Abstract
Objectives
To determine the prevalence, risk factors, treatments, and outcomes of bloodstream infections (BSIs) due to carbapenem-resistant Enterobacteriaceae (CRE) in adult neutropenic patients with hematologic malignancies.
Methods
We reviewed all BSIs between 2008–2012 in this population at two New York City oncology centers. A case-control study was conducted to identify CRE BSI risk factors, using three controls of non-CRE BSIs per case.
Results
CRE caused 43 (2.2%) of 1,992 BSIs overall and 4.7% of Gram-negative bacteremias. Independent risk factors for CRE BSI were prior β-lactam/β-lactamase inhibitor (adjusted odds ratio [aOR] 3.2; P=0.03) or carbapenem (aOR 3.0; P=0.05) use, current trimethoprim-sulfamethoxazole (aOR 24; P=0.001) or glucocorticoid (aOR 5.4, P=0.004) use, and having a prior CRE culture (aOR 12; P=0.03). Patients with CRE bacteremia had a median of 52 hours from culture collection until receipt of active therapy. They had a 51% BSI-related mortality rate, with a median of 4 days from bacteremia onset until death. CRE-active empirical therapy was associated with a lower 30-day mortality rate (17% vs. 59%; P=0.08).
Conclusions
CRE are lethal emerging causes of bacteremia in neutropenic patients. New strategies are needed to shorten the delay in administration of CRE-active agents and improve outcomes in this vulnerable population.
Keywords: carbapenem-resistant Enterobacteriaceae, neutropenic patients, hematologic malignancies, bacteremia, prevalence, risk factors, outcomes
INTRODUCTION
Neutropenic patients with hematologic malignancies are uniquely threatened by multidrug-resistant Gram-negative bacteria because they rely on immediate bactericidal therapy to combat Gram-negative infections. Enterobacteriaceae are the most common causes of Gram-negative bacteremia in this population and have historically been susceptible to recommended β-lactam agents for the treatment of fever and neutropenia [1, 2]. However, over the last decade, carbapenem-resistant Enterobacteriaceae (CRE) have emerged worldwide as lethal pathogens that are typically resistant to all β-lactam agents due to production of β-lactam-hydrolyzing enzymes such as Klebsiella pneumoniae carbapenemase (KPC) [3]. Given that currently recommended empirical therapies are inactive against CRE, and identification of CRE by culture typically takes 2–3 days, the spread of CRE into neutropenic patients with hematologic malignancies could have devastating consequences because effective therapy would be delayed [4].
We recently reported 18 patients with hematologic malignancies who developed bacteremia due to CRE [5]. Nine of 13 neutropenic patients in this study died, there were long delays until receipt of active therapy, and all deaths were related to CRE bacteremia. Given these preliminary findings, we sought to better understand the epidemiology of CRE in this patient population in an area where CRE are endemic nosocomial pathogens. Thus, we conducted a study at two large oncology centers in New York City, USA, a global epicenter for CRE, to determine the prevalence, risk factors, and outcomes of CRE bacteremia in neutropenic patients with hematologic malignancies.
METHODS
Study design
The study was approved by the Institutional Review Boards of Weill Cornell Medicine and Memorial Sloan Kettering Cancer Center (MSKCC). We first identified all episodes of bloodstream infection (BSI) in adult (age ≥18 years) neutropenic patients (absolute neutrophil count ≤500 cells/mm3) with hematologic malignancies at New York-Presbyterian Hospital/Weill Cornell Medical Center and MSKCC from 2008–2012. BSIs where the patient had a prior positive blood culture for the same organism(s) within the previous 30 days were excluded. Common skin commensals were designated causes of BSI if isolated from ≥2 blood culture sets from the same or consecutive days [6]. From this cohort, we determined the proportion of BSIs, Gram-negative bacteremias, and Enterobacteriaceae bacteremias that were due to CRE.
We performed two case-control analyses to identify risk factors for CRE bacteremia. In the primary analysis, we compared CRE cases to controls of BSIs due to pathogens other than carbapenem-resistant Gram-negative bacteria. In the secondary risk factor analysis, we designated BSI episodes due to carbapenem-susceptible Gram-negative bacteria as controls. For both control groups, we randomly selected three controls per case, matched by study center and year. Surveillance for detection of CRE colonization was not routinely performed at either study center.
We abstracted the following clinical data for cases and controls: demographics, comorbidities [7], malignancy characteristics, healthcare exposures, prior infections, presence of sepsis [8], Pitt bacteremia score [9], antimicrobial therapies, time until receipt of an active agent (an agent to which the bloodstream isolate tested susceptible in vitro), 30-day mortality from BSI onset, and BSI-related mortality (death in a patient with ongoing bacteremia from the initial pathogen or who never recovered from septic shock associated with the BSI episode). Lastly, we reviewed episodes of monomicrobial bacteremia included in the case-control analyses and compared the 30-day survival by pathogen type.
Microbiologic methods
Species identification and antimicrobial susceptibility testing were performed by Vitek II (bioMérieux, Durham, NC, USA) or Microscan (Beckman Coulter, Brea, CA, USA). Carbapenem resistance was defined as resistance to any carbapenem based on updated Clinical and Laboratory Standards Institute interpretive breakpoints [10]. Extended-spectrum-β-lactamase (ESBL)/AmpC-producing Enterobacteriaceae were defined as being ceftriaxone-resistant and meropenem-susceptible, or testing positive for ESBL production [10].
For available isolates, we performed broth microdilution testing using TREK panels (Thermo Fisher Scientific, Oakwood Village, OH, USA) and Etest (bioMérieux) for susceptibility to ceftazidime-avibactam. These results were used instead of automated susceptibility testing results, when available. CRE with polymyxin B or tigecycline minimum inhibitory concentrations (MICs) ≤2 μg/mL were considered susceptible to these agents.
In order to characterize the genetic basis of carbapenem resistance, we used a multiplexed PCR on available isolates to detect KPC, NDM, VIM, IMP, OXA-48-type, and CTX-M β-lactamase genes, followed by gene sequencing of positive results [11–13]. For carbapenem-resistant Klebsiella pneumoniae isolates, we also sequenced outer membrane porin genes [14].
Statistical analysis
Categorical variables were compared using chi-square or Fisher’s exact tests and continuous variables were compared using the Wilcoxon rank-sum test. P values ≤0.05 (two tailed) were considered statistically significant. For both case-control analyses, we constructed multivariable conditional logistic regression models to identify independent risk factors for CRE bacteremia. Thirty-day mortality for monomicrobial BSIs were compared by pathogen type using Kaplan-Meier survival curves and the log-rank test. We then constructed a multivariable Cox proportional hazards model to assess the association between pathogen and mortality. All variables with P≤0.05 in univariate analyses were entered into the multivariable models and a backward stepwise selection process was applied until only variables with P≤0.1 were retained in the final models. STATA, version 12.0 (StataCorp, College Station, TX) was used for statistical analyses.
RESULTS
Prevalence and characteristics of CRE
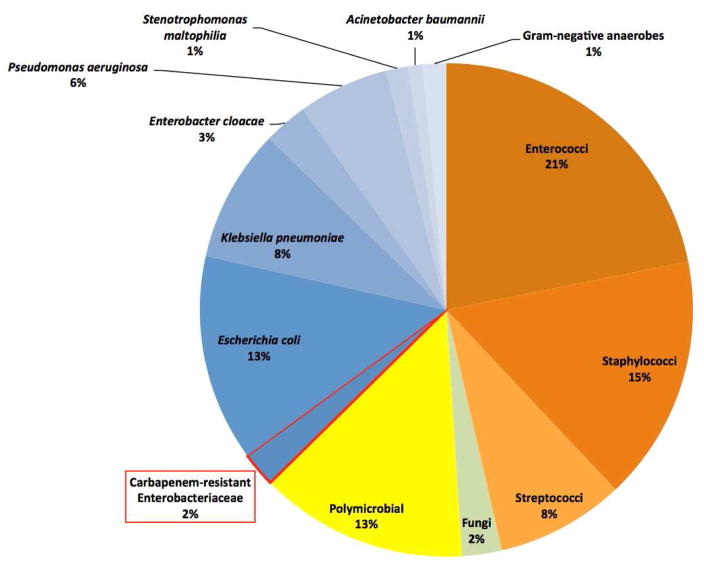
We identified 1,992 BSI episodes in neutropenic patients with hematologic malignancies during the study period, of which 43 (2.2%; 95% confidence interval [CI]: 1.6%-2.9%) were caused by CRE (Figure 1). The proportion of BSIs due to CRE at each hospital was 2.4% and 1.9%, respectively, and varied from 0.8%-3.0% by study year, with no clear trend over time. CRE caused 4.7% of bacteremias that included a Gram-negative bacterium and 6.5% of bacteremias that included an Enterobacteriaceae. Eighteen percent of Klebsiella pneumoniae bacteremias were carbapenem-resistant. Hospital-wide data were only available from 2011–2012, and during these years the proportion of BSIs due to CRE was greater in neutropenic patients with hematologic malignancies (1.8%) than in other types of hospitalized patients (0.7%; P=0.003).
Figure 1.
Microbial characteristics of 1,992 bloodstream infections in adult neutropenic patients from 2008–2012.
Forty-four CRE isolates caused 43 CRE bacteremia episodes. Klebsiella pneumoniae was the most common species (n=30; 68%), followed by Enterobacter cloacae (n=8; 18%), Escherichia coli (n=2; 5%), and Enterobacter aerogenes, Enterobacter gergoviae, Klebsiella oxytoca, and Serratia marcescens (n=1). Eleven (26%) CRE bacteremia episodes were polymicrobial. No isolates were susceptible to piperacillin-tazobactam, aztreonam, ceftriaxone, ceftazidime, or ertapenem. Other antimicrobial susceptibilities were as follows: tigecycline (86% susceptible), polymyxin B (82%), amikacin and gentamicin (48%), ciprofloxacin and levofloxacin (23%), cefepime (18%), meropenem (12%), imipenem (7%), and tobramycin (7%).
Twenty-three isolates were available for additional testing, including 15 K. pneumoniae and four Enterobacter cloacae strains. All tested susceptible to ceftazidime-avibactam (MIC ≤4/4 μg/mL). BlaKPC was identified in 21 (91%) of these isolates (16: KPC-3; 5: KPC-2). No other carbapenemase genes were detected. Two imipenem- and meropenem-resistant Klebsiella pneumoniae isolates lacked a carbapenemase, but possessed blaCTX-M-15 and mutations in a K. pneumoniae outer membrane porin gene.
Risk factors for CRE bacteremia
The following factors were associated with CRE bacteremia in univariate analysis using the primary control group of BSI episodes caused by pathogens other than carbapenem-resistant Gram-negative bacteria (Table 1): allogeneic hematopoietic stem cell transplantation, concurrent solid tumor, recent or current ICU admission, prior antimicrobial exposures, prior CRE isolate or BSI during neutropenia, presence of a urinary catheter, receipt of mechanical ventilation or glucocorticoids, septic shock, diarrhea, and hypoalbuminemia. In multivariable analysis (Table 2), factors independently associated with CRE bacteremia were use of a β-lactam/β-lactamase inhibitor (BLBLI, adjusted odds ratio [aOR] 3.2, P=0.03) or carbapenem (aOR 3.0, P=0.05) within the previous 30 days, receipt of trimethoprim-sulfamethoxazole (TMP-SMX, aOR 24, P=0.001) or glucocorticoids (aOR 5.4, P=0.004) at BSI onset, and having a CRE isolate within the previous 90 days (aOR 12, P=0.03). These variables were also independent risk factors for CRE bacteremia using the secondary control group of BSIs due to carbapenem-susceptible Gram-negative bacteria (Table 2).
Table 1.
Comparison of cases of CRE bacteremia to controls.
| Characteristic | Cases (n=43) | Primary control group1 (n=129) | P2 | Secondary control group3 (n=129) | P4 |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 57 (45–66) | 60 (48–66) | 0.38 | 61 (49–69) | 0.06 |
| Male gender | 18 (42) | 72 (56) | 0.11 | 76 (59) | 0.05 |
| Underlying disease | |||||
| Acute myeloid leukemia | 24 (56) | 74 (57) | 0.86 | 80 (62) | 0.47 |
| Acute lymphoid leukemia | 8 (19) | 21 (16) | 0.72 | 14 (11) | 0.19 |
| Lymphoma | 8 (19) | 20 (16) | 0.63 | 18 (14) | 0.47 |
| Other | 3 (7) | 14 (11) | 0.46 | 18 (14) | 0.29 |
| HSCT recipients | |||||
| Allogeneic | 15 (35) | 24 (19) | 0.03 | 28 (22) | 0.08 |
| Autologous | 3 (7) | 10 (8) | 1.00 | 13 (10) | 0.76 |
| Comorbidities | |||||
| Charlson comorbidity index score | 2 (2–3) | 2 (2–3) | 0.77 | 2 (2–3) | 0.53 |
| Myocardial infarction | 1 (2) | 12 (9) | 0.19 | 11 (9) | 0.30 |
| Congestive heart failure | 4 (9) | 8 (6) | 0.50 | 10 (8) | 0.75 |
| Cerebrovascular disease | 0 | 2 (2) | 1.00 | 4 (3) | 0.57 |
| Chronic pulmonary disease | 2 (5) | 4 (3) | 0.64 | 9 (7) | 0.73 |
| Peptic ulcer disease | 1 (2) | 2 (2) | 1.00 | 5 (4) | 1.00 |
| Liver disease | 2 (5) | 4 (3) | 0.64 | 1 (1) | 0.16 |
| Diabetes | 5 (12) | 17 (13) | 0.79 | 10 (8) | 0.53 |
| Renal disease | 7 (16) | 9 (7) | 0.12 | 3 (2) | 0.003 |
| Solid tumor | 0 | 13 (10) | 0.04 | 9 (7) | 0.11 |
| HIV infection | 0 | 0 | 1.00 | 1 (1) | 1.00 |
| Healthcare exposures within 30 days of BSI | |||||
| ICU | 16 (37) | 12 (9) | <0.001 | 6 (5) | <0.001 |
| Outside hospital | 5 (12) | 11 (9) | 0.55 | 10 (8) | 0.53 |
| Long-term care facility | 1 (2) | 4 (3) | 1.00 | 0 | 0.25 |
| Chemotherapy | 37 (86) | 122 (95) | 0.07 | 117 (91) | 0.40 |
| Radiation therapy | 3 (7) | 7 (5) | 0.71 | 10 (8) | 1.00 |
| Surgery | 4 (9) | 3 (2) | 0.07 | 2 (2) | 0.04 |
| Antibiotic therapy | |||||
| Any antibiotic | 40 (93) | 96 (74) | 0.009 | 82 (64) | <0.001 |
| Use at time of BSI | 38 (88) | 71 (55) | <0.001 | 45 (35) | <0.001 |
| Penicillin without BLI | 1 (2) | 6 (5) | 0.68 | 6 (5) | 0.68 |
| Use at time of BSI | 1 (2) | 4 (3) | 1.00 | 3 (2) | 1.00 |
| β-lactam/BLI | 14 (33) | 15 (12) | 0.002 | 9 (7) | <0.001 |
| Use at time of BSI | 8 (19) | 9 (7) | 0.03 | 7 (5) | 0.01 |
| Cephalosporin | 18 (42) | 42 (33) | 0.27 | 31 (24) | 0.03 |
| Use at time of BSI | 6 (14) | 16 (12) | 0.79 | 3 (2) | 0.003 |
| Carbapenem | 17 (40) | 20 (16) | 0.001 | 21 (16) | 0.001 |
| Use at time of BSI | 7 (16) | 8 (6) | 0.06 | 0 | <0.001 |
| Fluoroquinolone | 14 (33) | 47 (36) | 0.65 | 21 (16) | 0.02 |
| Use at time of BSI | 12 (28) | 26 (20) | 0.29 | 10 (8) | 0.001 |
| Aminoglycoside | 5 (12) | 7 (5) | 0.18 | 4 (3) | 0.04 |
| Use at time of BSI | 2 (5) | 5 (4) | 1.00 | 0 | 0.06 |
| Vancomycin (IV) | 19 (44) | 29 (22) | 0.006 | 34 (26) | 0.028 |
| Use at time of BSI | 9 (21) | 17 (13) | 0.22 | 14 (11) | 0.09 |
| Vancomycin (oral) | 5 (12) | 7 (5) | 0.18 | 6 (5) | 0.15 |
| Use at time of BSI | 4 (9) | 3 (2) | 0.07 | 3 (2) | 0.07 |
| Linezolid | 10 (23) | 7 (5) | 0.002 | 12 (9) | 0.018 |
| Use at time of BSI | 5 (12) | 4 (3) | 0.044 | 8 (6) | 0.32 |
| Metronidazole | 8 (19) | 15 (12) | 0.24 | 13 (10) | 0.14 |
| Use at time of BSI | 3 (7) | 12 (9) | 0.76 | 8 (6) | 1.00 |
| TMP-SMX | 11 (26) | 6 (5) | <0.001 | 7 (5) | <0.001 |
| Use at time of BSI | 10 (23)5 | 2 (2) | <0.001 | 2 (2) | <0.001 |
| Prior infections | |||||
| Clostridium difficile infection within last 30 days | 9 (21) | 13 (10) | 0.07 | 14 (11) | 0.12 |
| Prior CRE bacteremia within last 90 days | 3 (7) | 0 | 0.02 | 0 | 0.02 |
| Prior CRE isolate at any site within last 90 days | 6 (14) | 1 (1) | 0.001 | 0 | <0.001 |
| Prior BSI during episode of neutropenia | 18 (42) | 28 (22) | 0.01 | 25 (19) | 0.003 |
| Characteristics at time of BSI | |||||
| Central venous catheter | 33 (77) | 117 (91) | 0.02 | 113 (88) | 0.09 |
| Mechanical ventilation | 5 (12) | 4 (3) | 0.04 | 2 (2) | 0.01 |
| Receiving TPN | 6 (14) | 7 (5) | 0.09 | 6 (5) | 0.08 |
| Receiving enteric tube feeds | 3 (7) | 2 (2) | 0.10 | 0 | 0.02 |
| Urinary catheter | 13 (30) | 18 (14) | 0.02 | 8 (6) | <0.001 |
| Patient location | |||||
| Outpatient | 5 (12) | 22 (17) | 0.40 | 17 (13) | 0.79 |
| Inpatient, non-ICU | 30 (70) | 102 (79) | 0.21 | 110 (85) | 0.02 |
| ICU | 8 (19) | 5 (4) | 0.004 | 2 (2) | <0.001 |
| Receiving glucocorticoids | 17 (40) | 9 (7) | <0.001 | 14 (11) | <0.001 |
| Receiving calcineurin or mTOR inhibitor | 6 (14) | 9 (7) | 0.21 | 9 (7) | 0.21 |
| Serum creatinine (mg/dL) | 1.0 (0.6–2.0) | 0.9 (0.7–1.1) | 0.38 | 0.8 (0.7–1.1) | 0.30 |
| Serum albumin (g/dL) | 2.6 (2.1–3.1) | 3.0 (2.4–3.5) | 0.02 | 3.0 (2.6–3.4) | 0.002 |
| Mucositis | 8 (19) | 33 (26) | 0.35 | 27 (21) | 0.74 |
| Diarrhea | 16 (37) | 25 (19) | 0.02 | 33 (26) | 0.17 |
| Duration of hospitalization | 15 (8–30) | 12 (3–18) | 0.10 | 11 (3–17) | 0.08 |
| Duration of neutropenia | 10 (4–26) | 8 (3–28) | 0.51 | 6 (2–24) | 0.09 |
| Presentation of BSI | |||||
| Sepsis category | |||||
| None | 2 (5) | 10 (8) | 0.73 | 1 (1) | 0.16 |
| Sepsis | 18 (42) | 65 (50) | 0.33 | 72 (56) | 0.11 |
| Severe sepsis | 15 (35) | 45 (35) | 1.00 | 46 (36) | 0.93 |
| Septic shock | 8 (19) | 9 (7) | 0.04 | 10 (8) | 0.08 |
| Fever | 36 (84) | 105 (81) | 0.73 | 120 (93) | 0.12 |
| Pitt bacteremia score | 2 (0–3) | 1 (0–3) | 0.17 | 1 (0–3) | 0.38 |
| Polymicrobial | 11 (26) | 19 (15) | 0.10 | 25 (19) | 0.39 |
| Treatments | |||||
| Empirical (within 12h) antibacterial regimen active against BSI pathogen(s) | 6 (14) | 72 (56) | <0.001 | 114 (88) | <0.001 |
| Hours until receipt of active therapy | 52 (32–69)6 | 5 (0–21) | <0.001 | 0 (0–2) | <0.001 |
| Outcomes | |||||
| 30-day mortality | 23 (53) | 29 (22) | <0.001 | 24 (19) | <0.001 |
| Mortality during index hospitalization | 23 (53) | 41 (32) | 0.01 | 26 (20) | <0.001 |
| BSI-related mortality | 22 (51) | 19 (15) | <0.001 | 8 (6) | <0.001 |
Values are expressed in N (%) and median (interquartile range).
Abbreviations: BLI, β-lactamase inhibitor; BSI, bloodstream infection; CRE, carbapenem-resistant Enterobacteriaceae; HIV, human immunodeficiency virus; HSCT, hematopoietic stem cell transplant; ICU, intensive care unit; IV, intravenous; mTOR, mammalian target of rapamycin; TPN, total parenteral nutrition; TMP-SMX, trimethoprim-sulfamethoxazole.
Primary controls (BSIs due to pathogens other than carbapenem-resistant Gram-negative bacteria) were matched to cases by study site and year. This control group consisted of 110 monomicrobial BSIs, including Enterococcus faecium (16%), Escherichia coli (14%), Staphylococcus aureus and Klebsiella pneumoniae (10%, each), Pseudomonas aeruginosa (9%), and viridans group streptococci and Enterobacter spp. (7%, each). Nineteen BSIs in this group were polymicrobial.
P value comparing cases to the primary control group. P values <0.05 are bolded.
Secondary controls (BSI due to carbapenem-susceptible Gram-negative bacteria) consisted of 105 monomicrobial Gram-negative bacteremias, including Escherichia coli (25%), Klebsiella pneumoniae (23%), Enterobacter spp. (16%), and Pseudomonas aeruginosa (11%). Twenty-four BSIs in this group were polymicrobial.
P value comparing cases to the secondary control group.
Nine of the 10 patients were receiving TMP-SMX for prophylaxis against Pneumocystis jiroveci.
Nine of the 43 cases never received active therapy because they died prior to the availability of antimicrobial susceptibility testing results.
Table 2.
Multivariable analysis of factors associated with CRE bacteremia in neutropenic patients compared to controls.
| Variable | Adjusted OR using primary control group (95% CI)1, 2 | P | Adjusted OR using secondary control group (95% CI)3 | P |
|---|---|---|---|---|
| β-lactam/β-lactamase inhibitor within previous 30 days | 3.2 (1.1–9.3) | 0.03 | 5.0 (1.2–22) | 0.03 |
| Carbapenem within previous 30 days | 3.0 (1.0–9.2) | 0.05 | 7.0 (1.9–26) | 0.004 |
| Receiving TMP-SMX at BSI onset | 24 (3.8–151) | 0.001 | 33 (4.2–256) | 0.001 |
| Receiving glucocorticoids at BSI onset | 5.4 (1.7–17) | 0.004 | 4.9 (1.4–17) | 0.01 |
| Prior CRE at any site within the previous 90 days | 12 (1.2–113) | 0.03 | Unable to include4 | N/A |
| ICU stay within previous 30 days | 2.7 (0.9–7.9) | 0.07 | 11 (2.8–42) | 0.001 |
| Receiving cephalosporin at BSI onset | Not significant | N/A | 7.7 (1.1–54) | 0.04 |
| Receiving fluoroquinolone at BSI onset | Not significant | N/A | 6.4 (1.5–27) | 0.01 |
| Renal disease5 | Not significant | N/A | 21.0 (3.9–111) | <0.001 |
Abbreviations: BSI, bloodstream infection; CI, confidence interval; CRE, carbapenem-resistant Enterobacteriaceae; ICU, intensive care unit; OR, odds ratio; TMP-SMX, trimethoprim-sulfamethoxazole.
The primary control group consisted of BSIs due to pathogens other than carbapenem-resistant Gram-negative bacteria.
The C statistic for this multivariable model was 0.83.
The secondary control group consisted of BSIs due to carbapenem-susceptible Gram-negative bacteria.
This variable could not be entered into the multivariate model because no episodes in the control group had a prior CRE at any site.
Renal disease was defined as having a serum creatinine > 3 mg/dL, receiving dialysis, or history of a kidney transplant [7].
Treatments and outcomes
Active empirical antibacterial therapy was administered within 12 hours of BSI onset in 14% of CRE bacteremia episodes, compared to 56% and 88% in controls, respectively (P<0.001, Table 1). Nine (21%) of 43 patients with CRE bacteremia never received active therapy because they died prior to the availability of susceptibility testing results. In the remaining patients, there was a median of 52 hours (interquartile range [IQR] 33–69) from BSI onset until receipt of active therapy.
Thirty-day mortality was 53% in cases of CRE bacteremia, compared to 22% and 19%, respectively, in the control groups (P<0.001, Table 1). BSI-related mortality was also higher in cases of CRE bacteremia (51%) than in either of the control groups (15% and 6%, P<0.001). There was a median of four days (IQR: 2–8) from CRE bacteremia onset until death. Only one of six patients (17%) with CRE bacteremia who received active empirical therapy died within 30 days, compared to 22 of 37 patients (59%) who did not receive active empirical therapy (P=0.08; Table 3). Definitive antimicrobial regimens and associated mortality rates are outlined in Table 3.
Table 3.
Treatments and 30-day mortality rates of 43 episodes of CRE bacteremia
| Variable | N (% of total) | 30-day mortality (%) |
|---|---|---|
| All episodes of CRE bacteremia | 43 (100) | 23/43 (53) |
| Empirical therapy within 12 hours of BSI onset | ||
| Active empirical therapy1 | 6 (14) | 1/6 (17) |
| Inactive empirical therapy | 37 (86) | 22/37 (59) |
| Definitive antimicrobial regimen2,3 | ||
| Died before ever received active therapy | 9 (21) | 9/9 |
| Single Gram-negative agent | 10 (23) | 4/10 |
| Aminoglycoside monotherapy | 5 (12) | 4/5 |
| Fluoroquinolone monotherapy | 3 (7) | 0/3 |
| Two Gram-negative agents | 16 (37) | 6/16 |
| Polymyxin B and tigecycline | 5 (12) | 2/5 |
| Aminoglycoside and tigecycline | 3 (7) | 0/3 |
| Aminoglycoside and carbapenem | 3 (7) | 3/3 |
| Three Gram-negative agents | 8 (19) | 4/8 |
| Polymyxin B, aminoglycoside and carbapenem | 3 (7) | 2/3 |
| Bacteremia characteristics | ||
| Polymicrobial | 11 (26) | 9/11 (82) |
| Monomicrobial | 32 (74) | 14/32 (44) |
| Klebsiella pneumoniae | 21 (49) | 11/21 (52) |
| Other types of CRE | 11 (26) | 3/11 (27) |
Active therapy was defined as receipt of an antimicrobial agent to which the bloodstream isolate tested susceptible in vitro.
Definitive therapy was defined as antimicrobial agents administered on the day after final antimicrobial susceptibility testing results were reported.
30-day mortality represented as # of patients who died within 30 days / total # of patients.
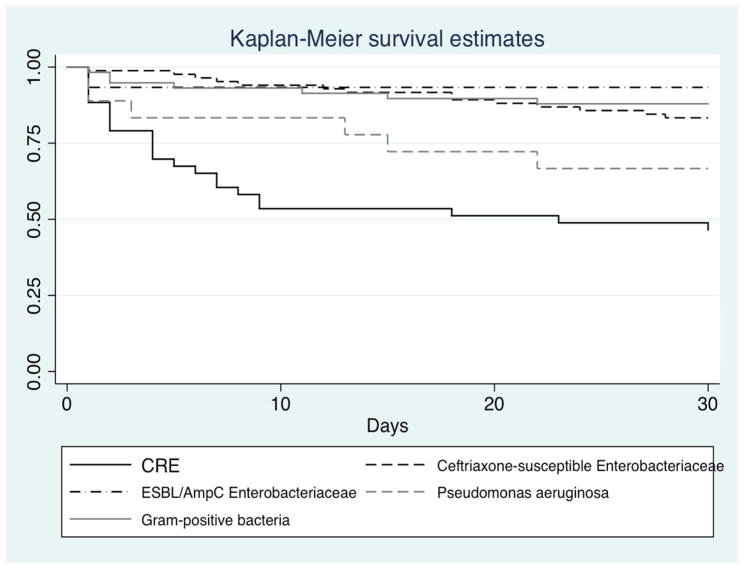
Monomicrobial BSI episodes in the control groups included BSIs due to ceftriaxone-susceptible Enterobacteriaceae (n=84), ESBL/AmpC-producing Enterobacteriaceae (n=15), Pseudomonas aeruginosa (n=18), and Gram-positive bacteria (total: n=59; Enterococcus faecium: n=19; Staphylococcus aureus: n=11; coagulase-negative staphylococci: n=10; viridans group streptococci: n=8). Thirty-day mortality rates by pathogen were 17% for ceftriaxone-susceptible Enterobacteriaceae, 7% for ESBL/AmpC-producing Enterobacteriaceae, 33% for Pseudomonas aeruginosa, and 14% for Gram-positive bacteria. Survival analysis demonstrated increased mortality with CRE bacteremia compared to Gram-positive bacteremia and bacteremia due to ceftriaxone-susceptible and ESBL/AmpC-producing Enterobacteriaceae and a trend towards increased mortality compared to P. aeruginosa bacteremia (Figure 2). In a multivariable analysis that adjusted for age, Pitt Bacteremia score, patient location, serum albumin, and duration of hospitalization at BSI onset, CRE bacteremia was independently associated with increased mortality (adjusted hazard ratio 2.9, 95% CI 1.3–6.4, P=0.01) compared to bacteremia due to ceftriaxone-susceptible Enterobacteriaceae.
Figure 2.
30-day survival after onset of monomicrobial BSI, by pathogen type. CRE (n=32) vs. ceftriaxone-susceptible Enterobacteriaceae (n=84; P<0.001); vs. ESBL/AmpC-producing Enterobacteriaceae (n=15; P=0.004); vs. Pseudomonas aeruginosa (n=18; P=0.16); vs. Gram-positive bacteria (n=59, P<0.001).
DISCUSSION
To our knowledge, this is the first reported study to assess the prevalence, risk factors, and outcomes of CRE bacteremia in neutropenic patients with hematologic malignancies. We conducted this study at two large oncology centers in New York City, where CRE have been endemic for over a decade [15], and found that CRE represent 2.2% of all BSIs and 4.7% of Gram-negative bacteremias in this population. The number of CRE bacteremia cases was similar to that of candidemia and greater than that of BSIs caused by Acinetobacter baumannii or Stenotrophomonas maltophilia.
The 30-day mortality rate of 53% after CRE bacteremia was substantially greater than the 13–18% mortality rates typically reported after Gram-negative bacteremia in neutropenic patients with hematologic malignancies [1, 16]. Furthermore, all but one of the deaths in this study was directly related to CRE bacteremia and the median time from bacteremia onset until death was only 4 days. This rapid demise is similar to what was observed in neutropenic patients with Gram-negative bacteremia prior to recognition of fever and neutropenia as a medical emergency, where antimicrobial therapy was delayed until culture positivity [17].
In order to identify patients at high-risk of CRE bacteremia, we conducted risk factor analyses using two control groups: patients who were bacteremic with a pathogen other than carbapenem-resistant Gram-negative bacteria and those bacteremic with carbapenem-susceptible Enterobacteriaceae. We believe that the first control group is more representative of the entire population of febrile neutropenic patients, and thus is preferable for the primary analysis. The purpose of the second control group was to ensure that independent risk factors identified in the primary analysis were not merely risk factors for Gram-negative bacteremia.
Although prior carbapenem use was a risk factor for CRE bacteremia, 60% of patients with CRE bacteremia had not recently received a carbapenem. Thus, the absence of carbapenem exposure does not preclude CRE bacteremia. Identification of BLBLI exposure as a risk factor for CRE bacteremia may be related to a similar broad-spectrum antimicrobial effect that alters the intestinal microbiome and selects for CRE. TMP-SMX, which was typically administered for Pneumocystis jiroveci prophylaxis, and glucocorticoids were also independent risk factors, although it is possible that they served as markers of immunosuppression and chronic illness. Given that these medications are commonly used in neutropenic patients and absence of these exposures does not preclude CRE bacteremia, these risk factors may have limited utility at the bedside for clinicians who are deciding on whether to administer CRE-active empirical therapy to a febrile neutropenic patient.
The limited predictive capabilities of this risk factor-based approach support investigation into alternative strategies to identify neutropenic patients at high risk for CRE bacteremia. One strategy to consider in high prevalence areas is to perform active surveillance of the gastrointestinal tract to identify CRE colonization and administer CRE-targeted empirical therapy to colonized patients who develop signs of infection. High rates of subsequent CRE bacteremia have been observed in ICU patients with asymptomatic CRE colonization [18–19]. However, data on the risk of CRE bacteremia in colonized neutropenic patients are needed to better assess the merits of this strategy.
The 52-hour delay until administration of effective therapy in patients with CRE bacteremia is alarming because these delays are associated with increased mortality in neutropenic patients [20–21]. Guidelines of the Infectious Diseases Society of America recommend that patients with fever and neutropenia receive empirical antibacterial therapy within two hours of presentation [2]. Furthermore, 21% of our patients died prior to the availability of susceptibility data and never received active therapy. Notably, only one of 6 patients who received active empirical therapy died, compared to a 59% mortality rate among patients who did not receive active empirical therapy. This finding suggests that rapid administration of CRE-active therapy in infected patients might improve outcomes.
Rapid diagnostic tools are essential to shorten the delay in administering CRE-active therapies to infected neutropenic patients. Two multiplex PCR systems have now been approved for use in Europe and the U.S.: the Verigene Gram-negative blood culture assay (Nanosphere, Northbrook, IL, USA) and the FilmArray blood culture identification assay (BioFire Diagnostics, Salt Lake City, UT, USA). These assays detect carbapenemase-encoding genes, as well as most of the common bacterial and fungal bloodstream pathogens, directly from blood culture broth within two hours of culture positivity [22–23]. Routine implementation of these assays should decrease the time until detection of CRE to less than 24 hours, which should lead to more timely appropriate therapy. In CRE-endemic areas where these technologies are not available, the detection of Gram-negative bacteremia in a patient with multiple risk factors for CRE bacteremia warrants consideration of CRE-active therapy while awaiting organism identification.
CRE-active agents, such as polymyxins and tigecycline, have major limitations of toxicity and suboptimal efficacy [24–26]. Ceftazidime-avibactam, which was recently approved by the U.S. Food and Drug Administration and is under review by the European Medicines Agency, offers a potential advance in treatment as the first BLBLI with in vitro activity against KPC-producing Enterobacteriaceae [27]. Ceftazidime-avibactam was active against all tested isolates in our study, which was a collection dominated by KPC-producers. Importantly, ceftazidime-avibactam is not active against metallo-β-lactamase-producing Enterobacteriaceae, and thus its usefulness will be diminished in areas where these organisms predominate. Furthermore, clinical trials of ceftazidime-avibactam have largely not included patients infected with CRE or neutropenic patients. Data evaluating the clinical effectiveness of this agent in neutropenic patients with CRE bacteremia are urgently needed.
Several study limitations warrant consideration. CRE are highly prevalent in New York City and our prevalence rates may not apply to other locations. We did not have access to all bloodstream CRE isolates to determine the carbapenem resistance mechanisms, but KPC production predominated among the strains that were evaluated. Given the modest number of cases, we also had limited power to detect all risk factors for CRE bacteremia.
In summary, CRE have emerged as lethal causes of bacteremia in neutropenic patients with hematologic malignancies. The inability to rapidly identify neutropenic patients who are bacteremic with CRE and reliance on traditional diagnostics leads to a 2–3 day delay until administration of appropriate therapy and in turn, high bacteremia-related mortality rates. Rapid diagnostic assays and active surveillance for colonization may be required to provide more timely CRE-active therapy. Earlier administration of appropriate therapy, combined with the use of new agents such as ceftazidime-avibactam, may improve outcomes for these patients.
HIGHLIGHTS.
CRE caused 2.2% of all BSIs and 4.7% of Gram-negative BSI in neutropenic patients.
Multiple antibiotics, steroids and a prior CRE culture were CRE BSI risk factors.
Patients with CRE BSI had a 2–3-day delay until receipt of CRE-active therapy.
The CRE BSI-related mortality rate was 51%, with a median of 4 days until death.
New strategies are needed to mitigate this threat in this vulnerable population.
Acknowledgments
FUNDING
This work was partially supported by the National Institute of Allergy and Infectious Diseases [K23 AI114994 to M.J.S., R01 AI090155 to B.N.K., and R21 AI117338 to L.C.] and the National Center for Advancing Translational Science [UL1 TR000457] at the National Institutes of Health (NIH), the NIH/National Cancer Institute Cancer Center Support Grant [P30 CA008748 to N.C., Z.G., S.K.S], and Save Our Sick Kids Foundation and Sharp Family Foundation [to T.J.W].
Footnotes
AUTHOR CONTRIBUTIONS
M.J.S., N.C., T.J.W., and S.K.S. contributed to the conception and design of the study. M.J.S., N.C., K.M., Z.G., S.K.S. contributed to acquisition of data. M.S., N.C., R.S., G.A., L.C., B.N.K., T.J.W., and S.K.S. contributed to the analysis and interpretation of the data.
Potential conflicts of interest. M.J.S. has received research grants/contracts from Allergan and Achaogen. T.J.W. has received research grants/contracts from Allergan and Merck. All other authors report no potential conflicts of interest.
This study was presented, in part, at IDWeek 2014, Philadelphia, PA, USA (abstract #434). Nine of the 43 CRE bacteremia episodes in this study were included in a previously published manuscript [5].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael J. Satlin, Email: mjs9012@med.cornell.edu.
Nina Cohen, Email: cohenn@mskcc.org.
Kevin C. Ma, Email: kcm9006@nyp.org.
Zivile Gedrimaite, Email: gedrimaz@mskcc.org.
Rosemary Soave, Email: rsoave@med.cornell.edu.
Gülce Askin, Email: gua2004@med.cornell.edu.
Liang Chen, Email: chen11@njms.rutgers.edu.
Barry N. Kreiswirth, Email: kreiswba@njms.rutgers.edu.
Thomas J. Walsh, Email: thw2003@med.cornell.edu.
Susan K. Seo, Email: seos@mskcc.org.
References
- 1.Klastersky J, Ameye L, Maertens J, et al. Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents. 2007;(Suppl 1):S51–9. doi: 10.1016/j.ijantimicag.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e36–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 3.Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Inf Dis. 2011;17:1791–8. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satlin MJ, Jenkins SG, Walsh TJ. The global challenge of carbapenem-resistant Enterobacteriaceae in transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;58:1274–83. doi: 10.1093/cid/ciu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satlin MJ, Calfee DP, Chen L, et al. Emergence of carbapenem-resistant Enterobacteriaceae as causes of bloodstream infections in patients with hematologic malignancies. Leuk Lymphoma. 2013;54:799–806. doi: 10.3109/10428194.2012.723210. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. [Accessed 10 Nov 2015];Device-associated module: Bloodstream Infection Event. Available at http://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf.
- 7.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 8.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 9.Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications for extended-spectrum beta-lactamase production in nosocomial infections. Ann Intern Med. 2004;140:26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. CLSI M100-S22. Wayne, PA: CLSI; 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. [Google Scholar]
- 11.Woodford N, Fagan EJ, Ellington MJ. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum (beta)-lactamases. J Antimicrob Chemother. 2006;57:154–55. doi: 10.1093/jac/dki412. [DOI] [PubMed] [Google Scholar]
- 12.Poirel L, Walsh TR, Cuvillier V, et al. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70:119–23. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Mediavilla JR, Endimiani A, et al. Multiplex real-time PCR assay for detection and classification of Klebsiella pneumoniae carbapenemase gene (blaKPC) variants. J Clin Microbiol. 2011;49:579–85. doi: 10.1128/JCM.01588-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaczmarek FM, Dib-Hajj F, Shang W, Gootz TD. High-level carbapenem resistance in a Klebsiella pneumoniae clinical isolate is due to the combination of bla(ACT-1) beta-lactamase production, porin OmpK35/36 insertional inactivation, and down-regulation of the phosphate transport porin phoe. Antimicrob Agents Chemother. 2006;50:3396–406. doi: 10.1128/AAC.00285-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med. 2005;165:1430–5. doi: 10.1001/archinte.165.12.1430. [DOI] [PubMed] [Google Scholar]
- 16.Marin M, Gudiol C, Ardanuy C, et al. Factors influencing mortality in neutropenic patients with haematologic malignancies or solid tumors with bloodstream infection. Clin Microbiol Infect. 2015;21:583–90. doi: 10.1016/j.cmi.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 17.Schimpff SC, Greene WH, Young VM, Wiernik PH. Pseudomonas septicemia: incidence, epidemiology, prevention and therapy in patients with advanced cancer. Eur J Cancer. 1973;9:449–55. doi: 10.1016/0014-2964(73)90110-2. [DOI] [PubMed] [Google Scholar]
- 18.Calfee D, Jenkins SG. Use of active surveillance cultures to detect asymptomatic colonization with carbapenem-resistant Klebsiella pneumoniae in intensive care unit patients. Infect Control Hospital Epidemiol. 2008;29:966–8. doi: 10.1086/590661. [DOI] [PubMed] [Google Scholar]
- 19.Debby BD, Ganor O, Yasmin M, et al. Epidemiology of carbapenem-resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis. 2012;31:1811–7. doi: 10.1007/s10096-011-1506-5. [DOI] [PubMed] [Google Scholar]
- 20.Lin MY, Weinstein RA, Hota B. Delay of active antimicrobial therapy and mortality among patients with bacteremia: impact of severe neutropenia. Antimicrob Agents Chemother. 2008;52:3188–94. doi: 10.1128/AAC.01553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trecarichi EM, Tumbarello M, Spanu T, et al. Incidence and clinical impact of extended-spectrum-beta-lactamase (ESBL) production and fluoroquinolone resistance in bloodstream infections caused by Escherichia coli and in patients with hematological malignancies. J Infect. 2009;58:299–307. doi: 10.1016/j.jinf.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Ledeboer NA, Lopansri BK, Dhiman N, et al. Identification of Gram-negative bacteria and genetic resistance determinants from positive blood culture broths by use of the Verigene Gram-negative blood culture multiplex microarray-based molecular assay. J Clin Microbiol. 2015;53:2460–72. doi: 10.1128/JCM.00581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blaschke AJ, Heyrend C, Byington CL, et al. Rapid identification of pathogens from positive blood cultures by multiplex polymerase chain reaction using the FilmArray system. Diagn Microbiol Infect Dis. 2012;74:349–55. doi: 10.1016/j.diagmicrobio.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis. 2011;53:879–84. doi: 10.1093/cid/cir611. [DOI] [PubMed] [Google Scholar]
- 25.Rigatto MH, Behle TF, Falci DR, et al. Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: a multicenter prospective cohort study. J Antimicrob Chemother. 2015;70:1552–7. doi: 10.1093/jac/dku561. [DOI] [PubMed] [Google Scholar]
- 26.MacGowan AP. Tigecycline pharmacokinetic/pharmacodynamic update. J Antimicrob Chemother. 2008;62(suppl 1):11–6. doi: 10.1093/jac/dkn242. [DOI] [PubMed] [Google Scholar]
- 27.Castanheira M, Mills JC, Costello SE, Jones RN, Sader HS. Ceftazidime-avibactam activity tested against Enterobacteriaceae isolates from U.S. hospitals (2011 to 2013) and characterization of β-lactamase-producing strains. Antimicrob Agents Chemother. 2015;59:3509–17. doi: 10.1128/AAC.00163-15. [DOI] [PMC free article] [PubMed] [Google Scholar]