Abstract
Background and Goal
Infectious complications after ischemic stroke are frequent and lead to neurologic deterioration, poor functional outcomes, and higher mortality. Local and systemic inflammatory responses to brain ischemia differ between males and females, but little is known about differences in post-stroke susceptibility to infection by sex. The purpose of this study was to compare sex-related differences in the risk of hospital-acquired sepsis and pneumonia after acute ischemic stroke (AIS).
Materials and Methods
This was a retrospective, secondary analysis of the 2010–2011 California State Inpatient Database. Previously validated ICD-9 codes were used to identify adult hospitalizations for AIS. The primary outcome was hospital-acquired sepsis or pneumonia, also identified using ICD-9 codes. Associations between sex and hospital-acquired sepsis or pneumonia were adjusted for baseline characteristics and comorbidities using multivariable logistic regression.
Findings
There were 91,643 visits for AIS included in this analysis, of which 1,027 had hospital-acquired sepsis and 1,225 had hospital-acquired pneumonia. The in-hospital mortality without infection was 4.6%; the presence of hospital-acquired infections was associated with higher mortality for sepsis (32.7%) and pneumonia (21.9%). Female (vs. male) sex was associated with a lower adjusted odds of hospital-acquired sepsis (OR 0.74, 95%CI 0.65–0.84) and pneumonia (OR 0.69, 95%CI 0.62–0.78). This difference was similar across age strata. Among visits with either hospital-acquired sepsis or pneumonia, sex did not influence mortality.
Conclusions
Female sex was associated with a lower risk of hospital-acquired sepsis and pneumonia after AIS. Further investigation is needed to determine the mechanisms underlying this clinical observation.
Keywords: Ischemic stroke, Sepsis, Pneumonia, Sex, Epidemiology
Introduction
Acute ischemic stroke (AIS) is a leading cause of death and adult neurologic disability worldwide. Sepsis and pneumonia are common, and highly morbid, infectious complications in stroke patients (1). The development of post-stroke infection leads to multiple poor outcomes including neurologic deterioration (2), prolonged hospital length of stay (3), poor functional outcome, and death (4). The high clinical impact and limited effective strategies for prevention or treatment of post-stroke infection indicate the need for further investigation into underlying mechanisms and potential therapeutic options.
The etiology for increased susceptibility to infection during the post-stroke period is multifactorial, however, compelling evidence indicates that brain ischemia leads to clinically important changes in immune function (5). In addition to local brain inflammatory responses contributing to the evolution of injury, emerging evidence indicates that depression of immune function is a natural defense mechanism to minimize excessive inflammation in the injured brain. (5). While likely limiting inflammation-related brain injury after stroke, this immunosuppression also leads to increased susceptibility to infection. This pathway is mediated by dysregulated autonomic signaling and changes in peripheral immune cell numbers and function (6).
Sex-related differences in ischemic stroke outcomes have been the topic of prior epidemiologic research (7), but little has been focused on post-stroke infectious complications. One prior retrospective study reviewed 568 admissions for AIS to develop a scoring system to predict the likelihood of post-stroke infection. They identified age, diabetes, and stroke severity as predictors of infection, while sex was not found to be predictive of this outcome (8). Rodent studies have shown sexual dimorphisms in functional outcomes and inflammatory response after experimental brain ischemia suggesting that post-stroke infectious complications may also be sex dependent (9). The objective of this study was to compare sex-related differences in the risk of hospital-acquired sepsis and pneumonia after AIS.
Materials and Methods
Study Design
This was a secondary analysis of the 2010–2011 California State Inpatient Database, which includes data from all hospitalizations in California. We chose California because it has a large and diverse population, and equivalent national data does not contain a ‘present on admission’ indicator variable, considered key to our analysis. Detailed methodology for the State Inpatient Databases is provided by the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project (www.hcup-us.ahrq.gov). The analysis of these de-identified data was approved by the Colorado Multiple Institutional Review Board as “not human subjects” research.
Cohort Definition
We included adult (age ≥18 years) hospitalizations with explicit International Classification of Disease-9 (ICD-9) diagnosis codes for AIS present on admission (433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91. 434.0, 436.0) in any of the 25 diagnosis fields using a previously validated approach, which is a modified version of the AHA/ASA classification system (10). We excluded outside hospital transfers to focus the analysis on acute hospital presentations.
Primary Predictor
Sex.
Outcome Variables
The primary outcome variable of interest was hospital-acquired sepsis or pneumonia defined by ICD-9 diagnosis. We used Clinical Classification Software grouping of ICD-9 diagnoses in any of the 25 listed fields to define sepsis (code 2) and pneumonia (code 122). The infections were considered hospital-acquired when coded as “not present on admission”. Urinary tract infections were not included in this analysis due to the well-known association between female sex and frequency of urinary tract infections. Secondary outcomes included sepsis or pneumonia that was present on admission as well as clinical outcomes (in-hospital mortality, hospital length of stay, and hospital discharge to post-acute care facility).
Covariates
We included other demographic characteristics (ages and race/ethnicity) and relevant comorbidities (end stage renal disease, diabetes mellitus, congestive heart failure, and cancer diagnosis).
Statistical Analysis
We summarized characteristics of our cohort using descriptive statistics, stratified by a diagnosis of sepsis or pneumonia. We used multivariable logistic regression models to calculate odds ratios (ORs) and 95% confidence intervals (CI) for the association between sex and outcomes of interest, adjusting for demographic and clinical characteristics. The analysis was also stratified by age (<50 years, 50–69 years, and ≥70 years) to evaluate for potential age-sex interaction. All analyses were performed using Stata 12.1 (StataCorp LP, College Station, TX), and two-tailed p <0.05 was considered statistically significant.
Results
We identified 91,643 California hospitalizations for AIS in 2010–2011, of which 1,027 met our case definition for hospital-acquired sepsis and 1,225 for hospital-acquired pneumonia. Table 1 shows the baseline characteristics and comorbidities of hospitalizations with diagnoses of sepsis or pneumonia stratified by whether the diagnosis code was hospital acquired or present on admission. As previously described, the presence of hospital acquired infection markedly increased in-hospital mortality, median inpatient length of stay, and likelihood of discharge to post-acute care setting (Table 2).
Table 1.
Association between Patient Characteristics and Infection (Hospital Acquired or Present on Admission), Among Ischemic Stroke Hospitalizations in California, 2010–2011
| Characteristics | Hospital Acquired | Present on Admission | ||
|---|---|---|---|---|
| Sepsis | Pneumonia | Sepsis | Pneumonia | |
| Overall (n=91643) | 1027 (1.12%) | 1225 (1.34%) | 3087 (3.37%) | 3364 (3.67%) |
| Age, years | ||||
| <50 (n=7275) | 89 (1.22%) | 116 (1.59%) | 209 (2.87%) | 152 (2.09%) |
| 50–59 (n=12573) | 135 (1.07%) | 161 (1.28%) | 369 (2.93%) | 292 (2.32%) |
| 60–69 (n=17959) | 200 (1.11%) | 238 (1.33%) | 553 (3.08%) | 537 (2.99%) |
| 70–79 (n=20796) | 268 (1.29%) | 298 (1.43%) | 720 (3.46%) | 796 (3.83%) |
| 80–89 (n=27131) | 291 (1.07%) | 352 (1.30%) | 1015 (3.74%) | 1267 (4.67%) |
| ≥90 (n=5871) | 44 (0.75%) | 60 (1.02%) | 221 (3.76%) | 320 (5.45%) |
| Sex | ||||
| Male (n=44135) | 582 (1.32%) | 710 (1.61%) | 1425 (3.23%) | 1622 (3.68%) |
| Female (n=47326) | 445 (0.94%) | 515 (1.09%) | 1662 (3.51%) | 1740 (3.68%) |
| Race/ethnicity | ||||
| NH White (n=51124) | 465 (0.91%) | 634 (1.24%) | 1634 (3.20%) | 1895 (3.71%) |
| NH Black (n=8886) | 122 (1.37%) | 100 (1.13%) | 386 (4.34%) | 297 (3.34%) |
| Hispanic (n=17675) | 261 (1.48%) | 273 (1.54%) | 614 (3.47%) | 677 (3.83%) |
| Asian (n=8671) | 120 (1.38%) | 142 (1.64%) | 278 (3.21%) | 298 (3.44%) |
| Other (n=1991) | 24 (1.21%) | 32 (1.61%) | 63 (3.16%) | 70 (3.52%) |
| Diabetes | ||||
| Yes (n=33043) | 407 (1.23%) | 478 (1.45%) | 1328 (4.02%) | 1269 (3.84%) |
| No (n=58600) | 620 (1.06%) | 747 (1.27%) | 1759 (3.00%) | 2095 (3.58%) |
| Cancer | ||||
| Yes (n=3686) | 66 (1.79%) | 53 (1.44%) | 247 (6.70%) | 265 (7.19%) |
| No (n=87957) | 961 (1.09%) | 1172 (1.33%) | 2840 (3.23%) | 3099 (3.52%) |
| Heart Failure | ||||
| Yes (n=13584) | 322 (2.37%) | 357 (2.63%) | 945 (6.96%) | 1153 (8.49%) |
| No (n=78059) | 705 (0.90%) | 868 (1.11%) | 2142 (2.74%) | 2211 (2.83%) |
| Renal Failure | ||||
| Yes (n=15014) | 305 (2.03%) | 305 (2.03%) | 982 (6.54%) | 879 (5.85%) |
| No (n=76629) | 722 (0.94%) | 920 (1.20%) | 2105 (2.75%) | 2485 (3.24%) |
Table 2.
Association between Infection, Hospital Acquired or Present on Admission, and Clinical Outcomes Among Ischemic Stroke Hospitalizations in California, 2010–2011
| Incident Infections | Mortality N (%) |
Hospital LOS Median Days (IQR) |
Discharge to PAC facility* N (%) |
|---|---|---|---|
| None (n=73497) | 3385 (4.61%) | 3 (2–5) | 25644 (36.6%) |
| Hospital-Acquired Infection | |||
| Sepsis (n=1027) | 336 (32.7%) | 16 (9–27) | 474 (68.6%) |
| Pneumonia (n=1225) | 268 (21.9%) | 15 (8–26) | 667 (69.7%) |
| Present on Admission Infection | |||
| Sepsis (n=3087) | 921 (29.8%) | 7 (4–13) | 1237 (57.1%) |
| Pneumonia (n=3364) | 635 (18.9%) | 6 (4–11) | 1470 (53.8%) |
among those that survive to hospital discharge
LOS, length of stay; PAC, post-acute care
Table 3 shows the adjusted associations between demographic and clinical characteristics and the presence of sepsis or pneumonia diagnoses among AIS hospitalizations. The most notable finding is the lower adjusted odds of hospital-acquired sepsis (OR 0.74, 95% CI 0.65–0.84) and pneumonia (OR 0.69, 95% CI 0.62–0.78) for female sex. However, this association did not extend to present on admission sepsis or pneumonia. Minority race/ethnicity and several medical comorbidities were also associated with more frequent hospital-acquired sepsis or pneumonia. Interestingly, older age and diabetes, which are common risk factors for infection, were not predictive of increased risk of hospital-acquired sepsis or pneumonia in our AIS cohort.
Table 3.
Adjusted Associations between Characteristics and Presence of Infection Diagnoses
| Characteristics | Hospital Acquired | Present on Admission | ||
|---|---|---|---|---|
| Sepsis OR (95%CI) |
Pneumonia OR (95%CI) |
Sepsis OR (95%CI) |
Pneumonia OR (95%CI) |
|
| Age per ↑10 years | 0.94 (0.90–0.99) | 0.93 (0.89–0.97) | 1.01 (0.98–1.04) | 1.18 (1.15–1.21) |
| Female Sex | 0.74 (0.65–0.84) | 0.69 (0.62–0.78) | 1.11 (1.03–1.20) | 0.93 (0.87–1.00) |
| Race/ethnicity | ||||
| NH White | Referent | Referent | Referent | Referent |
| NH Black | 1.36 (1.10–1.67) | 0.81 (0.66–1.01) | 1.25 (1.11–1.41) | 0.98 (0.86–1.12) |
| Hispanic | 1.62 (1.39–1.90) | 1.21 (1.04–1.40) | 1.09 (0.99–1.20) | 1.20 (1.09–1.32) |
| Asian | 1.55 (1.27–1.91) | 1.34 (1.11–1.61) | 0.99 (0.87–1.13) | 1.00 (0.88–1.13) |
| Other | 1.35 (0.89–2.05) | 1.29 (0.90–1.85) | 1.02 (0.79–1.33) | 1.07 (0.84–1.37) |
| Diabetes | 0.93 (0.81–1.06) | 0.95 (0.84–1.08) | 1.18 (1.10–1.28) | 1.02 (0.95–1.11) |
| Cancer | 1.70 (1.32–2.20) | 1.08 (0.82–1.44) | 2.19 (1.91–2.52) | 2.08 (1.82–2.39) |
| CHF | 2.53 (2.19–2.91) | 2.39 (2.10–2.73) | 2.20 (2.02–2.39) | 2.72 (2.51–2.94) |
| Renal Failure | 1.80 (1.56–2.08) | 1.45 (1.26–1.67) | 2.03 (1.87–2.21) | 1.41 (1.30–1.54) |
UTI, urinary tract infection; OR, odds ratio; CI, confidence interval
Bolded text denotes p<0.05
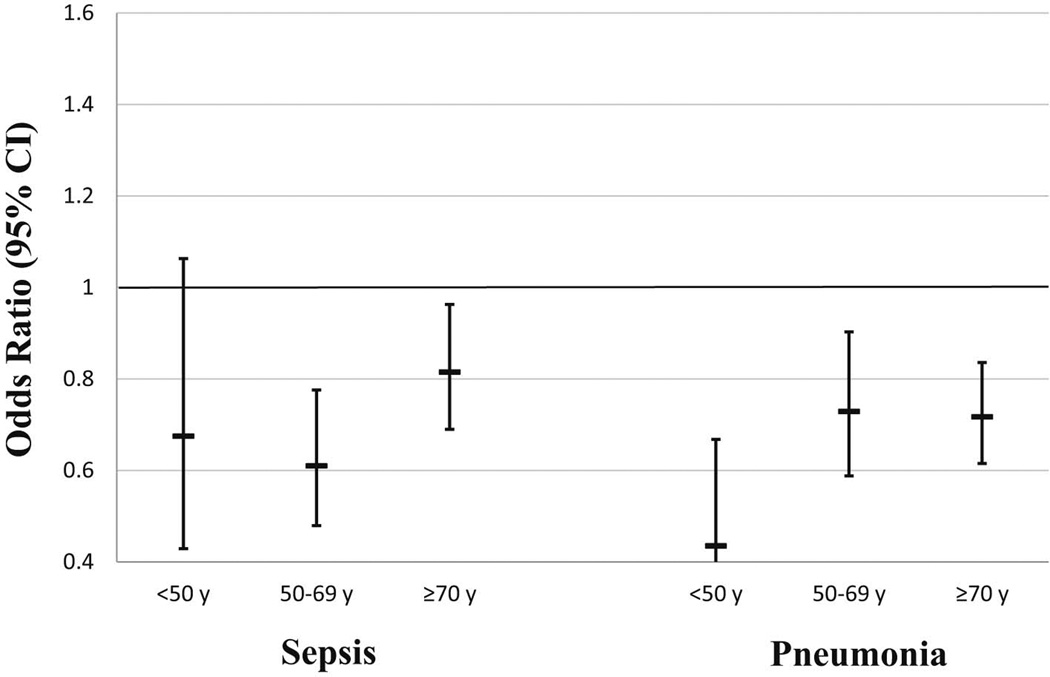
Because there is often an interaction between age and sex differences in other conditions, we next looked at sex differences across age strata (Figure 1). The adjusted odds of hospital-acquired sepsis or pneumonia for females (vs. males) were similar across age strata (all p for trend >0.05).
Figure 1.
Adjusted association between sex and hospital infection stratified by age. Estimates represent odds ratios for females (vs. males) within the age strata. Models adjusted for age, race/ethnicity, diabetes, cancer, heart failure, renal failure.
Discussion
In this large, diverse cohort, female sex was independently associated with a markedly lower risk of hospital-acquired sepsis and pneumonia after AIS. This difference was present across all age groups, suggesting that these sex-related differences in infection risk might not depend on variation in sex hormones throughout the female lifespan. Our data also confirm prior observations of increased morbidity and mortality associated with the development of hospital-acquired sepsis or pneumonia after AIS. Among the group with hospital-acquired infections, sex was not associated with morbidity or mortality suggesting that sex may influence the incidence, but not the severity of these infections.
Stroke-induced immunosuppression has been described in both clinical and experimental studies, with the most common findings being lymphopenia and impaired T-cell function (11–13). Mouse studies have demonstrated that adoptive transfer of T and natural killer cells after induced stroke reduces bacterial infections (12). Most recently, Wong et al have published an in-depth analysis of post-stroke immunosuppression due to changes in hepatic invariant NKT (iNKT) cells that were regulated by noradrenergic input (14). Indeed, several studies have implicated steroidal regulation of post-stroke immunosuppression, suggesting potential immunomodulatory therapeutic options in post-stroke patients to decrease the risk of infection (15). Further understanding these mechanisms may help explain the observed clinical data, and may have important clinical utility, as prior clinical interventions have not been successful. For example, a recent randomized trial showed no benefit in terms of mortality or functional outcomes with prophylactic antibiotic therapy (16).
Variation in sex hormones could be an explanation for the differences between male and female susceptibility to post-stroke infection. Extensive experimental data indicates that circulating estrogens provide neuroprotection, leading to significantly smaller ischemic injury in female animals compared to age-matched males (17). Estrogen related neuroprotection leading to smaller brain injury among females could therefore result in a lower risk of post-stroke infection. Additionally, estrogens may directly regulate the post-stroke immune response, as sex steroid receptors are present on all cell types in the brain, including microglia, astrocytes and various circulating immune cells (9). Our data, however, implicate additional sex-specific mechanisms that are independent of sex steroids given that we found reduced infection among females even in the advanced age-group, when post-menopausal women have low estrogen levels (similar to males). Of note, traditional risk factors for infection such as older age and diabetes were not predictive of infection in this post-stroke cohort. Thus, our data implicate complex interactions between sex, steroids, and age on the impact of stroke on the immune system.
The strength of this analysis was using a large representative dataset, which allowed us to adjust for potential confounders and detect subtle differences in the incidence and timing of infectious complications after AIS, which would not be possible in most stroke databases or single center studies. There are several limitations to this study with regards to its generalizability and applicability. As with all retrospective studies, there is the potential for misclassification and unmeasured confounders that are driving the differences between groups. Thus, we can infer only association but not causation. We were also unable to account for the size or location of infarct, timing of brain ischemia, and clinical severity of stroke. These data are not available in administrative databases but could impact the incidence and severity of infections after AIS, including sex differences. Finally, this database does not contain medication records; thus we were unable to ascertain if chronic outpatient hormone therapy such as contraceptives or hormone replacement therapy were being used. Therefore, while the large sex differences in post-stroke infection are intriguing, they should be considered hypothesis-generating and further investigation is needed to confirm these results, uncover mechanisms, and understand clinical implications.
Summary/Conclusions
Female sex was associated with a strikingly lower risk of hospital-acquired sepsis and pneumonia after AIS. The mechanisms underlying this difference will require further investigation, but may be due to local and systemic inflammatory response driven by sex hormones or sex differences in noradrenergic input. Understanding this physiology and potential therapeutic implications are important for reducing the incidence and severity of infectious complications after stroke, particularly given the failure of other clinical strategies such as prophylactic antibiotics to improve these outcomes.
Acknowledgments
Source of Funding: None
Grant Support:
Dr. Colbert was supported by NIH grant 5T32AG000279
Dr. Ginde was supported by NIH grant 1K23AG040708
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
James F Colbert, University of Colorado School of Medicine, Aurora CO
Richard J Traystman, University of Colorado School of Medicine, Aurora CO
Sharon N Poisson, University of Colorado School of Medicine, Aurora CO.
Paco S Herson, University of Colorado School of Medicine, Aurora CO
Adit A Ginde, University of Colorado School of Medicine, Aurora CO.
References
- 1.Westendorp WF, Nederkoorn PJ, Vermeij JD, et al. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boehme AK, Kumar AD, Dorsey AM, et al. Infections present on admission compared with hospital-acquired infections in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. 2013;22:e582–e589. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George AJ, Boehme AK, Siegler JE, et al. Hospital-acquired infection underlies poor functional outcome in patients with prolonged length of stay. ISRN Stroke. 2013;2013 doi: 10.1155/2013/312348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popović N, Stefanović-Budimkić M, Mitrović N, et al. The frequency of poststroke infections and their impact on early stroke outcome. J Stroke Cerebrovasc Dis. 2013;22:424–429. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Smith CJ, Lawrence CB, Rodriguez-Grande B, et al. The immune system in stroke: Clinical challenges and their translation to experimental research. J Neuroimmune Pharmacol. 2013;8:867–887. doi: 10.1007/s11481-013-9469-1. [DOI] [PubMed] [Google Scholar]
- 6.Chamorro Á, Meisel A, Planas AM, et al. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–410. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- 7.Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: A systematic review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 8.Friedant AJ, Gouse BM, Boehme AK, et al. A simple prediction score for developing a hospital-acquired infection after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24:680–686. doi: 10.1016/j.jstrokecerebrovasdis.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Won SJ, Xu Y, Swanson RA. Targeting microglial activation in stroke therapy: Pharmacological tools and gender effects. Curr Med Chem. 2014;21:2146–2155. doi: 10.2174/0929867321666131228203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones SA, Gottesman RF, Shahar E, et al. Validity of hospital discharge diagnosis codes for stroke: The atherosclerosis risk in communities study. Stroke. 2014;45:3219–3225. doi: 10.1161/STROKEAHA.114.006316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: Experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- 12.Prass K, Meisel C, Höflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke t helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogelgesang A, Dressel A. Immunological consequences of ischemic stroke: Immunosuppression and autoimmunity. J Neuroimmunol. 2011;231:105–110. doi: 10.1016/j.jneuroim.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Wong CH, Jenne CN, Lee WY, et al. Functional innervation of hepatic inkt cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 15.Meisel C, Meisel A. Suppressing immunosuppression after stroke. N Engl J Med. 2011;365:2134–2136. doi: 10.1056/NEJMcibr1112454. [DOI] [PubMed] [Google Scholar]
- 16.Westendorp WF, Vermeij JD, Zock E, et al. The preventive antibiotics in stroke study (pass): A pragmatic randomised open-label masked endpoint clinical trial. Lancet. 2015;385:1519–1526. doi: 10.1016/S0140-6736(14)62456-9. [DOI] [PubMed] [Google Scholar]
- 17.Roof RL, Hall ED. Gender differences in acute cns trauma and stroke: Neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]