Abstract
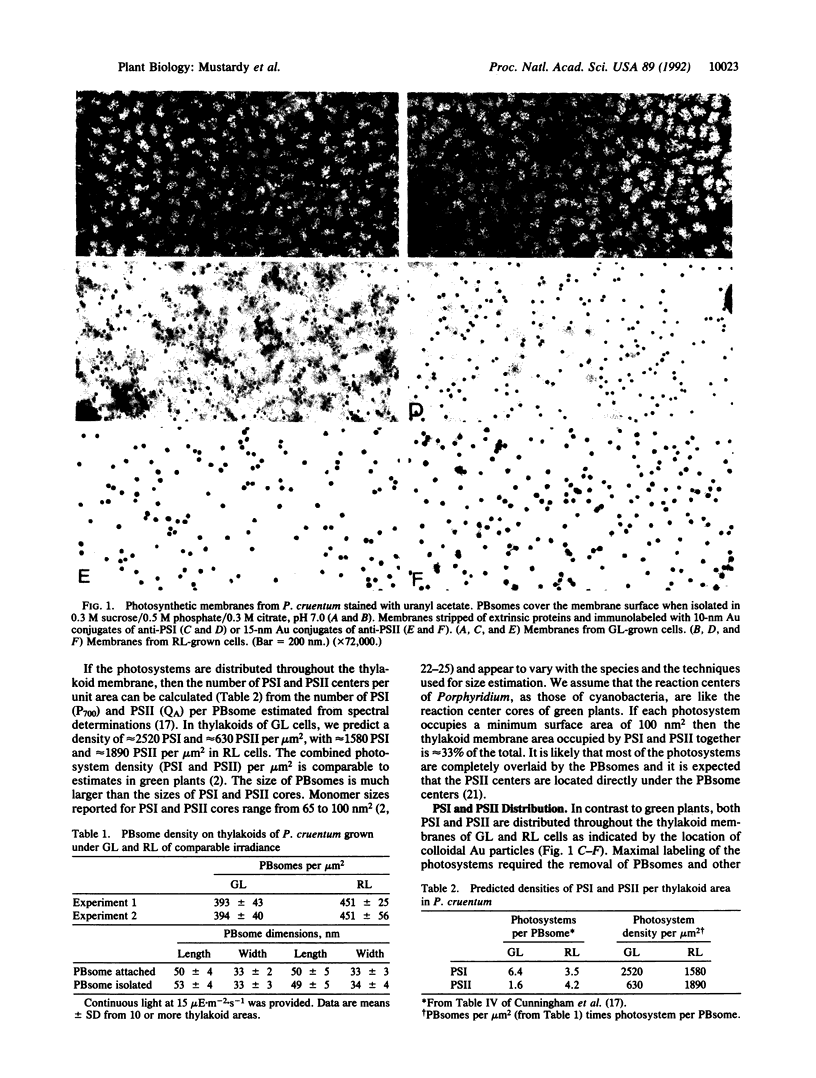
An immunolabeling approach was developed for quantitative in situ labeling of photosystems I and II (PSI and PSII). Photosynthetic membranes from the phycobilisome-containing red alga Porphyridium cruentum were isolated from cells in which different photosystem compositions were predetermined by growing cells in green light (GL) or red light (RL). Based on phycobilisome densities per membrane area of 390 per m2 (GL) and 450 per m2 (RL) and the PSI reaction center (P700) and PSII reaction center (QA) content, the photosystem densities per m2 of membrane were calculated to be 2520 PSI in GL and 1580 in RL and 630 PSII in GL and 1890 in RL. PSI was detected in the membranes with 10-nm Au particles conjugated to affinity-purified anti-PSI, and PSII was detected with 15-nm Au particles conjugated to anti-PSII. Distribution of Au particles appeared relatively uniform, and the degree of labeling was consistent with the calculated photosystem densities. However, the absolute numbers of Au-labeled sites were lower than would be obtained if all reaction center monomers were labeled. Specific labeling of PSI was 25% in GL and RL membranes, and PSII labeling was 33% in GL but only 17% in RL membranes. An IgG-Au particle is larger than a monomer of either photosystem and could shield several closely packed photosystems. We suggest that clustering of photosystems exists and that the cluster size of PSI is the same in GL and RL cells, but the PSII cluster size is 2 times greater in RL than in GL cells. Such variations may reflect changes in functional domains whereby increased clustering can maximize the cooperativity between the photosystems, resulting in enhancement of the quantum yield.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. R., Staehelin L. A. Lateral Distribution of the Cytochrome b(6)/f and Coupling Factor ATP Synthetase Complexes of Chloroplast Thylakoid Membranes. Plant Physiol. 1985 May;78(1):199–202. doi: 10.1104/pp.78.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almog O., Shoham G., Michaeli D., Nechushtai R. Monomeric and trimeric forms of photosystem I reaction center of Mastigocladus laminosus: crystallization and preliminary characterization. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5312–5316. doi: 10.1073/pnas.88.12.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F. X., Dennenberg R. J., Jursinic P. A., Gantt E. Growth under Red Light Enhances Photosystem II Relative to Photosystem I and Phycobilisomes in the Red Alga Porphyridium cruentum. Plant Physiol. 1990 Jul;93(3):888–895. doi: 10.1104/pp.93.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F. X., Dennenberg R. J., Mustardy L., Jursinic P. A., Gantt E. Stoichiometry of Photosystem I, Photosystem II, and Phycobilisomes in the Red Alga Porphyridium cruentum as a Function of Growth Irradiance. Plant Physiol. 1989 Nov;91(3):1179–1187. doi: 10.1104/pp.91.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. F., Gantt E. Phycobilisome-thylakoid Topography on Photosynthetically Active Vesicles of Porphyridium cruentum. Plant Physiol. 1981 Apr;67(4):608–612. doi: 10.1104/pp.67.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford R. C., Hefti A., Engel A. Ordered arrays of the photosystem I reaction centre after reconstitution: projections and surface reliefs of the complex at 2 nm resolution. EMBO J. 1990 Oct;9(10):3067–3075. doi: 10.1002/j.1460-2075.1990.tb07503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings T. H., Wasmann C., Staehelin L. A. Structure of the Thylakoids and Envelope Membranes of the Cyanelles of Cyanophora paradoxa. Plant Physiol. 1983 Feb;71(2):409–419. doi: 10.1104/pp.71.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw J. E., Miller K. R. Localization of light-harvesting complex II to the occluded surfaces of photosynthetic membranes. J Cell Biol. 1989 Oct;109(4 Pt 1):1725–1731. doi: 10.1083/jcb.109.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrgang K. D., Boekema E. J., Vater J., Renger G. Structural determination of the photosystem II core complex from spinach. Eur J Biochem. 1988 Dec 1;178(1):209–217. doi: 10.1111/j.1432-1033.1988.tb14445.x. [DOI] [PubMed] [Google Scholar]
- Lavergne J., Joliot P. Restricted diffusion in photosynthetic membranes. Trends Biochem Sci. 1991 Apr;16(4):129–134. doi: 10.1016/0968-0004(91)90054-y. [DOI] [PubMed] [Google Scholar]
- Lefort-Tran M., Cohen-Bazire G., Pouphile M. Les membranes photosynthétiques des algues à biliproteines observées apres cryodécapage. J Ultrastruct Res. 1973 Aug;44(3):199–209. doi: 10.1016/s0022-5320(73)80056-5. [DOI] [PubMed] [Google Scholar]
- Ley A. C. Effective Absorption Cross-Sections in Porphyridium cruentum: Implications for Energy Transfer between Phycobilisomes and Photosystem II Reaction Centers. Plant Physiol. 1984 Feb;74(2):451–454. doi: 10.1104/pp.74.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustardy L., Cunningham F. X., Gantt E. Localization and quantitation of chloroplast enzymes and light-harvesting components using immunocytochemical methods. Plant Physiol. 1990 Sep;94(1):334–340. doi: 10.1104/pp.94.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]



