Abstract
Background
Although emergence from general anesthesia is clinically treated as a passive process driven by the pharmacokinetics of drug clearance, agents that hasten recovery from general anesthesia may be useful for treating delayed emergence, emergence delirium, and post-operative cognitive dysfunction. Activation of central monoaminergic neurotransmission with methylphenidate has been shown to induce reanimation (active emergence) from general anesthesia. Cholinergic neurons in the brainstem and basal forebrain are also known to promote arousal. The objective of this study was to test the hypothesis that physostigmine, a centrally acting cholinesterase inhibitor, induces reanimation from isoflurane anesthesia in adult rats.
Methods
The dose-dependent effects of physostigmine on time to emergence from a standardized isoflurane general anesthetic were tested. It was then determined whether physostigmine restores righting during continuous isoflurane anesthesia. In a separate group of rats with implanted extradural electrodes, physostigmine was administered during continuous inhalation of 1.0% isoflurane, and the electroencephalogram changes were recorded. Finally, 2.0% isoflurane was used to induce burst suppression, and the effects of physostigmine and methylphenidate on burst suppression probability (BSP) were tested.
Results
Physostigmine delayed time to emergence from isoflurane anesthesia at doses ≥0.2 mg/kg (n=9). During continuous isoflurane anesthesia (0.9% ± 0.1%), physostigmine did not restore righting (n=9). Blocking the peripheral side effects of physostigmine with the co-administration of glycopyrrolate (a muscarinic antagonist that does not cross the blood-brain barrier) produced similar results (n=9 each). However, during inhalation of 1.0% isoflurane, physostigmine shifted peak electroencephalogram power from δ (<4 Hz) to θ (4-8 Hz) in 6/6 rats. During continuous 2.0% isoflurane anesthesia, physostigmine induced large, statistically significant decreases in BSP in 6/6 rats, whereas methylphenidate did not.
Conclusions
Unlike methylphenidate, physostigmine does not accelerate time to emergence from isoflurane anesthesia, and does not restore righting during continuous isoflurane anesthesia. However, physostigmine consistently decreases BSP during deep isoflurane anesthesia, whereas methylphenidate does not. These findings suggest that activation of cholinergic neurotransmission during isoflurane anesthesia produces arousal states that are distinct from those induced by monoaminergic activation.
Introduction
Although emergence from general anesthesia is clinically treated as a passive process driven by the pharmacokinetics of drug clearance, agents that hasten recovery from general anesthesia may be useful for the treatment of delayed emergence, emergence delirium, and post-operative cognitive dysfunction.1 Numerous ascending arousal pathways and their corresponding neurotransmitters have been identified in the brain,2-4 and it is becoming increasingly evident that these pathways are important for emergence from general anesthesia.4-8 The pharmacological modulation of arousal states can provide considerable insight into the neuronal pathways and circuit mechanisms of general anesthesia. However, the brain states produced by activating arousal circuits during general anesthesia remain largely uncharacterized.
Methylphenidate (an inhibitor of dopamine and norepinephrine reuptake transporters) induces reanimation, or active emergence (behaviorally defined by restoration of righting in rodents) during continuous general anesthesia with isoflurane9 or propofol.10 Reanimation also occurs with the administration of dextroamphetamine11 and a D1 dopamine receptor agonist,12 as well as electrical stimulation of the ventral tegmental area (VTA), a major dopamine nucleus in the brain.1 These findings suggest that a dopaminergic arousal pathway projecting from the VTA promotes active emergence from general anesthesia.
Arousal-promoting cholinergic neurons project from the laterodorsal tegmental area and pedunculopontine tegmental area in the brainstem to the thalamus, upper brainstem, midbrain, and regions of the prefrontal cortex and basal forebrain, while a separate group of arousal-promoting cholinergic neurons in the basal forebrain project mainly to cortex.13 Unlike neostigmine, physostigmine is a cholinesterase inhibitor that crosses the blood-brain barrier and therefore stimulates central cholinergic neurotransmission.14 Some studies suggest that physostigmine may be clinically useful to enhance recovery from general anesthesia,15,16 while others suggest limited efficacy.17,18
This study was performed to test the hypothesis that physostigmine induces reanimation from isoflurane anesthesia in adult rats. Using the same experimental protocols used previously in our laboratory, these experiments allowed us to perform a direct comparison to our prior results with methylphenidate.9 We also performed additional experiments to compare the effects of physostigmine and methylphenidate at a high dose of isoflurane that induced burst suppression on the electroencephalogram.
Materials and Methods
Ethics Statement
All studies were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee, and were performed in strict accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Invasive procedures and experiments were always performed under general anesthesia, and appropriate analgesia was provided after surgery. All efforts were made to minimize animal suffering.
Animal Care and Use
In our previous study using the same experimental methods with methylphenidate,9 the standard deviation for time to emergence was 153 sec for the normal saline group, and 32 sec for the methylphenidate group. We made the initial prediction that physostigmine would decrease the standard deviation for time to emergence similar to methylphenidate, but to a lesser extent. Therefore, for our power calculation we estimated the standard deviation of the physostigmine group to be 80 sec. In order to detect an effect size of 90 seconds with a Type I error of 0.05 and power of 0.8, we calculated that a sample size of 9 rats in each group would be required.
Twenty-four male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used for this study. One group of 9 rats was used in random order for all behavioral experiments without glycopyrrolate, and a second group of 9 rats was used for all behavioral experiments with glycopyrrolate. In addition, a third group of 6 rats was used in random order for all experiments involving electroencephalogram recordings. Each animal was provided with at least 3 days of rest between experiments. Animals were kept on a standard day-night cycle (lights on at 7:00 AM and off at 7:00 PM), and all experiments were performed during the day.
Anesthetizing Protocol
After inducing general anesthesia with isoflurane (2% to 3%) in oxygen, a 24-gauge IV catheter was placed in a lateral tail vein, a rectal temperature probe was inserted, and the animal was placed in a cylindrical acrylic anesthetizing chamber as previously described.9 A heating pad was placed under the chamber to maintain rectal temperature between 36.5°C and 37.4°C. Gas was sampled from the distal end of the chamber, and isoflurane, oxygen, and carbon dioxide concentrations were continuously monitored with a calibrated Ohmeda 5250 anesthetic agent analyzer (GE Healthcare, Waukesha, WI).
Preparation and Delivery of Drugs
Isoflurane, physostigmine and glycopyrrolate were obtained from Henry Schein (Melville, NY), Akorn (Lake Forest, IL) and American Regent (Shirley, NY), respectively. Methylphenidate was purchased from Sigma–Aldrich (St. Louis, MO), dissolved in normal saline to a final volume of 0.5 ml, and sterile filtered prior to IV administration via the lateral tail vein catheter. The intravenous tubing (approximate volume 0.6 ml) was always flushed with 2 ml of normal saline after drug administration to ensure complete drug delivery.
Time to Emergence after a Standardized Isoflurane General Anesthetic
After placement of the lateral tail vein catheter, the inhaled concentration of isoflurane was fixed at 1.5%, and normothermia was maintained with a warming pad as described previously.9 After 40 minutes, rats (n=9) received normal saline or physostigmine IV (0.1 mg/kg or 0.2 mg/kg). The same 9 rats were used for all 3 conditions, in random order, with at least 3 days of rest between experiments. Isoflurane was continued for 5 additional minutes, after which the rat was taken from the chamber and the temperature probe removed. The animal was placed supine on a warming pad and inspired room air. Time to emergence was defined as the time from termination of isoflurane to return of righting (prone position with all four paws touching the floor). In order to test whether the peripheral muscarinic side effects of physostigmine affected time to emergence, additional experiments were conducted with the same anesthesia protocol in a separate group of rats (n=9) that received normal saline or physostigmine (0.2 mg/kg or 0.4 mg/kg IV) with glycopyrrolate (0.2 mg per 1 mg of physostigmine, e.g. 0.04 mg/kg of glycopyrrolate for 0.2 mg/kg of physostigmine). The same 9 rats were used for all 3 conditions, in random order, with at least 3 days of rest between experiments.
Test for Reanimation during Continuous Isoflurane General Anesthesia
The isoflurane concentration was held at a dose that produced loss of righting with no purposeful movement for 40 consecutive minutes, as described previously.9 The IV catheter was then flushed with 2 ml of normal saline to ensure patency of the IV, and to confirm that the injection of normal saline did not induce an arousal response. Five minutes after this control injection, physostigmine alone (0.2 mg/kg), or physostigmine (0.4 mg/kg) with glycopyrrolate (0.08 mg/kg) was administered (n=9 each). After IV drug administration, each animal continued to inhale the same dose of isoflurane for 20 minutes.
Electroencephalogram Electrode Placement and Recording
Extradural electroencephalogram electrodes were implanted surgically at least 7 days before recording, as detailed previously.9,10 On the day of an experiment, the potential difference between electrodes A0L0 and A6L3 (right somatosensory cortex) or between electrodes A0L0 and A6L-3 (left somatosensory cortex) was recorded based on which signal gave less motion artifact. The signal was referenced to A10L2 and recorded using a QP511 Quad AC Amplifier System (Grass Instruments, West Warwick, RI) and a USB-6009 14-bit data acquisition board (National Instruments, Austin, TX). The sampling rate was 512 Hz, and no line filter was used. The electroencephalogram signal was filtered between 0.3 Hz and 50 Hz.
Rats were anesthetized with isoflurane and placed in the chamber, with the inhaled isoflurane concentration fixed at 1.0% or 2.0%. The electroencephalogram was recorded continuously, and the prone position was used in all electroencephalogram experiments to minimize motion artifacts.9 After a minimum isoflurane exposure of 40 minutes, normal saline was administered IV as a control injection, and 5 minutes later physostigmine (0.2 mg/kg IV) or methylphenidate (5 mg/kg IV) was administered. Isoflurane anesthesia was continued at the same dose for an additional 20 minutes.
Spectral Analysis of the Electroencephalogram
Multitaper methods from the Chronux toolboxes in MATLAB 8.3 were used to construct time-frequency domain spectrograms, and perform frequency domain spectral analysis.19,20 Spectrograms were constructed from electroencephalogram data using a 3-second window with a stepsize of 0.25 seconds. The spectrogram frequency band was from 0 to 35 Hz, the half-bandwidth was 1 Hz, and 5 tapers were used in its calculation.
Power spectral densities were computed from 2-minute time windows pre- and post-drug administration. For all experiments, the pre-drug window was 150 to 30 seconds before injection, and the post-drug window was 30 to 150 seconds after injection. Group power spectra were created using multitaper methods, and the time-frequency toggle bootstrap.21 The time-frequency toggle bootstrap was used to obtain independent and identically distributed time-domain samples from the electroencephalogram. This allowed us to construct confidence intervals using a percentile bootstrap. The power spectral density within each bootstrapped electroencephalogram sample was constructed by calculating the spectral density for the first 2 seconds of bootstrapped electroencephalogram sample, and then by stepping 2 seconds until the end of the time window. Each power spectral density had a frequency range of 0 to 35 Hz (141 discrete frequencies), and a half-bandwidth of 1 Hz, and was constructed using 3 tapers. For electroencephalogram recordings that had periods of burst suppression, the suppression component was omitted from the spectral analysis. To account for uncertainty in group spectral densities, 95% confidence intervals were constructed around the mean using the percentile bootstrap.22
Classification of Electroencephalographic Bursts and Suppressions
Bursts and suppressions were classified for electroencephalogram experiments where animals continuously inhaled 2.0% isoflurane. Electroencephalograms were detrended by convoluting the electroencephalogram with a Gaussian function and then subtracting the convolution from the original signal. The electroencephalogram was transformed to the energy domain using the discrete version of the non-linear energy operator.23 The energy domain was additionally convoluted with a Gaussian function for smoothing, and then a manual threshold was set to separate high energy bursts from the lower energy suppressions. Electroencephalogram values that fell above the threshold were classified as bursts, and values that fell below the threshold were classified as suppressions.
Calculation of the Burst-Suppression Probability
Electroencephalogram data that were classified as bursts were given a value of 0, and data that were classified as suppressions were given a value of 1. This binary time-series was used by the burst-suppression probability (BSP) algorithm to find the probability of burst-suppression, with 0 indicating no probability of suppression and 1 indicating an isoelectric electroencephalogram. Unlike the burst-suppression ratio (BSR), the BSP gives the instantaneous probability of burst-suppression, as well as a corresponding measure of uncertainty in the form of confidence intervals on a one-second timescale.24,25
Statistical Analysis
Prism 5.04 (Graphpad Software, San Diego, CA) or MATLAB 8.4 was used for statistical analysis and, when possible, results are reported in terms of 95% confidence intervals based on bootstrap analysis. Confidence intervals constructed using the percentile bootstrap were used to test whether physostigmine accelerates emergence from isoflurane anesthesia.
A Bayesian Monte Carlo procedure was used to compute Bayesian 95% CI (credibility intervals). The CIs were used to determine the efficacy of methylphenidate or physostigmine to restore righting, as previously described.9 We computed the posterior probability that the propensity to restore righting in the methylphenidate group was greater than in the physostigmine group. We considered the difference to be statistically significant if the posterior probability was greater than 0.95.
The effects of methylphenidate and physostigmine during isoflurane-induced burst suppression were analyzed by comparing the BSP before and after drug injection. We compared “pre-drug” time points (from 5 minutes before injection to the time of injection) with “post-drug” time points (from 2 minutes after injection to 7 minutes after injection). We did not use time points during the first 2 minutes after drug administration in order to allow the drugs sufficient time to take effect. Comparisons were made using a Bayesian Monte Carlo procedure. 10,000 time points were randomly chosen from the “pre-drug” and “post-drug” time periods. The joint distribution of the state process was estimated for these 10,000 pairs, and the difference between the state processes was found. We then calculated 95% confidence intervals for these 10,000 differences. If the overall confidence bounds were negative, this was considered a statistically significant decrease in BSP. Conversely, if the overall bounds were positive, this was considered to be a statistically significant increase in BSP. If the bounds were both positive and negative, it was concluded that there was no statistically significant difference in BSP.
Results
Physostigmine Increases Time to Emergence from Isoflurane General Anesthesia
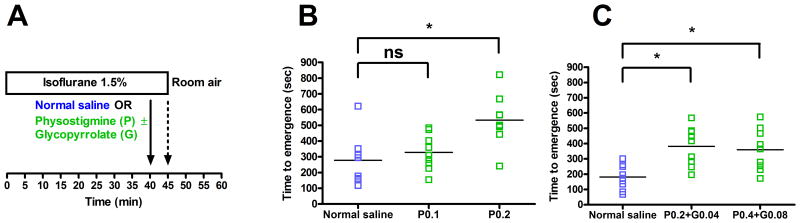
Figure 1A illustrates the experimental protocol used to test for time to emergence from isoflurane general anesthesia. Time to emergence was defined as the time from termination of general anesthesia until return of righting. As shown in Figure 1B (n=9), rats that received normal saline had a mean time to emergence of 278 sec (95% CI: 192 to 383 sec). Rats that received 0.1 mg/kg of physostigmine had a small increase in mean time to emergence to 328 sec (95% CI: 261 to 397 sec), but this increase was not statistically significant (mean difference: 50 sec; 95% CI: -75 to 161 sec). However, rats that received 0.2 mg/kg of physostigmine had a mean time to emergence of 533 sec (95% CI: 436 to 633 sec), and the difference between this group and the normal saline group was statistically significant (mean difference: 255 sec; 95% CI: 114 to 384 sec).
Figure 1. Physostigmine increases time to emergence from isoflurane general anesthesia.
(A) Rats inhaled 1.5% isoflurane in oxygen for 40 min, and then received normal saline or physostigmine ± glycopyrrolate IV (solid arrow). After 5 additional minutes of isoflurane anesthesia, rats were removed from the anesthetizing chamber (dashed arrow) and emergence was defined as return of righting. (B) Scatter plots showing emergence times (n=9). P0.1 = Physostigmine 0.1 mg/kg, P0.2 = physostigmine 0.2 mg/kg. For each data set, the horizontal line represents the mean. (C) Scatter plots showing emergence times for a separate cohort of rats (n=9). P0.2+G0.04 = physostigmine 0.2 mg/kg and glycopyrrolate 0.04 mg/kg, P0.4+G0.08 = physostigmine 0.4 mg/kg and glycopyrrolate 0.08 mg/kg. * Significant difference between groups with 95% confidence.
In order to test whether the peripheral cholinergic effects of physostigmine were affecting emergence time, in a separate group of rats (n=9) the antimuscarinic agent glycopyrrolate was co-administered with physostigmine. As shown in Figure 1C, in this group the mean time to emergence after normal saline (control) was 181 sec (95% CI: 133 to 240 sec). Rats that received physostigmine (0.2 mg/kg IV) with glycopyrrolate (0.04 mg/kg IV) had an increase in mean time to emergence to 382 sec (95% CI: 302 to 458 sec), and this increase was statistically significant (mean difference: 201 sec; 95% CI: 109 to 293 sec). Rats that received an even higher dose of physostigmine (0.4 mg/kg) with glycopyrrolate (0.08 mg/kg) had a mean time to emergence of 360 sec (95% CI: 280 to 444 sec), and the difference between this group and the normal saline group was also statistically significant (mean difference: 179 sec; 95% CI: 84 to 276 sec).
Physostigmine Does Not Induce Reanimation during Continuous Isoflurane Anesthesia
The continuous dose of isoflurane required to maintain loss of righting (0.9% ± 0.1%, mean ± SD) was the same as in our previous study.9 None of the rats had return of righting during the 20 minute observation period after the administration of physostigmine alone (0.2 mg/kg IV, n=9). Although one animal had occasional head and limb movements after physostigmine administration, 8/9 animals did not exhibit any behavioral signs of arousal. Righting attempts, grooming, or escape behaviors that were commonly observed with methylphenidate in our previous study9 were not observed with physostigmine.
In order to determine whether physostigmine did not restore righting due to insufficient dosing or peripheral cholinergic effects, we used a separate group of rats (n=9) under the same anesthetizing conditions, but co-administered high doses of physostigmine (0.2 mg/kg or 0.4 mg/kg IV) with glycopyrrolate (0.2 mg per 1 mg of physostigmine). At both doses, 0/9 rats had return of righting within 20 minutes of physostigmine/glycopyrrolate co-administration. In the animals that received the highest dose of physostigmine (0.4 mg/kg IV) with glycopyrrolate (0.08 mg/kg IV), increased muscle twitching was observed, but righting was not restored in any of the animals.
Physostigmine Decreases Electroencephalogram Power during Continuous Inhalation of 1.0% Isoflurane
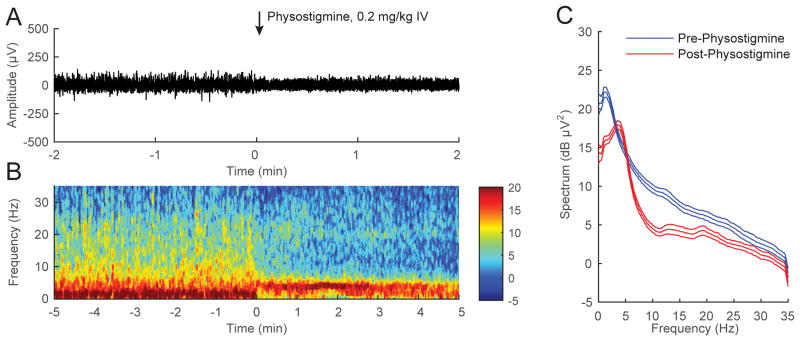
A representative electroencephalogram recorded during continuous inhalation of 1.0% isoflurane (Figure 2A) shows that the administration of physostigmine (0.2 mg/kg IV) decreased the overall amplitude of the signal. A spectrogram computed from the same animal (Figure 2B) shows that physostigmine decreased δ (<4 Hz) power and increased θ (4-8 Hz) power. The combined power spectra computed from all 6 animals (Figure 2C) show that physostigmine increased θ power, decreased δ power and α (8-12 Hz) power, and shifted peak power from δ to θ. The non-overlapping 95% confidence intervals in the power spectra indicate that these changes in electroencephalogram power were statistically significant.
Figure 2. Physostigmine increases θ power during continuous inhalation of 1.0% isoflurane.
(A) A representative electroencephalogram recorded from a rat inhaling 1.0% isoflurane, with time=0 indicating the start of physostigmine (0.2 mg/kg IV) injection. There was a decrease in overall amplitude shortly after drug administration. (B) A spectrogram computed from the same animal in (A), with warm colors (e.g. red) indicating high power and cool colors (e.g. blue) indicating low power at any given frequency. Physostigmine (0.2 mg/kg IV) was administered at time=0. A rapid shift in peak power from δ to θ occurred after drug injection. (C) The pre-physostigmine power spectral density (computed from a two-minute window -150 sec to -30 sec before drug injection) and the post-physostigmine power spectral density (computed from a two-minute window 30 sec to 150 sec after drug injection) and their 95% confidence intervals (with Bonferroni correction) were constructed around the mean spectra across all rats (n=6). Physostigmine increased θ power, decreased δ and α power, and shifted peak power from δ to θ.
Physostigmine Decreases BSP during Continuous Inhalation of 2.0% Isoflurane
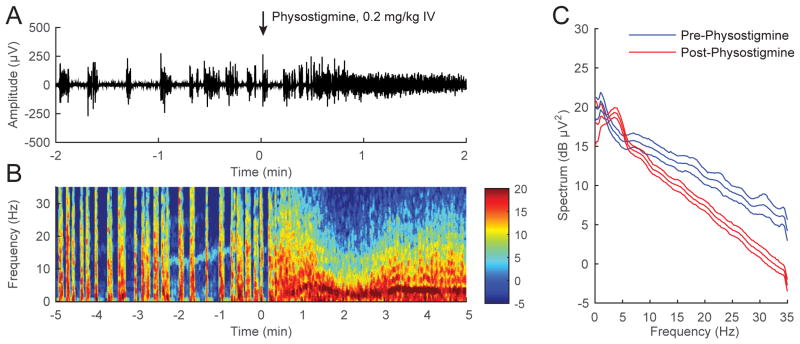
A representative electroencephalogram recorded during continuous inhalation of 2.0% isoflurane (Figure 3A) shows that the administration of physostigmine (0.2 mg/kg IV) changed the burst suppression pattern to a new rhythm without isoelectric (suppression) periods. A spectrogram computed from the same animal in Figure 3B shows that the new rhythm after physostigmine administration had high δ, θ and α power. The combined power spectra computed from burst periods in all 6 animals (Figure 3C) show that power at higher frequencies was significantly decreased after physostigmine administration.
Figure 3. Physostigmine reverses burst suppression during continuous inhalation of 2.0% isoflurane.
(A) A representative electroencephalogram recorded from a rat inhaling 2.0% isoflurane, with time=0 indicating the beginning of physostigmine (0.2 mg/kg IV) injection. The electroencephalogram changed from a burst suppression pattern to a new rhythm without isoelectric (suppression) periods after physostigmine administration. (B) A spectrogram computed from the same animal in (A) shows that physostigmine increased δ, θ and α power. (C) Power spectral densities computed from burst periods 2-minutes pre- and post-physostigmine, and their 95% confidence intervals (with Bonferroni correction), were constructed around the mean spectra across all rats (n = 6). Power at higher frequencies was reduced after the administration of physostigmine.
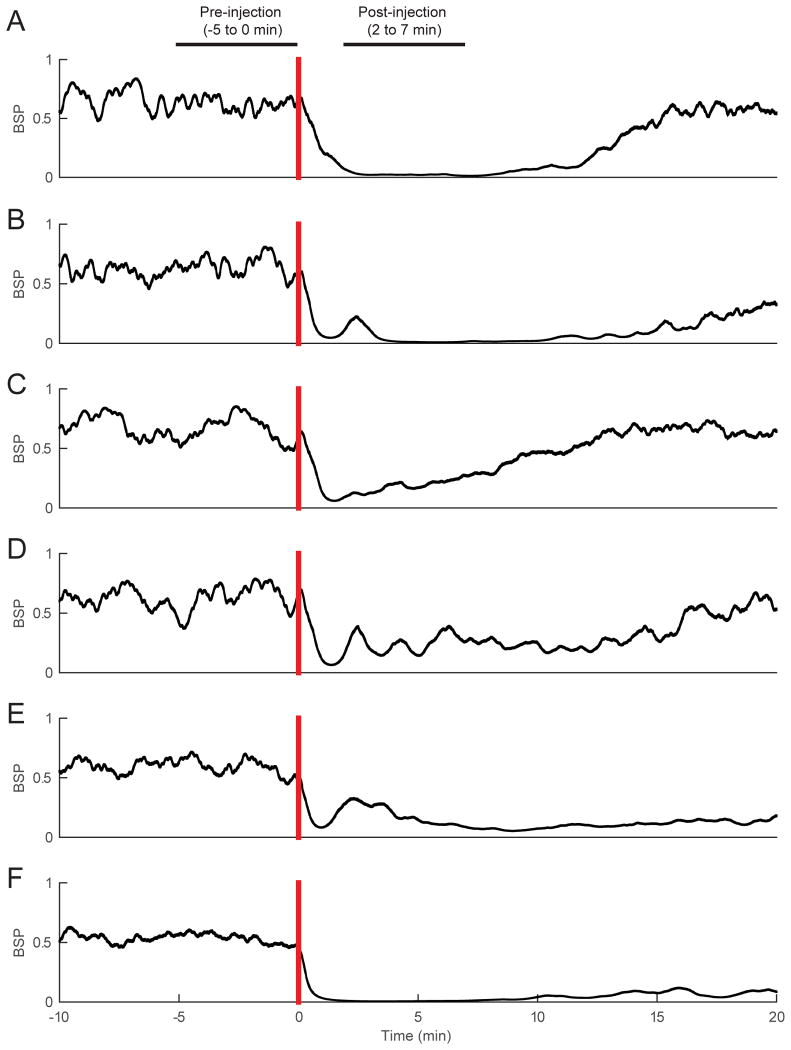
Figure 4 shows the computed BSP for each of the 6 rats before and after physostigmine administration. The administration of physostigmine (0.2 mg/kg IV) induced a large decrease in BSP in 6/6 animals, and in 3/6 rats, the BSP became 0 (i.e. suppression periods were no longer present). The Bayesian Monte Carlo comparison between 10,000 randomly chosen time points before and after physostigmine administration yielded a statistically significant decrease in BSP for 6/6 rats.
Figure 4. Physostigmine decreases BSP during continuous inhalation of 2.0% isoflurane.
(A)-(F) The BSP computed for each of 6 rats during continuous inhalation of 2.0% isoflurane, from 10 minutes before physostigmine (0.2 mg/kg IV) to 20 minutes after physostigmine. All 6 rats had large reductions in BSP after physostigmine administration. When comparing the “pre-drug” and “post-drug” periods shown in the figure (indicated by the horizontal bars), physostigmine induced statistically significant reductions in BSP in 6/6 rats.
Methylphenidate Does Not Decrease BSP during Continuous Inhalation of 2.0% Isoflurane
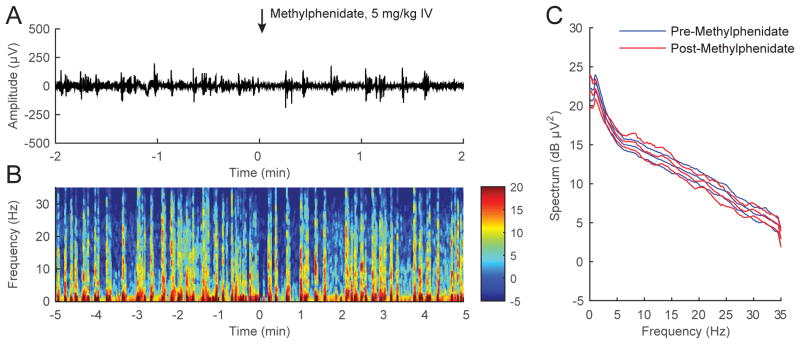
Figure 5A shows a representative electroencephalogram recorded from a rat that received methylphenidate during continuous inhalation of 2.0% isoflurane. Unlike physostigmine, methylphenidate did not induce an appreciable change in the electroencephalogram pattern, which remained in burst suppression. The spectrogram computed from the same animal (Figure 5B) demonstrates that methylphenidate did not induce obvious changes, and the combined analysis for all 6 rats (Figure 5C) shows that methylphenidate did not induce significant changes in the power spectrum.
Figure 5. Methylphenidate does not induce significant electroencephalogram changes during continuous inhalation of 2.0% isoflurane.
(A) A representative electroencephalogram recorded from a rat inhaling 2.0% isoflurane, with time=0 indicating the start of methylphenidate (5 mg/kg IV) injection. The electroencephalogram remained in a burst suppression pattern after methylphenidate was administered. (B) A spectrogram computed from the same animal shows that methylphenidate did not induce significant changes in spectral power. (C) Power spectral densities computed from burst periods 2-minutes pre- and post-methylphenidate, and their 95% confidence intervals (with Bonferroni correction), were constructed around the mean spectra across all rats (n = 6). Methylphenidate did not induce significant changes in the power spectrum.
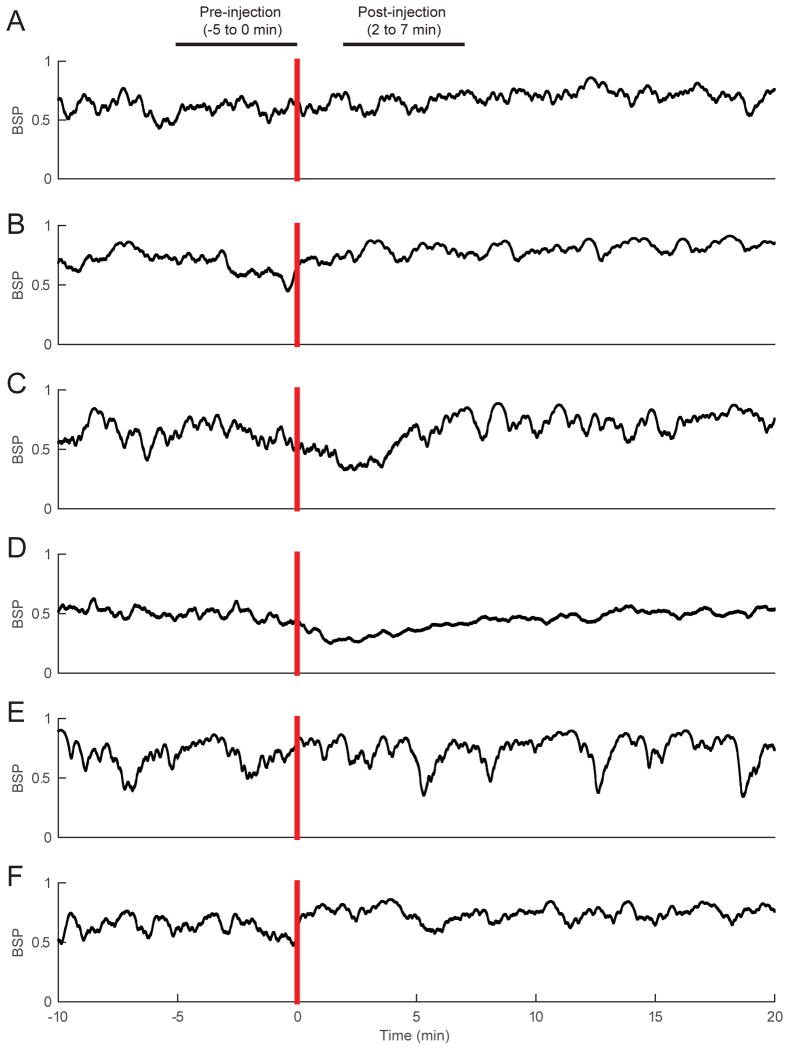
The BSP computed for each of the 6 rats (Figure 6) shows that, unlike physostigmine, methylphenidate induced relatively small (if any) changes in BSP. The Bayesian Monte Carlo comparison between 10,000 randomly chosen time points before and after methylphenidate administration yielded no statistically significant difference in BSP for 6/6 rats.
Figure 6. Methylphenidate does not induce significant changes in BSP during continuous inhalation of 2.0% isoflurane.
(A)-(F) The BSP computed for each of 6 rats during continuous inhalation of 2.0% isoflurane, from 10 minutes before methylphenidate (5 mg/kg IV) to 20 minutes after methylphenidate administration. When comparing the “pre-drug” and “post-drug” periods shown in the figure (indicated by the horizontal bars), methylphenidate did not induce statistically significant changes in BSP in any of the rats.
Discussion
In this study, we employed the same experimental protocols used previously in our laboratory to provide a direct comparison between the arousal effects of physostigmine and methylphenidate during isoflurane general anesthesia in rats. Unlike methylphenidate, physostigmine did not decrease time to emergence from isoflurane general anesthesia, and at doses ≥0.2 mg/kg (alone or co-administered with glycopyrrolate), physostigmine increased time to emergence. In addition, physostigmine did not restore righting during continuous isoflurane anesthesia, regardless of whether glycopyrrolate was co-administered.
As in our previous studies,1,9,10,12 we only used the righting reflex as our behavioral endpoint, and did not employ a formal arousal scoring system to document more subtle signs of arousal during continuous isoflurane anesthesia. This is because there is currently no standard arousal scoring system in the literature, although several groups have devised such systems in an effort to refine measures of the anesthetized state.8,26-28 Furthermore, because physostigmine can produce significant effects at the neuromuscular junction, it is difficult to distinguish whether minor peripheral movements in the absence of righting represent physostigmine-induced muscle twitches, or a true change in arousal state. Although the highest dose of physostigmine (0.4 mg/kg) co-administered with glycopyrrolate (0.08 mg/kg) caused more muscle twitching than lower doses of physostigmine (0.1-0.2 mg/kg), these movements were distinct from the purposeful movements (e.g. grooming, clawing, and righting attempts) observed with methylphenidate, 9 a D1 agonist,12 and VTA stimulation1 during isoflurane anesthesia.
The co-administration of glycopyrrolate with physostigmine did not decrease time to emergence when compared with saline controls, and did not cause restoration of righting during continuous isoflurane anesthesia. These findings suggest that the peripheral muscarinic side effects of physostigmine are not the reason for the lack of reanimation observed with physostigmine. The present results are consistent with the report by Reed et al.28 who found that the combination of physostigmine and glycopyrrolate (at the same doses used in this study) does not restore righting in rats during continuous propofol anesthesia. Because glycopyrrolate does not cross the blood-brain barrier,14 the presence or absence of glycopyrrolate should not affect the electroencephalogram results. Therefore we did not perform additional electroencephalogram experiments with glycopyrrolate.
However, it is likely that physostigmine produced some degree of muscle weakness due to high acetylcholine levels at the neuromuscular junction. Eikermann et al. previous reported that neostigmine dose-dependently causes a decrease in EMG activity in the genioglossus and diaphragm in isoflurane-anesthetized rats.29 Physostigmine and neostigmine are cholinesterase inhibitors that have similar peripheral effects, so it is reasonable to assume that physostigmine caused some degree of muscle weakness in our study as well, which may have contributed to the prolonged emergence times.
Several studies have suggested that physostigmine may be clinically useful as an arousal-promoting agent to reverse the effects of general anesthesia. In dogs, the administration of physostigmine during halothane anesthesia produced a rapid change in EEG to a low-amplitude pattern similar to the awake state, although no behavioral evidence of arousal was noted.30 In surgical patients, physostigmine reduced postoperative somnolence after halothane anesthesia.31
Hudetz et al.32 reported that the direct administration of neostigmine into the cerebral ventricles of rats during isoflurane anesthesia produced an increase in cross-approximate entropy of the electroencephalogram, and elicited behavioral signs of arousal, such as spontaneous limb movements and orofacial explorative movements. Alkire et al.6 reported that the microinjection of nicotine in the central medial thalamus (CMT) restored righting during continuous sevoflurane inhalation, providing evidence that cholinergic pathways that activate the thalamus induce arousal during general anesthesia. The latter group also showed that microinfusion of an antibody to a voltage-gated potassium channel in the CMT produces similar behaviors,33 suggesting that the CMT plays an important role in modulating arousal. These studies suggest that the route of administration is important for drugs that stimulate central cholinergic neurotransmission. Because peripheral cholinergic neurotransmission is involved in neuromuscular transmission as well as the parasympathetic nervous system, physostigmine can produce profound peripheral side effects that can be avoided when the drugs are administered ICV.
In studies involving volunteers, physostigmine reversed propofol-induced LOC in 9 of 11 subjects,15 and reversed sevoflurane-induced LOC in 5 of 8 subjects.34 Both of these studies reported that the administration of physostigmine produced significant increases in the auditory steady-state response and bispectral index. In a follow-up study by the same research group,35 8 of 11 subjects regained consciousness during propofol anesthesia with physostigmine administration, and positron emission tomography was used to demonstrate that increases in regional cerebral blood flow to the thalamus and precuneus correlated with physostigmine-induced arousal.
However, several studies have suggested that physostigmine does not antagonize the effects of general anesthesia. In dogs, physostigmine transiently increased halothane MAC, but then produced a dose-dependent decrease in MAC,36 and the authors concluded that physostigmine would not be effective for treating post-operative somnolence after halothane anesthesia. In patients undergoing breast surgery, Paraskeva et al. found that physostigmine does not accelerate time to recovery from sevoflurane anesthesia and does not change the bispectral index,37,38 even when sevoflurane is used as the sole anesthetic.17 Rohm et al. found that physostigmine does not accelerate emergence after desflurane anesthesia,18 and Zvosec et al. reported that physostigmine is not effective for treating gamma-hydroxybutyrate intoxication.39
During continuous inhalation of 1.0% isoflurane, we found that physostigmine induced a shift in peak electroencephalogram power from δ to θ, similar to the results of our previous study with methylphenidate.9 However, unlike methylphenidate, physostigmine failed to restore righting during isoflurane anesthesia, and did not evoke a significant behavioral arousal response. Our findings suggest that both drugs induce neurophysiological changes that lead to cortical activation during isoflurane anesthesia.
Although it is possible that the observed lack of behavioral arousal with physostigmine may have been due to muscle weakness, as noted above, several groups have reported similar dissociations between neurophysiological and behavioral evidence of arousal. Luo and Leung7 reported that the microinjection of histamine into the nucleus basalis magnocellularis of rats during deep isoflurane anesthesia caused a reduction in the burst suppression ratio, which did not correlate directly with behavioral evidence of arousal. Our group recently found that atomoxetine, a selective inhibitor of the norepinephrine transporter, induces a shift in spectral power from δ to θ during isoflurane anesthesia, but does not elicit signs of behavioral arousal.11 Conversely, McCarron et al. recently reported that dexmedetomidine microinjected into the ventrolateral preoptic area (VLPO) during isoflurane anesthesia induces behavioral arousal, but does not elicit spectral changes in the EEG.27 Similarly, we reported that the D1 agonist chloro-APB induces behavioral arousal including return of righting during continuous isoflurane anesthesia in rats, but only produces relatively small changes in EEG spectral power.12
Interestingly, physostigmine consistently induced a large, statistically significant decrease in BSP during inhalation of 2.0% isoflurane, whereas methylphenidate did not. Burst suppression is an electroencephalogram pattern indicating a state of markedly decreased metabolic activity,40 and the present results suggest that brain metabolic activity is significantly increased when physostigmine is administered during isoflurane anesthesia, whereas methylphenidate has little to no effect in this regard. These differences may be due to the distinct characteristics of cholinergic and monoaminergic arousal circuits in the brain. Future studies with intracranial recordings and circuit-specific manipulations are needed to shed more light on these mechanisms.
In conclusion, the results of the present study suggest that methylphenidate is more likely than physostigmine to promote active emergence from general anesthesia. However, physostigmine induces significant electroencephalogram changes during burst suppression that are consistent with neurophysiological antagonism of isoflurane anesthesia. Taken together, the current findings and the results of our previous studies suggest that cholinergic and monoaminergic stimulation produce distinct arousal states during isoflurane general anesthesia.
Acknowledgments
Funding: This work was supported by National Institutes of Health grant numbers TR01-GM104948, DP1-OD003646, and K08-GM094394.
Disclosures
Name: Jonathan D. Kenny*
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts: Jonathan D. Kenny reported no conflicts of interest.
Attestation: Jonathan D. Kenny has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Jessica J. Chemali, BS*
Contribution: This author helped conduct the study and analyze the data.
Conflicts: Jessica J. Chemali has filed a patent application for the use of intravenous methylphenidate to reverse general anesthesia in surgical patients.
Attestation: Jessica J. Chemali has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Joseph F. Cotten, MD, PhD
Contribution: This author helped design the study and conduct the study.
Conflicts: Joseph F. Cotten has filed a patent application for the use of intravenous methylphenidate to reverse general anesthesia in surgical patients.
Attestation: Joseph F. Cotten has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Christa J. Van Dort, PhD
Contribution: This author helped write the manuscript.
Conflicts: Christa J. Van Dort reported no conflicts of interest.
Attestation: Christa J. Van Dort has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Seong-Eun Kim, PhD
Contribution: This author helped analyze the data.
Conflicts: Seong-Eun Kim reported no conflicts of interest.
Attestation: Seong-Eun Kim has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Demba Ba, PhD
Contribution: This author helped analyze the data.
Conflicts: Demba Ba reported no conflicts of interest.
Attestation: Demba Ba has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Norman E. Taylor, MD, PhD
Contribution: This author helped write the manuscript.
Conflicts: Norman E. Taylor reported no conflicts of interest.
Attestation: Norman E. Taylor has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Emery N. Brown, MD, PhD
Contribution: This author helped design the study, analyze the data, and write the manuscript.
Conflicts: Emery N. Brown has filed a patent application for the use of intravenous methylphenidate to reverse general anesthesia in surgical patients.
Attestation: Emery N. Brown has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Ken Solt, MD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Conflicts: Ken Solt has filed a patent application for the use of intravenous methylphenidate to reverse general anesthesia in surgical patients.
Attestation: Ken Solt has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Footnotes
Reprints will not be available from the authors.
This report was previously presented, in part, at the Association of University Anesthesiologists
Contributor Information
Jonathan D. Kenny, Department of Anesthesia, Massachusetts General Hospital, Boston, Massachusetts.
Jessica J. Chemali, Department of Anesthesia, Massachusetts General Hospital, Boston, Massachusetts.
Joseph F. Cotten, Department of Anesthesia, Massachusetts General Hospital, Boston, Massachusetts.
Christa J. Van Dort, Department of Anesthesia, Massachusetts General Hospital, Boston, Massachusetts.
Seong-Eun Kim, Massachusetts Institute of Technology, Cambridge, Massachusetts.
Demba Ba, Massachusetts Institute of Technology, Cambridge, Massachusetts (current affiliation: Harvard School of Engineering and Applied Sciences, Cambridge, Massachusetts).
Norman E. Taylor, Department of Anesthesia, Massachusetts General Hospital, Boston, Massachusetts.
Emery N. Brown, Department of Anesthesia, Massachusetts General Hospital, Boston, Massachusetts.
Ken Solt, Department of Anesthesia, Massachusetts General Hospital, Boston, Massachusetts.
References
- 1.Solt K, Van Dort CJ, Chemali JJ, Taylor NE, Kenny JD, Brown EN. Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology. 2014;121:311–9. doi: 10.1097/ALN.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–95. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 4.Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–28. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, Dixon S, Thornton M, Funato H, Yanagisawa M. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105:1309–14. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107:264–72. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- 7.Luo T, Leung LS. Basal forebrain histaminergic transmission modulates electroencephalographic activity and emergence from isoflurane anesthesia. Anesthesiology. 2009;111:725–33. doi: 10.1097/ALN.0b013e3181b061a0. [DOI] [PubMed] [Google Scholar]
- 8.Pillay S, Vizuete JA, McCallum JB, Hudetz AG. Norepinephrine infusion into nucleus basalis elicits microarousal in desflurane-anesthetized rats. Anesthesiology. 2011;115:733–42. doi: 10.1097/ALN.0b013e31822c5ee1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology. 2011;115:791–803. doi: 10.1097/ALN.0b013e31822e92e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active Emergence from Propofol General Anesthesia Is Induced by Methylphenidate. Anesthesiology. 2012;116:998–1005. doi: 10.1097/ALN.0b013e3182518bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenny JD, Taylor NE, Brown EN, Solt K. Dextroamphetamine (but not atomoxetine) induces reanimation from general anesthesia: Implications for the roles of dopamine and norepinephrine in active emergence. PLoS One. 2015 doi: 10.1371/journal.pone.0131914. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor NE, Chemali JJ, Brown EN, Solt K. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology. 2013;118:30–9. doi: 10.1097/ALN.0b013e318278c896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 14.Miller RD, Cucchiara RF. Anesthesia. 5th. Philadelphia: Churchill Livingstone; 2000. [Google Scholar]
- 15.Meuret P, Backman SB, Bonhomme V, Plourde G, Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology. 2000;93:708–17. doi: 10.1097/00000542-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Plourde G, Belin P, Chartrand D, Fiset P, Backman SB, Xie G, Zatorre RJ. Cortical processing of complex auditory stimuli during alterations of consciousness with the general anesthetic propofol. Anesthesiology. 2006;104:448–57. doi: 10.1097/00000542-200603000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Paraskeva A, Petropoulos G, Siafaka I, Fassoulaki A. Sevoflurane as a single anesthetic and physostigmine failure to enhance arousal. Minerva anestesiologica. 2006;72:821–6. [PubMed] [Google Scholar]
- 18.Rohm KD, Riechmann J, Boldt J, Schollhorn T, Piper SN. Do patients profit from physostigmine in recovery from desflurane anaesthesia? Acta Anaesthesiol Scand. 2007;51:278–83. doi: 10.1111/j.1399-6576.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 19.Mitra P, Bokil H. Observed brain dynamics. Oxford, New York: Oxford University Press; 2008. [Google Scholar]
- 20.Thomson DJ. Spectrum estimation and harmonic analysis. Proceedings of the IEEE. 1982;70:1055–96. [Google Scholar]
- 21.Kirsh C, Politis DN. TFT-bootstrap: Resampling time series in the frequency domain to obtain replicates in the time domain. Ann Statist. 2011;39:1427–70. [Google Scholar]
- 22.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 23.Kaiser JF. On a simple algorithm to calculate the energy of a signal International Conference on Acoustics, Speech, and Signal Processing, 1988 (ICASSP-88) 1990:381–4. [Google Scholar]
- 24.Chemali J, Ching S, Purdon PL, Solt K, Brown EN. Burst suppression probability algorithms: state-space methods for tracking EEG burst suppression. J Neural Eng. 2013;10:056017. doi: 10.1088/1741-2560/10/5/056017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chemali JJ, Wong KF, Solt K, Brown EN. A state-space model of the burst suppression ratio. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:1431–4. doi: 10.1109/IEMBS.2011.6090354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devor M, Zalkind V. Reversible analgesia, atonia, and loss of consciousness on bilateral intracerebral microinjection of pentobarbital. Pain. 2001;94:101–12. doi: 10.1016/S0304-3959(01)00345-1. [DOI] [PubMed] [Google Scholar]
- 27.McCarren HS, Chalifoux MR, Han B, Moore JT, Meng QC, Baron-Hionis N, Sedigh-Sarvestani M, Contreras D, Beck SG, Kelz MB. alpha2-Adrenergic stimulation of the ventrolateral preoptic nucleus destabilizes the anesthetic state. J Neurosci. 2014;34:16385–96. doi: 10.1523/JNEUROSCI.1135-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed SJ, Plourde G, Tobin S, Chapman CA. Partial antagonism of propofol anaesthesia by physostigmine in rats is associated with potentiation of fast (80-200 Hz) oscillations in the thalamus. Br J Anaesth. 2013;110:646–53. doi: 10.1093/bja/aes432. [DOI] [PubMed] [Google Scholar]
- 29.Eikermann M, Fassbender P, Malhotra A, Takahashi M, Kubo S, Jordan AS, Gautam S, White DP, Chamberlin NL. Unwarranted administration of acetylcholinesterase inhibitors can impair genioglossus and diaphragm muscle function. Anesthesiology. 2007;107:621–9. doi: 10.1097/01.anes.0000281928.88997.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy RC, Stullken EH. Electroencephalographic evidence of arousal in dogs from halothane after doxapram, physostigmine, or naloxone. Anesthesiology. 1981;55:392–7. doi: 10.1097/00000542-198110000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Hill GE, Stanley TH, Sentker CR. Physostigmine reversal of postoperative somnolence. Can Anaesth Soc J. 1977;24:707–11. doi: 10.1007/BF03006714. [DOI] [PubMed] [Google Scholar]
- 32.Hudetz AG, Wood JD, Kampine JP. Cholinergic reversal of isoflurane anesthesia in rats as measured by cross-approximate entropy of the electroencephalogram. Anesthesiology. 2003;99:1125–31. doi: 10.1097/00000542-200311000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Alkire MT, Asher CD, Franciscus AM, Hahn EL. Thalamic microinfusion of antibody to a voltage-gated potassium channel restores consciousness during anesthesia. Anesthesiology. 2009;110:766–73. doi: 10.1097/aln.0b013e31819c461c. [DOI] [PubMed] [Google Scholar]
- 34.Plourde G, Chartrand D, Fiset P, Font S, Backman SB. Antagonism of sevoflurane anaesthesia by physostigmine: effects on the auditory steady-state response and bispectral index. Br J Anaesth. 2003;91:583–6. doi: 10.1093/bja/aeg209. [DOI] [PubMed] [Google Scholar]
- 35.Xie G, Deschamps A, Backman SB, Fiset P, Chartrand D, Dagher A, Plourde G. Critical involvement of the thalamus and precuneus during restoration of consciousness with physostigmine in humans during propofol anaesthesia: a positron emission tomography study. Br J Anaesth. 2011;106:548–57. doi: 10.1093/bja/aeq415. [DOI] [PubMed] [Google Scholar]
- 36.Horrigan RW. Physostigmine and anesthetic requirement for halothane in dogs. Anesth Analg. 1978;57:180–5. doi: 10.1213/00000539-197803000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Paraskeva A, Papilas K, Fassoulaki A, Melemeni A, Papadopoulos G. Physostigmine does not antagonize sevoflurane anesthesia assessed by bispectral index or enhances recovery. Anesth Analg. 2002;94:569–72. doi: 10.1097/00000539-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Paraskeva A, Staikou C, Diamadis M, Siafaka I, Fassoulaki A. Anesthesia with 1.5 minimum alveolar concentration sevoflurane is not altered by physostigmine as measured by bispectral and clinical indices. J Clin Anesth. 2005;17:581–5. doi: 10.1016/j.jclinane.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Zvosec DL, Smith SW, Litonjua R, Westfal RE. Physostigmine for gamma-hydroxybutyrate coma: inefficacy, adverse events, and review. Clinical toxicology. 2007;45:261–5. doi: 10.1080/15563650601072159. [DOI] [PubMed] [Google Scholar]
- 40.Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN. A neurophysiological-metabolic model for burst suppression. Proc Natl Acad Sci U S A. 2012;109:3095–100. doi: 10.1073/pnas.1121461109. [DOI] [PMC free article] [PubMed] [Google Scholar]