Abstract
The prefrontal cortex (PFC) regulates cognitive processes critical for goal-directed behavior. PFC cognitive dysfunction is implicated in multiple psychopathologies, including attention deficit hyperactivity disorder (ADHD). Although it has long been known that corticotropin-releasing factor (CRF) and CRF receptors are prominent in the PFC, the cognitive effects of CRF action within the PFC are poorly understood. The current studies examined whether CRF receptor activation in the PFC modulates cognitive function in rats as measured in a delayed response task of spatial working memory. CRF dose-dependently impaired working memory performance when administered either intracerebroventricularly (ICV) or directly into the PFC. The working memory actions of CRF in the PFC were topographically organized, with impairment observed only following CRF infusions into the caudal dorsomedial PFC (dmPFC). Additional studies examined whether endogenous CRF modulates working memory. Both ICV and intra-dmPFC administration of the nonselective CRF antagonist, D-Phe-CRF, dose-dependently improved working memory performance. To better assess the translational potential of CRF antagonists, we examined the cognitive effects of systemic administration of the CRF1 receptor selective antagonist, NBI 35965. Similar procognitive actions were observed in these studies. These results are the first to demonstrate that CRF acts in the PFC to regulate PFC-dependent cognition. Importantly, the ability of CRF antagonists to improve working memory is identical to that seen with all approved treatments for ADHD. These observations suggest that CRF antagonists may represent a novel approach for the treatment of ADHD and other disorders associated with dysregulated prefrontal cognitive function.
Introduction
The prefrontal cortex (PFC) plays a pivotal role in higher cognitive processes required for flexible goal-directed behavior (Miller and Cohen, 2001). PFC cognitive dysfunction is associated with a variety of behavioral disorders, including depression, schizophrenia, and attention-deficit hyperactivity disorder (ADHD; Millan et al, 2012). The majority of clinically efficacious drugs used in the treatment of prefrontal cognitive dysfunction target catecholamines, consistent with extensive evidence demonstrating an important role of these transmitters in the regulation of PFC-dependent cognition (Berridge and Arnsten, 2015). However, there is a serious need for improved treatments for PFC-related cognitive dysfunction. For example, currently available treatments lack full efficacy across the broader population of patients. Moreover, although psychostimulants are highly effective in a large proportion of ADHD patients, these drugs possess significant potential for misuse/abuse (Setlik et al, 2009). Unfortunately, the development of novel pharmacological treatments for PFC-dependent cognitive dysfunction is limited by a scarcity of alternative targets.
The neuropeptide, corticotropin-releasing factor (CRF), is prominent in the PFC (Swanson et al, 1983), and early correlative observations suggested a potential role of PFC CRF in PFC-related psychopathology (Nemeroff et al, 1988). However, despite extensive research into the neurobiology of CRF over the ensuing decades, the functional significance of CRF receptor signaling in the PFC is poorly understood. In the rat, CRF receptors are observed throughout the medial PFC (De Souza et al, 1985; Lovenberg et al, 1995; Van Pett et al, 2000), and limited observations indicate that CRF receptor activation in this region exerts behaviorally and physiologically relevant actions (Jaferi and Bhatnagar, 2007; Miguel et al, 2014). However, the cognitive effects of CRF in the PFC are currently unknown.
To address this issue, we first examined the effects of intracerebroventricular (ICV) and intra-PFC infusion of CRF on performance in a delayed response task of spatial working memory in rats. This task has been used extensively to study catecholamine modulation of PFC-dependent higher cognitive function and has been demonstrated to possess strong translational relevance (Arnsten and Pliszka, 2011; Berridge et al, 2012; Spencer et al, 2012). In particular, the pharmacology of performance in this task mirrors the pharmacology of ADHD, with all approved ADHD treatments improving task performance (Berridge and Arnsten, 2015). Our results demonstrate that CRF receptor activation, both globally in the brain and selectively within the PFC, elicits a dose-dependent impairment in working memory. The cognitive actions of CRF receptor activation in the PFC were topographically organized, with cognitive impairment only observed following CRF infusions into the caudal portion of the dorsomedial PFC (dmPFC). To assess the cognitive actions of endogenous CRF within the PFC, we examined the working memory effects of intra-PFC infusion of a nonselective CRF antagonist (D-Phe-CRF). CRF receptor blockade in the caudal dmPFC elicited a dose-dependent improvement in working memory. Similar actions were observed with both ICV D-Phe-CRF and systemic treatment with a CRF1 receptor selective antagonist (NBI 35965).
Collectively, these studies demonstrate that CRF receptor signaling in the PFC has a prominent role in higher cognitive function. Moreover, given all FDA-approved treatments for ADHD enhance working memory (Berridge and Arnsten, 2015), these results suggest the possibility that CRF receptor antagonists may represent a novel treatment strategy for ADHD and other disorders associated with PFC-dependent cognitive dysfunction.
Materials and methods
Animals
Male Sprague–Dawley rats (280–400 g; Charles River, Wilmington, Massachusetts) were pair-housed in opaque polycarbonate cages on a 13/11-hour light/dark cycle. Animals were fed ad libitum for the first 4–7 days after arrival. Subsequently, the amount of food was titrated for each animal (15–17 g of chow/day) to maintain motivation for food reward while avoiding weight loss. Rats were handled extensively before behavioral testing commenced. Training/testing was conducted between 0800 and 1600 h (5–6 days/week). All facilities and procedures were in accordance with the guidelines regarding animal use and care put forth by the National Institutes of Health of the United States and approved by the Institutional Animal Care and Use Committee of the University of Wisconsin–Madison.
Presurgery Behavioral Training
T-maze training and testing was conducted in rooms devoid of external spatial cues and/or in a dark room lit with a red light as previously described (Berridge et al, 2012). Briefly, animals were trained to enter the arm of a T-maze not chosen on the previous trial to receive a food reward (one chocolate chip or sucrose pellet per trial). Between trials, rats were placed in a start box at the base of the maze and prevented from exiting by a removable gate. Sessions consisted of 20 trials (one session per day).
Surgery
After completion of maze training, rats were anesthetized with isoflurane (1–1.5%) and stainless steel cannulae (26-gauge) were surgically implanted bilaterally over one of the following subregions: rostral (A +3.6–3.0; L±0.8; V −0.2 mm from dura and bregma) or caudal (A +2.8–+2.3) PFC. Dorsal (V −2.5 to −3 mm) and ventral (V −4 mm) aspects of the medial PFC were targeted via different needle projection length in different groups of animals (see below). For ICV infusions, cannulae were unilaterally aimed at the lateral ventricles (A −0.8, L±1.5, V −2.0; hemisphere counterbalanced). Only one region was targeted per animal. Given anatomical and behavioral evidence implicating the dmPFC in higher cognitive/behavioral processes, care was taken to maintain the structural integrity of the dmPFC, with cannulae lowered no more than 200 μm below the dura. Cannulae were secured to the skull with stainless steel screws and acrylic cement (Plastics One, Roanoke, Virginia). Stainless steel stylets prevented occlusion of cannulae and were replaced as needed.
Drugs
CRF (human/rat, Bachem, Torrance, CA) was dissolved in buffered artificial extracellular fluid (147 mmol/l NaCl, 1.3 mmol/l CaCl, 0.9 mmol/l MgCl, 2.5 mmol/L KCl; pH=7.4). The CRF antagonists, D-Phe-CRF (D-Phe12,Nle21.38,α-Me-Leu37)-CRF (12–41); human/rat, Bachem, Torrance, CA) and NBI 35965 (Tocris, Bristol, UK) were dissolved in 0.9% saline.
Infusions and Working Memory Testing
Following surgery, rats resumed T-maze testing until performance reached presurgery levels. Following this, a short delay of 10 s between each trial was introduced. Baseline performance levels of 80–92% and 70–85% were required for CRF and CRF antagonist studies, respectively. In this task, performance improves with repeated testing. Therefore, delays were increased when performance exceeded the desired range (Berridge et al, 2012; Spencer et al, 2012; Zahrt et al, 1997). To ensure task dependence on the PFC, animals requiring delays >60 s were excluded from further study. For all studies, the number of accurate trials was recorded per session. Run-time was also calculated, defined as: (total run duration)−(total delay duration).
Prior to testing, animals were given two mock infusions, consisting of an initial needle insertion followed by a second needle insertion with vehicle infusion 48 h later. This allowed animals to acclimate to the gentle handling associated with infusions and to minimize detrimental behavioral effects of tissue damage related to the initial needle insertion.
Bilateral intra-PFC infusions (500 nl) were made with 33-gauge needles. For the dmPFC, needles projected 2.5–3.0 mm. For ventromedial PFC (vmPFC), needles projected 4.0 mm. Intra-PFC infusions were performed using a microprocessor-controlled pump (Harvard Apparatus, South Natick, MA) at a rate of 250 nl/min for 2 min (500 nl total volume). ICV infusions were made through 33-gauge needles projecting 2.0 mm past the cannula at a rate of 1 μl/min for 2 min (2 μl total). Needles were kept in place for 2 min following the infusion, after which the stylets were replaced.
To limit tissue damage, the number of infusions was limited to four for intra-tissue infusions and six for ICV infusions (excluding mock infusions). Our prior experience demonstrates that this results in minimal tissue damage. In general, animals received only one type of treatment (CRF vs D-Phe-CRF vs NBI 35965). The one exception to this was for ICV-treated animals that were tested with CRF and D-Phe-CRF (on different days). This latter approach permitted aligning treatment with baseline performance to minimize animal usage (higher baselines for CRF treatment; lower baselines for antagonist treatment). This was feasible given the greater limit on the number of infusions relative to intra-tissue infusions (see above).
On the day of testing, rats were transported to the testing room in their home cage, infused with CRF or D-Phe-CRF and returned to their home cage for 15 min prior to testing. NBI 35965 was injected subcutaneously (1 ml/kg) 60 min before testing (Million et al, 2003). For all treatments, doses were counterbalanced.
Treatments were administered when stable baseline performance was observed (two consecutive days in which performance accuracy did not differ by >10%). Given performance improves over time, it is essential to confirm that posttreatment performance is in the same range of pretreatment baseline performance. This ensures that any change in performance on a treatment day reflects the treatment and not time-dependent changes in performance. For this reason, rats were tested at the same delay on the first two posttreatment days. Data were included in analyses only if performance was stable (pre- vs post-infusion performance difference <10%). To facilitate data collection, only the first posttreatment day was used to determine stable performance for studies involving the systemic CRF1 antagonist, NBI 35965.
Given the limits on delays and infusion number, not every animal received each dose of a given treatment.
Feeding Analyses
In the only study in which drug treatment affected run-time (ICV CRF), we additionally examined whether this might reflect changes in motivation for food reward. For these studies, testing occurred in the T-maze testing room in a 23 × 43 × 20 cm opaque Plexiglas cage that animals were habituated to for 2 days prior to the start of testing (20 min/day). During acclimation, animals were allowed to explore the cage and consume 20 sugar pellets scattered throughout the cage (same quantity as in T-maze testing). During testing, animals were placed in the testing cage, and the latency to initiate and time to complete consumption of all sugar pellets within a 20-minute period was recorded by an experimenter in the room. Animals received 2 μl infusions (1 μl/min) of vehicle and 0.1, 0.2, and 1 μg CRF 15 min before testing in a within-subjects design. All treatments were counterbalanced.
Histological Analyses
Rats were deeply anesthetized with isoflurane and transcardially perfused with 10% wt/vol formaldehyde and brains stored in formaldehyde for at least 24 h before sectioning. Injector needle placement was verified in 40 μm thick coronal sections stained with Neutral Red dye (Sigma-Aldrich, St Louis, MO).
Statistical Analyses
Data from a given experiment were included only when histological analyses verified accurate placement of injectors, minimal PFC damage, and stable performance before and after treatment. Given limits on delay and number of infusions, and the requirement for stable performance across pre and posttreatment testing days, it was not possible for all T-maze animals to receive all doses of a given treatment. For this reason, treatment effects could not be analyzed using between- or within-subjects analyses of variance. Therefore, we estimated a linear mixed-effects model using the lmer package in R to analyze the effect of treatments on performance accuracy and run-time. To assess the effects of ICV CRF on feeding, a one-way repeated measures analyses of variance was used. When statistical significance was indicated, comparisons between drug dose vs vehicle were determined using Bonferroni-corrected t-tests.
Results
Effects of CRF Receptor Activation on Working Memory Performance
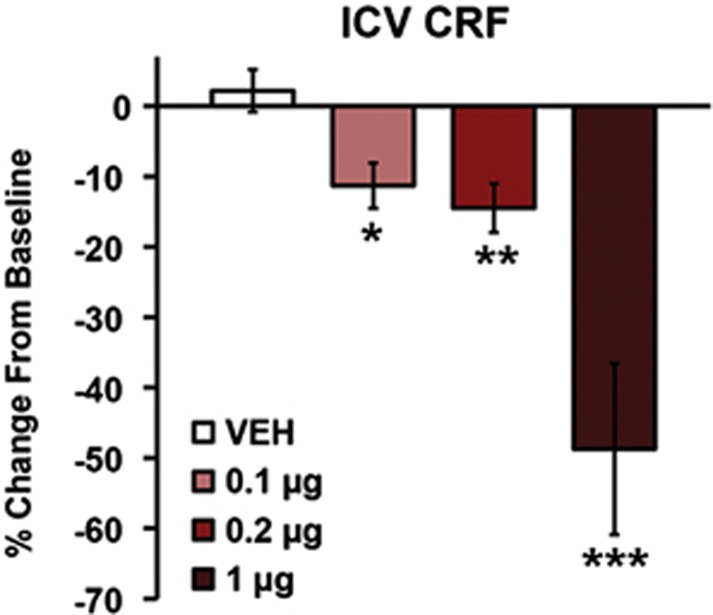
Initial studies examined whether ICV-administered CRF impairs working memory performance. For these studies, animals were treated with vehicle (n=9), 0.1 μg (n=7), 0.2 μg (n=8), or 1 μg (n=8) CRF. Doses were based on previous studies demonstrating behavioral/cognitive actions of ICV CRF (Cole et al, 2016; Dunn and Berridge, 1990; Snyder et al, 2011). As shown in Figure 1 and Supplementary Table S1, ICV CRF elicited dose-dependent impairments in working memory performance (F1,28.3=32.8; P<0.001), with significant impairment observed at all doses. Run-time was only significantly affected (increased) at the highest dose (Supplementary Table S1; F1,19.3=28.2; P<0.001). This effect on run-time does not appear to reflect decreased motivation for or ability to consume (due to competing behaviors) sugar, as CRF had no effect on latency to initiate (F3,24=0.67; P=1.0) or time to consume (F3,24=0.56; P=1.0) sugar (n=9; Supplementary Table S2).
Figure 1.
Intracerebroventricular (ICV) CRF impairs working memory. Shown are the effects of ICV-administered vehicle (n=9) and varying doses of CRF (0.1 μg CRF, n=7; 0.2 μg CRF, n=8; 1 μg CRF, n=8) on working memory performance as measured by percentage change in correct trials (accuracy) from baseline. Results represent mean±SEM percentage change in correct trials (accuracy) from baseline. *P<0.05, **P<0.01, ***P<0.001 vs vehicle.
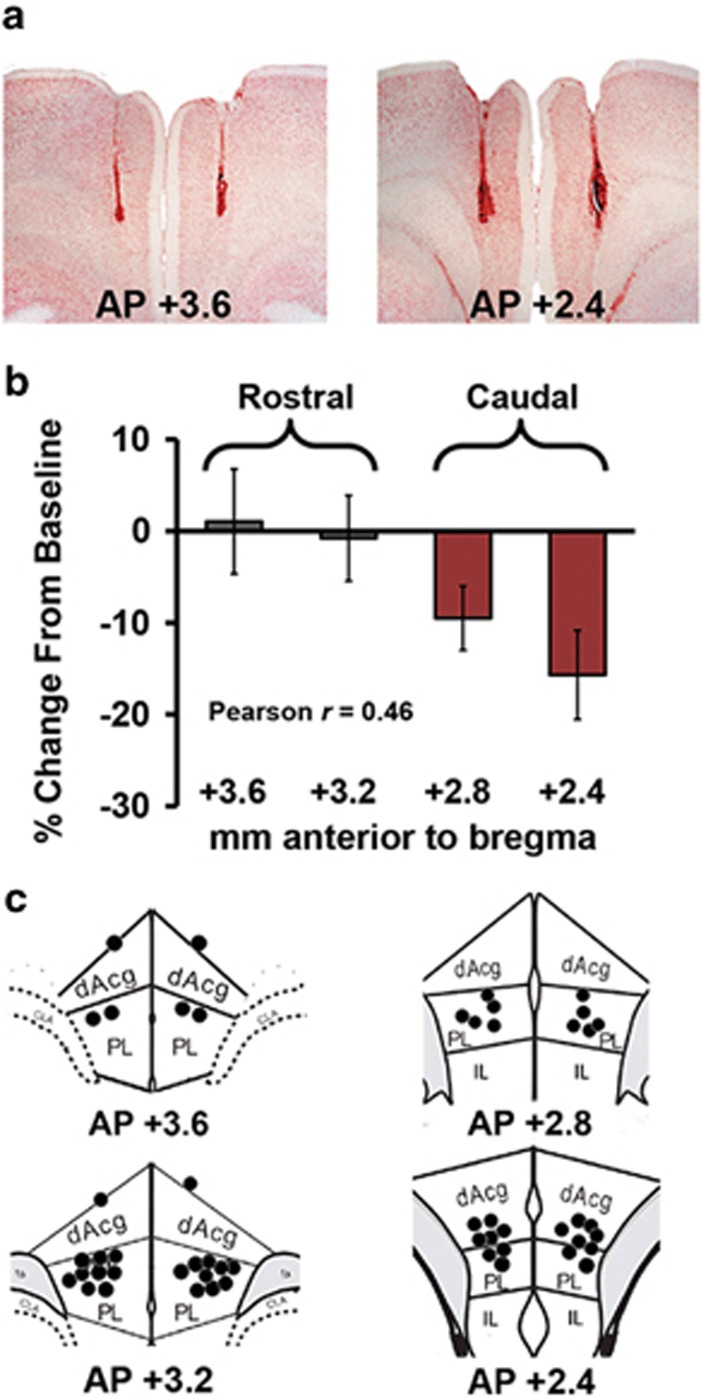
Additional studies examined the working memory effects of CRF receptor activation within the PFC. Given the topographic organization of the rat PFC reviewed above, we initially examined the effects of bilateral 100 ng CRF infusions into the dmPFC. Multiple studies demonstrate that this dose is sufficient to elicit significant behavioral and physiological actions when infused into the PFC and other brain regions (eg, Jaferi and Bhatnagar, 2007). As shown in Figure 2 and Supplementary Table S1, we observed a CRF-induced impairment in working memory that was topographically organized across the rostrocaudal axis. Specifically, CRF impaired performance when infused into the caudal dmPFC (+2.8 mm, n=6 to +2.4 mm, n=8 anterior to bregma), but not more rostrally (+3.6, n=6 to +3.2 mm, n=13). Consistent with this, correlation analyses indicated a significant relationship between the placement of infusion needles within the dmPFC and the magnitude of CRF-induced performance impairment (Pearson's r=0.46, P=0.008).
Figure 2.
CRF acts in the dmPFC to elicit a topographically organized impairment in working memory performance. (a) Representative micrograph depicting the main body of infusion sites into the rostral (left) and caudal (right) dmPFC. (b) Working memory effects of 100 ng/hemisphere CRF infused into varying rostrocaudal subfields of the dmPFC (AP +3.6, n=6; +3.2, n=13; +2.8, n=6; +2.4, n=8). There was a significant correlation between infusion needle placement and CRF-induced working memory impairment (Pearson's r=0.46, P<0.01). These results identify functionally distinct rostral and caudal portions of the dmPFC that were targeted in subsequent studies. Results represent mean±SEM percentage change in accuracy relative to baseline. (c) Schematic depiction of rostral (left) and caudal (right) dmPFC infusion sites.
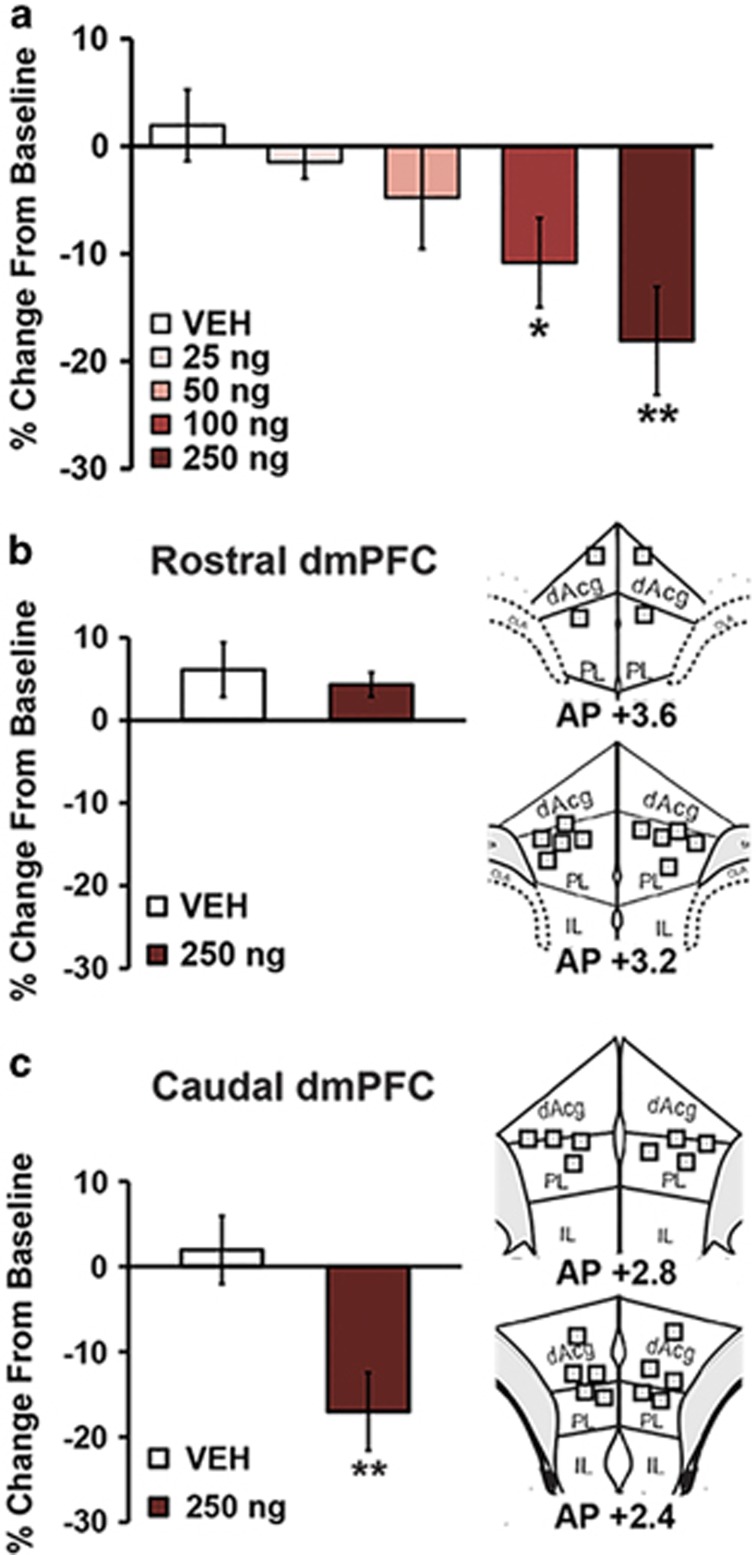
We then conducted a detailed dose–response analysis of the working memory effects of CRF when infused into the caudal dmPFC (AP +2.8 to +2.4; vehicle, n=10; 25 ng, n=6; 50 ng, n=8; 100 ng, n=15; 250 ng, n=8). CRF elicited a robust dose-dependent impairment in working memory performance, with significant impairment observed at the 100 and 250 ng doses (Figure 3a, Supplementary Table S1; F1,30.4=10.5, P=0.003). Given 250 ng CRF elicited a greater impairment in task performance than the 100 ng dose, we additionally examined the effects of 250 ng CRF infusions into the rostral dmPFC (n=7) to confirm insensitivity of this portion of the dmPFC to the working memory effects of CRF. In contrast to that seen in the caudal dmPFC, 250 ng CRF did not significantly affect performance when infused into the rostral dmPFC (Figure 3b vs Figure 3c; F1,16=0.19, P=0.7). As shown in Supplementary Table S1, intra-PFC infusions of CRF had no effect on run-times in the maze (rostral dmPFC: F1,31=0.11; P=0.74; caudal dmPFC: F1,16.2=0.0007; P=0.99).
Figure 3.
CRF acts in caudal dmPFC to elicit dose-dependent impairments in working memory. (a) Shown are the working memory effects of varying doses of vehicle (n=10) and CRF infused bilaterally into the caudal dmPFC (25 ng CRF, n=6; 50 ng, n=8; 100 ng, n=15; 250 ng, n=8). (b) Left panel: given 250 ng CRF elicited a more robust impairment than 100 ng CRF in the caudal dmPFC, the working memory effects of 250 ng CRF infusions into the rostral dmPFC were also examined (n=7). Within this region, this dose had no significant effects on working memory performance relative to vehicle (n=10). Right panel: schematic depiction of infusion sites shown to the left. (c) Left panel: effects of 250 ng CRF in the caudal dmPFC on performance. Right panel: schematics of infusion sites shown to the left. Results represent mean±SEM percentage change in correct trials (accuracy) relative to baseline performance. *P<0.05, **P<0.01 vs vehicle.
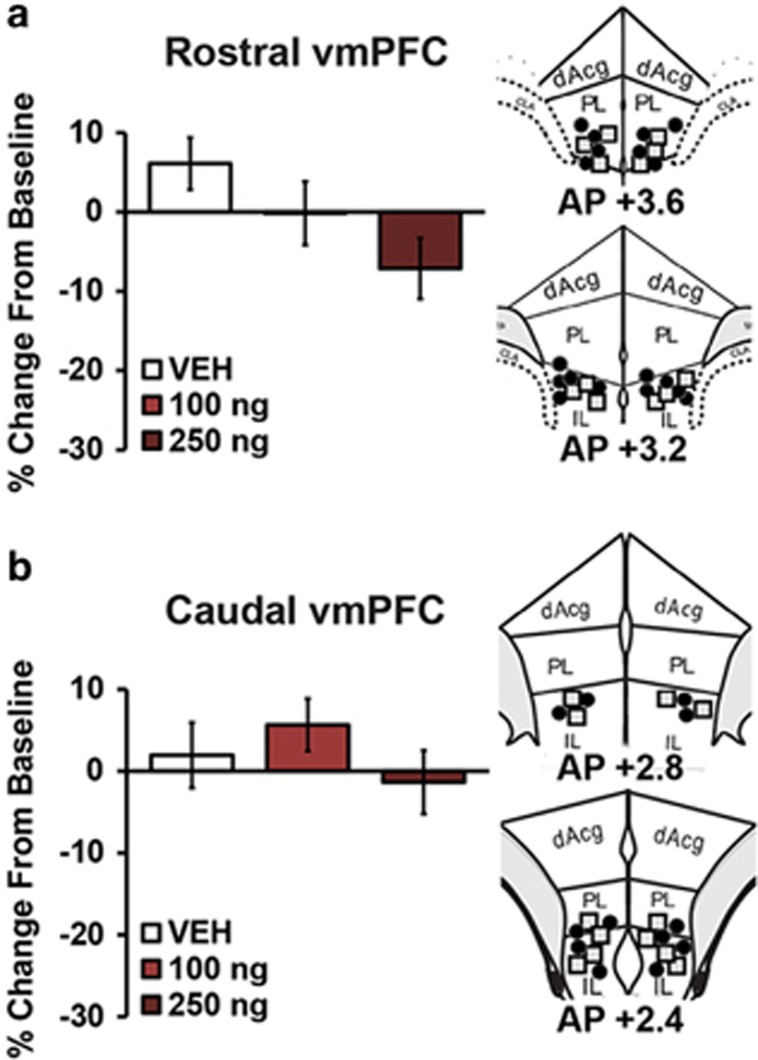
Although the dmPFC is most strongly implicated in higher cognitive function, both anatomical and functional studies argue for a role of the vmPFC in working memory/cognition (Heidbreder and Groenewegen, 2003). Thus, additional studies examined the working memory effects of intra-vmPFC infusions of CRF (100 ng, 250 ng). As shown in Figure 4 and Supplementary Table S1, CRF had no effect on working memory performance when infused into either the rostral vmPFC (vehicle, n=10; 100 ng, n=9; 250 ng, n=6; F1,16.3=3.7, P=0.08; Figure 4a) or caudal vmPFC (vehicle, n=10; 100 ng, n=6; 250 ng, n=6; F1,19.2=0.28; P=0.59; Figure 4b). Similarly, CRF infusions into both rostral and caudal subfields of the vmPFC had no effect on run-time (Supplementary Table S1; rostral vmPFC: F1,7.9=1.5; P=0.3; caudal vmPFC: F1,7.4=3.7; P=0.09).
Figure 4.
CRF does not act in the vmPFC to modulate working memory performance. (a) Left panel: working memory effects of bilateral vehicle (n=10) or 100 ng (n=9) or 250 ng (n=6) CRF infusions into the rostral vmPFC on task performance. Right: schematics of infusion sites: 100 ng CRF, black circles; 250 ng CRF, gray squares. (b) Left: effects of bilateral vehicle (n=10) or 100 ng (n=6) or 250 ng (n=6) CRF infusions into the caudal vmPFC on task performance. Right: schematics of infusion sites: 100 ng CRF, black circles; 250 ng CRF, gray squares. Results represent mean±SEM percentage change in correct trials (accuracy) relative to baseline performance.
Role of Endogenous CRF Actions within the PFC in Working Memory
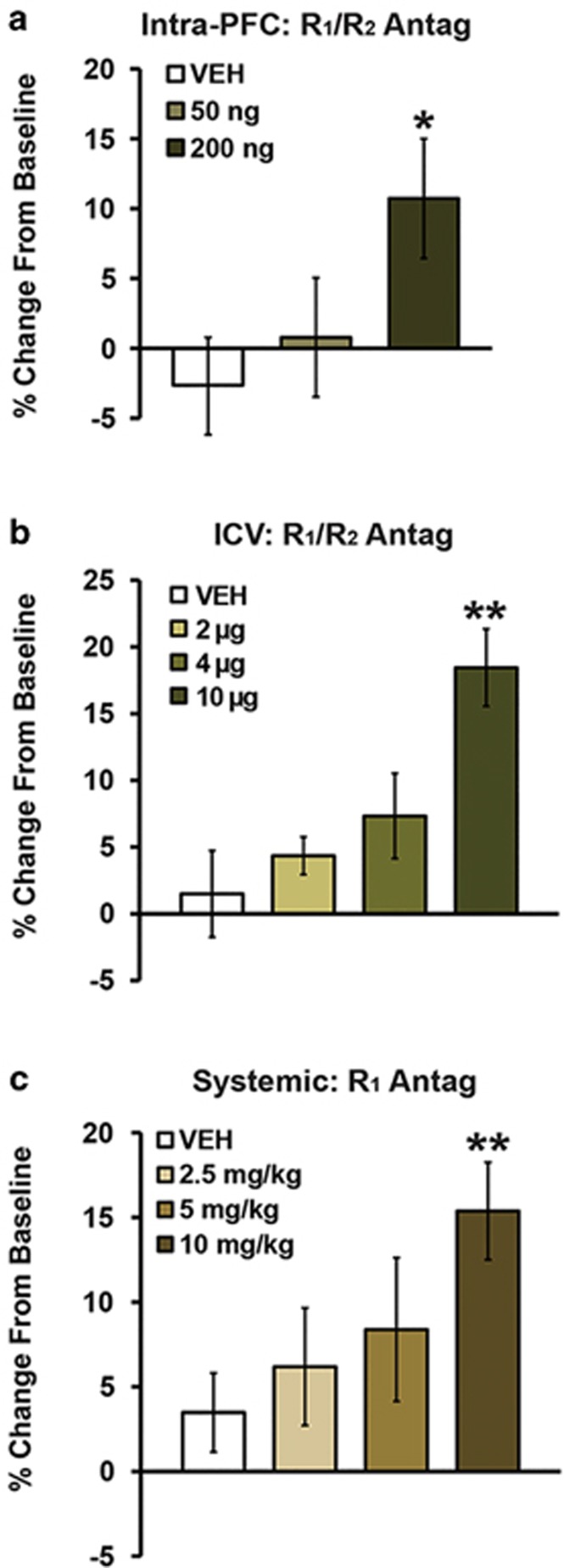
The above-described results indicate that CRF receptor activation, both globally and within the caudal dmPFC, impairs working memory. Additional studies examined the actions of endogenous CRF signaling in the PFC on working memory using bilateral intra-caudal dmPFC infusions of the nonselective CRF antagonist, D-Phe-CRF (vehicle, n=9; 50 ng, n=7; 200 ng, n=9). D-Phe-CRF elicited a dose-dependent improvement in working memory performance relative to vehicle (Figure 5a and Supplementary Table S1; F1,20.5=5.9, P=0.02) although lacking significant effects on run-time (F1,20.8=0.01, P=0.94; Supplementary Table S1).
Figure 5.
Cognition-enhancing effects of CRF receptor blockade. Shown are the working memory effects of (a) bilateral intra-caudal dmPFC infusions of the nonselective CRF1/2 receptor antagonist, D-Phe-CRF (vehicle, n=9; 50 ng, n=7; 200 ng, n=9) and (b) ICV infusion of D-Phe-CRF (vehicle, n=7; 2 μg, n=8; 4 μg, n=6; 10 μg, n=8) on working memory performance. For both routes of administration, this CRF antagonist dose-dependently improved working memory performance. (c) Systemic administration of the CRF1 receptor selective antagonist, NBI 35965, similarly improved working memory performance in a dose-dependent manner (vehicle, n=14; 2.5 mg/kg, n=6; 5 mg/kg, n=8; 10 mg/kg, n=7). Results represent mean±SEM percentage change in correct trials (accuracy) relative to baseline performance. *P<0.05, **P<0.01 vs vehicle.
The procognitive effects of intra-PFC CRF receptor blockade in this task are similar to that seen with all FDA-approved treatments for ADHD (Berridge and Arnsten, 2015). To better assess the potential clinical utility of CRF antagonists, we examined whether CRF antagonist distribution more globally in the brain improves working memory performance. Specifically, we tested the effects of vehicle (n=7) and varying doses of ICV-administered D-Phe-CRF (2 μg, n=8; 4 μg, n=6; 10 μg, n=8). As shown in Figure 5b and Supplementary Table S1, ICV infusion of this CRF antagonist elicited a dose-dependent improvement in working memory performance (F1,11.7=28.4, P<0.001) in the absence of significant changes in run-time (F1,10.8=2.3; P=0.2; Supplementary Table S1). At the highest dose tested, the magnitude of working memory improvement was comparable to that seen with systemic administration of clinically-relevant doses of the ADHD medication, methylphenidate (Ritalin; Berridge et al, 2006).
Finally, to assess the cognitive effects of selective CRF1 receptor antagonism, additional studies examined the working memory effects of systemic administration of the CRF1 receptor-selective antagonist, NBI 35965 (Million et al, 2003). As shown in Figure 5c and Supplementary Table S1, systemic treatment with this antagonist (2.5 mg/kg, n=6; 5 mg/kg, n=8; 10 mg/kg, n=7) also elicited a dose-dependent improvement in working memory performance relative to vehicle (n=14) that was comparable to that seen with methylphenidate (Berridge et al, 2012). Systemic administration of this antagonist had no effect on run-time (F1,24.9=0.05; P=0.8; Supplementary Table S1).
Discussion
These studies are the first to show that CRF acts in the PFC to impair higher cognitive function, as measured in a delayed response task of working memory. The cognitive effects of CRF receptor activation were topographically organized, such that working memory impairment was only observed following CRF infusions into the caudal dmPFC. The cognition-enhancing actions of CRF antagonists observed in these studies demonstrate a cognitive role of endogenous CRF and mimic that seen with all FDA-approved drugs for ADHD (Berridge et al, 2012; Berridge and Arnsten, 2015; Spencer et al, 2012). Combined, these observations demonstrate a prominent role of CRF in the PFC in the regulation of higher cognitive function and suggest CRF may represent a novel pharmacological target for the treatment of ADHD or other forms of PFC-dependent cognitive dysfunction.
The restriction of the working memory actions of CRF to the dmPFC is consistent with the well-documented dorsoventrally organized functional topography of the rodent medial PFC. Specifically, the dmPFC is more closely associated with higher cognitive function, whereas the vmPFC is more closely associated with affect and motivation (Gabbott et al, 2005; Heidbreder and Groenewegen, 2003; Voorn et al, 2004). However, our results also highlight a less-well-studied rostrocaudally organized topography. The restriction of the working memory effects of CRF to the caudal dmPFC is identical to that observed for noradrenergic modulation of sensorimotor gating (Alsene et al, 2011). In contrast, catecholamine-dependent modulation of working memory in rodents is more closely linked to the rostral dmPFC (Arnsten et al, 1999; Zahrt et al, 1997). The neural bases for the rostrocaudal topography observed in the current study are unclear. CRF receptors are distributed throughout the dorsoventral and rostrocaudal medial PFC (Van Pett et al, 2000). Thus, preferential sensitivity of the caudal dmPFC to CRF does not reflect a differential distribution of CRF receptors. Instead, this topography may reflect functional differences in PFC efferent connectivity within corticostriatal and corticothalamic circuits. For example, the rostral prelimbic PFC sends denser projections to the rostral ventral striatum and innervates more medial regions of the dorsal striatum relative to the caudal prelimbic PFC (Gorelova and Yang, 1996; Sesack et al, 1989). In addition, the caudal prelimbic PFC is more strongly connected to the caudal ventral striatum and sends denser projections to the mediodorsal thalamic nucleus relative to the rostral prelimbic PFC (Gorelova and Yang, 1996; Sesack et al, 1989).
Currently, the cellular mechanisms underlying the cognition-impairing actions of CRF receptor activation in the PFC are unknown. In the rodent, the PFC predominantly expresses CRF1 receptors, whereas in the primate, both CRF1 and CRF2 receptor subtypes are present in the PFC (Sanchez et al, 1999; Van Pett et al, 2000). Limited evidence indicates that both CRF1 and CRF2 receptors can activate protein kinase A and phosphatidylinositol-protein kinase C pathways (Miguel et al, 2014; Tan et al, 2004). Evidence demonstrates that both protein kinase A and phosphatidylinositol-protein kinase C signaling modulate working memory via regulation of PFC pyramidal neuron activity (Arnsten, 2009). Moreover, evidence indicates that at least in rodents, cortical pyramidal neurons express CRF1 receptors (Gallopin et al, 2006). Thus, CRF-dependent modulation of working memory may involve activation of both protein kinase A and phosphatidylinositol-protein kinase C cascades within PFC pyramidal neurons.
To date, the sources of PFC CRF have not been definitively identified. CRF-containing cell bodies are present in most layers of the rat PFC and at least some of these cell bodies are GABAergic interneurons (Helmeke et al, 2008; Mohila, 2004). However, whether interneurons comprise the entire population of CRF cell bodies within the PFC is unclear. In addition, CRF fibers within the PFC may arise from regions outside the PFC. For example, CRF neurons are found in regions known to project to the PFC, such as the bed nucleus of the stria terminalis (Hoover and Vertes, 2007; Swanson et al, 1983).
In the present study, distribution of CRF and CRF antagonists globally in the brain (eg, ICV, systemic) appeared to elicit larger cognitive effects relative to intra-PFC administration. This likely reflects the fact that CRF activates systems outside the PFC, many of which are known to modulate PFC function, including catecholamines (Dunn and Berridge, 1987; Valentino et al, 1983; Arnsten et al, 1999; Lapiz and Morilak, 2006) and glucocorticoids (Barsegyan et al, 2010; Roozendaal, 2004).
CRF has a pivotal role in regulating behavioral responding during stress (Bale and Vale, 2004; Dunn and Berridge, 1990). However, the cognition-enhancing actions of CRF antagonists we observed unlikely reflect solely an antistress effect. First, our animals were highly habituated to testing over many weeks. In addition, our subjects displayed relatively high performance accuracy, whereas stress is well-documented to impair working memory performance (Arnsten, 2009; Devilbiss et al, 2012). Moreover, evidence from humans and rodents demonstrates that stress impairs behavioral and PFC neuronal responses to rewards (Bogdan and Pizzagalli, 2006; Ossewaarde et al, 2011), whereas doses of CRF used in these studies had no effect on motivation to obtain sugar reward and perform the task. The fact that, in general, run-time was not altered indicates that CRF-induced performance impairment did not reflect the presence of a competing behavior, including stress-related behavior (eg, grooming and motor activation). Collectively, these observations suggest that CRF signaling in the PFC modulates higher cognitive function under nonstressful conditions associated with alert and high motivation goal-directed behavior. Thus, at least in the PFC, CRF may modulate arousal-dependent cognitive processes that include, but may not be limited to, stress. Whether PFC CRF contributes to stress-related cognitive impairment is an important question of future studies.
Finally, evidence indicates that CRF exerts differential actions in males vs females, with females displaying greater sensitivity to certain behavioral and cellular actions of CRF during stress (Valentino et al, 2012). The current study used male animals to (1) better compare the actions of CRF with the known modulatory actions of PFC catecholamines on PFC-dependent working memory and (2) to better align with a large literature documenting cognition-enhancing actions of ADHD medications. In recent studies, ICV CRF elicited larger impairments in some, but not all, aspects of sustained attention performance in females relative to males (Cole et al, 2016). Collectively, the available evidence suggests that CRF signaling in the PFC likely impairs working memory in females to a similar or greater degree than males, though this is an important topic for future studies.
Summary
These studies demonstrate that CRF exerts topographically organized cognitive effects within the rat medial PFC, with receptor activation impairing and receptor blockade improving working memory. Cognition-enhancing actions were similarly observed when CRF antagonists were administered ICV and systemically. The ability of CRF antagonists to improve working memory performance mimics that seen with all approved drugs for the treatment of ADHD. Thus, CRF antagonists may be a useful tool for treating PFC-dependent cognitive dysfunction associated with various disorders, including ADHD.
Funding and disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Institutes of Health grants MH081843, MH102211, MH107140, and GM001507.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Alsene KM, Rajbhandari AK, Ramaker MJ, Bakshi VP (2011). Discrete forebrain neuronal networks supporting noradrenergic regulation of sensorimotor gating. Neuropsychopharmacology 36: 1003–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10: 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Mathew R, Ubriani R, Taylor JR, Li BM (1999). α-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry 45: 26–31. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Pliszka SR (2011). Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav 99: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW (2004). CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44: 525–557. [DOI] [PubMed] [Google Scholar]
- Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B (2010). Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci USA 107: 16655–16660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Arnsten AF (2015). Catecholamine mechanisms in the prefrontal cortex: proven strategies for enhancing higher cognitive function. Curr Opin Behav Sci 4: 33–40. [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B et al (2006). Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60: 1111–1120. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Shumsky JS, Andrzejewski ME, McGaughy JA, Spencer RC, Devilbiss DM et al (2012). Differential sensitivity to psychostimulants across prefrontal cognitive tasks: differential involvement of noradrenergic α1- and α2-receptors. Biol Psychiatry 71: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA (2006). Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry 60: 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RD, Kawasumi Y, Parikh V, Bangasser DA (2016). Corticotropin releasing factor impairs sustained attention in male and female rats. Behav Brain Res 296: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ (1985). Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci 5: 3189–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Jenison RL, Berridge CW (2012). Stress-induced impairment of a working memory task: role of spiking rate and spiking history predicted discharge. PLoS Comput Biol 8: e1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW (1987). Corticotropin-releasing factor administration elicits a stress-like activation of cerebral catecholaminergic systems. Pharmacol Biochem Behav 27: 685–691. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW (1990). Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev 15: 71–100. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ (2005). Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492: 145–177. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Geoffroy H, Rossier J, Lambolez B (2006). Cortical sources of CRF, NKB, and CCK and their effects on pyramidal cells in the neocortex. Cereb Cortex 16: 1440–1452. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Yang CR (1996). The course of neural projection from the prefrontal cortex to the nucleus accumbens in the rat. Neuroscience 76: 689–706. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ (2003). The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27: 555–579. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Ovtscharoff W, Poeggel G, Braun K (2008). Imbalance of immunohistochemically characterized interneuron populations in the adolescent and adult rodent medial prefrontal cortex after repeated exposure to neonatal separation stress. Neuroscience 152: 18–28. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP (2007). Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212: 149–179. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S (2007). Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic–pituitary–adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Res 1186: 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz MD, Morilak DA (2006). Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137: 1039–1049. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB et al (1995). Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA 92: 836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel TT, Gomes KS, Nunes-de-Souza RL (2014). Tonic modulation of anxiety-like behavior by corticotropin-releasing factor (CRF) type 1 receptor (CRF1) within the medial prefrontal cortex (mPFC) in male mice: Role of protein kinase A (PKA). Horm Behav 66: 247–256. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS et al (2012). Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11: 141–168. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001). An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Million M, Grigoriadis DE, Sullivan S, Crowe PD, McRoberts JA, Zhou H et al (2003). A novel water-soluble selective CRF1 receptor antagonist, NBI 35965, blunts stress-induced visceral hyperalgesia and colonic motor function in rats. Brain Res 985: 32–42. [DOI] [PubMed] [Google Scholar]
- Mohila CA (2004). Increases in the density of parvalbumin-immunoreactive neurons in anterior cingulate cortex of amphetamine-withdrawn rats: evidence for corticotropin-releasing factor in sustained elevation. Cereb Cortex 15: 262–274. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M (1988). Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry 45: 577–579. [DOI] [PubMed] [Google Scholar]
- Ossewaarde L, Qin S, Van Marle HJ, van Wingen GA, Fernández G, Hermans EJ (2011). Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage 55: 345–352. [DOI] [PubMed] [Google Scholar]
- Roozendaal B (2004). The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci 24: 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR (1999). Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol 408: 365–377. [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS (1989). Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290: 213–242. [DOI] [PubMed] [Google Scholar]
- Setlik J, Bond GR, Ho M (2009). Adolescent prescription ADHD medication abuse is rising along with prescriptions for these medications. Pediatrics 124: 875–880. [DOI] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ (2011). Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology 37: 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RC, Klein RM, Berridge CW (2012). Psychostimulants act within the prefrontal cortex to improve cognitive function. Biol Psychiatry 72: 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983). Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36: 165–186. [DOI] [PubMed] [Google Scholar]
- Tan H, Zhong P, Yan Z (2004). Corticotropin-releasing factor and acute stress prolongs serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons. J Neurosci 24: 5000–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G (1983). Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res 270: 363–367. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D (2012). Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology 62: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C et al (2000). Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM (2004). Putting a spin on the dorsal–ventral divide of the striatum. Trends Neurosci 27: 468–474. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF (1997). Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17: 8528–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.