Abstract
MicroRNAs (miRNAs) are small, non-coding RNAs acting as post-transcriptional regulators of gene expression. Though implicated in multiple CNS disorders, miRNAs have not been examined in any psychiatric disease state in anterior cingulate cortex (AnCg), a brain region centrally involved in regulating mood. We performed qPCR analyses of 29 miRNAs previously implicated in psychiatric illness (major depressive disorder (MDD), bipolar disorder (BP) and/or schizophrenia (SZ)) in AnCg of patients with MDD and BP versus controls. miR-132, miR-133a and miR-212 were initially identified as differentially expressed in BP, miR-184 in MDD and miR-34a in both MDD and BP (although none survived multiple correction testing and must be considered preliminary). In silico target prediction algorithms identified putative targets of differentially expressed miRNAs. Nuclear Co-Activator 1 (NCOA1), Nuclear Co-Repressor 2 (NCOR2) and Phosphodiesterase 4B (PDE4B) were selected based upon predicted targeting by miR-34a (with NCOR2 and PDE4B both targeted by miR-184) and published relevance to psychiatric illness. Luciferase assays identified PDE4B as a target of miR-34a and miR-184, while NCOA1 and NCOR2 were targeted by miR-34a and 184, respectively. qPCR analyses were performed to determine whether changes in miRNA levels correlated with mRNA levels of validated targets. NCOA1 showed an inverse correlation with miR-34a in BP, while NCOR2 demonstrated a positive correlation. In sum, this is the first study to demonstrate miRNA changes in AnCg in psychiatric illness and validate miR-34a as differentially expressed in CNS in MDD. These findings support a mechanistic role for miRNAs in the regulation of stress-responsive genes disrupted in psychiatric illness.
Introduction
Known as melancholia at the time of Hippocrates, ‘depression’ is a general term that encompasses a large number of mood disorders. Two of these particularly debilitating disorders—major depressive disorder (MDD, or unipolar depression) and bipolar disorder (BP; bipolar depression)—are also extremely common, with a lifetime prevalence of 16.6% and 3.9%, respectively (Kessler et al., 2005). Though a genetic component has been established (due in part to a high degree of heritability (Bierut et al., 1999, Burton et al., 2007, Lohoff, 2010, McGuffin et al., 2003, Sklar et al., 2011, Smoller and Finn, 2003)), the genomic architecture of these disorders remains poorly understood.
In recent years, however, microRNAs (miRNAs)—small, 21–23 nt RNAs that canonically act as post-transcriptional regulators of gene expression—have become an increasing focus for understanding CNS processes. Greater than 40% of all protein-coding transcripts are predicted to be regulated by miRNAs (Tan et al., 2009, Xie et al., 2005). MiRNAs are also highly enriched within the CNS, with greater than two-thirds of identified miRNAs expressed in brain (Bak et al., 2008, Cao et al., 2006, Sempere et al., 2004). MiRNAs are also key governors of CNS processes at both the cellular level (e.g. synaptic plasticity, neuronal differentiation and neuronal migration (Cui et al., 2012, Makeyev et al., 2007, Morgado et al., 2014, Schratt et al., 2006)) and the systems level, with miRNAs linked to the regulation of HPA axis glucocorticoid negative feedback and complex behaviors such as responses to both acute and chronic stress as well as mood and anxiety (Bahi et al., 2014, Haramati et al., 2011, Honda et al., 2013, Katsuura et al., 2012, Muinos-Gimeno et al., 2011, Vreugdenhil et al., 2009).
The role of miRNAs in the regulation of stress responses is of particular interest given that chronic stress is not only a precipitant of mood and affective disorders (Breslau and Davis, 1986, Ilgen and Hutchison, 2005) but HPA axis disruption is one of the most commonly observed pathophysiologies in MDD patients, with symptomatic severity correlating with extent of hypercortisolemia (Gibbons and Mc, 1962, Vythilingam et al., 2004). Intriguingly, a number of studies have directly demonstrated dysregulation of the miRNA regulatory network in patients with a variety of mood and affective disorders, with the vast majority focusing on schizophrenia (SZ) (Beveridge et al., 2010, Beveridge et al., 2008, Kim et al., 2010, Miller et al., 2012, Moreau et al., 2011, Perkins et al., 2007, Santarelli et al., 2011, Shi et al., 2012, Smalheiser et al., 2014, Wan et al., 2015). Absent from these studies, however, has been analysis of the anterior cingulate cortex (AnCg), a brain region centrally involved in the regulation of mood, affect and cognition (Drevets et al., 2008, Ebert and Ebmeier, 1996, Mayberg et al., 1999, Posner and DiGirolamo, 1998). Alterations in AnCg function have been increasingly linked to mood disorders with AnCg activity previously demonstrated to differentiate patients with unipolar versus bipolar depression (Diler et al., 2014) and also to predict successful pharmaceutical and cognitive treatment response (Fujino et al., 2015, Mulert et al., 2007, Pizzagalli et al., 2001, Salvadore et al., 2009). Further work has also established alterations in various systems within AnCg in MDD and BP disorders, including dysregulation in the fibroblast growth factor (FGF) system and clock genes (Bunney et al., 2015, Cheng et al., 2007, Evans et al., 2004).
In the present study we assessed miRNA expression in the AnCg of both MDD and BP patients compared to controls. As miRNAs exert their regulatory effects by targeting mRNA transcripts, we employed bioinformatics approaches to identify mRNA targets of miRNAs whose expression varied due to disease and validated several mRNAs as direct targets. Finally, we examined the steady-state levels of a subset of validated mRNA targets and identified two that vary as a function of affective disease.
Materials and Methods
Postmortem brain tissue and RNA extraction
RNA samples derived from human post-mortem AnCg tissue were provided by the Pritzker Neuropsychiatric Research Consortium. The initial acquisition of tissue, microdissection of AnCg and subsequent RNA extraction that generated these samples is described in detail in (Evans et al., 2003). Briefly, brains were extracted during autopsy and sliced into coronal slabs approximately 0.75 cm thick. Slabs were then snap-frozen and stored at −80 degrees C until subsequent dissections. Anterior cingulate cortex (AnCg, corresponding to Brodmann’s Area 24) was identified and dissected from left hemisphere, with all dissections being performed with tissue slabs on dry ice. Following dissection, total RNA was extracted from each sample using TRIzol (Invitrogen).
Patient demographics and information—including gender, brain pH, post-mortem interval, medication history and agonal factor status—are listed in Supplementary Table 1. A total of 37 patients (n=8, BP; n=15, MDD; n=14, Control) were used for all miRNA and mRNA qPCR experiments. Patient diagnoses were based on criteria from the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, and were obtained from medical examiners, medical records and a family member. Patient samples were matched on two primary criteria, brain pH and agonal factor score. Given that our group has previously observed the significant impact of low brain pH on gene expression (Li et al., 2004) all patients were noted to have a brain pH ≥ 6.55 to mitigate gene expression variance. Additionally, as a prolonged agonal state (e.g. coma, multiple organ system failures, respiratory arrest, etc.) tended to be associated with lower brain pH, all patients included in this study had no agonal factors.
MicroRNA selection, reverse transcription and detection
29 MicroRNAs (Supplementary Table 2) were selected for qPCR analyses. These miRNAs were based upon several criteria including prior published association with psychiatric illness(es) at the time of miRNA selection, shared dysregulation between multiple psychiatric illnesses (e.g. SZ, BP and/or MDD), abundant expression and prior literature validating interactions with mRNAs previously implicated in mental illness (outlined in Supplementary Table 2). Total human RNA (7.5 ng) was reverse transcribed with the High Capacity RNA to cDNA Kit (Applied Biosystems Inc., Carlsbad, CA) as per manufacturer’s instructions using custom pooled RT primers corresponding to miRNAs selected for analysis. Following reverse transcription, first-strand cDNA was subjected to preamplification per manufacturer’s instructions (Applied Biosystems Inc., Carlsbad, CA) using custom pooled preamplification primers. The resulting preamplified material was diluted 1:4 in 0.1× TE buffer before being subjected to qPCR. qPCR was performed using custom TaqMan Low-Density Array (TLDA) cards (Applied Biosystems Inc., Carlsbad, CA). Each TLDA card accommodated 4 biological samples and measured 29 miRNAs, as well as RNU48 as a control, in technical triplicate. RNU48 showed no significant variability between patient cohorts (CTRL, MDD and/or BP). qPCR reactions were run and measured on a ViiA7 thermocycler (Applied Biosystems Inc., Carlsbad, CA) using the following conditions: 2 minutes at 50 C, 10 minutes at 95 C (1 repeat); 15 seconds at 95 C, 1 minute at 60 C (40 repeats). Following detection, miRNAs were analyzed for differential expression using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
In silico target prediction and vector construction
Putative mRNA targets of dysregulated miRNAs were identified based on predicted targeting in miRanda (Betel et al., 2010) (August 2010 Release) and/or TargetScan (Lewis et al., 2005) (Release 6.2) in silico target prediction algorithms using default parameters. A gene ontology analysis of predicted targets of miR-34a was performed using DAVID Bioinformatics (Huang da et al., 2009a, b) using default parameters (Supplementary Table 3). Following this, candidate mRNA targets were narrowed to those associated with terms in the top-scoring functional clusters. Targets were subsequently selected for validation based upon 1) a direct association (based upon prior literature) with neuropsychiatric illness and/or 2) function in a biological process implicated in the pathophysiology of mental illness (e.g. glucocorticoid signaling, synaptic plasticity, transcriptional regulation). Target genes were amplified via end-point PCR (Supplementary Table 4) and subsequently cloned into a previously described US2 plasmid expression vector encoding firefly luciferase driven by the human UBC promoter (US2-Luc) (Yu et al., 2008). Putative miRNA binding sites were identified via the aforementioned prediction algorithms and subsequently mutagenized using the QuikChange XL kit (Agilent Technologies, Santa Clara, CA) following manufacturer’s protocol. Mutagenesis primers (Supplementary Table 4) were designed using the web-based QuikChange Primer Design software (Agilent Technologies, Santa Clara, CA).
Cell culture, transfection and luciferase assays
96-well plates were coated for 15 minutes in 0.1 mg/mL poly-L-lysine (ThermoFisher Scientific, Waltham, MA) and washed with 1X PBS to aid in cell adherence. HEK293 cells were plated at 70–90% confluence in 75 µl DMEM media (Invitrogen, Carlsbad, CA) containing Pen-Strep (Life Technologies, Carlsbad, CA) and 10% FBS (ThermoFisher Scientific, Waltham, MA) per well. Cells were maintained at 37 C at 5% CO2.
24 hours following plating HEK293 cells were transfected with 0.5 µl Lipofectamine 2000 (Invitrogen, Carlsbad, CA), 300 ng US2-Firefly Luciferase (US2-Luc) plasmid containing the 3’ UTR of a gene of interest, 300 ng US2-Renilla Luciferase (US2-RL) plasmid and either 0.5 pmol miRNA mimic (MISSION MicroRNA Mimic) (Sigma-Aldrich, St. Louis, MO) (miRNA treated) or 1 µl sterile saline (vehicle treated) in 50 µl OPTI-MEM media (Life Technologies, Carlsbad, CA) per well (n=6 per treatment group). Cells were allowed to incubate for 24 hours before proceeding to luciferase assays.
Immediately prior to luciferase assays, OPTI-MEM media was aspirated and replaced with 75 µl of DMEM media per well. Protein lysates were prepared by adding 75 µl of Dual-Glo Luciferase Assay Reagent (Promega, Madison, WI) per well and transferring the resulting lysate to a 96-well microassay plate (Sigma-Aldrich, St. Louis, MO). Luciferase assays were then performed following manufacturer’s instructions (Dual-Glo® Luciferase Assay System (Promega, Madison, WI)). Luminescence was measured on a FluoStarOptima (BMG Labtech, Germany) with renilla luciferase serving as a transfection efficiency control.
Messenger RNA reverse transcription and qPCR detection
The same RNA samples used for the miRNA expression analyses were used for messenger RNA studies. Briefly, RNA samples (25 ng per sample) were converted to cDNA with random hexamer priming using SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA) following manufacturer’s instructions. The resulting cDNA was diluted 1:4 in sterile water before use in subsequent qPCR studies.
Individual TaqMan gene expression assays (Applied Biosystems Inc., Carlsbad, CA) were used to measure gene expression for NCOA1 (Assay ID# HS00186661_m1), NCOR2 (Assay ID# HS00196955_m1) and PDE4B (Assay ID# HS00963643_m1) with beta actin (Assay ID# HS99999903_m1) serving as a reference control. Messenger RNA qPCR detections were run on a ViiA7 thermocycler (Applied Biosystems Inc., Carlsbad, CA) using the previously described thermocycler conditions. All samples were run in technical triplicate and were analyzed for differential expression using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Data Analysis
For miRNA expression values, raw P-values were generated using a two-tailed Student’s T-test. Multiple correction testing was performed with the Benjamini-Hochberg False Discovery Rate (FDR; Benjamini and Hochberg, 1995) with FDR set at 15%. Luciferase assays were assayed for significance using a two-tailed Student’s T-test with significance set at p < 0.05.
Results
Differential expression of a subset of miRNAs in AnCg of patients with MDD or BP disorder
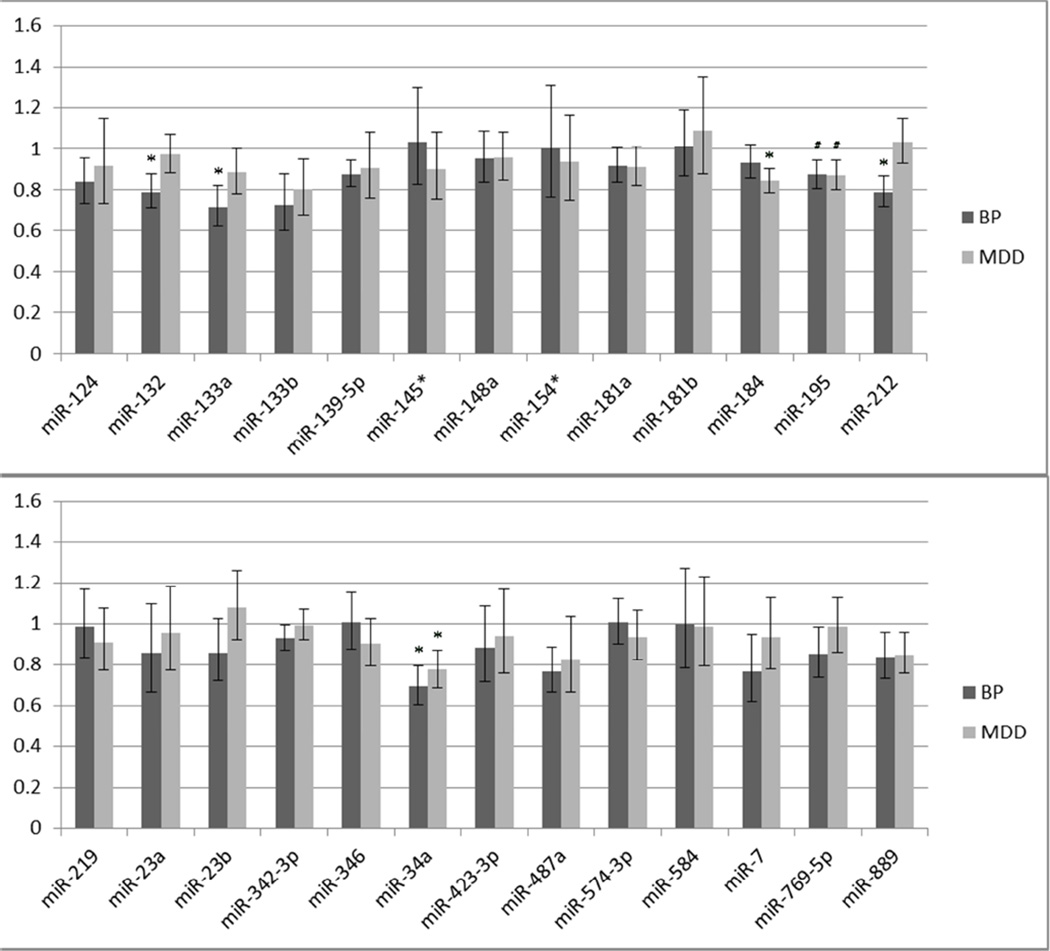
Following qPCR detection 3 miRNAs—miR-33a, miR-144 and miR-431*—were excluded from analysis due to high variability among technical replicates and cycle threshold values >30. After exclusion, 26 miRNAs were examined for differential expression in BP and MDD cohorts versus controls. Of these, 5 miRNAs—miR-132, miR-133a and miR-212 in the BP cohort; miR-184 in the MDD cohort, and miR-34a shared between cohorts—exhibited raw p-values < 0.05 (Figure 1) (although none passed multiple correction testing, e.g. FDR > 0.15).
Figure 1. A subset of miRNAs validate as differentially expressed in AnCg of BP and/or MDD patients.
Vertical axis represents linear fold change in BP or MDD cohorts versus control patients (BP: n=8; MDD: n=15; Control: n=14). Expression levels were measured using Taqman-chemistry-based qPCR using individual assays. Three miRNAs (miR-132, 133a and 212) were dysregulated in BP, one (miR-184) was dysregulated in MDD and one miRNA—miR-34a, previously linked to schizophrenia—was dysregulated in both groups. All samples were run in technical triplicate with the snoRNA RNU48 serving as a reference control. (*: P < 0.05, #: P ≤ 0.10; error bars represent SEM.)
Intriguingly, these miRNAs were dysregulated in a unidirectional fashion: all dysregulated miRNAs were repressed compared to control patients. While it also failed to achieve significance, miR-195—previously linked to regulation of brain-derived neurotrophic factor (BDNF) (Mellios et al., 2008)—exhibited a trend towards repression in both BP and MDD cohorts (p=0.10 and p=0.09, respectively). We note that three of these miRNAs—miR-132, miR-212 and miR-34a—have previously been shown to be dysregulated in PFC of SZ patients (Kim, Reimers, 2010, Miller, Zeier, 2012). These results suggest the shared dysregulation of several miRNAs across several neuropsychiatric conditions, with miR-34a serving as a consistently dysregulated miRNA in MDD, BP and SZ. Given this, along with the large number of validated miR-34a targets previously linked to multiple mood and affective disorders (Table 1), we elected to focus primarily on miR-34a for subsequent analyses.
Table 2.
Validated mRNA targets of miR-34a with linkage to neuropsychiatric illness. Gene names in bold represent novel mRNA targets.
| Gene | Gene Description | ||
|---|---|---|---|
| GRM7 | Metabotropic Glutamate Receptor 7 | Synaptic transmission | GWAS (BP)(Fleischhacker et al., 1992, Zhou et al., 2009) |
| ANK3 | Ankyrin G | Actin/spectrin adaptor protein | GWAS (BP)(Bavamian et al., 2015) |
| CACNB3 | Voltage-Dependent L-Type Clacium Channel Subunit Beta-3 | Synaptic transmission | GWAS (BP)(Bavamian et al., 2015) |
| VEGFA | Vascular Endothelial Growth Factor A | Growth factor signaling | Dysregulation of mRNA and protein levels, linkage analysis (MDD)(Berent et al., 2014, Tsai et al., 2009) |
| NCOA1 | Nuclear Receptor Co-Activator 1 | Modulation of gene transcription via glucocorticoid receptor | Dysregulation of mRNA levels (BP), modulation of stress responses and HPA axis activity. (Lachize et al., 2009, Winnay et al., 2006) |
| NCOR2 | Nuclear Receptor Co-Repressor 2 | Modulation of gene transcription via glucocorticoid receptor | Dysregulation of mRNA levels (MDD) |
| PDE4B | cAMP-specific 3',5'-cyclic Phosphodiesterase 4B | Regulates synaptic transmission via controlling cAMP levels | Dysregulation of protein levels (MDD), SNP (SZ and BP)(Fatemi et al., 2008, Pickard et al., 2007, Yuan et al., 2011) |
| SIRT1 | Sirtuin 1 | Histone deacetylase | Dysregulation of mRNA levels (BP, MDD)(Abe, 2011) |
In silico target prediction analyses of dysregulated miRNAs
Following the identification of miR-34a as differentially expressed, we performed a gene ontology analysis on the 655 putative miR-34a targets identified by the TargetScan algorithm (Lewis, Burge, 2005). Based upon the enriched terms in the two most significant GO clusters— specifically, ‘Synapse’ (Cluster 1) and ‘Transcription Regulator Activity’ (Cluster 2) (Supplementary Table 3)—we were able to identify 3 putative targets of miR-34a based upon our previously described selection criteria. Prior work has shown that two of these genes (NCOA1 and NCOR2) modulate the transcriptional activity of the glucocorticoid receptor (van der Laan et al., 2008), while PDE4B—a genetic risk factor for mental illness (Fatemi et al., 2008, Millar et al., 2005, Numata et al., 2009, Pickard et al., 2007)—regulates cAMP signaling and is enriched at the synapse (Bradshaw et al., 2008, Millar, Pickard, 2005). Further sequence analysis indicated that, in addition to putative miR-34a binding sites, both NCOR2 and PDE4B possess putative miR-184 binding sites, potentially indicating common regulatory targets for both miRNAs disrupted in MDD patients.
Target validations of dysregulated miRNAs
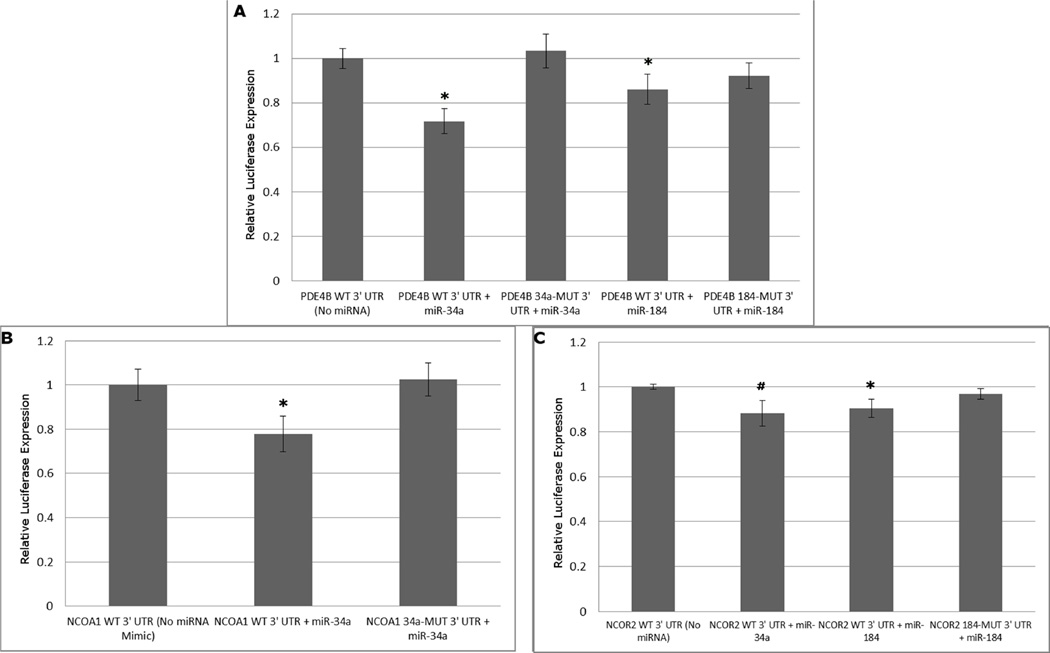
Following the identification of NCOA1, NCOR2 and PDE4B as putative mRNA targets of dysregulated miRNAs, luciferase vectors containing their respective 3’ UTRs were generated as previously described (Yu, Chung, 2008). HEK293 cells transfected with US2-Luc plasmids containing a wild-type 3’ UTR from PDE4B (PDE4B WT 3’ UTR) and either a miR-34a mimic or a miR-184 mimic yielded reduced luciferase values relative to control (Fig. 2a) (n=6 per treatment group). Similarly, HEK293 cells transfected with NCOA1 or NCOR2 WT 3’ UTR yielded reduced luciferase activity when transfected with miR-34a or miR-184 mimics, respectively (Fig. 2b and 2c). Though it exhibited a strong trend, treatment of HEK293 cells transfected with NCOR2 WT 3’ UTR and miR-34a did not result in a statistically significant repression of luciferase activity (Fig. 2c). The specificity of these mRNA/miRNA interactions were demonstrated when mutating predicted miR-34a or miR-184 binding sites (34a-MUT or 184-MUT, respectively) was sufficient to relieve repression in the presence of miRNA mimics (Fig. 2a–c). These findings demonstrate the direct regulation of these genes of interest by miRNAs dysregulated in BP and MDD patients.
Figure 2. PDE4B, NCOA1 and NCOR2 are targets of miRNAs dysregulated in psychiatric illness.
HEK293 cell cultures were plated and cotransfected with a miRNA mimic (either miR-34a or miR-184) and a firefly luciferase vector (Yu, Chung, 2008) containing either a wild-type 3’ UTR (WT 3’ UTR) or a 3’ UTR mutagenized at a predicted miRNA binding site (34a-MUT or 184-MUT 3’ UTR) (n=6/group). A vector encoding renilla luciferase was used to normalize luciferase activity. Significant reductions in luciferase activity were observed for PDE4B when cotransfected with either miR-34a or miR-184 (Fig. 2a), NCOA1 when cotransfected with miR-34a (Fig. 2b) and NCOR2 when cotransfected with miR-184 (Fig. 2c). Mutagenesis of the predicted binding sites was sufficient to relieve these 3’ UTR constructs of miRNA-induced inhibition. Mutagenesis of sites in the NCOR2 3’ UTR was not performed since this UTR showed no significant repression by miR-34a. (*: P < 0.05, #: P ≤ 0.10 ; error bars represent SEM.)
qPCR of validated miRNA targets
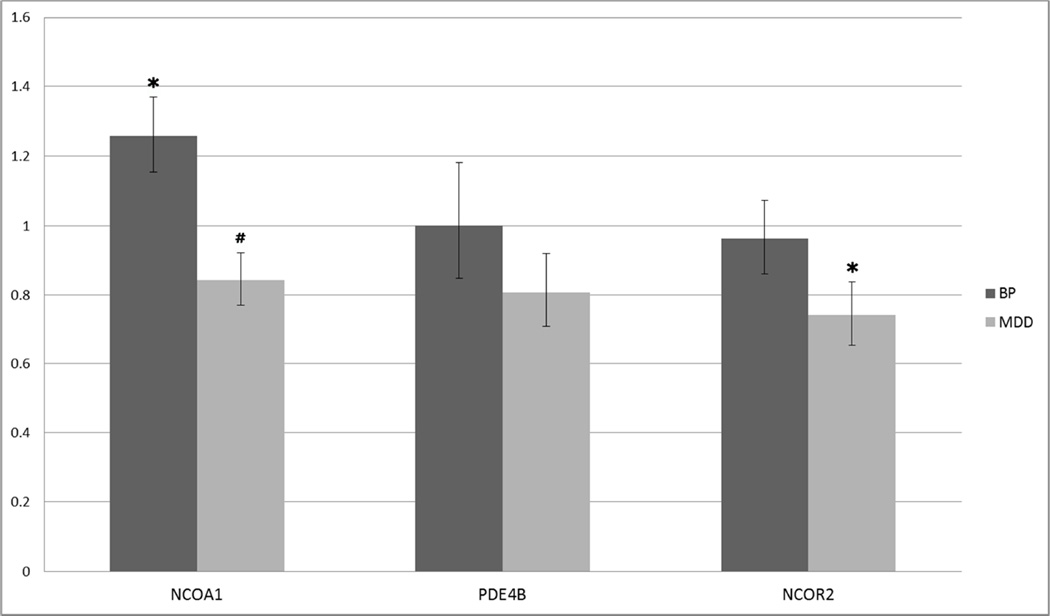
As one way that miRNAs can exert their regulatory influence is via the degradation of mRNA transcripts, we employed qPCR methodologies to examine steady-state levels of our validated targets of miR-34a and/or miR-184 (Fig. 3). Consistent with miRNAs’ canonical role as negative regulators of gene expression, we observed a significant increase in NCOA1 mRNA levels in the BP cohort but not the MDD cohort. In contrast, we observed a significant decrease in NCOR2 mRNA levels specific to the MDD cohort. Neither BP nor MDD cohorts showed significant alterations in PDE4B mRNA expression. Taken together, these results suggest the possibility that the reduction in miR-34a levels may influence steady-state levels of NCOA1 in BP patients.
Figure 3. Steady-state mRNA levels of validated targets are altered in BP and MDD patients.
The mRNA levels of NCOA1 and NCOR2 were dysregulated specifically in the BP and MDD cohorts, respectively. The same patient cohorts were used in both miRNA and mRNA expression analyses (BP: n=8; MDD: n=15 ; Control: n=14). Vertical axis represents linear fold change in BP or MDD patients versus controls. mRNA expression levels were analyzed using single-tube TaqMan qPCR assays specific to the genes of interest. Bars represent linear fold changes in BP and MDD cohorts compared to control patients with error bars representing SEM. (*: P < 0.05, #: P ≤ 0.10)
Discussion
Given their enrichment in the brain, their regulation of key CNS processes, their widespread regulation of protein-coding transcripts and their dysregulation in a number of illnesses, miRNAs are uniquely positioned to play a key role in the pathology of psychiatric illness. In this study we examined the expression of 26 miRNAs in the AnCg of MDD and BP patients versus controls. From this, we identified 5 miRNAs—3 in BP, 1 in MDD and 1 shared across both cohorts—that were differentially expressed in patients with psychiatric illness. Intriguingly, fold changes were unidirectional with all differentially expressed miRNAs reduced in MDD or BP versus controls. Additionally, we examined a subset of putative targets for differentially expressed miRNAs, and were able to validate NCOA1 as a target of miR-34a, NCOR2 as a target of miR-184 and PDE4B as a target of both miR-34a and miR-184. mRNA levels of NCOA1 showed an inverse correlation with miR-34a in the BP cohort, while mRNA levels of NCOR2 showed a positive correlation with miR-34a and miR-184 in the MDD cohort (with PDE4B showing no expression change in either). While we note that it is possible that repression may occur at the level of translation rather than transcription (Wilczynska and Bushell, 2015), patient samples were not initially processed for protein examination; as such, we were unable to investigate this line of inquiry. While we recognize that the observed changes in miRNA expression levels are relatively modest, we feel it important to note that cooperative repression by a miRNA in combination with other miRNAs or RNA-binding proteins allows a non-linear relationship between miRNA expression and extent of mRNA target repression (Broderick et al., 2011, Jacobsen et al., 2010, Mukherji et al., 2011), potentially allowing even minor changes in miRNA levels to act as ‘switches’ rather than simply fine-tuning gene expression.
These data add to our understanding of potential mechanisms underlying psychiatric disorders. While miRNA expression has been examined across a multitude of psychiatric illnesses, encompassing several brain regions, cell types and blood (Bavamian et al., 2015, Fan et al., 2014, Kim, Reimers, 2010, Miller, Zeier, 2012, Moreau, Bruse, 2011, Sun et al., 2015, Walker et al., 2015), to our knowledge this is the first work to examine miRNAs dysregulated as a function of psychiatric illness in AnCg. Additionally, comparing prior work examining miRNA dysregulation in the DLPFC—another brain region of intense interest in the pathology of mental illness—of BP and MDD patients to our results in AnCg, we see little overlap in the specific miRNA species dysregulated as a function of anatomy (e.g. brain region). Potential explanations for this lack of overlap across studies include the usage of different cohorts of patients across multiple studies (including our own which, in turn, severely limits our ability to draw parallels between miRNA expression between brain regions in the present study) and the heterogeneity of neuropsychiatric illness. However, given prior work revealing that the complement of transcripts dysregulated in mental illness is highly dependent on brain region, the notion of region-specific patterns in miRNA disruption is consistent with current knowledge.
Of further note is that this specificity extends not only to miRNA species (e.g. miR-34a versus miR-132) but also to individual miRNA isoforms. In a prior study, miR-133b was differentially expressed in DLPFC of BP patients (Kim, Reimers, 2010). In contrast, while miR-133b levels did not change in our cohort of MDD and BP patients versus controls, miR-133a (encoded by a different gene than miR-133b) was differentially expressed in the AnCg of our cohort of BP patients (Fig. 1). As the canonical mature forms of miR-133a and b differ in only one nucleotide (a U versus a G, respectively, at the final 3’ residue), this single-nucleotide difference could represent a more subtle (but potentially significant) shift in target recognition between the two isoforms. However, as canonical miRNAs represent one of several abundant isoforms of the mature miRNA (each of which may have different nucleotides at the 5’ and 3’ ends), this hypothesis remains speculative.
While miR-132 and miR-212 have been previously identified as differentially expressed in the DLPFC of SZ patients (Kim, Reimers, 2010, Miller, Zeier, 2012), we have identified these miRNAs—which are co-transcribed in the same primary transcript—as differentially expressed in a cohort of BP patients. This finding is intriguing as BP and SZ may share familial and genetic risk factors (Berrettini, 2003, Kim et al., 2015, Purcell et al., 2009, Shepherd et al., 2015) including miRNAs (Kim, Reimers, 2010, Miller, Zeier, 2012, Walker, Rybka, 2015) and, given the seed sequences of miR-132 and miR-212 are identical, these miRNAs may share a number of targets. Additionally, as miR-34a expression levels are dysregulated in cohorts of both SZ and BP patients (in DLPFC and cerebellum, respectively) (Bavamian, Mellios, 2015, Kim, Reimers, 2010), our finding that miR-34a is significantly differentially expressed in a cohort of patients with MDD identifies miR-34a as the first miRNA to be differentially expressed in the CNS across 3 psychiatric illnesses—BP, MDD and SZ. Unfortunately, the lack of SZ patients in the present study precludes the possibility of identifying whether miR-34a is regulated across BP, MDD and SZ specifically in AnCg: an intriguing possibility given the postulated linkage between BP and SZ (Berrettini, 2003, Purcell, Wray, 2009) and several shared symptoms of BP and MDD.
Given prior evidence that suicide may be a strong factor in influencing miRNA expression (Smalheiser, Lugli, 2014) we also performed miRNA expression analyses specifically in the suicide subgroups of our MDD and BP cohorts. Analyses of miRNA expression in the suicide subgroups of MDD and BP mirrored the directionality, magnitude of fold-change and disease specificity compared to entire patient populations. For example, miR-184 demonstrated a linear fold change of 0.84 and 0.85, while miR-34a exhibited a linear fold change of 0.78 and 0.76 (both in suicide- and non-suicide MDD patients, respectively). While these miRNA expression analyses using only non-suicide patients revealed trends in the directionality of fold change and disease specificity identical to both suicide-subgroup and whole-group analyses, these changes also failed to achieve statistical significance. We note, however, that the total number of patients in the non-suicide groups are extremely small (n=2, BP; n=5, MDD) and, as such, we are unable to definitively conclude whether suicide defined a subgroup of BP or MDD subjects.
miR-34a and miR-184’s respective regulation of NCOA1 and NCOR2—which, in turn, can alter the glucocorticoid receptor’s (GR) transcriptional activity (Lachize et al., 2009, van der Laan, Lachize, 2008)—is intriguing for several reasons. NCOA1 has been identified as necessary for proper stress responses (Lachize, Apostolakis, 2009, Winnay et al., 2006) while prior literature demonstrates both NCOA1 and NCOR2 modulate the GR-mediated transcription of corticotropin releasing hormone (CRH), a psychiatric risk factor linked to MDD pathology (van der Laan, Lachize, 2008). Prior work has demonstrated the direct transcriptional repression of miR-184 by the psychiatric risk factor methyl CpG binding protein 2 (MeCP2) (Nomura et al., 2008). Additionally, the miR-34 family (including miR-34a) has previously been linked to stress (Haramati, Navon, 2011) and is strongly induced by the TP53 gene, a key cell-cycle control gene that also regulates expression of the mood-stabilizing Wip1 gene (Rokavec et al., 2014, Ruan et al., 2015). Chronic stress is thought to act as a precipitating environmental factor for a host of mental illnesses and induces chronic elevations in circulating glucocorticoids (e.g. hypercortisolemia). Altered HPA axis activity—particularly hypercortisolemia—is one of the most consistently observed pathophysiologies in MDD patients (Gibbons and Mc, 1962) and is observed in both BP and SZ (Altamura et al., 1999, Daban et al., 2005, Jakovljevic et al., 1998). As glucocorticoids exert powerful transcriptional effects through the GR (with GR mRNA level significantly reduced in MDD versus control patients in a prior study (Qi et al., 2013)) and miR-34a exerts influence over a GR cofactor, these findings suggest a role for miR-34a in the transcriptional response to stress. The authors acknowledge the limitations inherent to proposing direct linkages between miRNA and mRNA function. Specifically, we note that the polygenic and heterogeneous molecular architecture of depressive illnesses make it extremely difficult to couple the impact of an individual miRNA with a specific mRNA. While our hypotheses are consistent with our present data, future studies are of the utmost importance to characterize the prospective impact(s) of miRNAs dysregulated in disease.
The shared regulation of PDE4B by miR-34a and miR-184 is also of note given PDE4B’s linkage to MDD, BP, SZ and anxiety (Fatemi, King, 2008, McGirr et al., 2015, Millar, Pickard, 2005, Numata, Iga, 2009, Padmos et al., 2008, Pickard, Thomson, 2007, Yuan et al., 2011). In addition to PDE4B binding to and being regulated by the psychiatric risk factor Disrupted in Schizophrenia 1 (DISC1) (Millar, Pickard, 2005), pharmacological inhibitors of PDE4B activity have previously been tested as atypical antidepressants (Fleischhacker et al., 1992, Zeller et al., 1984). While these drugs were never widely deployed (due primarily to negative side-effects at therapeutic dosage), the directionality suggested by this pharmacological treatment—i.e. that increased PDE4B is correlated with MDD—is substantiated by prior work demonstrating an increase in levels of PDE4B protein in cingulate cortex of MDD patients versus controls (Yuan, Tragon, 2011). This prior work is also consistent with our current data, in which decreases in miR-34a and miR-184 (Fig. 1)—which can negatively regulate PDE4B (Fig. 2)—should lead to an increase in PDE4B protein levels. While PDE4B mRNA levels were unchanged in either BP or MDD cohorts of the present study, it is important to note that miRNAs can exert their regulatory influence through translational inhibition as well as via mRNA degradation (reviewed in (Wilczynska and Bushell, 2015)).
We note that—in addition to the work presented here—several groups have validated additional targets of miR-34a that are either directly dysregulated or indirectly implicated in biological processes thought to be disrupted in psychiatric disease (Table 1). Given that mental illnesses are noted for their heterogeneity of causes, symptoms and treatments, it is noteworthy that miR-34a—representing the only shared miRNA dysregulated in MDD, BP and SZ—targets transcripts implicated in the pathology of all three of these illnesses (Table 1). Intriguingly, miR-34a has been linked to acute responses to stress while another miR-34 isoform—miR-34c—was differentially expressed in animal models of both acute and chronic stress (Haramati, Navon, 2011). As stress-induced HPA axis dysfunction is a common precipitating event for mood and affective disorders—and our work presented here identifies miR-34a as a regulator of NCOA1, itself previously identified as a key modulator of both stress responses and HPA axis activity (Lachize, Apostolakis, 2009, Winnay, Xu, 2006)—these findings suggest miR-34a as an attractive target for further investigation.
Finally, we note several additional limitations and caveats in our study. We note that none of the miRNAs we identified as differentially expressed have passed multiple correction testing and, as such, must be regarded as preliminary. Limitations when using the Student’s T-test in modest sample sizes (such as those employed here) have also been well described, including poor reliability and reproducibility (reviewed in Halsey et al, 2015). We also acknowledge that, as we employed an in vitro model system using HEK293 cells engineered to express both the 3’ UTR of our mRNA target and our miRNA(s) of interest, future studies employing human neural cell types will be necessary to assess the biological relevance of the interactions.
In sum: due to their enrichment in the brain, regulation of key CNS processes and the fact one miRNA can regulate hundreds of mRNA targets, miRNAs are hypothesized to play a key role in mood and affective disorders. While we acknowledge several caveats and limitations, this current work sheds light on (albeit prelimnary) putative miRNA dysregulation in AnCg (a brain region central to the regulation of mood and cognition) as well as the mRNA targets of these dysregulated miRNAs. We also identify miR-34a in particular as a miRNA dysregulated across multiple psychiatric illnesses and across multiple cortical brain regions known to participate in affect and whose role in the molecular architecture of these disorders remains to be fully described.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant R21 MH083175 (RCT). J. Azevedo was supported by NIH T-32-NS076401. This work was also funded by the Pritzker Neuropsychiatric Disorders Research Consortium, which is supported by the Pritzker Neuropsychiatric Disorders Research Fund L.L.C. A shared intellectual property agreement exists between this philanthropic fund and the University of Michigan, Stanford University, the Weill Medical College of Cornell University, the University of California at Irvine, and the Hudson Alpha Institute for Biotechnology to encourage the development of appropriate findings for research and clinical applications.
Role of the Funding Sources
No sponsors of this research played a role in study design, data acquisition or interpretation, writing of the report or in the decision to submit this article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of Contributors
Joshua A Azevedo: study design, data analysis, manuscript drafting and final revision of manuscript.
Bradley S Carter: early-stage technical feasibility, final revision of manuscript.
Fan Meng: bioinformatics analysis, final revision of manuscript.
David L Turner: bioinformatics analysis, final revision of manuscript.
Manhong Dai: bioinformatics analysis, final revision of manuscript.
Alan F Schatzberg: overall study design and post-mortem sample procurement, final revision of manuscript.
Jack D Barchas: overall study design and post-mortem sample procurement, final revision of manuscript.
Edward G Jones: supervised and organized neuroanatomical dissection of human post-mortem tissue.
William E Bunney: supervised sample collection, overall study design and post-mortem sample procurement,
Richard M Myers: overall study design and post-mortem sample procurement, final revision of manuscript.
Huda Akil: overall study design and post-mortem sample procurement, final revision of manuscript.
Stanley J Watson: overall study design and post-mortem sample procurement, final revision of manuscript.
Robert C Thompson: study design, data analysis and final revision of manuscript.
All authors have approved the final version of this manuscript.
References
- Abe N, Uchida S, Otsuki K, Hobara T, Yamagata H, Higuchi F, Shibata T, Watanabe Y. Altered sirtuin deacetylase gene expression in patients with a mood disorder. J Psychiatr Res. 2011;45(8):1106–1112. doi: 10.1016/j.jpsychires.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Altamura AC, Boin F, Maes M. HPA axis and cytokines dysregulation in schizophrenia: potential implications for the antipsychotic treatment. Eur Neuropsychopharmacol. 1999;10:1–4. doi: 10.1016/s0924-977x(99)00017-6. [DOI] [PubMed] [Google Scholar]
- Bahi A, Chandrasekar V, Dreyer JL. Selective lentiviral-mediated suppression of microRNA124a in the hippocampus evokes antidepressants-like effects in rats. Psychoneuroendocrinology. 2014;46:78–87. doi: 10.1016/j.psyneuen.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Bak M, Silahtaroglu A, Moller M, Christensen M, Rath MF, Skryabin B, et al. MicroRNA expression in the adult mouse central nervous system. RNA. 2008;14:432–444. doi: 10.1261/rna.783108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakathiresan NS, Chandran R, Bhomia M, Jia M, Li H, Maheshwari RK. Serum and amygdala microRNA signatures of posttraumatic stress: fear correlation and biomarker potential. J Psychiatr Res. 2014;57:65–73. doi: 10.1016/j.jpsychires.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Bavamian S, Mellios N, Lalonde J, Fass DM, Wang J, Sheridan SD, et al. Dysregulation of miR-34a links neuronal development to genetic risk factors for bipolar disorder. Mol Psychiatry. 2015 doi: 10.1038/mp.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetatos L, Hatzimichael E, Londin E, Vartholomatos G, Loher P, Rigoutsos I, et al. The microRNAs within the DLK1-DIO3 genomic region: involvement in disease pathogenesis. Cellular and molecular life sciences : CMLS. 2013;70:795–814. doi: 10.1007/s00018-012-1080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berent D, Macander M, Szemraj J, Orzechowska A, Galecki P. Vascular endothelial growth factor A gene expression level is higher in patients with major depressive disorder and not affected by cigarette smoking, hyperlipidemia or treatment with statins. Acta Neurobiol Exp (Wars) 2014;74(1):82–90. doi: 10.55782/ane-2014-1974. [DOI] [PubMed] [Google Scholar]
- Berrettini W. Evidence for shared susceptibility in bipolar disorder and schizophrenia. Am J Med Genet C Semin Med Genet. 2003;123C:59–64. doi: 10.1002/ajmg.c.20014. [DOI] [PubMed] [Google Scholar]
- Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry. 2010;15:1176–1189. doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Human molecular genetics. 2008;17:1156–1168. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Heath AC, Bucholz KK, Dinwiddie SH, Madden PA, Statham DJ, et al. Major depressive disorder in a community-based twin sample: are there different genetic and environmental contributions for men and women? Arch Gen Psychiatry. 1999;56:557–563. doi: 10.1001/archpsyc.56.6.557. [DOI] [PubMed] [Google Scholar]
- Bradshaw NJ, Ogawa F, Antolin-Fontes B, Chubb JE, Carlyle BC, Christie S, et al. DISC1, PDE4B, and NDE1 at the centrosome and synapse. Biochem Biophys Res Commun. 2008;377:1091–1096. doi: 10.1016/j.bbrc.2008.10.120. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC. Chronic stress and major depression. Arch Gen Psychiatry. 1986;43:309–314. doi: 10.1001/archpsyc.1986.01800040015003. [DOI] [PubMed] [Google Scholar]
- Broderick JA, Salomon WE, Ryder SP, Aronin N, Zamore PD. Argonaute protein identity and pairing geometry determine cooperativity in mammalian RNA silencing. RNA. 2011;17:1858–1869. doi: 10.1261/rna.2778911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BG, Li JZ, Walsh DM, Stein R, Vawter MP, Cartagena P, et al. Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder. Mol Psychiatry. 2015;20:48–55. doi: 10.1038/mp.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, et al. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Yeo G, Muotri AR, Kuwabara T, Gage FH. Noncoding RNAs in the mammalian central nervous system. Annu Rev Neurosci. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, et al. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–829. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Xiao Z, Han J, Sun J, Ding W, Zhao Y, et al. MiR-125b orchestrates cell proliferation, differentiation and migration in neural stem/progenitor cells by targeting Nestin. BMC Neurosci. 2012;13:116. doi: 10.1186/1471-2202-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daban C, Vieta E, Mackin P, Young AH. Hypothalamic-pituitary-adrenal axis and bipolar disorder. Psychiatr Clin North Am. 2005;28:469–480. doi: 10.1016/j.psc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Diler RS, Pan LA, Segreti A, Ladouceur CD, Forbes E, Cela SR, et al. Differential Anterior Cingulate Activity during Response Inhibition in Depressed Adolescents with Bipolar and Unipolar Major Depressive Disorder. J Can Acad Child Adolesc Psychiatry. 2014;23:10–19. [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D, Ebmeier KP. The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biol Psychiatry. 1996;39:1044–1050. doi: 10.1016/0006-3223(95)00320-7. [DOI] [PubMed] [Google Scholar]
- Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, et al. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A. 2004;101:15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SJ, Choudary PV, Vawter MP, Li J, Meador-Woodruff JH, Lopez JF, et al. DNA microarray analysis of functionally discrete human brain regions reveals divergent transcriptional profiles. Neurobiol Dis. 2003;14:240–250. doi: 10.1016/s0969-9961(03)00126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HM, Sun XY, Guo W, Zhong AF, Niu W, Zhao L, et al. Differential expression of microRNA in peripheral blood mononuclear cells as specific biomarker for major depressive disorder patients. J Psychiatr Res. 2014;59:45–52. doi: 10.1016/j.jpsychires.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, King DP, Reutiman TJ, Folsom TD, Laurence JA, Lee S, et al. PDE4B polymorphisms and decreased PDE4B expression are associated with schizophrenia. Schizophr Res. 2008;101:36–49. doi: 10.1016/j.schres.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, Smoller JW, Grozeva D, Stone J, Nikolov I, Chambert K, Hamshere ML, Nimgaonkar VL, Moskvina V, Thase ME, Caesar S, Sachs GS, Franklin J, Gordon-Smith K, Ardlie KG, Gabriel SB, Fraser C, Blumenstiel B, Defelice M, Breen G, Gill M, Morris DW, Elkin A, Muir WJ, McGhee KA, Williamson R, MacIntyre DJ, MacLean AW, St CD, Robinson M, Van Beck M, Pereira AC, Kandaswamy R, McQuillin A, Collier DA, Bass NJ, Young AH, Lawrence J, Ferrier IN, Anjorin A, Farmer A, Curtis D, Scolnick EM, McGuffin P, Daly MJ, Corvin AP, Holmans PA, Blackwood DH, Gurling HM, Owen MJ, Purcell SM, Sklar P, Craddock N. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40(9):1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhacker WW, Hinterhuber H, Bauer H, Pflug B, Berner P, Simhandl C, et al. A multicenter double-blind study of three different doses of the new cAMP-phosphodiesterase inhibitor rolipram in patients with major depressive disorder. Neuropsychobiology. 1992;26:59–64. doi: 10.1159/000118897. [DOI] [PubMed] [Google Scholar]
- Fujino J, Yamasaki N, Miyata J, Sasaki H, Matsukawa N, Takemura A, et al. Anterior cingulate volume predicts response to cognitive behavioral therapy in major depressive disorder. J Affect Disord. 2015;174:397–399. doi: 10.1016/j.jad.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Gardiner E, Beveridge NJ, Wu JQ, Carr V, Scott RJ, Tooney PA, et al. Imprinted DLK1-DIO3 region of 14q32 defines a schizophrenia-associated miRNA signature in peripheral blood mononuclear cells. Mol Psychiatry. 2012;17:827–840. doi: 10.1038/mp.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons JL, Mc HP. Plasma cortisol in depressive illness. J Psychiatr Res. 1962;1:162–171. doi: 10.1016/0022-3956(62)90006-7. [DOI] [PubMed] [Google Scholar]
- Halsey LG, Curran-Everett D, Vowler SL, Drummond GB. The fickle P value generates irreproducible results. Nat Methods. 2015;12(3):179–185. doi: 10.1038/nmeth.3288. [DOI] [PubMed] [Google Scholar]
- Haramati S, Navon I, Issler O, Ezra-Nevo G, Gil S, Zwang R, et al. MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. J Neurosci. 2011;31:14191–14203. doi: 10.1523/JNEUROSCI.1673-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Kuwano Y, Katsuura-Kamano S, Kamezaki Y, Fujita K, Akaike Y, et al. Chronic academic stress increases a group of microRNAs in peripheral blood. PLoS One. 2013;8:e75960. doi: 10.1371/journal.pone.0075960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009a;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009b;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Ilgen MA, Hutchison KE. A history of major depressive disorder and the response to stress. J Affect Disord. 2005;86:143–150. doi: 10.1016/j.jad.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Jacobsen A, Wen J, Marks DS, Krogh A. Signatures of RNA binding proteins globally coupled to effective microRNA target sites. Genome Res. 2010;20:1010–1019. doi: 10.1101/gr.103259.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovljevic M, Muck-Seler D, Pivac N, Crncevic Z. Platelet 5-HT and plasma cortisol concentrations after dexamethasone suppression test in patients with different time course of schizophrenia. Neuropsychobiology. 1998;37:142–145. doi: 10.1159/000026493. [DOI] [PubMed] [Google Scholar]
- Katsuura S, Kuwano Y, Yamagishi N, Kurokawa K, Kajita K, Akaike Y, et al. MicroRNAs miR-144/144* and miR-16 in peripheral blood are potential biomarkers for naturalistic stress in healthy Japanese medical students. Neuroscience letters. 2012;516:79–84. doi: 10.1016/j.neulet.2012.03.062. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim AH, Reimers M, Maher B, Williamson V, McMichael O, McClay JL, et al. MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr Res. 2010;124:183–191. doi: 10.1016/j.schres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim JW, Koo TH, Yun HR, Won SH. Shared and distinct neurocognitive endophenotypes of schizophrenia and psychotic bipolar disorder. Clin Psychopharmacol Neurosci. 2015;13:94–102. doi: 10.9758/cpn.2015.13.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Yoshimura R, Kitajima T, Okochi T, Okumura T, Tsunoka T, Yamanouchi Y, Kinoshita Y, Kawashima K, Fukuo Y, Naitoh H, Umene-Nakano W, Inada T, Nakamura J, Ozaki N, Iwata N. SIRT1 gene is associated with major depressive disorder in the Japanese population. J Affect Disord. 2010;126(1–2):167–173. doi: 10.1016/j.jad.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Kocerha J, Faghihi MA, Lopez-Toledano MA, Huang J, Ramsey AJ, Caron MG, et al. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci U S A. 2009;106:3507–3512. doi: 10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachize S, Apostolakis EM, van der Laan S, Tijssen AM, Xu J, de Kloet ER, et al. Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proc Natl Acad Sci U S A. 2009;106:8038–8042. doi: 10.1073/pnas.0812062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CY, Yu SL, Hsieh MH, Chen CH, Chen HY, Wen CC, et al. MicroRNA expression aberration as potential peripheral blood biomarkers for schizophrenia. PLoS One. 2011;6:e21635. doi: 10.1371/journal.pone.0021635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li JZ, Vawter MP, Walsh DM, Tomita H, Evans SJ, Choudary PV, et al. Systematic changes in gene expression in postmortem human brains associated with tissue pH and terminal medical conditions. Human molecular genetics. 2004;13:609–616. doi: 10.1093/hmg/ddh065. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lohoff FW. Overview of the genetics of major depressive disorder. Curr Psychiatry Rep. 2010;12:539–546. doi: 10.1007/s11920-010-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McGirr A, Lipina TV, Mun HS, Georgiou J, Al-Amri AH, Ng E, et al. Specific Inhibition of Phosphodiesterase-4B Results in Anxiolysis and Facilitates Memory Acquisition. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- Mellios N, Huang HS, Grigorenko A, Rogaev E, Akbarian S. A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Human molecular genetics. 2008;17:3030–3042. doi: 10.1093/hmg/ddn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Miller BH, Zeier Z, Xi L, Lanz TA, Deng S, Strathmann J, et al. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc Natl Acad Sci U S A. 2012;109:3125–3130. doi: 10.1073/pnas.1113793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau MP, Bruse SE, David-Rus R, Buyske S, Brzustowicz LM. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol Psychiatry. 2011;69:188–193. doi: 10.1016/j.biopsych.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado AL, Xavier JM, Dionisio PA, Ribeiro MF, Dias RB, Sebastiao AM, et al. MicroRNA-34a Modulates Neural Stem Cell Differentiation by Regulating Expression of Synaptic and Autophagic Proteins. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8794-6. [DOI] [PubMed] [Google Scholar]
- Muinos-Gimeno M, Espinosa-Parrilla Y, Guidi M, Kagerbauer B, Sipila T, Maron E, et al. Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biol Psychiatry. 2011;69:526–533. doi: 10.1016/j.biopsych.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Mukherji S, Ebert MS, Zheng GX, Tsang JS, Sharp PA, van Oudenaarden A. MicroRNAs can generate thresholds in target gene expression. Nat Genet. 2011;43:854–859. doi: 10.1038/ng.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, et al. Rostral anterior cingulate cortex activity in the theta band predicts response to antidepressive medication. Clin EEG Neurosci. 2007;38:78–81. doi: 10.1177/155005940703800209. [DOI] [PubMed] [Google Scholar]
- Nomura T, Kimura M, Horii T, Morita S, Soejima H, Kudo S, et al. MeCP2-dependent repression of an imprinted miR-184 released by depolarization. Human molecular genetics. 2008;17:1192–1199. doi: 10.1093/hmg/ddn011. [DOI] [PubMed] [Google Scholar]
- Numata S, Iga J, Nakataki M, Tayoshi S, Taniguchi K, Sumitani S, et al. Gene expression and association analyses of the phosphodiesterase 4B (PDE4B) gene in major depressive disorder in the Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:527–534. doi: 10.1002/ajmg.b.30852. [DOI] [PubMed] [Google Scholar]
- Padmos RC, Hillegers MH, Knijff EM, Vonk R, Bouvy A, Staal FJ, et al. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch Gen Psychiatry. 2008;65:395–407. doi: 10.1001/archpsyc.65.4.395. [DOI] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, et al. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8:R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard BS, Thomson PA, Christoforou A, Evans KL, Morris SW, Porteous DJ, et al. The PDE4B gene confers sex-specific protection against schizophrenia. Psychiatr Genet. 2007;17:129–133. doi: 10.1097/YPG.0b013e328014492b. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Posner M, DiGirolamo G. Executive attention: Conflict, target detection, and cognitive control. In: Parasuraman R, editor. The Attentive Brain. MIT Press; 1998. [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XR, Kamphuis W, Wang S, Wang Q, Lucassen PJ, Zhou JN, et al. Aberrant stress hormone receptor balance in the human prefrontal cortex and hypothalamic paraventricular nucleus of depressed patients. Psychoneuroendocrinology. 2013;38:863–870. doi: 10.1016/j.psyneuen.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Rokavec M, Li H, Jiang L, Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014;6(3):214–230. doi: 10.1093/jmcb/mju003. [DOI] [PubMed] [Google Scholar]
- Ruan CS, Zhou FH, He ZY, Wang SF, Yang CR, Shen YJ, et al. Mice deficient for wild-type p53-induced phosphatase 1 display elevated anxiety- and depression-like behaviors. Neuroscience. 2015;293:12–22. doi: 10.1016/j.neuroscience.2015.02.037. [DOI] [PubMed] [Google Scholar]
- Rueckert EH, Barker D, Ruderfer D, Bergen SE, O’Dushlaine C, Luce CJ, Sheridan SD, Theriault KM, Chambert K, Moran J, Purcell SM, Madison JM, Haggarty SJ, Sklar P. Cis-acting regulation of brain-specific ANK3 gene expression by a genetic variant associated with bipolar disorder. Mol Psychiatry. 2013;18(8):922–929. doi: 10.1038/mp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli DM, Beveridge NJ, Tooney PA, Cairns MJ. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol Psychiatry. 2011;69:180–187. doi: 10.1016/j.biopsych.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AM, Quide Y, Laurens KR, O’Reilly N, Rowland JE, Mitchell PB, et al. Shared intermediate phenotypes for schizophrenia and bipolar disorder: neuroanatomical features of subtypes distinguished by executive dysfunction. J Psychiatry Neurosci. 2015;40:58–68. doi: 10.1503/jpn.130283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Du J, Qi Y, Liang G, Wang T, Li S, et al. Aberrant expression of serum miRNAs in schizophrenia. J Psychiatr Res. 2012;46:198–204. doi: 10.1016/j.jpsychires.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Sklar P, Ripke S, Scott LJ, Adreassen OA, Cichon S, Craddock N. et al. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43(10):977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, Lugli G, Zhang H, Rizavi H, Cook EH, Dwivedi Y. Expression of microRNAs and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects. PLoS One. 2014;9:e86469. doi: 10.1371/journal.pone.0086469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C:48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- Sun XY, Zhang J, Niu W, Guo W, Song HT, Li HY, et al. A preliminary analysis of microRNA as potential clinical biomarker for schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2015;168:170–178. doi: 10.1002/ajmg.b.32292. [DOI] [PubMed] [Google Scholar]
- Tan LP, Seinen E, Duns G, de Jong D, Sibon OC, Poppema S, et al. A high throughput experimental approach to identify miRNA targets in human cells. Nucleic Acids Res. 2009;37:e137. doi: 10.1093/nar/gkp715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Liou YJ, Chen TJ, Chen ML, Hou SJ, Yen FC, Yu YW. Haplotype analysis of single nucleotide polymorphisms in the vascular endothelial growth factor (VEGFA) gene and antidepressant treatment response in major depressive disorder. Psychiatry Res. 2009;169(2):113–117. doi: 10.1016/j.psychres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- van der Laan S, Lachize SB, Vreugdenhil E, de Kloet ER, Meijer OC. Nuclear receptor coregulators differentially modulate induction and glucocorticoid receptor-mediated repression of the corticotropin-releasing hormone gene. Endocrinology. 2008;149:725–732. doi: 10.1210/en.2007-1234. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil E, Verissimo CS, Mariman R, Kamphorst JT, Barbosa JS, Zweers T, et al. MicroRNA 18 and 124a down-regulate the glucocorticoid receptor: implications for glucocorticoid responsiveness in the brain. Endocrinology. 2009;150:2220–2228. doi: 10.1210/en.2008-1335. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Walker RM, Rybka J, Anderson SM, Torrance HS, Boxall R, Sussmann JE, et al. Preliminary investigation of miRNA expression in individuals at high familial risk of bipolar disorder. J Psychiatr Res. 2015;62:48–55. doi: 10.1016/j.jpsychires.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Liu Y, Wang X, Wu J, Liu K, Zhou J, et al. Identification of differential microRNAs in cerebrospinal fluid and serum of patients with major depressive disorder. PLoS One. 2015;10:e0121975. doi: 10.1371/journal.pone.0121975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczynska A, Bushell M. The complexity of miRNA-mediated repression. Cell Death Differ. 2015;22:22–33. doi: 10.1038/cdd.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnay JN, Xu J, O’Malley BW, Hammer GD. Steroid receptor coactivator-1-deficient mice exhibit altered hypothalamic-pituitary-adrenal axis function. Endocrinology. 2006;147:1322–1332. doi: 10.1210/en.2005-0751. [DOI] [PubMed] [Google Scholar]
- Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, et al. Systematic discovery of regulatory motifs in human promoters and 3’ UTRs by comparison of several mammals. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105(36):13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Lv Q, Wong CK, Hu S, Fu C, Hua Z, Cai G, Li G, Yang BB, Zhang Y. The effect of central loops in miRNA:MRE duplexes on the efficiency of miRNA-mediated gene regulation. PLoS One. 2008;3(3):e1719. doi: 10.1371/journal.pone.0001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, Chung KH, Deo M, Thompson RC, Turner DL. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res. 2008;314:2618–2633. doi: 10.1016/j.yexcr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Tragon T, Xia M, Leclair CA, Skoumbourdis AP, Zheng W, et al. Phosphodiesterase 4 inhibitors enhance sexual pleasure-seeking activity in rodents. Pharmacol Biochem Behav. 2011;98:349–355. doi: 10.1016/j.pbb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E, Stief HJ, Pflug B, Sastre-y-Hernandez M. Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry. 1984;17:188–190. doi: 10.1055/s-2007-1017435. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yuan P, Wang Y, Hunsberger JG, Elkahloun A, Wei Y, Damschroder-Williams P, Du J, Chen G, Manji HK. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology. 2009;34(6):1395–1405. doi: 10.1038/npp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Kalbfleisch T, Brennan MD, Li Y. A MicroRNA gene is hosted in an intron of a schizophrenia-susceptibility gene. Schizophr Res. 2009;109:86–89. doi: 10.1016/j.schres.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.