Abstract
Background:
Transforming growth factor-beta 1(TGF-β1) T869C (rs1800470, the same below) gene polymorphism is notably relative with the development of Diabetic Nephropathy (DN), and CC/CT genotype diabetic have higher frequency of than TT genotype diabetic. To find out individual risk factors in the two genotypes especially in susceptible genotype could provide more efficient and targeted prevention.
Methods:
This was a prospective cohort study. A total of 251 type 2 diabetes mellitus (T2DM) patients [53.4% male, 56(52–67) years] were enrolled in this cohort study. Multiple concerned factors were collected and the relationship of these risk factors and development of DN were evaluated by Cox regression analysis. Hazard ratios of development of DN were calculated by Kaplan-Meier curves and the Cox proportional hazards model for CC/CT genotype versus TT genotype patients.
Results:
TGF-β1 T869C gene polymorphism was an independent predictor of DN in T2DM patients (HR, 2.08; 95%CI, 1.18–3.66; P=0.012). Hyperlipemia (HR, 1.91; 95%CI, 1.19–3.08; P=0.007), age (HR, 0.95; 95%CI, 0.93–0.98; P=0.001) and smoking status (HR, 2.36; 95%CI, 1.07–5.21; P=0.033) were risk indictors of the development of DN in CC/CT genotype patients. HbA1c (HR, 2.8; 95%CI, 1.07–7.30; P=0.036), hypertension (HR, 7.46; 95%CI, 1.38–40.29; P=0.02), and hyperlipemia (HR, 12.33; 95%CI, 1.05–145.39; P=0.046) were risk indictors for the development of DN in TT genotype patients.
Conclusion:
TGF-β1 T869C gene polymorphism was an independent predictor of DN for T2DM patients and CC/CT genotype had higher susceptibility to DN. CC/CT genotype and TT genotype patients had different risk indictors of DN.
Keywords: TGF-β1, Diabetic nephropathy, T869C, Risk, Type 2 diabetes mellitus
Introduction
Diabetic Nephropathy (DN) is one of the most conventional microvascular complications of diabetes mellitus. Approximately 30%–40% of all patients with diabetes develop DN and it is the primary leading cause of end stage renal disease (ESRD) (1). Meanwhile, the ESRD is the main cause of death in type 2 diabetes mellitus (T2DM). Therefore, it is important to prevent diabetes developing into DN (2, 3).
Many factors, such as hypertension, hyperglycemia, the accumulation of advanced glycation products, dyslipidemia, and albuminuria/proteinuria, increase the risk of DN. Besides, diabetic patients may have increased susceptibility to DN due to genetic factors (4–6), such as transforming growth factor-β1 (TGF-β1) T869C (Leu 10 Pro) gene polymorphism. Transforming growth factor-beta (TGF-β) is a multifunctional regulator that modulates cell proliferation, differentiation, apoptosis, adhesion and migration of various cell types and induces the production of extracellular matrix proteins (7). “It is synthesized by many renal cell types and exerts its biological functions through a variety of signalling pathways, including the Smad and MAPK pathways” (8). “In renal diseases, TGF-β is upregulated and induces renal cells to produce extracellular matrix proteins leading to glomerulosclerosis as well as tubulointerstitial fibrosis, which ultimately result in renal dysfunction” (9). T869C gene polymorphism was notable different between patients with DN and diabetic patients without DN, and the DN group had higher frequency of CC/CT genotype than the diabetes without DN group (5). Similar results were also found in other studies (10, 11). These conclusions were in line with our previous findings (12). T869C gene polymorphism has not yet been studied in prospective cohort study in T2DM patients. We thereby conducted a study to confirm whether T869C gene polymorphism contributes to the established predictor of DN, and secondly, to find out different risk factors to provide a better and possibly different treatment for susceptible populations (T869C CC/CT genotype) and non-susceptible populations (T869C TT genotype).
Materials and Methods
Study patients
This was a prospective, observational cohort study. All of the patients admitted met the diagnostic criteria of DM proposed by WHO in 1999, the Chinese Medical Association, and the diagnostic criteria of T2DM in “China Guideline for Diabetes (Trial Version)”(13), and DN was defined in accordance with the internationally recognized Mogensen staging criteria (14). All of the cases were recruited from January 2010 to May 2011, diagnosed as T2DM. Patients were followed every half year until May 2013. All of the patients were recruited in the Hangzhou Red Cross Hospital, with a DM duration >4 yr and no limit of sex and age. Individuals with a history of malignancy, receiving a kidney transplant, cerebral vascular accidents, or myocardial infarction were eliminated from this study. A total of 251 patients were enrolled finally. Informed consent was obtained from all the patients before enrollment in the study. This study was performed and approved by the institutional Ethics Committee of our hospital.
Data Collection and laboratory measurements
The following demographic and clinical data were collected for each patient: age, gender, height, weight, primary renal disease, duration of DM, smoking status, drinking status, glycated hemoglobin (HbA1c) level, and comorbid conditions including hypertension, hyperlipemia. Fasting blood samples were checked for glucose, hemoglobin (HbA1c), and lipids. Arterial blood pressure was measured using a standard sphygmomanometer and hypertension was recorded if the patient was taking antihypertensive drugs or had two separated measured blood pressures >140/90 mmHg. Total cholesterol and low-density lipoprotein cholesterol (LDL-C) were measured by enzymatic method. Hyperlipidemic subjects had total cholesterol ≥200 mg/dL and LDL-C ≥100 mg/dL. HbA1c levels were measured by high-performance liquid chromatography.
We divided patients into CC/CT and TT group according to TGF-β1 T869C genotype. For all participants, TGF-β1 T869C genotype were identified, and the experimental method were as same as previously study (12). For all the subjects, 3 ml of venous blood were collected to take DNA samples using a blood DNA extraction kit (TaKaNa Co., Japan). PCR-RFLP was used to detect transforming growth factor (TGF)-β1 (T869C) gene polymorphisms, PCR amplification to amplify genes, and Primer 5.0 software to design the primers: TGF-β1 gene T869C upstream primer 5′-TTCCCTCGAGGCCCTCCTA and downstream primer 5′-GCCGCAGCTTGGACAGGATC. The synthesis was conducted by Sangon Biotech (Shanghai) Co., Ltd.
Statistical Analysis
Variables are presented in this study as median (interquartile range), or number (proportion) where appropriate. Differences in variables between the CC/CT and TT group were tested using the non-parametric Mann-Whitney test or the chi-square test where appropriate. Spearman rank correlation matrix was created to evaluate the relationships among clinical variables. Cox regression analysis was used to evaluate the prognostic risks of DN. Two different approaches were applied: 1) univariate Cox regression analysis was selected from the following variables: age, gender, smoking status, drinking status, HbA1C level, hypertension, and hyperlipemia for the development of DN, and 2) the multivariate Cox regression analysis model using covariates by an entry selection procedure. Statistical significance was assumed for P< 0.05. Data were analyzed using SPSS 19.0 (Chicago, IL, USA).
Results
Patient characteristics at baseline
Baseline characteristics of the patients, when divided into groups according to TGF-β1 T869C genotype, can be seen in Table 1. Median (interquartile range) age at baseline was 56 (52–67) yr. Diabetes duration at baseline was 9 (7–11) yr. Compared with TT genotype patients, CC/CT genotype patients had longer duration of diabetes [(9 (7–11) vs. 9 (5–11); P=0.008)], more frequently hypertension (76.0% vs. 33.3%; P<0.001), and smoking (79.8% vs. 19.4%; P<0.001). Successfully follow up were defined as development of DN or year 2013 without DN.
Table 1:
Baseline characteristics of 251 Diabetes mellitus patients
| Characteristics | Total | CC/CTgenetype | TTgenetype | P value |
|---|---|---|---|---|
| All, n(%) | 251(100) | 158 (62.9) | 93 (37.1) | |
| Age (yr) | 56 (52–67) | 55 (49–67) | 56 (54–67) | 0.545 |
| Male, n(%) | 134 (53.4) | 86 (54.4) | 48 (51.6) | 0.97 |
| Smoking, n(%) | 144 (57.4) | 126 (79.8) | 18 (19.4) | <0.001 |
| Drinking, n(%) | 138 (55) | 94 (59.5) | 44 (47.3) | 0.354 |
| HbA1c (%) | 6.9 (6.3–7.4) | 6.9 (6.3–7.4) | 7.0 (6.4–7.5) | 0.462 |
| Hypertension, n(%) | 151(60.2) | 120 (76.0) | 31(33.3) | <0.001 |
| hyperlipemia, n(%) | 120 (47.8) | 76 (48.1) | 44 (47.3) | 0.547 |
| Diabetes duration (yr) | 9 (7–11) | 9 (7–11) | 9 (5–11) | 0.008 |
Data are median (25th, 75th percentiles), or percentage.
Risk indicators of DN in CC/CT or TT genotype
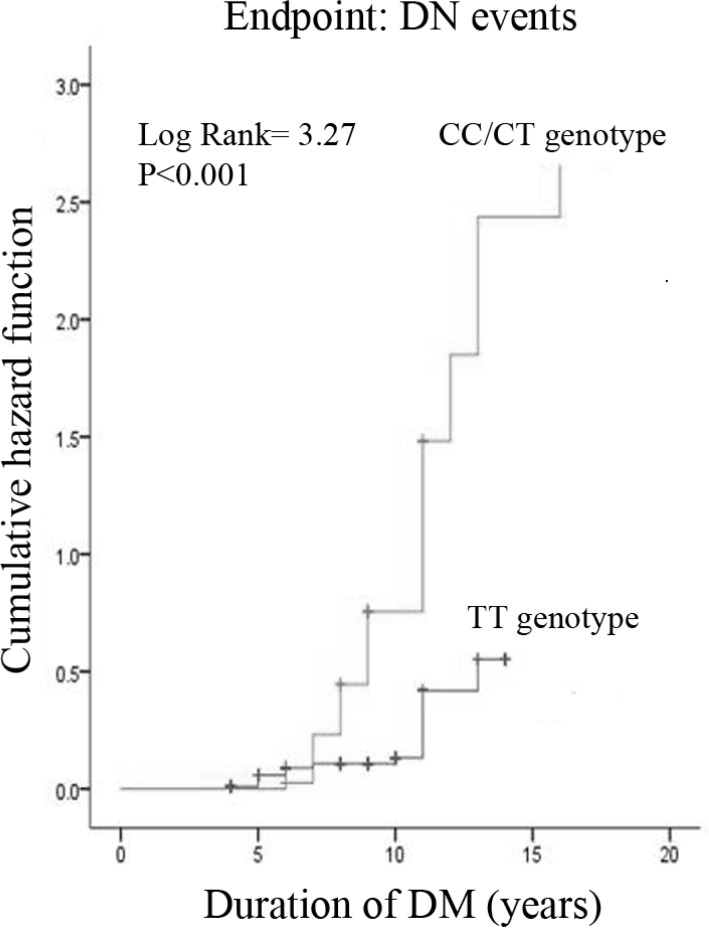
During follow-up to 2013, 11 patients and 87 patients had developed DN in TT genotype group and CC/CT genotype group, respectively. DN incidence rates were 55.06% higher in the CC/CT genotype group than that of 11.83% in the TT genotype group (P< 0.001). Obviously, the cumulative hazard of developing DN was significantly higher in CC/CT genotype group than that in TT genotype group (HR, 3.27; 95% CI, 1.99–5.37; P< 0.001) (Fig. 1).
Fig. 1:
Cumulative prevalence of DN events according to T869C polymorphism
The predictors of DN for the CC/CT genotype group are summarized in Table 2. In a univariate analysis, age, HbA1c, and smoking status were associated with risk of DN. However, in the multivariate model after adjusting, hyperlipemia (HR, 1.91; 95%CI, 1.19–3.08; P=0.007) took the place of HbA1c, along with age (HR, 0.95; 95%CI, 0.93–0.98; P=0.001) and smoking status (HR, 2.36; 95%CI, 1.07–5.21; P=0.033) became predictors of DN. In addition, hypertension another traditional risk indicators of DN, was not significant, singly or in combination.
Table 2:
Univariate and multivariate Cox regression analysis for the risk indicators of DN in 158 Diabetes mellitus patients with CC/CT genetype
| Hazard ratio (95%CI) | P value | |
|---|---|---|
| univariate Cox regression analysis | ||
| Age (yr) | 0.95 (0.93–0.97) | <0.001 |
| Male | 1.38 (0.96–1.97) | 0.08 |
| HbA1c | 1.15 (1.01–1.318) | 0.036 |
| Smoking | 2.13 (1.11–4.09) | 0.023 |
| Drinking | 0.93 (0.65–1.32) | 0.667 |
| Hypertension | 1.05(0.64–1.74) | 0.851 |
| Hyperlipemia | 1.29(0.90–1.87) | 0.167 |
| multivariate Cox regression analysis | ||
| Age (yr) | 0.95(0.93–0.98) | <0.001 |
| Smoking | 2.36(1.07–5.21) | 0.033 |
| Hyperlipemia | 1.91(1.19–3.08) | 0.007 |
The predictors of DN for the TT genotype group are summarized in Table 3. Significant variables included age, HbA1c, smoking status and hypertension by univariate Cox regression analysis. While HbA1c (HR, 2.80; 95%CI, 1.07–7.30; P=0.036), hypertension (HR, 7.46; 95%CI, 1.38–40.29; P=0.02), and hyperlipemia (HR, 12.33; 95%CI, 1.05–145.3; P=0.046) were significant in the multivariate model after adjusting for age, smoking status, and drinking status.
Table 3:
Univariate and multivariate Cox regression analysis for the risk indicators of DN in 93 Diabetes mellitus patients with TT genetype
| Hazard ratio (95%CI) | P value | |
|---|---|---|
| univariate Cox regression analysis | ||
| Age (yr) | 1.08 (1.01–1.15) | 0.03 |
| Male | 0.77 (0.29–2.04) | 0.597 |
| HbA1c | 1.99 (1.22–3.24) | 0.006 |
| Smoking | 3.56 (1.35–9.43) | 0.011 |
| Drinking | 0.34 (0.11–1.03) | 0.057 |
| Hypertension | 13.93 (3.12–62.20) | 0.001 |
| Hyperlipemia | 21.19 (2.70–166.35) | 0.004 |
| multivariate Cox regression analysis | ||
| HBA1C | 2.80 (1.07–7.30) | 0.036 |
| Hypertension | 7.46 (1.38–40.29) | 0.02 |
| Hyperlipemia | 12.33 (1.05–145.39) | 0.046 |
Discussion
In our prospective cohort study of subjects with T2DM, the main findings are that: risk indictors of DN were age, smoking, and hyperlipemia were predictors of DN in CC/CT genotype patients, whereas HbA1c, hypertension and hyperlipemia in TT genotype patients.
TGF-β1 is a pleiotropic cytokine and has been considered as an essential risk factor in the development of DN. Elevated concentration of TGF-b1 could induce renal hypertrophy and promote excessive accumulation of extracellular matrix (15–17). Exactly these pathological changes contribute to the initiation and progression of DN. During the past few decades TGF-β1 gene polymorphism was found associated with hepatic carcinoma (18), lung cancer (19), colorectal cancer (20), and various fibrosis disease (21, 22) in many studies. TGF-β T869C gene polymorphism was associated with DN in Chinese (5). Similar results were also found in Egyptian (23). These results were in line with our findings in this prospective cohort study.
The exact mechanism underlying the effect of TGF-β1 T869C polymorphism on DN susceptibility is not clear. To date, we know that the TGF-β1 gene is located on chromosome 19 (q13.1–13.3), including 7 exons and 6 introns (24). There are several common known single nucleotide polymorphisms (SNPs) in this gene. Among them, T869C (rs1800470; Leu10/Pro10; T29C, codon10) in exon1 of the TGF-β1 gene is located at position 10 in the signal peptide. It was supposed that due to substitute C allele for T in T869C, Leucine was replaced by Proline, which accelerated the hydrolysis of TGF-β1 precursor to a mature TGF-β1 protein. Consequently, the concentration of TGF-β1 protein was elevated. In accord with this, several studies have been reported that the number of allele C was positively correlated with serum TGF-β1 concentration (25, 26). However, further studies will be necessary.
Smoking, hyperglycemia, hypertension, and hyperlipemias are conventional risk factors of developing diabetic nephropathy (27–29). However, we found that some people with serious comorbid conditions would not develop DN. Therefore, we presumed that patients with different genetic background may have different risk factors of developing DN. To confirm this presumption, we evaluated the prognostic risks of DN by Cox regression analysis. In this study, we found that age, smoking, and hyperlipemia was predictors of DN in CC/CT genotype patients, whereas HbA1c, hypertension and hyperlipemia were risk indictors of DN in TT genotype patients. These results were in keeping with our presumption.
Elevated serum levels of low-density lipoprotein cholesterol and triglycerides and low levels of high-density lipoprotein cholesterol are strongly associated with increased risk for macrovascular events (e.g., diabetic nephropathy, myocardial infarction, ischemic stroke, and coronary mortality) among patients with T2DM (29–31). These results are in keeping with our study, that hyperlipemia was a risk predictor of DN in CC/CT genotype group or TT genotype group as well as in total subjects.
In this study, the HbA1c levels and hypertension, two traditional risk indicators of DN were not the risk indictors of DN in CC/CT genotype patients. HbA1c levels are associated with concentrations of plasma glucose, which could stimulate TGF-β1 expression with a high concentration level (17, 32), and TGF-β1 protein increase the risk of developing DN. According to preceding hypothesis, T869C, CC/CT genotype is related to high TGF-β1 protein level without stimulated by high concentration of glucose. In other words, T869C CC/CT genotype is equivalent to the stimulation by glucose. Maybe it is the reason why HbA1c is not the risk indictor of DN for T869C CC/CT genotype patients. Hypertension is a common risk factor of DN (28, 33), whereas it is not risk indictor of DN in CC/CT genotype. It may be explained by the following reasons. First, the TGF-β1 T869C genotype polymorphism exerts more marked effects on development of DN than hypertension, and the two factors were correlated in CC/CT genotype subjects (Spearman correlation coefficient = 0.42, P<0.001). When all factors are taken into consideration, the value of the hypertension to predict DN was not superior to that of T869C genotype polymorphism. Secondly, T869C CC/CT genotype weakened the effects of hypertension on developing DN through by TGF-β1 signal pathway and/or an unknown way by now. In T869C CC/CT genotype group, the samples were relatively small, which might not have sufficient power to detect a small effect size.
In addition, other independent risk factors in our study for the presence of DN in CC/CT genotype patients were older age, smoking. Similar observations were previously made (27, 34–36).
Conclusion
The conclusion was based on relatively small study samples. Larger well-designed studies, involving more relevant variables, should be warranted in future to elucidate the mechanism of the TGF-β1 T869C genotype polymorphism in DN susceptibility. Despite its limitations, our study may have practical implications. We found that age, smoking, and hyperlipemia were risk indictors for DN in T2DM patients with TGF-β1 T869C CC/CT genotype, which suggested that these patients should pay more attention to avoid high-fat diet and smoking less. Furthermore, these findings might prompt initiation of appropriate treatment for T2DM patients with CC/CT genotype or TT genotype, respectively.
Ethical Considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 30801467, 81273623), Technology Commission of Zhejiang Province (Grant No. 2013C33212), and Medical Scientific Commission of Zhejiang Province (Grant No. 2010KYB087). The authors declare that there is no conflict of interest.
References
- 1.Collins AJ, Kasiske B, Herzog C, et al. (2007). Excerpts from the United States Renal Data System 2006 Annual Data Report. American Journal of Kidney Diseases, 49(1 Suppl 1): A6–A7. [DOI] [PubMed] [Google Scholar]
- 2.Yang XL, So WY, Kong AP, et al. (2006). End-stage renal disease risk equations for Hong Kong Chinese patients with type 2 diabetes: Hong Kong Diabetes Registry. Diabetologia, 49(10): 2299–2308. [DOI] [PubMed] [Google Scholar]
- 3.Telishevka M, Chenett L, McKee M. (2001). Towards an understanding of the high death rate among young people with diabetes in Ukraine. Diabet Med, 18(1): 3–9. [DOI] [PubMed] [Google Scholar]
- 4.Pettitt DJ, Saad MF, Bennett PH, Nelson RG, Knowler WC. (1990). Familial predisposition to renal disease in two generations of Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia, 33(7): 438–443. [DOI] [PubMed] [Google Scholar]
- 5.Wong TY, Poon P, Chow KM, Szeto CC, Cheung MK, Li PK. (2003). Association of transforming growth factor-beta (TGF-beta) T869C (Leu 10Pro) gene polymorphisms with type 2 diabetic nephropathy in Chinese. Kidney Int, 63(5): 1831–1835. [DOI] [PubMed] [Google Scholar]
- 6.Xu K, Liu X, Yang F, et al. (2013). PAI-1-675 4G/5G polymorphism in association with diabetes and diabetic complications susceptibility: a meta-analysis study. PloS One, 8(11): e79150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennler S, Gouman MJ, ten Dijke P. (2002). Transforming growth factor signal transduction. J Leukoc Biol, 71: 731–740. [PubMed] [Google Scholar]
- 8.Barnette DN, Hulin A, Ahmed AS, Colige AC, Azhar M, Lincoln J. (2013). Tgfbeta-Smad and MAPK signaling mediate scleraxis and proteoglycan expression in heart valves. J Mol Cell Cardiol, 65: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WA BTY, NA N. (1996). Transforming growth factor beta in diabetic nephropathy. Diabetes Metab Rev, 12(4): 309–339. [DOI] [PubMed] [Google Scholar]
- 10.Buraczynska M, Baranowicz-Gaszczyk I, Borowicz E, Ksiazek A. (2007). TGF-beta1 and TSC-22 gene polymorphisms and susceptibility to microvascular complications in type 2 diabetes. Nephron Physiol, 106(4): pp69–p75. [DOI] [PubMed] [Google Scholar]
- 11.Jia H, Yu L, Gao B, Ji Q. (2011). Association between the T869C polymorphism of transforming growth factor-beta 1 and diabetic nephropathy: a meta-analysis. Endocrine, 40(3): 372–378. [DOI] [PubMed] [Google Scholar]
- 12.Mou X, Liu WH, Zhou DY, et al. (2011). Association of Chinese medicine constitution susceptibility to diabetic nephropathy and transforming growth factor-beta1 (T869C) gene polymorphism. Chin J Integr Med, 17(9): 680–684. [DOI] [PubMed] [Google Scholar]
- 13.The Chinese Diabetes Society of the Chinese Medical Association China Guideline for Diabetes. Beijing: Peking University Medical Press; 2003: 10. [Google Scholar]
- 14.Mogensen CE. Early Diabetic Renal Involvelent and Nephropathy: Elsevier Science publishers; 1987: 306. [Google Scholar]
- 15.Kopp JB, Factor VM, Mozes M, et al. (1996). Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest, 74(6): 991–1003. [PubMed] [Google Scholar]
- 16.Mason RM, Wahab NA. (2003). Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol, 14(5): 1358–1373. [DOI] [PubMed] [Google Scholar]
- 17.Sharma K, Ziyadeh FN. (1994). Renal hypertrophy is associated with upregulation of TGF-beta 1 gene expression in diabetic BB rat and NOD mouse. Am J Physiol, 267(6 Pt 2): F1094–1001. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Wu H, Song C. (2013). TGF-beta1 -509C/T (or +869T/C) polymorphism might be not associated with hepatocellular carcinoma risk. Tumor Biology, 34(5): 2675–2681. [DOI] [PubMed] [Google Scholar]
- 19.Guerra JL, Gomez D, Wei Q, et al. (2012). Association between single nucleotide polymorphisms of the transforming growth factor beta1 gene and the risk of severe radiation esophagitis in patients with lung cancer. Radiother Oncol, 105(3): 299–304. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Yang H, Li L, Xia X. (2013). An updated meta-analysis on the association of TGF-beta1 gene promoter -509C/T polymorphism with colorectal cancer risk. Cytokine, 61(1): 181–187. [DOI] [PubMed] [Google Scholar]
- 21.Chiu CJ, Chang ML, Chiang CP, Hahn LJ, Hsieh LL, Chen CJ. (2002). Interaction of collagen-related genes and susceptibility to betel quid-induced oral submucous fibrosis. Cancer Epidemiol Biomarkers Prev, 11(7): 646–653. [PubMed] [Google Scholar]
- 22.Son JY, Kim SY, Cho SH, et al. (2013). TGF-beta1 T869C polymorphism may affect susceptibility to idiopathic pulmonary fibrosis and disease severity. Lung, 191(2): 199–205. [DOI] [PubMed] [Google Scholar]
- 23.El-Sherbini SM, Shahen SM, Mosaad YM, Abdelgawad MS, Talaat RM. (2013). Gene polymorphism of transforming growth factor-beta1 in Egyptian patients with type 2 diabetes and diabetic nephropathy. Acta Biochim Biophys Sin (Shanghai), 45(4): 330–338. [DOI] [PubMed] [Google Scholar]
- 24.Celedon JC, Lange C, Raby BA, et al. (2004). The transforming growth factor-beta1 (TGFB1) gene is associated with chronic obstructive pulmonary disease (COPD). Hum Mol Genet, 13(15): 1649–1656. [DOI] [PubMed] [Google Scholar]
- 25.Suthanthiran M, Li B, Song JO, et al. (2000). Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: A novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci U S A, 97(7): 3479–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada Y, Miyauchi A, Takagi Y, Tanaka M, Mizuno M, Harada A. (2001). Association of the C-509-->T polymorphism, alone of in combination with the T869-->C polymorphism, of the transforming growth factor-beta1 gene with bone mineral density and genetic susceptibility to osteoporosis in Japanese women. J Mol Med (Berl), 79(2–3): 149–156. [DOI] [PubMed] [Google Scholar]
- 27.Hovind P, Rossing P, Tarnow L, Parving HH. (2003). Smoking and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care, 26(3): 911–916. [DOI] [PubMed] [Google Scholar]
- 28.UK Prospective Diabetes Study Group (1998). Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ, 317(7160): 703–713. [PMC free article] [PubMed] [Google Scholar]
- 29.Misra A, Kumar S, Kishore Vikram N, Kumar A. (2003). The role of lipids in the development of diabetic microvascular complications: implications for therapy. Am J Cardiovasc Drugs, 3(5): 325–338. [DOI] [PubMed] [Google Scholar]
- 30.Leiter LA. (2005). The prevention of diabetic microvascular complications of diabetes: is there a role for lipid lowering? Diabetes Res Clin Pract, 68 Suppl 2: S3–14. [DOI] [PubMed] [Google Scholar]
- 31.Toth PP, Simko RJ, Palli SR, Koselleck D, Quimbo RA, Cziraky MJ. (2012). The impact of serum lipids on risk for microangiopathy in patients with type 2 diabetes mellitus. Cardiovasc Diabetol, 11: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman BB, Sharma K, Zhu Y, Ziyadeh FN. (1998). Transcriptional activation of transforming growth factor-beta1 in mesangial cell culture by high glucose concentration. Kidney Int, 54(4): 1107–1116. [DOI] [PubMed] [Google Scholar]
- 33.Barzilay J, Warram JH, Bak M, Laffel LM, Canessa M, Krolewski AS. (1992). Predisposition to hypertension: risk factor for nephropathy and hypertension in IDDM. Kidney Int, 41(4): 723–730. [DOI] [PubMed] [Google Scholar]
- 34.Chuahirun T, Wesson DE. (2002). Cigarette smoking predicts faster progression of type 2 established diabetic nephropathy despite ACE inhibition. Am J Kidney Dis, 39(2): 376–382. [DOI] [PubMed] [Google Scholar]
- 35.O’Meara ES, White M, Siscovick DS, Lyles MF, Kuller LH. (2005). Hospitalization for pneumonia in the Cardiovascular Health Study: incidence, mortality, and influence on longer-term survival. J Am Geriatr Soc, 53(7): 1108–1116. [DOI] [PubMed] [Google Scholar]
- 36.Yende S, Angus DC, Ali IS, et al. (2007). Influence of comorbid conditions on long-term mortality after pneumonia in older people. J Am Geriatr Soc, 55(4): 518–525. [DOI] [PubMed] [Google Scholar]