Abstract
Error processing is impaired in psychosis, and numerous event-related potential studies have found reductions in the error-related negativity (ERN) and, more recently, the error positivity (Pe). The stability of reduced ERN/Pe in psychosis, however, is unknown. In a previous cross-sectional report, reduced ERN was associated with negative symptom severity and reduced Pe with a diagnosis of schizophrenia versus other psychosis. Here, we test the stability of impaired error processing over a four-year follow-up and relationships with subdimensions of negative symptoms. The ERN and Pe were recorded from individuals with psychotic disorders twice: 79 individuals were assessed 15 years after first hospitalization, and 69 were assessed at 19 years; 59 (26 with schizophrenia, 33 with other psychotic disorders) had data at both assessments. At 19 years the Pe was blunted in schizophrenia. The ERN and Pe exhibited temporal stability over the four years (r=.59 and .60, respectively). Reduced ERN and Pe correlated with the negative symptom subdimensions of inexpressivity and avolition, respectively, and not with psychotic or disorganized symptoms. Moreover, 15-year ERN predicted an increase in inexpressivity by year 19. No evidence was found for the reverse: negative symptoms did not predict change in ERN/Pe. Similar to non-clinical samples, the ERN and Pe show impressive four-year stability in late-phase psychosis. The ERN and Pe are promising neural measures for capturing individual differences in psychotic disorders, particularly with regard to negative symptomatology. They may prove to be useful clinically for forecasting illness course and as treatment targets.
Keywords: EEG, ERP, error-related negativity, error positivity, schizophrenia
1. Introduction
Executive function is impaired in schizophrenia (Kerns et al., 2008) and is a proposed cognitive mechanism for poor functioning (Bowie et al., 2008). One key aspect of executive function is error processing, which entails the evaluation of errors as salient events followed by the dynamic adjustment of cognitive control to improve performance (Kerns et al., 2008). Event-related potential (ERP) studies of error processing in schizophrenia have focused on the error-related negativity (ERN), which occurs 0-100 ms following errors on speeded tasks (Falkenstein et al., 1991; Gehring et al., 1993) and reflects error-related activation of the anterior cingulate cortex (ACC) (Holroyd and Coles, 2002). Numerous studies have observed a blunted ERN in schizophrenia (Alain et al., 2002; Bates et al., 2002; Bates et al., 2004; Foti et al., 2012; Foti et al., 2013; Horan et al., 2012; Kansal et al., 2014; Kopp and Rist, 1999; Mathalon et al., 2002; Morris et al., 2006), which is related to poor executive function (Kim et al., 2006). A blunted ERN has also been observed in other psychotic disorders (Foti et al., 2012; Foti et al., 2013; Minzenberg et al., 2014), high-risk individuals (Laurens et al., 2010; Perez et al., 2012) and unaffected siblings (Simmonite et al., 2012).
Studies have also examined the error positivity (Pe), which peaks 200-400 ms following errors. The ERN and Pe track distinct stages of error processing: early, automatic error detection and later, conscious error recognition, respectively, (Hughes and Yeung, 2011; Nieuwenhuis et al., 2001). Pe relates to post-error adjustment at the between-subjects level (Hajcak et al., 2003), whereas ERN relates to trial-by-trial adjustment (Cavanagh and Shackman, 2015). Initial studies in schizophrenia failed to find group differences in Pe amplitude versus controls (Alain et al., 2002; Horan et al., 2012; Kim et al., 2006; Mathalon et al., 2002; Morris et al., 2006), perhaps due to limited sample sizes and signal filters that attenuated the Pe (but not the ERN). By contrast, three recent studies have observed a blunted Pe specifically in schizophrenia, both early (Perez et al., 2012) and later in the course of illness (Foti et al., 2012; Kansal et al., 2014).
The ERN and Pe are promising measures of impaired error processing, with the ERN reduced in psychotic illness broadly and the Pe reduced specifically in schizophrenia. Previous studies have primarily been cross-sectional, however, leaving it unclear how impaired error processing relates to course of illness. In non-clinical samples, the ERN and Pe are highly stable, (Weinberg and Hajcak, 2011). Similarly high temporal stability among patients would indicate chronic neural deficits rather than indicators of current clinical state. In this case, it is possible that reduced ERN/Pe amplitudes may relate to long-term illness course. On the other hand, if the ERN and Pe fluctuate over time in patients (i.e., show low temporal stability), this would raise the question of what is responsible for fluctuations over time (e.g., whether changes in ERN/Pe amplitudes map onto concurrent changes in symptoms and functioning). In one study, ERN amplitude partially normalized following antipsychotic treatment over a six-week follow-up, suggesting short-term improvement with clinical sate (Bates et al., 2004); longer-term assessment is necessary to more fully capture the stability of these neural indices.
An additional question is which specific illness features relate to impaired error processing. We previously observed that reduced ERN mapped onto concurrent negative but not positive or disorganized symptoms, independent of diagnosis (Foti et al., 2012), which is consistent with neuropsychological research (Ventura et al., 2009) and proposals that negative symptoms and cognitive deficits are related aspects of psychotic illness (Harvey et al., 2006). A longitudinal approach is necessary, however, to clarify the direction of effects (i.e., whether neural deficits predict or are a consequence of negative symptoms). Moreover, recent structural research indicates that negative symptoms are comprised of two distinct dimensions—inexpressivity and avolition (Blanchard and Cohen, 2006; Kring et al., 2013; Strauss et al., 2013)—yet links between error processing and these subdimensions have yet to be examined.
To begin to address these gaps, we present data from a four-year follow-up in which the ERN and Pe were reassessed in the previously-reported patient sample (Foti et al., 2012). The current study had two aims: (a) We assessed the long-term temporal stability of the ERN and Pe within the full patient sample. (b) We evaluated the longitudinal associations between these ERPs and symptom dimensions, testing whether ERPs predict subsequent symptoms or vice versa. We expected that the ERN would relate to trajectories of negative symptoms, and we further tested for specificity with regard to the subdimensions of inexpressivity and avolition.
2. Methods and Materials
2.1. Participants
The sample was drawn from the Suffolk County Mental Health Project (Bromet et al., 2011; Bromet et al., 1992), an epidemiologic study of first-admission psychosis. Participants were recruited from inpatient psychiatric facilities from 1989-1995; eligibility criteria were the presence of psychosis, age 15-60, and ability to provide informed consent. Face-to-face assessments were conducted by master’s-level interviewers using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 2001). Consensus DSM-IV diagnosis was formulated based on 19 years of observation. This sample included 104 patients, 52 with a schizophrenia spectrum disorder (SZ; schizophrenia, schizoaffective disorder) and 52 with other psychotic disorders (OP; 29 bipolar, 8 depression, 9 substance-induced, 6 not otherwise specified).
The present study includes data from the 15- and 19-year assessments. Cross-sectional analysis of 15-year ERP data has been published previously (Foti et al., 2012; Foti et al., 2013; Jackson et al., 2014; Perlman et al., 2015). Of the 104 patients who completed the 15-year EEG, 87 (83.7%) completed the 19-year EEG. Patients were retained for analyses if they had usable ERP data (>50% artifact-free trials) and adequate task performance (>75% correct). Seventy-nine had usable data at year 15 (37 SZ, 42 OP) (Foti et al., 2012). At year 19, 18 of 87 were excluded from analysis (10 for poor performance, 5 for poor quality data, 3 for committing zero errors), yielding 69 with useable data (32 SZ, 37 OP). Fifty-nine had available ERP data at both assessments (26 SZ, 33 OP). The delay between assessments varied (M=41.0 months, SD=6.9). Sample characteristics at year 19 are presented in Table 1.
Table 1. Sample characteristics at the 19-year assessment.
| Schizophrenia (n = 32) |
Other Psychosis (n = 37) |
Comparison | ||||
|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | d | 95% CI |
|
| ||||||
| Age | 46.56 | 10.36 | 48.03 | 9.20 | −.15 | −2.42-2.12 |
|
| ||||||
| N | % | N | % | Odds Ratio | 95% CI | |
| Gender | ||||||
| Male | 21 | 65.6 | 21 | 56.8 | 1.46 | .55-3.97 |
| Female | 11 | 34.4 | 16 | 43.2 | ||
| Ethnicity | ||||||
| White | 23 | 71.9 | 32 | 86.5 | .40 | .12-1.35 |
| Other | 9 | 28.1 | 5 | 13.5 | ||
| Medication | ||||||
| Antipsychotic | 28 | 87.5 | 12 | 32.4 | 14.58*** | 4.16-51.08 |
| Antidepressant | 14 | 43.8 | 13 | 35.1 | 1.44 | .54-3.79 |
| Mood stabilizer | 12 | 37.5 | 10 | 27.0 | 1.62 | .59-4.49 |
| Benzodiazepine | 5 | 15.6 | 5 | 13.5 | 1.19 | .31-4.53 |
Note: Medication variables are past-month prescription status (yes/no).
p<.001
2.2. Task and Materials
2.2.1. Symptoms
Past-month symptoms were rated using the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1983b) and the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983a). Each was scored as two-factor analytically derived subscales: the SAPS as psychotic (hallucination, delusions) and disorganized (bizarre behavior, thought disorder) (Kotov et al., 2010), and the SANS as inexpressivity (affective flattening, alogia) and avolition (apathy, anhedonia, asociality) (Blanchard and Cohen, 2006; Kring et al., 2013; Strauss et al., 2013).
2.2.2. Task
An arrow flankers task was used to assess error processing (Eriksen and Eriksen, 1974). Five arrowheads were presented on each trial, with half of trials compatible (<<<<< or >>>>>) and half incompatible (<<><< or >><>>). Arrows were presented for 200 ms and followed by an inter-trial interval of 2300-2800 ms. Participants were instructed to press the left or right mouse button corresponding to the center arrow, and to maximize both speed and accuracy. Participants completed 11 blocks of 30 trials. Blockwise feedback was used to keep performance between 75-90%.
2.3. Procedure
The procedure was identical across assessments. Written informed consent was obtained at each. Patients completed interviews and then the EEG assessment. They performed multiple tasks, and task order was counterbalanced. Patients received financial compensation for their participation. This research was approved annually by Institutional Review Board at Stony Brook University.
2.4. EEG Recording, Processing, and Data Reduction
The EEG was recorded using the ActiveTwo BioSemi System (BioSemi), sampled at 1024 Hz. Recordings were taken from 34 scalp electrodes and two mastoid electrodes. The electro-oculogram was recorded from four facial electrodes. Offline analysis was performed using Brain Vision Analyzer software (Brain Products). Data were re-referenced to the mastoid average and filtered from .1-30 Hz. The EEG was segmented for each trial, spanning −400 to 800 ms relative to the response, and corrected for blinks and eye movements (Gratton et al., 1983). Channels were rejected trial-wise using a semi-automated procedure, with artifacts defined as a step of 50 μV, a difference of 300 μV within a trial, or a difference of less than .50 μV within 100-ms intervals; additional artifacts were identified visually. ERP averages were created for correct and incorrect responses, with a baseline of −400 to −200 ms. The ERN was scored as the mean from 0-100 ms at Cz, and the Pe from 200-400 ms at Pz.
2.5. Data Analysis
Temporal stability and cross-sectional associations were described using Pearson’s r. Stability was examined among patients with available data at both time points (N=59); cross-sectional associations were conducted with all available patients at each time point. Cross-sectional associations between ERPs and symptoms were further evaluated using partial correlations to adjust for demographics (age, gender, ethnicity), medication status (past month; coded as yes/no for each of antipsychotic, antidepressant, mood stabilizer, and benzodiazepine), diagnosis (SZ vs. OP), and task accuracy (% correct). These results were used to select candidate pairs of ERP and symptom variables for longitudinal analyses, for which we used structural equation modeling to simultaneously test both directions of effects (i.e., ERP→Clinical and Clinical→ERP). We specified a cross-lagged model in which 19-year variables were jointly predicted by relevant 15-year ERP and symptom variables (i.e., predicting change from 15-19 years). The model was estimated in Mplus (Version 7) (Muthén and Muthén, 1998-2012) using WLMSV estimator.
3. Results
3.1. Cross-Sectional Associations
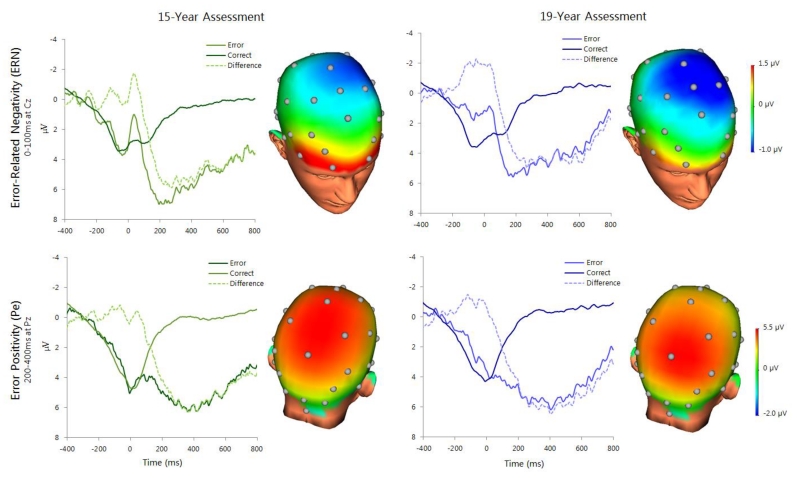
ERP data are presented in Figure 1. Modulation of the Pe on error versus correct trials was significant at both assessments (15-year: t(78)=8.09, p<.001; 19-year: t(68)=8.75, p<.001); modulation of the ERN was weaker and non-significant (15-year: t(78)= −1.57, p=.12; 19-year: t(68)= −1.94, p=.06). Group comparisons (SZ vs. OP) of ERP variables are in Table 2. ΔPe amplitude (error minus correct) was blunted in the SZ group at both assessments. ΔERN amplitude was blunted in the SZ group at year 15, but did not differ by year 19.
Figure 1.
ERP waveforms and headmaps depicting error-related neural activity at the 15-year (left; n=79) and 19-year (right; n=69) assessments. Headmaps depict the difference between error and correct trials from 0-100 ms (ERN, top) and 200-400 ms (Pe, bottom).
Table 2. Effects of diagnosis on ERN and Pe amplitude.
| Schizophrenia | Other Psychosis | Comparison | |||||
|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | t | d | 95% CI |
| 15-year ERPs | |||||||
| ERN | 1.87 | 5.45 | 2.30 | 4.53 | t(77) = −.38 | −.09 | −1.17-1.00 |
| CRN | 1.58 | 4.31 | 3.90 | 3.43 | t(77) = −2.67** | −.60 | −1.45-.23 |
| ΔERN | .30 | 4.12 | −1.61 | 3.78 | t(77) = 2.15* | .48 | −.37-1.35 |
| Pe | 3.03 | 6.09 | 8.01 | 6.33 | t(77) = −3.55*** | −.80 | −2.17-.54 |
| Pc | −.41 | 3.35 | .70 | 2.77 | t(77) = −1.61 | −.36 | −1.03-.30 |
| ΔPe | 3.44 | 5.47 | 7.31 | 6.00 | t(77) = −2.99** | −.68 | −1.94-.57 |
| 19-year ERPs | |||||||
| ERN | .52 | 3.83 | 2.76 | 4.39 | t(67) = −2.24* | −.55 | −1.51-.41 |
| CRN | 1.11 | 3.46 | 4.26 | 4.36 | t(67) =−3.29** | −.81 | −1.73-.12 |
| ΔERN | −.59 | 4.30 | −1.50 | 4.90 | t(67) = .82 | .20 | −.88-1.28 |
| Pe | 3.08 | 5.15 | 7.55 | 5.76 | t(67) = −3.38** | −.82 | −2.10-.45 |
| Pc | −.95 | 2.88 | .39 | 3.56 | t(67) = −1.70† | −.42 | −1.18-.34 |
| ΔPe | 4.03 | 4.93 | 7.16 | 5.47 | t(67) = −2.49* | −.60 | −1.82-.61 |
Note: Difference is error trials minus correct trials (i.e., ΔERN, ΔPe). Diagnoses are from the 19-year assessment. At 15 years, N = 37 (schizophrenia) and 42 (other psychosis). At 19 years, N = 32 (schizophrenia) and 37 (other psychosis).
p<.10,
p<.05,
p<.01
p<.001
Cross-sectional associations between ERPs and symptoms are in Table 3. Trends were observed between reduced ΔERN and Inexpressivity at both assessments; associations were significant after adjusting for covariates. Psychotic and Disorganized symptoms were not associated with ERP amplitudes at either assessment. Reduced ΔPe was associated with Avolition, but relationships were non-significant after adjusting for covariates, largely due to effects of diagnosis and antipsychotic medication.
Table 3. Cross-sectional associations between neural and clinical variables.
| 15-Year ΔERN (n = 78) | 19-Year ΔERN (n = 69) | |||
|---|---|---|---|---|
| Symptom Measure | Correlation (r) | Adjusted (rp) | Correlation (r) | Adjusted (rp) |
| SAPS Psychotic | .14 | .06 | .02 | −.07 |
| SAPS Disorganized | .09 | .10 | −.02 | −.10 |
| SANS Inexpressivity | .20† | .30* | .21† | .25* |
| SANS Avolition | .18 | .22† | −.10 | −.17 |
| 15-Year ΔPe (n = 78) | 19-Year ΔPe (n = 69) | |||
|---|---|---|---|---|
| Correlation (r) | Adjusted (rp) | Correlation (r) | Adjusted (rp) | |
| SAPS Psychotic | −.15 | −.07 | −.18 | −.05 |
| SAPS Disorganized | −.10 | −.04 | −.04 | .10 |
| SANS Inexpressivity | −.04 | .22† | −.15 | −.00 |
| SANS Avolition | −.26* | −.03 | −.32** | −.11 |
Note: Adjusted values include age, gender, ethnicity, diagnosis (schizophrenia vs. other psychosis) medication status (antipsychotic, antidepressant, mood stabilizer, and benzodiazepine), and task accuracy as covariates. Symptom variables are from the Scales for the Assessment of Positive (SAPS) and Negative Symptoms (SANS).
p<.10,
p<.05,
p<.01
3.2. Four-Year Stability
Mean-level changes and retest correlations from years 15-19 are presented in Table 4; scatterplots are in Figure 2. There were no significant mean-level changes across assessments for ERP variables or task accuracy; participants were slower overall at year 19. Symptom severity increased across assessments in each domain.
Table 4.
Four-year stability of neural, behavioral, and clinical measures
| Variable | 15-Year Assessment |
19-Year Assessment |
Change from Years 15-19 |
Test-retest Correlation |
|||
|---|---|---|---|---|---|---|---|
|
| |||||||
| M | SD | M | SD | t(58) | d | r | |
| ERP Amplitudes | |||||||
| ERN | 1.66 | 4.14 | 1.86 | 4.17 | −.06 | .05 | .61*** |
| CRN | 2.39 | 3.56 | 2.84 | 3.90 | 2.06 | .18 | .79*** |
| ΔERN | −.73 | 3.70 | −.98 | 4.57 | .47 | −.07 | .59*** |
| Pe | 5.63 | 6.71 | 5.45 | 5.39 | .01 | −.03 | .57*** |
| Pc | −.13 | 2.64 | −.41 | 2.85 | .23 | −.11 | 54*** |
| ΔPe | 5.77 | 6.40 | 5.86 | 5.55 | .13 | .02 | .60*** |
| Flankers Task Performance | |||||||
| Accuracy (% correct) | 93.15 | 4.98 | 92.70 | 5.35 | .56 | −.10 | .62*** |
| Correct RT (ms) | 536.12 | 111.54 | 562.60 | 114.48 | 4.82* | .30 | .70*** |
| Error RT (ms) | 396.93 | 99.87 | 452.27 | 136.01 | 11.31** | .47 | .51*** |
| Symptoms | |||||||
| SAPS Psychotic | 2.28 | 5.50 | 5.33 | 11.00 | 11.38** | .37 | .51*** |
| SAPS Disorganized | 2.12 | 3.86 | 5.28 | 6.75 | 24.68*** | .67 | .61*** |
| SANS Inexpressivity | 2.40 | 4.34 | 5.14 | 7.30 | 16.78*** | .48 | .51*** |
| SANS Avolition | 8.79 | 8.39 | 12.69 | 8.56 | 24.46*** | .69 | .78*** |
Note: N = 59 participants completed both assessments. Symptom variables come from the Scales for the Assessment of Positive (SAPS) and Negative Symptoms (SANS).
p<.05,
p<.01,
p<.001
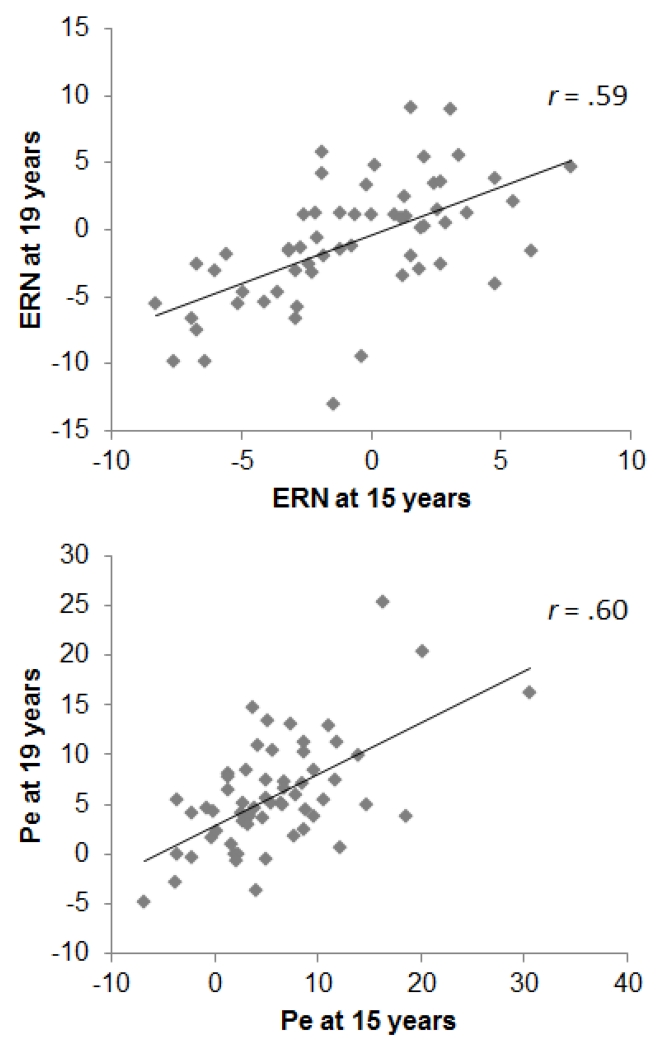
Figure 2.
Scatterplots depicting the association between 15- and 19-year ERPs.
Temporal stability was high for ERP and task performance variables (r’s ≥.50). The ERN and Pe exhibited greater temporal stability than either Psychotic or Inexpressivity symptom scores, but lower than Avolition scores. For the ΔERN, temporal stability estimates were similar for the SZ (r=.55, p<.01) and OP groups (r=.60, p<.001; comparison: z=.28, p=.78). For the ΔPe, temporal stability was higher in the SZ (r=.78, p<.001) than the OP group (r=.39, p<.05; comparison: z=2.30, p<.05).
Past-month antipsychotic medication status was highly stable across assessments: 53 patients (89.8%) reported no change, 4 (6.8%) were prescribed at year 19 but not 15, and 1 (1.7%) was prescribed at year 15 but not 19. Changes in ΔERN and ΔPe across assessments were not correlated with 15-year medication status, nor with change in medication status across assessments (p’s>.20).
3.3. Longitudinal Associations
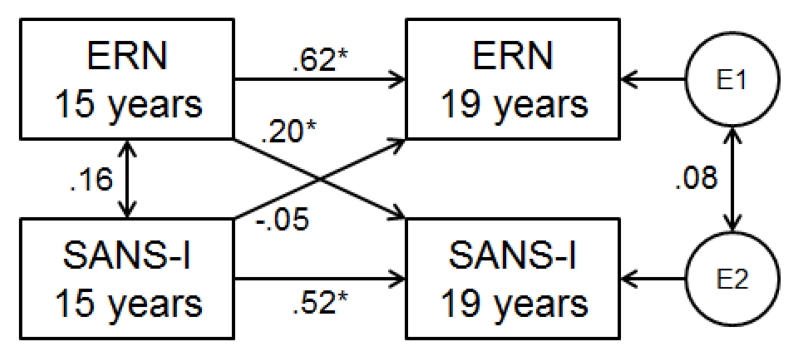
We followed-up cross-sectional findings by examining directional effects in longitudinal analyses (ERN with Inexpressivity, Pe with Avolition). The cross-lagged model testing longitudinal associations between ΔERN and Inexpressivity is shown in Figure 3. Blunted 15-year ΔERN predicted higher Inexpressivity scores four years later, over and above 15-year Inexpressivity (β=.20, p<.05, 95% CI=.02-.38), but Inexpressivity did not predict future ΔERN amplitude (β= −.05, p>.05, 95% CI=−.25-.14). Longitudinal relationships between ΔPe and Avolition scores were not significant (Pe→Avolition: β= −.11, Avolition→Pe: β= −.09, p’s>.05).
Figure 3.
Cross-lagged model testing the longitudinal association between ΔERN amplitude and SANS inexpressivity scores. *p<.05
4. Discussion
The current results are notable for three reasons: (1) Extending findings from a non-clinical sample (Weinberg and Hajcak, 2011), the long-term temporal stabilities of the ERN and Pe are high in late-phase psychosis. (2) We extend findings linking reduced ERN amplitude to negative symptoms (Foti et al., 2012), showing specificity with the sub-dimension of inexpressivity. Reduced Pe, meanwhile, correlated with avolition, although this was not significant after taking diagnosis and medication into account. (3) Blunted ERN amplitude predicted an increase in inexpressivity symptoms four years later, but not vice versa. Together, these results provide evidence supporting the temporal stability and potential predictive validity of ERP indices of impaired error processing in late-phase psychosis.
Long-term longitudinal studies of psychiatric biomarkers are necessary to establish the relative stability of neural abnormalities and track changes in relation to symptoms. Here, we demonstrate that the ERN and Pe both have high temporal stability in late-phase psychosis. Rank-order stability was on par with that of symptoms and task performance. Mean-level stability was higher, as sample means remained unchanged even as symptom severity increased. These data complement studies showing a diminished ERN in high-risk individuals (Laurens et al., 2010; Perez et al., 2012) and unaffected siblings (Simmonite et al., 2012), suggesting that reduced ERN may reflect liability for psychosis: ERN impairment is heritable, precedes symptom onset, and persists throughout course of illness.
The ERN may also provide prognostic information about illness course. Diminished ERN predicted more severe negative symptoms of inexpressivity four years later, over and above baseline severity. No evidence was found for the reverse effect—symptom severity did not predict future ERN/Pe amplitude—and medication status did not predict change in ERPs. This suggests that impaired error processing is not a consequence of illness course or treatment; rather, these ERPs may potentially be leveraged to help forecast illness trajectory. Insofar as effective treatments for negative symptoms are lacking (Fusar-Poli et al., 2015), impaired ERN/Pe may also represent a novel treatment target (Carter et al., 2012).
The link between ERN and inexpressivity warrants further evaluation. The ERN is thought to reflect preconscious, automatic error detection (Hajcak et al., 2003; Nieuwenhuis et al., 2001). One possibility is that inexpressivity may reflect a similar dysfunction in the automatic self-monitoring of expressive behavior, perhaps through common neural circuitry. In healthy populations, expressive behavior activates the ACC (Lindenberg et al., 2012)—the generator of the ERN. Moreover, ACC dysfunction has been proposed as a pathophysiological factor for negative symptoms (Bersani et al., 2014). ERN amplitude is also known to be sensitive to motivation and negative affect, with proposals that it reflects threat sensitivity (Proudfit et al., 2013). The impact of psychotic disorders on emotion, meanwhile, is complex (Kring and Elis, 2013; Strauss and Gold, 2012). Given the interplay between cognitive impairment, negative symptomatology, and functional outcome in psychosis (Ventura et al., 2009), future research should consider impaired error processing in the context of other social and affective deficits.
Replicating our cross-sectional findings at year 15 (Foti et al., 2012) within new follow-up data at year 19, Pe amplitude was reduced in schizophrenia, whereas the ERN did not reliably distinguish diagnostic groups. The ERN appears to be impaired in psychotic illness broadly, while reduced Pe is relatively specific to schizophrenia. In fact, stability of the Pe was higher in the schizophrenia group, whereas stability of the ERN was comparable across groups. Diagnoses were based on two decades of observation, enabling precise estimates of diagnostic effects on error-related ERPs. Indeed, we found that initial SCID-based consensus diagnoses misclassified many cases compared to 10-year gold-standard diagnoses (κ = .47 for schizophrenia; Bromet et al., 2011), partially masking differences in other neural measures (Perlman et al., 2015).
Whereas a reduced ERN related to inexpressivity, reduced Pe related to avolition, suggesting a dissociation between error-related ERPs and negative symptom sub-dimensions. This is tempered by the finding that Pe-avolition associations were not significant after taking into account covariates, including diagnosis and medication status. It is possible that the link with avolition, therefore, may be explained by the diagnostic differences in Pe amplitude. A theme across these findings is that the ERN and Pe map onto distinct phenotypes within psychotic disorders, consistent with the notion that they capture functionally distinct stages of error processing (Hajcak et al., 2003; Nieuwenhuis et al., 2001).
The current findings are qualified by several limitations. Longitudinal data from a control sample was not available. It would be valuable for research studies to compare ERN/Pe trajectories across patients and controls. Our patient sample, while larger than most studies of its kind, was modest in size and may have precluded the detection of other associations. We were primarily interested in negative symptoms (Foti and Hajcak, 2012; Foti et al., 2013), yet it would be informative to consider a more comprehensive profile of other illness features, such as functional impairment. Further, patients were not treatment-naïve, and we could not test ERN/Pe stability early in illness course. This cohort represents the majority of patients with psychotic disorders, however, insofar as few people in treatment settings are new onset.
The current study advances the literature by considering prospective relationships between ERP indices of error processing and course of psychotic illness. The ERN and Pe are stable neural measures in late-phase psychosis. The ERN also exhibits long-term predictive validity regarding negative symptoms. In this way, the ERN and Pe have the potential to someday be integrated into clinical care as neurophysiological assessment tools to improve judgments of diagnosis and prognosis. The present effects are modest, but a panel of similarly informative measures can offer sufficient predictive power to be clinically useful. Ultimately, the ERN and Pe might prove useful as targets for treatment development, helping to address the paucity of interventions for negative symptoms. The present study is a step in this direction.
Acknowledgments
We want to thank Evelyn Bromet, cohort founder, and Al Hamdy, study interviewer.
Funding
This work was supported by the National Institutes of Health (MH094398 to R.K.), Stony Brook University (Clinical Research Scholar Award to R.K.), and the Brain and Behavior Research Foundation (Young Investigator Grant to G.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that they have no conflicts of interest in relation to the subject of this study.
Contributors
R.K. and G.H. designed the study. D.F. and G.P. undertook the statistical analysis, and D.F. wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
References
- Alain C, McNeely HE, He Y, Christensen BK, West R. Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cereb Cortex. 2002;12(8):840–846. doi: 10.1093/cercor/12.8.840. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City: 1983a. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; Iowa City: 1983b. [Google Scholar]
- Bates AT, Kiehl KA, Laurens KR, Liddle PF. Error-related negativity and correct response negativity in schizophrenia. Clin Neurophysiol. 2002;113(9):1454–1463. doi: 10.1016/s1388-2457(02)00154-2. [DOI] [PubMed] [Google Scholar]
- Bates AT, Liddle PF, Kiehl KA, Ngan ET. State dependent changes in error monitoring in schizophrenia. J Psychiatr Res. 2004;38(3):347–356. doi: 10.1016/j.jpsychires.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Bersani FS, Minichino A, Fojanesi M, Gallo M, Maglio G, Valeriani G, Biondi M, Fitzgerald PB. Cingulate Cortex in Schizophrenia: its relation with negative symptoms and psychotic onset. A review study. Eur Rev Med Pharmacol Sci. 2014;18(22):3354–3367. [PubMed] [Google Scholar]
- Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32(2):238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63(5):505–511. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, Chang SW. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168(11):1186–1194. doi: 10.1176/appi.ajp.2011.11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet EJ, Schwartz JE, Fennig S, Geller L, Jandorf L, Kovasznay B, Lavelle J, Miller A, Pato C, Ram R, et al. The epidemiology of psychosis: the Suffolk County Mental Health Project. Schizophr Bull. 1992;18(2):243–255. doi: 10.1093/schbul/18.2.243. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM, Committee CE. Imaging biomarkers for treatment development for impaired cognition: report of the sixth CNTRICS meeting: Biomarkers recommended for further development. Schizophr Bull. 2012;38(1):26–33. doi: 10.1093/schbul/sbr109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence. J Physiol Paris. 2015;109(1-3):3–15. doi: 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16(1):143–149. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders--Patient Edition (SCID-I/P 2/2001 Revision) Biometrics Research Department, New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- Foti D, Hajcak G. Genetic variation in dopamine moderates neural responses during reward anticipation and delivery: Evidence from event-related potentials. Psychophysiology. 2012;49(5):617–626. doi: 10.1111/j.1469-8986.2011.01343.x. [DOI] [PubMed] [Google Scholar]
- Foti D, Kotov R, Bromet EJ, Hajcak G. Beyond the broken error-related negativity: Functional and diagnostic correlates of error processing in psychosis. Biol Psychiatry. 2012;71(10):864–872. doi: 10.1016/j.biopsych.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Kotov R, Hajcak G. Psychometric considerations in using error-related brain activity as a biomarker in psychotic disorders. J Abnorm Psychol. 2013;122(2):520–531. doi: 10.1037/a0032618. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr Bull. 2015;41(4):892–899. doi: 10.1093/schbul/sbu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4(6):385. [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40(6):895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative symptoms and cognitive deficits: what is the nature of their relationship? Schizophr Bull. 2006;32(2):250–258. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Horan WP, Foti D, Hajcak G, Wynn JK, Green MF. Impaired neural response to internal but not external feedback in schizophrenia. Psychol Med. 2012;42(8):1637–1647. doi: 10.1017/S0033291711002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G, Yeung N. Dissociable correlates of response conflict and error awareness in error-related brain activity. Neuropsychologia. 2011;49(3):405–415. doi: 10.1016/j.neuropsychologia.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson F, Foti D, Kotov R, Perlman G, Mathalon DH, Proudfit GH. An incongruent reality: the N400 in relation to psychosis and recovery. Schizophr Res. 2014;160(1-3):208–215. doi: 10.1016/j.schres.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansal V, Patriciu I, Kiang M. Illness insight and neurophysiological error-processing deficits in schizophrenia. Schizophr Res. 2014;156(1):122–127. doi: 10.1016/j.schres.2014.03.023. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biol Psychiatry. 2008;64(1):26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kang SS, Shin KS, Yoo SY, Kim YY, Kwon JS. Neuropsychological correlates of error negativity and positivity in schizophrenia patients. Psychiatry Clin Neurosci. 2006;60(3):303–311. doi: 10.1111/j.1440-1819.2006.01506.x. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F. An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. J Abnorm Psychol. 1999;108(2):337–346. doi: 10.1037//0021-843x.108.2.337. [DOI] [PubMed] [Google Scholar]
- Kotov R, Guey LT, Bromet EJ, Schwartz JE. Smoking in schizophrenia: diagnostic specificity, symptom correlates, and illness severity. Schizophr Bull. 2010;36(1):173–181. doi: 10.1093/schbul/sbn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Elis O. Emotion deficits in people with schizophrenia. Annu Rev Clin Psychol. 2013;9:409–433. doi: 10.1146/annurev-clinpsy-050212-185538. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170(2):165–172. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens KR, Hodgins S, Mould GL, West SA, Schoenberg PL, Murray RM, Taylor EA. Error-related processing dysfunction in children aged 9 to 12 years presenting putative antecedents of schizophrenia. Biol Psychiatry. 2010;67(3):238–245. doi: 10.1016/j.biopsych.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Uhlig M, Scherfeld D, Schlaug G, Seitz RJ. Communication with emblematic gestures: shared and distinct neural correlates of expression and reception. Hum Brain Mapp. 2012;33(4):812–823. doi: 10.1002/hbm.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J Abnorm Psychol. 2002;111(1):22–41. [PubMed] [Google Scholar]
- Minzenberg MJ, Gomes GC, Yoon JH, Swaab TY, Carter CS. Disrupted action monitoring in recent-onset psychosis patients with schizophrenia and bipolar disorder. Psychiatry Res. 2014;221(1):114–121. doi: 10.1016/j.pscychresns.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Yee CM, Nuechterlein KH. Electrophysiological analysis of error monitoring in schizophrenia. J Abnorm Psychol. 2006;115(2):239–250. doi: 10.1037/0021-843X.115.2.239. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7th ed. Muthén & Muthén; Los Angeles, CA: 1998-2012. [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38(5):752–760. [PubMed] [Google Scholar]
- Perez VB, Ford JM, Roach BJ, Woods SW, McGlashan TH, Srihari VH, Loewy RL, Vinogradov S, Mathalon DH. Error monitoring dysfunction across the illness course of schizophrenia. J Abnorm Psychol. 2012;121(2):372–387. doi: 10.1037/a0025487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman G, Foti D, Jackson F, Kotov R, Constantino E, Hajcak G. Clinical significance of auditory target P300 subcomponents in psychosis: Differential diagnosis, symptom profiles, and course. Schizophr Res. 2015;165(2-3):145–151. doi: 10.1016/j.schres.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH, Inzlicht M, Mennin DS. Anxiety and error monitoring: the importance of motivation and emotion. Front Hum Neurosci. 2013;7:636. doi: 10.3389/fnhum.2013.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonite M, Bates AT, Groom MJ, Jackson GM, Hollis C, Liddle PF. Error processing-associated event-related potentials in schizophrenia and unaffected siblings. Int J Psychophysiol. 2012;84(1):74–79. doi: 10.1016/j.ijpsycho.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169(4):364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Horan WP, Kirkpatrick B, Fischer BA, Keller WR, Miski P, Buchanan RW, Green MF, Carpenter WT., Jr. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47(6):783–790. doi: 10.1016/j.jpsychires.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr Res. 2009;113(2-3):189–199. doi: 10.1016/j.schres.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Longer term test-retest reliability of error-related brain activity. Psychophysiology. 2011;48(10):1420–1425. doi: 10.1111/j.1469-8986.2011.01206.x. [DOI] [PubMed] [Google Scholar]