Abstract
Objective
The microbiome is recognized as a new frontier in medicine with connections to a variety of diseases. We aimed to evaluate the association of lipopolysaccharide (LPS), a key proinflammatory product of the microbiome, with severity of inflammation, symptoms and radiographic abnormalities of knee osteoarthritis (OA).
Design
LPS was measured using a recombinant factor C assay, carefully optimized for systemic and synovial fluid (SF) analyses. LPS binding protein (LBP) was tested in both serum and synovial fluid of 25 patients (31 knees) from the Etarfolatide cohort for association with OA phenotypic outcomes. Models were adjusted for age, gender and body mass index.
Results
Based on LPS spike-and recovery, both serum and synovial fluid dilutions of 0.1% were required to achieve recovery rates of at least 75% in all test specimens. Low coefficients of variation (<10%) were achieved with both serum and synovial fluid dilutions <0.2%. Serum LPS and LBP were associated with the abundance of activated macrophages in the knee joint capsule and synovium. SF LPS and LBP were associated with the abundance of activated macrophages in the synovium. Serum LPS, LBP and SF LPS were associated with knee osteophyte severity. SF LPS was positively associated with knee joint space narrowing severity and total WOMAC score. SF LBP was positively associated with self-reported knee pain score.
Conclusion
These data strongly support a role for LPS in the pathogenesis and severity of structural abnormalities and symptoms of knee OA.
Keywords: lipopolysaccharide, LPS, knee osteoarthritis, inflammation, LPS binding protein, biomarker
Introduction
Osteoarthritis (OA) is a heterogeneous disease with differing predisposing conditions including aging, trauma, genetics and obesity [1, 2]. One means by which obesity could predispose to OA is through elevations of circulating lipopolysaccharide (LPS), also known as endotoxin [3]. LPS is now known to contribute to low-grade inflammation with a number of clinical conditions, including cardio-metabolic dysfunction, acceleration of artherosclerosis, and diabetes [4–7]. Based on these newly gained insights, we believe LPS might play an important role in the pathogenesis of OA and explain in large part the link between obesity and OA. Recently, we hypothesized a two-hit model of OA pathogenesis whereby LPS primes joint tissue macrophages via toll-like receptor 4 [3]. OA-related disease associated molecular patterns (DAMPs), released as a result of structural joint damage, activate macrophage inflammasome pathways [3, 8]. These complementary mechanisms, LPS and DAMPs thereby synergistically activate innate immune driven inflammation characterized by IL-1β production and other inflammatory mediators. However, quantifying LPS in complex protein samples, such as serum or plasma, has been a challenge for a long time because of LPS inhibitors that interfere with reliable quantification in biospecimens. Studies have shown that inhibitors such as esterase [9], lysozyme [10] and bactericidal/permeability-increasing protein (BPI) [11] can combine with LPS to form complexes that mask LPS detection.
The goal of this study was to evaluate the clinical relevance of this two-hit hypothesis. To achieve this goal we optimized the methodology of LPS quantification to ensure reliable analyses of human serum and synovial fluid samples. We then evaluated the associations of both systemic and local LPS and LPS binding protein (LBP) with other biomarkers in knee OA patients. To our knowledge, this is the first study attempting to reveal the possible pathological mechanism of LPS in OA.
Methods
Human serum and synovial fluid samples
Human samples were derived from two sources. The first source was human sera and synovial fluid from 5 patients meeting American College of Rheumatology criteria for symptomatic knee OA that presented to a community Rheumatology practice. These samples were used for optimizing the LPS detection methodology. The second source was human sera (N=25) and synovial fluid (N=31) from all participants (N=25) enrolled through Duke University in the Etarfolatide imaging study [12]. All these patients had radiographic knee OA (unilateral or bilateral Kellgren/Lawrence [K/L] grade 1–4 [13]). The mean age of these patients was 62.4±15.8 years. There were 18 females of the total 25 patients and the mean BMI was 29.2±4.8 kg/m2. Study approval was obtained from the Duke Institutional Review Boards and all subjects participated under informed consent.
LPS detection
LPS was measured using the EndoZyme Assay (recombinant factor C based) (Hyglos GmbH, Bernried, Germany). To optimize reliability of both serum and synovial fluid (SF) LPS measurements, we diluted samples (n=5 separate individuals) in LPS-free water (Hyglos GmbH, Bernried, Germany) from 1:10 (10% concentration) to 1:1000 (0.1% concentration). Per the manufacturer’s recommendations, samples required heating to 75°C for 10–15 minutes. Samples were then transferred to LPS-free flat-bottomed 96-well plates (Hyglos GmbH, Bernried, Germany) incubated at 37°C. The assay was performed according to the manufacturer’s protocol and coefficients of variation determined. For spike-and-recovery, exogenous LPS (0.1 EU/ml) was added to serum and SF of different dilutions and LPS measured. LPS concentrations of “naive” (no added LPS) serum were subtracted from spiked samples to calculate LPS recovery rates. LPS measurements of Etarfolatide study samples were all performed at dilutions of 0.1%, a level of dilution that we identified to be the optimal concentration for LPS measurements for overcoming natural inhibitors in the types of biospecimens we used (serum and SF).
Other biochemical analyses
Concentrations of LBP in the serum and SF were measured with a commercial sandwich ELISA kit for human LBP (Hycult Biotech, Uden, the Netherlands) according to the manufacturer’s instructions using a 1:1000 dilution. Plates were read at 450 nm on a microplate spectrophotometer (Infinite M200 pro, Tecan) within 30min. The detection range of LBP was between 4.4 ng/mL and 50 ng/mL. The OD values of samples were all within the range of the standard curve. The intra-assay coefficients of variation (CVs) ranged from 0.5% to 9.7%.
Soluble CD14 (sCD14), a marker of pro-inflammatory macrophages, was analyzed in baseline serum and SF from the Etarfolatide study as described previously [14].
Image scoring
As described previously, the intensity of scintigraphic radiolabel on Etarfolatide SPECT/CT scans of the knee was scored semiquantitatively as normal (score 0), mild (score 1), moderate (score 2), or intense (score 3) by an experienced nuclear medicine radiologist with high intrarater reliability [12]. Scores were summed across medial and lateral knee compartments by site of tissue localization (tibiofemoral knee capsule or synovium) [12].
Posteroanterior fixed-flexion weight-bearing knee radiographs were obtained with a SynaFlexer lower limb positioning frame (Synarc) and a 10° caudal x-ray beam angle [15]. Each knee radiograph was scored for K/L grade (0–4) and individual radiographic OA features of joint space narrowing (JSN; score 0–3) as well as osteophyte severity (score 0–3) in the medial and lateral compartments using the Osteoarthritis Research Society International standardized atlas [16]. Total JSN scores of 0–6 were possible for each knee and total osteophyte scores of 0–12 were possible as all 4 margins of the knee joint were scored.
Pain and function assessment
In the Etarfolatide study, knee pain was ascertained by the First National Health and Nutrition Examination Survey (NHANES-I) criterion of pain, aching, or stiffness in participants’ individual knees on most days of any one month in the last year. Pain was graded as none (score 0), mild (score 1), moderate (score 2), or severe (score 3). Symptoms and disability were also assessed using the Western Ontario and McMaster Osteoarthritis Index (WOMAC) questionnaire [17].
Statistical analysis
Results are presented as means±SD. Spearman correlations were used to evaluate the association of background and adjusted final absorbance. The proportion of inhibited samples at different concentrations was compared using McNemar’s exact test for non-parametric data. The correlations of LPS, LBP and serum (s) CD14 were assessed by Spearman correlation. Multivariable linear regression models using generalized estimating equations (GEEs), which account for correlation within knees, were performed, adjusted for age, gender and BMI, to evaluate associations of SF LPS concentrations with the following: intensity of Etarfolatide uptake in the tibiofemoral knee joint capsule and synovium (representing abundance of activated macrophages reflecting synovitis), and JSN and osteophyte severity. The parameter estimate (β), which represents the estimated mean for the slope, was reported along with the 95% confidence interval and p value. A p value (two-tailed) <0.05 was considered significant. Statistical analysis was performed with SPSS version 22.0 software (SPSS, Chicago, IL, USA) and Prism GraphPad v6.0 (La Giolla, CA, USA).
Results
Optimizing the methodology of LPS quantification in human serum samples
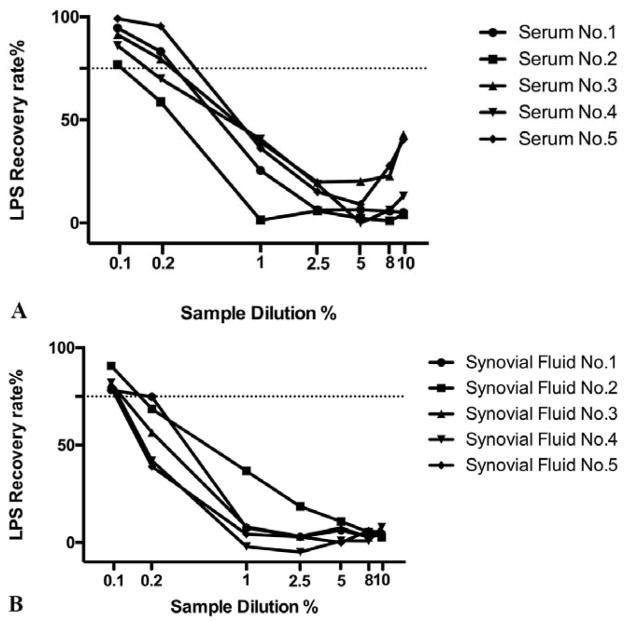
At serum concentrations of >1%, 0.2% and 0.1%, at least 75% recovery of spiked in LPS was achieved in 0%, 60% and 100% of specimens, respectively (Figure 1A). A significant difference (p<0.001) was detected in average recovery rate between 0.1% serum and all other concentrations except 0.2% (Supplemental Figure 1A). The CVs ranged from 1.3% to 5.8%.
Figure 1. Recovery rate of LPS in serum (A) and synovial fluid (SF) (B) specimens (n=5).
A fixed amount of exogenous LPS (0.1EU) from a reference E. coli standard was spiked into both serum and SF samples from 5 knee OA subjects that were diluted from 10% to 0.1%. A recovery rate of at least 75% (dotted black line) was achieved for all samples at a dilution of 1:1000 (0.1%).
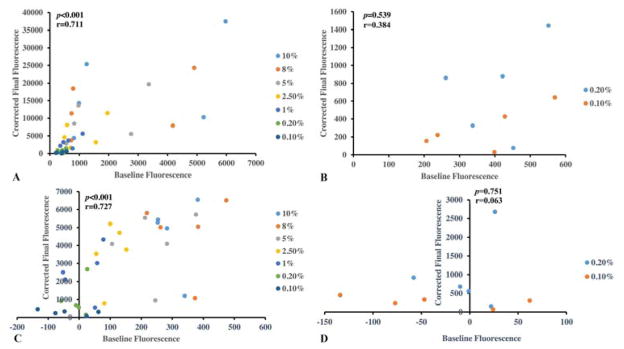
The existence of a systematic difference between the measurements (i.e., fixed bias) was evaluated by Bland-Altman plot. Above serum concentrations of 0.2%, the background fluorescence was correlated with the final LPS results (fluorescence values corrected by subtracting background values) (r=0.711, p<0.001, Figure 2A). However, this proportional bias was overcome at serum concentrations of <0.2% demonstrated by the lack of association of background and final corrected absorbance values at sample dilutions of 0.1% (r=0.384, p=0.539, Figure 2B).
Figure 2. Bland-Altman plots to evaluate for fixed bias in LPS measurements in test samples.
Using test serum samples (n=5) (A), or synovial fluid samples (n=5) (C) the corrected final fluorescence (y-axis) resulting from the LPS assay showed a systematic positive correlation with background fluorescence (x-axis) (r=0.711, p<0.001 for serum; r=0.727, p<0.001 for synovial fluid) demonstrating a systematic or fixed bias when all LPS results (all sample dilutions) were considered. No systematic or fixed bias could be discerned in LPS results derived from serum (B) or synovial fluid (D) samples diluted >0.2% (p=0.54 for serum; p=0.75 for synovial fluid).
Optimizing the methodology of LPS quantification in human synovial fluid samples
At SF concentrations of >1%, 0.2% and 0.1%, at least 75% recovery of spiked in LPS was achieved in 0%, 25% and 100% of specimens, respectively (Figure 1B). A significant difference (p<0.001) was detected in average recovery rate between 0.1% SF and all other concentrations (Supplemental Figure 1B). The intra-assay CVs ranged from 2.2% to 9.6%.
Bland-Altman plots were also used to evaluate for a systematic difference between the measurements. Results for synovial fluid were similar to serum; namely, evaluating all the sample dilutions, the background fluorescence was correlated with final LPS results (r=0.727, p<0.001, Figure 2C). However, when the concentrations above 0.2% were excluded, this correlation was appropriately eliminated (r=0.063 p=0.751, Figure 2D).
LPS and LBP in Etarfolatide study samples
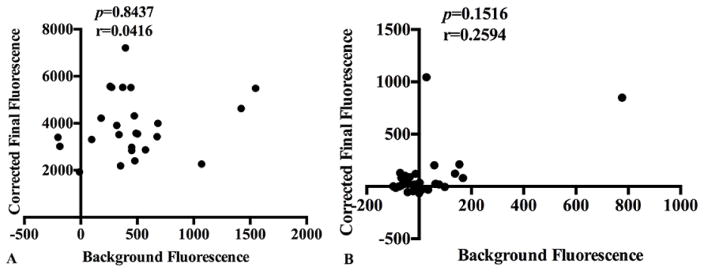
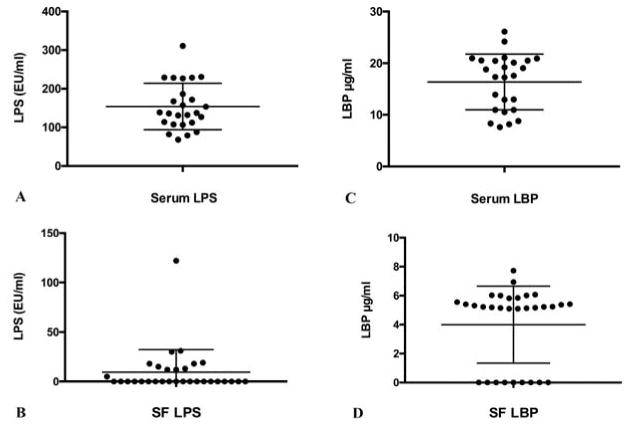
Based on the results described above, Etarfolatide cohort serum and SF samples were diluted 0.1% for LPS quantification. As expected at this dilution, no fixed bias in either serum or synovial fluid LPS measurements was observed for these samples (r=0.0416, p=0.8437; r=0.2594, p=0.1516, respectively, Figure 3). Serum LPS concentrations ranged from 68.0 EU/mL to 310.7 EU/mL, with a mean concentration of 154.0 (59.7) EU/mL (n=25) (Figure 4A). SF LPS concentrations ranged from 0 EU/mL to 122 EU/mL, with a mean concentration of 2.7 (1.8) EU/mL (n=31) (Figure 4B). Serum LBP concentrations ranged from 8.2 μg/mL to 26.1 μg/mL, with a mean concentration of 16.4 (5.3) μg/mL (n=25) (Figure 4C). SF LBP concentrations ranged from 0 μg/mL to 7.7 μg/mL, with a mean concentration of 9.58 (22.9) μg/mL (n=31) (Figure 4D). Both LPS and LBP concentrations were significantly lower (p<0.0001) in SF than in paired serum. Serum LPS and LBP concentrations were highly correlated (r=0.529, p<0.001) and individually correlated with BMI (r=0.436, p=0.017; r=0.424, p=0.017, respectively) and plasma sCD14 (r=0.392, p=0.006; r=0.605, p<0.001, respectively), a soluble marker of pro-inflammatory macrophages. Similarly, SF LPS and LBP concentrations were highly correlated (r=0.478, p=0.002) and individually correlated with SF sCD14 (r=0.501, p=0.004; r=0.377, p=0.036, respectively). However, no significant correlations were detected between SF LPS and LBP with BMI (r=−0.021, p=0.884; r=0.015, p=0.917, respectively). The correlations among the measured biomarkers are summarized in Table 1.
Figure 3. Bland-Altman plots to evaluate for fixed bias in LPS measurements in the Etarfolatide study samples.
A) Using Etarfolatide study serum samples (n=25), at 0.1% dilution, the corrected final fluorescence (y-axis) resulting from the LPS assay results no systematic positive correlation with background fluorescence (x-axis) (p=0.8437) demonstrating no fixed bias could be discerned in LPS results derived from samples diluted 0.1%. B) Using Etarfolatide study synovial fluid samples (n=31), at 0.1% dilution, the corrected final fluorescence (y-axis) resulting from the LPS assay showed no systematic positive correlation with background fluorescence (x-axis) (p=0.1516) demonstrating no fixed bias could be discerned in LPS results derived from samples diluted 0.1%.
Figure 4. Concentrations of LPS and LBP in the Etarfolatide study samples.
Dot plots of the mean ± SD of: A) Serum LPS (154 ±59.7 EU/ml). B) Synovial fluid LPS (2.67 ± 1.78 EU/ml, n=31). C) Serum LBP (16.4 ±5.3 μg/ml). D) Synovial fluid LBP (9.58±22.9 μg/ml). n=25 sera and n=31 synovial fluids.
Table 1.
Correlations serum and synovial fluid LPS, LBP and soluble CD14 in the Etarfolatide cohort*.
| Synovial fluid LPS | Synovial fluid LBP | Synovial fluid CD14 | Serum LPS | Serum LBP | Plasma CD14 | |
|---|---|---|---|---|---|---|
| Synovial fluid LPS | 1 | - | - | - | - | - |
| Synovial fluid LBP | 0.478 (0.002) | 1 | - | - | - | - |
| Synovial fluid CD14 | 0.501 (0.004) | 0.377 (0.036) | 1 | - | - | - |
| Serum LPS | 0.019 (0.921) | 0.177 (0.24) | 0.325 (0.026) | 1 | - | - |
| Serum LBP | 0.069 (0.711) | 0.358 (0.015) | 0.494 (<0.001) | 0.529 (<0.001) | 1 | - |
| Plasma CD14 | −0.035 (0.874) | 0.201 (0.180) | 0.325 (0.021) | 0.392 (0.006) | 0.605 (<0.001) | 1 |
LPS=lipopolysaccharide; LBP=LPS binding protein; CD14=cluster of differentiation 14
Values are Spearman’s r (p)
Association of LPS and LBP with the presence of activated macrophages in OA
Both LPS and LBP were significantly associated with activated macrophages in the joints of patients with knee OA in the Etarfolatide study (Table 2). Systemic serum LPS and LBP were significantly associated with the presence of macrophages in the knee joint capsule (parameter estimateβ=0.019, p<0.001 and β=0.160, p=0.001, respectively) and synovium (β=0.014, p<0.001 and β=0.118, p=0.036, respectively). In the SF, LPS and LBP were significantly associated with the presence of activated macrophages in the knee synovium (β=0.028, p=0.001 and β=0.464, p=0.021, respectively) (Table 2).
Table 2.
Associations of serum LPS and LBP concentrations with OA phenotypes^.
| OA phenotype | Serum LPS (EU/mL) | Serum LBP (μg/mL) | Synovial fluid LPS (EU/mL) | Synovial fluid LBP (μg/mL) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| β (95% CI)† | p | β (95% CI)† | p | β (95% CI)† | p | β (95% CI)† | p | |
| Activated macrophages in joint capsule | 0.019 [0.012, 0.025] | <0.001 | 0.160 [0.068, 0.252] | 0.001 | 0.009 [−0.012, 0.030] | 0.683 | 0.261 [−0.145, 0.667] | 0.207 |
| Activated macrophages in synovium | 0.014 [0.006, 0.022] | 0.001 | 0.118 [0.008, 0.228] | 0.036 | 0.028 [0.011, 0.045] | 0.001 | 0.464 [0.069, 0.859] | 0.021 |
| Osteophyte severity | 0.017 [0.002, 0.033] | 0.030 | 0.072 [0.013, 0.131] | 0.017 | 0.063 [0.041, 0.084] | <0.001 | −0.367 [−1.042, 0.308] | 0.286 |
| JSN severity | 0.006 [−0.001, 0.013] | 0.103 | 0.142 [−0.027, 0.311] | 0.099 | 0.014 [0.007, 0.021] | <0.001 | −0.088 [−0.249, 0.073] | 0.284 |
| WOMAC pain | −0.002 [−0.022, 0.017] | 0.816 | 0.210 [−0.022, 0.441] | 0.076 | 0.019 [−0.001, 0.040] | 0.063 | 0.304 [−0.210, 0.819] | 0.246 |
| WOMAC total | −0.058 [−0.145, 0.030] | 0.196 | 0.331 [−0.945, 1.607] | 0.611 | 0.177 [0.046, 0.309] | 0.008 | 0.275 [−2.638, 3.188] | 0.853 |
| Self-reported knee pain score | 0.000 [−0.002, 0.002] | 0.785 | 0.006 [−0.012, 0.024] | 0.518 | 0.002 [−0.001, 0.005] | 0.126 | 0.108 [0.005, 0.211] | 0.039 |
Multivariable linear regression models using GEEs (Generalized estimating equations) were used.
Adjusted for age, gender, and body mass index; values in bold are significantly different at p<0.05
LPS=lipopolysaccharide; LBP=LPS binding protein; JSN=joint space narrowing; WOMAC= Western Ontario and McMaster Universities Arthritis Index
Association of LPS and LBP with radiographic knee OA severity
Serum LPS and LBP were significantly associated with osteophyte severity (β =0.017, p=0.030 and β=0.072, p=0.017, respectively). In SF, only LPS was significantly associated with both osteophyte (β=0.028, p=0.001) and JSN severity (β=0.041, p<0.001).
Association of LPS and LBP with joint symptoms
Systemically, only serum LBP showed a trend toward association with WOMAC pain score (β=0.210, p=0.076) (Table 2). In SF, LPS was significantly associated with total WOMAC score (β=0.177, p=0.008) and showed a trend toward association with WOMAC pain score (β=0.019, p=0.063) (Table 2). SF LBP was significantly associated with self-reported knee pain score (β=0.108, p=0.039) (Table 2).
Discussion
LPS is an endotoxin associated with the outer membrane of a broad array of gram-negative pathogens [18] and a classical activator of the innate immune system that activates immune cells, such as macrophages and neutrophils in the host by binding to the Toll-like receptor 4 (TLR4) complex [18]. Because of its pathophysiological properties, LPS has been used to induce arthritis in conjunction with collagen in animal models [19, 20]. However, only recently, researchers have started to connect LPS with the pathogenesis of OA. Substantial evidence from both clinical and basic studies reveal bacterially produced products can make their way from the gut to the systemic circulation and organs to cause low-grade inflammation under certain conditions such as obesity. Since then, connections have been hypothesized and since proven between the microbiome and a variety of different diseases such as inflammatory bowel disease [21], type 2 diabetes [4] and atherosclerosis [22].
To evaluate possible associations of LPS with OA, we first had to optimize the methodology for LPS detection to ensure high reliability. The classical LPS assay utilizes the Limulus Amebocyte Lysate (LAL) test [23]. However, because of drawbacks related to lack of specificity, relatively complex sample preparation and endangerment of the horseshoe crab, many attempts have been made to develop a new method of LPS detection. Until recently, a breakthrough in genetic engineering with molecular expression of recombinant Factor C (rFC) with molecular expression of recombinant Factor C (rFC) has been described [24–26], yielding an assay with a remarkable sensitivity of 0.001EU/ml (equivalent to 0.01 ng/ml) [24–27]. However, manufacturers of classical LAL and rFC assays do not recommend them for use on sera due to the presence of assay inhibitors.
In the current study, we confirm that serum and SF LPS detection using the rFc assay can be challenging due to masking by inhibitors in both serum and SF. Some known and likely unknown proteins in the body fluid are the cause of this masking phenomenon and impact the rFC assay as shown in the current study, and the LAL assay [9]. Some of these inhibitors are heat sensitive; thus heating the sample in a 70°C water bath for 10 minutes is used routinely as a way to eliminate some of the inhibitory substances [28, 29]. However, other inhibitors that bind LPS cannot be displaced by heating but only by significant sample dilution [30]. Despite many attempts, the exact identities of these non-heat sensitive assay-inhibitors have not yet been determined. The present study illustrates that concentrations at least as low as 0.2% are required for reliable quantification of LPS in serum. This finding corresponds with the methodology used by Balagopal et al. although they used the LAL assay [30]. Although dilution of samples by 0.1% increases the likelihood of a result below the lower limit of detection of the assay, this can be offset, at least in part, by the increased sensitivity of detection of the rFC assay.
The most common concentrations used in previous published studies ranged from 1% to 10% [6, 18, 31]. Given that serum dilutions below 1% in our study uniformly raised the total measurable amount of LPS in the serum, previous studies may have underestimated the circulating LPS levels [6, 31–36]. If inhibition occurs to the same degree between samples at a particular dilution, relative differences in LPS concentrations might still be meaningful. However, if the amount of inhibition is sample-dependent, relative difference across individuals would be less interpretable. In the current study, we found that the range of LPS inhibition in human serum samples was sample dependent, ranging from 60% to 90% inhibition in moderately dilute serum (1%–10%), but only 1% to 23% inhibition in very dilute serum (0.1%). In SF samples, the range of LPS inhibition ranged from 64% to 100% in moderately dilute samples (1%–10%), but only 10% to 22% inhibition in very dilute SF (0.1%). These findings suggest that relative differences in LPS concentrations at moderate serum dilutions cannot fully reflect actual LPS concentrations that are more closely approximated at greater serum dilutions.
Levin et al. [37] first used heat to eliminate inhibitors interfering with quantification of serum LPS. Thereafter, heating rapidly became a method of choice, and has been used in a large number of studies up to the present [5, 6, 9, 30]. Although heating is easy to accomplish, until recently a consensus was not reached regarding the ideal temperature to use. Roth et al. [28] found little difference in the recovery for samples heated between 60–100°C. However, one drawback of using a temperature higher than 80°C was the necessity of centrifugation to remove an assay-interfering precipitate. Thus, heating under 70–75°C for 10–15 minutes is now considered routine practice [4, 30]. We employed this method to minimize interfering factors in the current study.
Since its discovery and identification in the 1980s, LBP has long been considered a potential diagnostic marker in sepsis and invasive infections because of its pathophysiological role in the innate immune system. LBP is involved in the macrophage response to LPS; it enhances formation of CD14-LPS complexes and increases LPS sensitivity of the host [38, 39]. Also, as suggested by some researchers, LBP is a marker of “effective” LPS and the innate immune response triggered by LPS [40, 41]. The most important findings of the current study were the associations of LPS and LBP in the Etarfolatide study samples with the presence of activated macrophages in the knee, and with radiographic OA severity and joint symptoms. These results are consistent with a role for LPS in the pathogenesis of OA by activating macrophages. Mehta et al. [42] showed that a single intravenous injection of very small amounts of LPS (42 ng) could cause acute inflammation without overt clinical responses such as fever, tachycardia or flu-like symptoms. Based on a blood volume of 5L, this amount would equate to a serum concentration of 8.4 pg/ml. All of our subjects in the Etarfolatide study had serum LPS levels above this threshold and 11 of the synovial fluids (35% of knees) had concentrations above this threshold.
The current study has several strengths. First, compared with previous studies, we optimized the methodology of LPS detection. Second, we analyzed both LPS and LBP, recognized as a surrogate for “effective” LPS burden. One minor limitation was that the analytes were measured in samples that had been stored frozen for several years. Although LPS is quite hardy and stable to conditions of heating and freezing, to the best of our knowledge, there has been no available information about the stability of LBP in frozen samples over time. It must be noted however, that our results were in a similar range to those reported in studies in different populations using fresh samples.
Conclusion
In conclusion, our study indicates that a masking phenomenon impacts serum and SF LPS quantification using the rFc assay but this can be overcome by diluting samples to 0.1%–0.2%. Our findings in the Etarfolatide study indicate the presence of LPS and LBP in both serum and SF of individuals with OA. Moreover, we report the correlation of these two markers with the presence of activated macrophages in the knee, radiographic OA severity and joint symptoms. These data support a role of LPS in the pathogenesis of OA through the activation of macrophages. Strategies for lowering LPS could be important treatments for OA in a subset of individuals with measurable concentrations. Further studies are warranted to further evaluate the role of LPS in OA and to test the impact of LPS lowering on OA progression.
Supplementary Material
Box plots (mean, box 25th/75th percentiles and whiskers 5th/95th percentiles) of LPS concentrations are depicted at different sample dilutions ranging from of 0.1% to 10% dilution. Specimens diluted >0.2% yielded mean recovery rates of 75% and above.
Acknowledgments
We wish to acknowledge funding to support the effort of ZH by the China Health Ministry Program (201302007). We also wish to acknowledge support for the effort of VBK by NIH/NIA OAIC P30-AG-028716.
Footnotes
Author contributions
All authors contributed equally to the research of data for the article; all authors contributed substantially to discussion of the content, drafting of the article, reviewing and revising the manuscript before submission. All authors approved the final version for submission.
Conflict of Interest
None of the authors have competing interests to disclose. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
ZeYu Huang, Email: zey.huang@gmail.com.
Thomas Stabler, Email: tvs@duke.edu.
FuXing Pei, Email: peifuxing@vip.163.com.
Virginia Byers Kraus, Email: vbk@duke.edu.
References
- 1.Karsdal MA, Christiansen C, Ladel C, Henriksen K, Kraus VB, Bay-Jensen AC. Osteoarthritis--a case for personalized health care? Osteoarthritis Cartilage. 2014;22:7–16. doi: 10.1016/j.joca.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 3.Huang Z, Kraus VB. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat Rev Rheumatol. 2016;12:123–129. doi: 10.1038/nrrheum.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creely SJ, McTernan PG, Kusminski CM, Fisherf M, Da Silva NF, Khanolkar M, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E740–747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 5.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palu G, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 6.Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014;63:1513–1521. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 8.Stabler T, Montell E, Verges J, Kraus VB. Attentuation of hyaluronan fragment induced inflammatory response in macrophages by chondroitin sulphate. Osteoarthritis and Cartilage. 23:A263–A264. [Google Scholar]
- 9.Hurley JC, Tosolini FA, Louis WJ. Quantitative Limulus lysate assay for endotoxin and the effect of plasma. J Clin Pathol. 1991;44:849–854. doi: 10.1136/jcp.44.10.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petsch D, Deckwer W-D, Anspach F. Proteinase K Digestion of Proteins Improves Detection of Bacterial Endotoxins by theLimulusAmebocyte Lysate Assay: Application for Endotoxin Removal from Cationic Proteins. Analytical biochemistry. 1998;259:42–47. doi: 10.1006/abio.1998.2655. [DOI] [PubMed] [Google Scholar]
- 11.Elsbach P, Weiss J. The bactericidal/permeability-increasing protein (BPI), a potent element in host-defense against gram-negative bacteria and lipopolysaccharide. Immunobiology. 1993;187:417–429. doi: 10.1016/S0171-2985(11)80354-2. [DOI] [PubMed] [Google Scholar]
- 12.Kraus VBGM, Shipes S, Perty NA, Low PS, Shen J, MCNearney TA, Mitchell P. Direct in vivo evidence of activated macrophages in human osteoarthritis ( NCT01237405) Osteoarthritis Cartilage. 2016 doi: 10.1016/j.joca.2016.04.010. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daghestani HN, Pieper CF, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67:956–965. doi: 10.1002/art.39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–132. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 16.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 18.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz W, Buhrmann C, Mobasheri A, Lueders C, Shakibaei M. Bacterial lipopolysaccharides form procollagen-endotoxin complexes that trigger cartilage inflammation and degeneration: implications for the development of rheumatoid arthritis. Arthritis Res Ther. 2013;15:R111. doi: 10.1186/ar4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caccese RG, Zimmerman JL, Carlson RP. Bacterial lipopolysaccharide potentiates type II collagen-induced arthritis in mice. Mediators Inflamm. 1992;1:273–279. doi: 10.1155/S0962935192000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardiner KR, Halliday MI, Barclay GR, Milne L, Brown D, Stephens S, et al. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897–901. doi: 10.1136/gut.36.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caesar R, Fak F, Backhed F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J Intern Med. 2010;268:320–328. doi: 10.1111/j.1365-2796.2010.02270.x. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong PB, Rickles FR. Endotoxin-induced degranulation of the Limulus amebocyte. Exp Cell Res. 1982;140:15–24. doi: 10.1016/0014-4827(82)90150-1. [DOI] [PubMed] [Google Scholar]
- 24.Ding JL, Navas MA, 3rd, Ho B. Two forms of factor C from the amoebocytes of Carcinoscorpius rotundicauda: purification and characterisation. Biochim Biophys Acta. 1993;1202:149–156. doi: 10.1016/0167-4838(93)90076-4. [DOI] [PubMed] [Google Scholar]
- 25.Ding JL, Navas MA, 3rd, Ho B. Molecular cloning and sequence analysis of factor C cDNA from the Singapore horseshoe crab, Carcinoscorpius rotundicauda. Mol Mar Biol Biotechnol. 1995;4:90–103. [PubMed] [Google Scholar]
- 26.Ding JL, Ho B. A new era in pyrogen testing. Trends Biotechnol. 2001;19:277–281. doi: 10.1016/s0167-7799(01)01694-8. [DOI] [PubMed] [Google Scholar]
- 27.Thorne PS, Perry SS, Saito R, O’Shaughnessy PT, Mehaffy J, Metwali N, et al. Evaluation of the Limulus amebocyte lysate and recombinant factor C assays for assessment of airborne endotoxin. Appl Environ Microbiol. 2010;76:4988–4995. doi: 10.1128/AEM.00527-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth RI, Levin FC, Levin J. Optimization of detection of bacterial endotoxin in plasma with the Limulus test. J Lab Clin Med. 1990;116:153–161. [PubMed] [Google Scholar]
- 29.Harthug S, Bjorvatn B, Osterud B. Quantitation of endotoxin in blood from patients with meningococcal disease using a limulus lysate test in combination with chromogenic substrate. Infection. 1983;11:192–195. doi: 10.1007/BF01641194. [DOI] [PubMed] [Google Scholar]
- 30.Balagopal A, Gama L, Franco V, Russell JN, Quinn J, Higgins Y, et al. Detection of microbial translocation in HIV and SIV infection using the Limulus amebocyte lysate assay is masked by serum and plasma. PLoS One. 2012;7:e41258. doi: 10.1371/journal.pone.0041258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. 2011;34:392–397. doi: 10.2337/dc10-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 33.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 34.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani PD, Burcelin RG, et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22:2416–2426. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- 36.Miller MA, McTernan PG, Harte AL, Silva NF, Strazzullo P, Alberti KG, et al. Ethnic and sex differences in circulating endotoxin levels: A novel marker of atherosclerotic and cardiovascular risk in a British multi-ethnic population. Atherosclerosis. 2009;203:494–502. doi: 10.1016/j.atherosclerosis.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Levin J, Tomasulo PA, Oser RS. Detection of endotoxin in human blood and demonstration of an inhibitor. J Lab Clin Med. 1970;75:903–911. [PubMed] [Google Scholar]
- 38.Schumann RR. Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem Soc Trans. 2011;39:989–993. doi: 10.1042/BST0390989. [DOI] [PubMed] [Google Scholar]
- 39.Tobias PS, Soldau K, Ulevitch RJ. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem. 1989;264:10867–10871. [PubMed] [Google Scholar]
- 40.Lepper PM, Schumann C, Triantafilou K, Rasche FM, Schuster T, Frank H, et al. Association of lipopolysaccharide-binding protein and coronary artery disease in men. J Am Coll Cardiol. 2007;50:25–31. doi: 10.1016/j.jacc.2007.02.070. [DOI] [PubMed] [Google Scholar]
- 41.Albillos A, de la Hera A, Gonzalez M, Moya JL, Calleja JL, Monserrat J, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208–217. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 42.Mehta NN, Heffron SP, Patel PN, Ferguson J, Shah RD, Hinkle CC, et al. A human model of inflammatory cardio-metabolic dysfunction; a double blind placebo-controlled crossover trial. J Transl Med. 2012;10:124. doi: 10.1186/1479-5876-10-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Box plots (mean, box 25th/75th percentiles and whiskers 5th/95th percentiles) of LPS concentrations are depicted at different sample dilutions ranging from of 0.1% to 10% dilution. Specimens diluted >0.2% yielded mean recovery rates of 75% and above.