Abstract
IFN responses control acute HSV infection, but their role in regulating HSV latency is poorly understood. To address this we used mice lacking IFN signaling specifically in neural tissues. These mice supported a higher acute viral load in nervous tissue and delayed establishment of latency. While latent HSV-1 genome copies were equivalent, ganglia from neuronal IFN signaling-deficient mice unexpectedly supported reduced reactivation. IFNβ promoted survival of primary sensory neurons after infection with HSV-1, indicating a role for IFN signaling in sustaining neurons. We observed higher levels of latency associated transcripts (LATs) per HSV genome in nice lacking neuronal IFN signaling, consistent with a role for IFN in regulating LAT expression. These data show that neuronal IFN signaling modulates the expression of LAT and may conserve the pool of neurons available to harbor latent HSV-1 genome. The data also show that neuronal IFN signaling is dispensable for the establishment of latency.
Herpes simplex virus 1 (HSV-1) is a highly prevalent neurotropic virus, which cycles between lytic and latent phases of infection (Smith and Robinson, 2002; Xu et al., 2006). HSV-1 establishes latency in the neurons of sensory ganglia, and reactivation from latency can result in disease pathologies ranging from herpes labialis to herpes simplex encephalitis (HSE) (Rowe et al., 2013; Whitley and Gnann, 2002). The mechanisms governing the establishment of, maintenance of and reactivation from latency are unclear, particularly at the level of the sensory neuron. A global interferon (IFN)-driven antiviral response is critical for protection against HSV-1 infection in mice and humans (Andersen et al., 2015; Casrouge et al., 2006; Dupuis et al., 2003; Leib et al., 1999; Luker et al., 2003; Menachery et al., 2010; Zhang et al., 2007). Moreover, neuronal IFN signaling plays a pivotal role in controlling acute HSV-1 replication and pathogenesis (Rosato and Leib, 2015). Accordingly, patients with genetic defects in antiviral signaling suffer from recurrent HSE and neurons derived from these patients are more permissive to HSV infection (Lafaille et al., 2012). We therefore hypothesized that neuronal IFN signaling is important for the establishment and maintenance of HSV-1 latency.
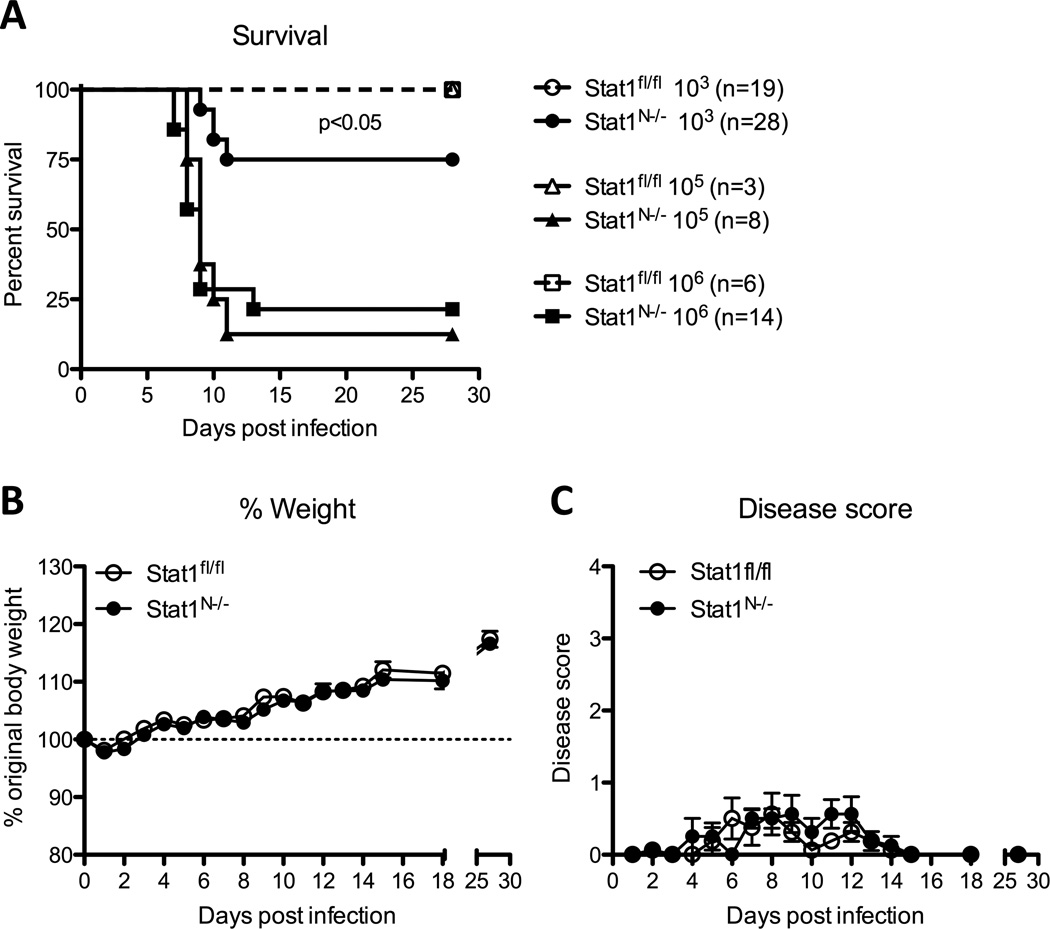
Corneal HSV infection of mice lacking IFNαβγ receptors or STAT1, a critical transcription factor mediating IFN receptor signaling, results in 100% mortality (Pasieka et al., 2011). While these models demonstrate the importance of IFN signaling, the high mortality renders them unsuitable for studying latency. To address this, we used a conditional knock-out mouse, Stat1N−/−, lacking STAT1 specifically in neural tissues (Rosato and Leib, 2015). We infected Stat1N−/− and Stat1fl/fl littermate control mice with WT HSV-1 strain KOS via the cornea (Smith, 1964). While infection of Stat1N−/− mice with 105 or 106 PFU HSV-1 led to near 100% lethality, approximately 75% of Stat1N−/− mice survived an inoculum of 103 PFU (Fig 1A). Therefore, to maximize post-infection survival, we performed all following experiments using this inoculum. Disease scores and weight changes in the surviving Stat1N−/− mice were identical to those observed in Stat1fl/fl controls (Fig 1 B,C).
Figure 1. Mouse model to study neuronal IFN signaling and HSV-1 latency.
Mice expressing CRE recombinase under the neuron-specific nestin promoter were crossed with Stat1fl/fl mice to yield progeny with a neural-specific Stat1 deletion (Stat1N−/−). A) Survival of mice infected via the cornea with 103 (circles), 105 (triangles) or 106 (squares) PFU/eye. Mice were euthanized upon reaching endpoint criteria, here stated as survival. Significance was determined by Log-rank test. B) The percentage of original body weight in infected Stat1N−/− or Stat1fl/fl mice over time. C) Clinical score of HSV-1-infected Stat1N−/− or Stat1fl/fl mice over time. Mice were scored on a scale of 1–4 clinical severity of HSV-1 disease by a masked observer (Jiang et al., 2015). All data are collected from ≥2 independent experiments.
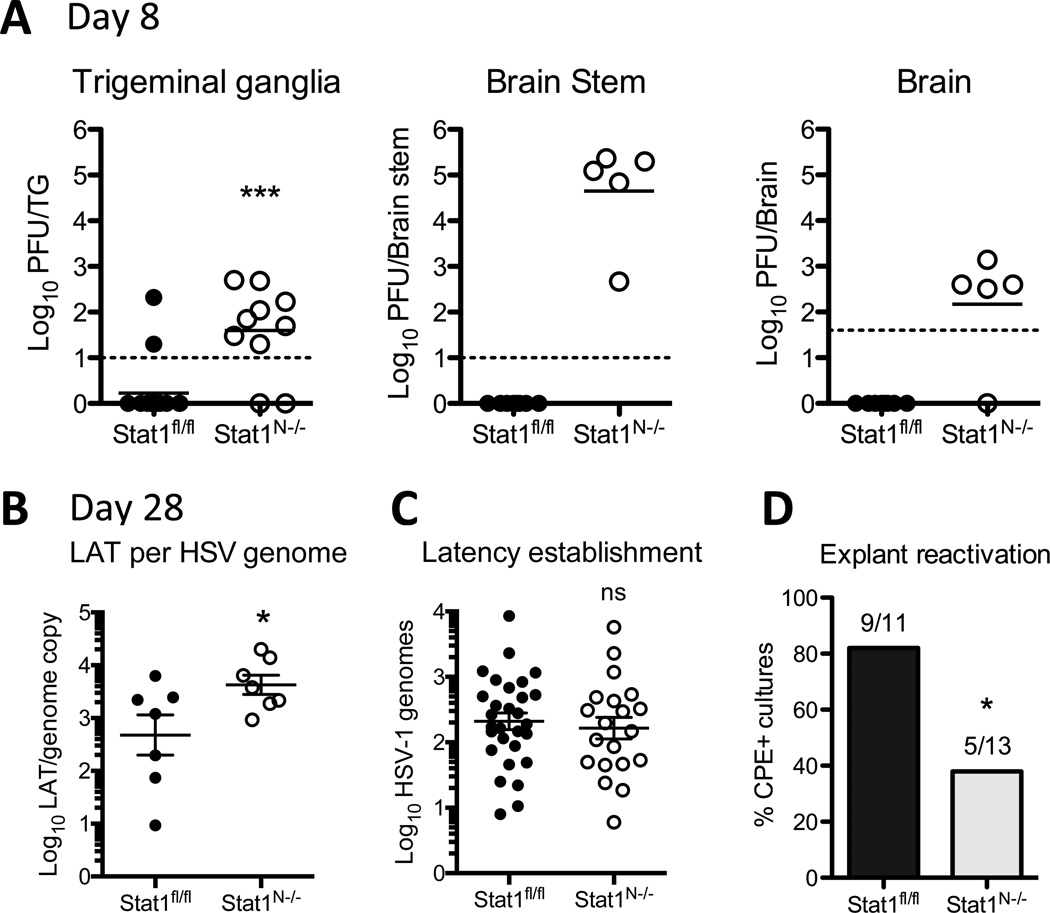
We previously observed increased viral titers in nervous system tissues of Stat1N−/− compared to control mice when infected with strain 17, a more neurovirulent strain of HSV-1 (Rosato and Leib, 2015). In the current model, at 8dpi, titers of strain KOS were low or undetectable in the trigeminal ganglia (TG), brain stem and brain of Stat1fl/fl mice (Fig. 2A). There were, however, significantly increased viral titers in the nervous system of Stat1N−/− mice, consistent with previous data (Rosato and Leib, 2015). This demonstrates that without STAT1 signaling there is prolonged acute replication and therefore delayed establishment of latency.
Figure 2. Stat1N−/− mice support increased acute viral load, yet equivalent latent genomes and reduced reactivation.
A) Viral titers in the trigeminal ganglia, brain stem and brain of Stat1N−/− or Stat1fl/fl mice 8 days post infection with 1×103 PFU KOS. Each datapoint represents one tissue sample from ≥2 independent experiments. Significance was determined by Student’s t-test where *** p<0.001. B) Copies of latency associated transcript (LAT) per HSV genome in Stat1fl/fl or Stat1N−/− TGs at 28dpi. LAT intron was quantified by RT-PCR and normalized to GAPDH. Significance determined by Student’s t-test where * p<0.05. C) HSV-1 genome copy number at 28 dpi in ≥2 independent experiments. Samples with no detectable genomes were omitted from the graph and statistical analysis (Stat1fl/fl 1, Stat1N−/−, 3). Copy number was determined by qPCR for viral TK and normalized to mouse adipsin. Each datapoint represents one TG. Ns=not significant by Student’s t-test. D) Reactivation of HSV-1 in TG explant cultures. TGs from day 28 infected mice were removed, cut into 8 pieces and cultured on a monolayer of Vero cells for 5 days. Cultures were then harvested and supernatant was assessed for CPE on Vero cells in ≥2 independent experiments. Bars depict the percent of TGs with CPE. Significance was determined by Chi-squared analysis where * p<0.05.
We next tested whether neuronal IFN signaling impacts the establishment or maintenance of HSV-1 latency. To assess establishment of latency, we quantified HSV-1 genomes at 28dpi by qPCR, measuring HSV TK copies normalized to the single-copy adipsin gene (Kramer and Coen, 1995). We detected genomes in 20/23 Stat1N−/− TGs, and 29/30 Stat1fl/fl TGs. Additionally, infectious virus was undetectable in both Stat1fl/fl and Stat1N−/− mice, demonstrating that, by 28dpi, latency had been fully established in both groups of mice. To determine whether IFN signaling regulates the latent state, we measured the expression of the latency-associated transcript (LAT) by RT-qPCR (Pan et al., 2014). We found significantly more LAT transcripts per HSV genome in Stat1N−/− mice (Fig 2B), consistent with the hypothesis that that IFN signaling may downregulate the expression of LAT (Catez et al, 2012). Taken together, these data demonstrate that neuronal IFN signaling is dispensable for the establishment of HSV-1 latency, but may play a role in its regulation.
Given the crucial role that IFN signaling plays in controlling HSV lytic replication in neurons, we expected to find more latent genomes and greater reactivation in Stat1N−/− relative to Stat1fl/fl TGs. Unexpectedly, in the samples with detectable genomes, we found no significant difference in HSV genome levels between Stat1N−/− and Stat1fl/fl TGs (Fig 2C). Moreover, using a TG explant model of reactivation (Leib et al., 1989), we detected significantly fewer reactivation events from Stat1N−/− TGs, compared to controls (Fig 2D). This demonstrates that despite a 10–100 fold higher viral load during acute timepoints there is equivalent establishment of latency and reduced reactivation in TGs lacking IFN signaling.
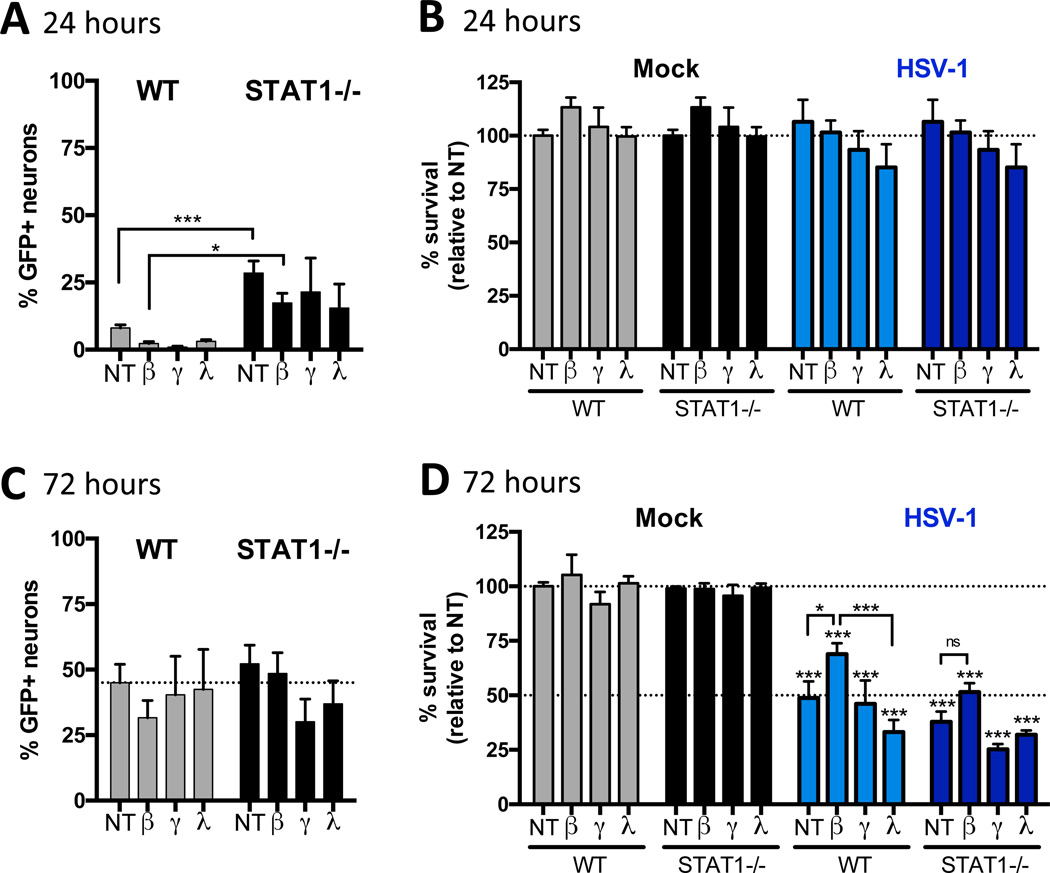
IFNβ is required for long-term neuronal homeostasis even in the absence of infection (Ejlerskov et al, 2015), and can promote viability of neuron cultures after virus infection (Low-Calle et al., 2013; Samuel and Diamond, 2005). We therefore hypothesized that IFN signaling prevents neuronal loss during the initial infection, thereby sustaining the population of neurons that harbors latent HSV-1 genome and reactivation events. Due to the technical challenges of quantifying total numbers of neurons from latently infected TGs, we instead cultured TG neurons from 129SVEV (WT) or STAT1 null (STAT1−/−) adult mice to test this hypothesis (Bertke et al., 2011; Durbin et al., 2002; Rosato and Leib, 2014). Since STAT1 is involved in IFNα/β (Type I), IFNγ (Type II) and IFNλ (Type III) signaling, we tested the ability of all three IFNs to promote neuronal survival after HSV infection. Adult TG neuron cultures were pre-treated with 100U/mL IFNβ, or 100ng/mL IFNγ or IFNλ for 18 hours and then infected with HSV-1 64-GFP KOS, a virus that expresses GFP under the CMV IE promoter (Van Heyningen and Leib, unpublished), to facilitate visualization and quantification of infected neurons. Since adult neurons are fully differentiated and do not undergo division, we were able to examine neuronal survival by quantifying the number of neurons remaining in the cultures, assessed by staining for the neuronal marker NeuN. At 24hpi, we found significantly fewer GFP-positive neurons in WT relative to STAT1−/− cultures in untreated and IFNβ treated groups (Fig. 3A). At that timepoint, there was no change in neuronal survival (Fig. 3B), and by 72hpi, the numbers of infected neurons were comparable in all groups, regardless of IFN status (Fig. 3C). Neuronal survival was also comparable in all uninfected cultures, regardless of IFN treatment (Fig. 3D). However, in the WT infected cultures, IFNβ, but not IFNγ or IFNλ, significantly increased neuronal survival while there was no significant difference between untreated and IFN treated STAT1−/− cultures (Fig 3D). These data are consistent with the hypothesis that there is increased neuron death in the TGs of Stat1N−/− infected mice, resulting in a decreased reservoir of neurons capable of supporting latent infection and reactivation. Taken together, given the equivalent genome load between control and Stat1N−/− mice, these data also imply that there is more viral DNA per neuron in mice lacking neuronal IFN signaling.
Figure 3. IFNβ signaling promotes neuronal survival.
A) Percent of infected neurons in TG neuron cultures isolated from 6–10 week old 129SVEV (WT) or STAT1−/− mice in ≥2 independent experiments. Cells were untreated (NT) or pre-treated with 100 U/mL IFNβ, 100ng/mL IFNγ or 100ng/mL IFNλ for 18 hours then infected with KOS expressing GFP under the CMV promoter (64-GFP). Cultures were fixed at indicated timepoints and stained for the neuronal marker, NeuN. A and C) Quantification of the percent of infected (GFP+) neurons (NeuN+) out of total neurons. B and D) Survival of neurons relative to mock infected cultures. The total number of NeuN+ cells was quantified using Fiji (ImageJ) and expressed as a percent relative to mock-infected, untreated cultures. Significance was determined by two-way analysis of variance followed by Tukey’s multiple comparisons test where * p<0.05, ***p<0.001 compared to mock, unless noted by brackets. Dotted lines represent Mock NT mean (A,B,C and D) and HSV-1 NT mean (D).
Overall, our data are consistent with a model in which neuronal STAT1 signaling facilitates the transition of HSV-1 to a latent state by resolving acute infection and supporting neuronal survival. That being said, HSV-1 latency is established, albeit delayed, in the absence of neuronal STAT1 signaling. We show that neuronal STAT1 signaling may modulate LAT expression, consistent with previous studies (Kriesel et al, 2004). This could also occur through upregulation of the IFN-inducible promyelocytic leukemia (PML) nuclear bodies (Catez et al., 2012), potentially facilitating reactivation from latency (Nicoll et al., 2016). Our findings are consistent with studies demonstrating the ability of exogenous IFNβ to promote HSV latency in vitro (De Regge et al., 2010; Wigdahl et al., 1983). These results also suggest that neuronal IFN signaling is important during reactivation in vivo, to sustain the neuronal population and promote re-establishment of latency. Thus, this study adds to our understanding of the factors governing HSV-1 latency and the neuronal IFN response.
Highlights.
Neuronal IFN responses are dispensable for the establishment of HSV latency.
HSV-1 expresses increased levels of LAT in the absence of IFN signaling.
IFN promotes the survival of neurons following HSV infection.
Acknowledgments
This study was supported by National Institutes of Health grant to D.L. (RO1 EY09083) and D.M.C. (P01 AI098681). The project was also supported by P20RR016437 from the National Center for Research Resources to Dartmouth. Training Grant support from the Geisel School of Medicine Microbiology and Molecular Pathogenesis Program Training Grant (5T32AI007519) to P.R. We also acknowledge David Knipe for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen LL, Mørk N, Reinert LS, Kofod-Olsen E, Narita R, Jørgensen SE, Skipper KA, Höning K, Gad HH, Østergaard L, Ørntoft TF, Hornung V, Paludan SR, Mikkelsen JG, Fujita T, Christiansen M, Hartmann R, Mogensen TH. Functional IRF3 deficiency in a patient with herpes simplex encephalitis. J. Exp. Med. 2015;212:1371–1379. doi: 10.1084/jem.20142274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertke AS, Swanson SM, Chen J, Imai Y, Kinchington PR, Margolis TP. A5-Positive Primary Sensory Neurons are Non-Permissive for Productive Infection with Herpes Simplex Virus 1 In Vitro. J. Virol. 2011 doi: 10.1128/JVI.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casrouge A, Zhang S-Y, Eidenschenk C, Jouanguy E, Puel A, Yang K, Alcais A, Picard C, Mahfoufi N, Nicolas N, Lorenzo L, Plancoulaine S, Sénéchal B, Geissmann F, Tabeta K, Hoebe K, Du X, Miller RL, Héron B, Mignot C, de Villemeur TB, Lebon P, Dulac O, Rozenberg F, Beutler B, Tardieu M, Abel L, Casanova J-L. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- Catez F, Picard C, Held K, Gross S, Rousseau A, Theil D, Sawtell N, Labetoulle M, Lomonte P. HSV-1 Genome Subnuclear Positioning and Associations with Host-Cell PML-NBs and Centromeres Regulate LAT Locus Transcription during Latency in Neurons. PLOS Pathog. 2012;8:e1002852. doi: 10.1371/journal.ppat.1002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Regge N, Van Opdenbosch N, Nauwynck HJ, Efstathiou S, Favoreel HW. Interferon Alpha Induces Establishment of Alphaherpesvirus Latency in Sensory Neurons In Vitro. PLoS ONE. 2010;5:e13076. doi: 10.1371/journal.pone.0013076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, Yang K, Chapgier A, Eidenschenk C, Eid P, Ghonaium AA, Tufenkeji H, Frayha H, Al-Gazlan S, Al-Rayes H, Schreiber RD, Gresser I, Casanova J-L. Impaired response to interferon-[alpha]/[beta] and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- Durbin JE, Johnson TR, Durbin RK, Mertz SE, Morotti RA, Peebles RS, Graham BS. The Role of IFN in Respiratory Syncytial Virus Pathogenesis. J. Immunol. 2002;168:2944–2952. doi: 10.4049/jimmunol.168.6.2944. [DOI] [PubMed] [Google Scholar]
- Ejlerskov P, Hultberg JG, Wang J, Carlsson R, Ambjørn M, Kuss M, Liu Y, Porcu G, Kolkova K, Friis Rundsten C, Ruscher K, Pakkenberg B, Goldmann T, Loreth D, Prinz M, Rubinsztein DC, Issazadeh-Navikas S. Lack of Neuronal IFN-β-IFNAR Causes Lewy Body- and Parkinson's Disease-like Dementia. Cell. 2015;163:324–339. doi: 10.1016/j.cell.2015.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Yin X, Stuart PM, Leib DA. Dendritic Cell Autophagy Contributes to Herpes Simplex Virus-Driven Stromal Keratitis and Immunopathology. mBio. 2015;6:e01426–e01415. doi: 10.1128/mBio.01426-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MF, Coen DM. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 1995;69:1389–1399. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriesel JD, Jones BB, Dahms KM, Spruance SL. STAT1 binds to the herpes simplex virus type 1 latency associated transcript promoter. J. Neurovirol. 2004;10:12–20. doi: 10.1080/13550280490261680. [DOI] [PubMed] [Google Scholar]
- Lafaille FG, Pessach IM, Zhang S-Y, Ciancanelli MJ, Herman M, Abhyankar A, Ying S-W, Keros S, Goldstein PA, Mostoslavsky G, Ordovas-Montanes J, Jouanguy E, Plancoulaine S, Tu E, Elkabetz Y, Al-Muhsen S, Tardieu M, Schlaeger TM, Daley GQ, Abel L, Casanova J-L, Studer L, Notarangelo LD. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature. 2012;491:769–773. doi: 10.1038/nature11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Coen DM, Bogard CL, Hicks KA, Yager DR, Knipe DM, Tyler KL, Schaffer PA. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. Interferons Regulate the Phenotype of Wild-type and Mutant Herpes Simplex Viruses In Vivo. J. Exp. Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low-Calle AM, Prada-Arismendy J, Castellanos JE. Study of interferon-β antiviral activity against Herpes simplex virus type 1 in neuron-enriched trigeminal ganglia cultures. Virus Res. 2013 doi: 10.1016/j.virusres.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Luker GD, Prior JL, Song J, Pica CM, Leib DA. Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. J. Virol. 2003;77:11082–11093. doi: 10.1128/JVI.77.20.11082-11093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menachery VD, Pasieka TJ, Leib DA. Interferon regulatory factor 3-dependent pathways are critical for control of herpes simplex virus type 1 central nervous system infection. J. Virol. 2010;84:9685–9694. doi: 10.1128/JVI.00706-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll MP, Hann W, Shivkumar M, Harman LER, Connor V, Coleman HM, Proença JT, Efstathiou S. The HSV-1 Latency-Associated Transcript Functions to Repress Latent Phase Lytic Gene Expression and Suppress Virus Reactivation from Latently Infected Neurons. PLoS Pathog. 2016;12:e1005539. doi: 10.1371/journal.ppat.1005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Flores O, Umbach JL, Pesola JM, Bentley P, Rosato PC, Leib DA, Cullen BR, Coen DM. A neuron-specific host microRNA targets herpes simplex virus-1 ICP0 expression and promotes latency. Cell Host Microbe. 2014;15:446–456. doi: 10.1016/j.chom.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasieka TJ, Collins L, O’Connor MA, Chen Y, Parker ZM, Berwin BL, Piwnica-Worms DR, Leib DA. Bioluminescent Imaging Reveals Divergent Viral Pathogenesis in Two Strains of Stat1-Deficient Mice, and in αβγ Interferon Receptor-Deficient Mice. PloS One. 2011;6:e24018. doi: 10.1371/journal.pone.0024018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato PC, Leib DA. Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis. PLoS Pathog. 2015;11:e1005028. doi: 10.1371/journal.ppat.1005028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato PC, Leib DA. Intrinsic innate immunity fails to control herpes simplex virus and vesicular stomatitis virus replication in sensory neurons and fibroblasts. J. Virol. 2014;88:9991–10001. doi: 10.1128/JVI.01462-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL. Herpes keratitis. Prog. Retin. Eye Res. 2013;32:88–101. doi: 10.1016/j.preteyeres.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Diamond MS. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 2005;79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Robinson NJ. Age-Specific Prevalence of Infection with Herpes Simplex Virus Types 2 and 1: A Global Review. J. Infect. Dis. 2002;186:S3–S28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- Smith KO. RELATIONSHIP BETWEEN THE ENVELOPE AND THE INFECTIVITY OF HERPES SIMPLEX VIRUS. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. N. Y. N. 1964;115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Gnann JW. Viral encephalitis: familiar infections and emerging pathogens. Lancet. 2002;359:507–513. doi: 10.1016/S0140-6736(02)07681-X. [DOI] [PubMed] [Google Scholar]
- Wigdahl BL, Ziegler RJ, Sneve M, Rapp F. Herpes simplex virus latency and reactivation in isolated rat sensory neurons. Virology. 1983;127:159–167. doi: 10.1016/0042-6822(83)90380-x. [DOI] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- Zhang S-Y, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku C-L, Casrouge A, Zhang X-X, Barreiro L, Leonard J, Hamilton C, Lebon P, Héron B, Vallée L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova J-L. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]