Abstract
Background
Treatment for osteonecrosis of the femoral head (ONFH) remains controversial. Current reviews include low-level evidence studies evaluating the treatment of both pre-collapse and collapse stages of the disease.
Questions/Purposes
The purpose of the current study is to systematically review the literature evaluating core decompression (CD) with bone marrow mesenchymal cells (BMMCs), CD alone, and bisphosphonate treatment in pre-collapse ONFH by focusing just on randomized clinical trials (RCTs) reporting functional and radiologic outcomes. We aim to determine if the literature provides evidence supporting any single approach.
Methods
Using PubMed and EMBASE databases, we reviewed the clinical evidence of treatments for pre-collapse ONFH following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Twelve RCTs met the inclusion criteria.
Results
Results showed that CD with BMMCs has lower risk of femoral head collapse when compared to the CD alone excluding hips lost to follow-up (relative risk (RR) [95% CI]:0.25 [0.11, 0.60]; p = 0.002) and when assumed that hips lost to follow-up experienced collapse (RR [95% CI]: 0.11 [0.03, 0.47]; p = 0.003). Neither CD nor bisphosphonate treatments showed lower risk to femoral head collapse when compared to control treatments (p = 0.46 and 0.31, respectively)
Conclusion
Current literature shows that there is a lower risk of femoral head collapse in patients with ONFH treated with CD combined with BMMCs when compared to CD alone; however, there is no robust evidence to determine the effect on functional outcomes. More RCTs assessing new combination therapies and using standardized outcome measures are required.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-016-9505-9) contains supplementary material, which is available to authorized users.
Keywords: osteonecrosis, femoral head, pre-collapse treatment
Introduction
Osteonecrosis of the femoral head (ONFH) has become an increasingly common disease with an incidence of 20,000–30,000 in the USA [52]. Although both traumatic and atraumatic factors have been identified as risk factors, the etiology of ONFH remains unclear. The most frequent risk factors for ONFH in the USA include alcohol (20–40%), corticosteroid therapy (35–40%), and idiopathic (20–40%) [47]. One of several theories proposes that ONFH is a disease resulting from impaired blood supply to the femoral head, which leads to osteocyte death, defective bone repair, and collapse of the architectural bone structure [10, 37]. Impaired vascular supply can result from external or internal vascular insult caused by direct injury, thrombotic intravascular occlusion, or extravascular compression [19, 21, 35]. There are several mechanisms that can lead to vascular occlusion resulting in ONFH. High-dose steroid administration and alcohol abuse have been found to alter circulating lipids leading to resultant microemboli in the arteries supplying the bone [22]. Disease processes that increase intravascular coagulation and thrombus formation can also lead to vascular damage. Occlusion can result from red blood cell sickling as seen in hemoglobinopathies, accumulations of cerebroside-filled cells within the bone marrow as seen in Gaucher’s disease, and nitrogen bubble formation as seen in Caisson disease [20]. Trauma such as fracture or dislocation primarily in the subcapital region of the femoral neck can also lead to compromised blood flow to the femoral head [20]. In some cases where no cause can be identified, it is considered of idiopathic origin [5, 35, 50].
ONFH commonly affects patients between 30 and 50 years of age and progresses to complete collapse in 80% of untreated patients [31, 52]. Therefore, early diagnosis is critical to halt or reverse the progression of the disease, ultimately preventing collapse and the need for total hip replacement (THR). Clinically, patients can initially present asymptomatically but, as the disease progresses, can develop groin pain radiating to the knee or buttock as well as limited hip range of motion and pain with forced internal rotation [20]. Diagnostic modalities available include radiography, scintigraphy, functional bone evaluation, magnetic resonance imaging (MRI), computer-assisted tomography, and histological studies. Current ONFH diagnosis is dependent upon plain AP and frog-leg lateral radiographs of the hip, followed by MRI. Pre-collapse joint-preserving treatments include nonweight bearing (NWB) therapy, physical therapy, extracorporeal shock wave therapy (ESWT), hyperbaric oxygen (HBO), bisphosphonates, core decompression (CD) with or without concentrated stem cells, bone grafting, and osteotomy procedures [35, 43, 50]. Unfortunately, the optimal treatment for pre-collapse ONFH has not yet been identified [52].
Current reviews in ONFH include studies with level I to level IV evidence evaluating different treatment options for pre-collapse and post-collapse lesions [12, 20, 28, 30, 40, 50]. To our knowledge, there is no systematic review of high-level evidence studies that attempts to quantitatively analyze the outcomes of the different treatments for pre-collapse stage ONFH. The purpose of the current study was to systematically review the literature evaluating the clinical evidence for CD with bone marrow mesenchymal cells (BMMCs), CD alone, and bisphosphonate treatment in pre-collapse ONFH by focusing on randomized clinical trials (RCTs) reporting both radiologic and functional outcomes. Our primary comparative outcomes were rates of femoral head collapse and need for THR. We aimed to determine if there is literature support for a treatment that delays progression to femoral head collapse and THR and improves functional outcomes when compared to other treatment regimens.
Methods
A systematic review of two electronic databases, PubMed and EMBASE, was conducted using the following search terms: “(avn OR avascular necrosis OR osteonecrosis OR osteonecrosis of femoral head OR onfh) AND (femoral head OR hip OR femur) AND (bisphosphonates OR forteo OR pth OR parathyroid hormone OR teriparatide OR coring OR core decompression OR stem cells OR drilling OR free vascularized fibular graft OR bone graft)”. Limits used were English and humans. The search was performed on February 10, 2016.
Inclusion criteria included (1) RCTs on treatments for pre-collapse ONFH, (2) reported functional and radiologic outcomes, (3) human studies, (4) published in a peer-reviewed journal, (5) comparison groups of ≥10 hips, and (6) those written in English. No publication date restrictions were imposed. Exclusion criteria included (1) in vitro studies, (2) post-collapse treatment, (3) studies performed in the pediatric population, (4) studies reporting subchondral insufficiency fracture or transient marrow edema, and (5) studies comparing different surgical techniques or different grafts (vascularized fibular grafts versus nonvascularized fibular grafts).
Two independent reviewers systematically performed the eligibility assessment (*** and ***). Reference lists of selected articles were also reviewed to ensure that no pertinent articles were omitted. Disagreements between reviewers were resolved through consensus, and a third reviewer (***) was consulted if no consensus was reached.
A data extraction sheet was developed and pilot-tested for five randomly selected included studies and refined accordingly. Two reviewers performed data extraction independently in included trials on (1) demographic characteristics of participants, (2) type of intervention, (3) radiologic and functional outcomes, and (4) length of follow-up. Disagreements were resolved by discussion. If agreement was not reached, a third author made the final decision (***). A fourth reviewer with experience in ONFH performed a final check on all data (***).
To evaluate validity of eligible RCTs, two reviewers (***and ***) determined adequacy of randomization, concealment of allocation, blinding, and the extent of loss of follow-up according to Cochrane Collaboration’s Risk of Bias Tool for randomized controlled trials [17].
Studies were categorized into the following groups: (1) CD with BMMCs versus CD alone, (2) CD alone versus conservative treatment, (3) bisphosphonates versus control (conservative treatment, placebo, or electroshock wave therapy [ESWT]), or (4) CD with alendronate versus CD alone. Qualitative and quantitative analysis for radiologic and functional outcomes in each group was done if possible. Treatment complications were also reported.
Statistical Analysis
Effect sizes are expressed as risk ratios with 95% confidence intervals for each study. Random-effects method was calculated for each treatment comparison using the Mantel-Haenszel method. Study-level and pooled risk ratios are presented in forest plots. When patients were excluded or lost to follow-up in trials after randomization, the study effect size was calculated without these hips and, as a sensitivity analysis, with these hips by considering them as worst possible outcome (i.e., femoral head collapse or THR). Analyses were performed using Revman V.5.3 (Nordic Cochrane Centre, Copenhagen) [41].
Results
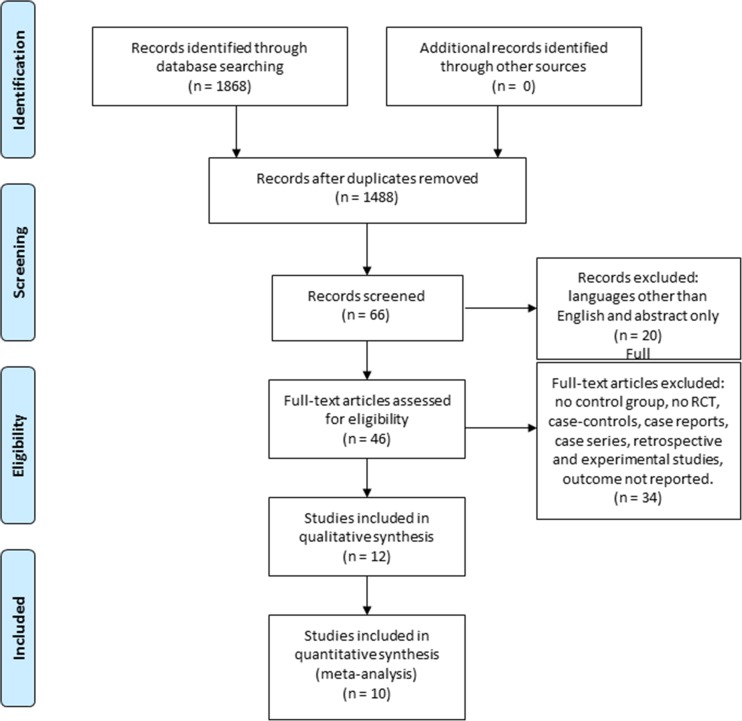
The results of the literature search are presented in a flow chart according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [29, 33]. No additional studies were identified by searching the references. Twelve studies were included in the qualitative synthesis and ten in the quantitative analysis (Fig. 1).
Fig. 1.
PRISMA flow diagram.
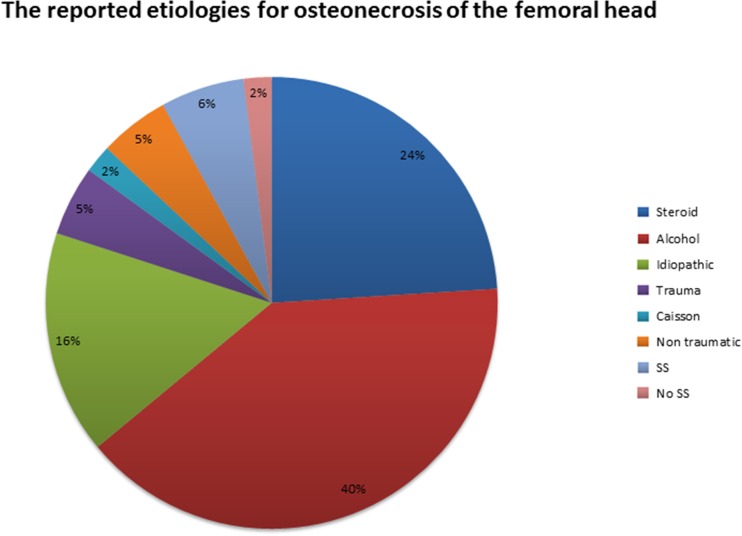
All studies [9, 11, 18, 23, 24, 26, 27, 38, 45, 49, 51, 53] reported the stage of ONFH, 592 hips (76%) were classified as stages I and II (Steinberg [48], ARCO [6], or FICAT [7]), and 186 hips (24%) were classified as stage III according with Steinberg classification. One study used Mitchell [32] classification (Table 1). Alcohol was the most common etiology (Fig. 2). Functional and radiologic outcomes are presented in Table 2.
Table 1.
Baseline characteristics of randomized control trials
| Study | Study type | Group 1 | Group 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Patients (hips) | Mean age | Gender (M/F) | Steinberg class (hips) | Treatment | Patients (hips) | Mean age | Gender (M/F) | Steinberg class (hips) | ||
| Gangji, 2011 [11] | RCT | CD + BMMCs | 13 hips | 42.2 | a | I (2), II (11)b | CD | 11 hips | 45.7 | a | I (2), II (9)b |
| Zhao, 2012 [54] | RCT | CD + BMMCs | 53 hips | 32.7 | 26:24 | I C (3), IIA (15), IIB (23), IIC (10)b | CD | 51 hips | 33.8 | 27:23 | IC (2), IIA (15), IIB (22), IIC (12)b |
| Sen, 2012 [45] | RCT | CD + BMMCs | 26 hips | – | 19:7 | Mitchell–A (7), B (4), C (8), D (1) | CD | 25 hips | – | 18:7 | Mitchell–A (6), B (9), C (11), D (1) |
| Koo, 1995 [24] | RCT | CD | 18 hips | 44.83 | 17:1 | I (10), II (7), III (1) | Conservative | 19 hips | 48.31 | 17:1 | I (12), II (4), III (3) |
| Neumayr, 2006 [38] | RCT | CD | 17 (17) | 24.67 | 8:9 | I (2), II (5), III (10) | Conservative | 21 (21) | 26.41 | 11:10 | I (10), II (11), III (17) |
| Stulberg, 1991 [49] | RCT | CD | 19 (29) | 38.6 | _ | I (10), II (7), III (11)c | Conservative | 17 (26) | 38.6 | – | 1(5), II (7), III (10)c |
| Chen, 2012 [9] | RCT | Alendronate | 26 (32) | 48.4 | 22:4 | IIC (20), IIIC (12) | Placebo | 26 (33) | 44.2 | 19:7 | IIC (25), IIIC (8) |
| Lai, 2005 [26] | RCT | Alendronate | 20 (29) | 42.6 | 15:5 | II (17), III (20) | Placebo | 20 (25) | 42.4 | 15:5 | II (13), III (20) |
| Lee, 2015 [27] | RCT | Zoledronate | 55 (55) | 43.8 | 39:16 | I (29), II (26) | Conservative | 55 (55) | 45.2 | 41:14 | I (31), II (24) |
| Hsu, 2010 [18] | RCT | Alendronate + ESWT | 28 (50) | 39.1 | 18:10 | I (2) II (35) III (13) | EWST | 35 (48) | 39.6 | 27:8 | I (2) II (27) III (19) |
| Wang, 2008 [51] | RCT | Alendronate + ESWT | 23 (30) | 38.6 | 20:5 | I and II (19), III (11)b | EWST | 25 (30) | 35.7 | 13:10 | I and II (25), III (5)b |
| Kang, 2012 [23] | RCT | Alendronate + CD | 55 hips | 43.8 | 37:18 | IIA (17), IIB (25), III (13)c | CD | 52 hips | 45.3 | 35:17 | IIA (15), IIB (24), III (13)c |
RCT randomized control trials, CD core decompression, BMMCs bone marrow mesenchymal cells, ESWT electroshock wave therapy
aGender is reported for all population: M 9; F 10
bARCO classification
cFICAT classification
Fig. 2.
The reported etiologies for osteonecrosis of the femoral head. SS sickle cell disease.
Table 2.
Functional and clinical outcomes
| Study | Functional assessment | Outcome | Follow-up time (months) | Radiologic assessment | Outcome | Mean follow-up time (months) |
|---|---|---|---|---|---|---|
| Gangji, 2011 [11] | VAS | + | 24 | MRI | Delay to collapse: + | NA |
| Lesquene | + | Delay to THR: ND | ||||
| WOMAC | + | |||||
| Zhao, 2012 [54] | HHS | + | 24 | MRI | Delay to collapse: + | NA |
| Delay to THR: ND | ||||||
| Sen, 2012 [45] | HHS | − | 24 | MRI, X-rays | Delay to THR: − | |
| Koo, 1995 [24] | MAP | + | 13 | MRI, X-rays | Delay to collapse: ND | 24 |
| Delay to THR: ND | ||||||
| Neumayr, 2006 [38] | CHOHES | I | 27 | MRI, X-rays | Delay to THR: ND | CD group: 37.2 |
| Control group: 36 | ||||||
| Delay to collapse: ND | ||||||
| Stulberg, 1991 [49] | HHS | + | 13 | MRI, X-rays | Delay to THR: + | 26.8 |
| Chen , 2012 [9] | HHS | ND | 24 | MRI, X-rays | Delay to collapse: ND | NA |
| SF-36 | ND | Delay to THR: ND | ||||
| Lai, 2005 [26] | HHS | I | 24 | MRI, X-rays | Delay to collapse: + | 8 |
| Delay to THR: + | ||||||
| Lee, 2015 [27] | HHS | − | 24 | MRI, X-rays | Delay to collapse: ND | 24 |
| WOMAC | − | 24 | Delay to THR: ND | |||
| Hsu, 2010 [18] | HHS | − | 26 | MRI, X-rays | Progression: ND | NA |
| MAP | – | Delay to THR: ND | ||||
| WOMAC | – | |||||
| SF-12 | – | |||||
| Wang, 2008 [51] | HHS | − | 6.5 | MRI, X-rays | Progression: ND | 24.87–26.14 |
| Kang, 2012 [23] | HHS | − | 15.75 | MRI, X-rays | Delay to collapse (+) | 62.5 |
Outcomes: (+) significant improvement, (−) no significant improvement; (ND) no difference, and (I) statistics not reported
MAP Multidimensional Assessment Pain Scale, WOMAC Western Ontario and McMaster Universities Arthritis, CHOHES Children’s Hospital Oakland Hip Evaluation Scale, HHS Harris Hip Scores, VAS visual analogue score, SF-36 Short-Form 36 Health Survey, SF-12 Short-Form 12 Health Survey
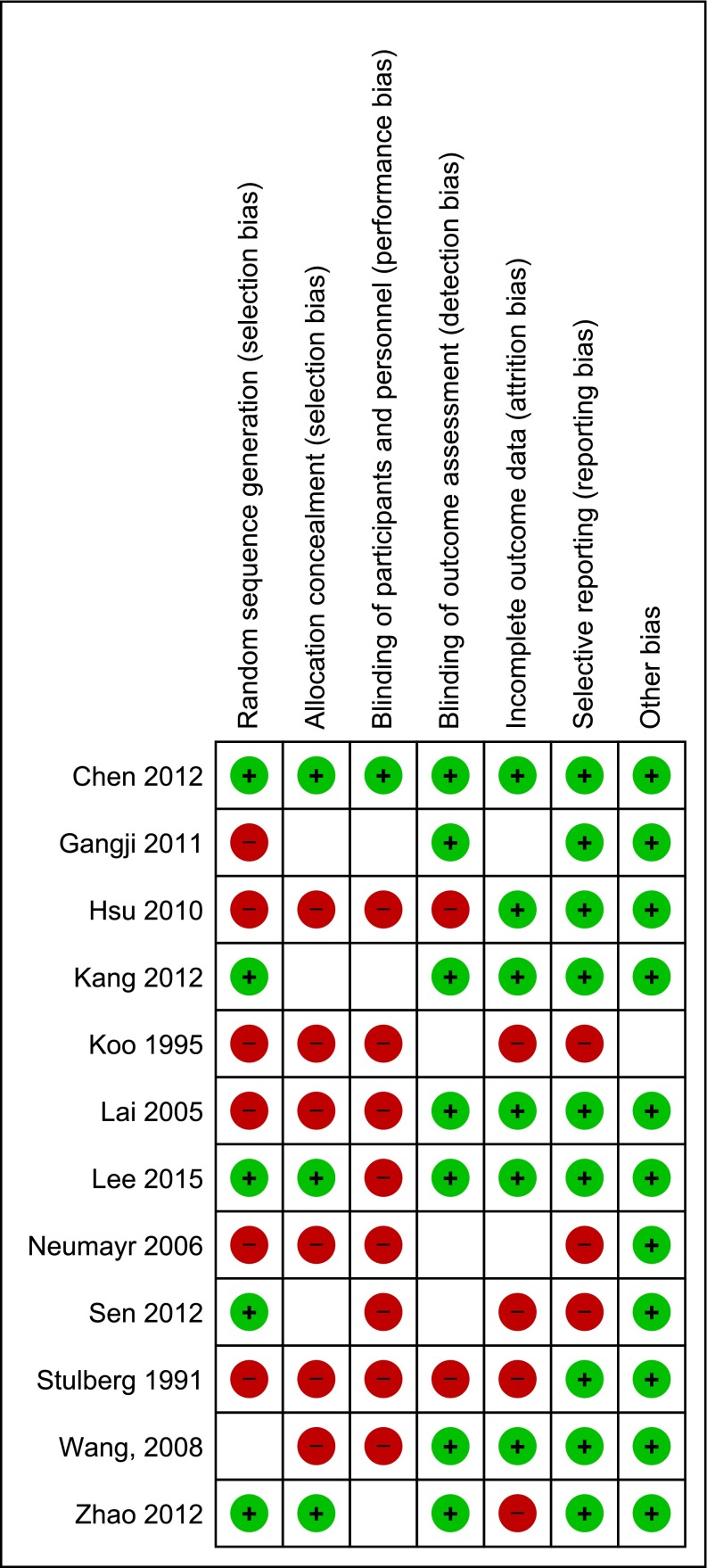
All studies were assessed for risk of bias in their Methods. One study had low risk of bias for all elements [9]. Eight studies had high risk for blinding of participants and personnel [18, 27, 45, 49, 51], and six studies were judged to be high risk of bias for random sequence generation [11, 18, 24, 26, 38, 49]. The randomization methods were described as adequate in five trials. The specific reviewers’ judgments for risk of bias are detailed in Fig. 3.
Fig. 3.
Risk of bias summary: review of authors’ judgments about each risk of bias item for each included study. (+): low risk of bias; (−): high risk of bias; ( ): unknown risk of bias.
Synthesis of Results
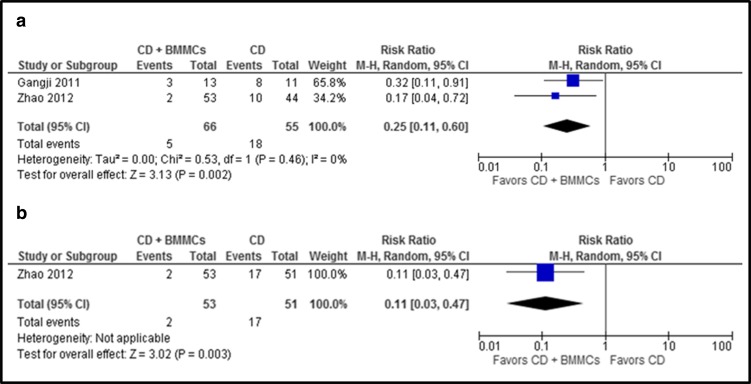
Core decompression (CD) plus BMMCs versus CD alone was compared. CD with BMMC treatment showed a lower risk of femoral head collapse when excluding hips lost to follow-up (relative risk (RR) [95% CI]:0.25 [0.11, 0.60]; p = 0.002) and when assuming that hips lost to follow-up experienced collapse (RR [95% CI]: 0.11 [0.03, 0.47]; p = 0.003) (Fig. 4a, b).
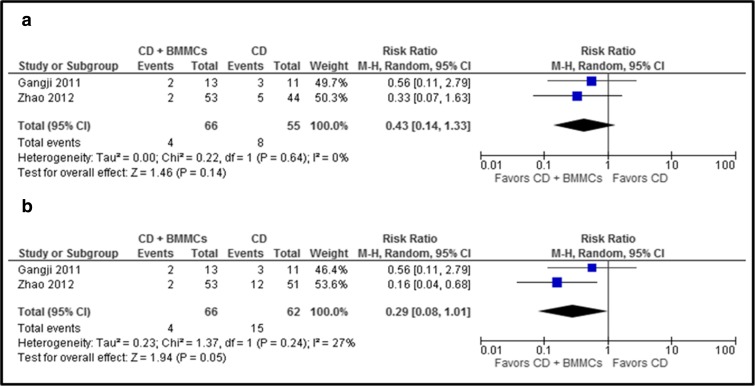
Fig. 4.
a, b The graph shows the risk ratio for femoral head collapse for CD plus BMMCs versus CD. a Excluding hips lost to follow-up or excluded from analysis. b Assuming that all hips lost to follow-up or hips excluded from analysis experienced femoral head collapse (worst-case scenario). Number of events represents the number of hips that progressed to the fractural stage of osteonecrosis (ARCO stage III or more).
No significant difference was seen in the risk ratio for THR when hips lost to follow-up were excluded (RR [95% CI]:0.43[0.14, 1.33]; p = 0.14)] or when assuming that hips lost to follow-up and excluded from analysis required THR (RR [95% CI]:0.29 [0.08, 1.01]; p = 0.05)] (Fig. 5a, b). No complications were reported in either of the groups.
Fig. 5.
a, b The graph shows the risk ratio for THR in CD with BMMCs versus CD alone. a Excluding hips lost to follow-up or excluded from analysis. b Assuming that all hips lost to follow-up or hips excluded from analysis underwent THR (worst-case scenario).
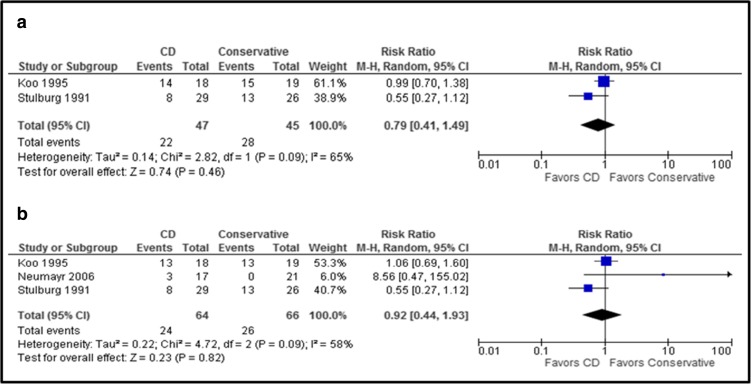
When core decompression (CD) was compared to conservative treatment, quantitative analysis showed no significant difference in the risk of femoral head collapse for CD versus conservative treatment (RR [95% CI]: 0.79 [0.41, 1.49]; p = 0.46). No significant difference was seen in the risk of THR for CD versus conservative treatment (RR [95% CI]: 0.92 [0.44, 1.93]; p = 0.82) (Fig. 6a, b). No hips were lost to follow-up or excluded from analysis [24, 38, 49]. Two cases of infection were reported and were treated with antibiotics (CD group).
Fig. 6.
a, b The upper graph (a) shows the risk ratio for femoral head collapse for CD versus conservative treatment. The lower graph (b) shows the risk ratio for THR in comparing CD versus conservative treatment (no hips were lost to follow-up).
Bisphosphonate treatment was also compared with control treatment (conservative treatment, placebo, or ESWT).
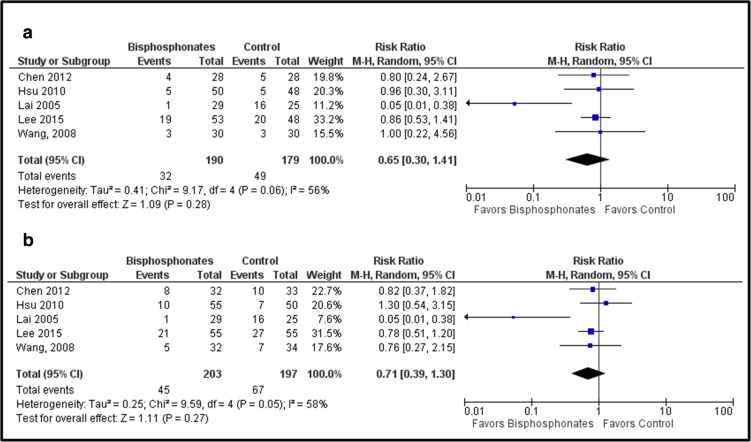
Group analysis did not show a difference in the risk of femoral head collapse when excluding hips lost to follow-up or excluded from the analysis (RR [95% CI]:0.59 [0.22, 1.61]; p = 0.31) or when assuming that all hips lost to follow-up or excluded from analysis progressed to collapse (RR [95% CI]: 0.72 [0.37, 1.42]; p = 0.35) (Fig. 7a, b). Similar results in risk of THR were seen when excluding hips lost to follow-up (RR [95% CI]: 0.65 [0.30, 1.41]; p = 0.28) and when assuming that all hips lost to follow-up or excluded from analysis progressed to THR (RR [95% CI]: 0.71 [0.39, 1.30]; p = 0.27) (Fig. 8a, b).
Fig. 7.
a, b The graph shows the risk ratio for femoral head collapse when comparing bisphosphonates versus controls. a Excluding hips lost to follow-up or excluded from analysis. b Assuming that all hips lost to follow-up or hips excluded from analysis experienced femoral head collapse (worst-case scenario).
Fig. 8.
a, b The graph shows the risk ratio for THR when comparing bisphosphonates versus controls. a Excluding hips lost to follow-up or excluded from analysis. b Assuming that all hips lost to follow-up or hips excluded from analysis underwent THR (worst-case scenario).
There was no significant difference in mean HHS at 24-month follow-up (RR [95% CI]: 3.85 [−7.43, 15.13]; p = 0.50) (Fig. 9). Local complications (swelling and ecchymosis) after ESWT, transient dizziness after HBO, and dyspepsia following alendronate treatment were reported.
Fig. 9.
Analysis for mean difference in follow-up HHS score when comparing bisphosphonates versus control.
Discussion
The goal in treatment of ONFH is to delay progression of the disease and ultimately prevent collapse and THR, especially in the younger population. However, the optimal treatment for pre-collapse ONFH has not yet been identified [52]. This systematic review of RCTs quantitatively analyzes radiologic and functional outcomes on CD with BMMCs, CD alone, and bisphosphonate treatment for pre-collapse stage ONFH. We found that CD combined with BMMCs is the only compared treatment that decreased the risk of femoral head collapse in patients with pre-collapse ONFH.
The analysis showed significant difference in the risk for femoral head collapse when comparing CD combined with BMMCs versus CD alone (RR 0.11; p = 0.003). CD plus BMMC treatment did not reduce the time to THR, even under assumption that all hips lost to follow-up required a THR (RR = 0.29; p = 0.05). It is important to note that for all studies, THR was not the standard treatment for hip collapse, indications for THR were not specified, and one study was excluded from the analysis because failure incidence was not reported [45]. Similar results were reported in previous studies; however, those reviews included level II to level IV evidence studies [28, 35, 40]. It has been reported that the addition of stem cells to CD accelerates fracture healing by differentiating in osteoprogenitor cells required for bone formation [14, 16, 23, 30]. Direct instillation of stem cells through the core tract was the method reported in these studies [11, 53]. Gangji et al. [11] harvested the bone marrow from the iliac crest obtaining a final volume of 49.7 ± 2.3 ml and 1.9 ± 0.2 × 109 mononuclear cells, while Zhao et al. [54] harvested the bone marrow cells from the CD tunnels of the subtrochanteric femoral region, cultured for 2 weeks, and injected a total of 2 × 106 BMMCs. Although results proved to be good in both cases, previous studies have shown that a number of mononuclear cells over 5 × 107 are essential in order for this method to be effective [15]. CD plus BMMCs also appears to be a safe procedure that has not carried complications in the RCTs done at the time of this systematic review.
In this study, CD alone showed no significant difference in the risk of collapse or time to THR when compared with conservative treatment at 24-month follow-up. Results are limited by the fact that outcomes were reported as hip survival rate [38, 49] using the end point of no further intervention (THR for patients in the CD group, and CD or THR for patients in the control group). Neither the indication for additional surgery nor the definition of failure was reported. Furthermore, the CD technique was different in each study. Koo et al. [24] used the CD technique as described by Steinberg et al. [48] and filled decompression tunnels with cancellous bone from the trochanteric area. Neuymar et al. [38] used one drill hole directed at the necrotic lesion defined on preoperative MRI, followed by curettage and irrigation. Lastly, Stulberg et al. [49] continuously monitored intra-osseous pressure drilling. However, there is no reported evidence of statistical delay to collapse with any of these techniques when compared with conservative treatment [42, 46]. These negative results using CD alone may be due to destructive resorption without effective consecutive bone formation, as a consequence of decreased proliferation of progenitors [14]. One previously published meta-analysis of 24 studies demonstrated that the best results of CD were in the treatment of early-stage lesions, as 84% of patients with Ficat stage I disease and 65% of patients with stage II disease had successful results; therefore, reported outcomes may be affected by stage of disease [21, 34].
Bisphosphonate treatment inhibits the resorptive activity of mature osteoclasts and reduces the apoptosis in osteoblasts and osteocytes [44]. Since osteoclasts resorb the necrotic bone and weaken the cancellous bone between the viable and the necrotic area [26], bisphosphonates have been used to prevent micro fractures and collapse in cases of ONFH in several studies [1–4]. However, pooled risk ratio of studies comparing bisphosphonates versus control treatments (conservative treatment, placebo, and ESWT) did not show any improvement on radiologic or functional outcomes at 24-month follow-up. Lai et al. [26] reported positive results after alendronate treatment but included hips that had CD at the beginning or during the study. Contrarily, it was found that neither alendronate [9] nor zolendronate [27] prevents collapse, reduces the incidence of THR, or improves HSS outcome after 2-year follow-up. Alendronate treatment is administered 10 mg per day or 70 mg per week for 24 weeks, while zolendronate treatment is administered 5 mg intravenously annually [27]. Both studies [9, 27] included patients with large osteonecrosis lesions (≥30%) at high risk of collapse. Contrarily to what has been reported in a prospective comparative study by Nishii et al. [39], our results suggest that in large size lesions, substitution of the necrotic bone cannot be enhanced by bisphosphonate treatment alone. As shown in previous reviews, there is a lack of controlled and double-blind studies about the efficacy of bisphophonates in the treatment of ONFH [8, 30]. However, noncontrolled studies appeared to demonstrate favorable results, particularly in diminishing pain, improving mobility, and lowering the incidence of collapse [8]. The controversy over the effectiveness of bisphosphonate use justifies the necessity for further research in this area. Outcomes of biophysical treatments were not analyzed in this study. ESWT groups were used as controls to compare the efficacy of alendronate in the treatment of ONFH [18, 51].
With regard to combining bisphosphonates and CD, Kang et al. [23] found that multiple drilling plus alendronate can delay the progression of ONFH and improve HSS scores when compared with multiple drilling alone at 4-year follow-up. These results suggest that combination of these two treatments enhances their efficacy. CD improves vascularity by decreasing intra-osseous pressure [23]. Probably, the increase in blood flow to the femoral head also increases the amount of bisphosphonate reaching the area between the viable and the necrotic bone where osteoclast inhibition prevents the weakening of the cancellous bone [26, 27]. A previous review by Ma et al., however, does not recommend any adjunctive therapy (bisphosphonates, ESWT, iloprost, etc.) because studies combining adjunctive therapies and CD are limited by sample size, questionable efficacy, and low-level evidence [30]. It is to the authors’ belief that further RCTs are necessary on this topic. Studies using zolendronate are of interest since this nitrogen-bound bisphosphonate has the highest potency and affinity to the bone [23]. We also suggest more studies in large-size osteonecrosis lesions since it is known that small-size lesion will not progress without any intervention [13, 25, 36].
Several limitations are present in this study. Limitations related with the included studies are the heterogeneity of study population in terms of risk factors, lesion size and stage, bilateral versus unilateral disease, surgical techniques, indications for THR, and follow-up time. Furthermore, included studies reported different classification systems (X-ray based and MRI based) decreasing the reliability to assess the status of avascular necrosis. Just three of the included studies reported ARCO classification, accounting for lesion location and area of head involvement; further RCTs using ARCO classification are encouraged. Another limitation is the inadequate reporting with regard to definition of “failure,” lost to follow-up patients, hazard ratio, and 95% confidence interval, which was seen in some studies. Limitations of the analysis included small number of RCTs in each comparative group and the assumption that hips that were lost or excluded from the analysis had the worst outcome. The last factor may have influenced the results but was necessary to analyze the data due to the lack of reported hazard ratios. The variability in functional outcome assessments did not allow to determine whether CD plus BMMCs or CD alone improves functional outcomes in patients with ONFH.
In conclusion, the current literature has demonstrated that there is a decrease in the risk to femoral head collapse in patients with ONFH treated with CD combined with BMMCs when compared to CD alone; however, there is no robust evidence to determine the effect on functional outcomes. Neither CD nor bisphosphonate treatment showed improvement on radiologic or functional outcomes. This study shows that there is a lack of RCTs assessing treatments for femoral head AVN. New studies comparing treatments as a function of ONFH stage when treatment is started, describing lesion sizes and using standardized surgical techniques and outcome measures, are required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1224 kb)
Compliance with Ethical Standards
Conflict of Interest
Jordan C. Villa, MD, Solomon Husain, BS, Jelle P. van der List, MD, Arianna Gianakos, DO, have declared that they have no conflict of interest. Joseph M. Lane, MD, reports other from Agnovos, Amgen, Bone Therapeutics, SA, Merck, Inc. CollPlant, Inc., Graftys, Kuros, and Lilly, Inc., outside the work.
Human/Animal Rights
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed Consent
N/A
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Agarwala S, Shah S, Joshi VR. The use of alendronate in the treatment of avascular necrosis of the femoral head: follow-up to eight years. J. Bone Joint Surg. Br. 2009;91:1013–1018. doi: 10.1302/0301-620X.91B8.21518. [DOI] [PubMed] [Google Scholar]
- 2.Agarwala S, Shah SB. Ten-year follow-up of avascular necrosis of femoral head treated with alendronate for 3 years. J. Arthroplasty. 2011;26:1128–1134. doi: 10.1016/j.arth.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Agarwala S, Sule A, Pai BU, et al. Study of alendronate in avascular necrosis of bone. J. Assoc. Physicians India. 2001;49:949–950. [PubMed] [Google Scholar]
- 4.Agarwala S, Sule A, Pai BU, et al. Alendronate in the treatment of avascular necrosis of the hip. Rheumatology (Oxford) 2002;41:346–347. doi: 10.1093/rheumatology/41.3.346-a. [DOI] [PubMed] [Google Scholar]
- 5.Aldridge JM 3rd, Berend KR, Gunneson EE, Urbaniak JR. Free vascularized fibular grafting for the treatment of postcollapse osteonecrosis of the femoral head. Surgical technique. J. bone Jt. surgery. American Vol. 2004;86-A Suppl:87–101. [DOI] [PubMed]
- 6.Anon. ARCO Committee on Terminology and Staging. The ARCO perspective for reaching one uniform staging system of osteonecrosis. In: Schoutens A, Arlet J, Gardeniers JWM, Huges SPF, editors. Bone circulation and vascularization in normal and pathological . Plenum Press. 1993:375–80.
- 7.Arlet JFRP. Forage-biopsie de la tete femorale dans l’osteonecrose primitive. Observations histopathologiques portant sur huit foranes. Rev Rhum. 1964;31:257–64. [Google Scholar]
- 8.Cardozo JB, Andrade DMS, Santiago MB. The use of bisphosphonate in the treatment of avascular necrosis: a systematic review. Clin. Rheumatol. 2008;27:685–688. doi: 10.1007/s10067-008-0861-9. [DOI] [PubMed] [Google Scholar]
- 9.Chen CH, Chang JK, Lai KA, et al. Alendronate in the prevention of collapse of the femoral head in nontraumatic osteonecrosis: A two-year multicenter, prospective, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64:1572–1578. doi: 10.1002/art.33498. [DOI] [PubMed] [Google Scholar]
- 10.Cui Q, Botchwey EA. Emerging ideas: treatment of precollapse osteonecrosis using stem cells and growth factors. Clin. Orthop. Relat. Res. 2011;469:2665–2669. doi: 10.1007/s11999-010-1738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangji V, De Maertelaer V, Hauzeur J-P. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year follow-up of a prospective controlled study. Bone. 2011;49:1005–1009. Available at: doi:10.1016/j.bone.2011.07.032 [DOI] [PubMed]
- 12.Giusti A, Hamdy N a T, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: A cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone. 2011;48:966–971. Available at: doi:10.1016/j.bone.2010.12.033 [DOI] [PubMed]
- 13.Ha Y-C, Jung WH, Kim J-R, et al. Prediction of collapse in femoral head osteonecrosis: a modified Kerboul method with use of magnetic resonance images. J. Bone Joint Surg. Am. 2006;88(Suppl 3):35–40. doi: 10.2106/JBJS.F.00535. [DOI] [PubMed] [Google Scholar]
- 14.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin. Orthop. Relat. Res. 2002;405:14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Hernigou P, Manicom O, Poignard A. Core decompression with marrow stem cells. Oper Tech Orthop. 2004;14:68. doi: 10.1053/j.oto.2004.03.001. [DOI] [Google Scholar]
- 16.Hernigou P, Poignard A, Manicom O, et al. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint. Surq Br. 2005;87:896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu SL, Wang CJ, Lee MS, et al. Cocktail therapy for femoral head necrosis of the hip. Arch. Orthop. Trauma Surg. 2010;130:23–29. doi: 10.1007/s00402-009-0918-5. [DOI] [PubMed] [Google Scholar]
- 19.Hungerford DS, Jones LC. Asymptomatic osteonecrosis: should it be treated? Clin. Orthop. Relat. Res. 2004;429:124–130. doi: 10.1097/01.blo.0000150275.98701.4e. [DOI] [PubMed] [Google Scholar]
- 20.Joaquin JMM-A, Ariana G, Jordan C V, Amelia N, Lane, Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J. Orthop. 2015;6:590. Available at: http://www.wjgnet.com/2218-5836/full/v6/i8/590.htm [DOI] [PMC free article] [PubMed]
- 21.Johnson AJ, Mont MA, Tsao AK, et al. Treatment of femoral head osteonecrosis in the United States: 16-year analysis of the Nationwide Inpatient Sample. Clin. Orthop. Relat. Res. 2014;472:617–623. doi: 10.1007/s11999-013-3220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones JPJ. Fat embolism and osteonecrosis. Orthop. Clin. North Am. 1985;16:595–633. [PubMed] [Google Scholar]
- 23.Kang P, Pei F, Shen B, et al. Are the results of multiple drilling and alendronate for osteonecrosis of the femoral head better than those of multiple drilling? A pilot study. Joint. Bone. Spine. 2012;79:67–72. doi: 10.1016/j.jbspin.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Koo KH, Kim R, Ko GH, et al. Preventing collapse in early osteonecrosis of the femoral head. A randomised clinical trial of core decompression. J Bone Joint Surq Br. 1995;77:870–874. [PubMed] [Google Scholar]
- 25.Koo KH, Kim R. Quantifying the extent of osteonecrosis of the femoral head. A new method using MRI. J. Bone Joint Surg Br. 1995;77:875–880. [PubMed] [Google Scholar]
- 26.Lai KA, Shen WJ, Yang CY, et al. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surq Am. 2005;87:2155–2159. doi: 10.2106/JBJS.D.02959. [DOI] [PubMed] [Google Scholar]
- 27.Lee YK, Ha YC, Cho YJ, et al. Does Zoledronate Prevent Femoral Head Collapse from Osteonecrosis? A Prospective, Randomized, Open-Label, Multicenter Study. J Bone Joint. Surq Am. 2015;97:1142–1148. doi: 10.2106/JBJS.N.01157. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Xu X, Wu W. Comparison of bone marrow mesenchymal stem cells and core decompression in treatment of osteonecrosis of the femoral head : a meta-analysis. 2014;7:5024–5030. [PMC free article] [PubMed]
- 29.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Ma M, Jj C, Rj S, Lc J, Jr L. Nontraumatic Osteonecrosis of the Femoral Head : Where Do We Stand Today ? 2015:1604–1627. [DOI] [PubMed]
- 31.Min BW, Song KS, Cho CH, et al. Untreated asymptomatic hips in patients with osteonecrosis of the femoral head. Clin. Orthop. Relat. Res. 2008;466:1087–1092. doi: 10.1007/s11999-008-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell DG, Rao VM, Dalinka MK, et al. Femoral head avascular necrosis: correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology. 1987;162:709–715. doi: 10.1148/radiology.162.3.3809484. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin. Orthop. Relat. Res. 1996:169–178 [DOI] [PubMed]
- 35.Mont MA, Marulanda GA, Jones LC, et al. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint. Surq Am. 2006;88(Suppl 3):16–26. doi: 10.2106/JBJS.F.00457. [DOI] [PubMed] [Google Scholar]
- 36.Mont MA, Zywiel MG, Marker DR, et al. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J. Bone Joint Surg. Am. 2010;92:2165–2170. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 37.Mwale F, Wang H, Johnson AJ, et al. Abnormal vascular endothelial growth factor expression in mesenchymal stem cells from both osteonecrotic and osteoarthritic hips. Bull. NYU Hosp. Jt. Dis. 2011;69(Suppl 1):S56–61. [PubMed] [Google Scholar]
- 38.Neumayr LD, Aguilar C, Earles AN, et al. Physical therapy alone compared with core decompression and physical therapy for femoral head osteonecrosis in sickle cell disease. Results of a multicenter study at a mean of three years after treatment. J Bone Joint. Surq Am. 2006;88:2573–2582. doi: 10.2106/JBJS.E.01454. [DOI] [PubMed] [Google Scholar]
- 39.Nishii T, Sugano N, Miki H, et al. Does alendronate prevent collapse in osteonecrosis of the femoral head? Clin. Orthop. Relat. Res. 2006;443:273–279. doi: 10.1097/01.blo.0000194078.32776.31. [DOI] [PubMed] [Google Scholar]
- 40.Papakostidis C, Tosounidis TH, Jones E, Peter V. The role of “cell therapy” in osteonecrosis of the femoral head The role of “cell therapy” in osteonecrosis of the femoral head A systematic review of the literature and meta-analysis of 7 studies. 2016;3674. [DOI] [PMC free article] [PubMed]
- 41.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998;17:2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Phemister DB. Treatment of the necrotic head of the femur in adults. J Bone Joint. Surq Am. 1949;31A:55–66. [PubMed] [Google Scholar]
- 43.Pinggera. K KWSNAO. Interthrocanteric osteotomy for avascular necrosis of the head of the femur. Survival probability of two different methods. J Bone Jt. Surg Br. 84(6):817–24. [DOI] [PubMed]
- 44.Reis ND, Schwartz O, Militianu D, et al. Hyperbaric oxygen therapy as a treatment for stage-I avascular necrosis of the femoral head. J Bone Joint. Surq Br. 2003;85:371–375. doi: 10.1302/0301-620X.85B3.13237. [DOI] [PubMed] [Google Scholar]
- 45.Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early Results of Core Decompression and Autologous Bone Marrow Mononuclear Cells Instillation in Femoral Head Osteonecrosis. A Randomized Control Study. J. Arthroplasty. 2012;27:679–686. Available at: doi:10.1016/j.arth.2011.08.008 [DOI] [PubMed]
- 46.Soohoo NF, Vyas S, Manunga J, et al. Cost-effectiveness analysis of core decompression. J. Arthroplasty. 2006;21:670–681. doi: 10.1016/j.arth.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 47.Steinberg ME, Larcom PG, Strafford B, Hosick WB, Corces A, Bands RE, Hartman KE. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin. Orthop. Relat. Res. 2001:71–78. [DOI] [PubMed]
- 48.Steinberg ME. Core decompression of the femoral head for avascular necrosis: indications and results. Can. J surq. 1995;38(Suppl 1):S18–24. [PubMed] [Google Scholar]
- 49.Stulberg BN, Davis a W, Bauer TW, et al. Osteonecrosis of the femoral head: A prospective randomized treatment protocol. Clin. Orthop. Relat. Res. 1991;268:140–151. [PubMed] [Google Scholar]
- 50.Wang C, Peng J, Lu S. Summary of the various treatments for osteonecrosis of the femoral head by mechanism: A review. Exp. Ther. Med. 2014;8:700–706. doi: 10.3892/etm.2014.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang CJ, Wang FS, Yang KD, et al. Treatment of osteonecrosis of the hip: Comparison of extracorporeal shockwave with shockwave and alendronate. Arch. Orthop. Trauma Surg. 2008;128:901–908. doi: 10.1007/s00402-007-0530-5. [DOI] [PubMed] [Google Scholar]
- 52.Zalavras CG, Lieberman JR. Osteonecrosis of the femoral head: evaluation and treatment. J. Am. Acad. Orthop. Surg. 2014;22:455–464. doi: 10.5435/JAAOS-22-07-455. [DOI] [PubMed] [Google Scholar]
- 53.Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, Liu B, Yu X. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50:325–330. Available at: doi:10.1016/j.bone.2011.11.002 [DOI] [PubMed]
- 54.Zhao D, Cui D, Wang B, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50:325–330. doi: 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1224 kb)