Abstract
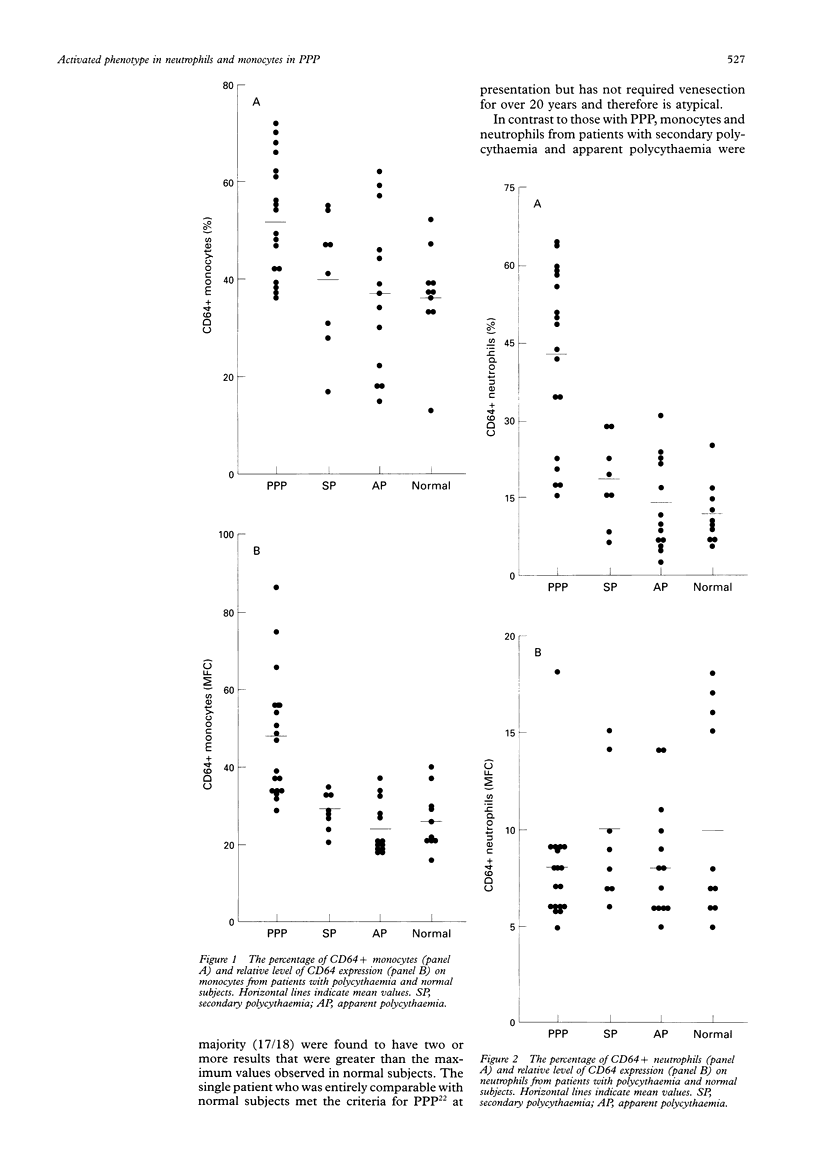
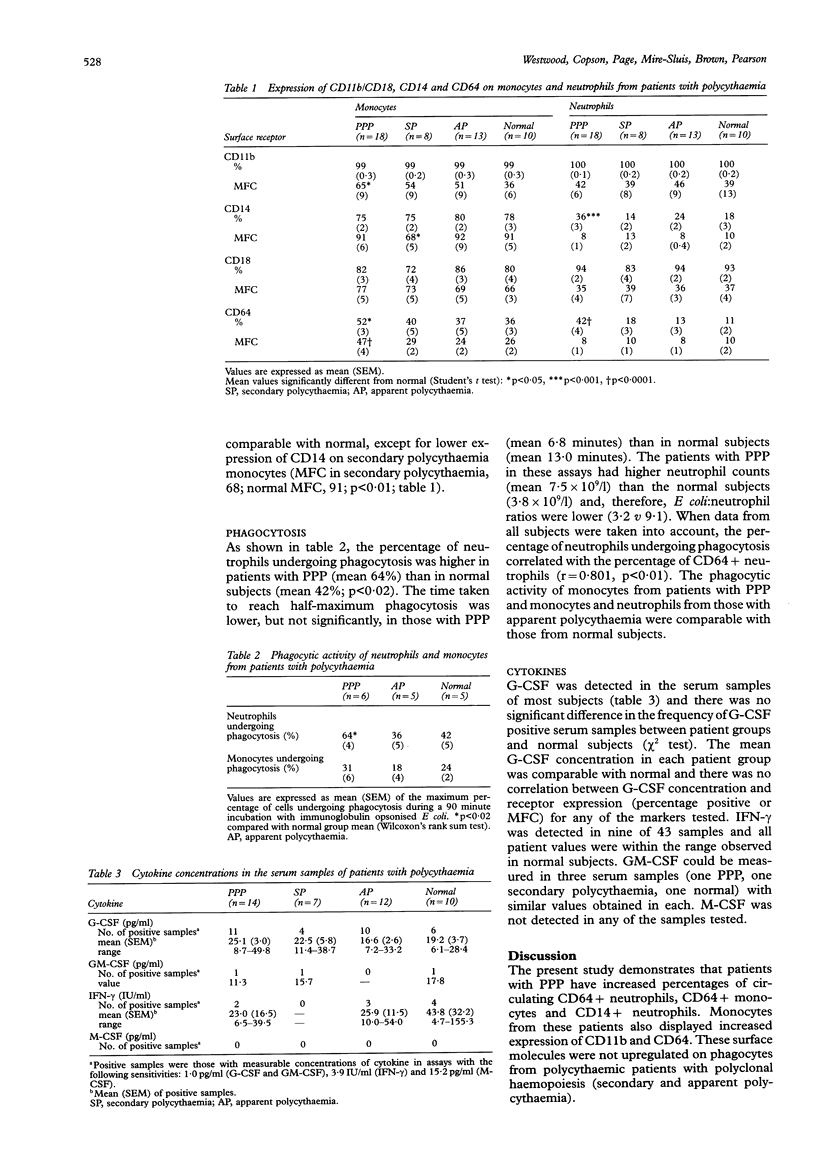
AIM--To investigate whether monocytes and neutrophils from patients with primary proliferative polycythaemia (PPP) exhibit increased expression of markers of cell activation and, if so, whether they are associated with the phagocytic activity of these cells and concentrations of circulating cytokines. METHODS--Expression of CD11b, CD14, CD18, and CD64 on monocytes and neutrophils was assessed by flow cytometry. Phagocytosis was analysed using immunoglobulin opsonised Escherichia coli. Serum concentrations of granulocyte colony stimulating factor (G-CSF), granulocyte-macrophage CSF (GM-CSF) and macrophage CSF (M-CSF) were determined by bioassays, and interferon-gamma (IFN-gamma) by enzyme linked immunosorbent assay (ELISA). RESULTS--Patients with PPP (n = 18), when compared with normal subjects (n = 10), had increased percentages of CD64+ monocytes (52% v 36%) and neutrophils (42% v 11%) and of CD14+ neutrophils (36% v 18%). Monocytes from patients with PPP exhibited increased expression of CD64 (47 v 26) and of CD11b (65 v 36). These abnormalities were not found in patients with secondary (n = 8) or apparent (n = 13) polycythaemia. The percentage of neutrophils undergoing phagocytosis was higher in patients with PPP (mean 64%; n = 6) than in normal subjects (mean 42%; n = 5). G-CSF, GM-CSF and IFN-gamma concentrations in patients' serum samples were comparable with normal; M-CSF was not detected in any of the samples. There was no correlation between cytokine concentrations and the expression of CD11b, CD14, CD18, and CD64 on patients' phagocytes. CONCLUSIONS--Increased expression of CD11b and CD64 by monocytes, increased percentages of CD14+ and CD64+ neutrophils and the high phagocytic activity of neutrophils suggests that these cells are activated in vivo in patients with PPP. The phenotypic changes of PPP phagocytes were not associated with increased concentrations of circulating cytokines and probably reflect intrinsic abnormalities within the neoplastic PPP clone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson J. W., Fialkow P. J., Murphy S., Prchal J. F., Steinmann L. Polycythemia vera: stem-cell and probable clonal origin of the disease. N Engl J Med. 1976 Oct 21;295(17):913–916. doi: 10.1056/NEJM197610212951702. [DOI] [PubMed] [Google Scholar]
- Akerley W. L., 3rd, Guyre P. M., Davis B. H. Neutrophil activation through high-affinity Fc gamma receptor using a monomeric antibody with unique properties. Blood. 1991 Feb 1;77(3):607–615. [PubMed] [Google Scholar]
- Anderson C. L., Shen L., Eicher D. M., Wewers M. D., Gill J. K. Phagocytosis mediated by three distinct Fc gamma receptor classes on human leukocytes. J Exp Med. 1990 Apr 1;171(4):1333–1345. doi: 10.1084/jem.171.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990 Mar 1;75(5):1037–1050. [PubMed] [Google Scholar]
- Berlin N. I. Diagnosis and classification of the polycythemias. Semin Hematol. 1975 Oct;12(4):339–351. [PubMed] [Google Scholar]
- Blanchard K. L., Gilliland D. G., Bunn H. F. Clonality in myeloproliferative disorders. Am J Med Sci. 1992 Aug;304(2):125–130. doi: 10.1097/00000441-199208000-00007. [DOI] [PubMed] [Google Scholar]
- Campbell A. D., Wicha M. S. Extracellular matrix and the hematopoietic microenvironment. J Lab Clin Med. 1988 Aug;112(2):140–146. [PubMed] [Google Scholar]
- Cooper M. R., DeChatelet L. R., McCall C. E., Spurr C. L. The activated phagocyte of polycythemia vera. Blood. 1972 Sep;40(3):366–374. [PubMed] [Google Scholar]
- Corberand J., LaHarrague P., De Larrard B., Nguyen F., Pris J. Phagocytosis in myeloproliferative disorders. Am J Clin Pathol. 1980 Sep;74(3):301–305. doi: 10.1093/ajcp/74.3.301. [DOI] [PubMed] [Google Scholar]
- Faint R. W. Platelet-neutrophil interactions: their significance. Blood Rev. 1992 Jun;6(2):83–91. doi: 10.1016/0268-960x(92)90010-n. [DOI] [PubMed] [Google Scholar]
- Ghosh M. L., Hudson G., Blackburn E. K. Skin window studies in polycythaemia rubra vera. Br J Haematol. 1975 Mar;29(3):461–467. doi: 10.1111/j.1365-2141.1975.tb01843.x. [DOI] [PubMed] [Google Scholar]
- Gilbert H. S., Goldberg R., Ward L. Increased circulating neutrophils with surface receptor activity for immunoglobulin G in polycythemia vera and myeloid metaplasia. Blood. 1979 Jun;53(6):1106–1113. [PubMed] [Google Scholar]
- Gilbert H. S., Praloran V., Stanley E. R. Increased circulating CSF-1 (M-CSF) in myeloproliferative disease: association with myeloid metaplasia and peripheral bone marrow extension. Blood. 1989 Sep;74(4):1231–1234. [PubMed] [Google Scholar]
- Guyre P. M., Campbell A. S., Kniffin W. D., Fanger M. W. Monocytes and polymorphonuclear neutrophils of patients with streptococcal pharyngitis express increased numbers of type I IgG Fc receptors. J Clin Invest. 1990 Dec;86(6):1892–1896. doi: 10.1172/JCI114921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopen G. Granulocyte and platelet adhesiveness in malignant paraproteinaemia, leukaemia and myeloproliferative diseases. Scand J Haematol. 1981 Nov;27(5):339–345. doi: 10.1111/j.1600-0609.1981.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Huizinga T. W., Van der Schoot C. E., Roos D., Weening R. S. Induction of neutrophil FC-gamma receptor I expression can be used as a marker for biologic activity of recombinant interferon-gamma in vivo. Blood. 1991 May 1;77(9):2088–2090. [PubMed] [Google Scholar]
- Jayaram Y., Buckle A. M., Hogg N. The Fc receptor, FcRI, and other activation molecules on human mononuclear phagocytes after treatment with interferon-gamma. Clin Exp Immunol. 1989 Mar;75(3):414–420. [PMC free article] [PubMed] [Google Scholar]
- Kerst J. M., van de Winkel J. G., Evans A. H., de Haas M., Slaper-Cortenbach I. C., de Wit T. P., von dem Borne A. E., van der Schoot C. E., van Oers R. H. Granulocyte colony-stimulating factor induces hFc gamma RI (CD64 antigen)-positive neutrophils via an effect on myeloid precursor cells. Blood. 1993 Mar 15;81(6):1457–1464. [PubMed] [Google Scholar]
- LUGANOVA I. S., SEITS I. F. GLYCOGEN CONTENT AND METABOLISM IN NORMAL AND LEUKEMIC HUMAN LEUKOCYTES. Fed Proc Transl Suppl. 1963 Nov-Dec;22:1058–1062. [PubMed] [Google Scholar]
- MITUS W. J., BERGNA L. J., MEDNICOFF I. B., DAMESHEK W. Alkaline phosphatase of mature neutrophils in chronic forms of the myeloproliferative syndrome. Am J Clin Pathol. 1958 Oct;30(4):285–294. doi: 10.1093/ajcp/30.4.285. [DOI] [PubMed] [Google Scholar]
- McCall C. E., Caves J., Cooper R., DeChatlet L. Functional characteristics of human toxic neutrophils. J Infect Dis. 1971 Jul;124(1):68–75. doi: 10.1093/infdis/124.1.68. [DOI] [PubMed] [Google Scholar]
- Moore A., Nachman R. L. Platelet Fc receptor. Increased expression in myeloproliferative disease. J Clin Invest. 1981 Apr;67(4):1064–1071. doi: 10.1172/JCI110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz P., Gomez F., Lopez R., Chien P., Rossman M. D., Schreiber A. D. Granulocyte Fc gamma receptor recognition of cell bound and aggregated IgG: effect of gamma-interferon. Am J Hematol. 1992 Apr;39(4):257–263. doi: 10.1002/ajh.2830390405. [DOI] [PubMed] [Google Scholar]
- Schwager I., Jungi T. W. Effect of human recombinant cytokines on the induction of macrophage procoagulant activity. Blood. 1994 Jan 1;83(1):152–160. [PubMed] [Google Scholar]
- Shen L., Guyre P. M., Fanger M. W. Polymorphonuclear leukocyte function triggered through the high affinity Fc receptor for monomeric IgG. J Immunol. 1987 Jul 15;139(2):534–538. [PubMed] [Google Scholar]
- Thomas H., Balkwill F. R. Effects of interferons and other cytokines on tumors in animals: a review. Pharmacol Ther. 1991 Dec;52(3):307–330. doi: 10.1016/0163-7258(91)90030-p. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Ulevitch R. J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993 Mar;14(3):121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]


