Cytokinesis occurs along the cell's short axis midway between the 2 nuclei in numerous organisms across all 3 domains of life. Many of these organisms assemble a ring-like structure at the equatorial cell division plane; bacteria and some archaeal species assemble an FtsZ ring, whereas amoebas, fungi, and animals assemble an actomyosin contractile ring.1 In many of the early divergent protozoa, including Trypanosoma brucei, cell division via binary fission occurs along the cell's longitudinal axis.1 Trypanosomes divide uni-directionally, along the cell division plane that is defined by the newly assembled flagellum and its associated flagellum attachment zone (FAZ) filament, from the anterior toward the posterior between the 2 flagella.2 Prior to cleavage furrow ingression, an invagination of the cell body occurs between the 2 flagella, forming a division fold that extends from the anterior end of the new flagellum daughter cell to the posterior end of the old flagellum daughter cell.3,4 Subsequently, furrow ingression is initiated from the anterior tip of the new FAZ filament, and proceeds, along the division fold, to the posterior. Therefore, the new FAZ tip constitutes the cytokinesis initiation site in trypanosomes. Notably, trypanosomes do not assemble an actomyosin contractile ring,5 suggesting that trypanosomes have evolved distinct cytokinesis machineries.
Two evolutionarily conserved kinases, the Polo-like kinase and the Aurora B kinase, are required for cytokinesis initiation in trypanosomes.6,7 The 2 kinases localize to the anterior tip of the new FAZ filament sequentially during the cell cycle, but they never meet with each other at any cell cycle stages, suggesting that there might be a third protein interconnecting the 2 kinases at the new FAZ tip. Through yeast 2-hybrid screening, we identified a trypanosome-specific protein named CIF1 (Cytokinesis Initiation Factor 1), which interacts with Polo-like kinase during early cell cycle stages and with Aurora B kinase during late cell cycle stages (Fig. 1). CIF1 is a substrate of both kinases, but only phosphorylation by Polo-like kinase is required to target CIF1 to the new FAZ tip. Once targeted to the new FAZ tip, CIF1 recruits Aurora B kinase to the new FAZ tip during late anaphase. These results delineate a novel signaling cascade from Polo-like kinase through CIF1 to Aurora B kinase, and demonstrate a concerted action of the 3 proteins at the new FAZ tip for cytokinesis initiation.4 This cytokinesis signaling pathway is totally different from that in fungi and animals. Given that trypanosomes diverged from the last eukaryotic common ancestor before fungi and animals, it is likely that the trypanosome cytokinesis regulatory pathway may reflect certain features of the ancestral cytokinesis pathway.
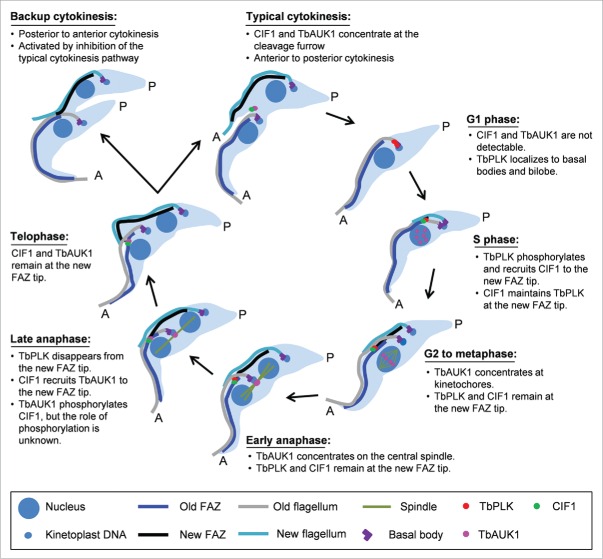
Figure 1.
Regulation of cytokinesis initiation by Polo-like kinase, CIF1, and Aurora B kinase in trypanosomes and the activation of a backup cytokinesis pathway by inhibiting normal cytokinesis. Depicted are trypanosome cells at different cell cycle stages and the dynamic localizations of T. brucei Polo-like kinase homolog TbPLK, Aurora B kinase homolog TbAUK1, and CIF1. A, anterior; P, posterior.
The most surprising discovery from the investigation of CIF1 function is that depletion of CIF1 activates a backup cytokinesis pathway that promotes cell division from the posterior end of the cell toward the anterior tip of the new FAZ filament, which is exactly the opposite direction of the typical anterior-to-posterior cytokinesis (Fig. 1). The two cytokinesis pathways appear to use the same division fold that is pre-formed prior to cytokinesis initiation, albeit operating in opposite directions. This unexpected discovery is significant in that it demonstrates that trypanosomes have a fail-safe mechanism to maintain genomic integrity and cell survival after failed cytokinesis. It also demonstrates that trypanosomes possess the capability to initiate cytokinesis furrowing from the posterior end of the division fold, despite that this capability is kept inert under normal growth conditions by unknown mechanisms. It is unclear why trypanosomes do not take advantage of this capability to initiate furrowing from both ends of the division fold, which, in theory, would take the least time to accomplish cell division and thus minimize the chance of potential defects. However, given that the FAZ filament does not extend to the posterior end, it might be challenging for the cell to target the FAZ tip-localized cytokinesis machinery to the posterior for furrowing due to structural constraints. Indeed, in CIF1 RNAi cells, the regulator from the typical cytokinesis pathway is not targeted to the posterior end. Therefore, the backup cytokinesis apparently utilizes a distinct set of regulators.
Our discovery opens the door to a new research field that we know nothing about. For example, what are the regulators in this backup pathway? How are they activated? Are they expressed under normal growth conditions? Given that trypanosomes do not have transcriptional control of gene expression, it is likely that they are transcribed constitutively. But are they post-transcriptionally regulated? Where do they localize under normal growth conditions and are they targeted to the cell posterior to initiate the alternative cytokinesis when normal cytokinesis is inhibited? No matter which end of the division fold is used for cytokinesis initiation, we believe that both cytokinesis pathways will need the membrane abscission machinery to sever the plasma membrane site and subsequently the membrane fusion machinery to seal the plasma membrane between the 2 daughter cells. It remains unclear whether the same membrane fission and fusion machineries are employed by both cytokinesis pathways, and, if they are, how are they targeted to opposite cell ends and how are they activated by distinct cytokinesis regulators?
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Oliferenko S, Chew TG, Balasubramanian MK. Positioning cytokinesis.. Genes Dev 2009; 23:660-74; PMID:19299557; http://dx.doi.org/ 10.1101/gad.1772009 [DOI] [PubMed] [Google Scholar]
- [2].Li Z, Umeyama T, Wang CC. The aurora kinase in Trypanosoma brucei plays distinctive roles in metaphase-anaphase transition and cytokinetic initiation.. PLoS Pathog 2009; 5:e1000575; PMID:19750216; http://dx.doi.org/ 10.1371/journal.ppat.1000575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wheeler RJ, Scheumann N, Wickstead B, Gull K, Vaughan S. Cytokinesis in Trypanosoma brucei differs between bloodstream and tsetse trypomastigote forms: implications for microtubule-based morphogenesis and mutant analysis. Mol Microbiol 2013; 90:1339-55; PMID:24164479; http://dx.doi.org/ 10.1111/mmi.12436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhou Q, Gu J, Lun Z-R, Ayala FJ, Li Z. Two distinct cytokinesis pathways drive trypanosome cell division initiation from opposite cell ends.. Proc Natl Acad Sci U S A 2016; 113:3287-92; PMID:26929336; http://dx.doi.org/ 10.1073/pnas.1601596113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Garcia-Salcedo JA, Pérez–Morga D, Gijón P, Dilbeck V, Pays E, Nolan DP. A differential role for actin during the life cycle of Trypanosoma brucei. EMBO J 2004; 23:780-9; PMID:14963487; http://dx.doi.org/ 10.1038/sj.emboj.7600094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tu X, Kumar P, Li Z, Wang CC. An aurora kinase homologue is involved in Regulating Both mitosis and cytokinesis in Trypanosoma brucei.. J Biol Chem 2006; 281:9677-87; PMID:16436376; http://dx.doi.org/ 10.1074/jbc.M511504200 [DOI] [PubMed] [Google Scholar]
- [7].Kumar P, Wang CC. Dissociation of cytokinesis initiation from mitotic control in a eukaryote.. Eukaryot Cell 2006; 5:92-102; PMID:16400171; http://dx.doi.org/ 10.1128/EC.5.1.92-102.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]