Abstract
Objective
To test cognitive behavioral therapy (CBT) for persistent ADHD symptoms in a sample of medication treated adolescents.
Methods
46 adolescents (ages 14-18), with clinically significant ADHD symptoms despite stable medication treatment were randomly assigned to receive CBT for ADHD or wait list control in a cross-over design. 24 were randomized to CBT, 22 to wait list, and 15 crossed-over from wait list to CBT. A blind independent evaluator (IE) rated symptom severity on the ADHD Current Symptom Scale, by adolescent and parent report, and rated each subject using the Clinical Global Impression Severity Scale (CGI), a global measure of distress and impairment. These assessments were performed at baseline, 4-months (post-CBT or post-wait list), and 8-months (post-treatment for those originally assigned to the wait list condition and 4-month follow-up for those originally assigned to CBT). Trial Registration: http://clinicaltrials.gov/show/NCT01019252.
Results
Using all available data, mixed effects modeling, and pooling for the wait list crossover, participants who received CBT received a mean score 10.93 lower on the IE-rated parent assessment of symptom severity (95% CI: -12.93, -8.93; p<.0001), 5.24 lower on the IE-rated adolescent assessment of symptom severity (95% CI: -7.21, -3.28; p<.0001), and 1.17 lower IE-rated CGI (95% CI:-1.39, -.94; p<.0001). Results were consistent across 100 multiple imputations (all p-values < 0.0001). There was a greater proportion of responders after CBT by parent (50% vs 18%, p=.00) and adolescent (58% vs. 18% p=.02) report.
Conclusions
This study demonstrates initial efficacy of CBT for adolescents with ADHD who continued to exhibit persistent symptoms despite medications.
Keywords: ADHD, Adolescence, Behaviour therapy, Cognitive therapy
INTRODUCTION
Between 5% and 10% of adolescents have attention deficit hyperactivity disorder (Centers for Disease Control and Prevention (CDC), 2010; Fergusson, Horwood, & Lynskey, 1993; Murphy & Barkley, 1996; Verhulst, van der Ende, Ferdinand, & Kasius, 1997) a disorder characterized by impairing levels of inattention, hyperactivity, and impulsivity (American Psychiatric Association, 2013). Despite extensive research on ADHD in younger children, ADHD in adults and adolescents has been less studied. However, 50% to 80% of children with ADHD continue to meet criteria for ADHD as adolescents (Barkley, 1990; Barkley, Fischer, Edelbrock, & Smallish, 1991; Gittelman, Mannuzza, Shenker, & Bonagura, 1985). This affects school performance, repeating grades, dropping out of school, social relationships, and relationships at home (Barkley, Anastopoulos, Guevremont, & Fletcher, 1991; Barkley, Fischer, Edelbrock, & Smallish, 1990; Tercyak, Peshkin, Walker, & Stein, M.A., 2002); ADHD in adolescence can place youth on a trajectory towards negative outcomes such as school failure (Barkley, Anastopoulos, et al., 1991; Barkley et al., 1990) substance use, risky driving (Barkley, Guevremont, Anastopoulos, DuPaul, & Shelton, 1993) and high risk behaviors (Barkley, Murphy, & Fischer, 2007).
Although medication can be an effective treatment for individuals of all ages with ADHD, additional efforts are necessary for many with the disorder. This may be particularly true for adolescents (Wilens et al., 2006). Responders to ADHD medications (i.e., those who are rated as “much improved” or “very much improved”), can still have significant residual symptoms and impairment post-medication treatment (Chronis, Jones, & Raggi, 2006; Wilens et al., 2006). Additional relevant clinical issues with pharmacotherapy in adolescents include side- effects and long-term safety concerns of medication. As clinicians weigh the pros and cons of long-term medication use for each individual child or adolescent (Lerner & Wigal, 2008) they share with families the desire for complementary treatments for this age group.
Many authors have noted the need for additional research on psychosocial treatments for adolescents with ADHD (Chronis et al., 2006; Evans, Owens, & Bunford, 2013). In a recent review by Evans et al. (2013), the authors differentiate between behavioral treatments in which parents or teachers manipulate contingencies and training interventions in which skills are taught directly to the individual. Among behavior management interventions, they note that behavioral parent training, behavioral classroom management and behavioral peer interventions meet criteria as well-established treatments. In terms of training interventions, however, they point to the fact that organizational skills training meets criteria for being a well-established treatment, yet there is only one study of this treatment in adolescents. The authors further go on to say that training interventions may be the preferred mode of treatment for adolescents due to the fact that adolescents encounter numerous teachers, parents monitor adolescents less closely and it is sometimes difficult to come up with salient rewards for adolescents. Promising results have been published on a middle school-based treatment program for students with ADHD (Evans, Axelrod, & Langberg, 2004; Evans, Langberg, Raggi, Allen, & Buvinger, 2005), an after school program for young adolescents with ADHD (Evans, Schultz, Demars, & Davis, 2011), and a homework, organization and planning skills intervention with middle school students (Langberg, Epstein, & Becker, 2012). Additional research has been done on family therapy for adolescents with ADHD and oppositional defiant disorder (ODD), comparing problem-solving communication training (PSCT) with behavior management training plus PSCT(Barkley, Edwards, Laneri, Fletcher, & Metevia, 2001). Although some families exhibited normalization of symptoms and the percentage did not differ between the two groups, the authors note that the vast majority of families who participated in this study did not demonstrate reliable change. They go on to suggest that future research may need to focus on alternative methods of service delivery to these high risk teens and their families.
Sibley and colleagues (Sibley, Kuriyan, Evans, Waxmonsky, & Smith, 2014) reviewed the literature on the treatment of adolescent ADHD since 1999. They identified 22 studies of behavior therapy for ADHD published since 1999, including six controlled trials. They point to data suggesting that many teens on medication for ADHD discontinue the medication before graduating from high school (Molina et al., 2009). This underscores the importance of psychosocial treatments as well as the importance of including an adult collaborator (parent or teacher) in the treatment to increase the likelihood of success. Sibley et al. (2014) further note that the addition of motivational interviewing directed towards adult stakeholders may be useful.
Given the need for different types of psychosocial interventions for adolescents with ADHD, the present study sought to evaluate an adapted cognitive behavioral therapy (CBT) for adolescents with ADHD. The treatment is based on our approach used successfully in adults with ADHD (Safren, Otto, et al., 2005; Safren, Perlman, Sprich, & Otto, 2005), which was tested in randomized controlled trials with adults who were treated with medications but still had residual symptoms. In our initial pilot randomized controlled trial, we demonstrated that individuals who received CBT had superior outcomes compared to those who continued on medications but did not receive CBT (Safren, Otto, et al., 2005). In our second trial, an efficacy study, we showed that CBT had superior outcomes compared to those who had an active, credible, time-matched control, relaxation with educational support (Safren et al., 2010). An uncontrolled study of CBT based on this work was conducted by Antshel, Faraone, and Gordon (Antshel, Faraone, & Gordon, 2012) who found that a number of variables were improved at post-treatment, including adolescent reported self-esteem, and parent and teacher ratings of inattentive symptoms.
Given the positive findings of our approach with adults, we hypothesized that, in a 2-arm randomized cross-over design, CBT for residual ADHD symptoms would be superior to wait-list in adolescent participants.
RESEARCH DESIGN AND METHODS
Design and procedures
This was an 8-month 2-arm cross-over randomized controlled efficacy trial. The two arms were 1) CBT, and 2) a 4 month wait list, followed by CBT. Assessments were at baseline, 4 months, and 8 months, and took place at Massachusetts General Hospital (Behavioral Medicine Service). The period of recruitment was 05/12/2009 to 11/17/2011; the 8 month follow-up period extended until 8/30/12.
All procedures were reviewed and approved by the Institutional Review Board at Massachusetts General Hospital. All participants completed an informed consent process with a trained study staff member, including parents signing an informed consent form and adolescents signing an assent form. Trial Registration: http://clinicaltrials.gov/show/NCT01019252.
Participants
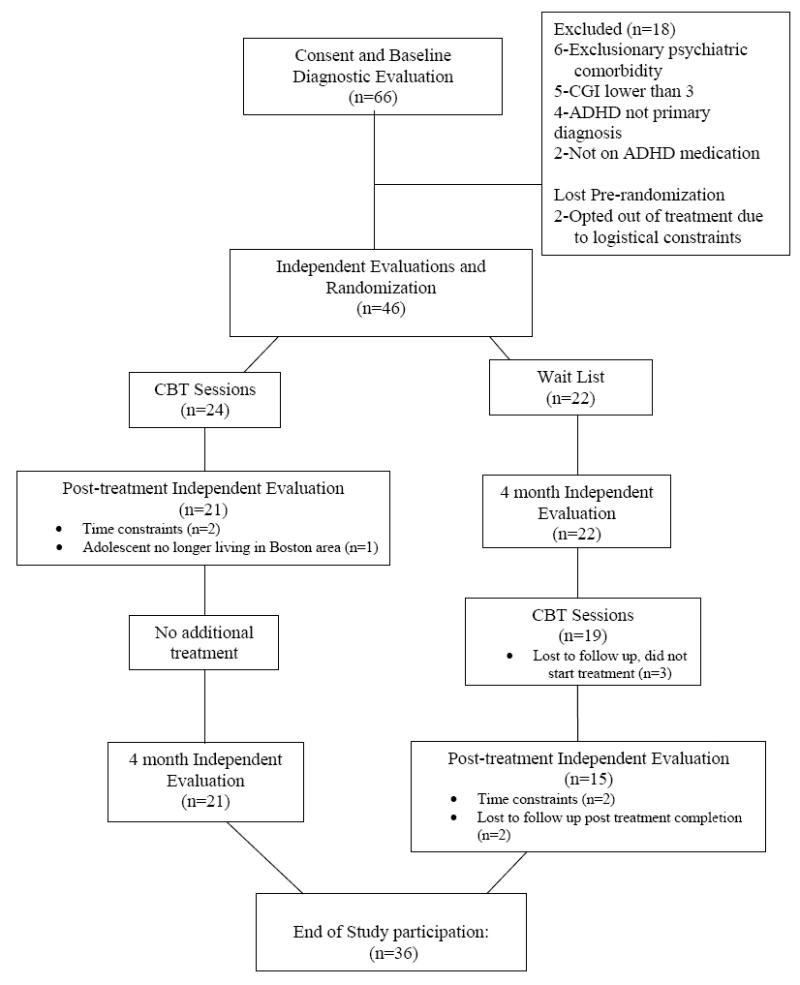
Participants were 46 adolescents between the ages of 14-18, with a principal diagnosis of ADHD, with a Clinical Global impression Severity Rating (CGI) of 3 (moderate severity) or greater at baseline, and on a stable dose (defined as no change in dose for at least two months) of an FDA approved medication. See Figure 1 for participant flow. Participants who temporarily discontinued their medication for the summer, but resumed treatment for the school year, were not required to wait 2 months in order to enter the study (N=1). All participants were on a stable dose of medication for ADHD at the time of randomization into the study. However, some participants changed the type or dosage of medication during the course of the study (N=12), and were allowed to continue their participation following intent-to-treat principals of clinical trials. Two participants who were originally assigned to the wait list condition discontinued their medication during the time between the baseline assessment and the 4-month assessment. These participants were allowed to cross-over to the CBT condition after the 4-month assessment. Participants were recruited from the Pediatric Psychopharmacology Service, the Child Psychiatry Clinic, and the Pediatric Clinics at Massachusetts General Hospital. Recruitment strategies included letters to doctors, IRB-approved flyers, and advertising via radio and Facebook. After a phone screen, interested families were invited to complete the in-person study screening visit. Of the 66 subjects who completed the in-person screen for the study, 16 were referred by physicians and 50 were self-referred.
Figure 1. Participant Flow.
Principal diagnosis of ADHD and psychiatric comorbidity was confirmed by the Kiddie-Schedule for Affective Disorders and Schizophrenia-Epidemiologic Version (Orvaschel, 1985) in separate interviews with the adolescent and parent. The combined report was used to establish diagnoses. We required that participants meet criteria for ADHD at the time of the assessment for inclusion in the study. The K-SADS were administered by a study clinician (Psychology Fellow or Doctoral level psychologist trained via audio-tape supervision in the treatment and assessment protocols).
Exclusion criteria included severe comorbid disorders that would interfere with participation (no one was excluded for this), active suicidality, conduct disorder, active substance abuse or dependence (<3 months remission), organic mental disorder, mental retardation, pervasive developmental disorder, or a history of CBT for ADHD. 56.4 % of the participants had at least one current comorbid condition. These included oppositional defiant disorder (N=12), specific phobia (N=6), social phobia (N=6), generalized anxiety disorder (N=3), tic disorder (N=2), and dysthymia (N=1).
Independent Assessment Visits
The independent evaluator (IE) met with all participants and parents for baseline, 4-month and 8-month assessments. The IE was blind to treatment status (CBT vs. wait list). The IE reminded all participants not to reveal anything about their treatment condition during assessments.
Medication Adherence
We assessed for medication adherence on a weekly basis by asking patients to complete a self-report measure of medication adherence that asked participants to rate their ability to take all of their medications as prescribed in the past week on a 6 point scale that ranged from 1 (very poor) to 6 (excellent) and what percent of the time the participant took his or her ADHD medications in the past week (Lu et al., 2008).
Intervention
CBT
The intervention delivered was the same regardless of point of delivery (pre or post waiting period). All participants completed seven modules of treatment over twelve sessions, ten of which were 1:1 with the therapist and adolescent, and two of which also included the parent. Ninety-four percent of the study completers participated in all twelve sessions because we offered to reschedule all sessions that were missed or cancelled. We did consider participants to be “completers” if they completed at least 9 sessions. We tried to minimize cancellations by offering sessions in the late afternoon and evening hours to make the visits more convenient for subjects and families. The average time for completers to complete the 12 sessions was 17.31 weeks. Two additional optional parent-only sessions were offered as well. For each 1:1 session the parent was included for approximately ten minutes, generally at the end of the session, to discuss progress, the course of intervention content, and how the parent could assist with any take-home practice.
Modules included 1) Psychoeducation and Organization/Planning (4 sessions): orienting adolescents to the CBT model, psychoeducation about ADHD, and organizing and planning skills. Skills such as keeping a centralized task list and task prioritization were taught in these initial sessions. 2) Distractibility (2 sessions): Participants were taught to use the “distractibility delay,” a technique that involves writing down distractions while working and coming back to them after a set amount of time. Cue-control procedures were also taught which involved instructing the subject to check in with him or herself regularly to ask him or herself if he or she was still working on the task at hand or if he or she had gotten distracted. 3) Adaptive Thinking (2 sessions): cognitive restructuring skills were taught so that each participant could maximize adaptive thinking. This included both helping adolescents respond to “negative thoughts” typically associated with anxiety and depression as well as “overly positive thoughts” that are often associated with ADHD (Mitchell, Anastopoulos, Knouse, Kimbrel, & Benson, 2008). 4) Procrastination (1 session): skills were taught that built on previously learned organizational skills, such as breaking down tasks into manageable steps, learning to set realistic goals for completing tasks, and rethinking beliefs about perfectionism. 5) Parent-Adolescent Sessions (2 sessions): These sessions consisted of psychoeducation about ADHD for the parents, with the goal of the parents being able to help to extend the treatment outside of the sessions and upon completion of the formal treatment. 6) Parent-only sessions (2 optional sessions): These sessions included an assessment of parenting style and a discussion of contingency management systems. Parents also received suggestions about interacting with schools and advocating for their child with ADHD. We added the parent-optional sessions midway through the study. They were not offered to the first 19 families. Of the 27 families who were offered the optional sessions, 21 families (78%) attended at least one and 18 (67%) completed both optional sessions. 7) Relapse prevention (1 session): all skills were reviewed and each participant was asked to rate the usefulness of each skill. Adolescents were also provided with a “troubleshooting form” to help associate potential future difficulties with skills learned in the treatment, and were asked to schedule a self-check-in one month after the last treatment session. For a more detailed description of our intervention, please see (Sprich, Burbridge, Lerner, & Safren, 2015).
Wait List
Participants initially assigned to the wait list received no psychosocial treatment for 4 months, although they did continue to receive psychopharmacological treatment during this time period. The research assistant contacted those families who were interested in crossing-over to receive CBT to schedule the 4-month assessment visit with the independent evaluator followed by the first CBT session.
Fidelity Ratings
All sessions were audio taped. Five percent of the tapes were reviewed by a second study therapist and rated for fidelity to the protocol. The average general session fidelity rating was 97.5% and the average session specific fidelity rating was 94.3%.
Outcomes
IE-Rated ADHD Severity Ratings
The independent evaluator administered the ADHD rating scale to parents and adolescents separately (Barkley, 1990; DuPaul, Power, Anastopoulos, & Reid, 1998). The IE read the items aloud and asked the parent or adolescent to respond. If the response was not clear, the IE asked for additional information and then used his clinical judgment to make a rating. This scale, updated for DSM-IV (DuPaul et al., 1998), assesses each of 18 individual symptoms of ADHD using an identical four-point severity grid (0 = not present up to 3 = severe; minimum total score = 0, maximum total score =54). This scale has been shown to be correlated with ADHD in adolescence, and has been shown to be sensitive to medication effects in pediatric (Barkley, 1990) and adult samples (Faries, Yalcin, Harder, & Heiligenstein, 2001; Spencer et al., 1995; Wilens et al., 2006). For the purposes of the present study, we had two separate summary scores, one for the adolescent and one for the parent.
IE Rated Clinical Global Impression (CGI)
The Clinical Global Impression (CGI) Scale (N.I.M.H., 1985) is a widely used rating scale to measure overall distress and impairment related to ADHD symptoms. The Global Severity rating ranges from; 1=not ill, to 7= extremely ill. This scale has been used extensively in psychopharmacology research and has been shown to be adequately sensitive in drug trials (N.I.M.H., 1985). This CGI rating was made by the independent evaluator at the end of each independent evaluation.
Categorical Responder Status
Following procedures used in psychopharmacology studies, we used a 30% reduction in symptoms as a cutoff for a “treatment responder”(Steele, Jensen, & Quinn, 2006). To do this, we report on both parent and adolescent ADHD symptom ratings.
Power Considerations and Sample Size
Our adult data, with a treatment as usual control group (Safren, Otto, et al., 2005), had strikingly-large, between-group effect sizes: 1.19 for Independent Evaluator ADHD Current Symptoms Scale change scores (post-treatment minus pre-treatment). For the current study, we adopted Cohen’s effect size d = 0.78 as our smallest target effect size (i.e., treatment difference) to detect statistical significance. With a total of 40 subjects, the proposed design was powered to detect this target effect size with an 80% power at a two-tailed p = 0.05 significance level for the primary analysis using the ADHD Current Symptoms Scale.
Randomization
We stratified randomization based on sex and CGI score (cutoff = ADHD CGI severity rating of >=5) in blocks of 2. We randomized based on CGI score to protect against having a baseline difference in severity in the two groups that could have impacted the study outcome. This method was used in our two adult studies of CBT for ADHD as well (Safren et al., 2005, 2010). The randomization sequence was generated by the study research assistant flipping a coin to determine the next assignment and then assigning the following participant to the alternate condition. The randomization sequence was generated prior to the initiation of the study and was not shared with the interventionists prior to subject assignment. Study interventionists conducted the enrollment visits.
Statistical Methods
All analyses were performed using all available data of all randomized study subjects, including those who completed and did not complete the protocol, i.e., intention-to-treat (ITT). Data were summarized by mean ± standard deviation unless otherwise specified. A longitudinal general linear mixed effects model (using SAS, version 9.2) was applied, with the variance components error correlation structure among the repeated measures over 0, 4, and 8 months. The mixed model contained a random subject specific intercept, fixed months variable (0, 4, or 8), fixed time varying treatment (1=on CBT; 0=Wait List), and months by treatment interaction term. Treatment efficacy was estimated by contrasting the longitudinal mean changes between the CBT and Wait List conditions at each time point. The interaction term tested whether the efficacy was attenuated if subjects were assigned to receive CBT at the beginning of the study versus being assigned to the Wait List first and receiving CBT later. Analyses were repeated over 100 multiply imputed data sets in order to examine the impact of the missing data. The proportion of responders after CBT versus after wait list was calculated also using all available data and a chi square test.
RESULTS
Participant Characteristics and Flow
Table 1 depicts baseline demographic characteristics for those randomized, and a CONSORT-style diagram of participant flow is depicted in Figure 1. (See online appendix S1 for the CONSORT checklist) None of the demographic or outcome data differed by treatment arm. Of the 46 participants randomized, at 4-month assessment, retention was 93%, and 78% at the 8-month assessment. There were no study-related serious adverse events.
Table 1.
Baseline Demographics by Study Condition
| CBT | Waitlist | |
|---|---|---|
| n | 24 | 22 |
| Sex, n (%) | ||
| Male | 18 (75%) | 18 (81.8%) |
| Female | 6 (25%) | 4 (18.2%) |
| Mean Age, years (SD) | 15.17 (1.01) | 15.09 (1.11) |
| Race n (%) | ||
| African American | 0 (0%) | 1 (4.5%) |
| Asian | 0 (0%) | 0 (0%) |
| Native American | 1 (4.2%) | 0 (0%) |
| Other | 1 (4.2%) | 0 (0%) |
| White | 22 (91.7%) | 21 (95.5%) |
| Ethnicity n (%) | ||
| Not Hispanic or Latino | 21 (87.5%) | 21 (95.5%) |
| Hispanic or Latino | 3 (12.5%) | 1 (4.5%) |
| CGI (SD) | 4.75 (.68) | 4.64 (.85) |
| IE-Rated ADHD Severity Ratings (SD) | ||
| Parent | 25.25 (9.2) | 28.05 (9.57) |
| Adolescent | 16.46 (10.91) | 14.36 (8.31) |
Abbreviations: CBT-Cognitive Behavioral Therapy, SD-Standard Deviation, CGI-Clinical Global, Impression, IE-Independent Evaluator
At baseline, the average participant rating of medication adherence in the past week was 4.59 which falls in between the “good” and “very good” categories and at post-treatment, the average participant rating of medication adherence in the past week was 5.17 which falls between the “very good” and “excellent” categories. Similarly, the self-reported percentage of medication use was 78% at baseline and 84% at post-treatment.
Formal SES was not calculated, but information was obtained on parent occupational status. 81.9% of families had principal wage-earners who fell into the top three job categories on the SES scale (higher executive/major professional, administrator/lesser professional/proprietor of medium-sized business, or smaller business owners/farm owners/managers/minor professional; Hollingshead, 1975).
Continuous ADHD Outcomes
Receipt of CBT resulted in improvements on the three outcomes. CBT resulted in 10.93 lower points on the IE-rated parent assessment of symptom severity mean score (95% CI: -12.93, -8.93; p<.0001), 5.24 lower points on the IE-rated adolescent assessment of symptom severity mean score (95% CI: -7.21, -3.28; p<.0001), and 1.17 lower IE-rated CGI mean score (95% CI:-1.39, -.94; p<.0001). Results were consistent across 100 multiple imputations with all p-values less than .0001.
Categorical Responder Status
A 30% reduction on the ADHD rating scale was used to calculate categorical responder status. Using the parent report data, there were more responders to CBT (18 of the 36, 50%) compared to those originally assigned to wait list (4 of the 22, 18%) (Chi Sq (1) = 8.98, p=.00). The pattern was similar using adolescent report data with 21 of the 36 (58%) who received CBT compared to 4 of the 22 (18%) originally assigned to wait list (Chi Sq (1) = 5.87, p=.02).
DISCUSSION
The primary finding of this study was that adding CBT to medication treatment in adolescents with ADHD was superior to medications alone in this randomized controlled wait-list design. Our finding provides further evidence for the utility of this therapeutic approach, consistent with adult samples (Safren et al., 2010; Safren, Otto, et al., 2005). The results are also consistent with findings from another research group who adapted our adult treatment for use in adolescent high school students. Antshel et al.(2012) in an uncontrolled trial of a downward extension of our adult treatment in 68 adolescents with ADHD, found that a number of variables were improved at post-treatment, including adolescent self-report of self-esteem, as well as parent and teacher ratings of inattentive symptoms. Our current study builds off of that open trial in that we utilized a randomized design.
One limitation of the present study was that as an initial trial, the sample size was relatively small. Replication is needed with a larger sample. Also consistent with our adult studies, we selected subjects who were on stable doses of medication for ADHD. Therefore, although it provides data about a next-step treatment approach, we cannot conclude whether or not this treatment would be similarly effective in adolescents with ADHD who are not on medications. This sample may not be representative in that the participants were on medication, had primarily professional parents, were largely self-referred (i.e., responded to study advertisements), were seen at an urban teaching hospital and contained few minority participants. These factors may limit the generalizability of the findings to community samples as our sample likely had more resources available to enable the subjects to respond to the treatment than would more typical community samples. A further limitation that may limit generalizability was that we had comorbid conduct disorder as a rule-out and we had many participants who presented with comorbid conditions. Future studies on this approach with larger sample sizes should examine the relationship between comorbidity and treatment response. It would also be helpful to measure IQ in future studies, so that the relationship between IQ and treatment response could be examined.
In terms of study design, the cross-over design did not allow for follow-up data on all subjects, in that the 8-month assessment was immediately post-treatment for subjects who first received the wait list condition. It will be important to examine whether treatment effects are maintained over a longer time period with adolescent samples, as was observed in our adult trial (Safren et al., 2010). Future studies with a longer follow-up period should assess quality of life outcomes. We hypothesize that quality of life outcomes would have a delayed response to treatment. Accordingly, after symptom reduction for some period of time, one would later see functional improvements. This pattern of delayed functional improvement was recently found in a study by Young, et al. (2015), where they found that symptom improvement after CBT was maintained at 3 month follow-up while secondary outcomes, including quality of life measures, continued to improve. For example, if symptom reduction yielded greater to ability to do homework, it would take some time for repeated homework to yield a better scholastic outcome. Other authors have suggested that multimodal approaches that combine medication treatment with psychosocial treatment may be effective in improving functional outcomes (Arnold, et al, 2015; Emilsson et al., 2011; Hinshaw & Arnold, 2015; Young et al., 2015).
In addition, it would be helpful to compare the CBT condition with a time- and attention-matched control group, as opposed to a wait list condition, to examine whether the effects are the result of the specific treatment or simply due to time with a therapist. Additionally, the current study relied on the self-report of the study participants as well as parent-report of the parents of the subjects. Obviously, these individuals were aware that they were receiving CBT and this may have biased their reports. Having a time- and attention-matched control condition could help minimize this potential bias, if the participants and parents were unaware of the hypothesis that CBT would prove more beneficial than the comparison condition. Another methodological limitation of the present study was that we did not validate the use of the self-report measure on medication adherence and hence some participants may have been more or less adherent to their medications. Although this is a psychosocial trial, it would be helpful to validate this measure, or, if adherence may be a confounder, develop and use objective measures for future research studies. Further, we did not systematically gather data on family structure and parental age. This information would have been useful, given that we used parent ratings as a source of information about participant improvement.
In conclusion, this study provides preliminary support for the extension of our CBT program for adult ADHD to the adolescent population. CBT targeted therapy for adolescent ADHD may serve as a much-needed alternative or complement to medication, for this highly common and impairing condition. Additionally, the findings from this trial are consistent with what has been recommended in the literature with respect to an intervention for the individual client versus for the parents or teachers (Evans et al., 2013), and may be useful for clinicians and/or families who do not have access to optimal school-based approaches. However, the findings must be interpreted with caution, given the limitations noted above.
Supplementary Material
Table 2.
CBT for Adolescent ADHD
| Observed Data | Multiple Imputationsa | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Outcomes | Time-Months | Initial Study Assignment
|
Estimated Effect of CBT on Outcomes (95% Confidence Interval) p-value | Estimated Effect of CBT on Outcomes (95% Coverage Intervalb) | |||
| Wait List | CBT | ||||||
|
| |||||||
| WL/CBT | mean +/- SD (n) | WL/CBT | mean +/- SD (n) | ||||
| CGI | 0 | WL | 4.64 +/- 0.85 (22) | WL | 4.75 +/- 0.68 (24) | -1.18 (-1.31, -1.05)c | |
| 4 | WL | 4.55 +/- 0.80 (22) | CBT | 3.71 +/- 0.85 (21) | -1.17 (-1.39, -0.94) | ||
| p<.0001 (***) | |||||||
| 8 | CBT | 3.20 +/- 0.86 (15) | CBT | 3.71 +/- 0.96 (21) | |||
|
| |||||||
| Parent Rating-Total | 0 | WL | 28.05 +/- 9.57 (22) | WL | 25.30 +/- 9.17 (24) | -11.47 (-12.50, -10.44-)c | |
| 4 | WL | 24.18 +/- 8.23 (22) | CBT | 16.76 +/- 10.40 (21) | -10.93 (-12.93,-8.93) | ||
| p<.0001 (***) | |||||||
| 8 | CBT | 12.82 +/- 6.51 (15) | CBT | 13.71 +/- 9.65 (21) | |||
|
| |||||||
| Adolescent Rating-Total | 0 | WL | 14.41 +/- 8.35 (22) | WL | 16.46 +/- 10.90 (24) | -5.81 (-6.50, -5.11)c | |
| 4 | WL | 13.86 +/- 8.12 (22) | CBT | 11.95 +/- 8.89 (21) | -5.24 (-7.21, -3.28) | ||
| p<.0001 (***) | |||||||
| 8 | CBT | 7.42 +/- 3.71 (15) | CBT | 9.71 +/- 7.21 (21) | |||
Notes:
This set of results is from a mixed model using multiple imputations to estimate the main effects of treatment and group assignment on the outcome.
The estimated 95% coverage interval is the 2.5th and 97.5th percentiles of the sampling distribution of the estimated effects.
In an analysis of 100 data sets with imputed values for cases with missing data, there was a statistically significant (p<0.0001) effect of CBT on this outcome in all 100 iterations.
Abbreviations: WL-Waitlist, CBT-Cognitive Behavioral Therapy, SD-Standard Deviation, CGI-Clinical Global Impression
Key Points.
Between 5% and 10% of adolescents have attention deficit hyperactivity disorder (ADHD). Adolescents with ADHD on medication can still have significant residual symptoms and impairment post-medication treatment.
This study is a 2-arm cross-over randomized controlled efficacy trial of cognitive behavioral therapy for medication treated adolescents with ADHD. A blind independent evaluator (IE) rated symptom severity on the ADHD Current Symptom Scale, and rated each subject using the Clinical Global Impression Severity Scale (CGI).
Participants who received CBT received a significantly lower mean score on the IE-rated CGI, and IE-rated parent and adolescent assessments of symptom severity.
This randomized controlled trial demonstrates initial efficacy of CBT for adolescents with ADHD who continued to exhibit persistent symptoms despite medications.
Acknowledgments
This project was supported by NIMH Grant R34MH083063; Additional support for data analysis came from the Harvard Catalyst, Harvard Clinical and Translational Science Center by NIH Grant 1 UL1 RR025758-03. S.A.S. and S.E.S receive royalties from Oxford University Press for their published therapist guide and client workbook. S.A.S receives royalties from Guilford Publications for authored books. S.E.S receives royalties from Springer for an edited book.
The authors thank the following individuals who assisted with the project: Christine Cooper-Vince, Meghan Groves Cromer, and Aleksandra Margolina, for research assistance; Jennifer Burbridge, Aude Henin, Laura Knouse, and Jonathan Lerner, for study intervention and assessing; Steven Evans, for consultation at the start of the study on the design and implementation of the trial; Hang Lee, for consulting on the data analysis.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article: Appendix S1: CONSORT checklist
The rest of the authors have declared that they have no potential or competing conflicts of interest in relation to the work reported.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. American Psychiatric Publishing; 2013. [Google Scholar]
- Antshel KM, Faraone SV, Gordon M. Cognitive Behavioral Treatment Outcomes in Adolescent ADHD. Journal of Attention Disorders. 2012 doi: 10.1177/1087054712443155. http://doi.org/10.1177/1087054712443155. [DOI] [PubMed]
- Arnold LE, Hodgkins P, Caci H, Kahle J, Young S. Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: A systematic review. PloS one. 2015;10(2) doi: 10.1371/journal.pone.0116407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment. New York, NY: Guilford Press; 1990. [Google Scholar]
- Barkley RA, Anastopoulos AD, Guevremont DC, Fletcher KE. Adolescents with ADHD: patterns of behavioral adjustment, academic functioning, and treatment utilization. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30(5):752–761. doi: 10.1016/s0890-8567(10)80010-3. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. The efficacy of problem-solving communication training alone, behavior management training alone, and their combination for parent-adolescent conflict in teenagers with ADHD and ODD. Journal of Consulting and Clinical Psychology. 2001;69(6):926–941. [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Edelbrock C, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria--III. Mother-child interactions, family conflicts and maternal psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1991;32(2):233–255. doi: 10.1111/j.1469-7610.1991.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;29(4):546–557. doi: 10.1097/00004583-199007000-00007. http://doi.org/10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Guevremont DC, Anastopoulos AD, DuPaul GJ, Shelton TL. Driving-related risks and outcomes of attention deficit hyperactivity disorder in adolescents and young adults: a 3- to 5-year follow-up survey. Pediatrics. 1993;92(2):212–218. [PubMed] [Google Scholar]
- Barkley RA, Murphy K, Fischer M. ADHD in adults: What the science says. New York, NY: Guilford; 2007. [Google Scholar]
- Emilsson, et al. Cognitive Behaviour Therapy in Medication-Treated Adults with ADHD and Persistent Symptoms: A randomized controlled trial. BMC psychiatry. 2011;11(1):116. doi: 10.1186/1471-244X-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children --- United States, 2003 and 2007. MMWR Morbidity and Mortality Weekly Report. 2010;59(44):1439–1443. [PubMed] [Google Scholar]
- Chronis AM, Jones HA, Raggi VL. Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder. Clinical Psychology Review. 2006;26(4):486–502. doi: 10.1016/j.cpr.2006.01.002. http://doi.org/10.1016/j.cpr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD rating scale-IV: Checklists, norms, and clinical interpretations. New York, NY: Guilford Press; 1998. [Google Scholar]
- Evans SW, Axelrod J, Langberg JM. Efficacy of a school-based treatment program for middle school youth with ADHD: pilot data. Behavior Modification. 2004;28(4):528–547. doi: 10.1177/0145445503259504. http://doi.org/10.1177/0145445503259504. [DOI] [PubMed] [Google Scholar]
- Evans SW, Langberg J, Raggi V, Allen J, Buvinger EC. Development of a school-based treatment program for middle school youth with ADHD. Journal of Attention Disorders. 2005;9(1):343–353. doi: 10.1177/1087054705279305. http://doi.org/10.1177/1087054705279305. [DOI] [PubMed] [Google Scholar]
- Evans SW, Owens JS, Bunford N. Evidence-Based Psychosocial Treatments for Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2013 doi: 10.1080/15374416.2013.850700. http://doi.org/10.1080/15374416.2013.850700. [DOI] [PMC free article] [PubMed]
- Evans SW, Schultz BK, Demars CE, Davis H. Effectiveness of the Challenging Horizons After-School Program for young adolescents with ADHD. Behavior Therapy. 2011;42(3):462–474. doi: 10.1016/j.beth.2010.11.008. http://doi.org/10.1016/j.beth.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faries DE, Yalcin I, Harder D, Heiligenstein JH. Validation of the ADHD Rating Scale as a clinician administered and scored instrument. Journal of Attention Disorders. 2001;5:107–115. [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Prevalence and comorbidity of DSM-III-R diagnoses in a birth cohort of 15 year olds. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32(6):1127–1134. doi: 10.1097/00004583-199311000-00004. http://doi.org/10.1097/00004583-199311000-00004. [DOI] [PubMed] [Google Scholar]
- Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up. I. Psychiatric status. Archives of General Psychiatry. 1985;42(10):937–947. doi: 10.1001/archpsyc.1985.01790330017002. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Arnold LE. Attention-deficit hyperactivity disorder, multimodal treatment, and longitudinal outcome: evidence, paradox, and challenge. Wiley Interdisciplinary Reviews: Cognitive Science. 2015;6(1):39–52. doi: 10.1002/wcs.1324. http://doi.org/10.1002/wcs.1324. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status. Yale University; 1975. [Google Scholar]
- Langberg JM, Epstein JN, Becker SP. Evaluation of the Homework, Organization, and Planning Skills (HOPS) Intervention for Middle School Students with Attention Deficit Hyperactivity Disorder as Implemented by School Mental Health Providers. School Psychology Review. 2012;41(3):342–364. [PMC free article] [PubMed] [Google Scholar]
- Lerner M, Wigal T. Long-term safety of stimulant medications used to treat children with ADHD. Journal of Psychosocial Nursing and Mental Health Services. 2008;46(8):38–48. doi: 10.3928/02793695-20080801-06. [DOI] [PubMed] [Google Scholar]
- Lu M, Safren SA, Skolnik PR, Rogers WH, Coady W, Hardy H, Wilson IB. Optimal recall period and response task for self-reported HIV medication adherence. AIDS and Behavior. 2008;12(1):86–94. doi: 10.1007/s10461-007-9261-4. http://doi.org/10.1007/s10461-007-92614. [DOI] [PubMed] [Google Scholar]
- Mitchell JT, Anastopoulos AD, Knouse LE, Kimbrel NA, Benson J. Evaluating potential mechanisms of change in the treatment of AD/HD in adulthood: An exploratory analysis of maladaptive thoughts. 2008 Presented at the ABCT, Orlando, FL. [Google Scholar]
- Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. MTA Cooperative Group. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(5):484–500. doi: 10.1097/CHI.0b013e31819c23d0. http://doi.org/10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Barkley RA. Attention deficit hyperactivity disorder adults: comorbidities and adaptive impairments. Comprehensive Psychiatry. 1996;37(6):393–401. doi: 10.1016/s0010-440x(96)90022-x. [DOI] [PubMed] [Google Scholar]
- N.I.M.H. CGI: Clinical Global Impression Scale - NIMH. Psychopharmacology Bulletin. 1985;21:839–844. [Google Scholar]
- Orvaschel H. Psychiatric interviews suitable for use in research with children and adolescents. Psychopharmacology Bulletin. 1985;21(4):737–745. [PubMed] [Google Scholar]
- Safren SA, Otto MW, Sprich S, Winett CL, Wilens TE, Biederman J. Cognitive-behavioral therapy for ADHD in medication-treated adults with continued symptoms. Behaviour Research and Therapy. 2005;43(7):831–842. doi: 10.1016/j.brat.2004.07.001. http://doi.org/10.1016/j.brat.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Safren SA, Perlman CA, Sprich S, Otto MW. Mastering your Adult ADHD: A cognitive behavioral treatment program, Therapist Guide. New York, NY: Oxford University Press; 2005. [Google Scholar]
- Safren SA, Sprich S, Mimiaga MJ, Surman C, Knouse L, Groves M, Otto MW. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA: The Journal of the American Medical Association. 2010;304(8):875–880. doi: 10.1001/jama.2010.1192. http://doi.org/10.1001/jama.2010.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Kuriyan AB, Evans SW, Waxmonsky JG, Smith BH. Pharmacological and psychosocial treatments for adolescents with ADHD: an updated systematic review of the literature. Clinical Psychology Review. 2014;34(3):218–232. doi: 10.1016/j.cpr.2014.02.001. http://doi.org/10.1016/j.cpr.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1995;52(6):434–443. doi: 10.1001/archpsyc.1995.03950180020004. [DOI] [PubMed] [Google Scholar]
- Sprich SE, Burbridge J, Lerner JA, Safren SA. Cognitive-Behavioral Therapy for ADHD in Adolescents: Clinical Considerations and a Case Series. Cognitive and Behavioral Practice. 2015;22(2):116–126. doi: 10.1016/j.cbpra.2015.01.001. http://doi.org/10.1016/j.cbpra.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele M, Jensen PS, Quinn DMP. Remission versus response as the goal of therapy in ADHD: a new standard for the field? Clinical Therapeutics. 2006;28(11):1892–1908. doi: 10.1016/j.clinthera.2006.11.006. http://doi.org/10.1016/j.clinthera.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Tercyak KP, Peshkin BN, Walker LR, Stein MA. Cigarette smoking among youth with attention-deficit/hyperactivity disorder: Clinical phenomenology, comorbidity, and genetics. Journal of Clinical Psychology in Medical Settings. 2002;9:35–50. [Google Scholar]
- Verhulst FC, van der Ende J, Ferdinand RF, Kasius MC. The prevalence of DSM-III-R diagnoses in a national sample of Dutch adolescents. Archives of General Psychiatry. 1997;54(4):329–336. doi: 10.1001/archpsyc.1997.01830160049008. [DOI] [PubMed] [Google Scholar]
- Wilens TE, McBurnett K, Bukstein O, McGough J, Greenhill L, Lerner M, Lynch JM. Multisite controlled study of OROS methylphenidate in the treatment of adolescents with attention-deficit/hyperactivity disorder. Archives of Pediatrics & Adolescent Medicine. 2006;160(1):82–90. doi: 10.1001/archpedi.160.1.82. http://doi.org/10.1001/archpedi.160.1.82. [DOI] [PubMed] [Google Scholar]
- Young S, Sedgwick O, Fridman M, Gudjonsson G, Hodgkins P, Lantigua M, González RA. Co-morbid psychiatric disorders among incarcerated ADHD populations: a meta-analysis. Psychological Medicine. 2015;45(12):2499–2510. doi: 10.1017/S0033291715000598. http://doi.org/10.1017/S0033291715000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S, Khonkoker M, Emilsson B, Sigurdsson J, Philli-Weignmann F, Baldursson G, Olafdottir H, Gudjonsson G. Cognitive-behavioral therapy in medication-treated adults with attendion-deficit/hyperactvity disorder and co-morbid psychopathology: a randomized controlled trial using multi-level analysis. Psychological Medicine. 2015;45:2793–2804. doi: 10.1017/S0033291715000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


