholar]
- Vesuna F, Lisok A, Kimble B, Domek J, Kato Y, van der Groep P, Artemov D, Kowalski J, Carraway H, van Diest P, et al. Twist contributes to hormone resistance in breast cancer by downregulating estrogen receptor-alpha. Oncogene. 2012;31:3223–3234. doi: 10.1038/onc.2011.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nature reviews. Cancer. 2014;14:233–247. doi: 10.1038/nrc3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Lv DD, Jiao S, Zhao W, Li X, Sun H, Yan B, Fan L, Hu RG, Fang J. pVHL mediates K63-linked ubiquitination of nCLU. PloS one. 2012;7:e35848. doi: 10.1371/journal.pone.0035848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nature cell biology. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends in cell biology. 2015;25:675–686. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Kang Y. Multilayer control of the EMT master regulators. Oncogene. 2014;33:1755–1763. doi: 10.1038/onc.2013.128. [DOI] [PubMed] [Google Scholar]
- Zhong J, Ogura K, Wang Z, Inuzuka H. Degradation of the transcription factor Twist, an oncoprotein that promotes cancer metastasis. Discovery medicine. 2013;15:7–15. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.