Abstract
Intestinal function is primarily controlled by an intrinsic nervous system of the bowel called the enteric nervous system (ENS). The cells of the ENS are neural crest derivatives that migrate into and through the bowel during early stages of organogenesis before differentiating into a wide variety of neurons and glia. Although genetic factors critically underlie ENS development, it is now clear that many non-genetic factors may influence the number of enteric neurons, types of enteric neurons, and ratio of neurons to glia. These non-genetic influences include dietary nutrients and medicines that may impact ENS structure and function before or after birth. This review summarizes current data about gene-environment interactions that affect ENS development and suggests that these factors may contribute to human intestinal motility disorders like Hirschsprung disease or irritable bowel syndrome.
Introduction
The enteric nervous system (ENS) is an integrated network of neurons and glia within the bowel wall that controls most aspects of bowel function (Furness, 2012; Wood, 2008). The complex ENS circuitry permits the bowel to operate largely autonomously so we can eat and enjoy the finer things in life, without having to think about mixing food with digestive enzymes, facilitating contact with the epithelium to enhance nutrient uptake, coordinating motility to avoid excessive bowel dilation, moving luminal contents toward the end of the bowel for elimination, regulating epithelial secretion and proliferation, or altering regional blood flow in response to metabolic needs. To perform these tasks the bowel contains about as many neurons as the spinal cord and diverse neuron subtypes produce and respond to the full spectrum of neurotransmitters found in the central nervous system. In addition to neurons, there are several types of enteric glia (Gulbransen and Sharkey, 2012; Sharkey et al., 2004) with diverse morphology and function. This beautiful nervous system needs to respond to a wide array of dietary patterns to facilitate nutrient intake and growth, and to avoid dehydration. Although significant accommodation to varied diets may occur without changing the fundamental structure of the ENS, if non-genetic factors impacted the types of neurons and glia produced or other aspects of ENS development and maintenance, it would permit broader adaptation to diverse nutrient and fluid intake. Indeed, recent data suggest that many non-genetic factors influence ENS development as well as mature structure and function. This has important implications for birth defects affecting the human enteric nervous system and for acquired intestinal motility disorders.
ENS development
The more complex the machine, the more ways it can go wrong!
The ENS forms from enteric neural crest-derived precursor cells (ENCDC) that originate primarily in the vagal region of the neural tube with minor contributions from sacral and upper thoracic ENCDC (Avetisyan et al., 2015a; Goldstein et al., 2013; Lake and Heuckeroth, 2013; Newgreen et al., 2013; Sasselli et al., 2012). Vagal ENCDC exit the neural tube at about embryonic day 8.5 (E8.5) in mice and at about week three of human gestation. These vagal ENCDC then migrate to the bowel and colonize the bowel in a rostral to caudal progression migrating through gut mesenchyme to reach the end of the bowel by E13.5 in mice and week eight of gestation in humans. During migration ENCDC proliferate vigorously, and then exit the cell cycle, differentiate into neurons or glia, cluster into ganglia, and form an extensive interconnected network that extends all the way along the bowel. This process is controlled by many known genetic factors discussed in more detail in other articles in this Special Issue of Developmental Biology. A few key molecules are briefly described in Table 1. This article highlights how non-genetic factors may affect the ENS before and after birth and explains links to human intestinal motility disorders.
Table 1.
Simplified list of genes that impact ENS development
| Gene | Role in ENS development | Protein function/Comments |
|---|---|---|
| RET | Supports ENS precursor survival, proliferation, migration, neuronal differentiation, neurite growth and axon patterning |
-Transmembrane tyrosine kinase receptor -Most commonly inactivated gene in people with HSCR. |
| GDNF | RET activating ligand | -Neurotrophic factor -Rarely mutated in people with HSCR |
| EDNRB | Prevents premature differentiation of ENCDC Facilitates colon colonization by ENCDC |
-G-protein coupled receptor -Mutated in 5% of people with HSCR -Also causes hearing loss and pigmentation defects (Waarderburg-Shah, WS4)) |
| EDN3 | EDNRB activating ligand | -Peptide -Rarely mutated in people with HSCR |
| SOX10 | Required for bowel colonization by ENCDC Activates RET expression |
-Transcription factor -Mutations cause HSCR plus hearing loss and pigmentation defects (WS4) |
| PHOX2B | Required for bowel colonization by ENCDC Activates RET expression |
Transcription factor Mutations cause HSCR plus congenital central hypoventilation (Haddad syndrome) |
The complex cellular processes that occur during ENS development are supported by a wide array of cell surface proteins, extracellular ligands, intracellular signaling molecules, transcriptonal regulators, and non-genetic factors. These pathways have recently been reviewed in detail by our group and other investigators (Amiel et al., 2008; Avetisyan et al., 2015a; Goldstein et al., 2013; Lake and Heuckeroth, 2013; Newgreen et al., 2013; Sasselli et al., 2012).
Hirschsprung disease (HSCR)
HSCR is a disorder where the ENS is missing from the end of the bowel. The region without enteric neurons is called “aganglionic bowel”. In the absence of all enteric neurons, the bowel tonically contracts causing functional obstruction (Amiel et al., 2008; Heuckeroth, 2013; Skinner, 1996). Symptoms of HSCR include abdominal distension, vomiting, severe constipation, growth failure, a predisposition to bowel inflammation (enterocolitis) and early death. HSCR occurs in about 1:5000 children as a result of the failure of bowel colonization by ENCDC during fetal development. Because vagal ENCDC have a very long migratory route and most children with HSCR (80%) have only a “short-segment” of bowel with missing enteric neurons (i.e., rectum an sigmoid colon aganglionosis), a marginal increase (e.g. 10%) in fetal bowel colonization by vagal ENCDC would have prevented HSCR from occurring in many of these children. This may explain why the male to female ratio for short segment human HSCR is 4:1 while the male to female ratio for long segment HSCR is 2:1 (Badner et al., 1990). In long segment HSCR, small increases in bowel colonization by ENCDC will shorten the aganglionic zone, but do not prevent HSCR. In short segment HSCR, a 10% increase in bowel colonization by ENCDC could have prevented HSCR. These data suggest that in human females, ENCDC colonize fetal bowel slightly more efficiently than in males, consistent with observations in mouse models (Bergeron et al., 2015; Cantrell et al., 2004; McCallion et al., 2003; Vohra et al., 2007b). The minimal improvement in bowel colonization by ENCDC needed to prevent HSCR in most affected children also suggests that many genetic and non-genetic factors could influence HSCR risk even if they only marginally affect bowel colonization efficiency.
Bowel colonization by ENCDC is driven by cell proliferation
ENCDC must form a network of neurons and glia along the entire length of the bowel suggesting that the entire migratory route is hospitable for ENCDC. Full bowel colonization by ENCDC, however, appears to be driven by competition for available space and trophic factors as ENCDC proliferate, instead of by trophic factor gradients (Gianino et al., 2003; Landman et al., 2007; Newgreen et al., 2013; Wang et al., 2010). Although gradients of some trophic factors like glial cell line-derived neurotrophic factor (GDNF) exist in fetal bowel and might drive migration (Natarajan et al., 2002), individual ENCDC can actually migrate through the bowel in either direction (and do so during normal development) (Burns et al., 2002; Druckenbrod and Epstein, 2007; Young et al., 2014). Because proliferation drives bowel colonization, anything that reduces the number of ENCDC or the proliferation of these cells during development should predispose to HSCR. Consistent with this hypothesis, removing parts of the vagal neural tube to reduce neural crest-derived cells that enter the bowel can cause HSCR-like disease (Barlow et al., 2008). Reducing cell proliferation with mycophenolic acid, a drug that blocks the rate limiting step in de novo guanine nucleotide synthesis, also caused distal bowel aganglionosis in mice and reduced ENCDC colonization of distal bowel in fish (Lake et al., 2013). Consistent with this hypothesis, inactivating mutations in the GDNF receptor RET (a transmembrane tyrosine kinase) are the most commonly identified cause of human HSCR (Amiel et al., 2008), and RET signaling is needed for ENCDC survival and proliferation (Schuchardt et al., 1994). Inactivating mutations in the G-protein coupled receptor EDNRB also cause HSCR (Puffenberger et al., 1994) and may permit early differentiation of ENCDC within the colon (Barlow et al., 2003), reducing proliferation that normally drives bowel colonization by ENCDC.
One prediction from these observations is that reduced activity of any of the signaling molecules that are needed to drive ENCDC proliferation should increase HSCR risk, especially in the context of other genetic changes that predispose to HSCR. Furthermore, fetal malnutrition and other causes of intrauterine growth retardation might increase HSCR occurrence if they occurred during the period of ENCDC migration, but this hypothesis has not yet been tested. Importantly, formation of some structures during development (like the ENS) depends on cell proliferation, so a global reduction in fetal cell proliferation may result in not only a smaller baby, but also a higher risk of structural birth defects. This hypothesis is supported by higher rates of major congenital anomalies in children born after placental abruption (OR 3.81, 95% confidence interval (CI) 1.34-2.37 for gastrointestinal anomalies and 1.92, 95% CI 1.6-2.52 for all anomalies) (Riihimaki et al., 2013).
HSCR genetics
HSCR is one of the best understood complex human genetic diseases (Alves et al., 2013; Amiel et al., 2008; Avetisyan et al., 2015a; Lake et al., 2013; McKeown et al., 2013; Panza et al., 2012). As might be predicted by the complex cellular mechanisms needed to form the ENS (i.e., proliferation, migration, controlled differentiation), many gene defects can increase HSCR risk. This includes mutations that reduce activity of cell surface receptors (RET, EDNRB), extracellular ligands (GDNF, NRTN, EDN3), and transcription factors (SOX10, PHOX2B, ZFHX1B), as well as specific chromosomal anomalies (Down syndrome). Work in model systems implicates many additional signaling molecules (Pik3, MEK, PLCγ, PKCζ, GSK3), enzymes (RALDH2, EDN3), extracellular matrix proteins (laminin, fibronectin, collagen VI), integrins (ITGB1), synaptic vesicle proteins (Synaptobrevin, SNAP25), morphogens (BMP2/BMP4, Shh, Ihh), and small molecules (retinoic acid, serotonin) influence ENS development. Undoubtedly there is more to learn since the known molecules are inadequate to explain how the ENS forms. Non-genetic factors may influence ENS development by modifying the expression levels or activity of these molecules already known to guide ENS development. This means that there are many targets through which non-genetic factors could affect the developing ENS.
Gene-environment interactions and the ENS
Human development is largely driven by genetics, but gene products do not work in isolation (Figure 1). Intrauterine growth requires energy, building blocks for protein, ions that act as enzyme cofactors or are essential for membrane electrical properties and vitamins that play diverse roles in intermediary metabolism. Oxygen is essential for efficient energy production from the electron transport chain in mitochondria, but reactive oxygen species reduce bowel colonization by ENCDC in some settings (e.g. TCOF1 mutation) (Barlow et al., 2012) and low intrauterine oxygen levels relative to post-natal levels appear to be optimal for ENS stem cell proliferation (Hegewald et al., 2011). Many of the signaling molecules needed for ENS development are also common drug targets and some vitamins (vitamin A, folate), nutrients and small metabolites (butyrate) can influence gene expression. Given the large number of potential targets and non-genetic factors that might impact proteins critical for ENS morphogenesis, it is not surprising that non-genetic factors impact ENS development. This is especially important since known genetic changes that predispose to HSCR are partially penetrant (Alves et al., 2013). This means that a child with HSCR typically has more than one predisposing genetic change or a combination of genetic and non-genetic risk factors led to the disease. If non-genetic factors can be identified and eliminated, some cases of HSCR might be prevented.
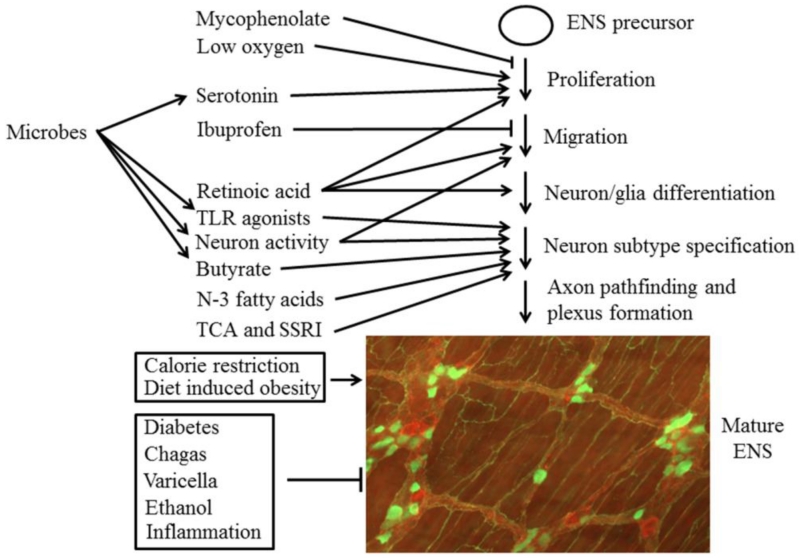
Figure 1.
This schematic shows many of the non-genetic factors affect ENS development or the mature ENS. The mature ENS is an intricate structure as represented in the image that shows the adult mouse myenteric plexus stained with antibodies to calretinin (green) and substance P (red) (courtesy of Marina Avetisyan). Arrows indicate aspects of ENS development that are “modulated” (i.e., changed) by a specific factor, or indicate that the factor acts on the mature ENS. The symbol “—l” indicates that these factors inhibit a specific process or damage the mature ENS.
Non-HSCR motility disorders
In contrast to HSCR, the underlying problems that cause other types of bowel motility disorders are relatively poorly understood. These disorders include achalasia (Castell, 2013), gastroparesis (Camilleri et al., 2013), chronic intestinal pseudoobstruction syndrome (CIPO) (Di Lorenzo and Youssef, 2010; Schappi et al., 2013), slow transit constipation, and irritable bowel syndrome (IBS) (Camilleri, 2013; Heuckeroth, 2014; Knowles et al., 2010; Panza et al., 2012; Wood, 2013) (Table 2).
Table 2.
Human intestinal motility disorders where ENS defects my cause symptoms (simplified)
| Hirschsprung disease (HSCR) | Absence of enteric neurons in distal bowel causes tonic contraction and functional obstruction leading to abdominal distension, vomiting, constipation, growth failure and early death |
| Achalasia | Defective esophageal peristalsis and poor relaxation of the lower esophageal sphincter causes swallowing problems |
| Gastroparesis | Defective stomach emptying or accommodation in association with visceral hypersensitivity causes nausea, abdominal pain, vomiting and weight loss |
| Chronic intestinal pseudoobstruction (CIPO) |
Abnormal intestinal contractility as a result of defects in the ENS, intestinal smooth muscle or pacemaker cells (Interstitial cells of Cajal) causes abdominal distension, vomiting, and the inability to survive solely on enteral feeding |
| Slow transit constipation | Constipation do to slow movement of luminal content through the colon |
| Irritable bowel syndrome (IBS) | Abnormal bowel motility and visceral hypersensitivity causes diarrhea and/or constipation and abdominal pain. IBS affects about 25% of the adult population and can be uncomfortable, but does not affect nutrition or longevity |
Also, in contrast to HSCR where the ENS is completely missing from distal bowel, these disorders may be caused by altered numbers of enteric neurons and glia, by changes in the types of neurons present, by disruptions in neuronal circuitry or by altered neuronal function. Damage to the ENS may be induced by destructive effects of systemic illness (e.g. diabetic gastroparesis) (Thazhath et al., 2013), infection (achalasia and colon dysmotility in Chagas disease (Machado et al., 2012), varicella zoster (Chen et al., 2011; Holland-Cunz et al., 2006)), toxins (e.g., ethanol) (Krecsmarik et al., 2006), or local inflammation (causing inflammatory bowel disease associated dysmotility (Mawe, 2015; Mawe et al., 2009; Vasina et al., 2006) and post-infectious irritable bowel syndrome (Spiller and Garsed, 2009)). In part, post-inflammatory changes in the ENS may be mediated by cytokines that induce GDNF and NGF synthesis in enteric glia or alter glial phenotypes (von Boyen et al., 2006a; von Boyen et al., 2004, 2006b). Enteric glia modulate neuronal function to regulate motility, and can affect neuron survival and bowel epithelial barrier function, among other roles (Brown et al., 2016; Ochoa-Cortes et al., 2016; Sharkey, 2015). Furthermore, inflammation can destroy the ENS, change neurochemical content or alter enteric neuron and glial function through a variety of complex mechanisms (Brierley and Linden, 2014; MacEachern et al., 2015; Moynes et al., 2014; Poole et al., 2015). Remarkably, early life emotional distress (e.g., maternal separation model) and adult stress (e.g. water immersion in rodents) can also cause long-term changes in enteric glia and neurons, as well as changes in colon and gastric function in ways that may be relevant for gastroparesis and IBS (Bian et al., 2011; Fujikawa et al., 2015; Li et al., 2015; Moloney et al., 2015; Tominaga et al., 2016). Non-genetic factors may also protect the ENS from injury. For example, quercetin, a flavonol antioxidant found in fruits, vegetables and grains, reduced enteric neuron and glia loss in a diabetic rat model (Lopes et al., 2012). Clearly we need to find additional non-genetic factors that reduce ENS injury if we hope to prevent serious intestinal motility disorders.
Evidence in model systems that non-genetic factors affect HSCR risk
Although data that non-genetic factors cause human HSCR is limited, population based surveys are underpowered to uncover these links (i.e., even if you had good data about 100,000 pregnancies, only about 20 children with HSCR would be expected) and little work has been done in this area. To find evidence that non-genetic factors alter HSCR risk, a dedicated case-control study focused on early pregnancy events, maternal health, nutrition and exposures would be needed. One epidemiologic study suggested that maternal coffee consumption and first trimester fever increased HSCR risk in children with Down syndrome (Torfs and Christianson, 1999), but this has not been replicated. The results are plausible, at least for coffee, since caffeine increases cAMP by blocking phosphodiesterase (Salzman et al., 1972) and reduced cAMP-dependent Protein kinase A (PKA) activity is an important aspect of EDNRB signaling (Barlow et al., 2003; Fuchs et al., 2001). Studies of first trimester maternal fever and HSCR have yielded inconsistent results (Larsson et al., 1989; Torfs and Christianson, 1999), but there is little data about the duration or intensity of fever during pregnancy and the period of ENCDC migration lasts five weeks in humans (a long time for a febrile illness).
In the absence of robust human data, model systems powerfully support the hypothesis that non-genetic factors may alter HSCR risk. A drug screen in zebrafish using only a single drug concentration identified nine medicines that reduce ENCDC colonization of fish bowel including artesunate, lovastatin and mycophenolic acid (Lake et al., 2013). Artesunate is a commonly used antimalarial whose mechanisms is poorly understood (Haynes et al., 2013). Lovastatin blocks 3-hydroxy-3-methylglutaryl coenzyme A reductase, the rate limiting step in de novo cholesterol synthesis (Alberts, 1988). Mycophenolate reduces cellular GTP levels (and reduces other guanine nucleotides) as detailed above. The reason that lovastatin reduces ENCDC colonization of zebrafish bowel is not known, but hedgehog proteins are cholesterol modified morphogens that affect the ENS (Fu et al., 2004; Nagy et al., 2015; Porter et al., 1996; Sukegawa et al., 2000), and lipid rafts are cholesterol rich membrane domains that appear to be important for efficient RET signaling (Pierchala et al., 2006; Tansey et al., 2000). Since only a single drug concentration was used for the zebrafish screen described above, it is reasonable to expect that other medicines would be found to affect ENCDC colonization of developing bowel if additional drug concentrations were tested (i.e., since the concentration tested may not be near the active concentration for many tested drugs). In support of this hypothesis, when drugs used by >0.5% of U.S. women during early pregnancy were tested at a range of concentrations on zebrafish, ibuprofen was found to slow ENCDC migration by altering actin cytoskeletal dynamics (Schill et al., 2015). Interestingly, inactivation of the cyclooxygenase enzymes that make prostaglandins (the primary therapeutic targets of ibuprofen) did not slow ENCDC colonization of fetal bowel in mice, suggesting that “off target” effects of ibuprofen lead to ENCDC bowel colonization defects. The concentration of ibuprofen needed to slow ENCD migration and the magnitude the effect differs among species tested (zebrafish, chick and mouse), emphasizing the need for data in human populations about maternal medicine use and HSCR occurrence.
Maternal nutrition is also likely to influence HSCR risk. The most powerful data to support this hypothesis is that relatively mild vitamin A deficiency causes HSCR-like disease in mice (Fu et al., 2010). Vitamin A is the precursor for retinoic acid (RA), a small molecule that alters transcription by binding to and regulating the RAR and RXR transcription factors that control many aspects of development (D’Ambrosio et al., 2011; di Masi et al., 2015). Interestingly, RALDH1, RALDH2, and RALDH3, the retinaldehyde dehydrogenases that make RA, also appear to affect ENS development (Wright-Jin et al., 2013), with RALDH2 activity absolutely essential for formation of the ENS (Niederreither et al., 2003). Furthermore, retinol binding protein 4 (RBP4) deficiency causes HSCR-like disease in mice that are also heterozygous for Ret, a major HSCR risk allele (Fu et al., 2010). The mechanism for RA-deficiency induced HSCR-like disease in mice is not completely understood since there appear to be retinoid effects on the abundance of multiple proteins at various stages. Early in development as ENCDC migrate from the neural tube to the bowel, RA signaling is essential to induce Ret expression within ENCDC (Simkin et al., 2013) and at later stages RA supports neuronal precursor proliferation and neuronal differentiation (Sato and Heuckeroth, 2008). In contrast, when the wavefront of migrating ENCDC has reached the mid-colon, RA reduces PTEN protein levels in ENCDC at the leading edge of the migration wavefront (Fu et al., 2010). This is important because PTEN reverses the action of PI-3 kinase, a major RET regulated protein required for ENCDC survival, proliferation and migration. Indeed, a balance of PTEN/PI-3 kinase activity is likely to be important for ENS development since cell-autonomous PTEN deficiency within ENCDC causes bowel hyperganglionosis and a CIPO phenotype (i.e., intestinal distension and weight loss) that results in early death (Puig et al., 2009). This phenotype is reminiscent of the hyperganglionosis and dysmotility that occurs with mutations in a Sprouty2 (Taketomi et al., 2005), a protein that reduces RET activity. These data suggest that vitamin A deficiency, one of the most common micronutrient deficiencies in many parts of the world (West, 2002), may be a preventable cause of HSCR. Unfortunately, even though large studies of maternal vitamin A supplementation have been performed (as summarized in a recent Cochran review of 153,500 women), there is no data about HSCR frequency as a function of vitamin A supplementation (McCauley et al., 2015). Furthermore, because HSCR is often not apparent at birth and diagnosis requires sophisticated medical tests, ascertainment of HSCR cases is unlikely to be good in nutrient poor populations. Based on known biochemistry, it seems likely that other micronutrient deficiencies also predispose to ENS defects. For example, mycophenolate causes HSCR-like aganglionosis in mice because it blocks de novo guanine nucleotide synthesis via IMPDH inhibition. De novo guanine synthesis also requires folate, niacin, vitamin B6 and vitamin B12 dependent enzymes. Recent data also suggest that biotin may enhance migration of ENS precursors (Fattahi et al., 2016). Biotin is a nutrient required by a family of carboxylases that have important roles in fatty acid metabolism, amino acid metabolism, carbohydrate metabolism, polyketide synthesis, and urea utilization among other cellular processes.
Evidence that non-genetic factors may alter ENS structure without causing HSCR
ENS function depends on a balance of specific neuron subtypes (e.g. excitatory motor neurons, inhibitory motor neurons, intrinsic primary afferent neurons (IPANs, sensory), interneurons, etc.). These neuronal subtypes differ in morphology, neurotransmitters produced, receptors, axon number, axon trajectory and function (Furness, 2012; Hao and Young, 2009). The factors that guide neuronal subtype identity in the ENS remain poorly understood, but the ratio of neuronal subtypes is influenced by the timing of cell cycle exit (Avetisyan et al., 2015a; Bergner et al., 2014; Chalazonitis et al., 2008; D’Autreaux et al., 2011; Pham et al., 1991; Wang et al., 2010) and many factors impact the decision to exit the cell cycle (e.g., GDNF, EDN3, Shh, BMPs, RA) (Lake and Heuckeroth, 2013). One intriguing observation is that serotonin (5-HT) produced by early differentiating enteric neurons acts as a trophic factor for surrounding ENCDC and that altered serotonin levels influences neuron subtype ratios (Li et al., 2011). For this reasons, mice with a mutation in the 5-HT producing enzyme tryptophan hydroxylase 2 (TPH2) have fewer “late born” GABA and DAT (dopamine active transporter) immunoreactive neurons than WT animals. The norepinephrine reuptake transporter (NET) is also required for development of serotonergic neurons and reduced NET activity affects ENS development (Li et al., 2010). Interestingly, in postnatal bowel, serotonin also promotes new neurogenesis and ENS repair, at least partially via 5-HT4 receptor (Gershon, 2012; Matsuyoshi et al., 2010; Takaki et al., 2014). Consistent with these observations, human data based on 35,400 pregnancies suggest that first trimester exposure to tricyclic antidepressants (TCAs) and second or third trimester exposure to selective serotonin reuptake inhibitors (SSRIs) increases the use of laxatives in children after birth up to 10-fold for combined exposures (Nijenhuis et al., 2012). This epidemiologic observation may be explained by the ability of TCAs to block NET and of SSRIs to block serotonin reuptake from the synapse leading to increased norepinephrine and 5-HT signaling respectively.
Neuronal subtype specification also appears to be influenced by neuronal activity. Blocking neural activity not only slows migration of ENCDC through fetal bowel (Vohra et al., 2006), but also selectively reduces the number of NO producing neurons (Hao et al., 2010). In contrast, ENS precursor depolarization increases tyrosine hydroxylase and vasoactive intestinal peptide (VIP) producing neurons in culture without increasing NO producing cells (Chevalier et al., 2008). While the precise mechanisms underlying these observations are not known, many neuromodulatory medicines (antidepressants, antipsychotics, anti-epileptics, anti-cholinergics, anti-hypertensives) cross the placenta and could therefore affect enteric neuron subtype specification by altering neuron activity, leading to changes in post-natal bowel motility.
After birth, diet has complex effects on the ENS that may be due direct effects on enteric neuron activity or to changes in gut microbes that secondarily affect the ENS. In support of direct effects on the ENS, many nutrients activate enteric neurons (e.g., glucose, fatty acids, amino acids) and may induce long term changes in neurotransmitter expression (Neunlist and Schemann, 2014). As an example, the short chain fatty acids butyrate can alter the ratio of myenteric choline acetyltransferase (ChAT) and nNOS immunoreactive neurons (Soret et al., 2010; Suply et al., 2012). This might result from changes in neuron activity that can affect cell fate since mucosally projecting myenteric neurons undergo transient depolarization and late hyperpolarization in response to butyrate. This occurs via calcium release from intracellular stores that then acts on calcium dependent potassium channels (Hamodeh et al., 2004; Haschke et al., 2002; Neunlist et al., 1999). Alternatively, butyrate could alter gene expression within developing enteric neurons by inhibiting histone deacetylases (Soret et al., 2010; Steliou et al., 2012). In addition to the short chain fatty acid butyrate, feeding long chain N-3 polyunsaturated fatty acids to pigs during gestation and while nursing also altered enteric neuron subtype ratios, increasing ChAT and decreasing VIP immunoreactive neurons in the jejunal submucosal plexus of their piglets (De Quelen et al., 2011). The mechanism underlying this observation is not understood, but N-3 unsaturated fatty acids are known to compete with N-6 unsaturated fatty acids, inhibiting arachidonic acid metabolism and reducing inflammation (Yates et al., 2014). As noted above, inflammation can alter enteric neuron subtype ratios. Finally, calorie restriction reduced aging related neuron loss in the ileal ENS of rats (Thrasivoulou et al., 2006), while diet induced obesity reduced age associated antral nitrergic neuron loss (Baudry et al., 2012) possibly via altered trophic factor expression in the bowel wall that results from these dietary manipulations (Korsak et al., 2012; Saavedra et al., 2008). The impact of diet on the ENS is difficulty to separate from the role of gut microbes after birth. For example, butyrate may be ingested, or can be synthesized in the colon by anaerobic bacteria that ferment dietary fiber (Soret et al., 2010). Collectively these data suggest that neuron subtype ratios and therefore cell fate in the ENS is regulated in part by dietary nutrients. This adaptation may facilitate the bowel’s ability to digest a wide range of nutrients.
Strong support for microbial effects on the ENS comes from studies in germ free mice, since these animals have altered motility and changes in ENS structure including fewer myenteric neurons, an increased proportion of NO neurons, and fewer calbindin+ myenteric neurons compared to specific pathogen free or conventionally colonized animals (Collins et al., 2014; Dey et al., 2015; McVey Neufeld et al., 2015). The mechanisms underlying these observations are likely to be complicated. In addition to bacterial metabolites, microbial structural components may affect the ENS directly and indirectly via Toll-like receptors, a subset of pattern recognition receptors that bind to and are activated by microbial molecules. TLR activation stimulates intracellular signaling cascades that may result in the release of cytokines, chemokines and neurotrophic factors (Frosali et al., 2015). For example, TLRs 1-9 are expressed by intestinal smooth muscle, a prominent source of neurotrophic factors. Stimulation of TLR2, TLR4, TLR5 or TLR9 increased levels of GDNF, NGF, BDNF and LIF by intestinal muscle cells (Brun et al., 2015) and GDNF prominently supports the developing and mature ENS (Lake and Heuckeroth, 2013; Rodrigues et al., 2011). In contrast, TLR2−/− mice have reduced GDNF expression, altered intestinal motility, smaller ganglia and fewer neurons than wild type animals (Brun et al., 2013). NGF may also enhance neurite growth from enteric neurons (Dothel et al., 2015; Esteban et al., 1998) while BDNF supports enteric glia (Levanti et al., 2009). TLR2 can be activated by a wide range of bacterial, fungal and viral components and is expressed by enteric neurons and glia in addition to smooth muscle. Similarly, TLR3, TLR4, and TLR7 are expressed in the human ENS (Barajon et al., 2009). TLR4 recognizes lipopolysaccharide (LPS), a major gram negative bacteria cell wall component and TLR4−/− mice have reduced nitrergic neuron number in the colon myenteric plexus and delayed gastrointestinal motility (Anitha et al., 2012). This fits well with the observation that LPS enhances proliferation of ENS neural progenitor cells in culture and delays precursor differentiation (Schuster et al., 2014). Bacteria also release diverse neurotransmitters (e.g., GAGA, serotonin, acetylcholine) (Wall et al., 2014) and changes in ENS structure may reflect altered neuronal activity as discussed above. Similar mechanistic observations may underlie the increased galanin and calcitionin gene-related peptide (CGRP) immunoreactive submucosal neurons observed in piglets fed the probiotic Pediococcus acidilactici (di Giancamillo et al., 2010) or the reduced calbindin+ myenteric neurons in pig jejunum after feeding with Saccharomyces boulardii (Kamm et al., 2004).
Special mention should be made of breast milk, which provides not only a full spectrum of nutrients, but also many bioactive compound including neurotrophic factors (GDNF, BDNF, NT3, CNTF) and cytokines (tumor necrosis factor (TNF)-α, Interferon (IFN)-γ, RANTES, monocyte chemotactic protein (MCP)-1, MIP-1-a, IL-1, IL-6, IL-8, IL-10, ENA78, GRO-a, Leptin, IL-7 and IL-17) that could support enteric neurons and glia (Collado et al., 2015; Fichter et al., 2011). It is not clear if these compounds can get across the gut epithelium in the absence of injury, but at least during early postnatal stages the mucosal barrier partially permits transport of many macromolecules (Drozdowski et al., 2010). Interestingly, breast milk reduces occurrence of a deadly bowel disease of premature infants called necrotizing enterocolitis (NEC) (Good et al., 2014). Bowel injury during NEC should further increase translocation of neurotrophic factors and many other breast milk components (e.g., nitrite, L-arginine, glutamine, lactoferrin, or epithelial growth factors) across the epithelium where they may act in concert to prevent further injury and modulate bowel inflammation (Avetisyan et al., 2015b; Gershon, 2012; Savidge et al., 2007; Sigalet et al., 2007). The role of the ENS in NEC is under-investigated, but NEC clearly causes injury to the ENS and transplantation of enteric neural crest-derived stem cells prevents death in an experimental NEC model (Zhou et al., 2013).
Collectively these data show that dietary components may alter the ratio of neuron subtypes in the bowel, a plausible way to permit adaptation to varied diets and intestinal microbes. These changes may be mediated by altered neuronal activity, dietary nutrients, their metabolites, or microbial products that regulate transcription, modulate signal transduction, or regulate neurotrophic factor production. Much more work is needed to define mechanisms of subtype specification in the ENS, and to establish how neuromodulatory medicines, food and microbes affect ENS development.
Even “simple” observations have complex implications for ENS development when non-genetic factors impact protein abundance (“a tale of GDNF”)
GDNF is an essential trophic factor for ENCDC during fetal development because GDNF activates the RET transmembrane tyrosine kinase via the co-receptor GFRα1 (Cacalano et al., 1998; Durbec et al., 1996; Enomoto et al., 1998; Heuckeroth et al., 1998; Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996; Trupp et al., 1996). RET supports ENCDC survival, migration and proliferation during bowel colonization, but also enhances neuronal differentiation and neurite growth once cells stop dividing (Schafer and Mestres, 1999; Schuchardt et al., 1994; Vohra et al., 2007a). Because different enteric neuron subtypes exit the cell cycle at different stages of development (Bergner et al., 2014; Pham et al., 1991), the timing, location and intensity of GDNF production in the bowel affects enteric neuron subtype ratios. The location of GDNF production also affects patterning for NO producing neurites (Wang et al., 2010). Inactivating mutations in GDNF, RET or their co-receptor GFRα1 cause total intestinal aganglionosis (no neurons in the small bowel or colon) in mice. Furthermore, mice with GDNF heterozygosity have reduced enteric neuron number and abnormal bowel motility (Gianino et al., 2003). As might be expected, RET heterozygosity is a common cause of human HSCR (about 30% of all cases) producing about a 50% HSCR risk (i.e., partial penetrance) (Alves et al., 2013; Amiel et al., 2008), while homozygous RET inactivating mutations cause human total intestinal aganglionosis (Shimotake et al., 2001) similar to the murine phenotype. These observations powerfully suggest that altered RET activity as a result of changes in GDNF production should lead to changes in ENS structure and function during development and in adulthood, especially since GDNF appears to have a direct role in the peristaltic response (Grider et al., 2010).
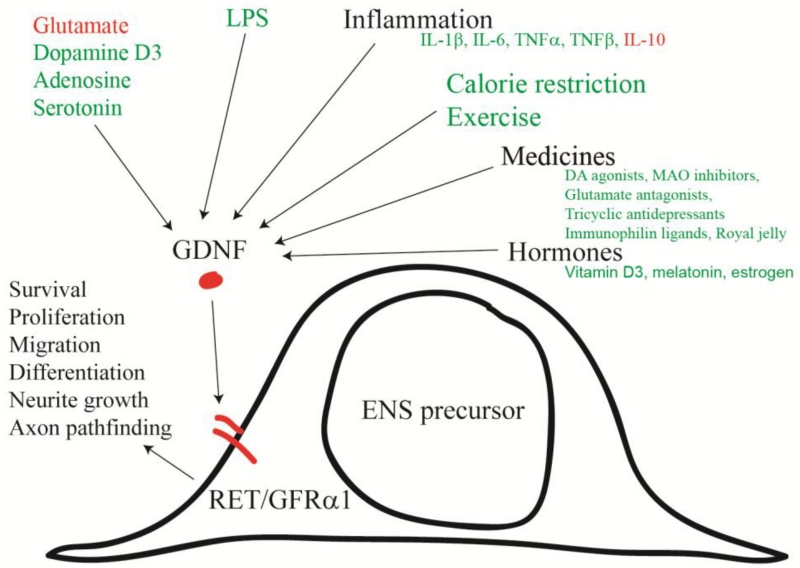
Regulation of GDNF expression is remarkably complex (Figure 2) (Saavedra et al., 2008) suggesting that some changes in ENS structure and function before and after birth might be induced by changes in GDNF in response to non-genetic signals. Regulators of GDNF expression in the bowel are incompletely understood, but in other tissues GDNF levels are influenced by many transcription factors (Six1, Six2, Pax3, Eya1, Sall1, Hox11, Foxc1, Foxc2, NF-κB, CREB), signaling proteins (MAPK, PKC, PKA, Ca++, PP2A, Sprouty1), neurotransmitters (glutamate, dopamine, adenosine, serotonin), extracellular ligands (GDF11, Slit2, BMP4, EDN1, FGF2), pro-inflammatory cytokines (IL-1β, IL-6, TNFα, TNFβ, IL-10,), bacterial products (LPS), lifestyle choices (calorie restriction, exercise), pharmacologic agents (dopamine receptor agonists, monoamine oxidase inhibitors, glutamate receptor antagonists, antidepressants, antipsychotics, mood stabilizers, anti-epileptics, anti-dementia drugs, immunophilin ligands), hormones (melatonin, estrogen), vitamin D3, and by some traditional medicines (Rehmannia glutinosa, Ibogaine, royal jelly). These GDNF regulatory mechanisms coupled with GDNF effects on ENS structure and function suggest that many non-genetic factors may affect the ENS simply by modifying GDNF levels in fetal or post-natal bowel.
Figure 2.
GDNF/GFRα1/RET signaling impacts many aspects of ENS development and has important roles in the adult ENS. For this reason, the timing, location and intensity of GDNF signaling can profoundly alter ENS structure and function. Remarkably, many non-genetic factors impact GDNF abundance, although most of these effects have not been well studied in the fetal or adult bowel. Based primarily on work in other systems, green text indicates factor that increase GDNF mRNA or protein levels. Red text indicates that the factor reduces GDNF mRNA or protein levels.
Summary
The ENS is an elegant nervous system in the bowel wall that controls most aspects of bowel function. Because the developmental pathways needed to form the ENS are complicated, it is not surprising that many molecular mechanisms critically influence ENS precursor colonization of fetal bowel and the differentiation of enteric neurons and glia. While the underlying genetics provides the infrastructure for successful fetal morphogenesis, maternal health, placental function, maternal nutrition, and maternal medicines impact development changing the risk of structural birth defects. Hirschsprung disease seems particularly likely to be impacted by gene-environment interactions because in most children with HSCR the bowel is almost fully colonized by ENCDC, and even a small increase in bowel colonization could mean the difference between health and life threatening disease. These observations provide new hope that some cases of HSCR may be preventable and that we can take advantage of observed non-genetic mediators to enhance bowel function in children and adults with a wide range of bowel motility disorders.
Highlights.
The enteric nervous system (ENS) controls most aspects of gut function.
The ENS forms from neural crest-derived precursors that colonize fetal bowel.
Genetic mechanisms that control ENS development are complicated involving many genes.
ENS development can be altered by diverse medicines, nutrients, and microbes.
Some Hirschsprung disease cases may be preventable by optimizing non-genetic factors.
Acknowledgements
We are grateful to Marina Avetisyan for thoughtful comments on this manuscript. This work was supported by Irma and Norman Braman Endowment (ROH), Suzi and Scott Lustgarten Center Endowment (ROH), The Children’s Hospital of Philadelphia Research Institute (ROH), The Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital MD-II-2013-269 (ROH), NIH grants RO1 DK087715 (ROH), Burroughs Wellcome Fund Clinical Scientist Award in Translational Research (grant no. 1008525) (ROH), German Research Foundation (DFG SCHA 878-1, 2 and 3), and by the German Ministry of Education and Research (BMBF, FKZ 17114X10).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts AW. Discovery, biochemistry and biology of lovastatin. Am J Cardiol. 1988;62:10J–15J. doi: 10.1016/0002-9149(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Alves MM, Sribudiani Y, Brouwer RW, Amiel J, Antinolo G, Borrego S, Ceccherini I, Chakravarti A, Fernandez RM, Garcia-Barcelo MM, Griseri P, Lyonnet S, Tam PK, van Ijcken WF, Eggen BJ, te Meerman GJ, Hofstra RM. Contribution of rare and common variants determine complex diseases-Hirschsprung disease as a model. Dev Biol. 2013;382:320–329. doi: 10.1016/j.ydbio.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, Brooks AS, Antinolo G, de Pontual L, Clement-Ziza M, Munnich A, Kashuk C, West K, Wong KK, Lyonnet S, Chakravarti A, Tam PK, Ceccherini I, Hofstra RM, Fernandez R. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. 2012;143:1006–1016. e1004. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avetisyan M, Schill EM, Heuckeroth RO. Building a second brain in the bowel. J Clin Invest. 2015a;125:899–907. doi: 10.1172/JCI76307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avetisyan M, Wang H, Schill EM, Bery S, Grider JR, Hassell JA, Stappenbeck T, Heuckeroth RO. Hepatocyte Growth Factor and MET Support Mouse Enteric Nervous System Development, the Peristaltic Response, and Intestinal Epithelial Proliferation in Response to Injury. J Neurosci. 2015b;35:11543–11558. doi: 10.1523/JNEUROSCI.5267-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badner JA, Sieber WK, Garver KL, Chakravarti A. A genetic study of Hirschsprung disease. Am J Hum Genet. 1990;46:568–580. [PMC free article] [PubMed] [Google Scholar]
- Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A, Rumio C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem. 2009;57:1013–1023. doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow A, de Graaff E, Pachnis V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron. 2003;40:905–916. doi: 10.1016/s0896-6273(03)00730-x. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Dixon J, Dixon MJ, Trainor PA. Balancing neural crest cell intrinsic processes with those of the microenvironment in Tcof1 haploinsufficient mice enables complete enteric nervous system formation. Hum Mol Genet. 2012;21:1782–1793. doi: 10.1093/hmg/ddr611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow AJ, Wallace AS, Thapar N, Burns AJ. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135:1681–1691. doi: 10.1242/dev.017418. [DOI] [PubMed] [Google Scholar]
- Baudry C, Reichardt F, Marchix J, Bado A, Schemann M, des Varannes SB, Neunlist M, Moriez R. Diet-induced obesity has neuroprotective effects in murine gastric enteric nervous system: involvement of leptin and glial cell line-derived neurotrophic factor. J Physiol. 2012;590:533–544. doi: 10.1113/jphysiol.2011.219717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron KF, Cardinal T, Toure AM, Beland M, Raiwet DL, Silversides DW, Pilon N. Male-biased aganglionic megacolon in the TashT mouse line due to perturbation of silencer elements in a large gene desert of chromosome 10. PLoS Genet. 2015;11:e1005093. doi: 10.1371/journal.pgen.1005093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner AJ, Stamp LA, Gonsalvez DG, Allison MB, Olson DP, Myers MG, Jr., Anderson CR, Young HM. Birthdating of myenteric neuron subtypes in the small intestine of the mouse. J Comp Neurol. 2014;522:514–527. doi: 10.1002/cne.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian ZX, Qin HY, Tian SL, Qi SD. Combined effect of early life stress and acute stress on colonic sensory and motor responses through serotonin pathways: differences between proximal and distal colon in rats. Stress. 2011;14:448–458. doi: 10.3109/10253890.2011.558604. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Linden DR. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol. 2014;11:611–627. doi: 10.1038/nrgastro.2014.103. [DOI] [PubMed] [Google Scholar]
- Brown IA, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cellular and molecular gastroenterology and hepatology. 2016;2:77–91. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun P, Giron MC, Qesari M, Porzionato A, Caputi V, Zoppellaro C, Banzato S, Grillo AR, Spagnol L, De Caro R, Pizzuti D, Barbieri V, Rosato A, Sturniolo GC, Martines D, Zaninotto G, Palu G, Castagliuolo I. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology. 2013;145:1323–1333. doi: 10.1053/j.gastro.2013.08.047. [DOI] [PubMed] [Google Scholar]
- Brun P, Gobbo S, Caputi V, Spagnol L, Schirato G, Pasqualin M, Levorato E, Palu G, Giron MC, Castagliuolo I. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol Cell Neurosci. 2015;68:24–35. doi: 10.1016/j.mcn.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Delalande JM, Le Douarin NM. In ovo transplantation of enteric nervous system precursors from vagal to sacral neural crest results in extensive hindgut colonisation. Development. 2002;129:2785–2796. doi: 10.1242/dev.129.12.2785. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang L-C, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A. GFRα1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Genetics of human gastrointestinal sensation. Neurogastroenterol Motil. 2013;25:458–466. doi: 10.1111/nmo.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. doi: 10.1038/ajg.2012.373. quiz 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell VA, Owens SE, Chandler RL, Airey DC, Bradley KM, Smith JR, Southard-Smith EM. Interactions between Sox10 and EdnrB modulate penetrance and severity of aganglionosis in the Sox10Dom mouse model of Hirschsprung disease. Hum Mol Genet. 2004;13:2289–2301. doi: 10.1093/hmg/ddh243. [DOI] [PubMed] [Google Scholar]
- Castell DO. Motility: Achalasia subtyping directs therapy. Nat Rev Gastroenterol Hepatol. 2013;10:202–203. doi: 10.1038/nrgastro.2013.40. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, Pham TD, Li Z, Roman D, Guha U, Gomes W, Kan L, Kessler JA, Gershon MD. Bone morphogenetic protein regulation of enteric neuronal phenotypic diversity: relationship to timing of cell cycle exit. J Comp Neurol. 2008;509:474–492. doi: 10.1002/cne.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Gershon AA, Li Z, Cowles RA, Gershon MD. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J Neurovirol. 2011;17:578–589. doi: 10.1007/s13365-011-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier J, Derkinderen P, Gomes P, Thinard R, Naveilhan P, Vanden Berghe P, Neunlist M. Activity-dependent regulation of tyrosine hydroxylase expression in the enteric nervous system. J Physiol. 2008;586:1963–1975. doi: 10.1113/jphysiol.2007.149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado MC, Santaella M, Mira-Pascual L, Martinez-Arias E, Khodayar-Pardo P, Ros G, Martinez-Costa C. Longitudinal Study of Cytokine Expression, Lipid Profile and Neuronal Growth Factors in Human Breast Milk from Term and Preterm Deliveries. Nutrients. 2015;7:8577–8591. doi: 10.3390/nu7105415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 2014;26:98–107. doi: 10.1111/nmo.12236. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients. 2011;3:63–103. doi: 10.3390/nu3010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Autreaux F, Margolis KG, Roberts J, Stevanovic K, Mawe G, Li Z, Karamooz N, Ahuja A, Morikawa Y, Cserjesi P, Setlick W, Gershon MD. Expression level of Hand2 affects specification of enteric neurons and gastrointestinal function in mice. Gastroenterology. 2011;141:576–587. 587, e571–576. doi: 10.1053/j.gastro.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Quelen F, Chevalier J, Rolli-Derkinderen M, Mourot J, Neunlist M, Boudry G. n-3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J Physiol. 2011;589:4341–4352. doi: 10.1113/jphysiol.2011.214056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey N, Wagner VE, Blanton LV, Cheng J, Fontana L, Haque R, Ahmed T, Gordon JI. Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell. 2015;163:95–107. doi: 10.1016/j.cell.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Giancamillo A, Vitari F, Bosi G, Savoini G, Domeneghini C. The chemical code of porcine enteric neurons and the number of enteric glial cells are altered by dietary probiotics. Neurogastroenterol Motil. 2010;22:e271–278. doi: 10.1111/j.1365-2982.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo C, Youssef NN. Diagnosis and management of intestinal motility disorders. Semin Pediatr Surg. 2010;19:50–58. doi: 10.1053/j.sempedsurg.2009.11.006. [DOI] [PubMed] [Google Scholar]
- di Masi A, Leboffe L, De Marinis E, Pagano F, Cicconi L, Rochette-Egly C, Lo-Coco F, Ascenzi P, Nervi C. Retinoic acid receptors: From molecular mechanisms to cancer therapy. Molecular aspects of medicine. 2015;41C:1–115. doi: 10.1016/j.mam.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Dothel G, Barbaro MR, Boudin H, Vasina V, Cremon C, Gargano L, Bellacosa L, De Giorgio R, Le Berre-Scoul C, Aubert P, Neunlist M, De Ponti F, Stanghellini V, Barbara G. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2015;148:1002–1011. e1004. doi: 10.1053/j.gastro.2015.01.042. [DOI] [PubMed] [Google Scholar]
- Drozdowski LA, Clandinin T, Thomson AB. Ontogeny, growth and development of the small intestine: Understanding pediatric gastroenterology. World J Gastroenterol. 2010;16:787–799. doi: 10.3748/wjg.v16.i7.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckenbrod NR, Epstein ML. Behavior of enteric neural crest-derived cells varies with respect to the migratory wavefront. Dev Dyn. 2007;236:84–92. doi: 10.1002/dvdy.20974. [DOI] [PubMed] [Google Scholar]
- Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M, Sariola H, Pachnis V. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381:789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Jr., Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- Esteban I, Levanti B, Garcia-Suarez O, Germana G, Ciriaco E, Naves FJ, Vega JA. A neuronal subpopulation in the mammalian enteric nervous system expresses TrkA and TrkC neurotrophin receptor-like proteins. The Anatomical Record. 1998;251:360–370. doi: 10.1002/(SICI)1097-0185(199807)251:3<360::AID-AR12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Fattahi F, Steinbeck JA, Kriks S, Tchieu J, Zimmer B, Kishinevsky S, Zeltner N, Mica Y, El-Nachef W, Zhao H, de Stanchina E, Gershon MD, Grikscheit TC, Chen S, Studer L. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016 doi: 10.1038/nature16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichter M, Klotz M, Hirschberg DL, Waldura B, Schofer O, Ehnert S, Schwarz LK, Ginneken CV, Schafer KH. Breast milk contains relevant neurotrophic factors and cytokines for enteric nervous system development. Mol Nutr Food Res. 2011;55:1592–1596. doi: 10.1002/mnfr.201100124. [DOI] [PubMed] [Google Scholar]
- Frosali S, Pagliari D, Gambassi G, Landolfi R, Pandolfi F, Cianci R. How the Intricate Interaction among Toll-Like Receptors, Microbiota, and Intestinal Immunity Can Influence Gastrointestinal Pathology. Journal of immunology research. 2015;2015:489821. doi: 10.1155/2015/489821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Lui VC, Sham MH, Pachnis V, Tam PK. Sonic hedgehog regulates the proliferation, differentiation, and migration of enteric neural crest cells in gut. J Cell Biol. 2004;166:673–684. doi: 10.1083/jcb.200401077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Sato Y, Lyons-Warren A, Zhang B, Kane MA, Napoli JL, Heuckeroth RO. Vitamin A facilitates enteric nervous system precursor migration by reducing Pten accumulation. Development. 2010;137:631–640. doi: 10.1242/dev.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S, Amiel J, Claudel S, Lyonnet S, Corvol P, Pinet F. Functional characterization of three mutations of the endothelin B receptor gene in patients with Hirschsprung’s disease: evidence for selective loss of Gi coupling. Mol Med. 2001;7:115–124. [PMC free article] [PubMed] [Google Scholar]
- Fujikawa Y, Tominaga K, Tanaka F, Tanigawa T, Watanabe T, Fujiwara Y, Arakawa T. Enteric glial cells are associated with stress-induced colonic hyper-contraction in maternally separated rats. Neurogastroenterol Motil. 2015;27:1010–1023. doi: 10.1111/nmo.12577. [DOI] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Transactions of the American Clinical and Climatological Association. 2012;123:268–280. discussion 280. [PMC free article] [PubMed] [Google Scholar]
- Gianino S, Grider JR, Cresswell J, Enomoto H, Heuckeroth RO. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development. 2003;130:2187–2198. doi: 10.1242/dev.00433. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Hofstra RM, Burns AJ. Building a brain in the gut: development of the enteric nervous system. Clin Genet. 2013;83:307–316. doi: 10.1111/cge.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M, Sodhi CP, Hackam DJ. Evidence-based feeding strategies before and after the development of necrotizing enterocolitis. Expert Rev Clin Immunol. 2014;10:875–884. doi: 10.1586/1744666X.2014.913481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider JR, Heuckeroth RO, Kuemmerle JF, Murthy KS. Augmentation of the ascending component of the peristaltic reflex and substance P release by glial cell line-derived neurotrophic factor. Neurogastroenterol Motil. 2010;22:779–786. doi: 10.1111/j.1365-2982.2010.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625–632. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- Hamodeh SA, Rehn M, Haschke G, Diener M. Mechanism of butyrate-induced hyperpolarization of cultured rat myenteric neurones. Neurogastroenterol Motil. 2004;16:597–604. doi: 10.1111/j.1365-2982.2004.00545.x. [DOI] [PubMed] [Google Scholar]
- Hao MM, Moore RE, Roberts RR, Nguyen T, Furness JB, Anderson RB, Young HM. The role of neural activity in the migration and differentiation of enteric neuron precursors. Neurogastroenterol Motil. 2010;22:e127–137. doi: 10.1111/j.1365-2982.2009.01462.x. [DOI] [PubMed] [Google Scholar]
- Hao MM, Young HM. Development of enteric neuron diversity. J Cell Mol Med. 2009;13:1193–1210. doi: 10.1111/j.1582-4934.2009.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschke G, Schafer H, Diener M. Effect of butyrate on membrane potential, ionic currents and intracellular Ca2+ concentration in cultured rat myenteric neurones. Neurogastroenterol Motil. 2002;14:133–142. doi: 10.1046/j.1365-2982.2002.00312.x. [DOI] [PubMed] [Google Scholar]
- Haynes RK, Cheu KW, N’Da D, Coghi P, Monti D. Considerations on the mechanism of action of artemisinin antimalarials: part 1--the ‘carbon radical’ and ‘heme’ hypotheses. Infectious disorders drug targets. 2013;13:217–277. doi: 10.2174/1871526513666131129155708. [DOI] [PubMed] [Google Scholar]
- Hegewald C, Alt R, Hetz S, Cross M, Acikgoez A, Till H, Metzger R, Metzger M. Reduced oxygen stress promotes propagation of murine postnatal enteric neural progenitors in vitro. Neurogastroenterol Motil. 2011;23:e412–424. doi: 10.1111/j.1365-2982.2011.01761.x. [DOI] [PubMed] [Google Scholar]
- Heuckeroth RO. Hirschsprung disease. In: Faure C, DiLorenzo C, Thapar N, editors. Pediatric neurogastroenterology: gastrointestinal motility and functional disorders in children. Springer; New York: 2013. pp. 271–283. [Google Scholar]
- Heuckeroth RO. Congenital and Acquired Disorders of the Enteric Nervous System. In: D.L. G, L.C. S, J.R. S, editors. Translational Gastroenterology: Organogenesis to Disease. John Wiley & Sons, Inc.; 2014. pp. 225–239. [Google Scholar]
- Heuckeroth RO, Lampe PA, Johnson EMJ, Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Developmental Biology. 1998;200:116–129. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- Holland-Cunz S, Goppl M, Rauch U, Bar C, Klotz M, Schafer KH. Acquired intestinal aganglionosis after a lytic infection with varicella-zoster virus. J Pediatr Surg. 2006;41:e29–31. doi: 10.1016/j.jpedsurg.2005.12.060. [DOI] [PubMed] [Google Scholar]
- Kamm K, Hoppe S, Breves G, Schroder B, Schemann M. Effects of the probiotic yeast Saccharomyces boulardii on the neurochemistry of myenteric neurones in pig jejunum. Neurogastroenterol Motil. 2004;16:53–60. doi: 10.1046/j.1365-2982.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, Geboes K, Lindberg G, Martin JE, Meier-Ruge WA, Milla PJ, Smith VV, Vandervinden JM, Veress B, Wedel T. The London Classification of gastrointestinal neuromuscular pathology: report on behalf of the Gastro 2009 International Working Group. Gut. 2010;59:882–887. doi: 10.1136/gut.2009.200444. [DOI] [PubMed] [Google Scholar]
- Korsak K, Dolatshad NF, Silva AT, Saffrey MJ. Ageing of enteric neurons: oxidative stress, neurotrophic factors and antioxidant enzymes. Chemistry Central journal. 2012;6:80. doi: 10.1186/1752-153X-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krecsmarik M, Izbeki F, Bagyanszki M, Linke N, Bodi N, Kaszaki J, Katarova Z, Szabo A, Fekete E, Wittmann T. Chronic ethanol exposure impairs neuronal nitric oxide synthase in the rat intestine. Alcohol Clin Exp Res. 2006;30:967–973. doi: 10.1111/j.1530-0277.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- Lake JI, Heuckeroth RO. Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol. 2013;305:G1–24. doi: 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JI, Tusheva OA, Graham BL, Heuckeroth RO. Hirschsprung-like disease is exacerbated by reduced de novo GMP synthesis. J Clin Invest. 2013;123:4875–4887. doi: 10.1172/JCI69781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman KA, Simpson MJ, Newgreen DF. Mathematical and experimental insights into the development of the enteric nervous system and Hirschsprung’s disease. Development, growth & differentiation. 2007;49:277–286. doi: 10.1111/j.1440-169X.2007.00929.x. [DOI] [PubMed] [Google Scholar]
- Larsson LT, Okmian L, Kristoffersson U. No correlation between hyperthermia during pregnancy and Hirschsprung disease in the offspring. Am J Med Genet. 1989;32:260–261. doi: 10.1002/ajmg.1320320226. [DOI] [PubMed] [Google Scholar]
- Levanti MB, Esteban I, Ciriaco E, Perez-Pinera P, Cabo R, Garcia-Suarez O, Pardo B, Silos-Santiago I, Cobo J, Vega JA. Enteric glial cells express full-length TrkB and depend on TrkB expression for normal development. Neurosci Lett. 2009;454:16–21. doi: 10.1016/j.neulet.2009.02.051. [DOI] [PubMed] [Google Scholar]
- Li S, Fei G, Fang X, Yang X, Sun X, Qian J, Wood JD, Ke M. Changes in enteric neurons of small intestine in a rat model of irritable bowel syndrome with diarrhea. J Neurogastroenterol Motil. 2015 doi: 10.5056/jnm15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Caron MG, Blakely RD, Margolis KG, Gershon MD. Dependence of serotonergic and other nonadrenergic enteric neurons on norepinephrine transporter expression. J Neurosci. 2010;30:16730–16740. doi: 10.1523/JNEUROSCI.2276-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Cote F, Mallet J, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes CR, Ferreira PE, Zanoni JN, Alves AM, Alves EP, Buttow NC. Neuroprotective effect of quercetin on the duodenum enteric nervous system of streptozotocin-induced diabetic rats. Dig Dis Sci. 2012;57:3106–3115. doi: 10.1007/s10620-012-2300-7. [DOI] [PubMed] [Google Scholar]
- MacEachern SJ, Patel BA, Keenan CM, Dicay M, Chapman K, McCafferty DM, Savidge TC, Beck PL, MacNaughton WK, Sharkey KA. Inhibiting Inducible Nitric Oxide Synthase in Enteric Glia Restores Electrogenic Ion Transport in Mice With Colitis. Gastroenterology. 2015;149:445–455. e443. doi: 10.1053/j.gastro.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado FS, Dutra WO, Esper L, Gollob KJ, Teixeira MM, Factor SM, Weiss LM, Nagajyothi F, Tanowitz HB, Garg NJ. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Semin Immunopathol. 2012;34:753–770. doi: 10.1007/s00281-012-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyoshi H, Kuniyasu H, Okumura M, Misawa H, Katsui R, Zhang GX, Obata K, Takaki M. A 5-HT(4)-receptor activation-induced neural plasticity enhances in vivo reconstructs of enteric nerve circuit insult. Neurogastroenterol Motil. 2010;22:806–813. e226. doi: 10.1111/j.1365-2982.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- Mawe GM. Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon. J Clin Invest. 2015;125:949–955. doi: 10.1172/JCI76306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe GM, Strong DS, Sharkey KA. Plasticity of enteric nerve functions in the inflamed and postinflamed gut. Neurogastroenterol Motil. 2009;21:481–491. doi: 10.1111/j.1365-2982.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallion AS, Stames E, Conlon RA, Chakravarti A. Phenotype variation in two-locus mouse models of Hirschsprung disease: tissue-specific interaction between Ret and Ednrb. Proc Natl Acad Sci U S A. 2003;100:1826–1831. doi: 10.1073/pnas.0337540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley ME, van den Broek N, Dou L, Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev. 2015;10:CD008666. doi: 10.1002/14651858.CD008666.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown SJ, Stamp L, Hao MM, Young HM. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley interdisciplinary reviews. Developmental biology. 2013;2:113–129. doi: 10.1002/wdev.57. [DOI] [PubMed] [Google Scholar]
- McVey Neufeld KA, Perez-Burgos A, Mao YK, Bienenstock J, Kunze WA. The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol Motil. 2015;27:627–636. doi: 10.1111/nmo.12534. [DOI] [PubMed] [Google Scholar]
- Moloney RD, Stilling RM, Dinan TG, Cryan JF. Early-life stress-induced visceral hypersensitivity and anxiety behavior is reversed by histone deacetylase inhibition. Neurogastroenterol Motil. 2015;27:1831–1836. doi: 10.1111/nmo.12675. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichart LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Moynes DM, Lucas GH, Beyak MJ, Lomax AE. Effects of inflammation on the innervation of the colon. Toxicol Pathol. 2014;42:111–117. doi: 10.1177/0192623313505929. [DOI] [PubMed] [Google Scholar]
- Nagy N, Barad C, Graham H, Hotta R, Cheng L, Fejszak N, Goldstein AM. Sonic hedgehog controls enteric nervous system development by patterning the extracellular matrix. Development. 2015 doi: 10.1242/dev.128132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan D, Marcos-Gutierrez C, Pachnis V, De Graaff E. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129:5151–5160. doi: 10.1242/dev.129.22.5151. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Dobreva G, Schemann M. Characteristics of mucosally projecting myenteric neurones in the guinea-pig proximal colon. J Physiol. 1999;517(Pt 2):533–546. doi: 10.1111/j.1469-7793.1999.0533t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Schemann M. Nutrient-induced changes in the phenotype and function of the enteric nervous system. J Physiol. 2014;592:2959–2965. doi: 10.1113/jphysiol.2014.272948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgreen DF, Dufour S, Howard MJ, Landman KA. Simple rules for a “simple” nervous system? Molecular and biomathematical approaches to enteric nervous system formation and malformation. Dev Biol. 2013;382:305–319. doi: 10.1016/j.ydbio.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Roux IL, Schuhbaur B, Chambon P, Dolle P. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development. 2003;130:2525–2534. doi: 10.1242/dev.00463. [DOI] [PubMed] [Google Scholar]
- Nijenhuis CM, ter Horst PG, van Rein N, Wilffert B, de Jong-van den Berg LT. Disturbed development of the enteric nervous system after in utero exposure of selective serotonin re-uptake inhibitors and tricyclic antidepressants. Part 2: Testing the hypotheses. Br J Clin Pharmacol. 2012;73:126–134. doi: 10.1111/j.1365-2125.2011.04081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Cortes F, Turco F, Linan-Rico A, Soghomonyan S, Whitaker E, Wehner S, Cuomo R, Christofi FL. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2016;22:433–449. doi: 10.1097/MIB.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza E, Knowles CH, Graziano C, Thapar N, Burns AJ, Seri M, Stanghellini V, De Giorgio R. Genetics of human enteric neuropathies. Prog Neurobiol. 2012;96:176–189. doi: 10.1016/j.pneurobio.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. Journal of comparative neurology. 1991;314:789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Hui SZ, Granholm A-C, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Pierchala BA, Milbrandt J, Johnson EM., Jr. Glial cell line-derived neurotrophic factor-dependent recruitment of Ret into lipid rafts enhances signaling by partitioning Ret from proteasome-dependent degradation. J Neurosci. 2006;26:2777–2787. doi: 10.1523/JNEUROSCI.3420-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DP, Lieu T, Pelayo JC, Eriksson EM, Veldhuis NA, Bunnett NW. Inflammation-induced abnormalities in the subcellular localization and trafficking of the neurokinin 1 receptor in the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2015;309:G248–259. doi: 10.1152/ajpgi.00118.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- Puffenberger EG, Hosoda K, Washington SS, Nakao K, deWit D, Yanagisawa M, Chakravart A. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung’s disease. Cell. 1994;79:1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Puig I, Champeval D, De Santa Barbara P, Jaubert F, Lyonnet S, Larue L. Deletion of Pten in the mouse enteric nervous system induces ganglioneuromatosis and mimics intestinal pseudoobstruction. J Clin Invest. 2009;119:3586–3596. doi: 10.1172/JCI39929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riihimaki O, Metsaranta M, Ritvanen A, Gissler M, Luukkaala T, Paavonen J, Nuutila M, Andersson S, Tikkanen M. Increased prevalence of major congenital anomalies in births with placental abruption. Obstet Gynecol. 2013;122:268–274. doi: 10.1097/AOG.0b013e31829a6f91. [DOI] [PubMed] [Google Scholar]
- Rodrigues DM, Li AY, Nair DG, Blennerhassett MG. Glial cell line-derived neurotrophic factor is a key neurotrophin in the postnatal enteric nervous system. Neurogastroenterol Motil. 2011;23:e44–56. doi: 10.1111/j.1365-2982.2010.01626.x. [DOI] [PubMed] [Google Scholar]
- Saavedra A, Baltazar G, Duarte EP. Driving GDNF expression: the green and the red traffic lights. Prog Neurobiol. 2008;86:186–215. doi: 10.1016/j.pneurobio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Salzman EW, Kensler PC, Levine L. Cyclic 3′,5′-adenosine monophosphate in human blood platelets. IV. Regulatory role of cyclic amp in platelet function. Ann N Y Acad Sci. 1972;201:61–71. doi: 10.1111/j.1749-6632.1972.tb16287.x. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol. 2012;366:64–73. doi: 10.1016/j.ydbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Sato Y, Heuckeroth RO. Retinoic acid regulates murine enteric nervous system precursor proliferation, enhances neuronal precursor differentiation, and reduces neurite growth in vitro. Dev Biol. 2008;320:185–198. doi: 10.1016/j.ydbio.2008.05.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Schafer KH, Mestres P. The GDNF-induced neurite outgrowth and neuronal survival in dissociated myenteric plexus cultures of the rat small intestine decreases postnatally. Experimental brain research. 1999;125:447–452. doi: 10.1007/s002210050702. [DOI] [PubMed] [Google Scholar]
- Schappi MG, Staiano A, Milla PJ, Smith VV, Dias JA, Heuschkel R, Husby S, Mearin ML, Papadopoulou A, Ruemmele FM, Vandenplas Y, Koletzko S. A practical guide for the diagnosis of primary enteric nervous system disorders. J Pediatr Gastroenterol Nutr. 2013;57:677–686. doi: 10.1097/MPG.0b013e3182a8bb50. [DOI] [PubMed] [Google Scholar]
- Schill EM, Lake JI, Tusheva OA, Nagy N, Bery S, Foster L, Avetisyan M, Johnson SL, Stenson WF, Goldstein AM, Heuckeroth RO. Ibuprofen slows migration and inhibits bowel colonization by enteric nervous system precursors in zebrafish, chick and mouse. Developmental Biology in press. 2015 doi: 10.1016/j.ydbio.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Constantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Schuster A, Klotz M, Schwab T, Di Liddo R, Bertalot T, Schrenk S, Martin M, Nguyen TD, Nguyen TN, Gries M, Fassbender K, Conconi MT, Parnigotto PP, Schafer KH. Maintenance of the enteric stem cell niche by bacterial lipopolysaccharides? Evidence and perspectives. J Cell Mol Med. 2014;18:1429–1443. doi: 10.1111/jcmm.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KA. Emerging roles for enteric glia in gastrointestinal disorders. J Clin Invest. 2015;125:918–925. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KA, Nasser Y, Ruhl A. Enteric glia. Gut. 2004;53:1390. [PMC free article] [PubMed] [Google Scholar]
- Shimotake T, Go S, Inoue K, Tomiyama H, Iwai N. A homozygous missense mutation in the tyrosine E kinase domain of the RET proto-oncogene in an infant with total intestinal aganglionosis. Am. J. Gastroenterology. 2001;96:1286–1291. doi: 10.1111/j.1572-0241.2001.03714.x. [DOI] [PubMed] [Google Scholar]
- Sigalet DL, Wallace LE, Holst JJ, Martin GR, Kaji T, Tanaka H, Sharkey KA. Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol. 2007;293:G211–221. doi: 10.1152/ajpgi.00530.2006. [DOI] [PubMed] [Google Scholar]
- Simkin JE, Zhang D, Rollo BN, Newgreen DF. Retinoic acid upregulates ret and induces chain migration and population expansion in vagal neural crest cells to colonise the embryonic gut. PLoS One. 2013;8:e64077. doi: 10.1371/journal.pone.0064077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. Hirschsprung’s Disease. Curr. Probl. Surg. 1996;33:391–461. doi: 10.1016/s0011-3840(96)80009-8. [DOI] [PubMed] [Google Scholar]
- Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–1782. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- Steliou K, Boosalis MS, Perrine SP, Sangerman J, Faller DV. Butyrate histone deacetylase inhibitors. BioResearch open access. 2012;1:192–198. doi: 10.1089/biores.2012.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–1980. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- Suply E, de Vries P, Soret R, Cossais F, Neunlist M. Butyrate enemas enhance both cholinergic and nitrergic phenotype of myenteric neurons and neuromuscular transmission in newborn rat colon. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1373–1380. doi: 10.1152/ajpgi.00338.2011. [DOI] [PubMed] [Google Scholar]
- Takaki M, Goto K, Kawahara I. The 5-hydroxytryptamine 4 Receptor Agonist-induced Actions and Enteric Neurogenesis in the Gut. J Neurogastroenterol Motil. 2014;20:17–30. doi: 10.5056/jnm.2014.20.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketomi T, Yoshiga D, Taniguchi K, Kobayashi T, Nonami A, Kato R, Sasaki M, Sasaki A, Ishibashi H, Moriyama M, Nakamura K, Nishimura J, Yoshimura A. Loss of mammalian Sprouty2 leads to enteric neuronal hyperplasia and esophageal achalasia. Nat Neurosci. 2005;8:855–857. doi: 10.1038/nn1485. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Baloh RH, Milbrandt J, Johnson EMJ. GFRa-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron. 2000;25:611–623. doi: 10.1016/s0896-6273(00)81064-8. [DOI] [PubMed] [Google Scholar]
- Thazhath SS, Jones KL, Horowitz M, Rayner CK. Diabetic gastroparesis: recent insights into pathophysiology and implications for management. Expert Rev Gastroenterol Hepatol. 2013;7:127–139. doi: 10.1586/egh.12.82. [DOI] [PubMed] [Google Scholar]
- Thrasivoulou C, Soubeyre V, Ridha H, Giuliani D, Giaroni C, Michael GJ, Saffrey MJ, Cowen T. Reactive oxygen species, dietary restriction and neurotrophic factors in age-related loss of myenteric neurons. Aging Cell. 2006;5:247–257. doi: 10.1111/j.1474-9726.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- Tominaga K, Fujikawa Y, Tanaka F, Kamata N, Yamagami H, Tanigawa T, Watanabe T, Fujiwara Y, Arakawa T. Structural changes in gastric glial cells and delayed gastric emptying as responses to early life stress and acute adulthood stress in rats. Life Sci. 2016 doi: 10.1016/j.lfs.2016.02.025. [DOI] [PubMed] [Google Scholar]
- Torfs CP, Christianson RE. Maternal risk factors and major associated defects in infants with Down syndrome. Epidemiology. 1999;10:264–270. [PubMed] [Google Scholar]
- Trupp M, Arenas E, Fainzilber M, Nilsson A-S, Sieber B-A, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, Arumae U, Sariola H, Saarma M, Ibanez CF. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature. 1996;381:785–789. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- Vasina V, Barbara G, Talamonti L, Stanghellini V, Corinaldesi R, Tonini M, De Ponti F, De Giorgio R. Enteric neuroplasticity evoked by inflammation. Auton Neurosci. 2006;126-127:264–272. doi: 10.1016/j.autneu.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Vohra BP, Fu M, Heuckeroth RO. Protein kinase Czeta and glycogen synthase kinase-3beta control neuronal polarity in developing rodent enteric neurons, whereas SMAD specific E3 ubiquitin protein ligase 1 promotes neurite growth but does not influence polarity. J Neurosci. 2007a;27:9458–9468. doi: 10.1523/JNEUROSCI.0870-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra BP, Planer W, Armon J, Fu M, Jain S, Heuckeroth RO. Reduced endothelin converting enzyme-1 and endothelin-3 mRNA in the developing bowel of male mice may increase expressivity and penetrance of Hirschsprung disease-like distal intestinal aganglionosis. Dev Dyn. 2007b;236:106–117. doi: 10.1002/dvdy.21028. [DOI] [PubMed] [Google Scholar]
- Vohra BP, Tsuji K, Nagashimada M, Uesaka T, Wind D, Fu M, Armon J, Enomoto H, Heuckeroth RO. Differential gene expression and functional analysis implicate novel mechanisms in enteric nervous system precursor migration and neuritogenesis. Dev Biol. 2006;298:259–271. doi: 10.1016/j.ydbio.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boyen GB, Steinkamp M, Geerling I, Reinshagen M, Schafer KH, Adler G, Kirsch J. Proinflammatory cytokines induce neurotrophic factor expression in enteric glia: a key to the regulation of epithelial apoptosis in Crohn’s disease. Inflamm Bowel Dis. 2006a;12:346–354. doi: 10.1097/01.MIB.0000219350.72483.44. [DOI] [PubMed] [Google Scholar]
- von Boyen GB, Steinkamp M, Reinshagen M, Schafer KH, Adler G, Kirsch J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut. 2004;53:222–228. doi: 10.1136/gut.2003.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boyen GB, Steinkamp M, Reinshagen M, Schafer KH, Adler G, Kirsch J. Nerve growth factor secretion in cultured enteric glia cells is modulated by proinflammatory cytokines. J Neuroendocrinol. 2006b;18:820–825. doi: 10.1111/j.1365-2826.2006.01478.x. [DOI] [PubMed] [Google Scholar]
- Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol. 2014;817:221–239. doi: 10.1007/978-1-4939-0897-4_10. [DOI] [PubMed] [Google Scholar]
- Wang H, Hughes I, Planer W, Parsadanian A, Grider JR, Vohra BP, Keller-Peck C, Heuckeroth RO. The timing and location of glial cell line-derived neurotrophic factor expression determine enteric nervous system structure and function. J Neurosci. 2010;30:1523–1538. doi: 10.1523/JNEUROSCI.3861-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KP., Jr. Extent of vitamin A deficiency among preschool children and women of reproductive age. J Nutr. 2002;132:2857S–2866S. doi: 10.1093/jn/132.9.2857S. [DOI] [PubMed] [Google Scholar]
- Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol. 2008;24:149–158. doi: 10.1097/MOG.0b013e3282f56125. [DOI] [PubMed] [Google Scholar]
- Wood JD. Taming the irritable bowel. Curr Pharm Des. 2013;19:142–156. doi: 10.2174/13816128130119. [DOI] [PubMed] [Google Scholar]
- Wright-Jin EC, Grider JR, Duester G, Heuckeroth RO. Retinaldehyde dehydrogenase enzymes regulate colon enteric nervous system structure and function. Dev Biol. 2013;381:28–37. doi: 10.1016/j.ydbio.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141:272–282. doi: 10.1016/j.pharmthera.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Young HM, Bergner AJ, Simpson MJ, McKeown SJ, Hao MM, Anderson CR, Enomoto H. Colonizing while migrating: how do individual enteric neural crest cells behave? BMC biology. 2014;12:23. doi: 10.1186/1741-7007-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yang J, Watkins DJ, Boomer LA, Matthews MA, Su Y, Besner GE. Enteric nervous system abnormalities are present in human necrotizing enterocolitis: potential neurotransplantation therapy. Stem cell research & therapy. 2013;4:157. doi: 10.1186/scrt387. [DOI] [PMC free article] [PubMed] [Google Scholar]