Introduction
Ozone is an unstable allotropic form of oxygen that imparts oxidative stress following reactions with various biological components. By exploiting this mechanism, clinicians have utilized “ozone therapy” as a topical and systemic agent, to treat an array of conditions such as skin lesions, abscesses, autoimmune disease, and more. In recent years, though, there has been a rise in the use of ozone therapy in the treatment of spinal disorders (e.g., disk herniation). A systematic review and meta-analysis of ozone therapy for low back pain secondary to herniated disc provides plausible evidence that ozone therapy applied intradiscally can provide long-term relief of lumbar back pain [16]. In the case of ozone injection directly into the nucleus pulposus, there is some evidence that ozone dissolves in the intradiscal water and reacts with the proteoglycans and glicosoamynoglycans. This reaction entails an oxidation of these substrates leading to disintegration of the disc structure. Its collapse frees the entrapped water that, after reabsorption, allows a decrease of intradiscal pressure and possibly a disappereance of pain due to the reduced pressure on the nervous root [6]. Despite the short-term success of ozone therapy in this context, however, its safety profile and overall effectiveness remain poorly understood and controversial worldwide [16].
Although lumbar- and thoracic-level spondylodiscitis is common, cervical spondylodiscitis is a rare finding, with less than 6% of overall spine infections arising at the cervical level [29]. Mechanical complications of cervical spondylodiscitis treatment are related to the potential instability caused by cervical laminoplasty that is usually required for abscess drainage. Notably, this procedure has resulted in development of postoperative kyphosis and late deterioration, characterized by persistence of pain and other debilitating symptoms [27].
Here, we present a case of a subaxial cervical spondylodiscitis following intradiscal ozone–oxygen (O3-O2) chemonucleolysis for cervical disc herniation in a patient who subsequently developed cervical instability with progressive kyphosis.
Case Report
In September 2005, a previously healthy 51-year-old female presented to our institution with severe sepsis secondary to C6–C7 spondylodiscitis. Ten days prior, the patient had undergone C6–C7 intradiscal O3-O2 chemonucleolysis at an outside hospital as treatment for a cervical disk herniation. The patient explained that the procedure was performed without any incidents using X-ray as a guidance to the injection. At admission, the patient was febrile and complained of diffuse neck pain.
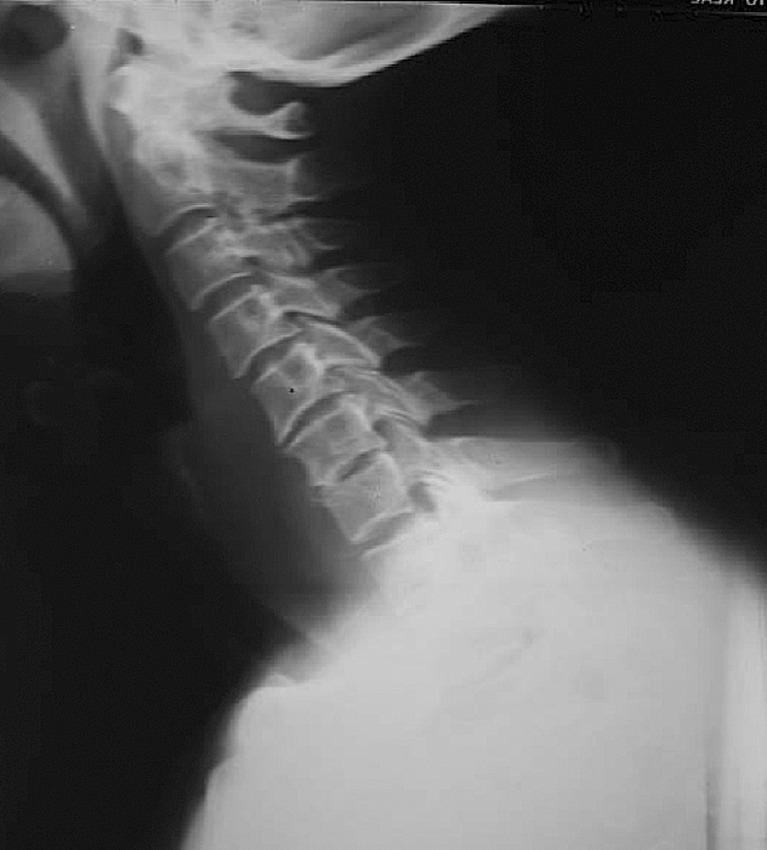
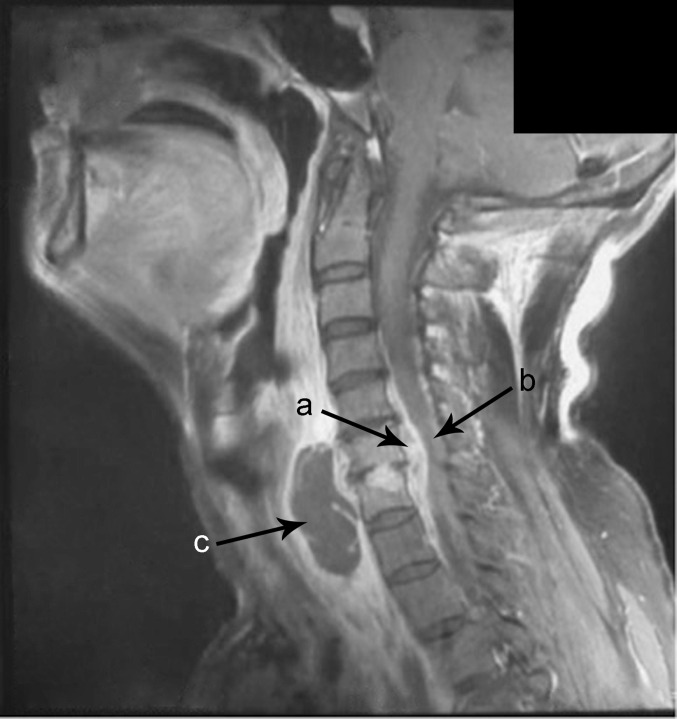
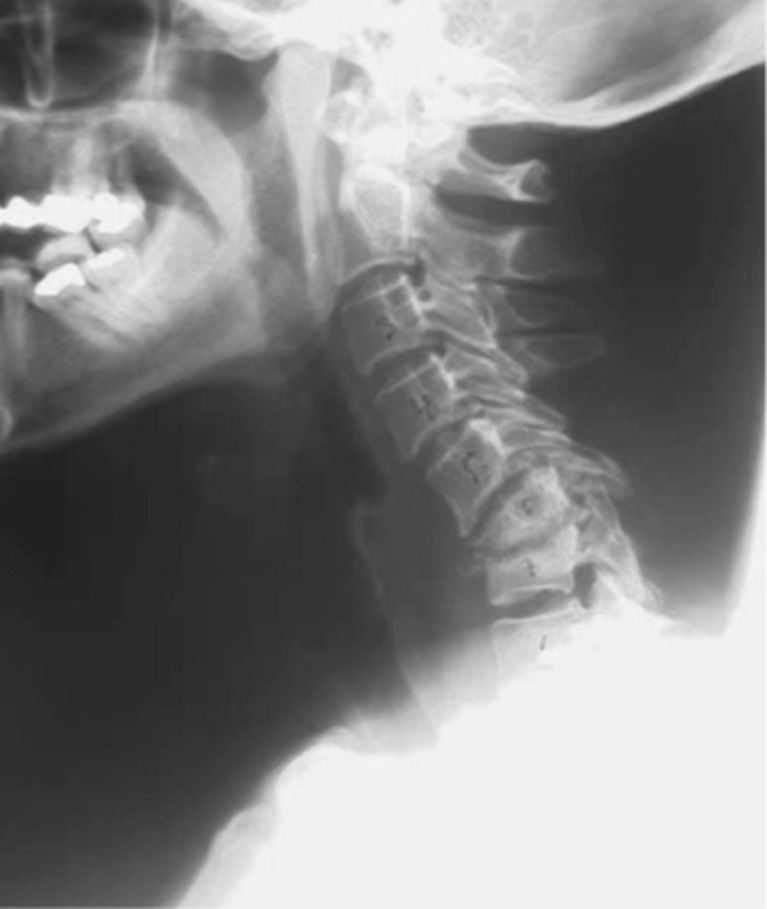
Physical examination revealed a temperature of 38.7°C and neck tenderness without neurological deficit. Hoffmann’s sign was positive and no sphincter disturbance was found. Laboratory findings showed leukocytosis (WBC, 57,000/mm3) with elevated erythrocyte sedimentation rate (ESR, 112 mm/h) and C-reactive protein (CRP, 21 mg/L). Plain radiographs of the cervical spine showed narrowing of the disc space at C6–C7 (Fig. 1), and magnetic resonance imaging (MRI) revealed extensive spinal epidural abscess (SEA) extending from C4–T1 anteriorly, C6–C7 spondylodiscitis, prevertebral abscess, and spinal cord compression (Fig. 2). The patient was admitted to the neurosurgical unit and was empirically started on 2 g IV ceftazidime, every 8 h.
Fig. 1.
Cervical radiograph performed on admission showing narrowing of the disc space at C6–C7.
Fig. 2.
Cervical sagittal T1-weighted gadolinium-enhanced MR image performed on admission shows (a) an extensive spinal epidural abscess (SEA) extending from C4 to T1 anterior to the spinal cord; (b) spinal cord myelopathy can be noted from C5 to C7 due to anterior compression by the lesion (black arrow); (c) also, it shows the infection of C6–C7 intervertebral disc linked with the epidural lesion and a prevertebral abscess with hypointense content and homogeneous enhancement of wall and septa.
On hospital day 2, the patient developed tachycardia, dyspnea, and hypoxemia. Physical exam demonstrated a progressive, global neurological deficit including diffuse motor weakness (grade 2–3/5). The neurosurgeon on call performed urgent abscess drainage and posterior decompressive laminectomies at levels C4–T1. Access and drainage of the wide prevertebral abscess were performed using a standard right-sided anterior cervical approach.
Beta-hemolytic streptococcus was isolated from the epidural infected tissue so a transesophageal puncture during disc ozone therapy was suspected. No bacterial growth was noted in the patient’s blood cultures. Following diagnosis, antibiotic therapy was converted to vancomycin according to local prococols and recommendations of the infectious diseases unit. From this point, the patient had a satisfactory postoperative course and a marked decrease in inflammatory biomarkers was observed. Given the trend, she was discharged home a few days later with an adjustable plastic Thomas collar.
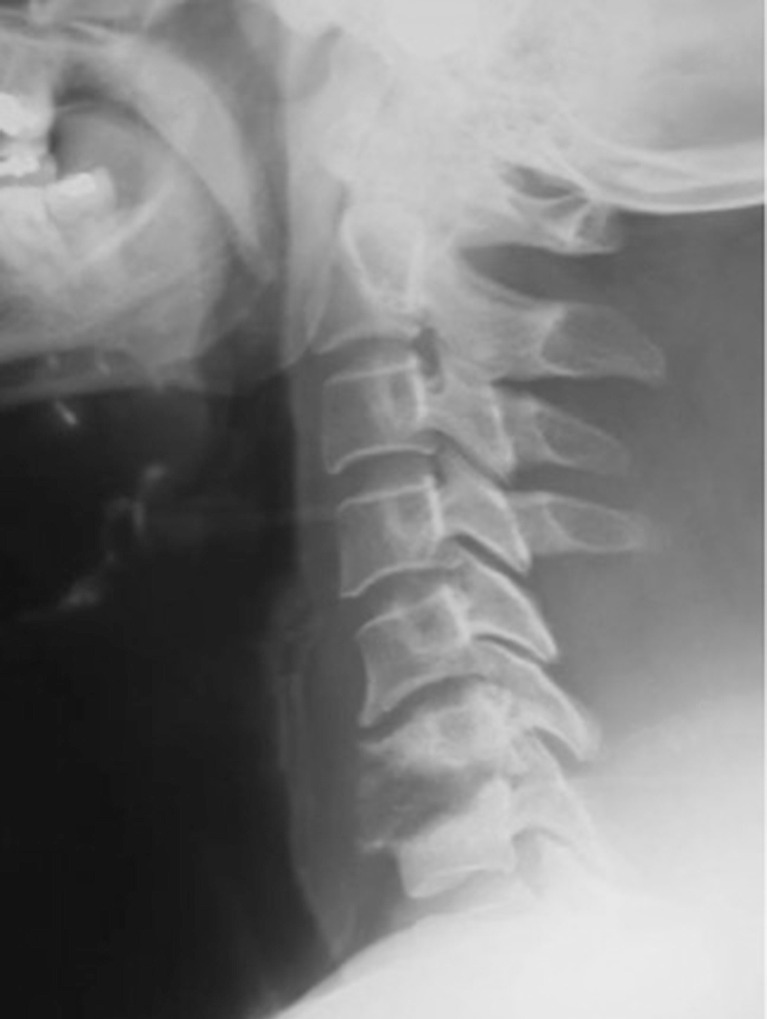
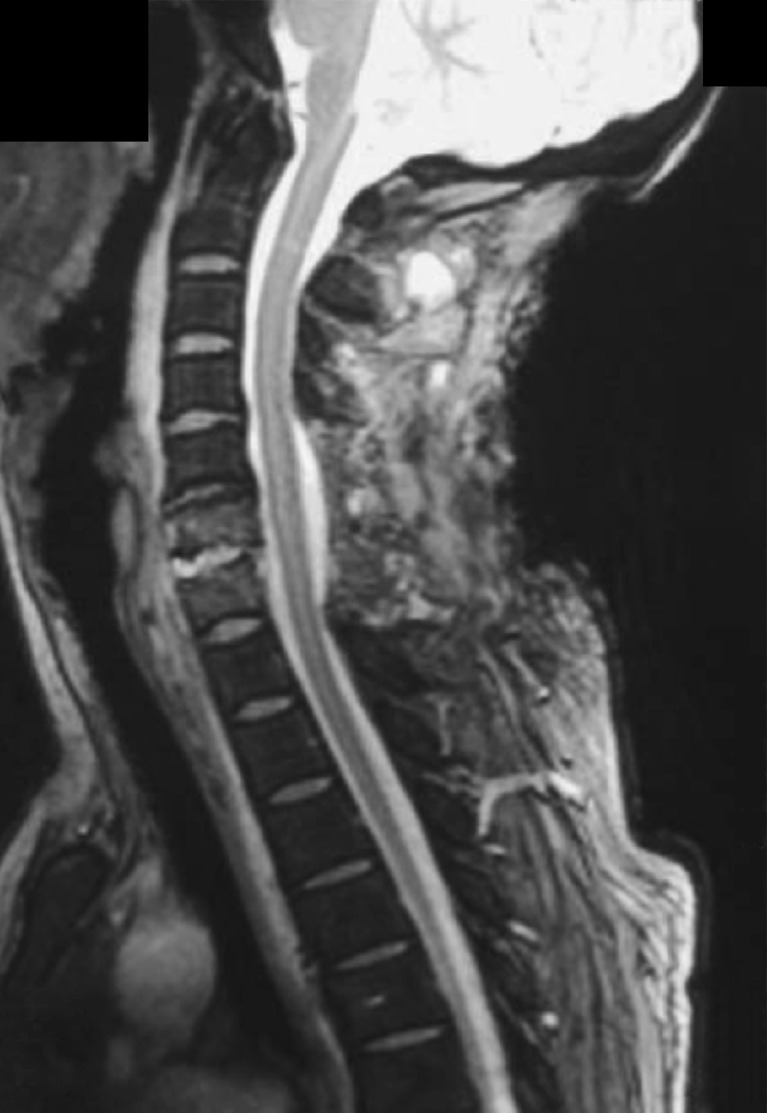
Plain radiograph (Fig. 3) and postoperative MR (Fig. 4) imaging performed at 1 week postoperation showed no further evidence of spinal cord compression, a partial resolution of the epidural mass, and a straight cervical spine without development of kyphotic deformity in sagittal view, as was seen in preoperative studies. Abnormal findings were limited to trace residual epidural enhancement.
Fig. 3.
Lateral cervical spine radiograph performed after surgery showing curettage of C6 and C7 vertebras and cervical laminectomy.
Fig. 4.
Cervical sagittal gradient recalled echo (GRE) T2-weighted imaging (T2WI) gadolinium-enhanced MR image performed 1 week after the surgery showing no further evidence of spinal cord compression, partial resolution of the epidural mass, and a straight cervical spine in sagittal alignment similar to that preoperatively, without development of kyphotic deformity.
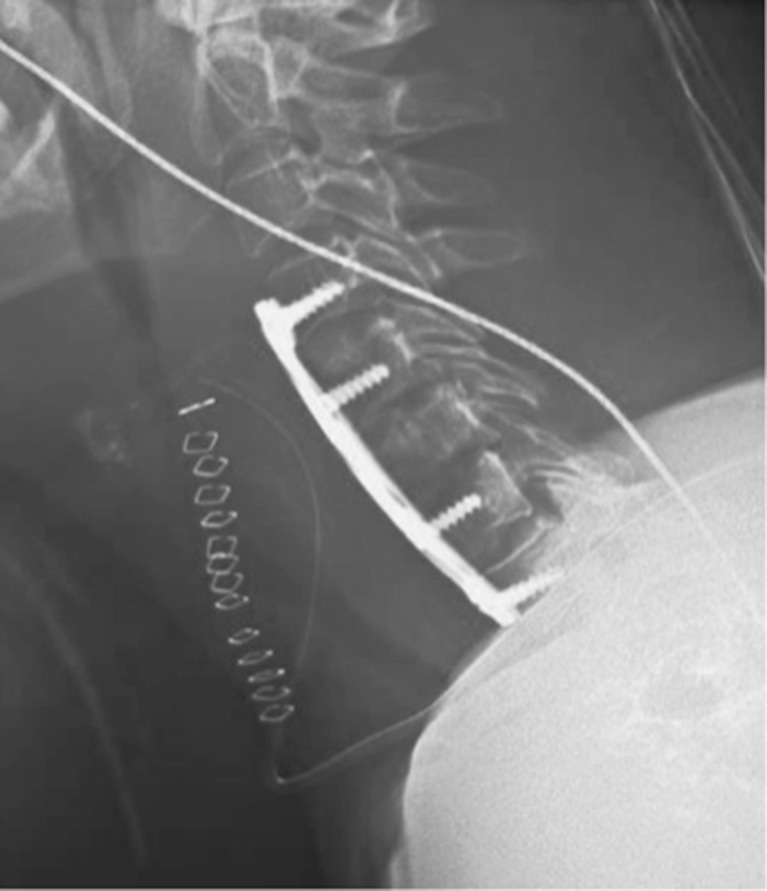
Two weeks later, however, the patient returned to the emergency department for significant neck pain without neurological deficit or fever. Interestingly, the plain radiograph of the cervical spine showed a kyphotic deformity (Fig. 5). Gardner–Wells skull traction was immediately performed and resulted in both clinical and radiologic improvement. The patient was consented for anterior cervical fusion using an anterior cervical approach (left side to avoid previous incision). An extensive curettage of the entire area of spondylodiscitis was performed and included multilevel discectomies, tricortical graft with autologous bone from the iliac crest, and C4–T1 stabilization with an Orion Anterior Cervical Plate System (Medtronic Sofamor-Danek Group, Memphis, TN, USA; Fig. 6). Postoperative course was uneventful and, at the short follow-up, the patient remained clinically satisfactory without evidence of a recurrent spinal infection.
Fig. 5.
Lateral cervical spine radiograph performed 2 weeks after surgery revealing a kyphotic deformity.
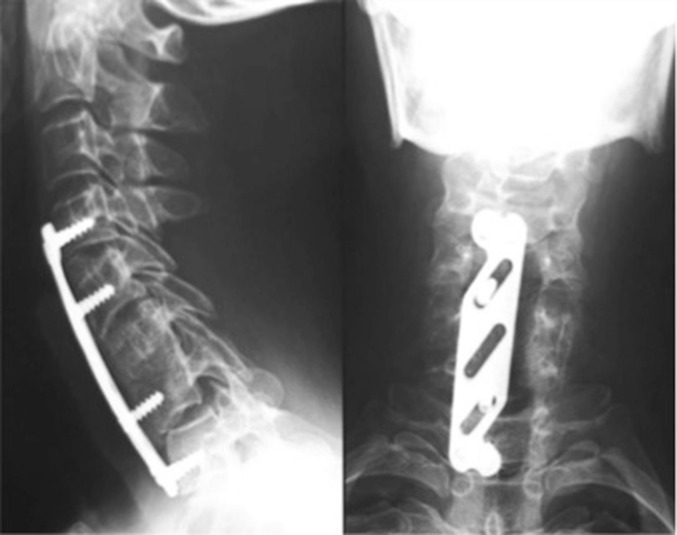
Fig. 6.
Lateral cervical spine radiograph performed after C4–T1 stabilization with an Orion Anterior Cervical Plate System (Medtronic Sofamor-Danek Group, Memphis, TN, USA) showing a slight improvement in the cervical spinal curve.
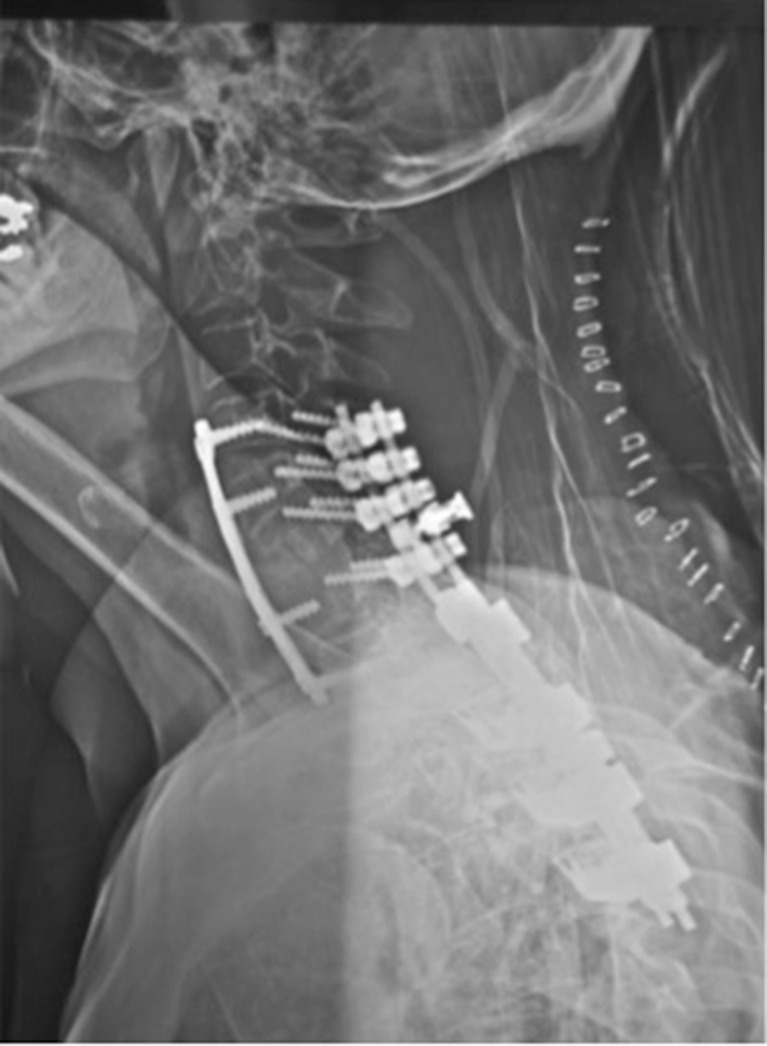
At 6-month follow-up, the patient still complained about neck pain. Plain radiograph was obtained and showed a nonconsolidation of the anterior C7–T1 fusion with kyphotic deformity (Fig. 7). Revision surgery was performed and consisted of a posterior cervicothoracic fusion (C4–T4) with Colorado 2 Spinal System and Vertex Reconstruction System (Medtronic Sofamor-Danek Group, Memphis, TN, USA; Figs. 8 and 9) with favorable clinical outcome.
Fig. 7.
Lateral and AP radiograph of cervical spine at nearly 6 months after anterior fusion showing a nonconsolidation of C7–T1 segment with mild kyphotic deformity.
Fig. 8.
Lateral radiograph of cervical spine after revision surgery; posterior cervicothoracic fusion (C4–T4) (Colorado 2 Spinal System and Vertex Reconstruction System [Medtronic Sofamor-Danek Group, Memphis, TN, USA]).
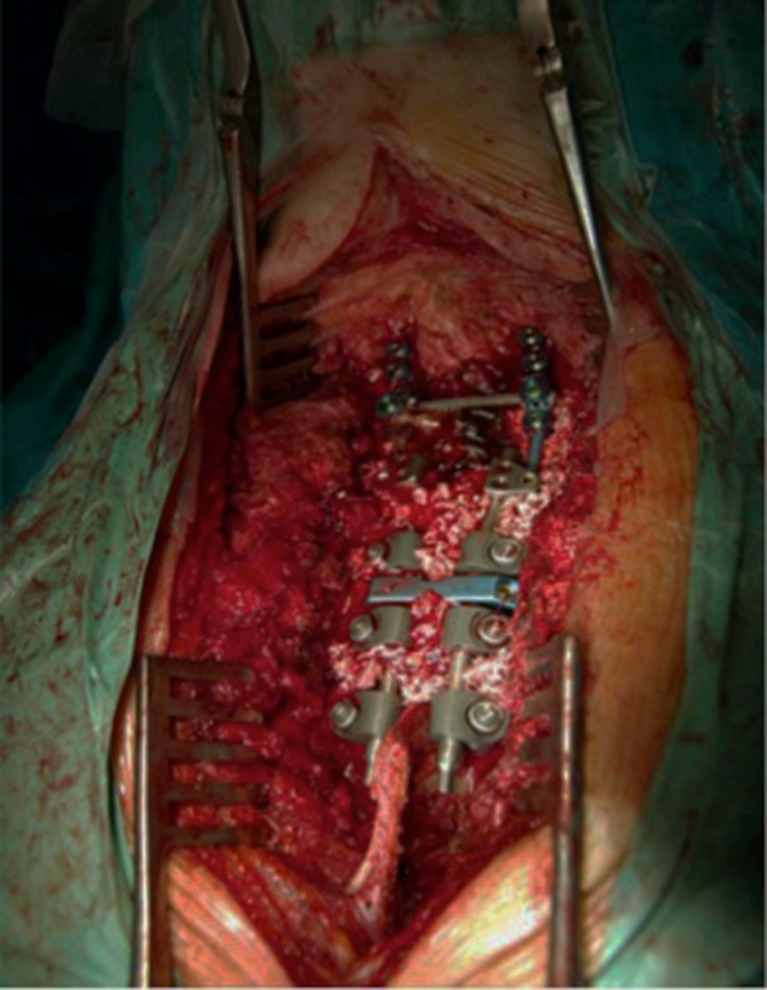
Fig. 9.
Intraoperative photography showing posterior instrumentation and autograft combined with demineralized bone matrix.
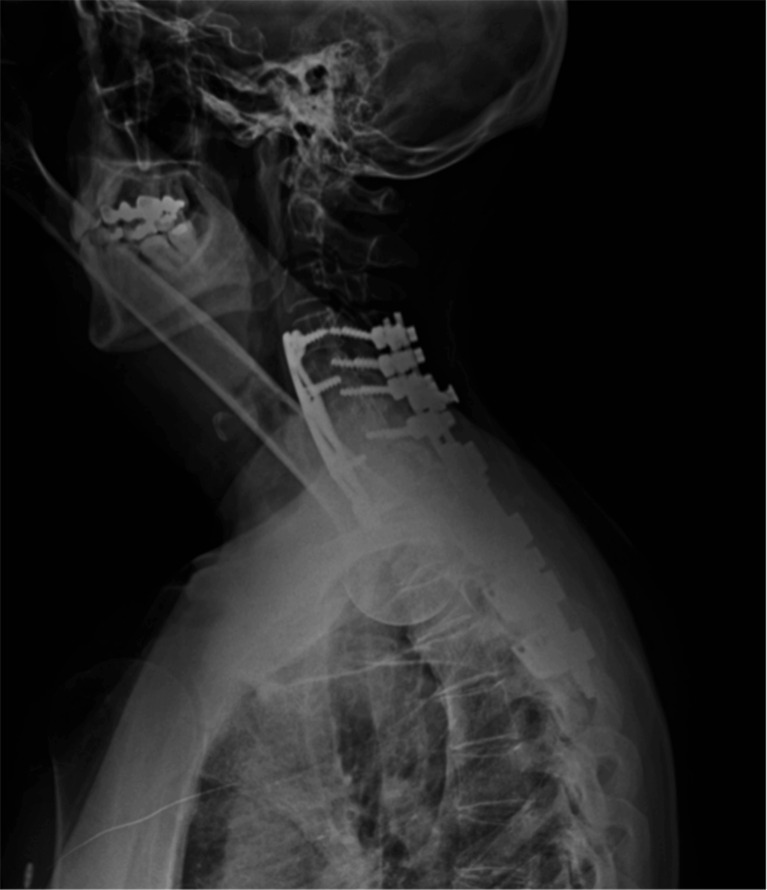
At 1-year follow-up, the patient reported improvement of her neurological function and follow-up radiograph revealed no deterioration of kyphotic deformity and a satisfactory cervical fusion (Fig. 10). The patient was satisfied with the result with occasional axial pain, including neck pain and shoulder stiffness. There was not any recurrence or deterioration of neurological status.
Fig. 10.
Lateral radiograph of cervicothoracic junction at nearly 1 year showing no deterioration of kyphotic deformity and a satisfactory cervical fusion.
Discussion
We present a case of cervical spondylodiscitis that occurred as a complication of O3-O2 therapy for a cervical disc herniation that manifested as cervical mechanic failure likely due to wide laminectomy.
The proposed therapeutic action of cervical intradiscal O3-O2 chemonucleolysis is multifold. It is reported to reduce herniated disc volume and in turn nerve root compression [13]. Second, disc size reduction reduces venous stasis caused by microvascular compression, so as to restore local microcirculation. Specifically, restoring normal flow ameliorates pain through a mechanism explained by Fernandez et al., who previously demonstrated that nerve roots are sensitive to hypoxia [14]. A third therapeutic claim is that it may trigger analgesic and anti-inflammatory effects, through molecular changes that block, or antagonize, so-called disc-induced pain [6].
Medical ozone is administered in the form of an ozone–oxygen gas mixture at nontoxic concentrations between 27 and 30 μg/mL. Placement of the needle inside cervical disc is usually administered under local anesthesia and by an antero-lateral approach with the patient in supine position under CT or fluoroscopic guidance, from the same side as the main location of symptoms. The unstable condition of medical ozone, which starts decaying (2 μg/mL) after about 20 s, allows a limited time for injection of no more than 15 s. Typically, no premedication is given, aseptic techniques are used, and the procedure is performed at an outpatient facility [1, 2, 25].
While ozone exposure has been linked to a number of adverse health effects [3], complications secondary to oxygen–ozone treatment are rare [5]. Nevertheless, its novel application as a percutaneous technique in treating spine disorders, especially at the lumbar spine, raises concerns about safety [30]. In a 2900-case experience, Muto et al. [20] reported no neurological or infectious complications during the short-term or long-term follow-up. Furthermore, only three local infectious complications are reported in the literature. Fort’s group reported a spondylodiscitis in the lumbar spine after an intradiscal injection of oxygen–ozone caused by Achromobacter xylosoxidans [10]; Menendez P et al. reported a Staphylococcus aureus paravertebral and intraabdominal abscess after six cycles of oxygen–ozone therapy [18]; and Bo et al. reported the first and so far only case reported of a cervical spondylodiscitis with associated spinal epidural abscess (SEA) secondary to oxygen–ozone therapy for cervical disc herniation; some similarities with our case are noted including development of cervical infection with SEA and instability after debridement, and some differences like a sterility technique problem instead of a transesophageal puncture [4]. Other systemic complications reported are fulminant septicemia, a vertebrobasilar stroke, bilateral vitreo-retinal hemorrhages, and an unexpected death caused by gas embolism [7, 11, 15, 17].
In the present report, beta-hemolytic streptococcus, a bacteria native to the respiratory tract, was isolated. This suggests a transesophageal puncture during disc ozone therapy, a complication not previously reported [8, 19]. Our patient did not have any predisposing risk factors, and due to rapid neurological worsening and failure of conservative measures, an emergent decompressive surgery was necessary.
Treatment of cervical spondylodiscitis includes (a) drainage of abscess to reverse sepsis and obtainment of additional material for culture and pathological analysis; (b) relief of compression from epidural sequestra, abscess formation, or kyphosis; (c) spinal stabilization with or without instrumentation; and (d) appropriate antibiotic therapy to eradicate the infection and reduce the chance of recurrence [24, 29]. Historically, most spinal epidural abscesses (SEAs) associated with spondylodiscitis are located ventral to the spinal cord, so surgical debridement is often performed first, via an anterior approach [12]. Cervical laminectomy alone results in a 6–47% incidence of postlaminectomy kyphosis that contributes to varying rates of late neurological deterioration (10–39%) [26]. In similarity with Bo et al. [4], our patient initially underwent wide laminectomy without fusion. This led to subsequent kyphotic deformity, necessitating cervicothoracic fusion. Fortunately, no severe neurologic deficit occurred. The follow-up radiograph at nearly 6 months after cervicothoracic fusion shows nonunion at C7–T1, probably due to the instability of the construct at this segment. This points to the need for posterior fusion or, in some cases, combined anterior and posterior fusion. With long cervical fusions ending at C7, we prefer extending the fusion across the cervicothoracic junction to reduce the potential for adjacent segment breakdown and an area of weakness in the arthrodesis level of the cervicothoracic junction that might favor a failure of fusion [9].
Following debridement of a cervical spine infection, reconstruction of the resulting defect and achievement of a stable construct with or without instrumentation are recommended to treat the infection and prevent kyphosis [31]. Shousha et al. in a review of 30 consecutive cervical spondilodyscitis reported good results following surgical treatment based on two principles: (1) meticulous surgical debridement including removal of all necrotic tissues, drainage of the SEA, and decompression of the spinal canal; and (2) reconstruction of the resulting defect and achievement of a stable construct with or without instrumentation [29]. On the other hand, Muzii et al. presented eight cases with cervical spondylodiscitis and SEA formation surgically treated (discectomy, debridement, and decompression of the spinal canal) without any reconstruction and without arthrodesis. Good outcomes of fusions without kyphosis were reported [21]. Shad et al. reported asymptomatic persistent bacterial colonization close to the removed anterior plates 1 year after surgery in four of five cases of cervical spondylodiscitis [28].
Important to mention is that most of these studies included a limited number of cases. Therefore, there is still controversy over the best surgical management of cervical spondylodiscitis, as both single-stage or two-stage have been proposed [22, 23]. No clear guidelines have addressed the utility of cervical instrumentation in treating cervical spine infections or proper course of antibiotic therapy and appear to vary according to clinician. A definitive recommendation regarding the optimal surgical strategy (one or two stages) is not possible given the current literature evidence and the heterogeneity among case series. An advantage of early mobilization in patients undergoing single-stage operations is to assume lower rates of complications such as pneumonia and deep venous thrombosis. Eradication of infection before a two-stage surgery should be verified by postoperative normalization of ESRs and CRP levels, and a relative advantage of this approach is a theoretically less risk of recurrence of infection and to achieve a satisfactory fusion.
In conclusion, this case illustrates that the safety of intradiscal oxygen–ozone therapy has not been established and related complications are increasingly reported in the literature. In contrast with other locations of spinal infections, cervical spondylodiscitis is rare but can carry devastating morbidity if neurologic and functional deficits result. According to our experience, a stable construct is necessary in treating a cervical spondylodiscitis, especially if cervical instability presents before or after surgical debridement; combined anterior/posterior debridement should be avoided (if possible) in this setting. Appropriate treatment of cervical instability depends on the number of levels involved, and surgical options include anterior, posterior, or combined fusions; a stable construct is necessary when there is any concern for instability.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
Compliance with Ethical Standards
Conflict of Interest
Pablo Andrés-Cano, MD; Tomás Vela, MD; Claudio Cano, MD; Gaspar García, MD; Juan Carlos Vera, MD; and Jose Antonio Andrés-García, MD, have declared that they have no conflicto of interest.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent
Informed consent was waived from all patients for being included in the study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Work performed at Orthopaedic Surgery and Traumatology Department, Hospital Universitario Puerta del Mar, Cádiz, Cádiz, Spain.
References
- 1.Andreula C, Muto M, Leonardi M. Interventional spinal procedures. Eur J Radiol. 2004;50:112–119. doi: 10.1016/j.ejrad.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Andreula CF, Simonetti L, De Santis F, Agati R, Ricci R, Leonardi M. Minimally invasive oxygen-ozone therapy for lumbar disk herniation. AJNR Am J Neuroradiol. 2003;24:996–1000. [PMC free article] [PubMed] [Google Scholar]
- 3.Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987-2000. JAMA. 2004;292:2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bo W, Longyi C, Jian T, Guangfu H, Hailong F, Weidong L, et al. A pyogenic discitis at c3-c4 with associated ventral epidural abscess involving c1-c4 after intradiscal oxygen-ozone chemonucleolysis: a case report. Spine (Phila Pa 1976) 2009;34:E298–304. doi: 10.1097/BRS.0b013e318195a87e. [DOI] [PubMed] [Google Scholar]
- 5.Bocci V, Borrelli E, Travagli V, Zanardi I. The ozone paradox: ozone is a strong oxidant as well as a medical drug. Med Res Rev. 2009;29:646–682. doi: 10.1002/med.20150. [DOI] [PubMed] [Google Scholar]
- 6.Borrelli E. Mechanism of action of oxygen ozone therapy in the treatment of disc herniation and low back pain. Acta Neurochir Suppl. 2011;108:123–125. doi: 10.1007/978-3-211-99370-5_19. [DOI] [PubMed] [Google Scholar]
- 7.Corea F, Amici S, Murgia N, Tambasco N. A case of vertebrobasilar stroke during oxygen-ozone therapy. J Stroke Cerebrovasc Dis. 2004;13:259–261. doi: 10.1016/j.jstrokecerebrovasdis.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Cullen JR, Primrose WJ, Vaughn CW. Osteomyelitis as a complication of a tracheo-oesophageal puncture. J Laryngol Otol. 1993;107:242–244. doi: 10.1017/S0022215100122753. [DOI] [PubMed] [Google Scholar]
- 9.Deen HG, Nottmeier EW, Reimer R. Early complications of posterior rod-screw fixation of the cervical and upper thoracic spine. Neurosurgery. 2006;59:1062–1067. doi: 10.1227/01.NEU.0000245592.54204.D0. [DOI] [PubMed] [Google Scholar]
- 10.Fort NM, Aichmair A, Miller AO, Girardi FP. L5-S1 Achromobacter xylosoxidans Infection Secondary to Oxygen-Ozone Therapy for the Treatment of Lumbosacral Disc Herniation: A Case Report and Review of the Literature. Spine (Phila Pa 1976) 2014;39:E413–416. doi: 10.1097/BRS.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 11.Gazzeri R, Galarza M, Neroni M, Esposito S, Alfieri A. Fulminating septicemia secondary to oxygen-ozone therapy for lumbar disc herniation: case report. Spine (Phila Pa 1976) 2007;32:E121–123. doi: 10.1097/01.brs.0000254125.85406.6e. [DOI] [PubMed] [Google Scholar]
- 12.Heyde CE, Boehm H, El Saghir H, Tschoke SK, Kayser R. Surgical treatment of spondylodiscitis in the cervical spine: a minimum 2-year follow-up. Eur Spine J. 2006;15:1380–1387. doi: 10.1007/s00586-006-0191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehnert T, Naguib NN, Wutzler S, Nour-Eldin NE, Bauer RW, Kerl JM, et al. Analysis of disk volume before and after CT-guided intradiscal and periganglionic ozone-oxygen injection for the treatment of lumbar disk herniation. J Vasc Interv Radiol. 2012;23:1430–1436. doi: 10.1016/j.jvir.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Leon Fernandez OS, Pantoja M, Diaz Soto MT, Dranguet J, Garcia Insua M, Viebhan-Hansler R, et al. Ozone oxidative post-conditioning reduces oxidative protein damage in patients with disc hernia. Neurol Res. 2012;34:59–67. doi: 10.1179/1743132811Y.0000000060. [DOI] [PubMed] [Google Scholar]
- 15.Lo Giudice G, Valdi F, Gismondi M, Prosdocimo G, de Belvis V. Acute bilateral vitreo-retinal hemorrhages following oxygen-ozone therapy for lumbar disk herniation. Am J Ophthalmol. 2004;138:175–177. doi: 10.1016/j.ajo.2004.02.059. [DOI] [PubMed] [Google Scholar]
- 16.Magalhaes FN, Dotta L, Sasse A, Teixera MJ, Fonoff ET. Ozone therapy as a treatment for low back pain secondary to herniated disc: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2012;15:E115–129. [PubMed] [Google Scholar]
- 17.Marchetti D, La Monaca G. An unexpected death during oxygen-ozone therapy. Am J Forensic Med Pathol. 2000;21:144–147. doi: 10.1097/00000433-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Menendez P, Garcia A, Pelaez R. Paravertebral and intra-abdominal abscess due to oxygen-ozone therapy for lower back pain. Rev Esp Cir Ortop Traumatol. 2014;58:125–127. doi: 10.1016/j.recot.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Metcalfe S, Morgan-Hough C. Cervical epidural abscess and vertebral osteomyelitis following non-traumatic oesophageal rupture: a case report and discussion. Eur Spine J. 2009;18(Suppl 2):224–227. doi: 10.1007/s00586-009-0889-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muto M, Ambrosanio G, Guarnieri G, Capobianco E, Piccolo G, Annunziata G, et al. Low back pain and sciatica: treatment with intradiscal-intraforaminal O(2)-O (3) injection. Our experience. Radiol Med. 2008;113:695–706. doi: 10.1007/s11547-008-0302-5. [DOI] [PubMed] [Google Scholar]
- 21.Muzii VF, Mariottini A, Zalaffi A, Carangelo BR, Palma L. Cervical spine epidural abscess: experience with microsurgical treatment in eight cases. J Neurosurg Spine. 2006;5:392–397. doi: 10.3171/spi.2006.5.5.392. [DOI] [PubMed] [Google Scholar]
- 22.Nakase H, Matsuda R, Tamaki R, Tei R, Park YS, Sakaki T. Two-stage management for vertebral osteomyelitis and epidural abscess: technical note. Neurosurgery. 2006;58:E1219. doi: 10.1227/01.NEU.0000215996.62828.76. [DOI] [PubMed] [Google Scholar]
- 23.Ogden AT, Kaiser MG. Single-stage debridement and instrumentation for pyogenic spinal infections. Neurosurg Focus. 2004;17:E5. doi: 10.3171/foc.2004.17.6.5. [DOI] [PubMed] [Google Scholar]
- 24.Ozkan N, Wrede K, Ardeshiri A, Hagel V, Dammann P, Ringelstein A, et al. Cervical spondylodiscitis--a clinical analysis of surgically treated patients and review of the literature. Clin Neurol Neurosurg. 2014;117:86–92. doi: 10.1016/j.clineuro.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 25.Rahimi-Movaghar V, Eslami V. The major efficient mechanisms of ozone therapy are obtained in intradiscal procedures. Pain Physician. 2012;15:E1007–1008. [PubMed] [Google Scholar]
- 26.Scheer JK, Tang JA, Smith JS, Acosta FL, Jr, Protopsaltis TS, Blondel B, et al. Cervical spine alignment, sagittal deformity, and clinical implications: a review. J Neurosurg Spine. 2013;19:141–159. doi: 10.3171/2013.4.SPINE12838. [DOI] [PubMed] [Google Scholar]
- 27.Schimmer RC, Jeanneret C, Nunley PD, Jeanneret B. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. 2002;15:110–117. doi: 10.1097/00024720-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Shad A, Shariff S, Fairbank J, Byren I, Teddy PJ, Cadoux-Hudson TA. Internal fixation for osteomyelitis of cervical spine: the issue of persistence of culture positive infection around the implants. Acta Neurochir (Wien) 2003;145:957–960. doi: 10.1007/s00701-003-0129-8. [DOI] [PubMed] [Google Scholar]
- 29.Shousha M, Boehm H. Surgical treatment of cervical spondylodiscitis: a review of 30 consecutive patients. Spine (Phila Pa 1976) 2012;37:E30–36. doi: 10.1097/BRS.0b013e31821bfdb2. [DOI] [PubMed] [Google Scholar]
- 30.Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine (Phila Pa 1976) 2009;34:49–59. doi: 10.1097/BRS.0b013e3181909558. [DOI] [PubMed] [Google Scholar]
- 31.Strowitzki M, Vastmans J, Vogel M, Jaksche H. Complex 360 degrees -reconstruction and stabilization of the cervical spine due to osteomyelitis. Eur Spine J. 2011;20(Suppl 2):S248–252. doi: 10.1007/s00586-010-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)