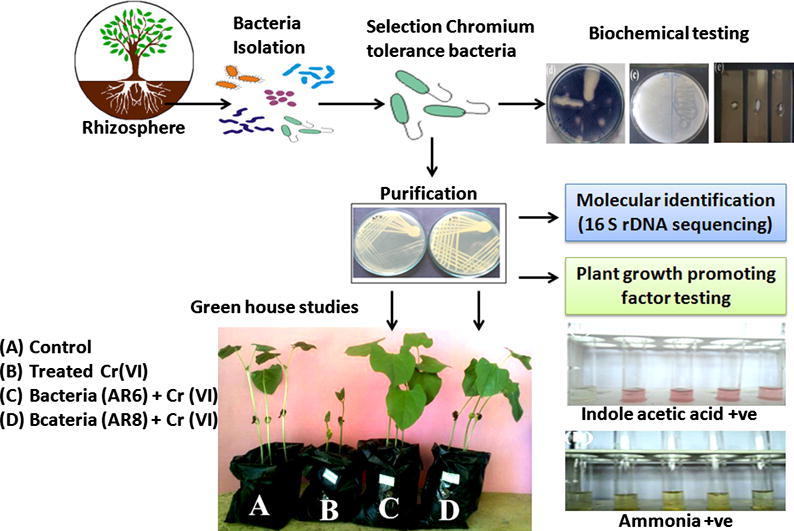
Graphical abstract
Keywords: Rhizosphere bacterial strains, Cr(VI) tolerance, Plant growth promoting substances, Antioxidant system
Abstract
Contamination of agriculture land by heavy metals is a worldwide risk that has sped up noticeably since the beginning of the industrial revolution. Hence, there arise the demands of heavy metal tolerant plant growth promoting bacterial strains for specific metal contaminated agricultural sites restoration. In this study, 36 bacterial isolates were screened out from the rhizospheric soil of Phaseolus vulgaris. Among these, two bacterial strains AR6 and AR8 were selected based on their higher Cr(VI) tolerance (1200 and 1100 μg/mL, respectively) and the maximum production of plant growth promoting substances. In the molecular characterization study, both the bacterial strains showed 99% homology with Cellulosimicrobium funkei KM032184. In greenhouse experiments, the exposure of Cr(VI) to P.vulgaris inhibited the growth and photosynthetic pigments and increased the enzymatic and non-enzymatic antioxidant expressions. However, rhizosphere bacterial inoculations alleviated the negative effect of Cr(VI) and enhanced the seed germination rate (89.54%), shoot (74.50%),root length (60%), total biomass (52.53%), chlorophyll a (15.91%), chlorophyll b (17.97%), total chlorophyll (16.58%) and carotenoid content (3.59%). Moreover, bacterial inoculations stabilized and modulated the antioxidant system of P. vulgaris by reducing the accumulation of Cr in plant tissues. The present finding shows the Cr(VI) tolerance and plant growth promoting properties of the rhizosphere bacterial strains which might make them eligible as biofertilizer of metal-contaminated soils.
Introduction
Heavy metal contamination is a serious environmental problem, limiting soil fertility and plant productivity and threatening human health. Maintenance of soil quality is important for sustainable agriculture. The soil may become contaminated with metals from a variety of industrial and anthropogenic sources. More than 12% of agricultural lands are polluted by heavy metals globally [1]. Chromium (Cr) is one of the predominant toxic heavy metals discharged by tanneries, textile, metallurgical, paint and other metal processing industries and anthropogenic activities. Approximately 2000–32,000 tons/year of elemental Cr are discharged into the environment from the tanning industries of India. Effluent contaminated water irrigation has severely affected crop productivity. About 35,000 ha of agricultural land has become unfit for cultivation due to tanneries in Vellore district, Tamil Nadu, India, which subsequently decreased the yield of paddy (75%), coconuts (52%) and sugarcane (48%) [2].
Chromium exists in nine valence states from −II to +VI. Among these, trivalent chromium (Cr III) and hexavalent chromium (Cr VI) are the predominant oxidation states of Cr that exist in the environment. Chromium(III) is an essential micronutrient for many organisms and also thousand times less toxic than Cr(VI). Chromium(VI) is the most toxic form due to its high solubility in water and rapid permeability through plasma membranes that can potentially interact with gene transcription, translation and protein expression [3]. High concentrations of Cr(VI) cause series of harmful effects to plants. These effects consist of plant growth inhibition, nutrient imbalance, leaf chlorosis with loss of plasma membrane integrity, alteration in bio-membranes permeability, impairment of photosynthesis and high reactive oxygen species (ROS) production. Chromium induced oxidative stress results in the overproduction of ROS such as superoxide and hydrogen peroxide. These free radical species are extremely harmful to plant cell growth and cause senescence [4].
Plant growth promoting rhizosphere (PGPR) bacteria have the ability to enhance the plant growth and yield through phosphate solubilization, synthesis of indole acetic acid (IAA), antimicrobial compounds and siderophores when applied on seeds or to soils [5]. Recently, the role of PGPR in metal stress attenuation of plants growing in metal contaminated soils has been recognized [6]. Improved nutritional status and altered metal uptake are among the most related benefits of PGPR association to host plants under metal stress [1]. Moreover, these PGPR also affect the availability of heavy metal by various metal tolerance mechanisms, including immobilization, chelation, exclusion, active removal, biosorption and bioaccumulation in external and intracellular spaces [7]. Thus, these PGPR act as metal sink, reducing local concentrations in soil and creating a more suitable stress free environment to plant in metal contaminated soils [6]. Therefore, such organisms endowed with Cr(VI) tolerant and plant growth promoting activities are of practically important for both the remediation of metal contaminated environment and plant growth promotion.
There has been little or no reports on Cr(VI) tolerant and plant growth promoting ability of Cellulosimicrobium funkei strain. However, reports related to the effect of PGPR on plant growth, physiological and biochemical aspects under the Cr(VI) stress are rare. With the impetus given for the year 2016 as the international year of pulses by Global Pulse Confederation (GPC), the study deems scientifically important in managing the agronomic gaps. In the present study, rhizosphere bacterial strains were isolated from P. vulgaris rhizosphere and screened for their Cr(VI) tolerance. The most effective strains were identified and investigated for their plant growth promoting traits to understand how they could confer plant growth and alleviation of Cr(VI) toxicity using a native P. vulgaris as a model plant. The mechanism for Cr(VI) stress alleviation in the host plant was evaluated by determining non-enzymatic (proline and melonoldehyde content) and enzymatic (catalase, peroxidase and polyphenol oxidase) antioxidant expression in the rhizosphere bacterial strains inoculated and uninoculated Cr(VI) treated plants.
Material and methods
Chemicals and reagents
The analytical chemicals were purchased from HiMedia, Mumbai, India. Molecular grade chemicals and PCR master mix were purchased from GeNeiTM, Bangalore, India, and PCR purification kit was purchased from Fermentas Life Science, Mumbai, India.
Seeds
Phaseolus vulgaris seeds were obtained from the district agriculture office, Omalur, Salem, Tamil Nadu, India. The seeds were physically analyzed and disease free, and healthy seeds were chosen for the greenhouse experiments.
Soil sample collection and characterization
Soil samples were collected from leather industry effluent discharging agricultural sites of Ambur, Tamil Nadu, India, and the control soil sample was collected from the experimental garden of Periyar University, Salem. The physicochemical properties such as texture, pH, total moisture content, macronutrients (N, P and K), calcium carbonate, electro conductivity and heavy metal concentrations of the soil samples were characterized by standard methods [8].
Isolation and purification of Cr(VI) tolerant rhizosphere bacterial strains
Soil samples were collected from rhizosphere of P. vulgaris, which was grown in leather industry effluents contaminated soil at the greenhouse of Periyar University. Approximately 10 g of rhizosphere soil sample was transferred to a conical flask containing 100 mL of sterile distilled water and shaken for 30 min at 200 rpm. Further, the homogenized soil sample was serially diluted and plated on 100 μg/mL of Cr(VI) (K2Cr2O7) amended Luria–Bertani (LB) medium (g/L: tryptone 10; yeast extract 5; NaCl 10; agar 20 and pH 7.0 ± 0.2). The culture plates were incubated at 35 ± 2 °C for 2–5 days. Bacterial colonies that grew on Cr(VI) amended medium were considered as Cr(VI) tolerant bacteria.
Assessment of bacterial strains for Cr(VI) tolerance
The rhizosphere bacterial strains were tested for their maximum tolerant level to Cr(VI) by the agar dilution method [3]. Freshly prepared LB plates were incorporated with increasing concentrations (0–1250 μg/mL) of Cr(VI) to determine the maximum Cr(VI) tolerance level of the strains. A loopful of bacterial colonies from bacterial grown on LB agar slant were spot inoculated on Cr(VI) amended agar plates. Plates were incubated at 35 ± 2 °C for 3–5 days to observe the bacterial growth. Two bacterial isolates AR6 and AR8 showed maximum tolerant against Cr(VI) and were selected for further studies.
Molecular identification of the rhizosphere bacterial strains
Molecular characterization of the rhizosphere bacterial strains AR6 and AR8 was carried out by 16S rDNA gene sequencing method using universal primers (27F 5′-AGA GTT TGA TCC TGG CTC AG-3′ and 1492R 5′-GGC TAC CTT GTT ACG ACT T-3′). For PCR, 1 μL of bacterial DNA and 2 μL of each primer were mixed with 10 μL of 2× PCR Master Mix (GeNeiTM, Bangalore, India) consisting of dNTPs, Taq DNA polymerase and PCR buffer. The final volume was made up to 20 μL with sterile double distilled water. PCR amplification was performed in a thermal cycler (cyber cycler-P series PCR peltier model p96+ USA) with the following cycling conditions: initial denaturation at 94 °C for 4 min and 30 cycles at 94 °C for 1 min, 62 °C for 1 min, 72 °C for 2 min with a final extension for 7 min at 72 °C. The amplified PCR product was purified by Gene Jet PCR purification kit (Fermentas life science, Mumbai, India) and sequencing was performed by an automated DNA sequencer with universal 16S rDNA primer (ABI 3730xl Genetic, Thermo Fisher Scientific, US) at Xcelris Labs Ltd., Gujarat, India. The sequence data were aligned and compared with the known nucleotide sequences in the GenBank database using BLASTn (http://www.ncbi.nlm.nih.gov/BLAST), to identify the bacterial strains. Phylogenetic tree was constructed using the MEGA 6 software [9].
Plant growth promoting ability of the rhizosphere bacterial strains
Indole acetic acid production and phosphate solubilization
The IAA producing ability of the rhizosphere bacterial strains was quantitatively analyzed by the method of Libbert et al. [10] with two different concentrations of l-tryptophan (50 and 100 μg/mL), as a precursor. The culture flasks were incubated at 35 ± 2 °C for 36 h with shaking at 200 rpm. After 36 h incubation, 2 mL of cell-free supernatant was collected by centrifugation (7500g for 10 min) and mixed with 2 mL of Salkowski’s reagent (2% 0.5 M FeCl3 in 35% perchloric acid). The reaction mixture was incubated in darkness at room temperature for 30 min. The absorbance of the developed pink color was read at 530 nm. The IAA concentration was determined using a calibration curve of pure IAA (HiMedia, India) as a standard. The phosphate solubilizing ability of the bacterial strains was assessed using Pikovskaya medium [11] that contains 0.5% of insoluble phosphate in the form of tricalcium phosphate (TCP) with bromothymol blue (0.05 g/L) as an indicator.
Exopolysaccharide (EPS) and ammonia production
Exopolysaccharide production was quantified as described by Mody et al. [12]. The bacterial strains were grown in 100 mL of LB broth supplemented with 5% sucrose. The inoculated culture flasks were incubated for 120 h at 35 ± 2 °C with shaking at 200 rpm. Culture broth was centrifuged at 12,000g for 30 min. The EPS was extracted by adding three volumes of ice cold acetone to one volume of the cell-free supernatant. The extracted EPS was rapidly washed thrice alternately with distilled water and acetone, transferred to a filter paper and weighed after overnight drying at room temperature. The ammonia producing ability of the rhizosphere bacterial strains was analyzed quantitatively by the method of Cappuccino and Sherman [13]. The rhizosphere bacterial strains were inoculated into the peptone water broth (g/L: Peptone 10; NaCl 5 and pH 7.0 ± 0.2) and incubated at 35 ± 2 °C for 96 h with shaking at 200 rpm. After incubation, 5 mL of liquid broth containing culture was centrifuged at 7500g for 10 min and 1 mL of cell-free supernatant was collected. It was mixed with 1 mL of Nessler’s reagent and volume of this mixture was made up to 10 mL by addition of ammonia free sterile distilled water. Optical density (OD) was measured by spectrophotometer at 450 nm with ammonium chloride as the standard.
Catalase and biosurfactant production
Catalase producing ability of the bacterial strains was studied following by Cappuccino and Sherman [14]. Overnight grown bacterial cultures were mixed with an appropriate amount of 3% hydrogen peroxide on a glass slide to observe the evolution of oxygen gas. The biosurfactant production was detected by an oil spreading method described by Morikawa et al. [15]. In brief, 10 mL of sterile distilled water was added to a watch glass followed by 20 μL crude oil to the surface of the water. On this surface, 10 μL of the cell free culture supernatant was added. The result was considered positive when the drop was flat.
Hydrolytic enzymes production
The production of hydrolytic enzymes such as protease, amylase and lipase by rhizosphere bacterial strains were studied qualitatively. For protease production, the bacterial strains were streaked on casein hydrolyzed medium and the plates were incubated at 35 ± 2 °C for 24 h. A clear zone around the bacterial colonies indicated the proteolytic activity of the strain. In case of amylase production, the strains were streaked on starch agar plates and incubated at 35 ± 2 °C for 24 h. After incubation, 1% of iodine solution was flooded on the starch agar plate for the production of a clear zone around the bacterial colonies, which indicated hydrolysis of starch by the produced amylase. The lipolytic activity of the bacterial strains was confirmed by tributyrin and tween 20 agar plate methods [16]. The bacterial strains were streaked on the tributyrin agar (TBA) and tween 20 agar plates. The inoculated plates were incubated at 35 ± 2 °C for 24 h. The formation of clear halos and precipitation around the colonies grown on TBA and tween 20 plates, respectively indicated the production of lipase enzyme.
Greenhouse experiments
Greenhouse study was conducted in polythene bags, each containing 2 kg of garden soil. Table 1 describes the physicochemical properties of the garden soil. The garden soil samples were sieved and sterilized by autoclaving at 121 °C for 30 min under 1.05 kg/cm2 pressure. Seeds were surface sterilized with 70% (v/v) ethanol and 3% (v/v) sodium hypochlorite for 3 min and then the seeds were washed thrice with sterile distilled water. The surface sterilized seeds were coated with rhizosphere bacterial culture by soaking the sterilized seeds in liquid culture media (108 cells/mL) for 2 h on a rotary shaker at 120 rpm. The uninoculated sterilized seeds were soaked in sterile distilled water, which served as control. The inoculated and uninoculated seeds (10 seeds per bags) were sown in polythene bags (25 cm high, 20 cm internal diameter) using different experimental conditions. These were: (1) P. vulgaris (control) (2) P. vulgaris + 50 mg/kg Cr(VI), (3) P. vulgaris + 50 mg/kg Cr(VI) with AR6 inoculum and (4) P. vulgaris + 50 mg/kg Cr(VI) with AR8 inoculum. The concentration of Cr(VI) (50 mg/kg) used in this study was higher than those found in leather industrial effluent contaminated soil, to study efficient plant growth promoting activity of the strains at the higher concentration of Cr(VI) from the lower prevailing concentration. All the treatments were set up in a fully randomized layout of greenhouse polythene bags with three replicates of each experiment. The plants were maintained in greenhouse at 28–36 °C (day time) and 20–28 °C (night time) with 16 h/8 h light/dark (photosynthetically active radiation of 400–700 nm). All the treated plants were watered every two days to maintain the soil moisture level (70–80%). Seed germination percentage was recorded every 24 h for 7 days. Three plants were maintained in each bag 1 week after emergence. To determine the P. vulgaris growth, the length of the longest root and shoot of each plant were recorded after 15 days of treatment. The total biomass of the plants was measured immediately after harvesting by electronic balance. To confirm the viability of the inoculated bacterial strains, rhizospheric soil sample was collected and serially diluted rhizospheric soil suspensions were plated on LB agar plate.
Table 1.
Physicochemical properties of the garden and leather industrial effluent contaminated soil.
| Properties | Garden soila | Leather industrial soil |
|---|---|---|
| Soil texture | Soil mixed with clay and sand | Grainy sand with clay |
| pH | 7.4 | 7.6 |
| Moisture (%) | 53.5 | 63.05 |
| Calcium carbonate | Medium | High |
| Electro conductivity (dsm−1) | 1.5 | 5.9 |
| Macro-nutrients (kg/ha) | ||
| Nitrogen (N) | 201 | 195 |
| Phosphorus (P) | 21.5 | 1.0 |
| Potassium (K) | 600 | 75 |
| Heavy metals (mg/kg) | ||
| Cr | 0.024 | 43.04 |
| Zn | BDL | BDL |
| Cu | 0.032 | 0.16 |
| Pb | BDL | 7.11 |
| Mn | 0.15 | 1.39 |
Mean of triplicate, BDL-below detection limit.
Estimation of photosynthetic pigments
In order to measure the photosynthetic pigments, fresh leaf samples (0.1 g) were used for extraction of pigments by adding 80% (v/v) ice cold acetone. The photosynthetic pigments were measured spectrophotometrically at 665, 649 and 470 nm for chlorophyll a, b and carotenoid contents, respectively, according to Arnon [17] and expressed in mg/g fresh weight (FW).
Starch accumulation
Starch granule accumulation was visualized by the Lugol’s reagent method described by Sehnke et al. [18]. The plant leaves were harvested and decolorized in 80% (v/v) of ethanol. After rinsing with double distilled water the leaves were stained with Lugol’s reagent. The starch granules were visualized using a light microscope (Magnus MLXi) and photographed using a Nikon camera.
Proline and lipid peroxidation determination
Proline content was determined by the acid ninhydrin method [19]. Approximately 0.5 g of leaf sample was homogenized using 3% of sulfosalicylic acid and filtered through Whatman no. 1 filter paper. Supernatant (2 mL) was transferred into fresh tubes to which 2 mL of freshly prepared acid–ninhydrin and glacial acetic acid were added. The reaction mixture was heated for 1 h at 100 °C in a water bath and 4 mL of toluene was added to it. This solution was mixed well and the absorbance was measured in a spectrophotometer at 520 nm using toluene as the blank. Proline content was calculated with a standard curve of proline (HiMedia, India) and results are expressed in mg/g FW. Lipid peroxidation was measured by determining the term of malondialdehyde (MDA) concentration by the thiobarbituric acid (TBA) reaction [20]. Briefly, fresh leaf tissues (0.2 g) were homogenized using 2 mL of 20% (w/v) trichloroacetic acid containing 0.5% (w/v) thiobarbituric acid (TBA) and then 8 mL of 20% TCA mixture was added. The homogenate was centrifuged at 12,000g for 10 min at 4 °C. The mixture was kept in boiling water bath for 30 min, then cooled and centrifuged. The absorbance of the supernatant was measured at 450, 532 and 600 nm. Malondialdehyde content was estimated using the formula given below: lipid peroxidation was expressed as malondialdehyde content in μmol/g FW.
where Vt = Total extraction liquid volume (10 mL),Vs = The extraction liquid volume used for measurement (2.0 mL),W = Fresh weight of sample (0.2 g).
Antioxidant enzyme assays
For enzyme assay, approximately 0.2 g of leaf tissues were homogenized with 2 mL of 0.1 M phosphate buffer (pH 7.8) containing 0.5 mM ethylenediaminetetraacetic acid (EDTA) and 1% (w/v) polyvinylpyrrolidone (PVP). The homogenates were centrifuged at 7500g for 10 min at 4 °C and the collected supernatants were used for estimation of protein and antioxidant enzymes. The protein content was estimated following the method of Bradford [21] using bovine serum albumin (BSA) as a standard. Catalase (CAT) activity was estimated according to Aebi [22], which measures the initial rate of disappearance of H2O2 at 240 nm. The reaction mixture contained 1.5 mL of 50 mM phosphate buffer (pH 7.0), 0.5 mL of 75 mM H2O2, 0.2 mL of enzyme extract and 0.8 mL of sterile distilled water. The decrease of the absorbance at 240 nm was recorded. Activity was calculated using an extinction coefficient of 39.04/mM/cm. Peroxidase (POX) activity was measured using the method of Polle et al. [23]. The reaction mixture contained 1 mL of 100 mM potassium phosphate buffer (pH 6.1), 0.5 mL of 96 mM guaiacol, 0.5 mL of 12 mM H2O2, 0.1 mL of enzyme extract and 0.4 mL of sterile distilled water were used for measurement of enzyme activity. The oxidation of guaiacol was measured by the increase in absorbance at 470 nm. The enzyme activity was calculated using the extinction coefficient 25.5/mM/cm. Polyphenol oxidase (PPO) activity was assayed by the method of Kumar and Khan [24]. The assay mixture contained 2 mL of 0.1 M potassium phosphate buffer (pH 6.6), 1 mL of 0.1 M guaiacol and 0.5 mL of enzyme extract. This was incubated for 5 min at 25 °C, after which the reaction was stopped by adding 1 mL of 2.5 N sulfuric acid. The absorbance of the purpurogallin was recorded at 495 nm. The activity of PPO was calculated using the extinction coefficient 1.35/mM/cm.
Determination of Cr uptake by P. vulgaris plants
For the determination of total Cr accumulation, the plant materials were oven dried at 80 °C for 24 h. The oven dried tissues were ground into fine powder and subjected to acid digestion following the method of Humphries [25]. The digested samples were used for Cr analysis by atomic absorption spectrophotometer (AAS).
Statistical analysis
All the experiments were carried out in triplicate (n = 3) and the data were recorded. Seed germination rate was analyzed by the Kruskal-Wallis test, plant growth promoting activities as well as greenhouse experiments were analyzed by one-way ANOVA and means were compared with the Tukey’s test, using the SPSS software (version 20, SPSS Inc., www.spss.com).
Results and discussion
Physicochemical properties of the soil samples
Contaminated soil samples were collected from leather industry effluent discharging sites of Ambur, Vellore district. Fig. 1 shows the soil samples collection site. This area was specifically selected for bacterial isolate because it was continuously exposed to leather industry effluents. Physicochemical properties of the contaminated and non-contaminated control soil sample were analyzed and are shown in Table 1. The soil pH, calcium carbonate and electro conductivity nature of the effluent contaminated soil were higher than those of control soil sample. However, parameters such as soil moisture, N, P and K were higher in the control soil than in the contaminated soil. Effluent contaminated soil contained higher concentrations of heavy metals such as Cr (43.04 mg/kg soil), Pb (6.01 mg/kg soil), Cu (0.21 mg/kg soil) and Mn (1.62 mg/kg soil) where metals such as Cr and Pb were observed above the permissible limits of the World Health Organization (WHO) in contaminated soil. However, Zn could not be estimated in contaminated soil because of its concentration below the detection limit of the instrument.
Fig. 1.
Location of soil sampling site in leather industrial effluent contaminated zone of Ambur, Tamil Nadu, India.
Rhizosphere bacterial isolation and evaluation of Cr(VI) tolerance
In the present study, 36 bacterial isolates were screened from rhizosphere of P. vulgaris, grown in leather industrial effluent contaminated soil. Among them, bacterial strains AR6 and AR8 displayed the maximum tolerance (1200 and 1100 μg/mL, respectively) to Cr(VI) and hence were selected for further studies. Such variations in the metal tolerance levels of microbial populations under in vitro conditions are probably due to the differences in genetic makeup and biochemical composition of bacteria and also due to the differences in media and growth conditions employed. Generally, microorganisms protect themselves against metal toxicity by restricting the entry of metal ions into cells or reducing the free ions in the cytosol. However, the reports on the effect of Cr(VI) on PGPR are conflicting. For instance, Pseudomonas strain CRB5, a gram-negative bacterium, tolerated chromium up to a concentration of 550 μg/mL [26], whereas Cr(VI) reducing strains of Bacillus isolated from rhizospheric soils of mustard and tomato showed tolerance levels of 400–550 μg/mL [27].
Molecular identification of rhizosphere bacterial strains
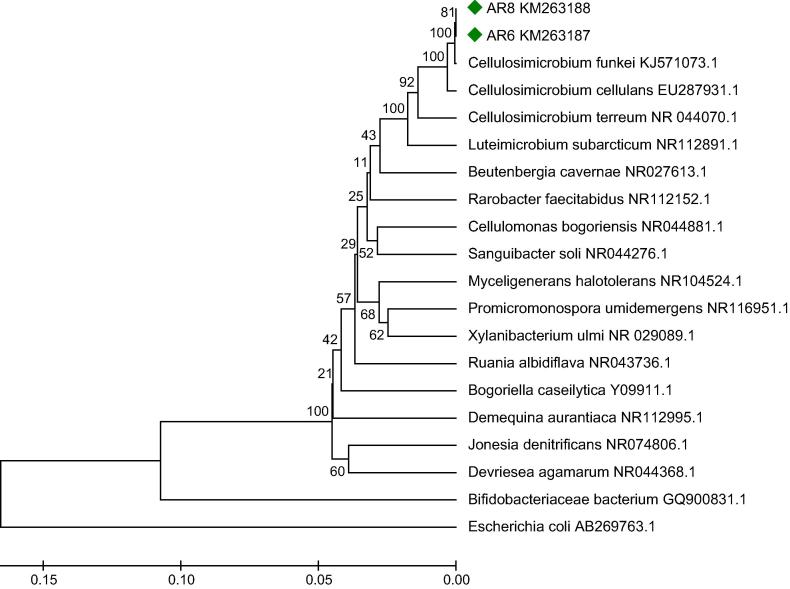
In the molecular characterization study, both bacterial strains showed a close relationship with Cellulosimicrobium funkei (KM032184). The 16S rDNA sequences of the strain AR6 and AR8 were submitted to GenBank with a unique accession number KM263187 and KM263188, respectively. The phylogenetic positions of the selected bacterial strains were asserted by phylogenetic analysis using the UPGMA method (Unweighted pair group method with arithmetic mean). The phylogenetic tree of the bacterial strains is shown in Fig. 2.
Fig. 2.
Phylogenetic tree showing relationship between the rhizosphere bacterial strains with NCBI obtained sequences. An algorithm with bootstrap values expressed as percentage of 1000 replication. Bar substitutions per nucleotide position.
Indole acetic acid production and phosphate solubilization
Both the bacterial strains produced a substantial quantity of IAA after 30 h of incubation and the results are presented in Table 2. While comparing the effect of various concentrations of l-tryptophan on the IAA synthesis, 100 μg/mL of l-tryptophan showed an increase of 25.76 and 32.85% in IAA over 50 μg l-tryptophan/mL by AR6 and AR8, respectively. The data revealed a l-tryptophan dependent increase in IAA production, both in the presence and absence of Cr(VI). Furthermore, the effect of Cr(VI) on IAA synthesis was quantitatively assayed and is represented in Table 2. Indole acetic acid producing ability of the bacterial strains under Cr(VI) stress did not differ significantly. Similar production of IAA by Cr(VI) tolerant bacteria has been reported by Wani et al. [27]. The IAA synthesized by bacterial strains can act as a signaling molecule during the plant growth and it enhances the physiological growth of plant such as root initiation, cell division, cell elongation, seed germination and root elongation [28]. In phosphate solubilization study, both the bacterial strains showed positive results in the presence and absence of Cr(VI) and results are represented in Table 2. From these results, it was concluded that the 50 μg/mL of Cr(VI) did not affect the phosphate solubilization ability of the strains. Phosphate solubilizing bacteria play a key role in plant growth promotion and root proliferation by making the availability of solubilized phosphate minerals in the soil [29].
Table 2.
Plant growth promoting activities of rhizosphere bacterial strains AR6 and AR8 in the presence of 50 μg/mL of Cr(VI).
| Bacterial strains | Cr(VI) conc. (μg/mL) | IAAa (μg/mL) |
EPSd (μg/mL) | Ammonia (μg/mL) | Pe solubilization | Catalase | Protease | Amylase | Biosurfactant | Lipase |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50Tb | 100Tc | TBAf | T20g | |||||||||
| (μg/mL) | (μg/mL) | |||||||||||
| AR6 | Control | 27.28 ± 1.43a | 36.75 ± 0.43a | 14.23 ± 0.86b | 60.40 ± 2.51a | + | + | + | + | − | + | + |
| 50 | 22.23 ± 0.76b | 30.13 ± 1.64b | 16.70 ± 1.34ab | 52.72 ± 0.79a | + | + | + | + | − | + | + | |
| AR8 | Control | 21.87 ± 0.76b | 32.57 ± 0.76a | 17.23 ± 0.76ab | 54.16 ± 0.76a | + | + | + | + | + | + | + |
| 50 | 19.14 ± 0.76b | 28.64 ± 0.76b | 18.31 ± 0.76a | 51.16 ± 0.76a | + | + | + | + | + | + | + | |
| F value | 14.02 | 10.69 | 5.92 | 2.70 | − | − | − | − | − | − | − | |
Note: ‘+’ positive for PGP activity, ‘−’ negative for PGP activity.
Indole acetic acid.
50 μg/mL of tryptophan.
100 μg/mL of tryptophan.
Exopolysaccharide.
Phosphate.
Tributyrin agar.
Tween 20. Results are expressed as the means of three replicates ± SE. Mean values followed by different letters are significantly different according to Tukey’s test at P < 0.05.
Exopolysaccharide and ammonia production
The influence of Cr(VI) on EPS production of the bacterial strains was investigated and the results are presented in Table 2. In Cr(VI) free control condition, bacterial strains secreted lower amount of EPS, which increased significantly to 14.79 and 5.89% in 50 μg/mL of Cr(VI) treated AR6 and AR8 strains, respectively. Oves et al. [3] reported that the EPS producing ability of the bacterial strains is strongly correlated with Cr(VI) concentrations in the production medium. The excess secretions of EPS produced a protective layer on bacterial cells, which could protect from the toxic heavy metals and pathogenic attack by masking the effects of pollutants and pathogens and hence, the bacteria can survive in the polymeric network of EPS [30]. Interestingly, EPS produced from PGPR could enhance the plant growth by direct and/or indirect mechanisms. For instance, the produced EPS directly stimulated the plant growth by altering the physiological processes of plants such as infection thread formation, bacteroid and nodule development during biological nitrogen fixation and invasion process. While in indirect mechanism, it enhanced the plant growth by increasing microbial activity in the rhizosphere and enhancing the soil organic content and thereby providing stability to soil aggregate with plant roots [31].
When the bacterial strains AR6 and AR8 were inoculated in Cr(VI) unamended medium, a maximum production of ammonia was observed by strain AR6 (60.40 μg/mL) after 48 h incubation and results are summarized in Table 2. In contrast to EPS production, ammonia production was reduced significantly at 50 μg/mL concentration of Cr(VI). Previous reports denoted that, the ammonia produced by bacteria acts as nitrogen source to the host plant and promotes root and shoot elongation, consequently increasing plant biomass [3]. Similar production of ammonia by Cr(VI) tolerant bacterial has also been reported by Wani et al. [1], [27].
Catalase and biosurfactant production
Catalase and biosurfactant producing ability of the bacterial strains were qualitatively analyzed and are presented in Table 2. Both the bacterial strains exhibited positive results for catalase production. However, strain AR6 showed negative result for biosurfactant production, both in the presence and absence of Cr(VI). It denoted the potency of the bacterial strain for the survival even in a stressed environment. The catalase production is associated with the resistance ability of the strains against oxidative, environmental, mechanical and chemical stress [32]. Therefore, the rhizosphere bacterial strain with catalase activity may help to make stress free rhizosphere, which indirectly involved in plant growth promotion in a contaminated environment.
Hydrolytic enzymes production
Both the bacterial strains showed positive results for protease, amylase and lipase production, without being affected by Cr(VI) stress and results are depicted in Table 2. Rhizosphere bacterial strains with the ability of hydrolytic enzyme production could lyse the cell wall of pathogenic fungi and protect the host from pathogens [32]. Previously, Moataza [33] also reported various types of mycolytic enzymes by different Pseudomonas strains that showed antagonistic activity on various plant pathogens such as Phytophthora capsici and Rhizoctonia solani.
Effect of bacterial inoculation on Plant growth
The seed germination rate of P. vulgaris was severely affected in uninoculated Cr(VI) treated seeds with 59.12%. Heavy metals interference changed the permeability of cell membranes, which decreased absorption and transport of metal and water as well as reduced the stress tolerance potential during germination. Additionally, heavy metals inhibited the expression of specific enzymes for germination, which are involved in the seed coat breakdown [34]. Similar to results reported in this study, Gao et al. [35] also reported decrease in seed germination and growth rate in plant grown under heavy metal stress. However, the seed germination rate was increased significantly in bacterial inoculated seeds, as displayed in Table 3. The maximum increase of the seed germination rate was observed in AR6 inoculated seeds (89.54%) followed by AR8 (85.94%). The inoculation of Cr(VI) tolerant rhizosphere bacterial strains increased the seed germination rate by providing a sufficient amount of IAA as phytohormones, which would have stimulated the activity of germination specific hydrolytic enzymes such as α-amylase and protease [28], [36].
Table 3.
Effects of rhizosphere bacterial inoculations on growth and photosynthetic pigment of P. vulgaris.
| Treatment | Seed germination (%)⁎ | Shoot length (cm)⁎⁎ | Root length (cm)⁎⁎ | Biomass (g/ FW)⁎⁎ | Photosynthetic pigments (mg/g FW)⁎⁎ |
|||
|---|---|---|---|---|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Total chlorophyll | Carotenoid | |||||
| Control | 82.11 ± 3.04a | 38.11 ± 0.71c | 9.33 ± 0.88a | 2.4 ± 0.17ab | 2.47 ± 0.15bc | 2.11 ± 0.06b | 4.58 ± 0.24b | 1.15 ± 0.09a |
| Cr(VI) | 57 ± 9.9b | 18.44 ± 0.65d | 4.66 ± 1.15b | 1.5 ± 0.26b | 2.11 ± 0.10c | 1.73 ± 0.05c | 3.84 ± 0.11c | 1.34 ± 0.04a |
| AR6 + Cr(VI) | 88.12 ± 6.78a | 72.33 ± 1.14a | 11.66 ± 0.39a | 3.16 ± 0.14a | 3.26 ± 0.10a | 2.57 ± 0.06a | 5.83 ± 0.20a | 1.39 ± 0.03a |
| AR8 + Cr(VI) | 86.32 ± 6.02a | 57.82 ± 2.73b | 10.34 ± 0.54a | 2.5 ± 0.29ab | 2.74 ± 0.06b | 2.11 ± 0.07b | 4.85 ± 0.05b | 1.16 ± 0.07a |
| F value | 41.86 | 227.50 | 14.01 | 9.036 | 18.26 | 27.87 | 45.99 | 3.64 |
Note: FW - fresh weight; values followed by different letters are significantly different at P < 0.05.
Kruskal-Wallis – median and interquartile range.
One-way ANOVA – mean ± standard error.
When uninoculated P. vulgaris plants were exposed to Cr(VI), growth parameters such as root and shoot length and biomass were decreased considerably. Chromium(VI) toxicity exerted severe effects on root growth and function, resulting in root damage, reduction in fresh weight, cell division, root elongation and reduced the uptake level of water and nutrients [5]. Moreover, accumulation of heavy metals in plant tissues may trigger water deficit, resulting in reduced growth and development of plants [35]. However, when seeds were inoculated with rhizosphere bacterial strains, the growth parameters of P. vulgaris plants were increased significantly. The maximum increase in shoot length (74.50%), root length (60%) and total biomass (52.53%) was observed in Cr(VI) treated AR6 inoculated plants when compared to uninoculated Cr(VI) treated plants, as displayed in Table 3. Plant growth promoting rhizobacteria can increase the growth and development of the plants either indirectly by reducing the toxic effects of metals or directly by producing the phytohormones and growth factors [5]. This study showed that rhizosphere bacterial strains possess Cr(VI) tolerant ability, which protected the P. vulgaris plants against the toxic effects of Cr(VI). Another reason for the growth, development and protection of the plants against metals could be the production of phytohormone and uptake of soil minerals by the host plant. Wani et al. [37] also reported similar results in Bacillus sp. PSB10 inoculated chickpea plants.
Effects of bacterial inoculations on photosynthetic pigments
The chlorophyll a, b and total chlorophyll contents were noticeably decreased in uninoculated Cr(VI) treated plants. This may be due to the inhibition of the enzymes responsible for the chlorophyll biosynthesis under the Cr(VI) stress. Heavy metals have a potency to alter the rate of photosynthesis by disturbing the structure of chloroplast leading to the changes in the fatty acid composition, inhibiting photosynthetic pigments and enzymes of the Calvin cycle [38]. Such biochemical alterations in chloroplast due to Cr(VI) stress are expected to prevent light harvesting and cause impairment of photosynthesis. Similar evidence of decreased photosynthetic pigment was reported in Cr(VI) treated Ocimum tenuiflorum plant by Rai et al. [4]. Nevertheless, the bacterial inoculations significantly increase in the photosynthetic pigments of P. vulgaris plants and results are depicted in Table 3. The maximum increase of chlorophyll a (15.91%), chlorophyll b (17.97%), total chlorophyll (16.58%) and carotenoids (3.59%) was observed in Cr(VI) treated AR6 inoculated plants when compared with uninoculated Cr(VI) treated plants. Different studies have found that plant inoculation with rhizosphere bacterial isolates under metal stress may improve chlorophyll synthesis [1], [3]. Moreover, the rhizosphere bacterial inoculation under stress might increase the chlorophyll by improving chlorophyll synthesis or slowing the process of chlorophyll degradation.
Effect of different bacterial inoculation on starch accumulation
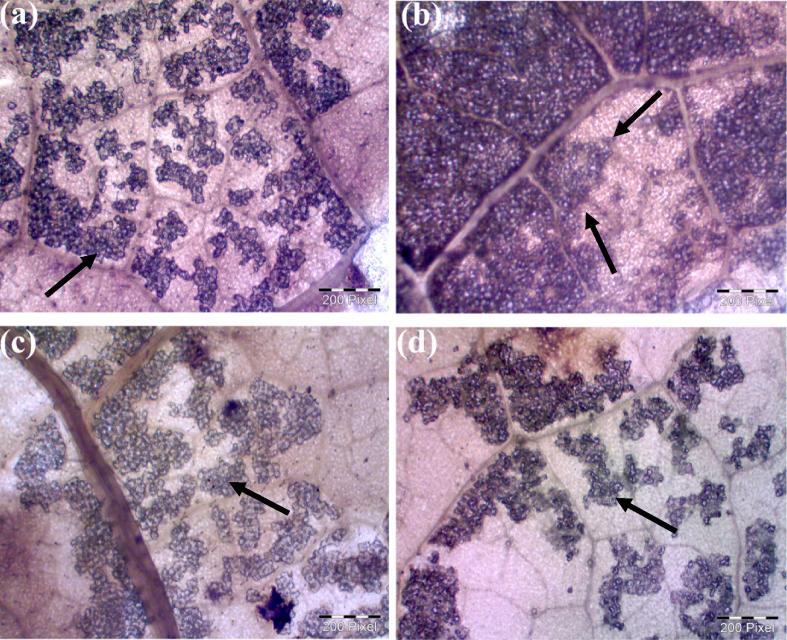
Influence of Cr(VI) and bacterial inoculation on starch accumulation was visualized and is shown in Fig. 3. The uninoculated Cr(VI) treated leaves showed visually more accumulation of starch granules. The increased level of starch is responsible to maintain the environmental stress, which can function as osmolytes to maintain cell turgor and have the ability to protect membranes and proteins from stress damage [39]. However, rhizosphere bacterial inoculated plants had lower starch accumulation even in the presence of Cr(VI). These results demonstrated that rhizosphere bacterial inoculation decreased the Cr(VI) toxicity and stabilized the plant metabolism under the Cr(VI) stress environment.
Fig. 3.
Microscopic observation of starch accumulation (arrowhead) (a) control plant, (b) uninoculated Cr(VI) treated plant, (c) Cr(VI) with AR6 inoculated plant and (d) Cr(VI) with AR8 inoculated plant.
Effect of bacterial inoculants on proline and MDA accumulation
Chromium(VI) toxicity influenced the accumulation of proline in plant tissues. Among the treatments, a maximum proline accumulation was observed in uninoculated Cr(VI) treated plant leaves (7.45 mg/g FW). Accumulation of a large quantity of free cellular proline is an adaptive response of plants to various biotic and abiotic stresses [4]. Proline seems to have a multifunctional protective role under osmotic stress condition, such as stabilization of protein, membranes and subcellular structures and stabilizing the cellular mechanisms by scavenging ROS [40]. In other studies, Zengin and Munzuroglu [41] also observed a similar result in various heavy metal treated P. vulgaris seedlings. However, Table 4 shows the reduced proline accumulation in Cr(VI) treated bacterial inoculated plant. Lower proline accumulated was observed in AR6 inoculated plants (84.56%). These results highlighted that inoculated plants were not affected by Cr(VI) toxicity, when grown in Cr(VI) amended soil. It indicated a positive correlation between proline accumulation and plant adaptation to stress [34]. This could be due to the bioreducing effect of the inoculants on Cr accumulation inside the plant tissues.
Table 4.
Effects of rhizosphere bacterial inoculations on non-enzymatic, enzymatic expression and chromium accumulation of P. vulgaris.
| Treatment | Proline (mg/g FW) | MDA (μmol/g FW) | Enzyme activity (μmol/min/mg protein) |
Chromium accumulation (μg/g) |
|||
|---|---|---|---|---|---|---|---|
| CAT | POX | PPO | Root | Shoot | |||
| Control | 2.18 ± 0.16c | 1.21 ± 0.02b | 2.26 ± 0.16a | 87.42 ± 1.42a | 7.52 ± 0.07b | BDL | BDL |
| Cr(VI) | 7.45 ± 0.63a | 1.74 ± 0.09a | 6.94 ± 0.09a | 91.52 ± 2.85a | 9.62 ± 0.12a | 7.92 ± 0.39a | 4.28 ± 0.09a |
| AR6 + Cr(VI) | 1.15 ± 0.12c | 1.26 ± 0.04b | 1.22 ± 0.10a | 64.71 ± 1.64b | 6.69 ± 0.18c | 2.26 ± 0.28b | 1.45 ± 0.24b |
| AR8 + Cr(VI) | 4.17 ± 0.10b | 1.04 ± 0.03b | 2.14 ± 0.17a | 63.66 ± 0.28b | 6.04 ± 0.15c | 2.35 ± 0.50b | 1.65 ± 0.20b |
| F value | 67.50 | 29.33 | 3.86 | 66.64 | 118.90 | 64.34 | 68.28 |
Note: BDL - below detection limit. Results are expressed as the means of three replicates ± SE. Mean values followed by different letters are significantly different according to Tukey’s test at P < 0.05.
The plant cell damages occurred due to oxidative stress by ROS generation in the P. vulgaris plant. The ROS generation was measured in terms of increase or decrease in endogenous levels of MDA contents [4]. In this study, increased accumulation of MDA content (1.74 μmol/g FW) was observed in uninoculated Cr(VI) treated plants. This result suggested that the cell membrane was damaged due to Cr(VI) induced oxidative stress in P. vulgaris plants. An increased level of lipid peroxidation may be attributed to the enhanced activity of antioxidant enzymes to reduce free radical generation levels in Cr(VI) treated plants. Therefore, the cell membrane damage could be minimized [34]. Nevertheless, higher MDA content was not observed in bacterial inoculated plants, which was decreased significantly. The maximum decrease was observed in AR8 inoculated plant (40.22%) and results are shown in Table 4. These results represented the amelioration of inoculation to oxidative stress that is related to the reduced Cr contents in root and shoot of these plants. This reduction in Cr content due to inoculation of rhizosphere bacterial strains resulted in reduced oxidative damage and ultimately promotes in growth. The most plausible explanation for such an effect is that the toxicity of the Cr is decreased due to exclusion and immobilization of Cr by inoculated rhizosphere bacterial strains. It indirectly removed the inhibitory effect of Cr induced oxidative stress on growth and enzymatic activities in P. vulgaris.
Effect of bacterial inoculations on antioxidant enzymes expression
The uninoculated Cr(VI) treated plant showed a noticeable increase in CAT (6.94 μmol/min/mg protein), POX (91.51 μmol/min/mg protein) and PPO (9.62 μmol/min/mg protein) expression. Antioxidant enzyme expressions are directly correlated with Cr(VI) stress. Chromium(VI) toxicity leads to trigger some key enzymes of the antioxidant defense system as a result of the overall balance between ROS production and enzyme level in P. vulgaris plant [35]. Similar increases in antioxidant enzyme expression have also been reported by Zhang et al. [34] and Socha et al. [42], whereas, above mentioned higher antioxidant enzyme activities were not observed in bacterial inoculated plants. Table 4 highlights the neutralization effect of rhizosphere bacterial inoculations on enzymatic antioxidant system of P. vulgaris. Lower CAT expression (82.32%) was observed in AR6 inoculated plants. On the other hand, lower POX (30.43%) and PPO (37.14%) expressions were documented in AR8 inoculated plants, when compared with uninoculated Cr(VI) treated P. vulgaris plants. These antioxidant enzyme expressions provided a clear idea about positive interaction between plant and microbes under the Cr(VI) stress. It is interesting to note that though a significant interaction between Cr(VI) stress and antioxidant enzyme activity was found, treatment with rhizosphere bacterial strains tends to reduce the toxic effect of Cr(VI) on expression of antioxidant enzymes.
Effects of rhizosphere bacterial inoculations on Cr accumulation
The maximum Cr accumulation was observed in uninoculated Cr(VI) treated root (7.92 μg/g) and shoot (4.28 μg/g) of P. vulgaris plants and the results are summarized in Table 4. In contrast, bacterial inoculated plant showed significantly lower Cr accumulation in AR6 inoculated root (71.08%) and shoot (66.12%) tissues when compared with uninoculated Cr(VI) treated P. vulgaris plants. The reduction of Cr accumulation in P. vulgaris tissues, thus exhibited the ability of inoculated rhizosphere bacterial strains to protect P. vulgaris against the inhibitory effect of Cr(VI). The lower accumulation of Cr in plant tissues may be due to the immobilization of Cr by bacterial inoculation. The rhizosphere bacterial strains may immobilize Cr in several ways, including adsorption, accumulation, secretion of cell surface associated polysaccharides and proteins. Similarly, Oves et al. [3] reported a lower accumulation of Cr in P. aeruginosa inoculated chickpea plant. Wani et al. [37] documented similar observable effects in Bacillus sp. inoculated Cicer arietinum plants when grown in chromium-amended soil. Moreover, the root tissues accumulated more Cr than shoots in both inoculated and uninoculated plants. The increased concentrations of Cr in the root could be due to the poor translocation of Cr from the root to upper plant parts.
The present findings suggested that rhizosphere bacterial strain AR6 was more effective than AR8 with respect to alleviating the negative effect of Cr(VI) on P. vulgaris. Moreover, very few research works have been carried out on C. funkei strain. This is the first research report, which elucidates the Cr(VI) tolerant and plant growth promotion ability of C. funkei strain under the Cr(VI) stress.
Conclusions
In this study, rhizosphere bacterial strains AR6 and AR8 isolated from rhizosphere of P. vulgaris, exhibited high tolerance to Cr(VI) and produced plant growth promoting substances under control and Cr(VI) stress, demonstrating their potential to contribute to beneficial plant–microbe interactions in the metal contaminated soil. This study provides clear evidence about the response of rhizosphere bacterial strains to Cr(VI) and enhanced P. vulgaris growth and antioxidant system under Cr(VI) stress. Therefore, inoculation of these rhizosphere bacterial strains may act as a sustainable factor for Cr phytostabilization and control Cr entry into the food chain.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics requirements
This article does not contain any studies with human or animal subjects.
Acknowledgment
We gratefully acknowledge the Department of Biotechnology, Periyar University, Tamil Nadu, India, for the support rendered through the University Research Fellowship (URF).
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Wani P.A., Khan M.S. Nickel detoxification and plant growth promotion by multi metal resistant plant growth promoting Rhizobium species RL9. Bull Environ Contam Toxicol. 2013;91:117–124. doi: 10.1007/s00128-013-1002-y. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy L. Cooperating for survival: tannery pollution and joint action in the Palar Valley, India. World Develop. 1999;27:1673–1691. [Google Scholar]
- 3.Oves M., Khan M.S., Zaidi A. Chromium reducing and plant growth promoting novel strain Pseudomonas aeruginosa OSG41 enhance chickpea growth in chromium amended soils. Eur J Soil Biol. 2013;56:72–83. [Google Scholar]
- 4.Rai V., Vajpayee P., Singh S.N., Mehrotra S. Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci. 2004;167:1159–1169. [Google Scholar]
- 5.Glick B.R. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gohre V., Paszkowski U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta. 2006;223:1115–1123. doi: 10.1007/s00425-006-0225-0. [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar M., Ae N., Prasad M.N.V., Freitas H. Potential of siderophore producing bacteria for improving heavy-metal phytoextraction. Trends Biotechnol. 2010;28:142–149. doi: 10.1016/j.tibtech.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Bhat M.S., Shaheen M., Zaman R., Muhee A. Mineral inter-relationship among soil, forage and dairy cattle in Kashmir India. Vet World. 2011;4:550–553. [Google Scholar]
- 9.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libbert E., Kaiser W., Kunert R. Interactions between plants and epiphytic bacteria regarding their auxin metabolism VI. The influence of the epiphytic bacteria on the content of extractable auxin in the plant. Physiol Plant. 2006;22:432–439. [Google Scholar]
- 11.Pikovskaya R.I. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya. 1948;17:362–370. [Google Scholar]
- 12.Mody B.R., Bindra M.O., Modi V.V. Extracellular polysaccharides of cowpea rhizobia: compositional and functional studies. Arch Microbiol. 1989;1:38–42. [Google Scholar]
- 13.Cappuccino J.C., Sherman N., editors. Microbiology: a laboratory manual. 3rd ed. Benjamin/Cummings Pub. Co; New York: 1992. pp. 125–179. [Google Scholar]
- 14.Cappuccino J.G., Sherman N. Addison-Wesley; New York: 1998. Microbiology: a laboratory manual. [Google Scholar]
- 15.Morikawa M., Hirata Y., Imanaka T.A. A study on the structure-function relationship of lipopeptide biosurfactants. Biochim Biophys Acta. 2000;1488:211–218. doi: 10.1016/s1388-1981(00)00124-4. [DOI] [PubMed] [Google Scholar]
- 16.Kumar D., Kumar L., Nagar S., Raina C., Parshad R., Gupta V.K. Screening, isolation and production of lipase/esterase producing Bacillus sp. strain DVL2 and its potential evaluation in esterification and resolution reactions. Arch Appl Sci Res. 2012;4:1763–1770. [Google Scholar]
- 17.Arnon D.J. Copper enzyme in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sehnke P.C., Chung H., Wu K., Ferl R.J. Regulation of starch accumulation by granule associated plant 14-3-3 proteins. PNAS. 2000;98:765–770. doi: 10.1073/pnas.021304198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates L., Waldren R.P., Teare I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- 20.Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 21.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Aebi H. Catalase in vitro. Methods Enzym Anal. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 23.Polle A., Otter T., Seifert F. Apoplastic peroxidases and lignification in needles of Norway (Picea abies L.) Plant Physiol. 1994;106:53–60. doi: 10.1104/pp.106.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar K.B., Khan P.A. Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Ind J Exp Bot. 1982;20:412–416. [PubMed] [Google Scholar]
- 25.Humphries E.C. Mineral component and ash analysis. In: Paech K., Traley M.Y., editors. Modern methods of plant analysis. Springer Verlag; Berlin, Gothigen: 1956. [Google Scholar]
- 26.McLean J., Beveridge T.J. Chromate reduction by a Pseudomonad isolated from a site contaminated with chromated copper arsenate. Appl Environ Microbiol. 2001;67:1076–1084. doi: 10.1128/AEM.67.3.1076-1084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wani P.A., Khan M.S., Zaidi A. Chromium reduction, plant growth–promoting potentials, and metal solubilizatrion by Bacillus sp. isolated from alluvial soil. Curr Microbiol. 2007;54:237–243. doi: 10.1007/s00284-006-0451-5. [DOI] [PubMed] [Google Scholar]
- 28.Tsakelova E.A., Klimova S.Y., Cherdyntseva T.A., Netrusov A.I. Microbial producers of plant growth stimulators and their practical use: a review. App Biochem Microbiol. 2006;42:117–126. [PubMed] [Google Scholar]
- 29.Lifshitz R., Kloepper J.W., Kozlowski M., Simonson C., Carlson J., Tipping E.M., Zalesca I. Growth promotion of canola (rapeseed) seedlings by a strain of Pseudomonas putida under gnotobiotic conditions. Can J Microbiol. 1987;33:390–395. [Google Scholar]
- 30.Tank N., Saraf M. Phosphate solubilization, exopolysaccharide production and indole acetic acid secretion by rhizobacteria isolated from Trigonella foenumgraecum. Ind J Microbiol. 2003;43:37–40. [Google Scholar]
- 31.Rashid M.I., Mujawar L.H., Shahzad T., Almeelbi T., Ismail I.M.I., Oves M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol Res. 2016;183:26–41. doi: 10.1016/j.micres.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Geetha K., Venkatesham E., Hindumathi A., Bhadraiah B. Isolation, screening and characterization of plant growth promoting bacteria and their effect on Vigna Radita (L.) R. Wilczek. Int J Curr Microbiol Appl Sci. 2014;3:799–809. [Google Scholar]
- 33.Moataza M.S. Destruction of Rhizoctonia solani and Phytophthora capsici causing tomato root-rot by Pseudomonas fluorescencences lytic enzymes. Res J Agric Biol Sci. 2006;2:274–281. [Google Scholar]
- 34.Zhang X.X., Chunjie L., Zhibiao N. Effects of cadmium stress on seed germination and seedling growth of Elymus dahuricus infected with the Neotyphodium endophyte. Sci Chin Life Sci. 2012;55:793–799. doi: 10.1007/s11427-012-4359-y. [DOI] [PubMed] [Google Scholar]
- 35.Gao S., Yang C., Tang L., Zhu J., Xu Y., Wang S., Chen F. Growth and antioxidant responses in Jatropha curcas seedling exposed to mercury toxicity. J Hazard Mater. 2010;182:591–597. doi: 10.1016/j.jhazmat.2010.06.073. [DOI] [PubMed] [Google Scholar]
- 36.Gholami A., Shahsavani S., Nezarat S. The effect of plant growth promoting rhizobacteria (PGPR) on germination, growth and yield of maize. World Acad Sci Eng Technol. 2009;29:19–24. [Google Scholar]
- 37.Wani P.A., Khan M.S. Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem Toxicol. 2010;48:3262–3267. doi: 10.1016/j.fct.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 38.Vazquez M.D., Poschenrieder C., Barcelo J. Chromium VI induced structural and ultra-structural changes in bush bean plants (Phaseolus vulgaris L.) Ann Bot. 1987;59:427–438. [Google Scholar]
- 39.Kaplan F., Guy C.L. Beta-amylase induction and the protective role of maltose during temperature shock. Plant Physiol. 2004;135:1674–1684. doi: 10.1104/pp.104.040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smirnoff N., Cumbes Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. [Google Scholar]
- 41.Zengin F.K., Munzuroglu O. Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol Cracov Ser Bot. 2005;47:157–164. [Google Scholar]
- 42.Socha A.N., Kafel A., Ciupa M.K., Gospodarek J., Raszka A.Z. Accumulation of heavy metals and antioxidant responses in Vicia faba plants grown on monometallic contaminated soil. Environ Sci Pollut Res Int. 2013;20:1124–1134. doi: 10.1007/s11356-012-1191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]