Abstract
Background
Schizophrenia is one of the most disabling psychiatric disorders with increased morbidity and mortality. Both schizophrenia and oxidative stress have been associated with accelerated aging. Previous studies found increased oxidative stress in individuals with schizophrenia, though only one study measured F2-isoprostanes and did so in urine. To our knowledge, the present study is the first to assess plasma F2-isoprostane levels, the putative gold standard measure of systemic oxidative stress in vivo, in schizophrenia.
Methods
We compared plasma F2-isoprostane levels in 134 stable outpatients with schizophrenia and 120 age- and gender-matched healthy comparison (HC) subjects. Sociodemographic and clinical data were collected in both groups.
Results
Plasma F2-isoprostane levels were significantly higher in the schizophrenia group than in the HC group. Women had higher F2-isoprostane levels compared to men, and those with higher body mass index (BMI) had higher levels, within each group. F2-isoprostane levels correlated with BMI, physical functioning, and medical comorbidity but not with severity of psychopathology or executive function. Linear models showed significant effects of diagnosis, gender, and BMI on F2-isoprostane levels, but no interactions.
Discussion
Our finding of increased oxidative stress in schizophrenia is consistent with reports of increased morbidity and mortality as well as accelerated aging in schizophrenia. The significant associations between F2-isoprostane levels and both gender and BMI warrant further study.
Keywords: F2-isoprostanes, schizophrenia, oxidative stress, gender, body mass index, aging
1. Introduction
Schizophrenia is one of the top ten global causes of disability (WHO, 2001). In addition to its debilitating psychiatric symptoms, schizophrenia is also associated with many physical illnesses, especially metabolic and cardiovascular diseases, as well as higher mortality (Hennekens et al., 2005; Jeste et al., 2011; Meyer and Nasrallah, 2009; Nasrallah et al., 2015; Saha et al., 2007). The increased medical comorbidity and mortality in all age groups suggests that people with schizophrenia age at an accelerated rate (Kirkpatrick et al., 2008). The rapid aging in schizophrenia may be at least partly attributable to the effects of greater stress, higher incidence of smoking, antipsychotic medications, lower physical activity, and worse management of medical illnesses.
An important contributor to accelerated aging is believed to be oxidative stress, the unopposed and widespread free radical-mediated damage at a molecular level (Finkel and Holbrook, 2000; von Zglinicki, 2002). In the general population, oxidative stress is associated with higher body mass index (BMI) (Keaney et al., 2003; Vincent and Taylor, 2006), cigarette smoking (Bachi et al., 1996; Morrow et al., 1995), alcohol use (Meagher et al., 1999), and cardiovascular and metabolic diseases (Basu, 2008; Davies and Roberts, 2011; Stephens et al., 2009). In some studies, oxidative stress differed between men and women, with greater oxidative stress being found in women (Fukui et al., 2011; Khadir et al., 2015; Peskind et al., 2014; Wiener et al., 2014). Three of these studies found significant gender differences that persisted when controlling for BMI or abdominal adiposity (Fukui et al., 2011; Khadir et al., 2015; Peskind et al., 2014). One study reported that both gender and BMI seemed to be equally significant contributors to oxidative stress (Keaney et al., 2003).
F2-isoprostanes are prostaglandin-like compounds formed from free fatty acids, and have roles in vasoconstriction, immune activation, and specific disease mechanisms (Montuschi et al., 2004; Morrow and Roberts, 1997; Patrono and FitzGerald, 1997). Elevated F2-isoprostane levels are linked to lifestyle risk factors including smoking, hypercholesterolemia, diabetes mellitus, hyperhomocysteinemia, fatty diet, and higher BMI (Davi et al., 2004; Dietrich et al., 2002; Montuschi et al., 2004). Most, but not all, published studies have shown that F2-isoprostane levels appear to increase with age in normal humans and in mice (Li et al., 2014; Roberts and Reckelhoff, 2001). Some investigators consider F2-isoprostane levels as a gold standard marker of oxidative stress in vivo, due to their high sensitivity and specificity, stability, and detectability in a variety of body fluids (Milne et al., 2015; Milne et al., 2007; Morrow and Roberts, 1997, 2002).
Since the mid-1950s, researchers have noted a strong association between oxidative stress and schizophrenia (Hoffer et al., 1954), and abnormalities in oxidative stress markers have been demonstrated in schizophrenia (Akyol et al., 2002; Ng et al., 2008; Wu et al., 2012; Yanik et al., 2003). Greater oxidative stress has also been linked to clinically more severe schizophrenia illness – i.e., with longer duration, earlier age of onset, and more severe symptoms as well as greater side effects of antipsychotic medications (Herken et al., 2001; Mukerjee et al., 1996; Pazvantoglu et al., 2009).
While certain gender differences in schizophrenia (i.e., later age of onset of schizophrenia in women) have been well-established (Aleman et al., 2003; Eranti et al., 2013; Hafner, 2003; Lindamer et al., 1999), gender differences in oxidative stress markers have not been adequately studied in people with schizophrenia. An interaction between gender and oxidative stress in schizophrenia has been shown in only two studies, where women had increased oxidative stress (Abdalla et al., 1986; Akyol et al., 2002), while other studies have not found significant gender differences (Herken et al., 2001; Kaiya et al., 1989; Padurariu et al., 2010; Reddy et al., 1991; Zhang et al., 2009; Zhang et al., 2003).
Different measures of oxidative stress (including superoxide dismutase activity, catalase activity, glutathione, nitric oxide and its metabolites) have been assessed in individual studies in schizophrenia. The limited literature on prostaglandin markers in schizophrenia is conflicting, with both positive (Kaiya et al., 1989; Mathe et al., 1980) and negative findings (Gerner and Merrill, 1983; Kaiya, 1984; Linnoila et al., 1983). To our knowledge, only one study examined F2-isoprostane levels by immunoassay in patients with schizophrenia and healthy comparison subjects (HCs), and found elevated isoprostane levels in urine in the schizophrenia group (Dietrich-Muszalska and Olas, 2009). The studied population was relatively young (ages ranging from 26 to 36 years) and the HCs were non-smokers. We did not find any studies of plasma F2-isoprostanes in people with schizophrenia.
The present investigation focused on evaluating oxidative stress in age- and gender-matched groups of people with versus without schizophrenia, using plasma levels of F2-isoprostanes. Previously we had reported a finding of significantly higher levels of high-sensitivity C-reactive protein (hs-CRP) in a smaller sample of patients with schizophrenia than in HCs from the same cohort (Joseph et al., 2015). In the present report, we hypothesized that the group with schizophrenia would have elevated F2-isoprostane levels compared to the HC group. We also hypothesized that there would be a gender difference in F2-isoprostane levels, with higher levels among women. We examined the relationship of age, gender, and group (schizophrenia vs. HC) and their interactions to better understand observed differences. We also explored the possible role of smoking and BMI, given their known associations with oxidative stress. Finally, within the schizophrenia group, we examined the relationship of F2-isoprostane levels to symptom severity, illness duration, and antipsychotic medication use.
2. Experimental/Materials and Methods
2.1 Study participants
We studied 134 adult outpatients with schizophrenia (n = 80) or schizoaffective disorder (n = 54), and 120 HCs with no history of major neuropsychiatric illness, matched by age group (26-35, 36-45, 46-55, and 56-65 years), in whom data on F2-isoprostanes were available. All the subjects were English-speaking, recruited from the greater San Diego community. The diagnoses of schizophrenia and schizoaffective disorder were based on the Structured Clinical Interview for the DSM-IV-TR (SCID) (First, November 2002). Several papers have demonstrated similarities between schizoaffective disorder and schizophrenia (Amann et al., 2016; Evans et al., 1999). Over 90% of individuals with schizophrenia were treated with antipsychotic medications. HCs completed the Mini-International Neuropsychiatric Interview (MINI) to rule out a history of major neuropsychiatric illnesses (Sheehan et al., 1998). Exclusion criteria were: 1) other current DSM-IV-TR Axis I diagnoses; 2) alcohol or other substance (other than tobacco) abuse or dependence within 3 months prior to enrollment; 3) diagnosis of dementia, intellectual disability disorder, or a major neurological disorder; 4) any medical disability that interfered with a subject's ability to complete the study procedures. The protocol was reviewed and approved by the UC San Diego Human Research Protections Program. All study participants gave written informed consent to participate.
2.2 Sociodemographic and clinical characteristics
Sociodemographic and clinical characteristics for the study sample were ascertained through participant interview and review of available research and medical records, with the appropriate HIPAA authorization from the study participants. Subjects completed several assessments of cognition (Delis-Kaplan Executive Function System), mental health (Patient Health Questionnaire-9, Scale for the Assessment of Positive Symptoms, Scale for the Assessment of Negative Symptoms, Short Form Health Survey - Mental), and physical health (Cumulative Illness Rating, Short Form Health Survey - Physical) (Andreasen and Olsen, 1982; Delis et al., 2001; Kroenke et al., 2001; van den Berg et al., 2012; Ware and Sherbourne, 1992). Medical comorbidity was measured with the total score from the Cumulative Illness Rating Scale (CIRS), which combines the presence and severity of common medical comorbidities (Linn et al., 1968). BMI was based on assessment of participant height and weight (kg/ m2). Assessments were performed by study staff members who underwent rigorous training and inter-rater reliability certification. Staff was trained in the administration of standardized assessments – shadowing senior raters and then being observed while performing assessments.
2.3 Plasma F2-isoprostane Assays
The lab assays were conducted at the Eicosanoid Laboratory at Vanderbilt University, using gas chromatography/negative ion chemical ionization mass spectrometry (GC/NICI-MS) methodology as described by Milne et al. (Milne et al., 2007) and Morrow et al. (Morrow and Roberts, 2002). Advantages of this method include high sensitivity and specificity, more accurate than the urine immunoassay which does not detect the glucoronidated F2-isoprostanes in the urine, up to 50% of the total F2-isoprostanes (Callewaert and Sloan, 2010). Normal plasma levels for this assay in healthy adults are 0.035 ± 0.006 ng/mL.
2.4 Statistical Analyses
All study variables were scrutinized for violation of distribution assumptions required for parametric analyses, and appropriate transformations were made. A number of variables including F2-isoprostane levels were log-transformed for all analyses.
Independent t-tests or chi-square tests were performed to determine differences in sociodemographic, clinical, and physical health measures between schizophrenia and HC groups. F2-isoprostane levels were compared by diagnostic group and gender. Unequal variances were assumed in the calculation of the t-statistics. Pearson bivariate correlations between F2-isoprostane levels and BMI and smoking variables were examined separately in the two groups. Pearson correlations between F2-isoprostane levels (log-transformed) and several clinical variables (i.e., duration of illness, severity of positive, negative, and depression symptoms, and daily antipsychotic dose) were also examined within the schizophrenia group.
To explore the associations of F2-isoprostane levels with age, gender, and group (schizophrenia vs. HC) along with any potential interactions, we conducted multiple linear regression analysis. These demographic variables were chosen because they are unmodifiable characteristics that might affect the interpretation of group differences observed in the univariate analyses. Also, our large samples that were matched on both age and gender and stratified well across these variables provided an opportunity to examine the role of these key variables.
After examining univariate correlations in the schizophrenia and healthy groups, we discovered that BMI was related to F2-isoprostanes levels, which raised the possibility that group differences in F2-isoprostanes levels might be driven by group differences in BMI. Thus, we further explored the effects of adding BMI and all relevant interactions to the model including group, age and gender, with a focus on changes in effect size for group relative to the univariate test.
Effect sizes are presented for all tests along with p-values. We interpreted results as meaningful when the effect sizes are medium or larger (i.e., Cohen's d > 0.45).
3. Results
3.1 Schizophrenia and Healthy Comparison (HC) Sample Characteristics
There were no significant differences between the participants with schizophrenia compared with those with schizoaffective disorder on mean age, duration or severity of mental illness, lifestyle factors (smoking, substance use), physical health (BMI, medical comorbidity) or cognition. We also looked at F2-isoprostane levels between the two groups and found no significant difference between the two groups (t(132) = -0.66, p = 0.51). Therefore, subsequent analyses combined these two groups. The sociodemographic and clinical characteristics of the schizophrenia and HC groups are summarized in Table 1. As expected, the two groups did not differ in age or gender distribution. Consistent with the psychopathology of schizophrenia, the schizophrenia group had a history of greater substance use and alcohol abuse, and worse psychotic symptoms as well as mental and physical wellness scores. As has been described in other studies (Goff et al., 1992; Hughes et al., 1986), the schizophrenia group had a more extensive smoking history and smoked more cigarettes currently. The schizophrenia group also had a significantly higher mean BMI; although the mean BMI values in both groups were in the overweight-obese range.
Table 1. Comparison of Study Participants With and Without Schizophrenia.
| Healthy Comparison (HC) | Schizophrenia | t | df | p | Cohen's d | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Std Dev | N | Mean | Std Dev | |||||
| Sociodemographic Factors | ||||||||||
| Age (years) | 120 | 48.6 | 11.6 | 134 | 48.1 | 10.1 | 0.34 | 237.4 | 0.731 | 0.044 |
| Gender (% women) | 54% | 45% | 2.23* | 1 | 0.167 | 1.49** | ||||
| Education (years) | 120 | 14.5 | 2.2 | 134 | 12.4 | 2.0 | 8.00 | 243.4 | <0.001 | |
| Current packs of cigarettes per day | 120 | 0.02 | 0.08 | 134 | 0.38 | 0.48 | -8.5 | 142.4 | <0.001 | -1.04 |
| Number of years smoked | 120 | 5.4 | 10.6 | 133 | 16.3 | 14.7 | -6.83 | 239.2 | <0.001 | -0.84 |
| Substance Abuse (Yes) | 19 | 15% | 55 | 41% | 19.85* | 1 | <0.001 | 4.45** | ||
| Clinical Factors | ||||||||||
| Duration of illness (years) | 133 | 25.1 | 11.2 | |||||||
| Antipsychotic dose*** | 134 | 1.8 | 1.5 | |||||||
| PHQ-9 Score | 116 | 2.0 | 2.9 | 130 | 7.6 | 6.6 | -8.72 | 182.0 | <0.001 | -1.29 |
| Positive symptoms† | 119 | 0.3 | 0.7 | 134 | 6.5 | 4.3 | -16.63 | 141.4 | <0.001 | -2.80 |
| Negative symptoms‡ | 118 | 1.4 | 2.3 | 134 | 7.4 | 4.4 | -13.93 | 203.6 | <0.001 | -1.95 |
| SF-36 Mental Composite score | 117 | 54.6 | 5.8 | 132 | 43.4 | 11.3 | 9.97 | 201.7 | <0.001 | 1.40 |
| Executive function | 120 | 0.4 | 0.6 | 134 | -0.5 | 0.7 | 11.63 | 249.9 | <0.001 | 1.47 |
| Physical Factors | ||||||||||
| SF-36 Physical Composite Score | 117 | 51.5 | 8.8 | 132 | 43.2 | 10.1 | 6.99 | 247.0 | <0.001 | 0.89 |
| CIRS Total score | 101 | 3.2 | 3.2 | 116 | 6.8 | 4.9 | -6.45 | 200.3 | <0.001 | -0.91 |
| BMI (kg/m2) | 117 | 27.7 | 7.0 | 132 | 32.2 | 7.4 | -4.90 | 245.7 | <0.001 | -0.63 |
| hs-CRP (mg/L) | 116 | 2.18 | 3.5 | 131 | 5.08 | 8.8 | -3.47 | 174.7 | 0.001 | -0.53 |
| Plasma F2-isoprostanes (ng/mL) | 120 | 0.032 | 0.015 | 134 | 0.040 | 0.021 | -3.31 | 243.4 | 0.001 | -0.43 |
X2 value
r value
Antipsychotic medication daily dosages were converted to WHO average daily doses based on published standards (WHO, 2009, 2010)
Scale for the Assessment of Positive Symptoms (SAPS) total score
Scale for the Assessment of Negative Symptoms (SANS) total score
PHQ-9 = patient health questionnaire
SF = Short Form Health Survey
CIRS = Cumulative Illness Rating
BMI = body mass index
hs-CRP = high sensitivity C-reactive protein
3.2 F2-isoprostane Levels
Plasma F2-isoprostane levels were significantly higher in the schizophrenia group compared to the HCs, with a medium effect size (Cohen's d = -0.43).
3.3 Correlates of F2-isoprostane Levels
Univariate Pearson correlation analysis showed that age and cigarette use were not significantly correlated with F2-isoprostane levels in either group (Table 2). F2-isoprostane levels were significantly related to gender, being higher in women than in men, in both diagnostic groups. F2-isoprostane levels were significantly positively correlated with BMI in both groups. F2-isoprostane levels also correlated with physical functioning (SF-36 Physical Composite score), and medical comorbidity (CIRS total score) but not with severity of psychopathology or executive function in schizophrenia.
Table 2. Pearson Correlations Between Key Demographic and Clinical Variables and F2-isoprostane Levels in Study Participants With and Without Schizophrenia.
| F2-Isoprostanes in Healthy Comparison Group | F2-Isoprostanes in Schizophrenia Group | |||||
|---|---|---|---|---|---|---|
| N | r | d | N | r | d | |
| Sociodemographic Factors | ||||||
| Age (years) | 120 | 0.07 | 0.14 | 134 | 0.06 | 0.12 |
| Gender | 120 | -0.29** | -0.61 | 134 | -0.36*** | -.77 |
| Education (years) | 120 | -.06 | -0.12 | 134 | -.03 | |
| Current packs of cigarettes per day | 120 | 0.06 | 0.12 | 134 | -0.12 | -0.24 |
| Number of years smoked | 120 | 0.09 | 0.18 | 133 | -0.00 | 0.01 |
| Substance abuse (yes) | 120 | 0.08 | 0.16 | 133 | -0.10 | |
| Clinical Factors | ||||||
| Duration of illness (years) | -- | -- | -- | 133 | 0.16 | 0.33 |
| Antipsychotic dose | -- | -- | -- | 134 | -0.07 | -0.14 |
| PHQ-9 Score | 116 | 0.02 | 0.04 | 130 | 0.14 | 0.28 |
| Positive symptoms† | -- | -- | -- | 134 | 0.06 | 0.12 |
| Negative symptoms‡ | -- | -- | -- | 134 | 0.05 | 0.10 |
| SF-36 Mental Composite score | 117 | -0.06 | -0.12 | 132 | -0.13 | -0.26 |
| Executive function | 120 | 0.06 | 0.12 | 134 | -0.16 | -0.33 |
| Physical Factors | ||||||
| SF-36 Physical Composite Score | 117 | -0.18 | -0.37 | 132 | -0.24** | -0.49 |
| CIRS Total score | 101 | 0.17 | 0.34 | 116 | 0.24** | 0.49 |
| BMI (kg/m2) | 117 | 0.23* | 0.47 | 132 | 0.24** | 0.49 |
| hs-CRP (mg/L) | 116 | 0.19* | 0.39 | 131 | 0.27** | 0.56 |
Scale for the Assessment of Positive Symptoms (SAPS) total score
Scale for the Assessment of Negative Symptoms (SANS) total score
PHQ-9 = patient health questionnaire
SF = Short Form Health Survey
CIRS = Cumulative Illness Rating
BMI = body mass index
hs-CRP = high sensitivity C-reactive protein
***,**, and *: Significant 2-tailed correlation coefficients at the 0.001, 0.01 and 0.05 levels, respectively
F2-isoprostane levels were significantly correlated with hs-CRP levels in the schizophrenia and HC groups. Within the schizophrenia group, F2-isoprostane levels were not significantly related to duration of illness, severity of positive symptoms, negative symptoms, depressive symptoms, or current daily antipsychotic dose.
3.4 Age, Gender, and Group Relationships with F2-isoprostane Levels
A general linear model that included age, gender, and group, and all interactions was significant with good model fit (F = 6.5, p < 0.001, R2 = 0.16), and revealed a main effect of group (F(1, 246) = 17.4, p < 0.001, Cohen's d = 0.53) with levels being higher in the schizophrenia group, and a main effect of gender (F(1,246) = 29.7, p < 0.001, Cohen's d = 0.70) with levels being higher in women (see Figure 1). There was no main effect of age, and no meaningful two-way or three-way interactions (all p's > 0.32, all Cohen's d's < 0.13).
Figure 1. F2-isoprostane levels by diagnostic group and gender.
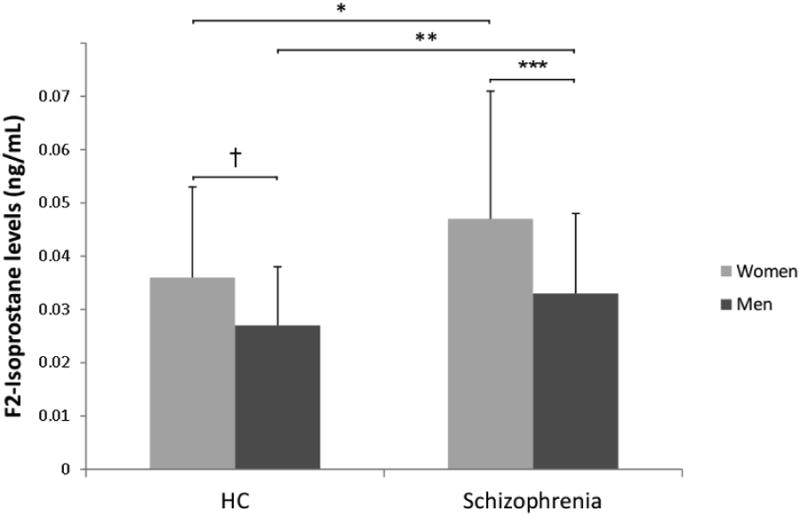
Bar graph depicting the mean levels of F2-isoprostanes (in ng/ml) in women (gray) and men (black) in the healthy comparison (HC) and schizophrenia group. Error bars depict the standard deviation around the mean.
*t = -3.01, df = 104.3, p = 0.003
**t = -2.36, df = 126.0, p = 0.02
***t = 4.06, df = 91.9, p < 0.001
†t= 3.35, df = 112.5, p = 0.001
3.5 Age, gender, group, and BMI relationships with F2-isoprostane levels
When we added BMI to the previous model containing age, gender, and group, along with all possible 2-, 3-, and 4-way interactions, the model was again significant and fit well (F = 4.4, p = <0.001, R2 = 0.22). The main effect of group (schizophrenia > HC) remained significant (F(1,233) = 9.1, p = 0.003, Cohen's d = 0.40), although the magnitude of the effect size was slightly smaller than in the model without BMI (d = 0.40 versus d = 0.53). Gender was still highly related to F2-isoprostane levels in the model containing BMI (F(1,233) = 28.3, p < 0.001, Cohen's d = 0.70), with women having higher levels than men. As suggested by the correlation analyses, BMI was significantly associated with F2-isoprostane levels in this model (F(1,233) = 6.8, p = 0.01, Cohen's d = 0.34), but with a somewhat lower effect size than in the univariate correlation analysis (d = 0.34 versus d = 0.47). Age was not strongly related to F2-isoprostane level (F(1,233) = 1.2, p = 0.27, Cohen's d = 0.14), nor were there any meaningful two-, three-, or four-way interactions (all p's > 0.07, all Cohen's d's < 0.23). Thus, we did not find evidence that BMI was differentially related to F2-isoprostane levels in individuals with schizophrenia compared to HCs, or in men relative to women.
4. Discussion
Consistent with our hypothesis, people with schizophrenia had higher levels of plasma F2-isoprostanes, indicating higher oxidative stress compared to HCs. Our exploratory analysis showed that F2-isoprostane levels were higher in women than in men and associated with higher BMI in both groups. Smoking was not significantly associated with F2-isoprostane levels in either group. The medium-sized effect of group persisted in models that accounted for age, gender, and BMI. F2-isoprostane levels did not correlate significantly with illness duration, symptom severity, or antipsychotic dose in the schizophrenia group.
This report of elevated F2-isoprostane levels in the schizophrenia group is consistent with several previous studies of oxidative stress. However, the literature is inconsistent and heterogeneous, with varied study populations and different markers of oxidative stress. Our use of F2-isoprostanes assayed by GC/NICI-MS is believed to provide an accurate measure of in vivo oxidative stress, and our results are consistent with the one other study of F2-isoprostanes in schizophrenia using a different assay and different body fluid – i.e., urine (Dietrich-Muszalska and Olas, 2009). Our larger age range allowed us to test for age associations (none were found), although the upper age limit was 65 years. Thus, this study broadens the generalizability of F2-isoprostane abnormalities to include young and middle-aged adults with schizophrenia.
We found, consistent with some previous studies (Fukui et al., 2011; Khadir et al., 2015; Peskind et al., 2014; Wiener et al., 2014), that women had higher levels of F2-isoprostanes. Antioxidant effects of estrogen have been demonstrated in cardiovascular and metabolic diseases, which might possibly account for the gender variance (Arias-Loza et al., 2013; Vassalle et al., 2012). The gender differences may also be related to differences in adiposity and inflammation (Joseph et al., 2015; Rossi et al., 2012).
We also found that individuals with higher BMI had more oxidative stress. This has been observed by other investigators in cross-sectional studies (Keaney et al., 2003; Sankhla et al., 2012; Vincent and Taylor, 2006), but the direction of causality is not clear. Obesity may place physiological stress on the body which engenders oxidative stress, or higher oxidative stress levels may promote weight gain. Interestingly, we did not see any evidence that relationships of F2-isoprostanes to gender and BMI were different in the two groups. Gender and BMI did not appear to interact, either, suggesting independent mechanisms of association with oxidative stress.
Although the schizophrenia group had a higher mean BMI than the HC group, this does not appear to entirely account for the findings of elevated F2-isoprostane levels in schizophrenia. The magnitude of the group difference was only slightly reduced (from Cohen's d = 0.5 to d = 0.4) in the model also containing age, gender, BMI, and all possible interactions. Thus, oxidative stress level alterations in schizophrenia, while related to BMI, are not completely explained by this factor. Anti-oxidant treatments such as vitamin C, vitamin E, and dehydroepiandrosterone have been tried without much improvement in symptoms of schizophrenia, though longer trials with broader outcomes including measures of relapse and functioning are needed (Magalhaes et al., 2016).
Cigarette use (either current or duration) was not significantly correlated with F2-isoprostane levels in either group. Cigarette use and oxidative stress have been associated in the general population (Bachi et al., 1996; Morrow et al., 1995). There were relatively low rates of smoking among our HCs, which may be reflective of the geographic location of the study sample. (California boasts one of the lowest smoking rates in the US.) However, our finding of a lack of association between smoking and increased F2-isoprostane levels in schizophrenia patients is similar to that of several other studies which reported no significant association in persons with schizophrenia (Yao et al., 2006; Yao et al., 2004; Yao et al., 2000). Some studies have found elevated oxidative stress in the schizophrenia group despite being matched for smoking with the HC group (Al-Asmari and Khan, 2014; Jorgensen et al., 2013; Padurariu et al., 2010; Sarandol et al., 2007; Yanik et al., 2003; Zhang et al., 2003).
Unlike previous studies using other markers of oxidative stress such as total antioxidant potentials, malondialdehyde, or superoxide dismutase (Mukerjee et al., 1996; Padurariu et al., 2010; Pazvantoglu et al., 2009; Wu et al., 2012), we did not find correlations of F2-isoprostane levels within the schizophrenia group with clinical variables such as illness duration, symptom severity, and antipsychotic dose. Longitudinal studies would be helpful to determine if fluctuations in severity of psychopathology or antipsychotic dose affect F2-isoprostane levels.
F2-isoprostane levels were significantly correlated with hs-CRP levels in both groups. This finding suggests that inflammation and oxidative stress are closely associated.
Several limitations of this study may affect the interpretation of these results. The cross-sectional study data cannot prove causality between oxidative stress and variables such as cigarette smoking and BMI. These findings are based on a sample of relatively stable outpatients with schizophrenia having mild to moderate level of psychopathology, and may not apply to inpatients or more acutely ill people with schizophrenia, treatment-resistant, or treatment-naïve patients. One limitation was the possibility of Type I error due to multiple comparisons and correlations.
Future work should examine how oxidative stress levels in plasma relate to those in the CSF or brain. Examining hormone levels may clarify the source of gender differences seen in F2-isoprostane levels. Longitudinal studies are warranted, as trajectories of change with age in oxidative stress may differ in those with and without schizophrenia, despite lack of cross-sectional interactions with age. Further investigation into the dysregulated oxidative stress system may provide novel targets for intervention. The association between obesity and cigarette use to oxidative stress underlines the clinical importance of lifestyle factors to the physical health of people with schizophrenia (McEvoy et al., 2005). Clinicians should focus on reducing BMI, modifying a sedentary lifestyle and utilizing a smoking cessation program to improve the health of patients with schizophrenia.
Acknowledgments
We thank all of the study participants and staff. We also thank Ginger Milne, who performed the F2-isoprostane assays in the Eicosanoid Laboratory at Vanderbilt University, and Rebecca Daly who helped with data management and analyses at UC San Diego.
Role of Funding Source: This study was supported, in part, by NIH R01MH094151-01 (PI: Dilip V. Jeste, MD), the NIMH T32 Geriatric Mental Health Program MH019934 (PI: Dilip V. Jeste, MD), and by the Stein Institute for Research on Aging at the University of California, San Diego.
Footnotes
Contributors: Ellen E. Lee conducted literature reviews, data interpretation, and manuscript preparation.
Lisa T. Eyler conducted data analyses, data interpretation, and manuscript preparation.
Owen Wolkowitz was involved with study design and manuscript preparation.
Averria Sirkin Martin was involved in data collection and manuscript preparation.
Chase Reuter was involved in data analyses and data interpretation.
Helena Kraemer was involved in data analyses and data interpretation.
Dilip V. Jeste designed the study and was involved in manuscript preparation.
Conflicts of Interest: The authors declare no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdalla DS, Monteiro HP, Oliveira JA, Bechara EJ. Activities of superoxide dismutase and glutathione peroxidase in schizophrenic and manic-depressive patients. Clin Chem. 1986;32(5):805–807. [PubMed] [Google Scholar]
- Akyol O, Herken H, Uz E, Fadillioglu E, Unal S, Sogut S, Ozyurt H, Savas HA. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients. The possible role of oxidant/antioxidant imbalance. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):995–1005. doi: 10.1016/s0278-5846(02)00220-8. [DOI] [PubMed] [Google Scholar]
- Al-Asmari AK, Khan MW. Inflammation and schizophrenia: alterations in cytokine levels and perturbation in antioxidative defense systems. Hum Exp Toxicol. 2014;33(2):115–122. doi: 10.1177/0960327113493305. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60(6):565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- Amann BL, Canales-Rodriguez EJ, Madre M, Radua J, Monte G, Alonso-Lana S, Landin-Romero R, Moreno-Alcazar A, Bonnin CM, Sarro S, Ortiz-Gil J, Gomar JJ, Moro N, Fernandez-Corcuera P, Goikolea JM, Blanch J, Salvador R, Vieta E, McKenna PJ, Pomarol-Clotet E. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2016;133(1):23–33. doi: 10.1111/acps.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39(7):789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- Arias-Loza PA, Muehlfelder M, Pelzer T. Estrogen and estrogen receptors in cardiovascular oxidative stress. Pflugers Arch. 2013;465(5):739–746. doi: 10.1007/s00424-013-1247-7. [DOI] [PubMed] [Google Scholar]
- Bachi A, Zuccato E, Baraldi M, Fanelli R, Chiabrando C. Measurement of urinary 8-Epi-prostaglandin F2alpha, a novel index of lipid peroxidation in vivo, by immunoaffinity extraction/gas chromatography-mass spectrometry. Basal levels in smokers and nonsmokers. Free Radic Biol Med. 1996;20(4):619–624. doi: 10.1016/0891-5849(95)02087-x. [DOI] [PubMed] [Google Scholar]
- Basu S. F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid Redox Signal. 2008;10(8):1405–1434. doi: 10.1089/ars.2007.1956. [DOI] [PubMed] [Google Scholar]
- Callewaert DM, Sloan C. Enzyme immunoassay of isoprostanes. Methods Mol Biol. 2010;610:435–449. doi: 10.1007/978-1-60327-029-8_26. [DOI] [PubMed] [Google Scholar]
- Davi G, Falco A, Patrono C. Determinants of F2-isoprostane biosynthesis and inhibition in man. Chem Phys Lipids. 2004;128(1-2):149–163. doi: 10.1016/j.chemphyslip.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Davies SS, Roberts LJ., 2nd F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic Biol Med. 2011;50(5):559–566. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function Scale (D-KEFS): Examiner's manual. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Dietrich-Muszalska A, Olas B. Isoprostenes as indicators of oxidative stress in schizophrenia. World J Biol Psychiatry. 2009;10(1):27–33. doi: 10.1080/15622970701361263. [DOI] [PubMed] [Google Scholar]
- Dietrich M, Block G, Hudes M, Morrow JD, Norkus EP, Traber MG, Cross CE, Packer L. Antioxidant supplementation decreases lipid peroxidation biomarker F(2)-isoprostanes in plasma of smokers. Cancer Epidemiol Biomarkers Prev. 2002;11(1):7–13. [PubMed] [Google Scholar]
- Eranti SV, MacCabe JH, Bundy H, Murray RM. Gender difference in age at onset of schizophrenia: a meta-analysis. Psychol Med. 2013;43(1):155–167. doi: 10.1017/S003329171200089X. [DOI] [PubMed] [Google Scholar]
- Evans JD, Heaton RK, Paulsen JS, McAdams LA, Heaton SC, Jeste DV. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60(12):874–882. [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Wiliams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: Nov, 2002. [Google Scholar]
- Fukui T, Yamauchi K, Maruyama M, Yasuda T, Kohno M, Abe Y. Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens Res. 2011;34(9):1041–1045. doi: 10.1038/hr.2011.76. [DOI] [PubMed] [Google Scholar]
- Gerner RH, Merrill JE. Cerebrospinal fluid prostaglandin E in depression, mania, and schizophrenia compared to normals. Biol Psychiatry. 1983;18(5):565–569. [PubMed] [Google Scholar]
- Goff DC, Henderson DC, Amico E. Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am J Psychiatry. 1992;149(9):1189–1194. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28(2):17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Herken H, Uz E, Ozyurt H, Sogut S, Virit O, Akyol O. Evidence that the activities of erythrocyte free radical scavenging enzymes and the products of lipid peroxidation are increased in different forms of schizophrenia. Mol Psychiatry. 2001;6(1):66–73. doi: 10.1038/sj.mp.4000789. [DOI] [PubMed] [Google Scholar]
- Hoffer A, Osmond H, Smythies J. Schizophrenia; a new approach. II. Result of a year's research. J Ment Sci. 1954;100(418):29–45. doi: 10.1192/bjp.100.418.29. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143(8):993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophr Bull. 2011;37(3):451–455. doi: 10.1093/schbul/sbr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A, Broedbaek K, Fink-Jensen A, Knorr U, Greisen Soendergaard M, Henriksen T, Weimann A, Jepsen P, Lykkesfeldt J, Poulsen HE, Balslev Jorgensen M. Increased systemic oxidatively generated DNA and RNA damage in schizophrenia. Psychiatry Res. 2013;209(3):417–423. doi: 10.1016/j.psychres.2013.01.033. [DOI] [PubMed] [Google Scholar]
- Joseph J, Depp C, Martin AS, Daly RE, Glorioso DK, Palmer BW, Jeste DV. Associations of high sensitivity C-reactive protein levels in schizophrenia and comparison groups. Schizophr Res. 2015;168(1-2):456–460. doi: 10.1016/j.schres.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiya H. Prostaglandin E1 treatment of schizophrenia. Biol Psychiatry. 1984;19(3):457–463. [PubMed] [Google Scholar]
- Kaiya H, Uematsu M, Ofuji M, Nishida A, Takeuchi K, Nozaki M, Idaka E. Elevated plasma prostaglandin E2 levels in schizophrenia. J Neural Transm. 1989;77(1):39–46. doi: 10.1007/BF01255817. [DOI] [PubMed] [Google Scholar]
- Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ, Framingham S. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- Khadir A, Tiss A, Kavalakatt S, Behbehani K, Dehbi M, Elkum N. Gender-specific association of oxidative stress and inflammation with cardiovascular risk factors in Arab population. Mediators Inflamm. 2015;2015:512603. doi: 10.1155/2015/512603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophr Bull. 2008;34(6):1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Millard SP, Peskind ER, Zhang J, Yu CE, Leverenz JB, Mayer C, Shofer JS, Raskind MA, Quinn JF, Galasko DR, Montine TJ. Cross-sectional and longitudinal relationships between cerebrospinal fluid biomarkers and cognitive function in people without cognitive impairment from across the adult life span. JAMA Neurol. 2014;71(6):742–751. doi: 10.1001/jamaneurol.2014.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindamer LA, Lohr JB, Harris MJ, McAdams LA, Jeste DV. Gender-related clinical differences in older patients with schizophrenia. J Clin Psychiatry. 1999;60(1):61–67. doi: 10.4088/jcp.v60n0114. quiz 68-69. [DOI] [PubMed] [Google Scholar]
- Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Whorton AR, Rubinow DR, Cowdry RW, Ninan PT, Waters RN. CSF prostaglandin levels in depressed and schizophrenic patients. Arch Gen Psychiatry. 1983;40(4):405–406. doi: 10.1001/archpsyc.1983.01790040059008. [DOI] [PubMed] [Google Scholar]
- Magalhaes PV, Dean O, Andreazza AC, Berk M, Kapczinski F. Antioxidant treatments for schizophrenia. Cochrane Database Syst Rev. 2016;2:CD008919. doi: 10.1002/14651858.CD008919.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathe AA, Sedvall G, Wiesel FA, Nyback H. Increased content of immunoreactive prostaglandin E in cerebrospinal fluid of patients with schizophrenia. Lancet. 1980;1(8158):16–18. doi: 10.1016/s0140-6736(80)90553-x. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, Meltzer HY, Hsiao J, Scott Stroup T, Lieberman JA. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Meagher EA, Barry OP, Burke A, Lucey MR, Lawson JA, Rokach J, FitzGerald GA. Alcohol-induced generation of lipid peroxidation products in humans. J Clin Invest. 1999;104(6):805–813. doi: 10.1172/JCI5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Nasrallah HA. Medical Illness and Schizophrenia. 2nd. American Psychiatric Publishing, Inc.; Arlington, VA: 2009. [Google Scholar]
- Milne GL, Dai Q, Roberts LJ., 2nd The isoprostanes--25 years later. Biochim Biophys Acta. 2015;1851(4):433–445. doi: 10.1016/j.bbalip.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2(1):221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18(15):1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332(18):1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36(1):1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes as indicators of oxidant stress. Methods Mol Biol. 2002;186:57–66. doi: 10.1385/1-59259-173-6:57. [DOI] [PubMed] [Google Scholar]
- Mukerjee S, Mahadik SP, Scheffer R, Correnti EE, Kelkar H. Impaired antioxidant defense at the onset of psychosis. Schizophr Res. 1996;19(1):19–26. doi: 10.1016/0920-9964(95)00048-8. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Harvey PD, Casey D, Csoboth CT, Hudson JI, Julian L, Lentz E, Nuechterlein KH, Perkins DO, Kotowsky N, Skale TG, Snowden LR, Tandon R, Tek C, Velligan D, Vinogradov S, O'Gorman C. The Management of Schizophrenia in Clinical Practice (MOSAIC) Registry: a focus on patients, caregivers, illness severity, functional status, disease burden and healthcare utilization. Schizophr Res. 2015;166(1-3):69–79. doi: 10.1016/j.schres.2015.04.031. [DOI] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Padurariu M, Ciobica A, Dobrin I, Stefanescu C. Evaluation of antioxidant enzymes activities and lipid peroxidation in schizophrenic patients treated with typical and atypical antipsychotics. Neurosci Lett. 2010;479(3):317–320. doi: 10.1016/j.neulet.2010.05.088. [DOI] [PubMed] [Google Scholar]
- Patrono C, FitzGerald GA. Isoprostanes: potential markers of oxidant stress in atherothrombotic disease. Arterioscler Thromb Vasc Biol. 1997;17(11):2309–2315. doi: 10.1161/01.atv.17.11.2309. [DOI] [PubMed] [Google Scholar]
- Pazvantoglu O, Selek S, Okay IT, Sengul C, Karabekiroglu K, Dilbaz N, Erel O. Oxidative mechanisms in schizophrenia and their relationship with illness subtype and symptom profile. Psychiatry Clin Neurosci. 2009;63(5):693–700. doi: 10.1111/j.1440-1819.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Li G, Shofer JB, Millard SP, Leverenz JB, Yu CE, Raskind MA, Quinn JF, Galasko DR, Montine TJ. Influence of lifestyle modifications on age-related free radical injury to brain. JAMA Neurol. 2014;71(9):1150–1154. doi: 10.1001/jamaneurol.2014.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R, Sahebarao MP, Mukherjee S, Murthy JN. Enzymes of the antioxidant defense system in chronic schizophrenic patients. Biol Psychiatry. 1991;30(4):409–412. doi: 10.1016/0006-3223(91)90298-z. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, 2nd, Reckelhoff JF. Measurement of F(2)-isoprostanes unveils profound oxidative stress in aged rats. Biochem Biophys Res Commun. 2001;287(1):254–256. doi: 10.1006/bbrc.2001.5583. [DOI] [PubMed] [Google Scholar]
- Rossi IA, Bochud M, Bovet P, Paccaud F, Waeber G, Vollenweider P, Taffe P. Sex difference and the role of leptin in the association between high-sensitivity C-reactive protein and adiposity in two different populations. Eur J Epidemiol. 2012;27(5):379–384. doi: 10.1007/s10654-012-9671-0. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Sankhla M, Sharma TK, Mathur K, Rathor JS, Butolia V, Gadhok AK, Vardey SK, Sinha M, Kaushik GG. Relationship of oxidative stress with obesity and its role in obesity induced metabolic syndrome. Clin Lab. 2012;58(5-6):385–392. [PubMed] [Google Scholar]
- Sarandol A, Kirli S, Akkaya C, Altin A, Demirci M, Sarandol E. Oxidative-antioxidative systems and their relation with serum S100 B levels in patients with schizophrenia: effects of short term antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(6):1164–1169. doi: 10.1016/j.pnpbp.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Stephens JW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202(2):321–329. doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- van den Berg E, Ruis C, Biessels GJ, Kappelle LJ, van Zandvoort MJ. The Telephone Interview for Cognitive Status (Modified): relation with a comprehensive neuropsychological assessment. J Clin Exp Neuropsychol. 2012;34(6):598–605. doi: 10.1080/13803395.2012.667066. [DOI] [PubMed] [Google Scholar]
- Vassalle C, Simoncini T, Chedraui P, Perez-Lopez FR. Why sex matters: the biological mechanisms of cardiovascular disease. Gynecol Endocrinol. 2012;28(9):746–751. doi: 10.3109/09513590.2011.652720. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30(3):400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- WHO. The World Health Report: Mental Health: New understanding, new hope. World Health Organization; Geneva: 2001. [Google Scholar]
- Wiener C, Rassier GT, Kaster MP, Jansen K, Pinheiro RT, Klamt F, Magalhaes PV, Kapczinski F, Ghisleni G, da Silva RA. Gender-based differences in oxidative stress parameters do not underlie the differences in mood disorders susceptibility between sexes. Eur Psychiatry. 2014;29(1):58–63. doi: 10.1016/j.eurpsy.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Wu Z, Zhang XY, Wang H, Tang W, Xia Y, Zhang F, Liu J, Fu Y, Hu J, Chen Y, Liu L, Chen da C, Xiu MH, Kosten TR, He J. Elevated plasma superoxide dismutase in first-episode and drug naive patients with schizophrenia: inverse association with positive symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36(1):34–38. doi: 10.1016/j.pnpbp.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Yanik M, Vural H, Kocyigit A, Tutkun H, Zoroglu SS, Herken H, Savas HA, Koylu A, Akyol O. Is the arginine-nitric oxide pathway involved in the pathogenesis of schizophrenia? Neuropsychobiology. 2003;47(2):61–65. doi: 10.1159/000070010. [DOI] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22(1-2):83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy RD. Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr Bull. 2004;30(4):923–934. doi: 10.1093/oxfordjournals.schbul.a007142. [DOI] [PubMed] [Google Scholar]
- Yao JK, Reddy R, van Kammen DP. Abnormal age-related changes of plasma antioxidant proteins in schizophrenia. Psychiatry Res. 2000;97(2-3):137–151. doi: 10.1016/s0165-1781(00)00230-4. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Chen da C, Xiu MH, Wang F, Qi LY, Sun HQ, Chen S, He SC, Wu GY, Haile CN, Kosten TA, Lu L, Kosten TR. The novel oxidative stress marker thioredoxin is increased in first-episode schizophrenic patients. Schizophr Res. 2009;113(2-3):151–157. doi: 10.1016/j.schres.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY. Elevated blood superoxide dismutase in neuroleptic-free schizophrenia: association with positive symptoms. Psychiatry Res. 2003;117(1):85–88. doi: 10.1016/s0165-1781(02)00303-7. [DOI] [PubMed] [Google Scholar]


