ABSTRACT
Astrocytes traditionally were thought to have merely a support function, but are now understood to be important regulators of neural development and function. The immature and mature astrocytes have stage-specific roles in neuronal development. However, it is largely unclear whether human astrocytes also serve stage-specific roles in oligodendroglial development. Owing to the broad and diverse roles of astroglia in the central nervous system, transplantation of astroglia also could be of therapeutic value in promoting regeneration after CNS injury or disease. Our recent study (Jiang et al., 2016) explores the developmental interactions between astroglia and oligodendroglia, using a human induced pluripotent stem cell (hiPSC) model. By generating immature and mature human astrocytes from hiPSCs, we reveal previously unrecognized effects of immature human astrocytes on oligodendrocyte development. Notably, tissue inhibitor of metalloproteinase-1 (TIMP-1) is differentially expressed in the immature and mature human astrocytes, and mediates at least in part the effects of immature human astrocytes on oligodendroglial differentiation. Furthermore, we demonstrate that hiPSC-derived astroglial transplants promote cerebral white matter regeneration and behavioral recovery in a neonatal mouse model of hypoxic-ischemic injury. Our study provides novel insights into the astro-oligodendroglial cell interaction and has important implications for possible therapeutic interventions for human white matter diseases.
KEYWORDS: human induced pluripotent stem cells, immature astroglia, myelination, oligodendrocytes, periventricular leukomalacia, white matter
Astrocytes are critical players in organizing and maintaining brain structure and function.1 Recent studies discover that astrocytes served stage-specific roles in assisting neuronal development, such as synapse stabilization and elimination.2,3 In our recent studies, we asked whether astrocytes, at specific immature and mature stages, differently regulate the development of oligodendrocytes, myelin-producing cells in the central nervous system (CNS), and whether astrocytes can be harnessed for regenerative medicine to promote myelination.4 Our study5 describes a novel method of differentiation of human induced pluripotent stem cells (hiPSCs) to astroglia for promoting oligodendrocyte regeneration and remyelination therapy. We first demonstrate that the hiPSC-derived immature, rather than mature, human astroglia promote cell lineage progression of the oligodendrocyte progenitor cells (OPCs) cultured from mouse brain tissue via increased secretion of tissue inhibitor of metalloproteinase-1 (TIMP-1) and further develop astroglia therapies to promote myelin regeneration from endogenous resident OPCs in the brain. We find that immature human astrocytes have greater capacity in promoting OPC proliferation than mature human astrocytes. Furthermore, immature human astrocytes, but not mature human astrocytes, robustly boost OPC differentiation to oligodendrocytes (Fig. 1). In addition, we provide both in vitro and in vivo evidence that TIMP-1 partially, but critically, mediates the effects of immature astrocytes on OPC differentiation (Fig. 1). This is the most exciting implication of this research, but TIMP-1 possibly could not be used as a therapy alone rather than stem cell implantation. However, in lieu of stem cell implantation, intranasal administration of the conditioned medium from immature hiPSC-derived astroglia (hiPSC-Astro) promotes oligodendrocyte maturation in a TIMP-1 dependent manner.
Figure 1.
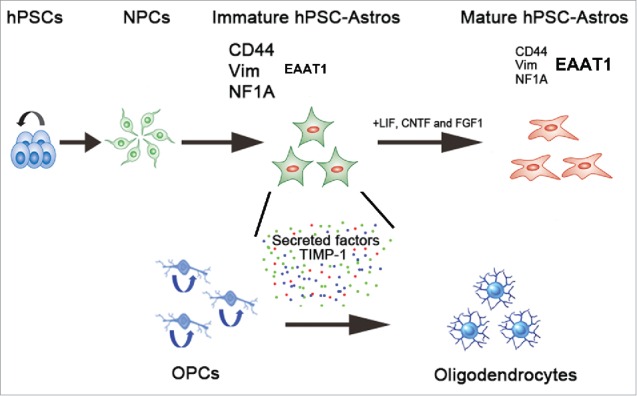
A conceptual diagram showing differences between immature and mature hPSC-astroglia in promoting oligodendrogenesis. By differentiating hPSCs into astrocytes at defined immature and mature stages, we investigate the effects of human astrocytes on oligodendrocyte development. The expression of CD44, vimentin and NF1A is higher in immature hPSC-Astros than that in mature hPSC-Astros. GLAST was expressed at higher level in mature hPSC-Astros than in immature hPSC-Astros. Compared to mature human astrocytes, the immature hPSC-Astros have stronger capability in regulating oligodendrocyte development, via released factors, such as TIMP-1. hPSC, human pluripotent stem cells; NPCs, neural progenitor cells; hiPSC-Astros, human pluripotent stem cell-derived astrocytes; Vim, vimentin; NF1A, nuclear factor-1A; GLAST, glutamate/aspartate transporter; LIF, leukemia inhibitory factor; CNTF, ciliary neurotrophic factor; FGF1, fibroblast growth factor 1; OPCs, oligodendrocyte progenitor cells; Colored dots, Secreted factors from immature hPSC-Astros that regulate oligodendrocyte development, such as tissue inhibitor of metalloproteinase-1 (TIMP-1).
Our study provides novel insights into the astroglial regulation of oligodendrogenesis and astroglia-based cell therapy for treating a number of CNS disorders including premature brain injury, cerebral palsy, multiple sclerosis, spinal cord injury, white matter stroke and neurodegenerative diseases, in which oligodendrocyte injury and myelin loss play an important role. Our findings suggest stage-specific developmental interactions between astroglia and oligodendroglia, with important therapeutic implications for promoting myelinogenesis.
White matter is the brain region underlying the gray matter cortex, composed of myelinated axons. White matter comprises more than 50% of the human brain, a far greater proportion than in other animals. The human brain has a low repair capacity, and white matter is part of the problem. White matter damage causes motor problems, as well as cognitive and behavioral problems. The estimated overall incidence of white matter diseases in children and adults is ∼1:1000. The financial costs for societies are great.
In particular, premature infants often suffer from hypoxic-ischemic damage, and the brunt of the damage is on periventricular white matter, termed periventricular leukomalacia (PVL), leading to life-long spasticity (cerebral palsy, CP) and cognitive deficits.6 An estimated 800,000 people in the United States have cerebral palsy, a neurological disorder that causes permanent loss of muscle coordination beginning early in life. Affected children may also have speech, hearing, and vision problems as well as cognitive impairment. Ongoing care for patients with the disorder—a potential consequence of premature birth—costs nearly $35 billion a year. The incidence of cerebral palsy is higher now than in the 1960s.
Recent studies reveal that up to 50 percent of the tiniest babies born between 24- and 26-week gestation (about 6 to 6 1/2 months into a pregnancy) (“the most vulnerable patients”) will have some degree of cognitive impairment. They are born quite early during a very important period of brain development. The field of neonatology has made real advances in treatments supporting heart and lung function, but when it comes to the brain we have no therapy to improve outcomes in babies born at these early stages. A significant barrier to improving outcomes and, ultimately, minimizing the occurrence of cerebral palsy is the lack of fundamental understanding of what goes wrong during brain development to cause the disorder. Cerebral palsy is usually attributed to an early episode of brain damage, but emerging evidence suggests that a major problem is the lack of novel mechanisms the brain can use to regenerate and repair. The textbook model for how the disease develops and how to treat the disease is inaccurate.
The limited endogenous capacity of the nervous system for self-repair is forcing the need to develop viable cell based replacement strategies for treating CNS injuries or diseases. Cell transplantation is a promising strategy for the therapy of a variety of CNS injuries or diseases. To date, the type of cells used for transplantation has ranged from neural stem cells that still have a degree of developmental plasticity, to more restricted neural cells, such as specific types of neurons, oligodendroglial progenitor cells, Schwann cells, olfactory ensheathing cells, and astrocytes. Emerging evidence indicates that, although astrocytes have long been called housekeeping cells, they can influence the actions of neurons in ways that have not been previously realized.7 An expanded role for astrocytes includes their ability to affect neuronal activity by modulating gliotransmitter chemicals, such as glutamate and ATP, and by quelling the firing of neurons and thus increasing synaptic fidelity. In addition, soluble factors from astrocytes can promote oligodendrocyte process extension and myelination of CNS axons.8 Astrocytes are thus integral to many sophisticated brain processes, contribute importantly to many CNS diseases, and may be ideal transplants for treating these disorders. Indeed, a number of previous studies have demonstrated beneficial effects from transplantation of astroglia in animal models of neurological disorders, including spinal cord injury, amyotrophic lateral sclerosis, Parkinson's disease, and ischemic stroke. Mechanistically, transplanted astroglia exerted these beneficial effects through multifaceted pathways, including release of neurotrophic factors, regulation of vascular remodeling, and modulation of immune responses in the CNS. For a detailed review, please refer to Chen et al., 2015.4
There is a strong rationale for pursuing cell therapy for white matter disease and specifically for choosing human stem cell-derived astroglia as a therapeutic candidate. There is increasingly strong evidence that neuronal injury or death is in part mediated by astrocytes and that healthy astrocytes can be neuroprotective. Human stem cells are the only obvious source of human astroglia-based replacement therapy. A previous study9 examines focal enrichment of normal astrocytes using transplantation of lineage-restricted astrocyte precursors, called glial-restricted precursors. These findings indicate the feasibility and efficacy of transplantation-based astrocyte replacement and show a proof-of-principle of astroglia transplants as a promising therapeutic strategy.10 However, to achieve optimal outcome, defined protocols need to be developed for generating human astroglial cells ideally suitable for cellular transplants.4,11 We believe that the human iPSC technology, combined with newly discovered information about the developing brain may be applied to patients and change the standard of care for children at risk for developmental disabilities and neurological problems.
The complexity and size of human astrocytes are among the few characteristics that differentiate human brains from rodent brains. Human astrocytes are much bigger in size and much more complex in both structure and function than rodent astrocytes.12,13 Our recent studies5,11 are thus aimed to develop stem cell derived human astroglial transplants for therapy. We present data showing that immature human iPSC-derived astroglial transplants are highly protective against hypoxic-ischemic white matter injury and improve functional outcome. Our recent study5 has emphasized the repair potential of astroglia and especially stage-specific differentiation of astroglia for optimal outcome in neural repair. We believe that this work has far-reaching implications in understanding the pathogenesis and treatment of white matter injury. Advancing techniques of isolating defined glial cells will have important implications for possible therapeutic interventions following CNS damage.
In clinical settings, intracerebral cell transplantation is not ideal because of its invasive procedure. We demonstrate that direct application of the concentrated factors collected from hiPSC-Astrocyte Conditioned Medium (ACM) containing concentrated factors from astrocytes like TIMP-1 via intranasal administration promote myelination regeneration. Therefore, this study puts forward a strategy of hiPSC-based cell-free therapy. It is tempting to suggest that this approach may be useful for many myelin disorders, e.g. multiple sclerosis, a chronic inflammatory demyelinating disease where inflamed environment may significantly compromise survival of transplanted cells,14 where administration of the cell-free, concentered factors that are released from human immature astrocytes may be effective in promoting remyelination. Considering that an array of factors released by immature astrocytes not only have effects on OPC maturation, but also on OPC proliferation, intranasal TIMP-1 alone may not have effects as potent as intranasal hiPSC-Astro ACM. Future secretomic studies may help further identify other key factors released from hiPSC-Astros and further promote the translational potential of hiPSC-based cell-free therapy.
In summary, our study presents a comprehensive set of data to show that immature human iPSC-derived astroglial transplants are highly protective against hypoxic-ischemic white matter injury and improve functional outcome. Our study highlights the repair potential of astroglia and especially stage-specific differentiation of astroglia for optimal outcome in neural repair. Studies comparing effects of different stages of astroglial transplants in different types of CNS damage are thus necessary in order to identify ideal candidates for cell replacement therapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was in part supported by grants from the National Institutes of Health (R01HD087566), the National Multiple Sclerosis Society and Shriners Hospitals for Children, as well as the California Institute of Regenerative Medicine.
Author contributions
P. J. and W. D. contributed equally to this work.
References
- [1].Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 2008; 60(3):430-40; PMID:18995817; http://dx.doi.org/ 10.1016/j.neuron.2008.10.013 [DOI] [PubMed] [Google Scholar]
- [2].Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 2013; 504(7480):394-400; PMID:24270812; http://dx.doi.org/ 10.1038/nature12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pfrieger FW. Role of glial cells in the formation and maintenance of synapses. Brain Res Rev 2010; 63(1–2):39-46; PMID:19931561; http://dx.doi.org/ 10.1016/j.brainresrev.2009.11.002 [DOI] [PubMed] [Google Scholar]
- [4].Chen C, Chan A, Wen H, Chung SH, Deng W, Jiang P. Stem and progenitor cell-derived astroglia therapies for neurological diseases. Trends Mol Med 2015; 21(11):715-29; PMID:26443123; http://dx.doi.org/ 10.1016/j.molmed.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jiang P, Chen C, Liu XB, Pleasure DE, Liu Y, Deng W. Human iPSC-derived immature astroglia promote oligodendrogenesis by increasing TIMP-1 secretion. Cell Rep 2016; 15(6):1303-15; PMID:27134175; http://dx.doi.org/ 10.1016/j.celrep.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Deng W. Neurobiology of injury to the developing brain. Nat Rev Neurol 2010; 6(6):328-36; PMID:20479779; http://dx.doi.org/ 10.1038/nrneurol.2010.53 [DOI] [PubMed] [Google Scholar]
- [7].Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 2012; 26(9):891-907; PMID:22549954; http://dx.doi.org/ 10.1101/gad.188326.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nash B, Ioannidou K, Barnett SC. Astrocyte phenotypes and their relationship to myelination. J Anat 2011; 219(1):44-52; PMID:21496013; http://dx.doi.org/ 10.1111/j.1469-7580.2010.01330.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci 2008; 11(11):1294-301; PMID:18931666; http://dx.doi.org/ 10.1038/nn.2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Noble M, Davies JE, Mayer-Proschel M, Proschel C, Davies SJ. Precursor cell biology and the development of astrocyte transplantation therapies: lessons from spinal cord injury. Neurotherapeutics 2011; 8(4):677-93; PMID:21918888; http://dx.doi.org/ 10.1007/s13311-011-0071-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jiang P, Chen C, Wang R, Chechneva OV, Chung SH, Rao MS, Pleasure DE, Liu Y, Zhang Q, Deng W. hESC-derived Olig2+ progenitors generate a subtype of astroglia with protective effects against ischaemic brain injury. Nat Commun 2013; 4:2196; PMID:23880652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci 2009; 29(10):3276-87; PMID:19279265; http://dx.doi.org/ 10.1523/JNEUROSCI.4707-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 2013; 12(3):342-53; PMID:23472873; http://dx.doi.org/ 10.1016/j.stem.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen L, Coleman R, Leang R, Tran H, Kopf A, Walsh CM, Sears-Kraxberger I, Steward O, Macklin WB, Loring JF, Lane TE. Human neural precursor cells promote neurologic recovery in a viral model of multiple sclerosis. Stem Cell Reports 2014; 2(6):825-37; PMID:24936469; http://dx.doi.org/ 10.1016/j.stemcr.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


