Abstract
Mephedrone (4-methylmethcathinone) is a novel psychoactive substance popular among drug users because it displays similar effects to MDMA (3,4-methylenedioxymethamphetamine, ecstasy). Mephedrone consumption has been associated with undesirable effects and fatal intoxications. At present, there is no research available on its pharmacological effects in humans under controlled and experimental administration. This study aims to evaluate the clinical pharmacology of mephedrone and its relative abuse liability compared with MDMA. Twelve male volunteers participated in a randomized, double-blind, crossover, and placebo-controlled trial. The single oral dose conditions were: mephedrone 200 mg, MDMA 100 mg, and placebo. Outcome variables included physiological, subjective, and psychomotor effects, and pharmacokinetic parameters. The protocol was registered in ClinicalTrials.gov (NCT02232789). Mephedrone produced a significant increase in systolic and diastolic blood pressure, heart rate, and pupillary diameter. It elicited stimulant-like effects, euphoria, and well-being, and induced mild changes in perceptions with similar ratings to those observed after MDMA administration although effects peaked earlier and were shorter in duration. Maximal plasma concentration values for mephedrone and MDMA peaked at 1.25 h and 2.00 h, respectively. The elimination half-life for mephedrone was 2.15 h and 7.89 h for MDMA. In a similar manner to MDMA, mephedrone exhibits high abuse liability. Its earlier onset and shorter duration of effects, probably related to its short elimination half-life, could explain a more compulsive pattern of use as described by the users.
INTRODUCTION
In recent years, new/novel psychoactive substances (NPS) have become increasingly popular in Europe and the United States (EMCDDA 2014a; Papaseit et al, 2014; Deluca et al, 2012). NPS are defined as substances which are not prohibited by the United Nations Drug Conventions of 1961 and 1971, or by the Misuse of Drugs Act 1971, but which may pose a public health threat comparable with that presented by substances listed in these conventions (ACMD, 2015). Mephedrone (4-methylmethcathinone, 4-MMC), also known as ‘M-Cat', ‘MC', ‘Meph' ‘Drone', ‘Bubbles', ‘Meow Meow', and ‘Meph' is a beta-keto analog of phenethylamine related to cathinone, the active stimulant present in khat leaves (Catha edulis) (Valente et al, 2014).
Marketed as ‘bath salts' or ‘plant-feed', mephedrone effects have been compared by users with other psychostimulants such as cocaine, amphetamine, and MDMA. The most frequently reported effects are euphoria, stimulation, alertness, empathy, sociability, talkativeness, intensification of sensory experiences, and light sexual arousal (Camí and Farré, 2003; Carhart-Harris et al, 2011; Vardakou et al, 2011; Winstock et al, 2011). Because of its extensive recreational use in the United Kingdom (UK) and other European countries, and its implication in a number of clinical adverse events and unexplained deaths, it was banned in the UK in April 2010 and some months later in the European Union (Schifano et al, 2011). Mephedrone adverse effects are typically consistent with a sympathomimetic toxidrome and include: teeth grinding, tachycardia, chest pain, sweating, blurred vision, agitation, brief psychosis, and hypertension (Wood et al, 2011). A number of intoxications and forensic cases after oral, inhaled, and injected mephedrone use have been reported (Busardò et al, 2015). In toxicological cases, mephedrone blood concentrations ranged from 1.330 to 5.500 ng/ml (Gerace et al, 2014; Adamowicz et al, 2013). It seems to have been directly involved in >100 cases of intoxication resulting in death in the UK during the period between 2009 and 2013 (Schifano et al, 2012).
Recent epidemiological data estimate a 0.5% use of mephedrone among the general population in the previous year (EMCDDA 2014a), whereas the prevalence among samples from club drug users ranges from 13.8% (Mixmag's Drug Survey: The Results, 2014) to 35.2% (González et al, 2013). Mephedrone is taken predominantly by nasal insufflation (15–125 mg) although markedly unwanted nasal effects have promoted oral use (bombs or tablets) and sometimes the combination of both routes. Recently, a new trend of mephedrone injection has been identified in some European countries among populations of high-risk drug users and young people (EMCDDA, 2014b).
After a recreational oral dose of ~200 mg (50–300 mg), the onset of desired effects appears within 15–45 min after ingestion and lasts ~2–3 h. To prolong the duration of these effects, users report consumption in one session of multiple doses ranging from 0.5 to 2 g (The vaults of Erowid, 2015).
Preclinical studies have reported that mephedrone pharmacology differs from that of MDMA and other amphetamines although it is structurally related to these substances (Liechti 2015; Green et al, 2014). Studies in animal models indicate that mephedrone acts as a monoamine releaser and a reuptake inhibitor (Simmler et al, 2013; Baumann et al, 2012; López-Arnau et al, 2012). It has, therefore, been shown to increase dopamine in a similar manner to amphetamine and produce more serotonin increase (5-HT) than MDMA (Baumann et al, 2012; Kehr et al, 2011). The profile of mephedrone action on monoamine receptors and transporters suggests it could have a high abuse liability, and several studies have found that it supports self-administration at a higher rate than MDMA (Green et al, 2014).
The pharmacokinetics and metabolism of mephedrone have been studied in rats (Martínez-Clemente et al, 2013; Meyer et al, 2010). Preliminary data on its pharmacokinetics in humans suggest a rather complex metabolic disposition (Pozo et al, 2015; Meyer et al, 2010). In vitro studies indicate that CYP2D6 is involved in oxidation reactions (Khreit et al, 2013; Pedersen et al, 2013). To date, it is unknown whether CYP2D6 polymorphism in a similar manner to that described in MDMA could have an important role in modulating the risk of mephedrone toxicity.
This study aims to evaluate the human pharmacology of mephedrone and its abuse liability compared with MDMA after oral administration in an experimental setting.
MATERIALS AND METHODS
Subjects
Twelve healthy male subjects were recruited (mean age 31 years, range 21–39 years; mean weight 75.2 kg, range 66–88 kg) by word of mouth. The subjects were recreational users of amphetamines, ecstasy, mephedrone, and cathinones with a lifetime exposure of at least six times without experiencing serious adverse reactions. The participants had no previous history of abuse or drug dependence according to the Diagnosis and Statistical Criteria for Mental Disorders for other substances except nicotine (in smokers). They have had previous experience with mephedrone (33%), MDMA (100%), amphetamines (100%), and cocaine (100%). All but four were smokers. The subjects drank an average of 1.4 units of alcohol per day.
Prior to their inclusion, the volunteers were submitted to a general medical examination, including blood laboratory tests, urinalysis, 12-lead electrocardiogram (ECG), and a psychiatric diagnostic examination (Psychiatric Research Interview for Substance and Mental Disorders). In addition, only subjects who were phenotypically CYP2D6 extensive metabolizers, determined by the dextromethorphan test in urine, were included to reduce the potential risk of developing MDMA acute toxicity, and possible mephedrone toxicity in the case that mephedrone was also metabolized by CYP2D6. A total of 22 subjects were selected for a complete screening, only one was a poor metabolizer (4.5%), a percentage similar to that described in a Caucasian population (5–10%) (Pardo-Lozano et al, 2012). Of the 21 subjects selected, nine participated in the pilot studies and 12 in the present study. The protocol was approved by the local Research Ethical Committee (CEIC-Parc de Salut Mar, Barcelona, Spain). The study was conducted in accordance with the Declaration of Helsinki and Spanish laws concerning clinical research. Volunteers were financially compensated. The study was registered in ClinicalTrials.gov (NCT02232789).
Drugs
Both synthetic drugs, mephedrone, and MDMA were supplied by the Spanish Ministry of Justice and the Ministry of Health. Placebo, mephedrone, and MDMA capsules were prepared as identically-appearing opaque, white, and soft gelatin capsules under the supervision of the Hospital del Mar Pharmacy Department.
Study Design
The study design was a double-blind, randomized, crossover, and controlled trial with placebo, MDMA, and mephedrone. Sessions were conducted with at least a 1-week washout period between them to minimize the influence of any carryover effect. The conditions were: 200 mg mephedrone, 100 mg MDMA, and placebo by oral route. The mephedrone dose was selected in a series of pilot studies that included doses of 50 mg, 100 mg, 150 mg, and 200 mg of mephedrone, and 100 mg MDMA. The selected dose of 200 mg was well tolerated and showed similar effects to MDMA (Farré et al, 2014).
Experimental Sessions
Prior to test days, participants completed a training session to familiarize themselves with procedures and questionnaires and to reach a steady performance in the psychomotor tasks.
Subjects were admitted to the Clinical Research Unit facilities at 0700 hours after an overnight fast. Upon arrival, they were questioned about any event that could affect their participation. They were requested to refrain from using any psychoactive drug, a minimum of 7 days prior to the study and throughout it, and from ingesting caffeinated products and alcohol in the 24 h prior to sessions. A urine sample was collected for drug testing (Instant-View, Multipanel 10 Test Drug Screen, Alfa Scientific Designs, Poway, CA, USA). Participants were required to be drug-free before inclusion in each experimental session.
The drug was administered between 0815 and 0830 hours, and the experimental sessions had a duration of 12h after administration. The subjects remained sitting/lying down in a calm and comfortable laboratory environment during the entire session.
At the beginning of each experimental session baseline measures were obtained. The participants received the drug in a fasting state with 250 ml of bottled water. Four, six, and ten hours after the administration, a light breakfast, meal, and snack were provided, respectively. A psychiatric evaluation was performed 8 h after dosing and adverse effects were assessed during each experimental session and the day after.
Physiological Measures
Noninvasive systolic blood pressure, diastolic blood pressure, heart rate, oral temperature (T), and pupillary function were repeatedly recorded at 45 and 15 min prior to predose (time 0, baseline), and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 h after drug administration. All assessments were made using a DinamapTM 8100-T vital signs monitor (Critikon, Tampa, FL, USA). Pupillary function was evaluated employing an infrared pupillometer (PRL-200, NeurOptics, Irvine, CA, USA) under standardized light conditions. The adapted maximal pupil diameter (PD MAX) and minimal pupil diameter (PD MIN) after a light stimulus were recorded (Hysek and Liechti, 2012). For safety reasons, electrocardiogram was continuously monitored for 12 h using a DinamapTM Plus vital signs monitor (Critikon).
Psychomotor Performance Measures
The psychomotor performance battery included a computerized version of the digit symbol substitution test and the Maddox-wing device. The digit symbol substitution test scores were based on the number of correct patterns keyed in during 90 s (correct responses) (Peiró et al, 2013; Farré et al, 2015; Camí et al, 2000; de la Torre et al, 2000). The Maddox wing device measures the balance of extraocular muscles and quantifies exophoria as an indicator of extraocular muscle relaxation. The results are expressed in diopters along the horizontal scale of the device. These measures have been previously used in the evaluation of psychostimulants and MDMA effects (Peiró et al, 2013; Farré et al, 2015; Camí et al, 2000).
The psychomotor performance battery was administered at −30 min (time 0, baseline), and at 1, 2, 3, 4, 6, 8, and 10 h after drug administration. Maddox-wing measures were recorded at −15 min, and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 h after drug administration.
Subjective Effects
Subjective effects were measured using a set of 23 visual analog scales (VAS), the Addiction Research Center Inventory (ARCI), the Evaluation of Subjective Effects of Substances with Abuse Potential questionnaire (VESSPA-SEE), and a pharmacological class identification questionnaire.
VAS (100mm) were labeled with different adjectives marked at opposite ends with ‘not at all' and ‘extremely' (Farré et al, 2014, 2015; Peiró et al, 2013). Subjects were asked to rate effects of ‘any effect', ‘stimulated', ‘high', ‘good effects', ‘bad effects', ‘liking', ‘changes in distances', ‘changes in colors', ‘changes in shapes', ‘changes in lights', ‘hallucinations seeing of lights or spots', ‘hallucinations seeing of animals, things, insects, or people', ‘changes in hearing', ‘hallucinations hearing of sounds or voices', ‘dizziness', ‘drowsiness', ‘confusion', ‘fear', ‘depression or sadness', ‘different or changed unreal body feeling', ‘unreal body feeling', ‘different surroundings', and ‘unreal surroundings'.
The Spanish validated version of the ARCI short form is a questionnaire that is sensitive to the effects of a variety of drugs of abuse and has five subscales: PCAG (pentobarbital-chlorpromazine-alcohol group); MBG (morphine-benzedrine group, a measure of euphoria); LSD (lysergic acid diethylamide group, a measure of dysphoria, and somatic symptoms); BG (benzedrine group, a stimulant scale relating to intellectual efficiency and energy); and A (amphetamine, a measure of d-amphetamine effects); (Farré et al, 2014, 2015).
The VESSPA-SEE is a questionnaire that measures changes in subjective effects caused by a number of drugs including MDMA. It includes six subscales: sedation (S), psychosomatic anxiety (ANX), changes in perception (CP), pleasure and sociability (SOC), activity and energy (ACT), and psychotic symptoms (PS) (González et al, 2015).
The pharmacological class identification questionnaire asks about the class of drug the participants believe they have been given (Rush et al, 1995). The options include placebo, benzodiazepine (eg, valium, diazepam, tranxilium, and rohypnol), alcohol, stimulant (eg amphetamine), designer drugs (ecstasy), opiate (eg morphine and heroin), cocaine, hallucinogen (eg LSD and mescaline), cannabinoids (eg marijuana and hashish), ketamine (special K), GHB (liquid ecstasy), and others.
VASs were administered at −30 min, and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 h after drug administration. ARCI and VESSPA-SEE were administered at −30 min, and at 1, 2, 3, 4, 6, 8, 10, and 12 h after drug administration. The pharmacological class identification questionnaire was given at 10 h after drug administration.
Pharmacokinetics
Blood samples for the determination of mephedrone and MDMA, and its respective metabolites (4-hydroxy-3-methoxymethamphetamine (HMMA), 4-hydroxy-3-methoxyamphetamine (HMA), and 3,4-methylenedioxyamphetamine (MDA)) were collected during each experimental session at −5 min (0 h, before administration), 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 h after drug administration. Urine was collected at various periods until 48 h (data not presented).
Mephedrone plasma concentrations were determined by gas chromatography-mass spectrometry. The method consisted of a liquid-liquid extraction with tert-butyl methyl ether and a further derivatization with N-Methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) of mephedrone. Mephedrone-D3 was used as internal standard. MDMA concentrations in plasma were measured by GC/MS (Pizarro et al, 2002).
STATISTICAL ANALYSIS
Effects
Values from physiological, psychomotor performance measures, and subjective effects were transformed to differences from baseline. The peak effects in the first 4 h after administration (maximum absolute change from baseline values, Emax) and the 4 h area under the curve (AUC) of effects vs time were calculated by the trapezoidal rule for each variable. These transformations were analyzed by one-way repeated-measures analysis of variance (ANOVA) with drug conditions as factor. When ANOVA results showed significant differences between treatment conditions, post hoc multiple comparisons were performed using the Tukey's test. Time course (T-C) of effects was analyzed using repeated-measures two-way ANOVA with drug condition and time (0–4 h) as factors. When drug condition or the drug condition × time interaction was statistically significant, multiple Tukey post hoc comparisons were performed at each time point. The difference in time to reach peak effects (Tmax) values between drug conditions was assessed using the nonparametric Friedman's test, and when significant results between conditions appeared, post hoc multiple comparison was performed applying the Wilcoxon signed rank test adjusting the p-value to three comparisons (p<0.016).
Pharmacokinetics
Peak concentration (Cmax), time to reach peak concentrations (Tmax), area under the concentration-time curve from 0 to 12 h (AUC0–12), from 0 to 24 h (AUC0–24), and from 0 to ∞ (AUC0–∞), elimination half-life (t1/2) and elimination constant (Ke), from mephedrone and MDMA plasma concentrations over time were determined using Pharmacokinetic Functions for Microsoft Excel (Joel Usansky, Atul Desai, and Diane Tang-Liu, Department of Pharmacokinetics and Drug Metabolism, Allergan, Irvine, CA, USA).
All statistical tests were performed at each time point using the PASW Statistics 18.0 (SPSS, Chicago, IL, USA). A value of p<0.05 was considered statistically significant.
RESULTS
Global Results
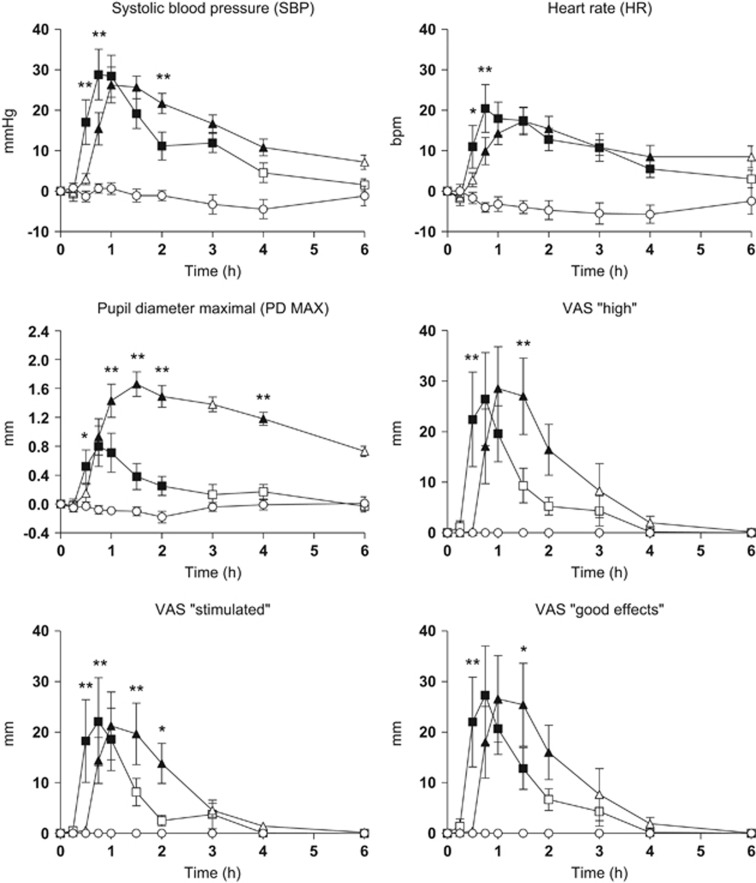
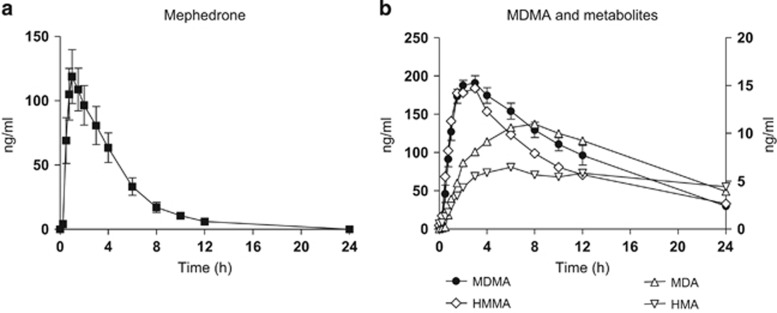
Table 1 presents a summary of the physiological, psychomotor, and subjective effects where at least one statistical difference was found in the ANOVA/Friedman's test and multiple comparison post hoc test analysis (peak effect, Tmax). In addition, Supplementary Table S1 includes AUC and T-C points that showed significant differences in ANOVA and the multiple comparison post hoc tests. Figure 1 summarizes the most relevant physiological effects, psychomotor performance, and subjective effects. Concentrations over time and pharmacokinetic parameters of mephedrone and MDMA, and metabolites in plasma are presented in Figure 2 and Table 2, respectively.
Table 1. Summary of Significant Statistical Result on the Physiological Parameters, Psychomotor Performance, and Subjective Effects (n=12) Observed after Administration of Mephedrone, MDMA, and Placebo.
| Variable | Parameter |
ANOVA |
Multiple comparison | Placebo | MDMA | Mephedrone | |
|---|---|---|---|---|---|---|---|
| F/X2 | p-value | Tukey/Wilcoxon | Mean±SD/median | Mean±SD/median | Mean±SD/median | ||
| Physiological | |||||||
| SBP | Peak (df=2.22) | 27.587 | <0.001 | A, B | −6.00±9.36 | 33.67±10.80 | 33.50±16.04 |
| Tmax | 8.318 | 0.016 | NS | 3.00 | 1.50 | 1.00 | |
| DBP | Peak (df=2.22) | 18.025 | <0.001 | A, B | −3.50±7.72 | 15.25±4.90 | 12.33±9.97 |
| Tmax | 2.087 | 0.352 | — | 1.50 | 1.00 | 0.75 | |
| HR | Peak (df=2.22) | 29.664 | <0.001 | A, B | 6.08±10.36 | 19.00±12.45 | 28.25±17.47 |
| Tmax | 15.136 | 0.001 | a, B | 2.50 | 1.25 | 0.88 | |
| T | Peak (df=2.22) | 9.768 | 0.001 | B, c | −0.23±0.33 | 0.49±0.48 | −0.01±0.45 |
| Tmax | 4.227 | 0.012 | — | 1.00 | 3.00 | 1.75 | |
| MAX PD | Peak (df=2.22) | 26.332 | <0.001 | A, B, C | −0.11±0.36 | 1.76±0.52 | 0.87±1.07 |
| Tmax | 1.682 | 0.431 | — | 2.00 | 1.50 | 1.00 | |
| MIN PD | Peak (df=2.22) | 11.510 | <0.001 | B,C | −0.02±0,41 | 1.38±0.60 | 0.37±1.20 |
| Tmax | 13.378 | 0.01 | A, C | 2.00 | 1.50 | 0.75 | |
| Psychomotor | |||||||
| DSST correct | Peak (df=2.22) | 8.801 | 0.002 | A, B | −3.58±2.87 | 2.00±4.35 | 1.50±3.94 |
| Tmax | 1.256 | 0.534 | — | 1.75 | 2.00 | 1.25 | |
| Visual analog scales | |||||||
| Any effect | Peak (df=2.22) | 9.781 | 0.001 | A, B | 0.00±0.00 | 38.25±25.90 | 30.58±32.89 |
| Tmax | 20.182 | <0.001 | a, B | 0.00 | 1.25 | 0.75 | |
| Stimulated | Peak (df=2.22) | 7.161 | 0.004 | A, b | 0.00±0.00 | 27.75±28.91 | 28.33±32.09 |
| Tmax | 17.721 | <0.001 | a, B | 0.00 | 1.00 | 0.75 | |
| High | Peak (df=2.22) | 12.171 | <0.001 | a, B | 0.00±0.00 | 39.67±26.83 | 34.00±32.14 |
| Tmax | 20.182 | <0.001 | a, B | 0.00 | 1.50 | 0.75 | |
| Good effects | Peak (df=2.22) | 11.452 | <0.001 | A, B | 0.00±0.00 | 36.17±27.57 | 36.50±32.33 |
| Tmax | 15.591 | <0.001 | a, b | 0.00 | 1.00 | 0.75 | |
| Liking | Peak (df=2.22) | 10.357 | 0.001 | A, B | 0.00±0.00 | 40.00±32.08 | 35.25±32.20 |
| Tmax | 14.683 | 0.001 | a, b | 0.00 | 0.75 | 0.75 | |
| Changes in distances | Peak (df=2.22) | 2.198 | 0.135 | — | 0.00±0.00 | 2.92±5.23 | 5.50±10.56 |
| Tmax | 5.810 | 0.055 | — | 0.00 | 0.00 | 0.00 | |
| Changes in lights | Peak (df=2.22) | 2.899 | 0.076 | NS | 0.00±0.00 | 2.75±5.21 | 10.92±20.92 |
| Tmax | 6.118 | 0.047 | NS | 0.00 | 0.00 | 0.00 | |
| Changes in hearing | Peak (df=2.22) | 2.111 | 0.145 | NS | 0.00±0.00 | 7.25±14.87 | 9.42±22.81 |
| Tmax | 7.053 | 0.029 | NS | 0.00 | 0.00 | 0.00 | |
| Dizziness | Peak (df=2.22) | 2.238 | 0.130 | NS | 0.00±0.00 | 3.33±6.15 | 1.92±6.64 |
| Tmax | 11.474 | 0.003 | a, b | 0.00 | 0.38 | 0.00 | |
| Confusion | Peak (df=2.22) | 2.034 | 0.155 | NS | 0.00±0.00 | 4.67±7.52 | 11.08±25.89 |
| Tmax | 11.200 | 0.004 | NS | 0.00 | 0.38 | 0.00 | |
| Different body sensation | Peak (df=2.22) | 8.953 | 0.001 | a, b | 0.00±0.00 | 25.08±19.73 | 32.08±34.36 |
| Tmax | 18.311 | <0.001 | a, b | 0.00 | 1.00 | 0.75 | |
| Different surroundings | Peak (df=2.22) | 1.640 | 0.217 | — | 0.00±0.00 | 9.83±21.94 | 15.58±28.27 |
| Tmax | 6.091 | 0.048 | NS | 0.00 | 0.00 | 0.00 | |
| ARCI questionnaire | |||||||
| ARCI-PCAG | Peak (df=2.22) | 3.474 | 0.049 | b | 0.00±0.00 | 2.83±3.19 | 0.83±3.27 |
| Tmax | 19.395 | <0.001 | A, B | 0.00 | 1.25 | 1.00 | |
| ARCI-MBG | Peak (df=2.22) | 14.347 | <0.001 | A, B | 0.00±0.00 | 5.42±4.19 | 6.08±4.48 |
| Tmax | 18.047 | <0.001 | A, b | 0.00 | 1.50 | 1.00 | |
| ARCI-LSD | Peak (df=2.22) | 13.008 | <0.001 | B, c | −0.08±0.29 | 4.08±3.23 | 2.00±2.49 |
| Tmax | 15.953 | <0.001 | a, B | 0.00 | 1.25 | 1.25 | |
| ARCI-BG | Peak (df=2.22) | 4.823 | 0.018 | a | 0.00±0.00 | 1.58±3.48 | 2.75±2.26 |
| Tmax | 20.140 | <0.001 | A, B | 0.00 | 1.00 | 1.00 | |
| ARCI-A | Peak (df=2.22) | 21.889 | <0.001 | A, B | 0.00±0.00 | 4.50±2.71 | 4.58±2.91 |
| Tmax | 20.762 | <0.001 | A, B | 0.00 | 1.00 | 1.00 | |
| VESSPA-SEE questionnaire | |||||||
| VESSPA-S | Peak (df=2.22) | 1.067 | 0.361 | — | 0.00±0.00 | 2.17±6.53 | 2.50±3.03 |
| Tmax | 15.297 | <0.001 | a, b | 0.00 | 1.00 | 1.00 | |
| VESSPA-ANX | Peak (df=2,22) | 14.648 | <0.001 | A, B | 0.00±0.00 | 7.50±4.72 | 8.42±7.33 |
| Tmax | 20.591 | <0.001 | A, B | 0.00 | 1.50 | 1.00 | |
| VESSPA-CP | Peak (df=2,22) | 1.737 | 0.199 | — | 0.00±0.00 | 0.50±1.17 | 0.80±0.23 |
| Tmax | 2.600 | 0.273 | — | 0.00 | 0.00 | 0.00 | |
| VESSPA-SOC | Peak (df=2,22) | 9.249 | 0.001 | A, B | 0.00±0.00 | 7.00±6.59 | 7.92±7.72 |
| Tmax | 15.050 | 0.001 | a, b | 0.00 | 1.50 | 1.00 | |
| VESSPA-ACT | Peak (df=2,22) | 10.984 | <0.001 | A, B | 0.00±0.00 | 7.33±6.75 | 7.67±6.27 |
| Tmax | 20.591 | <0.001 | A, B | 0.00 | 1.50 | 1.00 | |
| VESSPA-PS | Peak (df=2,22) | 5.699 | 0.010 | B | 0.00±0.00 | 2.17±2.69 | 0.83±1.99 |
| Tmax | 11.143 | 0.004 | b | 0.00 | 1.00 | 0.00 | |
For AUC and Time-Course, see Supplementary material (Supplementary Table S1). Peak=peak effects from 0 to 4 h measured by mm Hg (systolic blood pressure (SBP); diastolic blood pressure (DBP)), bpm (heart rate (HR)), °C (temperature (T)), mm (maximal diameter pupil (DP MAX); minimal diameter pupil maximal (DP MIN); visual analog scale (VAS)), and score (digit symbol substitution test (DSST), Addiction Research Center Inventory (ARCI), and Evaluation of Subjective Effects of Substances with Abuse Potential questionnaire (VESSPA-SEE)), and expressed as mean; Tmax, time to reach peak effects measured by hours and expressed as median; df, degree of freedom.
For peak, an ANOVA and multiple Tukey post hoc comparisons were used. Peak differences between conditions are presented as A: placebo vs mephedrone, p<0.01; a: placebo vs mephedrone, p<0.05; B: placebo vs MDMA, p<0.01; b: placebo vs MDMA, p<0.05; C: mephedrone vs MDMA, p<0.01; c: mephedrone vs MDMA, p<0.05.
For Tmax, a Friedman's test and multiple Wilcoxon post hoc comparisons were used. Differences between conditions are presented as A: placebo vs mephedrone, p<0.003; a: placebo vs mephedrone, p<0.016; B: placebo vs MDMA, p<0.003; b: placebo vs MDMA, p<0.016; C: mephedrone vs MDMA, p<0.003; c: mephedrone vs MDMA, p<0.016.
Figure 1.
T-C of physiological effects and subjective effects (n=12, mean, standard error); □, 200 mg of mephedrone; Δ, 100 mg of MDMA; ○, placebo. *p<0.05 and **p<0.01 indicate mephedrone significant differences from MDMA. Filled symbols indicate a significant difference from placebo (p<0.05). VAS, visual analog scale.
Figure 2.
Plasma concentrations over time curves of mephedrone (a), MDMA, and metabolites (b) (n=12, mean, standard error). In figure (b), right axis values correspond to MDMA and metabolite HMMA, and left axis values correspond to MDA and HMA metabolites.
Table 2. Pharmacokinetics Parameters of Mephedrone and MDMA in Plasma.
| Pharmacokinetic parameters |
Mephedrone |
MDMA |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Cmax (ng/ml) | 134.6 | 63.5 | 202.8 | 31.2 |
| AUC0–12 (ng/ml/h) | 519.5 | 287.0 | 1649.5 | 308.5 |
| AUC0–24 (ng/ml/h) | 556.2 | 320.2 | 2407.4 | 630.7 |
| AUC0–∞ (ng/ml/h) | 556.2 | 320.2 | 2795.9 | 843.5 |
| Tmax (h) | 1.25 | (0.5–4) | 2.00 | (1.5–4) |
| Ke (per h) | 0.33 | 0.07 | 0.09 | 0.03 |
| t1/2 (h) | 2.15 | 0.4 | 7.89 | 2.2 |
Abbreviations: AUC, area under the curve; Cmax, peak/maximal concentration; Ke, elimination constant; SD, standard deviation; t1/2, elimination half-life; Tmax, time to reach peak concentrations. Tmax is shown as median (range) values.
No serious adverse events, including hallucinations, psychotic episodes, or any other psychiatric symptoms were reported during the experimental sessions. None of the participants required specific therapy or special care, and all of them completed the study.
Physiological Effects
Regarding physiological effects, mephedrone 200 mg and MDMA 100 mg produced an increase in systolic blood pressure, diastolic blood pressure, heart rate, T, PD MAX, and PD MIN as compared with placebo (when considering the peak effects, AUC, or both). For cardiovascular effects (systolic blood pressure, diastolic blood pressure, and heart rate), differences among both active conditions were only significant in some T-C points, whereas statistically significant differences in peak effect was observed for T (Table 1, Figure 1, Supplementary Table S1). Differences between mephedrone and MDMA appeared in the PD. The effect of mephedrone on pupil size (PD MAX 0.87±1.07 mm; PD MIN 0.37±1.20 mm) peaked at 0.75 h after drug administration, whereas for MDMA, the peak effect (PD MAX 1.76±0.52 mm and PD MIN 1.38±0.60 mm) was at 1.5 h. In addition, significant differences in AUC and T-C after administration were detected among both active conditions (Supplementary Table S1). For T, only statistically significant differences were observed in peak effects between them (Table 1).
For physiological variables, mephedrone median Tmax values ranged from 0.75 to 1 h, whereas for MDMA, Tmax ranged from 1 to 3 h. Only significant differences were detected between placebo and each active drug condition (mephedrone and MDMA, respectively). Nevertheless, the majority of the subjects experimented mephedrone maximal effects earlier than those produced by MDMA and which disappeared earlier than those for MDMA (Table 1).
Psychomotor Performance
For the digit symbol substitution test task, mephedrone 200 mg and MDMA 100 mg produced an increase in correct responses as compared with placebo (when considering the peak effects, AUC, or both). However, no significant differences were found when comparing both active conditions (Table 1, Supplementary Table S1).
Compared with placebo, mephedrone and MDMA produced esophoria in the Maddox wing device with mephedrone having slightly higher levels. The mean peak difference for mephedrone was −1.24 transformed diopters, and this change was greatest at 0.38 h vs −1.17 transformed diopters at 1.25 h for MDMA. No significant differences between active conditions were observed in the AUC, peak effects, or T-C points.
Subjective Effects
Mephedrone and MDMA produced significant changes in subjective drug effects (VAS, ARCI, and VESSPA-SEE) compared with placebo (Table 1). Both substances caused an increase in VAS measures of stimulant-like effects and euphoria (VAS ‘stimulated', ‘high', ‘good effects', and ‘liking'), perception changes (VAS ‘change in distances', ‘change in lights', ‘change in hearing', ‘different body sensation', and ‘different surroundings') although significant differences were not observed in peak effects or AUC after comparing the two drugs (Table 1, Figure 1, Supplementary Table S1). However, marked differences between mephedrone and MDMA were reported at several T-C points (Supplementary Table S1). Similar results were observed for ‘VAS dizziness' and ‘VAS confusion' (Table 1, Figure 1,Supplementary Table S1).
Overall, the subjective effects produced by mephedrone, measured by VAS parameters, commenced at 0.25 h, peaked at ~0.75 h after administration (Tmax), returned to half-maximum at 1.5–2 h, and were close to predose values at 2–3 h after administration. In contrast, MDMA subjective effects appeared slightly later, approximately at 0.75 h, with maximum values observed between 1 and 1.5 h (Tmax), remaining until 2–3 h, and returning to almost predose values at 4 h after administration (Figure 1, Table 1).
With respect to the ARCI questionnaire, mephedrone and MDMA produced an increase in all the subscales compared with placebo. The most marked increases were observed in scores for the subscales MBG (euphoria), BG (intellectual efficiency and energy), and A (amphetamine). For mephedrone, MBG, BG, and A, the peak difference scores were higher compared with MDMA (6.08, 2.8, and 4.58 scores vs 5.42, 1.58, and 4.50 scores, respectively) although no statistical differences in peak effects, AUC, and T-C points were detected. Significant differences in LSD (dysphoria) were, however, found between both active treatment conditions. Moreover, MDMA increased the dysphoria and somatic symptom scores more than mephedrone, and statistical differences were found between them in peak effects, AUC, and T-C points (1–4 h) (Table 1, Figure 1, Supplementary Table S1).
Regarding the VESSPA-SEE questionnaire, mephedrone and MDMA increased all the subscales compared with placebo, but no statistical differences were observed in peak effects or AUC between the two active substances with the exception of AUC for the subscale S (sedation) (Table 1,Supplementary Table S1). In the rest of the VESSPA-SEE subscales, only statistical differences were shown in scores for mephedrone compared with MDMA in several T-C points between 1 h and 2 h post administration (Supplementary Table S1).
In the pharmacologic drug class identification questionnaire, 10 of the 12 subjects (83.3%) identified mephedrone as a designer drug (ecstasy: nine, mephedrone: one), one as a stimulant, and one as placebo, respectively. Eight subjects (66.7%) identified MDMA as a designer drug (ecstasy: seven, mephedrone: one), two subjects as a stimulant, one subject as ketamine, and one subject as a hallucinogen. The placebo was identified correctly by 11 patients with the exception of one subject who classified it as a benzodiazepine.
Pharmacokinetics
Pharmacokinetic parameters for mephedrone 200 mg and MDMA 100 mg are summarized in Table 2. Figure 2 represents plasma concentration over time curves of mephedrone and MDMA, including the metabolites of MDMA (HMMA, MDA, and HMA).
Mephedrone was quickly absorbed after administration and rapidly eliminated with an interindividual variability. Mephedrone concentrations peaked at 1.25 h (range 0.5 h and 4 h) after drug administration with a mean Cmax 134.6 ng/ml (range, 51.7–218.3 ng/ml). Following the absorption phase, concentrations declined to mean values of 6.1 ng/ml at 12 h to undetectable levels at 24 h. Mean t1/2 was 2.15 h.
With regard to MDMA, concentrations peaked at 2.00 h (range 1.5 and 4 h) after drug administration with a Cmax 202.8 ng/ml (range 160.1–262.4 ng/ml). Mean t1/2 was 7.89 h.
DISCUSSION
To the best of our knowledge, this is the first controlled human study evaluating the human pharmacology and comparative abuse liability of mephedrone. Our main finding is that mephedrone induced stimulant-like effects, euphoria, well-being, feelings of pleasure, and mild changes in perceptions, all similar to those produced by MDMA, but with shorter duration. Such effects are similar to those spontaneously reported by mephedrone recreational users and are the basis of its potential abuse. In addition, we described for first time in a controlled study, the pharmacokinetic parameters of mephedrone after a single oral dose.
Mephedrone at a dose of 200 mg under controlled administration produces marked, but short-lived, cardiovascular effects in comparison with MDMA. These results are consistent with previous studies in animal models, reporting that, it induces substantial increases in blood pressure, heart rate, and cardiac contractility (Meng et al, 2012), in addition to acute cardiovascular toxicity characterized by hypertension and tachycardia (Sivagnanam et al, 2013; Nicholson et al, 2010; Regan et al, 2011; Maskell et al, 2011; Wood et al, 2010). Moreover, mephedrone consistently results in mydriasis, a specific and acute effect also observed after MDMA and amphetamine administration (Farré et al, 2015; de la Torre et al, 2000, 2004; Mas et al, 1999), although slighter in comparison with MDMA.
Our findings show that although the profile of response attributable to mephedrone (hypertension, tachycardia, and mydriasis) is common to the sympathomimetic effects of stimulant-like drugs, its faster and shorter-lasting response is specific (Mas et al, 1999). Although its effects are similar to those induced by MDMA, they appear earlier and dissipate faster with a peak effect between 0.5 h and 0.75 h after administration and a return to close basal values at 2–3 h.
Regarding subjective effects, mephedrone produced increases in several VAS related to stimulant-like effects and changes in perceptions. Increases after mephedrone administration were also observed on the subscales of ARCI and VESSPA-SEE related to pleasurable effects and euphoria. All these euphoric-like feelings peaked at 0.75–1 h and returned to baseline levels at 3 h after administration. Thus, with respect to abuse potential, mephedrone induced positive effects with substantial similarities to MDMA in magnitude, but with a faster and shorter duration.
Furthermore, the results obtained agree with marked, but transient, psychostimulant, and euphoric effects described following oral cathinone administration, the parent compound of mephedrone, in an experimental study with humans (Brenneisen et al, 1990).
We observed that when administered by the oral route, the T-C of mephedrone pharmacological effects and its plasma concentrations rose, and fell with a similar profile. Both peak concentration and effects were observed between 0.5–1 h and returned to baseline 2–3 h after drug administration. An adequate pharmacological effect in relation to pharmacokinetics was, therefore, reported in spite of high interindividual variability among subjects. Mephedrone plasma concentrations peaked at 1.25 h in comparison with MDMA that peaked at 2 h. Elimination half-life of mephedrone was 2.15 h, considerably less than that of MDMA (around 8 h), amphetamine–methamphetamine (12 h), and cathinone (4 h). This short half-life could explain the briefer duration of its pharmacological effects in comparison with MDMA. Our results could be in agreement with the low oral bioavailability observed in animals (~10% of the administered dose in rats) suggesting that mephedrone undergoes an extensive first-pass effect after oral administration and an easy access to the central nervous system (Martínez-Clemente et al, 2013). They may also partially explain the fact that there is a strong tendency for recreational users, rather than employing the oral route, to use nasal insufflation for mephedrone (Winstock et al, 2011) or, more recently, intravenous injection (EMCDDA 2014b). It should also be taken into account the fact that mephedrone showed a shorter half-life and time to peak concentrations than that of MDMA, and that both drugs are often redosed (taking several pills in one session) by recreational users.
Our study has some limitations, the relatively scarce number of participants could justify a lack of power in the comparisons between both active drugs. Although the study was designed to explore the abuse liability of mephedrone in comparison with MDMA, the protocol only included one dose of mephedrone, and we cannot extrapolate our results to higher doses or demonstrate a dose–response relationship.
In conclusion, mephedrone presents an abuse potential profile similar to MDMA, but with some differences, a more rapid onset and a shorter duration of effects, probably related to its brief elimination half-life. Such differences could explain, in real life conditions, a more compulsive pattern of use to prolong the duration of the desired effects and, consequently, an increased risk of toxicity.
FUNDING AND DISCLOSURE
Funding is supported in part by grants from Instituto de Salud Carlos III (ISCIII, FIS-FEDER, PI11/01961), ISCIII-Red de Trastornos Adictivos (RTA RD12/0028/0009), and The European Commission (Drug Prevention and Information Programme 2014–16, Contract no. JUST/2013/DPIP/AG/4823, EU-MADNESS project and Drugs Policy Initiatives, Justice Programme 2014-2020, Contract no. HOME/2014/JDRU/AG/DRUG/7082, PREDICT Project). EP has a Rio Hortega fellowship (ISC-III, CM13/00016) and CP-M has a Juan Rodes fellowship (ISC-III, JR15/00005).
Acknowledgments
We are grateful to Esther Menoyo (RN), Marta Pérez (RN), Soraya Martín (RN), Clara Gibert (RN), and Joan Mestres (PsyD), for their valuable assistance throughout the clinical study.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adamowicz P, Tokarczyk B, Stanaszek R, Slopianka M (2013). Fatal mephedrone intoxication—a case report. J Anal Toxicol 37: 37–42. [DOI] [PubMed] [Google Scholar]
- Advisory Council on the Misuse of Drugs (2015). ACMD report on definitions for the Psychoactive Substances Bill. Report number 454039. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/454039/Definitions_report_final_14_august.pdf (last accessed date 5 January 2016).
- Brenneisen R, Fisch HU, Koelbing U, Geisshüsler S, Kalix P (1990). Amphetamine-like effects in humans of the khat alkaloid cathinone. Br J Clin Pharmacol 30: 825–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF et al (2012). The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37: 1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busardò FP, Kyriakou C, Napoletano S, Marinelli E, Zaami S (2015). Mephedrone related fatalities: a review. Eur Rev Med Pharmacol Sci 19: 3777–3790. [PubMed] [Google Scholar]
- Camí J, Farré M, Mas M, Roset PN, Poudevida S, Mas A et al (2000). Human pharmacology of 3,4-methylenedioxymethamphetamine ("ecstasy"): psychomotor performance and subjective effects. J Clin Psychopharmacol 20: 455–466. [DOI] [PubMed] [Google Scholar]
- Camí J, Farré M (2003). Drug addiction. N Engl J Med 349: 975–986. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, King LA, Nutt DJ (2011). A web-based survey on mephedrone. Drug Alcohol Depend 118: 19–22. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Roset PN, Pizarro N, Abanades S, Segura M et al (2004). Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit 26: 137–144. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Roset PN, Lopez CH, Mas M, Ortuño J et al (2000). Pharmacology of MDMA in humans. Ann N Y Acad Sci 914: 225–237. [DOI] [PubMed] [Google Scholar]
- Deluca P, Davey Z, Corazza O, Di Furia L, Farré M, Flesland LH et al (2012). Identifying emerging trends in recreational drug use; outcomes from the Psychonaut Web Mapping Project. Prog Neuropsychopharmacol Biol Psychiatry 39: 221–226. [DOI] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2014. a). European Drug Report 2014: Trends and developments. Catalog Number: TDAT14001ENN. Available at: http://www.emcdda.europa.eu/publications/edr/trends-developments/2014 (last accessed date 5 January 2016).
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2014. b). Perspective on drugs. Injection of synthetic cathinones. Catalog Number: 228233_EN_POD2014. Available at: http://www.emcdda.europa.eu/attachements.cfm/att_228233_EN_POD2014_Injection%20of%20synthetic%20cathinones.pdf. (last accessed date 5 January 2016).
- Farré M, Tomillero A, Pérez-Mañá C, Yubero S, Papaseit E, Roset PN et al (2015). Human pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) after repeated doses taken 4 h apart. Eur Neuropsychopharmacol 25: 1637–1649. [DOI] [PubMed] [Google Scholar]
- Farré M, Papaseit E, Pérez-Mañá C, Yubero-Lahoz S, Pujadas M, Fonseca F et al (2014). Pharmacokinetics and pharmacodynamics of mephedrone in humans. Res Adv Psychiatry (Suppl1): 19.
- Gerace E, Petrarulo M, Bison F, Salomone A, Vincenti M (2014). Toxicological findings in a fatal multidrug intoxication involving mephedrone. Forensic Sci Int 243: 68–73. [DOI] [PubMed] [Google Scholar]
- González D, Torrens M, Farré M (2015). Acute effects of the novel psychoactive drug 2C-B on emotions. Biomed Res Int 201: 643878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González D, Ventura M, Caudevilla F, Torrens M, Farré M (2013). Consumption of new psychoactive substances in a Spanish sample of research chemical users. Hum Psychopharmacol 28: 332–340. [DOI] [PubMed] [Google Scholar]
- Green AR, King MV, Shortall SE, Fone KC (2014). The preclinical pharmacology of mephedrone; not just MDMA by another name. Br J Pharmacol 171: 2251–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Liechti ME (2012). Effects of MDMA alone and after pretreatment with reboxetine, duloxetine, clonidine, carvedilol, and doxazosin on pupillary light reflex. Psychopharmacology (Berl) 224: 363–376. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F et al (2011). Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol 164: 1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khreit OI, Grant MH, Zhang T, Henderson C, Watson DG, Sutcliffe OB (2013). Elucidation of the Phase I and Phase II metabolic pathways of (±)-4'-methylmethcathinone (4-MMC) and (±)-4'-(trifluoromethyl)methcathinone (4-TFMMC) in rat liver hepatocytes using LC-MS and LC-MS. J Pharm Biomed Anal 72: 177–185. [DOI] [PubMed] [Google Scholar]
- Liechti M (2015). Novel psychoactive substances (designer drugs): overview and pharmacology of modulators of monoamine signaling. Swiss Med Wkly 145: w14043. [DOI] [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Pubill D, Escubedo E, Camarasa J (2012). Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol 167: 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Clemente J, López-Arnau R, Carbó M, Pubill D, Camarasa J, Escubedo E (2013). Mephedrone pharmacokinetics after intravenous and oral administration in rats: relation to pharmacodynamics. Psychopharmacology (Berl) 229: 295–306. [DOI] [PubMed] [Google Scholar]
- Mas M, Farré M, de la Torre R, Roset PN, Ortuño J, Segura J et al (1999). Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther 290: 136–145. [PubMed] [Google Scholar]
- Maskell PD, De Paoli G, Seneviratne C, Pounder DJ (2011). Mephedrone (4-methylmethcathinone)-related deaths. J Anal Toxicol 35: 188–191. [DOI] [PubMed] [Google Scholar]
- Meng H, Cao J, Kang J, Ying X, Ji J, Reynolds W et al (2012). Mephedrone, a new designer drug of abuse, produces acute hemodynamic effects in the rat. Toxicol Lett 208: 62–68. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Wilhelm J, Peters FT, Maurer HH (2010). Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal Bioanal Chem 397: 1225–1233. [DOI] [PubMed] [Google Scholar]
- Mixmag's Global Drug Survey: The results (2014). Available at: http://www.globaldrugsurvey.com/the-global-drug-survey-2014-findings/. (last accessed date 4 January 2016).
- Nicholson PJ, Quinn MJ, Dodd JD (2010). Headshop heartache: acute mephedrone 'meow' myocarditis. Heart 96: 2051–2052. [DOI] [PubMed] [Google Scholar]
- Papaseit E, Farré M, Schifano F, Torrens M (2014). Emerging drugs in Europe. Curr Opin Psychiatry 27: 243–250. [DOI] [PubMed] [Google Scholar]
- Pardo-Lozano R, Farré M, Yubero-Lahoz S, O'Mathúna B, Torrens M, Mustata C et al (2012). Clinical pharmacology of 3,4- methylenedioxymethamphetamine (MDMA, "ecstasy"): the influence of gender and genetics (CYP2D6, COMT, 5-HTT). PLoS One 7: e47599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AJ, Reitzel LA, Johansen SS, Linnet K (2013). In vitro metabolism studies on mephedrone and analysis of forensic cases. Drug Test Anal 5: 430–438. [DOI] [PubMed] [Google Scholar]
- Peiró AM, Farré M, Roset PN, Carbó M, Pujadas M, Torrens M et al (2013). Human pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) after repeated doses taken 2 h apart. Psychopharmacology (Berl) 225: 883–893. [DOI] [PubMed] [Google Scholar]
- Pizarro N, Ortuño J, Farré M, Hernandez-Lopez C, Pujadas M, Llebaria A et al (2002). Determination of MDMA and its metabolites in blood and urine by gas chromatography-mass spectrometry and analysis of enantiomers by capillary electrophoresis. J Anal Toxicol 26: 157–165. [DOI] [PubMed] [Google Scholar]
- Pozo ÓJ, Ibáñez M, Sancho JV, Lahoz-Beneytez J, Farré M, Papaseit E et al (2015). Mass spectrometric evaluation of mephedrone in vivo human metabolism: identification of phase I and phase II metabolites, including a novel succinyl conjugate. Drug Metab Dispos 43: 248–257. [DOI] [PubMed] [Google Scholar]
- Regan L, Mitchelson M, Macdonald C (2011). Mephedrone toxicity in a Scottish emergency department. Emerg Med J 28: 1055–1058. [DOI] [PubMed] [Google Scholar]
- Rush CR, Sullivan JT, Griffiths RR (1995). Intravenous caffeine in stimulant drug abusers: subjective reports and physiological effects. J Pharmacol Exp Ther 273: 351–358. [PubMed] [Google Scholar]
- Schifano F, Albanese A, Fergus S, Stair JL, Deluca P, Corazza O et al; Psychonaut Web Mapping; ReDNet Research Groups (2011). Mephedrone (4-methylmethcathinone; 'meow meow'): chemical, pharmacological and clinical issues. Psychopharmacology (Berl) 214: 593–602. [DOI] [PubMed] [Google Scholar]
- Schifano F, Corkery J, Ghodse AH (2012). Suspected and confirmed fatalities associated with mephedrone (4-methylmethcathinone, "meow meow") in the United Kingdom. J Clin Psychopharmacol 32: 710–714. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J et al (2013). Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168: 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivagnanam K, Chaudari D, Lopez P, Sutherland ME, Ramu VK (2013). "Bath salts" induced severe reversible cardiomyopathy. Am J Case Rep 14: 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The vaults of Erowid (2015). 4-methylmethcathinone/mephedrone. Available at: http://www.erowid.org/chemicals/4_methylmethcathinone/4_methylmethcathinone.shtml. (last accessed date 4 January 2016).
- Valente MJ, Guedes de Pinho P, de Lourdes Bastos M, Carvalho F, Carvalho M (2014). Khat and synthetic cathinones: a review. Arch Toxicol 88: 15–45. [DOI] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou Ch (2011). Drugs for youth via Internet and the example of mephedrone. Toxicol Lett 201: 191–195. [DOI] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J (2011). Mephedrone: use, subjective effects and health risks. Addiction 106: 1991–1996. [DOI] [PubMed] [Google Scholar]
- Wood DM, Davies S, Puchnarewicz M, Button J, Archer R, Ovaska H et al (2010). Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity. J Med Toxicol 6: 327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Greene SL, Dargan PI (2011). Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J 28: 280–282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.