Abstract
Background
Femoral stem fracture following total hip arthroplasty is an uncommon event that requires immediate revision surgery.
Questions/Purposes
We report on four patients who experienced stem fractures of one design and a review of the US Food and Drug Administration adverse event reports on this design.
Methods
Fracture surfaces of four EMPERION™ (Smith & Nephew, Memphis, TN) femoral stems were analyzed under optical and scanning electron microscopy. A search of the FDA’s Manufacturer and User Facility Device Experience (MAUDE) that reports on all EMPERION™ adverse events was completed.
Results
Fracture surfaces exhibited characteristics consistent with a fatigue fracture mechanism. Sixteen MAUDE reports claimed stem fracture or breakage of EMPERION™ stems.
Conclusion
The four cases of EMPERION™ stem fractures were likely driven by small stem diameter, high offset, and high patient weight. Modular stem-sleeve femoral systems are susceptible to fatigue failure under high stress and should only be used in appropriate patients, whom are not considered obese.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-016-9510-z) contains supplementary material, which is available to authorized users.
Keywords: total hip arthroplasty, stem fracture, uncemented, modular stem
Introduction
Modularity in total hip arthroplasty (THA) has become increasingly popular over the last decade. The theoretical advantages of modular implants include optimization of version, femoral offset, and limb length, in patients with diverse proximal femoral morphology [12]. The EMPERION™ Modular Hip System (Smith & Nephew, Memphis, TN) is a modular total hip system composed of two main femoral components, namely a sleeve and a stem. These components are assembled through a large taper junction intraoperatively. The stem is a polished, cylindrical titanium alloy (Ti6Al4V) implant. The rounded trapezoidal neck is wider in the medial/lateral direction than the anterior/posterior direction to accommodate higher flexion while resisting fatigue. The distal portion of the stem incorporates distal flutes to increase rotational stability and to enhance initial fixation, a coronal slot to ease insertion, reduces the overall stem stiffness, and reduces the risk of femoral fracture [1]. Primary stems are available in eight sizes, with three lengths and two offset options. The sleeve is also fabricated from Ti6Al4V with an outer porous coating covered with a hydroxyapatite layer (POROUS PLUS HA™). Sleeves come in two lengths (standard 40 or 60 mm tall); on the other hand, standard cones are available in four cone sizes (small, medium, large, and extra large) and three spout sizes (types 1, 2, and 3), while tall cones are only available in two cone sizes (small and medium) and two spout sizes (types 1 and 2). The combinations of stem and sleeve components allow a surgeon to independently achieve non-cemented proximal femoral fixation through the sleeve, while independently controlling for femoral stem version. The EMPERION™ system is meant to be highly versatile in both primary and revision total hip arthroplasty cases.
During a 4-month period, four surgeons from three institutions reported four EMPERION™ stem fractures that were subsequently revised. Stem fracture is a rare reason for failure of a THA and can cause extreme pain leading to the inability to ambulate. Modular femoral components allow for important intraoperative adjustments; however, all modular junctions introduce additional interfaces susceptible to crevice corrosion, fretting, wear debris generation, cold welding, and abnormal stress distributions [11]. Historically, stem fracture rates were reported ranging from 0.23% to 11%, but with improvements in metallurgy and fabrication techniques and improved surgical techniques, this complication has been markedly reduced [3, 4]. To date, several reports encompassing 11 cases of modular implant failures of a comparable design to the EMPERION™ (S-ROM®, DePuy Orthopaedics, Warsaw, IN) have been published [7, 9, 11, 12]. However, to our knowledge, no report of implant failures of the EMPERION™ Modular Hip System has been published. This report details our investigation into the four identified failures to determine the factors that may have predisposed these implants to fail.
To assess the extent of the problem, we also reviewed the FDA’s Manufacturer and User Facility Device Experience (MAUDE) reports on all EMPERION™ adverse events to identify additional failures of this device. The MAUDE database stores reports of suspected device-associated deaths, injuries, and failures submitted to the by device manufacturers, importers, device user facilities (e.g., hospitals), health care professionals, patients, and consumers.
Patients and Methods
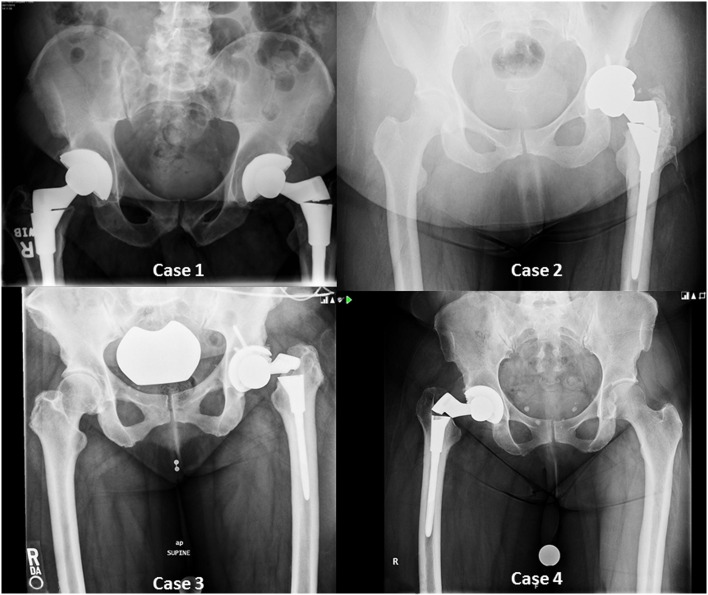
The four patients presented with EMPERION™ stem fractures (Fig. 1) between June 10 and September 15 2015. The patients were between 50 and 60 years of age (54.8 ± 4.6 years) with weights that ranged from 81 to 138 kg (103 ± 25; Table 1). The length of time that the devices had been implanted ranged from 1.2 to 8.3 years (4.43 ± 3.3 years). Three stems were implanted during primary total hip replacements, and one was used in a total hip revision. All four components had a high offset. No leg length or offset discrepancies existed between the operative and contralateral side, except for case 3, in which the operative side was 2 mm longer than the contralateral side prior to the fracture event. The surgeries to remove the four fractured implants were performed at an average of 3 ± 2 days following the date of fracture (range from 1 to 6 days).
Fig. 1.
Anteroposterior radiographs taken following fracture of four EMPERION™ femoral stems.
Table 1.
Patient demographic and specific stem details for the four fractured EMPERION™ cases
| Case | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age at index (years) | 60 | 52 | 50 | 57 |
| Weight (kg) | 138 | 91 | 104 | 81 |
| BMI (kg/m2) | 34 | 39 | 38 | 34 |
| Stem Size | 13 | 9 | 9 | 9 |
| Offset | High | Higha | Higha | Higha |
| Time in situ (years) | 8.3 | 5.0 | 1.2 | 1.5 |
| Trauma at time of fracture | Non-traumatic | Non-traumatic | Fall landing on knee | Non-traumatic |
| Occupation | Retired | Office manager | Corrections officer | Housekeeper |
| Activity level | Sedentary | Low | Moderate; low impact sports | Low |
aStem size 9 is available in two offset options named “reduced” and “standard.” Consequently, we refer to the standard offset as the high offset option
The four fractured EMPERION™ stems were retrieved for analysis following revision surgery. Implants were soaked in a 10% bleach solution for 20 min and washed with a mild detergent and warm tap water. Components were thoroughly rinsed with distilled water, then ethanol, and allowed to air dry overnight. Component sizing was provided by the revision surgeon and confirmed using the manufacturers’ catalog numbers on the components. Fracture surfaces were examined under an optical stereomicroscope (Wild Type 376788 Microscope, Heerbruug, Switzerland) at magnifications from ×6 to ×12 to determine the mechanism, origin, and direction of fracture. Scanning electron microscopy (ZIESS Supra 55, ZIESS, Oberkochen, Germany) was used to image the fracture surfaces at higher magnifications to further examine the fracture mechanism and to rule out any metallurgical defects in the titanium alloy [2].
The FDA MAUDE database search was conducted on September 17, 2015. The search was filtered by brand name (EMPERION™). No time limit was included.
Results
During three of the four revision procedures, extended trochanteric osteotomies (ETO) were required to remove and replace the fractured components. In the remaining patient, a small cortical window was created in the lateral cortex of the femur, immediately distal to the sleeve, exposing the lateral aspect of the stem. High-speed burrs and thin osteotomes were used around the ingrowth surface of the modular sleeve and the stem-sleeve composite was tapped out through the small lateral femoral window [13]. All four surgeons reported extensive bone ingrowth into the proximal sleeve porous coating and cold welding between the sleeve and the stem, both of which added to the difficulty of the revision procedures.
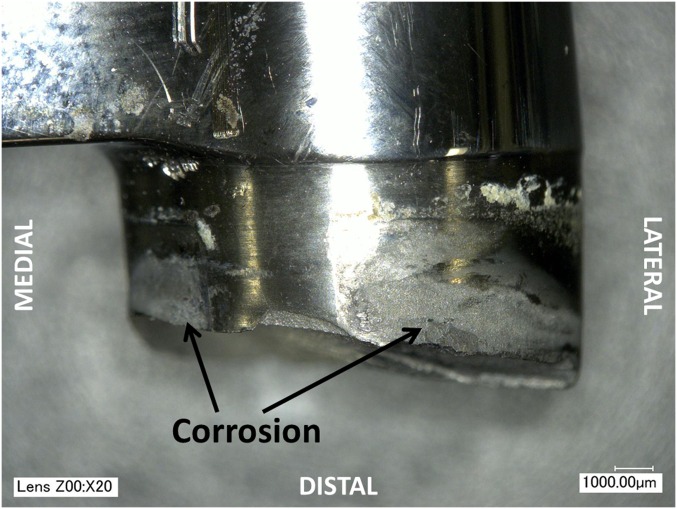
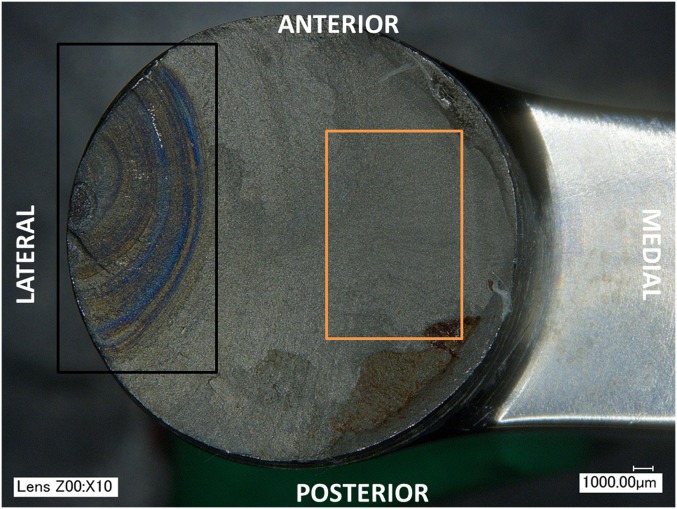
Analysis of the fracture surfaces under light microscopy and scanning electron microscopy (SEM) revealed fracture patterns consistent with fatigue due to a bending load. Fractures were initiated at the lateral aspect of the stem approximately 3 to 5 mm distal to the most proximal edge of the sleeve component (Fig. 2). Concentric beachmarks were consistent with fatigue fracture propagating from the lateral edge of the stem (Fig. 3). All four stems showed evidence of corrosion and pitting that extended across the origin of the fracture on the lateral aspect of the stem (Fig. 2), as well as discoloration in the initiation region of the fracture surface consistent with corrosion (Fig. 3). No metallurgical defects (e.g., poor microstructure and manufacturing flaws) that might have contributed to the fracture were evident in the titanium alloy at the region of fatigue crack initiation [2].
Fig. 2.
All four fractures initiated on the lateral aspect of the stem cross section. Regions of corrosion and pitting were visible on both the lateral and medial aspects of the stem, which were in contact with the sleeve in vivo.
Fig. 3.
All fractures exhibited concentric beachmarks moving outward from the fracture origin (black rectangle). Finger-like striations are visible (orange rectangle) moving through the region of the final spontaneous fracture, which covered about one third of the stem cross section.
Of the four fractured stems in our series, three were size 9 stems and one was a size 13 stem. Total stem offset (Do) ranged from 37 to 45 mm. The radius (R) of the stem at the region of fracture as measured with calipers, the Do, and patient weight were used to calculate femoral stem stress (σ) at the region of fracture based on previously established methods [7]:
where M is the moment, I is the area moment of inertia, F is the force, and A is the cross-sectional area. The average femoral stem stress of the four fractured EMPERION™ stems was 118.6 ± 12 MPa (range 104–133.5 MPa; Table 2).
Table 2.
Femoral stress at the lateral aspect of the stem was calculated for each fractured EMPERION™ stem in our series. Small stem diameter and high patient weight increased femoral stem stress, which reduced the time to fracture initiation
| Case | Stem size | Offset (mm) | Weight (kg) | Femoral stem stress (MPa) |
|---|---|---|---|---|
| 1 | 13 | 45 | 138 | 120.3 |
| 2 | 9 | 37 | 91 | 116.4 |
| 3 | 9 | 37 | 104 | 133.5 |
| 4 | 9 | 37 | 81 | 104.0 |
Thirty-four reports of adverse events were listed for the EMPERION™ device in the MAUDE database. Each event was reviewed for indication of femoral stem fracture; terms deemed indicative of fracture included “stem fracture” and “stem breakage.” Of the 34 reports, 16 unique reports described fracture of a modular EMPERION™ femoral stem; all sixteen cases required revision surgery. These 16 reports did not include the four cases reported in the current study. Component catalog numbers and lot numbers were available for 11 of the 16 stems identified in the MAUDE search. Four stems were size 9 stems, four were size 13 stems, and three were size 11. All size 11 stems were high offset stems. No information about the femoral heads or acetabular components was available via the MAUDE reports. One report cited a length of implantation of approximately 12 months before stem fracture. Dates of the adverse fracture events were listed in five reports and ranged from May 25, 2012 to March 25, 2014.
Discussion
We report four cases of EMPERION™ femoral stem fractures. To our knowledge, no such series has been previously reported. Stem fractures have been historically associated with high patient weight, component undersizing, varus positioning, retroversion, loss of medial calcar support, and metallurgy defects [2, 4, 8]. We examined the retrieved fractured components under light microscopy and SEM to determine mechanism of failure and conducted a MAUDE review to gain perspective on the prevalence of this problem.
This study has limitations. This is a retrospective review of four failed devices and may not represent the entire population of EMPERION™ components. The MAUDE database cannot be considered a comprehensive list of failed components because not all failures are reported to the FDA or the manufacturer. The reports usually lack patient demographic and radiographic information, so the fracture mode or the underlying cause of the fracture cannot be ascertained. Since the number of components that were implanted during the time frame of these reports is unknown, we cannot provide an estimate of the prevalence of stem fracture among all EMPERION™ stems implanted. However, of the four surgeons who revised the four fractured EMPERION™ stems in this report, only two surgeons regularly employ this system (SJ, AGDV). Between these two surgeons, a total of 134 EMPERION™ stems were implanted (in primary and revision cases), three of which (2.2%) have been revised to date (two for stem fracture as reported here and one for periprosthetic fracture). The remaining two surgeons (KC, SK) do not use this total hip system and only revised the fractured EMPERION™ stems reported here.
The fracture patterns and locations evident in our series of EMPERION™ stems were nearly identical to the patterns in four previous reports [7, 9, 11, 12] that described a total of 11 fractured S-ROM® (DePuy Orthopaedics, Inc, Warsaw, IN) stems. In these reports, high offset, high patient weight, and long lengths of implantations (6.5 ± 2.5 years; range 4–9.2 years) were found to predispose the stem-sleeve design of femoral stem to fatigue fracture. Our series was no exception. Risk of fatigue fracture in femoral stems increases with increased bending moment, which is dependent on the applied load and the lever arm. Our patients were obese, and thus high loads across the hip joint were coupled with the long lever arm of a high offset stem [7]. Thus, in our four EMPERION™ cases, high bending moments were applied cyclically, resulting in fatigue failure. Nonetheless, the prevalence of S-ROM® fractures appears to be relatively low, considering that the system has been on the market since 1982. The EMPERION™ stem was only introduced in 2006, which makes the cases we report extremely concerning.
In a retrieval study of 78 S-ROM® stems by Huot Carlson et al., seven stems were revised for fatigue fractures similar in presentation to those observed with this series of EMPERION™ stems. All seven stems were severely corroded at the stem-sleeve interface, under high stress in vivo, and had longer lengths of implantation, smaller stem diameters, and larger offsets than the non-fractured stems in the cohort. The surfaces of the fractured stems and their corresponding sleeves showed discoloration and/or black debris on between 65% and 100% of the total surface area. Discoloration of retrieved titanium alloy hip components has traditionally been considered to be a mild form of corrosion [6]. A small body of literature examining discoloration in titanium alloy dental implants following submersion in hydrogen peroxide solution attribute discoloration to alterations in the oxide film layer, suggesting corrosion [10, 14]. Corrosion, specifically crevice corrosion, occurring between the stem and the sleeve in these modular designs, may be a contributory factor to the stem fractures reported in the S-ROM® reports and our series of four EMPERION™ stems [7, 9, 11]. Corrosion may enhance fatigue crack initiation through the formation of pits on the stem surface, which can act as stress risers; this phenomenon is not present in non-modular stem systems [2]. Although two of our EMPERION™ stems fractured relatively quickly, after only 1 year in vivo, no drastic difference in the amount or severity of corrosion was found in these two cases compared to the two stems that experienced fracture after 5 and 8.3 years in vivo. Goldberg et al. suggested that increased bending could disrupt the passive layer, in turn speeding up the corrosion process [6]; however, in a previous study of 154 modular metal-on-polyethylene THA trunnion connections, we did not find an impact of length of implantation on corrosion at the head-neck junction [15]. Furthermore, in a study of retrieved modular neck stem connections, we found evidence of corrosion on the modular necks after only 4 weeks in vivo [5]. So, it appears that visual evidence of corrosion on retrievals is not time dependent.
Femoral stem stress was evaluated for the fractured and non-fractured S-ROM® stems in the Huot Carlson et al. series, and the fractured stems were found to be under significantly higher stress than the non-fractured stems [7]. As stress increases, the number of cycles to failure decreases [2, 7]. The four fractured EMPERION™ stems we examined experienced large cyclic stress, a combination of the large patient weights coupled with longer lever arms created by the large offset, and they subsequently fractured after only 1.2–8.3 years in vivo (Table 2). We suspect that the large bending moments were the driving factors causing the EMPERION™ stem fractures, and that fracture initiation occurred at the corroded lateral stem region that was weakened by pitting corrosion.
In summary, the variables that likely lead to an increased risk of fracture in modular stem-sleeve femoral systems include high-applied stress (due to increased weight and increased stem offset) and corrosion at the stem-sleeve interface. Fatigue fracture will always be a potential complication following THA; we urge surgeons choosing to use modular stem-sleeve femoral systems to consider the combined effect of patient weight, activity level, stem size, and stem offset when creating a preoperative plan to reduce the risk of stem fracture. Our cases, combined with those included in FDA MAUDE reports and those reported in the literature in similar modular stem-sleeve femoral systems, indicate that these designs are susceptible to fatigue failure under high stress and should be used cautiously in appropriate patients. We recommend being prepared to perform an extended trochanteric osteotomy in the event that a patient with this device presents with a stem fracture.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 3532 kb)
Acknowledgments
We acknowledge funding from the Mary and Fred Trump Institute for Implant Analysis, Mr. Glenn Bergenfield, and the Sidney, Milton, and Leoma Simon Foundation. We would like to thank Dr. Timothy Wright for his assistance in the retrieval analysis and manuscript preparation.
Compliance with Ethical Standard
Conflict of Interest
Chelsea N. Koch, BS, Laura Serrano Mateo, MD, and Kevin A. Cassidy, MD have declared that they have no conflict of interest. Stephen Kayiaros, MD reports personal fee from Depuy and other from Pfizer, outside the work. Seth A. Jerabek, MD reports personal fees from Stryker, outside the work. Alejandro Gonzalez Della Valle, MD reports personal fees from OrthoSensor, Ortho Development, and Link Bio, outside the work.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Therapeutic Study Level IV
References
- 1.Anon. EMPERION Modular Hip System: design rationale. www.smith-Nephew.com. 2006. Available at: http://www.smith-nephew.com/global/assets/pdf/products/surgical/emperion_design_45870401.pdf.
- 2.Becker W, Shipley R. ASM Handbook: Failure Analysis and Prevention. ASM International.
- 3.Charnley J. Fracture of femoral prostheses in total hip replacement. A clinical study. Clin Orthop. 1975;111:105–120. doi: 10.1097/00003086-197509000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Della Valle AG, Becksaç B, Anderson J, et al. Late fatigue fracture of a modern cemented forged cobalt chrome stem for total hip arthroplasty. J. Arthroplasty. 2005;20:1084–1088. doi: 10.1016/j.arth.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 5.De Martino I, Assini JB, Elpers ME, et al. Corrosion and fretting of a modular hip system: a retrieval analysis of 60 rejuvenate stems. J. Arthroplasty. 2015;30:1470–1475. doi: 10.1016/j.arth.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg JR, Gilbert JL, Jacobs JJ, et al. A multicenter retrieval study of the taper interfaces of modular hip prostheses. Clin. Orthop. 2002;401:149–161. doi: 10.1097/00003086-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Huot Carlson JC, Van Citters DW, Currier JH, et al. Femoral stem fracture and in vivo corrosion of retrieved modular femoral hips. J. Arthroplasty. 2012;27:1389–1396.e1. doi: 10.1016/j.arth.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Keaveny TM, Bartel DL. Mechanical consequences of bone ingrowth in a hip prosthesis inserted without cement. J Bone Jt. Surg Am. 1995;77:911–923. doi: 10.2106/00004623-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Mehran N, North T, Laker M. Failure of a modular hip implant at the stem-sleeve interface. Orthopedics. 2013;36:e978–e981. doi: 10.3928/01477447-20130624-33. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi T, Takemoto S, Hattori M, et al. Discoloration and dissolution of titanium and titanium alloys with immersion in peroxide- or fluoride-containing solutions. Dent. Mater. J. 2008;27:117–123. doi: 10.4012/dmj.27.117. [DOI] [PubMed] [Google Scholar]
- 11.Parisi T, Burroughs B, Kwon Y-M. Modular hip implant fracture at the stem-sleeve interface. Orthopedics. 2015;38:e234–e239. doi: 10.3928/01477447-20150305-91. [DOI] [PubMed] [Google Scholar]
- 12.Pearce S, Jenabzadeh A-R, Walter WL, et al. Spontaneous fracture of diaphyseal stem of S-ROM femoral prosthesis. BMJ Case Rep. 2014;2014:bcr2013202813. doi: 10.1136/bcr-2013-202813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellicci PM, Salvati EA, Robinson HJ. Mechanical failures in total hip replacement requiring reoperation. J. Bone Joint Surg. Am. 1979;61:28–36. [PubMed] [Google Scholar]
- 14.Takemoto S, Hattori M, Yoshinari M, et al. Discoloration of titanium alloy in acidic saline solutions with peroxide. Dent. Mater. J. 2013;32:19–24. doi: 10.4012/dmj.2012-194. [DOI] [PubMed] [Google Scholar]
- 15.Triantafyllopoulos GK, Elpers ME, Burket JC, et al. Otto Aufranc Award: large heads do not increase damage at the head-neck taper of metal-on-polyethylene total hip arthroplasties. Clin. Orthop. 2016;474:330–338. doi: 10.1007/s11999-015-4468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 3532 kb)