ABSTRACT
Fungal infections are a common cause of morbidity, mortality and cost in critical care populations. The increasing emergence of antimicrobial resistance necessitates the development of new therapeutic approaches for fungal infections. In the present study, we investigated the effectiveness of an innovative approach, antimicrobial blue light (aBL), for inactivation of Candida albicans in vitro and in infected mouse burns. A bioluminescent strain of C. albicans was used. The susceptibilities to aBL (415 nm) were compared between C. albicans and human keratinocytes. The potential development of aBL resistance by C. albicans was investigated via 10 serial passages of C. albicans on aBL exposure. For the animal study, a mouse model of thermal burn infected with the bioluminescent C. albicans strain was used. aBL was delivered to mouse burns approximately 12 h after fungal inoculation. Bioluminescence imaging was performed to monitor in real time the extent of infection in mice. The results obtained from the studies demonstrated that C. albicans was approximately 42-fold more susceptible to aBL than human keratinocytes. Serial passaging of C. albicans on aBL exposure implied a tendency of reduced aBL susceptibility of C. albicans with increasing numbers of passages; however, no statistically significant difference was observed in the post-aBL survival rate of C. albicans between the first and the last passage (P>0.05). A single exposure of 432 J/cm2 aBL reduced the fungal burden in infected mouse burns by 1.75-log10 (P=0.015). Taken together, our findings suggest aBL is a potential therapeutic for C. albicans infections.
KEYWORDS: antimicrobial blue light, bioluminescence imagining, burn, candida albicans, endogenous photosensitizer, mouse model
Introduction
Fungal infections, including fungal burn or wound infections, are a common cause of morbidity, mortality and cost in critical care populations.1-3 It is estimated that fungal infections occur in over a billion people each year, and recent evidence suggests the rate is increasing.4 Candida spp. are the most common causative species of fungal infections.5,6 Among Candida spp, Candida albicans is the major global pathogen, causing 50%–70% of candidiasis cases.7 The increasing emergence of antimicrobial drug resistance has significantly compromised the management of fungal infections.8-11 Recently, resistance to azole drugs, the most used class of antifungals, has been identified in C. albicans.12-14 As a result, there is a pressing need for the development of new approaches to tackle drug resistance in fungal infections.8
As a non-antibiotic approach, light-based antimicrobial therapies, including antimicrobial photodynamic therapy (aPDT) and ultraviolet-C (UVC) irradiation, have been extensively investigated as alternative therapeutics for infectious diseases, especially for localized infections.15,16 Advantages of light-based therapies include rapid action and equal inactivation effectiveness regardless of drug resistance.17-19 However, one major disadvantage of aPDT, as a two-part (photosensitizer + light) combination approach, is the challenge of introducing exogenous photosensitizers into certain pathogens and less than perfect selectivity for pathogens over host cells.20 The use of UVC, on the other hand, has limitations due to its detrimental effects on host cells.16
An innovative light-based antimicrobial approach, antimicrobial blue light (aBL), has attracted increasing attention due to its intrinsic ability to inactivate pathogens without the involvement of exogenous photosensitizers.21-23 The mechanism of action of aBL is still not fully understood. A common hypothesis is that aBL excites naturally occurring endogenous photosensitizers in the cells of pathogens and subsequently leads to the production of cytotoxic oxidative species.21,24 However, the use of aBL for treatment of actual infections has yet to become established.21,22 The majority of the publications on aBL have been confined to in vitro efficacy studies.25-38 There have been only three published reports to demonstrate the efficacy of aBL for infections in vivo.39-41 It has been demonstrated that aBL (415-nm) significantly reduced the bacterial burden (both Gram-positive and Gram-negative) in mouse wounds or burns,39-41 and was protective in a lethal mouse model of P. aeruginosa infection.40
In the present study, we investigated for the first time the use of aBL for C. albicans infections in mouse burns. Burns provide an ideal portal for invasive infections while also inducing substantial immune dysfunction; as a result, burn patients are cited as being among the highest risk groups for invasive fungal infections.3 The results obtained from the present study demonstrated that that C. albicans was much more susceptible to aBL than human keratinocytes. Serial passaging of C. albicans on aBL exposure implied a tendency of reduced aBL susceptibility of C. albicans with increasing numbers of passages; however, no statistically significant difference was observed in the post-aBL survival rate of C. albicans between the first and the last passage. A single exposure of aBL (432 J/cm2) significantly reduced the fungal burden in infected mouse burns.
Results
Susceptibility to aBL: C albicans vs. human keratinocytes
To ensure a selective aBL inactivation of fungal cells over host cells, in vitro study was conducted to compare aBL susceptibilities between C. albicans and human keratinocytes. The results of keratinocytes were obtained from our previous study,41 in which human keratinocytes (HaCaT) were exposed to aBL under the equivalent condition to that for aBL inactivation of C. albicans in the present study.
The aBL inactivation curves of both C. albicans and keratinocytes approximately followed the first-order kinetics, showing a linear relation between the log-transformed cell survival fraction log10 and aBL radiant exposure (Fig. 1).42 It can be seen that C. albicans cells are much more susceptible to aBL than keratinocytes. When an exposure of 70.2 J/cm2 light had been delivered, 5.42-log10 colony forming units (CFU) of C. albicans were inactivated on average, while only 0.11-log10 viability loss in keratinocytes was observed under the equivalent condition. The mean inactivation rate coefficients (kH) 43 of C. albicans and keratinocytes, which were estimated from the slopes of the inactivation curves, were 0.0795 and 0.0019 cm2/J, respectively, indicating an approximately 42-fold faster inactivation rate of C. albicans by aBL than keratinocytes.
Figure 1.
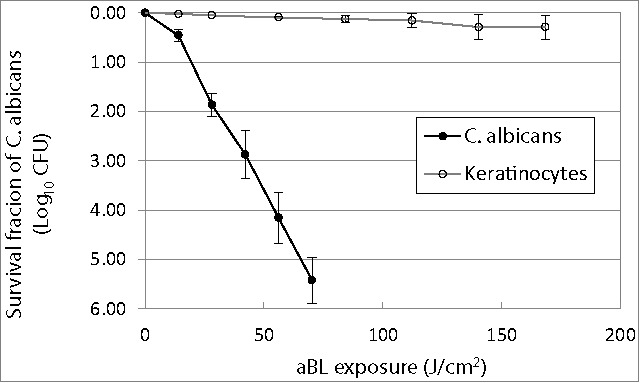
Antimicrobial blue light inactivation of C. albicans and human keratinocytes in vitro. Bars: standard deviation. The mean inactivation rate coefficients (kH) of C. albicans and keratinocytes were 0.0795 and 0.0019 cm2/J, respectively, indicating an approximately 42-fold faster inactivation rate of C. albicans by aBL than keratinocytes.
Transmission electron microscopy (TEM) imaging of C. albicans cells
Under TEM, untreated C. albicans cells showed typical morphology of C. albicans containing a nucleus, vacuoles and mitochondria, surrounded by cytoplasmic membrane and cell wall (Fig. 2A). When an exposure of 35.1 J/cm2 aBL had been delivered, disruption of inner organelles in some C. albicans cells with a deformed cell wall exhibited (Fig. 2B). In some cases, unusual vacuole morphology was observed, driving electron-dense structures to the cell periphery (Fig. 2C). When an exposure of 70.2 J/cm2 light had been delivered, complete decomposition of inner organelles with disrupted cell walls and almost complete loss of cytoplasmic components were seen in some C. albicans cells (Fig. 2D).
Figure 2.
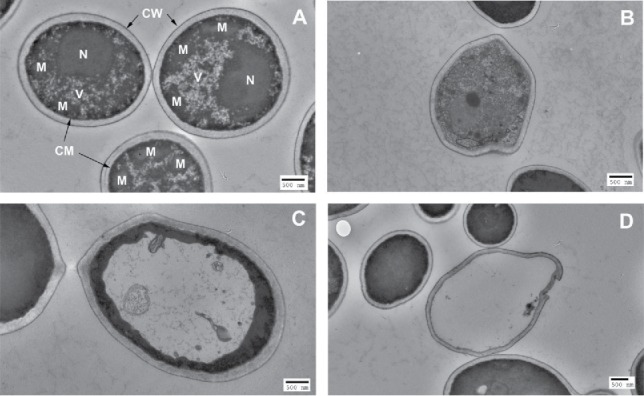
TEM images of C. albicans cells. (A) Untreated C. albicans cells: N=nucleus; V = vacuole; M=mitochondria; CM = cytoplasmic membrane; CW = cell wall. (B)-(D) aBL-treated C. albicans cells: (B) Decomposition of inner organelles with deformed cell wall (aBL radiant exposure = 35.1 J/cm2); (C) Unusual vacuole growth, driving electron-dense structures to the cell periphery (aBL radiant exposure = 35.1 J/cm2); and (D) Complete loss of cytoplasmic components with disrupted cell wall (aBL radiant exposure = 70.2 J/cm2).
Fluorescence spectroscopic measurements
We investigated the characteristic fluorescence emission spectra of C. albicans upon excitation at defined absorption peaks by using C. albicans cell lysates. Excitation at 405-nm, which is the absorption peak of porphyrin-like compounds, led to porphyrin-like dual fluorescence emission maxima at 631 and 667 nm (Fig. 3A); 44 while excitation at 470 nm, which is the absorption peak of flavin-like compounds, gave rise to a flavin-like emission maximum at 515 nm (Fig. 3B).45
Figure 3.
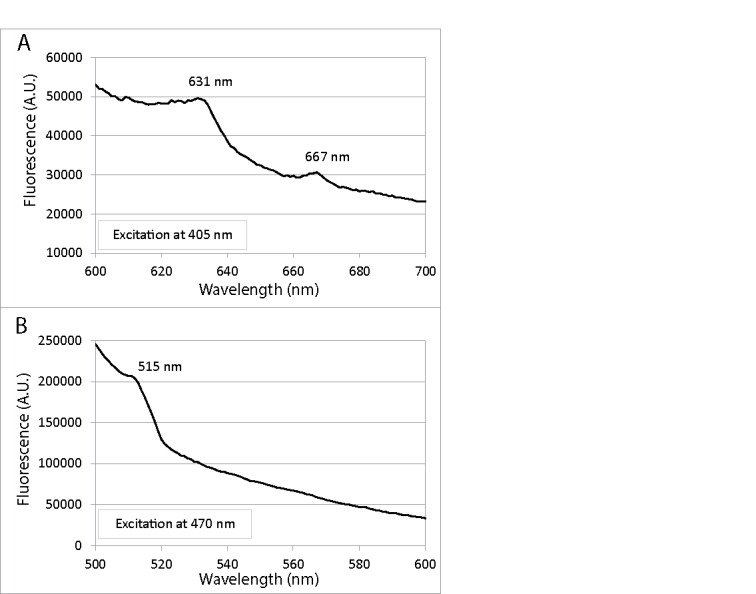
Fluorescence emission spectra of lysed C. albicans cells in NaOH/SDS: (A) Excitation at 405 nm led to porphyrin-like dual fluorescence emission maxima at 631 and 667 nm; (B) Excitation at 470 nm gave rise to a flavin-like emission maximum at 515 nm.
Serial passaging for C. albicans on aBL exposure
C. albicans suspensions were serially passaged 10 times on aBL exposure, and Figure 4 shows the changes in the extent of aBL inactivation of C. albicans with increasing numbers of passages. A correlation analysis indicated a tendency of decrease in aBL susceptibility with the numbers of passages (correlation coefficient = −0.64; P=0.045). However, statistical analysis revealed no significant difference in aBL inactivation extent between the 1st and 10th passage (P=0.09).
Figure 4.
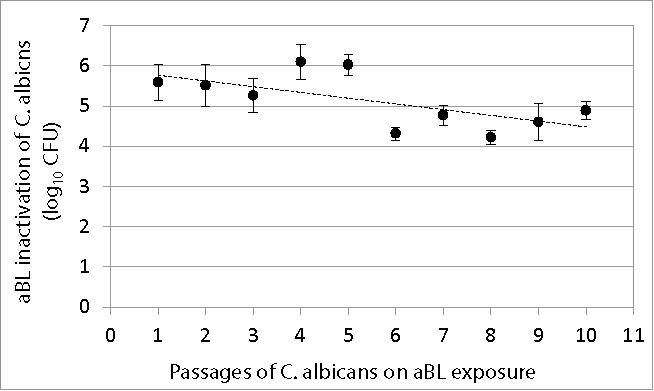
Change of extent of aBL inactivation of C. albicans (log10 CFU) with numbers of passages of C. albicans on aBL exposure. Bars: standard deviation. No statistically significant difference was observed in aBL inactivation extent between the 1st and 10th passage (P = 0.09).
aBL therapy for C. albicans infection in mouse burns
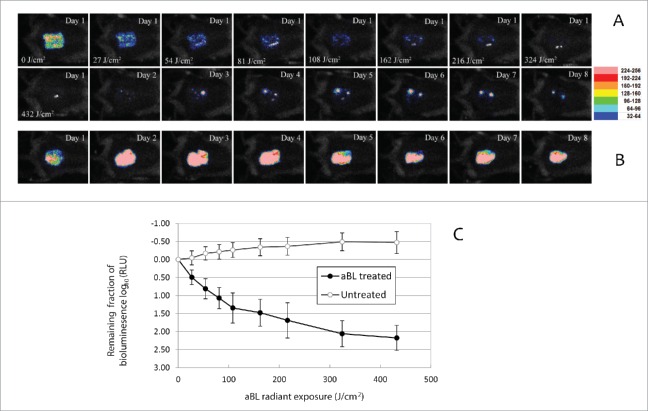
Our results indicated that aBL significantly reduced fungal burden in infected mouse burns. Figure 5A shows a set of bioluminescence images of a representative mouse burn infected with 105 CFU of C. albicans and treated with a single exposure of 432 J/cm2 aBL. aBL was delivered at 12 h after fungal inoculation. Immediately after aBL exposure, only a few pixels of remaining fungal luminescence were observed. Although recurrence of fungal luminescence (indicating infection regrowth) was observed during the following days in the aBL-treated mouse burn, the extent of infection (or fungal load) in the untreated mouse burn was always more than 1-log10 unit (10 fold) higher than that of the aBL-treated mouse burn during the whole period of experiment (at all the time points sampled). It can also be seen from Figure 5B that, in the untreated mouse burn, infection steadily developed with time and remained strong until 8 days after fungal inoculation.(Fig. 5B)
Figure 5.
(A-B) Successive fungal luminescence images of representative mouse burns infected with 105 CFU of bioluminescent C. albicans, with (panel A) and without aBL therapy (panel B), respectively. aBL irradiance = 90 mW/cm2. aBL was delivered at 12 h after fungal inoculation. In panel A, the Day 1-0 J/cm2 image was taken just prior to aBL exposure; Day 1-27 J/cm2, Day 1- 54 J/cm2, Day 1-81 J/cm2, Day 1-108 J/cm2, Day 1-162 J/cm2, Day 1-216 J/cm2, Day 1-324 J/cm2 and Day 1- 432 J/cm2 images were taken immediately after respective aBL exposure had been delivered; and the Day 1 to Day 8 images were taken at Day 1 to Day 8 after fungal inoculation, respectively. In panel B, the Day 1 to Day 8 images were taken at Day 1 to Day 8 after fungal inoculation, respectively. (C) Dose responses of mean fungal luminescence of mouse burns infected with 105 CFU of C. albicans, with (n = 8) and without (n = 8) aBL therapy, respectively. aBL was delivered at 12 h after fungal inoculation. Bars: standard deviation.
Quantitative analysis of the results supported the above findings from bioluminescence images. Figure 5C shows the dose-response curves of mean fungal luminescence (relative light units: RLU) from a group of mouse burns (n = 8) infected with 105 CFU C. albicans and treated with aBL at 12 h after fungal inoculation. Approximately 2.18-log10 (99.3%) inactivation of C. albicans in mouse burns was achieved after an exposure of 432 J/cm2 aBL had been delivered. The fungal luminescence of the mouse burns without being exposed to aBL (n = 8) increased by 0.39-log10 during the equivalent period of time (P<0.0001).
Figure 6A shows the time courses of the mean fungal luminescence (RLU) from day 2 (the next day after aBL therapy) to day 15 of mouse burns infected with 105 CFU of C. albicans followed by treatment with a single exposure of 432 J/cm2 aBL delivered at 12 h post-inoculation (n = 8) as well as infected mouse burns without aBL (n = 8). In the untreated mice, fungal luminescence remained strong until day 12. In both the aBL-treated and untreated mice, burn scabs started to peel off from the backs of mice as of approximately day 12. Very modest fungal luminescence remained in the burns after the scabs peeled off. Statistical analysis of the areas under the curves (AUC) of bioluminescence time course in the two-dimensional coordinate system in Figure 6A demonstrated that aBL significantly decreased fungal burden of the infected burns (Fig. 6B). The mean values of AUC of aBL-treated and untreated mice were 1.67×106 and 9.40×107 (P = 0.00005), respectively, indicating a 1.75-log10 (98.2%) reduction of fungal burden in mouse burns as a result of aBL therapy.
Figure 6.
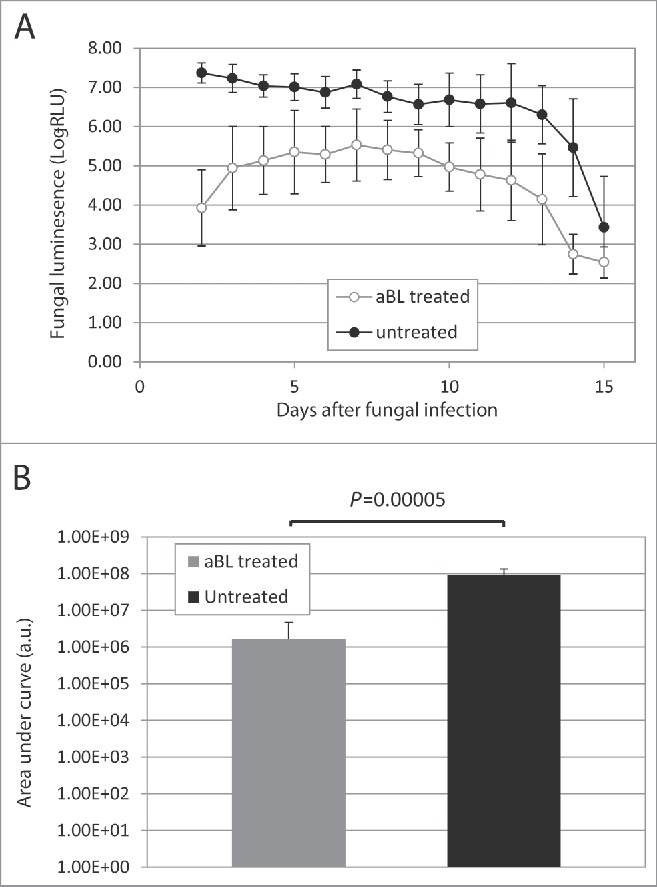
(A) Time courses of mean fungal luminescence (RLU, day 2 to day 11) of mouse burns infected with 105 CFU of C. albicans, with (n=8) and without (n=8) aBL therapy, respectively. aBL was delivered at 12 h after fungal inoculation. Bars: standard deviation. (B) Mean areas under the fungal luminescence versus time curves in the two-dimensional coordinate system in panel A, representing the overall fungal burden of infected mouse burns. Bars: standard deviation.
Discussion
Candida, a fungus that commonly causes serious illness, especially among hospital patients, is showing increasing resistance to the drugs used for treatment.8-11 This fact underlines the need for new treatments against Candida infections.8 In the present study, we investigated for the first time an innovative light-based approach, aBL, for treatment of C. albicans infections in mouse models. The study may serve as an initial effort in the pursuit of utilizing aBL therapy for fungal infections, especially those caused by drug-resistant strains. Thus, the first and most important impact of the study is the opening of an emerging area of study using a different therapeutic regimen for fungal infections. The results of the present study demonstrated that C. albicans was much more susceptible to aBL than human keratinocytes. Serial passaging of C. albicans on aBL exposure implied a tendency of reduced aBL susceptibility of C. albicans with increasing numbers of passages; however, no statistically significant difference was observed in the post-aBL survival rate of C. albicans between the first and the last passage. A single exposure of aBL (432 J/cm2) significantly reduced the fungal burden in infected mouse burns.
The results of the in vitro susceptibility study indicate that there exists a therapeutic window where C. albicans cells are selectively inactivated by aBL while host cells can be preserved. However, only one C. albicans strain and human keratinocytes were investigated in the present study. To confirm this finding, more C. albicans strains as well as other fungal species should be tested in the future to compare their aBL susceptibilities with those of host cells other than keratinocytes (i.e., fibroblasts, myoblasts, endothelial cells, etc.).
Fluorescence spectroscopic measurements suggested that C. albicans contains both endogenous porphyrins and flavins. In this study, aBL at 415 nm was delivered for inactivation of C. albicans both in vitro and in vivo. As 415-nm wavelength is close to the absorption peak of porphyrins (405 nm), it supports the hypothesis that the inactivation of C. albicans was due to the photo-excitation of endogenous porphyrins. On the other hand, the presence of flavins in C. albicans implies that C. albicans may also be susceptible to aBL at 470 nm. This is supported by a previous study showing aBL at 470 nm is fungicidal without the involvement of exogenous photosensitizers.46 Taken together, fluorescence spectroscopic measurements supported the hypothesis that endogenous porphyrins and/or flavins are associated with aBL inactivation of C. albicans. To further relate the action of aBL toxicity and porphyrins/flavins presence, future studies are needed to include the results from C. albicans strains deprived of the production of porphyrins and/or flavins. In addition, for better characterization of these endogenous photosensitizing substances, high performance liquid chromatography (HPLC) measurements should be performed in future studies.
To use aBL for inactivation of pathogens, one question that will have to be addressed is “Can pathogens develop aBL resistance?” An interesting finding in a previous study of our laboratory showed that a multidrug-resistant A. baumannii strain failed to develop aBL resistance after 10 serial passages on aBL exposure.41 On the contrary, there was a tendency of increased aBL susceptibility of A. baumannii with increasing numbers of passages.41 In the present study, we investigated the potential development of aBL resistance by C. albicans also by carrying out 10 serial passages of C. albicans on aBL exposure. Correlation analysis indicated a tendency for aBL susceptibility of C. albicans to decrease with increasing numbers of passages. However, statistical analysis did not show a significant difference in aBL inactivation extent of C. albicans between the 1st passage and the last (10th) passage. Further study (e.g., increase the number of passages of C. albicans on aBL exposure, i.e., up to 80 passages47) are imperative to investigate the aBL resistance development and, if aBL resistance does develop, to elucidate the molecular mechanism of reduced aBL susceptibility of C. albicans after repeated aBL inactivation.
The in vivo study demonstrated that a single exposure of aBL at 432 J/cm2 significantly reduced fungal burden in mouse burns in comparison to the untreated burns. Modest to mild recurrence of fungal luminescence (infection) was observed in some aBL-treated mouse burns. Future study will be carried out to counteract the recurrence of infection by, e.g., repeated aBL therapy.
A pitfall of the present study is that the effect of melanization by C. albicans during the infection was not investigated. C. albicans and many other fungi can produce melanin during infection. Melanin absorbs light and, subsequently, protects the fungi from being inactivated by aBL. In the present study, C. albicans cells were not grown in melanin inducing conditions. Future study is warranted to test the effects of melanization on aBL efficacy.
Another limitation of the present study lies in the fact that the mouse infection model used only represents the scenario of superficial infections. For deeply seated infections, topical application of aBL may not be able to reach the sites of infection. We are now investigating interstitial light delivery to treat deeply seated infections by using an optical needle array.48
Taken together, one can postulate that aBL is a potential alternative therapeutics for fungal infections. Future work is still needed to optimize this approach and introduce it to clinic.
Material and methods
Blue light source
In the cell culture study, aBL was delivered using an Omnilux clear-U™ light emitting diode (LED) array (Photo Therapeutics, Inc., Carlsbad, CA) with a central wavelength of 415-nm and a full-width half maximum of 20 nm. In the animal study, to avoid prolonged light exposure time, we used a more powerful prototype LED (Vielight Inc., Toronto, Canada) with the peak emission at 415 nm and the full-width half maximum of 10 nm. Irradiance of aBL was measured using a PM100D power/energy meter (Thorlabs, Inc., Newton, NJ).
Bioluminescent C. albicans strain and culture condition
The bioluminescent C. albicans strain used in the study was CEC 749, as described previously.49 In brief, the luciferase reporter was constructed by fusing a synthetic, codon-optimized version of the Gaussia princeps luciferase gene to C. albicans PGA59, which encodes a glycosylphosphatidylinositol-linked cell wall protein. Luciferase expressed from this PGA59-gLUC fusion was localized at the C. albicans cell surface,49 allowing the detection of luciferase in intact cells after the addition of the luciferase substrate, coelenterazine.
C. albicans was routinely grown at 30°C on yeast peptone dextrose (YPD) agar and sub-cultured in YPD medium to an optical density of 0.65 at 570 nm, which corresponds to 107 CFU/mL. The suspension was then centrifuged, washed with phosphate-buffered saline (PBS), and re-suspended in PBS at the cell density of 107 CFU/mL again.
aBL inactivation of C. albicans in vitro
Three (3) mL of C. albicans suspension in PBS at a cell density of ≈107 CFU/mL was placed into a 35-mm petri dish at room temperature (21°C). The suspension was exposed to aBL at an irradiance of 19.5 mW/cm2 (the maximum irradiance of this light source) with the lid of the Petri dish removed. During light exposure, the C. albicans suspension was gently stirred by a mini-magnetic bar (Fisher Scientific Co., Norcross, GA) at 20 rpm. Thirty (30) μL aliquots of the suspension were withdrawn at 0, 12, 24, 36, 48, and 60 min, respectively, when 0, 14.0, 28.1, 42.1, 56.2, and 70.2 J/cm2 light had been delivered. CFU were then determined by serial dilutions on YPD agar plates.50 Colonies were allowed to grow for 18-24 h at 30°C. The experiments were performed in triplicate.
Transmission electron microscopy (TEM)
aBL-treated or untreated C. albicans cells were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde mixture immediately after aBL exposure and stored for 24 h at 4°C. After centrifuge (1200 rpm, 10 min) and decanting the fixative, 0.1 M sodium cacodylate buffer (pH 7.2) was added to the pellets. After fixation, hot agar (2% in distilled water, heated to boiling) was immediately added to each pellet. Once the agar had hardly solidified, the cell pellets were then processed routinely for transmission electron microscope. The cell pellets were postfixed in 2% OsO4 in sodium cacodylate buffer, dehydrated in a graded alcohol series, and embedded in Epon t812 (Tousimis, Rockville, MD). Ultrathin sections (80-100 nm thick) were cut on a Reichert-Jung Ultracut E microtome (Vienna, Austria), collected on uncoated 200 mesh copper grids, stained with uranyl acetate (2 min) and lead citrate (2 min), and examined on a Philips CM-10 transmission electron microscope (Eindhoven, The Netherlands). The negatives were scanned on an Epson Perfection 3200 photo-scanner. Multiple parasite sections were microscopically analyzed and images representing the most typically observed morphologies were presented in the study.
Cell lysis of C. albicans and fluorescence spectroscopic measurement
To identify the presence and types of endogenous photosensitizers, C. albicans cells were lysed to investigate their fluorescence-emission characteristics.32 Briefly, an overnight C. albicans culture (3 mL) was centrifuged, washed with PBS, centrifuged again, and the supernatant removed. The C. albicans cells were then lysed by re-suspending the C. albicans pellets in 1 mL of a mixture of 0.1 M NaOH/1% sodium dodecyl sulfate (SDS), and the suspension was allowed to stand in the dark for 24 h. Fluorescence of the C. albicans lysates in NaOH/SDS (in a cuvette 1 cm thick) was measured on a fluorimeter (Fluoromax 3, SPEX Industries, Edison, NJ), with excitation at 405 or 470 nm and emission scanned from 580 to 700 nm.
Serial passaging of C. albicans on aBL exposure
To investigate the potential development of aBL resistance by C. albicans, C. albicans suspension was subjected to 10 serial passages on aBL exposure. In each passage, three independent cultures were tested. For each culture, 3 mL C. albicans suspension containing 107 CFU/mL in PBS was placed into a 35-mm petri dish. The suspension was then exposed to aBL at the irradiance of 19.5 mW/cm2. During aBL exposure, the C. albicans suspension was gently stirred with a miniature magnetic bar (20 rpm). In the 1st passage, the radiant exposure of aBL was adjusted to leave about 0.0004% fungal cells surviving (5.42-log10 inactivation) after aBL, and the same radiant exposure level of aBL was then used throughout the successive passages. Fungal CFU was determined by serial dilutions on YPD agar plates.50 The surviving fungal cells after aBL exposure were re-cultured for the next passage. This procedure was repeated until the 10th passage was reached. Fungal survival rates in different passages were compared using an one-way ANOVA.
Animals
Adult female BALB/c mice (Charles River Laboratories, Wilmington, MA), 6–8 week old and weighing 17–21 g, were used. Mice were given access to food and water ad libitum, and maintained on a 12-hour light/dark cycle under a room temperature of 21 C. All animal procedures in this study were approved by the Subcommittee on Research Animal Care (IACUC) of Massachusetts General Hospital and were in accordance with the guidelines of the National Institutes of Health (NIH).
C albicans infection in acute mouse burns
Before the infliction of burns, mice were anesthetized by intraperitoneal (I.P.) injection of a ketamine-xylazine cocktail and then shaved on the dorsal surfaces. Burns were inflicted by applying a preheated (≈95°C) brass block (Small Parts, Inc., Miami, FL) to the dorsal surfaces of mice for 3 s, resulting in nonlethal and third-degree thermal injuries. The brass block area was 12 mm × 12 mm, corresponding to 3.6% of the total body surface area 51 of mice. Immediately after burn injuries, mice were resuscitated with I.P. injections of 0.5 mL sterile saline to prevent dehydration.
Five (5) min after the infliction of burns (to allow the burns to cool down), a 50-μL aliquot of C. albicans in sterile PBS containing 105 cells was inoculated to the eschar of each mouse burn with a pipette tip and was then smeared onto the eschar with an inoculating loop. Mice were imaged with a bioluminescence camera immediately after the inoculation of C. albicans to ensure that the fungal inoculum applied to each mouse burn was consistent. Our previous studies showed that both conidia and hyphae were presented in the mouse wounds when infections established.52,53
aBL inactivation of C albicans in infected mouse burns
Sixteen (16) mice with infected burns were randomly divided into 2 groups of 8 mice each: one group was exposed to aBL and the other not (untreated controls). aBL was delivered at approximately 12 h (overnight) after fungal inoculation. Mice were given a total radiant exposure of up to 432 J/cm2 (80 min exposure time at the irradiance of 90 mW/cm2). To record the time course of the extent of infection, fungal luminescence of mouse burns was measured daily after aBL exposure until the infections resolved (characterized by the disappearance of fungal luminescence) or for up to 15 days after aBL therapy.
Bioluminescence imaging
We used an in vivo bioluminescence imaging technique to noninvasively monitor in real time the extent of infection in living mice.54 Our previous study demonstrated a perfectly linear correlation between C. albicans luminescence readout (RLU) and the corresponding CFU.52,53 This method is a significant improvement on the traditional use of animal survival or body fluid sampling and subsequent plating and colony counting. The setup consists of an ICCD camera (C2400-30H, Hamamatsu Photonics, Bridgewater, NJ), a camera controller, a specimen chamber, an image processor (C5510-50, Hamamatsu), and a color monitor (PVM 1454Q, Hamamatsu). White light LEDs are mounted inside the specimen chamber and supply the light required for obtaining dimensional imaging of the sample. Under photo-counting mode, a clear image can be obtained even under extremely low-light levels by detecting and integrating individual photons one by one.
Prior to imaging, mice were anesthetized by I.P. injections of a ketamine/xylazine cocktail. Twenty (20) μL coelenterazine (Gold Biotechnology, Inc., St. Louis, MO; 500 μg/mL in 1:9 methanol-PBS) was topically applied to the eschars of infected burns. Mice were then placed on a height-adjustable stage in the specimen chamber, and the infected mouse burns were positioned directly under the camera. A gray-scale background image of each mouse burn was made, and this was followed by a photon count of the same region. This entire burn photon count was quantified as RLU and was displayed in a false color scale ranging from pink (most intense) to blue (least intense).
Statistical analysis
Data were expressed as the mean ± SD. Differences were tested for significance using a one-way ANOVA. Values of P < .05 were considered statistically significant.
Disclosure of potential conflicts of interest
Y Zhang, Y Zhu, JC, YW, MES, MSV, DCH, MRH and TD declare that they have no conflicts of interest. CKM is an employee of the US government. The views expressed herein are those of the author and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government. This work was prepared as part of his official duties and, as such, there is no copyright to be transferred.
Acknowledgments
We thank Dr. Christophe d'Enfert (Unite´ Biologie et Pathoge´nicite´ Fongiques, F-75015 Paris, France) for providing the bioluminescent strain of C. albicans. We are also grateful to Dr. Tayyaba Hasan from the Wellman Center for her co-mentorship for Y Zhu, JC and YW.
Research was conducted in compliance with the Animal Welfare Act Regulations and other Federal statutes relating to animals and experiments involving animals and adheres to the principles set forth in the Guide for Care and Use of Laboratory Animals, National Research Council, 1996.
Part of the results was presented in SPIE DSS 2015, April, 2015, Baltimore, MD. Abstract number: 9268-13.
Funding
This work was supported by CIMIT under U.S. Army Medical Research Acquisition Activity Cooperative Agreement [CIMIT No. 14-1894]. The information contained herein does not necessarily reflect the position or policy of the Government, and no official endorsement should be inferred.
References
- [1].d'Enfert C. Hidden killers: persistence of opportunistic fungal pathogens in the human host. Curr Opin Microbiol 2009; 12:358-64; PMID:19541532; http://dx.doi.org/ 10.1016/j.mib.2009.05.008 [DOI] [PubMed] [Google Scholar]
- [2].Shoham S, Marwaha S. Invasive fungal infections in the ICU. J Intensive Care Med 2010; 25:78-92; PMID:19955115; http://dx.doi.org/ 10.1177/0885066609355262 [DOI] [PubMed] [Google Scholar]
- [3].Ballard J, Edelman L, Saffle J, Sheridan R, Kagan R, Bracco D, Cancio L, Cairns B, Baker R, Fillari P, et al.. Positive fungal cultures in burn patients: a multicenter review. J Burn Care Res 2008; 29:213-21; PMID:18182925 [DOI] [PubMed] [Google Scholar]
- [4].Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med 2012; 4:165rv13; PMID:23253612; http://dx.doi.org/ 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- [5].Vicente MF, Basilio A, Cabello A, Pelaez F. Microbial natural products as a source of antifungals. Clin Microbiol Infect 2003; 9:15-32; PMID:12691539; http://dx.doi.org/ 10.1046/j.1469-0691.2003.00489.x [DOI] [PubMed] [Google Scholar]
- [6].Segal E. Candida, still number one–what do we know and where are we going from there? Mycoses 2005; 48 Suppl 1:3-11; http://dx.doi.org/ 10.1111/j.1439-0507.2005.01103.x [DOI] [PubMed] [Google Scholar]
- [7].Nucci M, Queiroz-Telles F, Tobon AM, Restrepo A, Colombo AL. Epidemiology of opportunistic fungal infections in Latin America. Clin Infect Dis 2010; 51:561-70; PMID:20658942; http://dx.doi.org/ 10.1086/655683 [DOI] [PubMed] [Google Scholar]
- [8].Vandeputte P, Ferrari S, Coste AT. Antifungal resistance and new strategies to control fungal infections. Int J Microbiol 2012; 2012:713687; PMID:22187560; http://dx.doi.org/ 10.1155/2012/713687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 2012; 125:S3-13; PMID:22196207; http://dx.doi.org/ 10.1016/j.amjmed.2011.11.001 [DOI] [PubMed] [Google Scholar]
- [10].Kanafani ZA, Perfect JR. Antimicrobial resistance: resistance to antifungal agents: mechanisms and clinical impact. Clin Infect Dis 2008; 46:120-8; PMID:18171227; http://dx.doi.org/ 10.1086/524071 [DOI] [PubMed] [Google Scholar]
- [11].Barnes RA, Gow NA, Denning DW, May RC, Haynes K. Antifungal resistance: more research needed. Lancet 2014; 384:1427; PMID:25390325; http://dx.doi.org/ 10.1016/S0140-6736(14)61861-4 [DOI] [PubMed] [Google Scholar]
- [12].Chakrabarti A, Chatterjee SS, Rao KL, Zameer MM, Shivaprakash MR, Singhi S, Singh R, Varma SC. Recent experience with fungaemia: change in species distribution and azole resistance. Scand J Infect Dis 2009; 41:275-84; PMID:19229762; http://dx.doi.org/ 10.1080/00365540902777105 [DOI] [PubMed] [Google Scholar]
- [13].Mulu A, Kassu A, Anagaw B, Moges B, Gelaw A, Alemayehu M, Belyhun Y, Biadglegne F, Hurissa Z, Moges F, et al.. Frequent detection of ‘azole’ resistant Candida species among late presenting AIDS patients in northwest Ethiopia. BMC Infect Dis 2013; 13:82; PMID:23398783; http://dx.doi.org/ 10.1186/1471-2334-13-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tobudic S, Kratzer C, Presterl E. Azole-resistant Candida spp. – emerging pathogens? Mycoses 2012; 55:24-32; http://dx.doi.org/ 10.1111/j.1439-0507.2011.02146.x [DOI] [Google Scholar]
- [15].Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections–state of the art. Photodiagnosis Photodyn Ther 2009; 6:170-88; PMID:19932449; http://dx.doi.org/ 10.1016/j.pdpdt.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dai T, Vrahas MS, Murray CK, Hamblin MR. Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Exp Rev Anti-infective Therapy 2012; 10:185-95; http://dx.doi.org/ 10.1586/eri.11.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maisch T. A new strategy to destroy antibiotic resistant microorganisms: antimicrobial photodynamic treatment. Mini Rev Med Chem 2009; 9:974-83; PMID:19601890; http://dx.doi.org/ 10.2174/138955709788681582 [DOI] [PubMed] [Google Scholar]
- [18].Wainwright M, Phoenix DA, Laycock SL, Wareing DR, Wright PA. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol Lett 1998; 160:177-81; PMID:9532735; http://dx.doi.org/ 10.1111/j.1574-6968.1998.tb12908.x [DOI] [PubMed] [Google Scholar]
- [19].Conner-Kerr TA, Sullivan PK, Gaillard J, Franklin ME, Jones RM. The effects of UV radiation on antibiotic-resistant bacteria in vitro. Ostomy Wound Manage 1998; 44:50-6; PMID:9866596 [PubMed] [Google Scholar]
- [20].Wainwright M. Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother 1998; 42:13-28; PMID:9700525; http://dx.doi.org/ 10.1093/jac/42.1.13 [DOI] [PubMed] [Google Scholar]
- [21].Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP, Hamblin MR. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist Updat 2012; 15:223-36; PMID:22846406; http://dx.doi.org/ 10.1016/j.drup.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yin R, Dai T, Avci P, Jorge AE, de Melo WC, Vecchio D, Huang YY, Gupta A, Hamblin MR. Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr Opin Pharmacol 2013; 13:731-62; PMID:24060701; http://dx.doi.org/ 10.1016/j.coph.2013.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Enwemeka CS. Antimicrobial blue light: an emerging alternative to antibiotics. Photomed Laser Surg 2013; 31:509-11; PMID:24138170; http://dx.doi.org/ 10.1089/pho.2013.9871 [DOI] [PubMed] [Google Scholar]
- [24].Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob Agents Chemother 2005; 49:2822-7; PMID:15980355; http://dx.doi.org/ 10.1128/AAC.49.7.2822-2827.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Enwemeka CS, Williams D, Enwemeka SK, Hollosi S, Yens D. Blue 470-nm light kills methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomed Laser Surg 2009; 27:221-6; PMID:19196103; http://dx.doi.org/ 10.1089/pho.2008.2413 [DOI] [PubMed] [Google Scholar]
- [26].Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK. Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med 2008; 40:734-7; PMID:19065556; http://dx.doi.org/ 10.1002/lsm.20724 [DOI] [PubMed] [Google Scholar]
- [27].Maclean M, MacGregor SJ, Anderson JG, Woolsey G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl Environ Microbiol 2009; 75:1932-7; PMID:19201962; http://dx.doi.org/ 10.1128/AEM.01892-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guffey JS, Wilborn J. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed Laser Surg 2006; 24:684-8; PMID:17199466; http://dx.doi.org/ 10.1089/pho.2006.24.684 [DOI] [PubMed] [Google Scholar]
- [29].Lipovsky A, Nitzan Y, Gedanken A, Lubart R. Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers Surg Med 2010; 42:467-72; PMID:20662022; http://dx.doi.org/ 10.1002/lsm.20948 [DOI] [PubMed] [Google Scholar]
- [30].Murdoch LE, McKenzie K, Maclean M, Macgregor SJ, Anderson JG. Lethal effects of high-intensity violet 405-nm light on Saccharomyces cerevisiae, Candida albicans, and on dormant and germinating spores of Aspergillus niger. Fungal Biol 2013; 117:519-27; PMID:23931117; http://dx.doi.org/ 10.1016/j.funbio.2013.05.004 [DOI] [PubMed] [Google Scholar]
- [31].McKenzie K, Maclean M, Timoshkin IV, Endarko E, Macgregor SJ, Anderson JG. Photoinactivation of Bacteria Attached to Glass and Acrylic Surfaces by 405 nm Light: Potential Application for Biofilm Decontamination. Photochem Photobiol 2013; 89:927-35; PMID:23550978; http://dx.doi.org/ 10.1111/php.12077 [DOI] [PubMed] [Google Scholar]
- [32].Cieplik F, Spath A, Leibl C, Gollmer A, Regensburger J, Tabenski L, Hiller KA, Maisch T, Schmalz G. Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clin Oral Investig 2013; 18(7):1763-9; PMID:24297656; http://dx.doi.org/ 10.1007/s00784-013-1151-8 [DOI] [PubMed] [Google Scholar]
- [33].Chebath-Taub D, Steinberg D, Featherstone JD, Feuerstein O. Influence of blue light on Streptococcus mutans re-organization in biofilm. J Photochem Photobiol B 2012; 116:75-8; PMID:22982208; http://dx.doi.org/ 10.1016/j.jphotobiol.2012.08.004 [DOI] [PubMed] [Google Scholar]
- [34].Ramakrishnan P, Maclean M, MacGregor SJ, Anderson JG, Grant MH. Differential sensitivity of osteoblasts and bacterial pathogens to 405-nm light highlighting potential for decontamination applications in orthopedic surgery. J Biomed Opt 2014; 19:105001; PMID:25277146; http://dx.doi.org/ 10.1117/1.JBO.19.10.105001 [DOI] [PubMed] [Google Scholar]
- [35].Lafuente MT, Alferez F. Effect of LED Blue Light on Penicillium digitatum and Penicillium italicum Strains. Photochem Photobiol 2015; 91:1412-21; PMID:26288067; http://dx.doi.org/ 10.1111/php.12519 [DOI] [PubMed] [Google Scholar]
- [36].Bumah VV, Whelan HT, Masson-Meyers DS, Quirk B, Buchmann E, Enwemeka CS. The bactericidal effect of 470-nm light and hyperbaric oxygen on methicillin-resistant Staphylococcus aureus (MRSA). Lasers Med Sci 2015; 30:1153-9; PMID:25700768; http://dx.doi.org/ 10.1007/s10103-015-1722-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gupta S, Maclean M, Anderson JG, MacGregor SJ, Meek RM, Grant MH. Inactivation of micro-organisms isolated from infected lower limb arthroplasties using high-intensity narrow-spectrum (HINS) light. Bone Joint J 2015; 97-B:283-8; PMID:25628296; http://dx.doi.org/ 10.1302/0301-620X.97B2.35154 [DOI] [PubMed] [Google Scholar]
- [38].Imada K, Tanaka S, Ibaraki Y, Yoshimura K, Ito S. Antifungal effect of 405-nm light on Botrytis cinerea. Lett Appl Microbiol 2014; 59:670-6; PMID:25236427; http://dx.doi.org/; http://dx.doi.org/ 10.1111/lam.12330 [DOI] [PubMed] [Google Scholar]
- [39].Dai T, Gupta A, Huang YY, Sherwood ME, Murray CK, Vrahas MS, Kielian T, Hamblin MR. Blue Light Eliminates Community-Acquired Methicillin-resistant Staphylococcus aureus in Infected Mouse Skin Abrasions. Photomed Laser Surg 2013; 31(11):531-8; PMID:23406384; http://dx.doi.org/ 10.1089/pho.2012.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dai T, Gupta A, Huang YY, Yin R, Murray CK, Vrahas MS, Sherwood ME, Tegos GP, Hamblin MR. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother 2013; 57:1238-45; PMID:23262998; http://dx.doi.org/ 10.1128/AAC.01652-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS, Sherwood ME, Baer DG, Hamblin MR, Dai T. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii burn infection in mice: Implications for prophylaxis and treatment of combat-related wound infections. J Infect Dis 2014; 209:1963-71; PMID:24381206; http://dx.doi.org/ 10.1093/infdis/jit842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xiong R, Xie G, Edmondson AE, Sheard MA. A mathematical model for bacterial inactivation. Int J Food Microbiol 1999; 46:45-55; PMID:10050684; http://dx.doi.org/ 10.1016/S0168-1605(98)00172-X [DOI] [PubMed] [Google Scholar]
- [43].Sinton LW, Hall CH, Lynch PA, Davies-Colley RJ. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl Environ Microbiol 2002; 68:1122-31; PMID:11872459; http://dx.doi.org/ 10.1128/AEM.68.3.1122-1131.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Strauss WS, Sailer R, Schneckenburger H, Akgun N, Gottfried V, Chetwer L, Kimel S. Photodynamic efficacy of naturally occurring porphyrins in endothelial cells in vitro and microvasculature in vivo. J Photochem Photobiol B 1997; 39:176-84; PMID:9225460; http://dx.doi.org/ 10.1016/S1011-1344(97)00002-X [DOI] [PubMed] [Google Scholar]
- [45].Kotaki A, Yagi K. Fluorescence properties of flavins in various solvents. J Biochem 1970; 68:509-16; PMID:5488776 [DOI] [PubMed] [Google Scholar]
- [46].De Lucca AJ, Carter-Wientjes C, Williams KA, Bhatnagar D. Blue light (470 nm) effectively inhibits bacterial and fungal growth. Lett Appl Microbiol 2012; 55:460-6; PMID:23009190; http://dx.doi.org/ 10.1111/lam.12002 [DOI] [PubMed] [Google Scholar]
- [47].Alcantara-Diaz D, Brena-Valle M, Serment-Guerrero J. Divergent adaptation of Escherichia coli to cyclic ultraviolet light exposures. Mutagenesis 2004; 19:349-54; PMID:15388806; http://dx.doi.org/ 10.1093/mutage/geh039 [DOI] [PubMed] [Google Scholar]
- [48].Guimarães C, An J, Humar M, Goth W, Yun A. Biocompatible optical needle array for antibacterial blue light therapy. Proc SPIE 9341, Bioinspired, Biointegrated, Bioengineered Photonic Devices III, 2015:93410R-R-7 [Google Scholar]
- [49].Enjalbert B, Rachini A, Vediyappan G, Pietrella D, Spaccapelo R, Vecchiarelli A, Brown AJ, d'Enfert C. A multifunctional, synthetic Gaussia princeps luciferase reporter for live imaging of Candida albicans infections. Infect Immun 2009; 77:4847-58; PMID:19687206; http://dx.doi.org/ 10.1128/IAI.00223-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques 1997; 23:648-50; PMID:9343684 [DOI] [PubMed] [Google Scholar]
- [51].Gilpin DA. Calculation of a new Meeh constant and experimental determination of burn size. Burns 1996; 22:607-11; PMID:8982538; http://dx.doi.org/ 10.1016/S0305-4179(96)00064-2 [DOI] [PubMed] [Google Scholar]
- [52].Dai T, Kharkwal GB, Zhao J, St Denis TG, Wu Q, Xia Y, Huang L, Sharma SK, d'Enfert C, Hamblin MR. Ultraviolet-C light for treatment of Candida albicans burn infection in mice. Photochem Photobiol 2011; 87:342-9; PMID:21208209; http://dx.doi.org/ 10.1111/j.1751-1097.2011.00886.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dai T, Bil de Arce VJ, Tegos GP, Hamblin MR. Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrob Agents Chemother 2011; 55:5710-7; PMID:21930868; http://dx.doi.org/ 10.1128/AAC.05404-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis 2003; 187:1717-25; PMID:12751029; http://dx.doi.org/ 10.1086/375244 [DOI] [PMC free article] [PubMed] [Google Scholar]