Abstract
Vaccination strategies are among the most successful and cost-effective public health strategies for preventing disease and death. Until recently, most of the existing immunization programs targeted infants and children younger than 5 years which have successfully resulted in reducing global infant and child mortality. Adolescent immunization has been relatively neglected, leaving a quarter of world's population underimmunized and hence vulnerable to a number of preventable diseases. In recent years, a large number of programs have been launched to increase the uptake of different vaccines in adolescents; however, the recommended vaccination coverage among the adolescent population overall remains very low, especially in low- and middle-income countries. Adolescent vaccination has received significantly more attention since the advent of the human papillomavirus (HPV) vaccine in 2006. However, only half of the adolescent girls in the United States received a single dose of HPV vaccine while merely 43% and 33% received two and three doses, respectively. We systematically reviewed literature published up to December 2014 and included 23 studies on the effectiveness of interventions to improve immunization coverage among adolescents. Moderate-quality evidence suggested an overall increase in vaccination coverage by 78% (relative risk: 1.78; 95% confidence interval: 1.41–2.23). Review findings suggest that interventions including implementing vaccination requirement in school, sending reminders, and national permissive recommendation for adolescent vaccination have the potential to improve immunization uptake. Strategies to improve coverage for HPV vaccines resulted in a significant decrease in the prevalence of HPV by 44% and genital warts by 33%; however, the quality of evidence was low. Analysis from single studies with low- or very low–quality evidence suggested significant decrease in varicella deaths, measles incidence, rubella susceptibility, and incidence of pertussis while the impact was nonsignificant for incidence of mumps with their respective vaccines. Further rigorous evidence is needed to evaluate the effectiveness of strategies to improve immunization uptake among adolescents from low- and middle-income countries.
Keywords: Adolescent health, Immunization, Vaccination, School vaccination, National vaccination, Reminders, National permissive recommendation
Vaccination programs are among the most successful and cost-effective public health strategies for preventing infections. Until recently, most of the existing immunization programs targeted infants and children younger than 5 years which have successfully resulted in reducing global infant and child mortality [1]. As a result, adolescent immunization has been overshadowed, leaving a quarter of world's population vulnerable to a number of preventable diseases. Estimates suggest that around 35 million American adolescents fail to receive at least one recommended vaccine [2]. In 2012, only half of the adolescent girls in the United States received a single dose of human papillomavirus (HPV) vaccine while merely 43% and 33% received two and three doses, respectively [3]. Missed vaccination opportunities for adolescent vaccination against tetanus, diphtheria, pertussis (TDaP), tetravalent meningococcal conjugate vaccine, and HPV are also common in the United States since adolescents are less likely to utilize preventive care [4].
Infectious and vaccine-preventable diseases disproportionately affect the low- and middle-income countries (LMICs) and disadvantaged populations in high-income countries (HICs). There were an estimated 266,000 deaths from cervical cancer worldwide in 2012, accounting for 7.5% of all female cancer deaths, of which nearly 85% occurred in developing countries [5]. The worldwide prevalence of infection with HPV in women without cervical abnormalities is 11%–12% with higher rates in Sub-Saharan Africa (24%), Eastern Europe (21%), and Latin America (16%) [6]. The proportion of invasive cervical cancer cases is higher in the LMICs with a relatively higher mortality/incidence ratio compared to the HICs [7], [8]. In U.S. settings, African-American girls were less likely to have either initiated or completed the three-dose HPV vaccination series [9]. This warrants an additional focus on adolescents from LMICs and underprivileged populations in HICs as they also deserve a healthy transition into adulthood.
The recommended immunization during adolescence by the World Health Organization includes three doses of hepatitis B (for high-risk groups if not previously immunized), Td booster, one dose of rubella (adolescent girls and/or childbearing-aged women if not previously vaccinated), and two doses of HPV for females (9–14 years) and three doses for those aged 15 years and above [10]. Low immunization rates in adolescents have a wide array of implications: outbreaks of vaccine-preventable diseases, negative effects on quality of life, and increased disease associated costs. Importantly, low immunization rates establish reservoirs of disease in adolescents that can affect others, including high-risk infants, elderly persons, and persons with underlying medical conditions.
Adolescent vaccination is a growing topic that has received significantly more attention since the advent of the HPV vaccine in 2006. In recent years, large number of programs have been launched to increase the uptake of different vaccines in adolescent populations; however, the recommended vaccination coverage among adolescents still remains low. These changes reflect an increased emphasis on the importance of adolescent immunization, but by themselves they will not sufficiently increase awareness or immunization rates [1]. The American Academy of Pediatrics suggests implementing one or more of the strategies including reminder calls, prompts or standing orders, strong provider recommendation, including all recommended vaccination at every visit, provider feedback, educating patients and their parents, addressing costs, and setting up vaccination clinics to increase immunization coverage in adolescents [11].
This article is part of a series of reviews conducted to evaluate the effectiveness of potential interventions for adolescent health and well-being. Detailed framework, methodology, and other potential interventions have been discussed in separate articles [12], [13], [14], [15], [16], [17], [18]. In this article, we systematically reviewed published literature to ascertain the effectiveness of interventions to improve immunization coverage among adolescents.
Methods
We reviewed all literature published up to December 2014 to identify studies on interventions to improve vaccination coverage. We did not restrict our search to any time limits or geographical settings. For the purpose of this review, the adolescent population was defined as aged 11–19 years; however, since many studies targeted youth along with adolescents, exceptions were made to include studies targeting adolescents and youth. Based on the current recommended vaccines for adolescents [19], search was conducted to identify studies focusing on improving coverage for HPV; measles, mumps, rubella (MMR); TDaP; meningococcal conjugate vaccine; and varicella vaccines among adolescents and youth. Studies were excluded if they targeted age groups other than adolescents and youth or did not report segregated data for the age group of interest. Studies were excluded if the intervention was aimed at comparing the efficacy/effectiveness of different vaccine preparations, assessing changes in antibody titers in individual subjects, or comparing various modes of delivering vaccines without control or baseline data.
Our priority was to select existing randomized controlled trials (RCTs), quasitrials, and before–after studies in which the intervention was directed toward the adolescent and youth and reported immunization coverage outcomes. Search strategy was developed using appropriate keywords, medical subject heading, and free text terms. The following principal sources of electronic reference libraries were searched to access the available data: The Cochrane Library, Medline, PubMed, Popline, LILACS, CINAHL, EMBASE, World Bank's JOLIS search engine, CAB Abstracts, British Library for Development Studies at IDS, the World Health Organization regional databases, Google, and Google Scholar. The titles and abstracts of all studies identified were screened independently by two reviewers for relevance and matched. Any disagreements on selection of studies between these two primary abstractors were resolved by the third reviewer. After retrieval of the full texts of all the studies that met the inclusion/exclusion criteria, data from each study were abstracted independently and in duplicate into a standardized form. Studies that met the inclusion criteria were selected and double data abstracted on a standardized abstraction sheet. Quality assessment of the included RCTs was done according to the Cochrane risk of bias assessment tool. We conducted a meta-analysis for individual studies using the software Review Manager, version 5.3 (Cochrane Collaboration, London, United Kingdom). Pooled statistics were reported as the relative risk (RR) for categorical variables and standard mean difference for continuous variables between the experimental and control groups with 95% confidence intervals (CIs). A grade of “high,” “moderate,” “low,” and “very low” was used for grading the overall evidence indicating the strength of an effect on specific health outcome according to the Grading of Recommendations Assessment, Development and Evaluation criteria [20].
Results
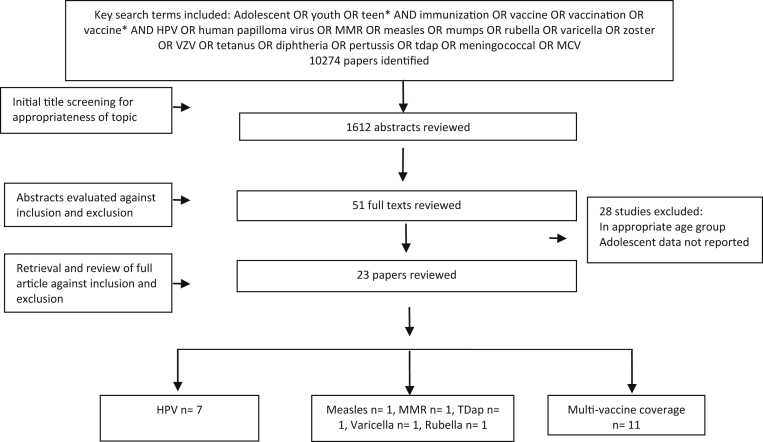
Figure 1 describes the search flow while characteristics of the included studies are detailed in Table 1. The search yielded 10,274 titles across all databases that were screened for the purpose of this review. Screening the relevant abstracts resulted in 51 full texts that were further screened after which 23 studies were included in this review [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], of which four were RCTs, three quasirandomized trials, and 16 before–after studies. Of the 23 included studies, seven [26], [27], [28], [29], [31], [32] focused on the HPV, 11 studies [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43] implemented interventions to improve coverage of multiple vaccines recommended for adolescents while measles [21], MMR [22], varicella [23], rubella [25], and TDaP [24] vaccines were assessed in one study each. All the studies were conducted in HICs of the United States, Canada, Australia, Denmark, and England. Included studies mainly focused on evaluating the impact of licensing and mass provision of vaccines as a part of national-level vaccination program to increase provision and coverage of adolescent vaccination, availability of free vaccines, implementing vaccination requirement before entry into school, reminder letters, telephone calls, and training of clinic staff on adolescent vaccines and strategies to improve immunization rates. Target populations in all studies were adolescents aged 11–19 years except three studies in the HPV vaccine category that targeted adolescents and youth till the age of 24 years.
Figure 1.
Search flow diagram. MCV = meningococcal conjugate vaccine; VZV = varicella zoster vaccine.
Table 1.
Characteristics of included studies
| Study | Study design | Country | Setting | Intervention | Target population | Control | Outcomes assessed |
|---|---|---|---|---|---|---|---|
| Measles | |||||||
| Zhuo et al. [21] | Before–after | China | Community | Supplementary immunization activities | All ages | No supplementary immunization activity | Incidence of measles |
| MMR | |||||||
| Ogbuanu et al. [22] | Before–after | United States | School | Selective school-based immunization | 9–14 years | No intervention | Incidence of mumps |
| Varicella | |||||||
| Nguyen et al. [23] | Before–after | United States | Nationwide vaccination | Universal childhood varicella vaccination program | All ages (outcome assessed for 10–19 years) | Before implementation of childhood varicella immunization | Number of deaths caused by varicella infection |
| TDaP | |||||||
| Quinn and McIntyre [24] | Before–after | Australia | School | School-based delivery of TDaP | 12–19 years | Nonavailability of school-based immunization | Incidence of pertussis |
| Rubella | |||||||
| Nelson et al. [25] | Before–after | United States | School | Vaccination requirement in school | Girls older than 10 years | Before the vaccination requirement | Rubella susceptibility |
| HPV | |||||||
| Baandrup et al. [26] | Before–after | Denmark | Countrywide provision | Licensing and mass provision of HPV as part of National HPV Program | 12–19 years | Absence of nationwide HPV availability | Incidence of genital warts |
| Bauer et al. [27] | Before–after | United States | Countrywide provision | Introduction population-level administration of HPV vaccine | <21 years | Nonavailability of population-level vaccination | Incidence of genital warts |
| Markowitz et al. [28] | Before–after | United States | Countrywide provision | Introduction of HPV vaccine into routine immunization schedule | 14- to 24-year-old females | HPV vaccine not included in routine immunization schedule | Prevalence of HPV |
| Mesher et al. [29] | Before–after | England | Countrywide provision | Introduction of National HPV Immunization Program | 16- to 24-year-old females | Nonavailability of population-level vaccination | Prevalence of HPV |
| Musto et al. [30] | Quasitrial | Canada | School and community | Within schools vaccination during Grades 1, 5, and 9 | 9- to 11- and 13- to 15-year-old females | Community-based vaccine availability at local community clinics by appointment | Vaccine uptake |
| Read et al. [31] | Before–after | Australia | Clinic | Introduction of National HPV Vaccination Program | 12- to 18-year-old females | HPV vaccine not included in national immunization schedule | Incidence of genital warts |
| Reiter et al. [32] | Before–after | United States | Nationwide recommendation | National permissive recommendation for HPV vaccine | 11- to 17-year-old males | No recommendation | Vaccine initiation |
| Multivaccine | |||||||
| Averhoff et al. [33] | Before–after | United States | School | Vaccination requirement in school | Fifth- through eighth-grade students | Students not subject to the requirement | Vaccine coverage |
| Bugenske et al. [34] | Quasitrial | United States | School | Vaccination requirement in school | 13–17 years | Students not subject to the requirement | Vaccine coverage |
| Carlson and Lewis [35] | Before–after | Canada | School | Vaccination requirement in school | Grades 7–13 | Students not subject to the requirement | Vaccine coverage |
| Fogarty et al. [36] | Before–after | United States | School | Vaccination requirement in school | Seventh-grade students | No control | Vaccine coverage |
| Harper and Murray [37] | Quasitrial | United States | Clinic | Clinic staff recommended vaccine on every visit | 11–18 years | No recommendation | Vaccine coverage |
| Kempe et al. [38] | RCT | United States | School | Recall reminders for vaccination | Sixth-grade male students | No recommendation | Vaccine coverage |
| Kharbanda et al. [39] | Before–after | United States | Hospital | Vaccination requirement in school | 11–14 years | Students not subject to the requirement | Vaccine coverage |
| Moss et al. [40] | Before–after | United States | Clinic | Clinic staff were invited to attend 1-hour one-on-one webinar on adolescent vaccines and strategies to improve immunization rates such as reviewing and flagging charts, decreasing missed opportunities, recalls, and establishing center guidelines for immunizations. | 12–17 years | No staff training | Vaccine coverage |
| Stockwell et al. [41] | RCT | United States | Community | Text message reminders for vaccination | 12–18 years | No reminder | Vaccine coverage |
| Suh et al. [42] | RCT | United States | Clinic | Reminders letters and calls for vaccination | 11–18 years | No reminder | Vaccine coverage |
| Szilagyi et al. [43] | RCT | United States | Clinic | Mail letters and telephone reminders for vaccination | 11–17 years | No reminder | Vaccine coverage |
HPV = human papillomavirus; MMR = measles, mumps, rubella; RCT = randomized controlled trial; TDaP = tetanus, diphtheria, pertussis.
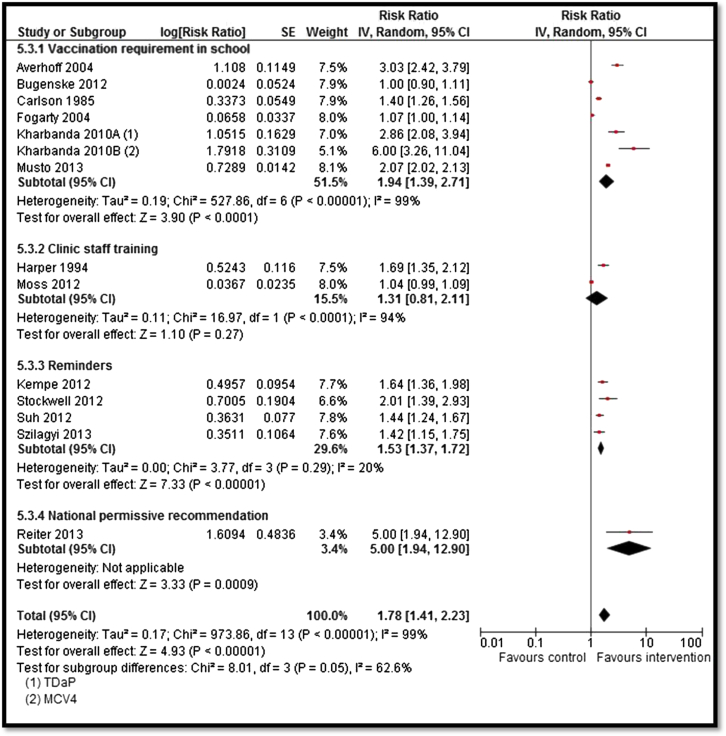
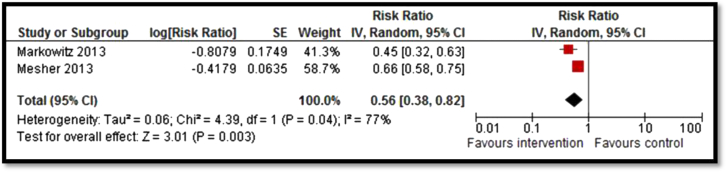
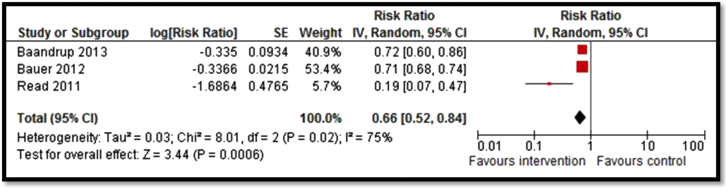
Moderate-quality evidence from 13 studies suggested an overall increase in vaccination coverage by 78% (RR: 1.78; 95% CI: 1.41–2.23; Figure 2) [30], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]. Subgroup analysis suggests that vaccination requirement in school, reminders, and national permissive recommendation had a significant impact on improving coverage while clinic staff training showed a nonsignificant impact. Strategies to improve coverage for HPV vaccines including countrywide provision and clinic-based delivery resulted in a significant decrease in the prevalence of HPV by 44% (RR: .56; 95% CI: .38–.82; Figure 3) [28], [29] and genital warts by 33% (RR: .66; 95% CI: .52–.84; Figure 4) [26], [27], [31]; however, the quality of evidence was low. Since only one study each was included for measles, mumps, pertussis, and varicella vaccines, it was not possible to pool results. Analysis from single studies with low or very low quality suggested significant decrease in varicella deaths (RR: .74; 95% CI: .56–.98), measles incidence (RR: .12; 95% CI: .03–.38), rubella susceptibility (RR: .27; 95% CI: .15–.46), and incidence of pertussis (RR: .24; 95% CI: .16–.36) while the impact was nonsignificant for incidence of mumps (RR: .96; 95% CI: .42–2.18).
Figure 2.
Forest plot for the impact of strategies on vaccination coverage. IV = inverse variance; MCV = meningococcal conjugate vaccine; SE = standard error.
Figure 3.
Forest plot for the prevalence of HPV. IV = inverse variance; SE = standard error.
Figure 4.
Forest plot for the prevalence of genital warts. IV = inverse variance; SE = standard error.
The outcome quality was rated to be “moderate” for vaccine coverage; “low” for the prevalence of HPV, genital warts, and mump incidence; and “very low” for varicella deaths, measles incidence, rubella susceptibility, and incidence of pertussis. The outcome quality was downgraded due to nonrobust designs, heterogeneity, and limited generalizability to HICs only. A summary of quality of evidence is provided in Table 2.
Table 2.
Summary of findings for the effect of interventions for improving immunization coverage among adolescents
| Quality assessment |
Summary of findings |
|||||||
|---|---|---|---|---|---|---|---|---|
| Number of studies | Design | Limitations | Consistency | Directness |
Number of events |
RR (95% CI) | ||
| Generalizability to population of interest | Generalizability to intervention of interest | Intervention | Control | |||||
| Vaccine coverage: moderate outcome-specific quality of evidence | ||||||||
| 13 studies (14 data sets) | RCT, quasi, and observational studies | Study designs not robust | Twelve studies suggest benefit | All studies targeted adolescents aged 11–19 years in developed countries | Interventions included vaccination requirement in school, reminders, and national permissive recommendation | 5,092 | 4,303 | 1.78 (1.41–2.23) |
| HPV prevalence: low outcome-specific quality of evidence | ||||||||
| Two studies | Observational studies | Study designs not robust | Both studies suggest benefit | Studies targeted adolescents aged 14–24 years in developed countries | Intervention included introducing HPV vaccine into routine immunization | 499 | 554 | .56 (.38–.82) |
| Incidence of genital warts: low outcome-specific quality of evidence | ||||||||
| Three studies | Observational studies | Study designs not robust | All three studies suggest benefit | All studies from developed countries targeting adolescents from age 12 to 21 years | All studies focused on increased provision of HPV vaccine through national HPV programs | 3,875 | 5,409 | .66 (.52–.84) |
| Varicella deaths: very low outcome-specific quality of evidence | ||||||||
| One | Observational study | Study design not robust | Only one study | Intervention targeted all age groups in the United States, outcomes reported for 10- to 19-year age group | Universal childhood varicella vaccination program | 77 | 104 | .74 (.56–.98) |
| Mumps incidence: low outcome-specific quality of evidence | ||||||||
| One | Quasitrial | No randomization (quasitrial) | Only one study | Adolescents 9–14 years in the United States | School-based immunization | 28 | 7 | .96 (.42–2.21) |
| Pertussis incidence: very low outcome-specific quality of evidence | ||||||||
| One | Observational study | Study design not robust | Only one study | Interventions targeted adolescents 12–19 years in Australia | School-based delivery of TDaP vaccine | 31 | 128 | .24 (.16–.36) |
| Rubella susceptibility: very low outcome specific quality of evidence | ||||||||
| One | Observational study | Study design not robust | Only one study | Interventions targeted adolescent girls >10 years in the United States | Vaccination requirement in school | 15 | 49 | .27 (.15–.46) |
| Measles incidence: very low outcome-specific quality of evidence | ||||||||
| One | Observational study | Study design not robust | Only one study | Interventions targeted all ages in China | Supplementary immunization activities | 3 | 26 | .12 (.03–.38) |
CI = confidence interval; HPV = human papillomavirus; RCT = randomized controlled trial; RR = relative risk; TDaP = Tetanus, diphtheria, pertussis.
Discussion
Our review findings suggest that strategies to increase HPV, TDaP, MMR, and varicella vaccination uptake among adolescents can significantly improve the coverage for these vaccines. Implementing vaccination requirement in school, sending reminders, and national permissive recommendation for adolescent vaccination has the potential to improve immunization uptake. These interventions have also led to significant decline in the prevalence of HPV and genital warts; incidence of measles and pertussis; rubella susceptibility; and varicella deaths. However, these findings should be interpreted with caution since these are from single studies with low or very low quality. Furthermore, these studies capture the incremental benefits of vaccination of those who may have missed earlier doses or failed to seroconvert to earlier doses since these vaccines are usually given at younger ages.
All the included studies were conducted in HICs depicting dearth of evidence evaluating the effectiveness of strategies to improve immunization uptake among adolescent from LMICs. This could also be attributable to the scope of review since our review was restricted to strict inclusion and exclusion criteria, and we did not include gray literature reporting various country case studies. Furthermore, recent state mandatory vaccination and exception policies could also have affected the vaccination coverage rates; however, these programs and policy interventions do not lend themselves to intervention studies. One of the limitations of the review was that the search terms were in English, and hence foreign language articles may not have been identified. There is lack of rigorously designed studies since most of the existing studies have utilized the pre- and postimplementation data after the approval of vaccine legislation or national launch of vaccination program without having a control site. Only a single study each for MMR, TDaP, varicella, and meningococcal vaccines were found, showing a lack of focus evaluating the impact of uptake for vaccines other than HPV. This highlights the need for further studies to assess the uptake and delivery platforms to deliver these vaccines in adolescent population. Included studies targeted various overlapping adolescent and youth age groups that might have led to variations in the outcome effect.
Despite the high burden of infectious diseases and low immunization coverage in LMICs, strategies to improve vaccine coverage for adolescent age group are minimal. Although there are existing data outlining what exists in LMICs for delivering adolescent immunization, primarily through school-based approaches; however, there are little data that have systematically been evaluated for the impact of strategies to increase coverage [44]. Various countries' case studies have documented experiences from LMICs with existing school-based immunization programs, for example, in Indonesia, Malaysia, Sri Lanka, and Tunisia; however, they lack rigorous evaluations [45]. For HPV, various national-level programs are in place especially in LMICs; Bhutan is the first LMIC to roll out a national HPV vaccination program, followed by Rwanda and Uganda. These programs suggest that vaccine uptake can be improved by providing evidence-based education and outreach; however, experiences in these countries underscore complex challenges and planning to ensure sustainability [46]. The number of LMICs that have introduced HPV vaccination is relatively low; however, the coverage levels in these countries are relatively higher than in some HIC. Enabling factors for improved coverage in LMICs include political will, nationwide sensitization campaign, school-based vaccination, and community involvement [44], [45], [46], [47].
Despite the availability of the HPV vaccine in HICs like the United States, the uptake remains low. Vaccine utilization is a multifactorial phenomenon which depends on several factors including vaccine acceptability, perceived disease susceptibility, perceived benefit of vaccination, and intention to receive the particular vaccine. A recent systematic review on barriers to HPV vaccination among adolescents in the United States suggests financial concerns and parental attitudes as barriers to HPV vaccination [48], [49]. Good understanding and knowledge of the factors and importance of vaccine in target population are important for tailoring vaccine improvement strategies and subsequent success of the program in achieving targeted vaccine coverage [50]. It is imperative to develop and test context-specific strategies to improve adolescent vaccine uptake and dose completion rates. Educational interventions could increase knowledge and clear misconceptions related to seriousness of vaccine-preventable infection and cervical cancer, susceptibility of adolescents to infection, and risk of infection. Such strategies would also address barriers to adolescent vaccine uptake and dose completion, such as parental concerns about vaccine safety, and effectiveness [51]. Very few of the included studies in our review utilized mHealth/eHealth technology for improving immunization coverage which could be one of the potentially effective strategies to target adolescent age group especially in LMIC settings owing to the higher use in this age group and recent explosion in Internet access in developing countries due to the emergence of mobile Internet [52]. One of the concerns with the introduction of HPV vaccine in low-resourced high-burden countries is lack of cost-effective data; however, some recent analysis suggests that HPV vaccination is likely to be cost-effective, especially in LMICs [53], [54].
Improving vaccination coverage to decrease the burden of these preventable diseases would require an integrated approach ranging from mass availability of vaccines at the national level to targeting adolescents in school and during health care visits to optimize the effectiveness of immunization programs. Besides these programs, there is a need for an increased emphasis on the importance of adolescent immunization by identifying and overcoming barriers to adolescent vaccination. Further research is needed to explore why missed vaccination opportunities occur and to develop evidence-based strategies to reduce missed opportunities and improve adolescent vaccination coverage.
Acknowledgments
All authors contributed to finalizing the manuscript.
Footnotes
Conflicts of Interest: The authors do not have any financial or nonfinancial competing interests for this review.
Disclaimer: Publication of this article was supported by the Bill and Melinda Gates Foundation. The opinions or views expressed in this supplement are those of the authors and do not necessarily represent the official position of the funder.
Funding Sources
The preparation and publication of these papers was made possible through an unrestricted grant from the Bill & Melinda Gates Foundation (BMGF).
References
- 1.National Foundation for Infectious Diseases: Adolescent vaccination bridging from a strong childhood foundation to a healthy adulthood: A report on strategies to increase adolescent immunization rates. Available at: http://www2.aap.org/immunization/pediatricians/pdf/ImmunizationofAdolescents_nfid.pdf. Accessed November 16, 2015.
- 2.National Foundation of Infectious Diseases . 2012. Adolescent vaccination: Bridging from a strong childhood foundation to a healthy adulthood.https://www.aap.org/en-us/Documents/immunization_Adolescents_nfid.pdf Available at: Accessed November 16, 2015. [Google Scholar]
- 3.Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2013—United States. Morbidity Mortality Weekly Rep. 2013 http://www2.aap.org/immunization/illnesses/hpv/HPV2013MMWR.pdf Available at: Accessed November 16, 2015. [PMC free article] [PubMed] [Google Scholar]
- 4.Wong C.A., Taylor J.A., Wright J.A. Missed opportunities for adolescent vaccination, 2006-2011. J Adolesc Health. 2013;53:492–497. doi: 10.1016/j.jadohealth.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . 2012. Globocan 2012: Estimated cancer incidence, mortality and prevalence worldwide.http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx Available at: Accessed November 16, 2015. [Google Scholar]
- 6.Forman D., de Martel C., Lacey C.J. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 7.WHO/ICO Information center on HPV and Cervical Cancer . 2010. Human papilomavirus and related cancers in Ethiopia.www.hpvcentre.net Summary report. Available at: Accessed November 16, 2015. [Google Scholar]
- 8.Fitzmaurice C., Dicker D., Pain A. The global burden of cancer 2013. JAMA Oncol. 2015;4:505. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessels S.J.M., Marshall H.S., Watson M. Factors associated with HPV vaccine uptake in teenage girls: A systematic review. Vaccine. 2012;30:3546–3556. doi: 10.1016/j.vaccine.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . 2015. WHO recommendations for routine immunization—Summary tables.http://www.who.int/immunization/policy/immunization_tables/en/ Available at: Accessed November 16, 2015. [Google Scholar]
- 11.American Academy of Pediatrics Immunization Resources . 2013. Adolescent immunizations: Strategies for increasing coverage rates.http://www2.aap.org/immunization/pediatricians/pdf/TopStrategiesforIncreasingCoverage.pdf Available at: Accessed November 16, 2015. [Google Scholar]
- 12.Salam R.A., Faqqah A., Sajjad N. Improving adolescent sexual and reproductive health: A systematic review of potential interventions. J Adolesc Health. 2016;59(Suppl. 4):S11–S28. doi: 10.1016/j.jadohealth.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salam R.A., Hooda M., Das J.K. Interventions to improve adolescent nutrition: A systematic review and meta-analysis. J Adolesc Health. 2016;59(Suppl. 4):S29–S39. doi: 10.1016/j.jadohealth.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das J.K., Salam R.A., Arshad A. Interventions for adolescent substance abuse: An overview of systematic reviews. J Adolesc Health. 2016;59(Suppl. 4):S61–S75. doi: 10.1016/j.jadohealth.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das J.K., Salam R.A., Lassi Z.S. Interventions for adolescent mental health: an overview of systematic reviews. J Adolesc Health. 2016;59(Suppl. 4):S49–S60. doi: 10.1016/j.jadohealth.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salam R.A., Arshad A., Das J.K. Interventions to prevent unintentional injuries among adolescents: A systematic review and meta-analysis. J Adolesc Health. 2016;59(Suppl. 4):S76–S87. doi: 10.1016/j.jadohealth.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salam R.A., Das J.K., Lassi Z.S., Bhutta Z.A. Adolescent health interventions: Conclusions, evidence gaps, and research priorities. J Adolesc Health. 2016;59(Suppl. 4):S88–S92. doi: 10.1016/j.jadohealth.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salam R.A., Das J.K., Lassi Z.S., Bhutta Z.A. Adolescent health and well-being: Background and methodology for review of potential interventions. J Adolesc Health. 2016;59(Suppl. 4):S4–S10. doi: 10.1016/j.jadohealth.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention . 2014. Vaccine recommendations of the ACIP.http://www.cdc.gov/vaccines/hcp/acip-recs/index.html Available at: Accessed November 16, 2015. [Google Scholar]
- 20.Walker N., Fischer-Walker C., Bryce J. Standards for CHERG reviews of intervention effects on child survival. Int J Epidemiol. 2010;39(Suppl. 1):i21–i31. doi: 10.1093/ije/dyq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuo J., Geng W., Hoekstra E.J. Impact of supplementary immunization activities in measles-endemic areas: A case study from Guangxi, China. J Infect Dis. 2011;204(Suppl. 1):S455–S462. doi: 10.1093/infdis/jir063. [DOI] [PubMed] [Google Scholar]
- 22.Ogbuanu I.U., Kutty P.K., Hudson J.M. Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics. 2012;130:e1567–e1574. doi: 10.1542/peds.2012-0177. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen H.Q., Jumaan A.O., Seward J.F. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N Engl J Med. 2005;352:450–458. doi: 10.1056/NEJMoa042271. [DOI] [PubMed] [Google Scholar]
- 24.Quinn H.E., McIntyre P.B. The impact of adolescent pertussis immunization, 2004-2009: Lessons from Australia. Bull World Health Organ. 2011;89:666–674. doi: 10.2471/BLT.11.086538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson D.B., Layde M.M., Chatton T.B. Rubella susceptibility in inner-city adolescents: The effect of a school immunization law. Am J Public Health. 1982;72:710–713. doi: 10.2105/ajph.72.7.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baandrup L., Blomberg M., Dehlendorff C. Significant decrease in the incidence of genital warts in young Danish women after implementation of a national human papillomavirus vaccination program. Sex Transm Dis. 2013;40:130–135. doi: 10.1097/OLQ.0b013e31827bd66b. [DOI] [PubMed] [Google Scholar]
- 27.Bauer H.M., Wright G., Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: An analysis of California public family planning administrative claims data, 2007-2010. Am J Public Health. 2012;102:833–835. doi: 10.2105/AJPH.2011.300465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markowitz L.E., Hariri S., Lin C. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010. J Infect Dis. 2013;208:385–393. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 29.Mesher D., Soldan K., Howell-Jones R. Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England. Vaccine. 2013;32:26–32. doi: 10.1016/j.vaccine.2013.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musto R., Siever J.E., Johnston J.C. Social equity in human papillomavirus vaccination: A natural experiment in Calgary Canada. BMC Public Health. 2013;13:640. doi: 10.1186/1471-2458-13-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Read T.R., Hocking J.S., Chen M.Y. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect. 2011;87:544–547. doi: 10.1136/sextrans-2011-050234. [DOI] [PubMed] [Google Scholar]
- 32.Reiter P.L., Gilkey M.B., Brewer N.T. HPV vaccination among adolescent males: Results from the National Immunization Survey-Teen. Vaccine. 2013;31:2816–2821. doi: 10.1016/j.vaccine.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Averhoff F., Linton L., Peddecord K.M. A middle school immunization law rapidly and substantially increases immunization coverage among adolescents. Am J Public Health. 2004;94:978–984. doi: 10.2105/ajph.94.6.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bugenske E., Stokley S., Kennedy A., Dorell C. Middle school vaccination requirements and adolescent vaccination coverage. Pediatrics. 2012;129:1056–1063. doi: 10.1542/peds.2011-2641. [DOI] [PubMed] [Google Scholar]
- 35.Carlson J.A., Lewis C.A. Effect of the immunization program in Ontario schools. Can Med Assoc J. 1985;133:215–216. [PMC free article] [PubMed] [Google Scholar]
- 36.Fogarty K.J., Massoudi M.S., Gallo W. Vaccine coverage levels after implementation of a middle school vaccination requirement, Florida, 1997-2000. Public Health Rep. 2004;119:163–169. doi: 10.1177/003335490411900209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harper P.G., Murray D.M. An organizational strategy to improve adolescent measles-mumps-rubella vaccination in a low socioeconomic population. A method to reduce missed opportunities. Arch Fam Med. 1994;3:257–262. doi: 10.1001/archfami.3.3.257. [DOI] [PubMed] [Google Scholar]
- 38.Kempe A., Barrow J., Stokley S. Effectiveness and cost of immunization recall at school-based health centers. Pediatrics. 2012;129:e1446–e1452. doi: 10.1542/peds.2011-2921. [DOI] [PubMed] [Google Scholar]
- 39.Kharbanda E.O., Stockwell M.S., Colgrove J. Changes in Tdap and MCV4 vaccine coverage following enactment of a statewide requirement of Tdap vaccination for entry into sixth grade. Am J Public Health. 2010;100:1635–1640. doi: 10.2105/AJPH.2009.179341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moss J.L., Reiter P.L., Dayton A., Brewer N.T. Increasing adolescent immunization by webinar: A brief provider intervention at federally qualified health centers. Vaccine. 2012;30:4960–4963. doi: 10.1016/j.vaccine.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 41.Stockwell M.S., Kharbanda E.O., Martinez R.A. Text4Health: Impact of text message reminder-recalls for pediatric and adolescent immunizations. Am J Public Health. 2012;102:e15–e21. doi: 10.2105/AJPH.2011.300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suh C.A., Saville A., Daley M.F. Effectiveness and net cost of reminder/recall for adolescent immunizations. Pediatrics. 2012;129:e1437–e1445. doi: 10.1542/peds.2011-1714. [DOI] [PubMed] [Google Scholar]
- 43.Szilagyi P.G., Albertin C., Humiston S.G. A randomized trial of the effect of centralized reminder/recall on immunizations and preventive care visits for adolescents. Acad Pediatr. 2013;13:204–213. doi: 10.1016/j.acap.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization . 2015. Immunization, vaccines and biologicals: Data, statistics and graphics.http://www.who.int/immunization/monitoring_surveillance/data/en/ Available at: Accessed November 16, 2015. [Google Scholar]
- 45.World Health Organization . 2015. Immunization, vaccines and biologicals: School-based immunization.http://www.who.int/immunization/programmes_systems/policies_strategies/school_based_immunization/en/ Available at: Accessed November 16, 2015. [Google Scholar]
- 46.Adams P. Reaching teenagers with three-times jab is a first for most countries. Bull World Health Organ. 2012;90:874. doi: 10.2471/BLT.12.021212. World Health Organization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Binagwaho A., Wagner C.M., Gatera M. Achieving high coverage in Rwanda's national human papillomavirus vaccination programme. Bull World Health Organ. 2012;90:623–628. doi: 10.2471/BLT.11.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holman D.M., Benar V., Roland K.B. Barriers to human papillomavirus vaccination among US Adolescents. A systematic review of the literature. JAMA Pediatr. 2014;168:76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cassidy B., Elizabeth A.S. Uptake of the human papillomavirus vaccine: A review of the literature and report of a quality assurance project. J Pediatr Health Care. 2012;26:92–101. doi: 10.1016/j.pedhc.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 50.Middleman A.B., Rosenthal S.L., Rickert V.I. Adolescent immunizations: A position paper of the Society for Adolescent Medicine. J Adolesc Health. 2006;38:321–327. doi: 10.1016/j.jadohealth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Tissot A.M., Zimet G.D., Rosenthal S.L. Effective strategies for HPV vaccine delivery: The views of pediatricians. J Adolesc Health. 2007;41:119–125. doi: 10.1016/j.jadohealth.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.John D Piette a, KC Lun b, Lincoln A Moura c, Hamish SF Fraser d, Patricia N Mechael e, John Powell f & Shariq R Khoja g. Impacts of e-health on the outcomes of care in low- and middle-income countries: Where do we go from here? Bulletin of the World Health Organization. Available at: http://www.who.int/bulletin/volumes/90/5/11-099069/en/index.html. Accessed November 16, 2015. [DOI] [PMC free article] [PubMed]
- 53.Jit M., Brisson M., Portnoy A., Hutubessy R. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: A PRIME modelling study. The Lancet Glob Health. 2014;2:e406–e414. doi: 10.1016/S2214-109X(14)70237-2. [DOI] [PubMed] [Google Scholar]
- 54.Fesenfeld M., Hutubessy R., Jit M. Cost-effectiveness of human papillomavirus vaccination in low and middle income countries: A systematic review. Vaccine. 2013;31:3786–3804. doi: 10.1016/j.vaccine.2013.06.060. [DOI] [PubMed] [Google Scholar]