Abstract
Detergent insoluble inclusions of TDP-43 protein are hallmarks of the neuropathology in over 90% of amyotrophic lateral sclerosis (ALS) cases and approximately half of frontotemporal dementia (FTLD-TDP) cases. In TDP-43 proteinopathy disorders, lesions containing aggregated TDP-43 protein are extensively post-translationally modified, with phosphorylated TDP-43 (pTDP) being the most consistent and robust marker of pathological TDP-43 deposition. Abnormally phosphorylated TDP-43 has been hypothesized to mediate TDP-43 toxicity in many neurodegenerative disease models. To date several different kinases have been implicated in the genesis of pTDP, but no phosphatases have been shown to reverse pathological TDP-43 phosphorylation. We have identified the phosphatase calcineurin as an enzyme binding to and catalyzing the removal of pathological C-terminal phosphorylation of TDP-43 in vitro. In C. elegans models of TDP-43 proteinopathy, genetic elimination of calcineurin results in accumulation of excess pTDP, exacerbated motor dysfunction, and accelerated neurodegenerative changes. In cultured human cells, treatment with FK506 (tacrolimus), a calcineurin inhibitor, results in accumulation of pTDP species. Lastly, calcineurin co-localizes with pTDP in degenerating areas of the central nervous system in subjects with FTLD-TDP and ALS. Taken together these findings suggest calcineurin acts on pTDP as a phosphatase in neurons. Furthermore, patient treatment with calcineurin inhibitors may have unappreciated adverse neuropathological consequences.
Keywords: Calcineurin, TDP-43, TARDBP, amyotrophic lateral sclerosis, frontotemporal lobar degeneration, FK506, tacrolimus, pTDP
Introduction
Neuronal cytoplasmic and nuclear aggregates of TAR DNA binding protein 43 (TDP-43) are the major pathological lesion in ~90% of patients with amyotrophic lateral sclerosis (ALS) and ~50% of patients with frontotemporal lobar degeneration (FTLDTDP) [2, 46, 47]. Since the first report of TDP-43 positive aggregates in ALS and FTLDTDP, other neurodegenerative diseases have been reported to have TDP-43 aggregates as a secondary pathologic feature in a subset of cases. These include Alzheimer’s disease (AD), chronic traumatic encephalopathy, traumatic brain injury, progressive supranuclear palsy, corticobasal degeneration, argyrophilic grain disease, post-encephalitic parkinsonism, dementia with Lewy bodies, and Huntington’s disease [1, 15, 19, 35, 40, 44, 52, 57, 61]. TDP-43 pathology has been correlated with severity of cognitive impairment and rate of cognitive decline in AD [23, 24, 59]. Further, numerous familial ALS causing mutations have been identified in TARDBP, the gene coding for TDP-43 protein, indicating that abnormal TDP-43 is not merely a hallmark of neurodegeneration, but that pathological TDP-43 drives disease [25, 31, 50, 56, 58].
Pathological TDP-43 exhibits a variety of post-translational modifications not observed in healthy neurons, including ubiquitination, acetylation, SUMOylation, and phosphorylation [2, 10, 46, 53]. Of these post-translational modifications, abnormal phosphorylation of TDP-43 is the most consistent and robust neuropathological marker of ALS/FTLD [45]. Specific sites of phosphorylation have been mapped and characterized in disease. These pathological phosphorylation sites cluster in the C-terminus of the TDP-43 protein, predominantly at serines 409 and 410 (S409/410), although phosphorylation at S379, S403, and S404 also correlate with disease pathology [18, 45]. Phosphorylation at S409/410 has been shown to promote decreased TDP-43 protein turnover, increased TDP-43 stabilization, cellular mislocalization of TDP-43, protein aggregation, and neurodegeneration [3, 7, 18, 32, 33, 62].
Several kinases have been identified that phosphorylate TDP-43, including CK1/2, CDC7, and TTBK1/2 [18, 33, 34]. These kinases may act in response to overlapping or unique sets of cellular or environmental triggers. Phosphatases catalyze the reversal of protein phosphorylation on their protein targets by removing phosphate groups from modified tyrosine, serine, and threonine residues. However, in the case of TDP-43, the specific phosphatase(s) responsible for reversing pathological phosphorylation have yet to be identified. Here we show the phosphatase calcineurin binds and dephosphorylates TDP-43 at pathological sites S409/410. Calcineurin activity on TDP-43 prevents accumulation of toxic TDP-43 species preserving neuronal homeostasis.
Materials and Methods
Yeast two-hybrid screen
Bait plasmids were constructed in the pLexA-N vector. Wild-type or M337V TDP-43 amino acids 260-414 were inserted under control of the LexA promoter. Cultures of TDP-43 bait plasmid yeast were grown overnight at 30°C, brought to log phase the next day, and transformed with the Clontech Matchmaker Human Fetal Brain Library (1.3 mg/mL), a cDNA library in the pACT2 vector, resulting in 1.2×106 (Wild-type) or 8.2×106 (M337V) transformants. Transformants were plated on 150mm plates of SD minimal media lacking leucine, tryptophan, and histidine and containing 25mM 3-Amino-1,2,4-Triazole (3-AT) and grown at 30°C. Colonies were picked and grown in SD broth lacking tryptophan and leucine. The candidate prey plasmid from the matchmaker library was isolated, transformed and amplified in E. coli, re-isolated, and identified by sequencing from primers within the pACT2 backbone.
Yeast spotting assay
L40 Ura− yeast were grown overnight and brought to log phase in YPAD medium, then co-transformed with combinations of LexA TDP-43(WT), (A315T), and (M337V) with the pACT2/ PPP3CC clone. pACT2/ MS2 was co-transformed as a negative control for PPP3CC interaction. Transformants were plated on SD minimal media lacking leucine and tryptophan and grown at 30°C. Colonies were isolated and grown in SD broth lacking tryptophan and leucine at 30°C overnight. One OD600 unit of yeast was collected, washed 3x in sterile water, diluted in series, then spotted on SD minimal media plates lacking leucine, tryptophan, and histidine with a titration series of 0, 5, 10, 15, 20, or 25 mM 3-AT. Images shown are at 10mM 3-AT after 6 days of growth.
In vitro dephosphorylation assays
Purified recombinant human calcineurin and calmodulin were purchased from Enzo Lifesciences (Catalog #s BML-SE325, BML-SE163). Phosphorylated TDP-43 was prepared by in vitro phosphorylation using recombinant CDC7 kinase and glutathione-bound GST-TDP-43 fusion protein as previously described [33]. Dephosphorylation was carried out in dephosphorylation reaction buffer [50 mm HEPES, pH 7.2, 50 mM NaCl, 2 mM MnCl2, 1 mM CaCl2, 1 mM DTT]. In an 40 μl reaction volume, approximately 400 units of Calcineurin and 1 μg of calmodulin were added to 2 μg of phosphorylated bead bound TDP-43 protein and incubated at 30°C for varying times up to 1 hour.
C. elegans strains and transgenics
Construction and characterization of TDP-43 transgenic (tg) strains used were described previously [32]. In brief, TDP-43 tg strains were generated by introducing familial ALS mutant human TDP-43 cDNAs driven by the pan neuronal snb-1 promoter (Psnb-1::TDP-43) into the C. elegans genome. TDP-43 tg strains used were CK426: TDP-43(A315T), CK423: TDP-43(M337V), CK410: TDP-43(WT), and CK507: TDP-43(M337V SS/AA). tax-6(p657);TDP-43 tg and tax-6(ok2065);TDP-43 tg animals were obtained by crossing PR675 tax-6(p657) or RB1667 tax-6(ok2065) with CK426, CK410 and CK507. cnb-1(jh103);TDP-43 tg animals were obtained by crossing KJ300 cnb-1(jh103) with CK423, CK410, and CK507.
Behavioral analysis
Assessments of C. elegans locomotion were carried out as previously described with minor modifications [32]. In brief, 15-20 animals were placed at the center of a 100mm plate of 5x concentrated peptone nematode growth media uniformly seeded with OP-50 bacteria. Animals were allowed to move freely for 30 minutes, and the radial distance traveled from the start point was recorded. Distance traveled was converted to micrometers per minute.
Neurodegeneration assays
Strains with GFP marked gamma-aminobutyric acid (GABA)-ergic motor neurons were generated by crossing tax-6(ok2065) with CK426, CK410, CK507, and the reporter strain EG1285 [Punc-47::GFP]. Animals were grown to day 1 adult. Living animals were immobilized on a 2% agarose pad with 0.01% sodium azide, and intact GABA-ergic neurons were scored under fluorescence microscopy on a DeltaVision Elite (GE, Issaquah, WA) imaging system using an Olympus 60x oil objective. Statistical significance was analyzed using Prism statistical software.
Cell culture
HEK 293 cells (from ATCC) were cultured under standard culture condition in Dulbecco’s modified Eagle medium (DMEM), 10% fetal bovine serum (FBS), penicillin (50 IU/ml)–streptomycin (50 mg/ml). For induced pTDP assays, cells were treated with FK506 (Prograf, Astellas Pharma) for 1.5 hours before addition of 150μM ethacrynic acid (EA) to induce production of phosphorylated TDP-43. Cells were harvested 3 hours after addition of EA, washed in PBS, and snap frozen in liquid N2. For FK506 treatment alone, cells were incubated with the indicated concentrations of FK506 for 24 hours, harvested, washed in PBS, and snap frozen in liquid N2. For Ni2+ metal ion activation of calcineurin, cells were incubated with 500μM NiCl2 for 3 hours, then harvested, washed in PBS, and snap frozen in liquid N2. For immunofluorescence assays, cells were grown on poly-D-lysine coated round cover slips (Neuvitro), treated with 10μM FK506 for 1.5 hours, followed by addition of 150μM EA for 3 hours. Cells were fixed in fresh 2% paraformaldehyde, followed by a 20 minute incubation in citrate buffer (10mM sodium citrate, 0.05% Tween 20, pH 6.0) at 80°C, and a 5 minute incubation in 0.1% Triton X-100. Cells were then incubated with the monoclonal antibody anti-phospho-TDP-43 (pS409/410) (CosmoBio TIP-PTD-M01) at 1:1000 dilution, with AlexaFluor 594 anti-mouse secondary antibody (Molecular Probes), followed by DAPI staining.
Immunoblotting
Samples were loaded and resolved on precast 4-15% gradient SDS-PAGE gels and transferred to PVDF membrane as recommended by the manufacturer (Bio-Rad). On immunoblots, human TDP-43 was detected by anti-TDP-43 (Abcam ab57105). TDP-43 phosphorylated at S409/S410 was detected by a monoclonal antibody anti-phospho TDP-43 (pS409/410) (Cosmobio TIP-PTD-M01). C. elegans β-tubulin levels were measured using monoclonal antibody E7 (Developmental Studies Hybridoma Bank) as a loading control. Human calcineurin was detected using anti-calcineurin antibody (Abcam ab82325). Dilutions were: 1:5000 for all primary antibodies. HRP labeled goat anti-mouse IgG was the secondary antibody (Jackson ImmunoResearch) and used at a dilution of 1:5000.
Protein extraction
Total protein fractions were obtained by lysing samples in RAB high salt buffer by homogenization as previously described [17]. For extraction of insoluble TDP-43 protein aggregates, fractions were obtained using methods previously described for analysis of FTLD-U and ALS brain samples [46]. Briefly, packed worm or cell pellets were resuspended in twice the amount (w/v) of Low Salt buffer (LS: [10 mM Tris, 5 mM EDTA, 10% Sucrose, pH 7.5]). Samples were completely lysed by sonication and homogenates centrifuged at 25,000 x g for 30 min. The supernatant constitutes the LS fraction. The pellet was re-extracted with non-ionic detergent containing buffer (TX: [10 mM Tris, 5 mM EDTA, 1% Triton X-100, 10% Sucrose, pH 7.5], centrifuged 20 min at 180,000 x g, with the supernatant constituting the TX fraction. The resulting pellet was extracted with ionic detergent containing buffer (SARK: [10 mM Tris, 5 mM EDTA, 1% Sarkosyl, 10% Sucrose, pH 7.5] and 180,000 x g for 20 minutes. The supernatant is the SARK fraction. The final pellet was solubilized in urea containing buffer (UREA: [30 mM Tris, 7M urea, 2M thiourea, 4% CHAPS, pH8.5]). All buffers contained Completetm Protease Inhibitor Cocktail (Roche) and 0.5 mM phenylmethylsulfonylfluoride to block proteolysis.
Post mortem human tissue
De-identified post-mortem brain tissue used in this study was determined to be exempt from IRB review by the VA Puget Sound Health Care System Human Research Protection Program Director on December 29, 2011. Tissue used for these studies was obtained from the University of Washington Alzheimer’s Disease Research Center brain bank (Seattle, WA), and the Indiana Alzheimer Disease Center brain bank (Indianapolis, IN), where consent for autopsy and permission for use of tissue in scientific experiments was obtained. FTLD-TDP (n = 10) and ALS (n= 2) cases were selected on the basis of having an autopsy-confirmed diagnosis of FTLD and FTLD-related disorders or ALS. Control samples (n = 8) were from Center participants who were neurologically healthy, lacked pTDP or tau containing lesions, and were of a similar age. Neither cases nor controls showed neuropathologic changes of Lewy body accumulation, ischemic injury, or hemorrhage in consensus screening sections [20, 41].
Immunohistochemistry and immunofluorescence analysis
Immunohistochemistry was performed on paraffin embedded frontal cortex and hippocampal sections from 10 FTLD-TDP cases and 8 normal control cases. Primary antibodies used were mouse monoclonal anti-Calcineurin A antibody CC-6 (Abcam, 1:500) and rat monoclonal anti-phospho TDP-43 409/410 (Antibody 1D3, gift of Manuela Neumann, 1:400). In order to minimize variability, sections from all cases (normal and affected subjects) were stained simultaneously for each antibody. Briefly, 5 μm sections were deparaffinized in xylene, rehydrated through graded alcohols, and an antigen retrieval step consisting of autoclaving sections in citrate buffer (1.8 mM citric acid/ 8.2 mM sodium citrate) was performed. Sections were treated for endogenous peroxidases with 3% hydrogen peroxide, blocked in 5% milk, incubated with primary antibody overnight at 4°C, followed by biotinylated secondary antibody for 45 minutes at room temperature. Finally, sections were incubated in an avidin-biotin complex (Vector’s Vectastain Elite ABC kit, Burlingame, CA) and the reaction product was visualized with 0.05% diaminobenzidine (DAB)/0.01% hydrogen peroxide in PBS. Nickel enhancement (DAB with 0.2% Ni) was utilized for phospho TDP-43 visualization. For double labeling experiments, sections were simultaneously immunostained with anti-phospho TDP-43 and calcineurin, while secondary antibody application and detection was performed consecutively. For double label immunofluorescence experiments on FTLD-TDP and ALS cases, AlexaFluor 488 goat anti-mouse and AlexaFluor 594 goat anti-rat secondary antibodies (Molecular Probes) were used and autofluorescence was quenched with 0.1% Sudan Black. Primary antibodies were tested singly to confirm staining patterns and avoid misinterpretation of double label staining. Fluorescent secondary antibodies were validated for specificity using a spotted antibody assay (dot blot) and were confirmed to have no immunoreactivity on tissue in the absence of primary antibody. Colocalization plots for statistical analysis (Pearson’s coefficient of correlation) were generated using softWoRX 5.0 software (Applied Precision, Issaquah, WA). Colocalization analysis was performed on panel images taken using a 60x oil immersion objective.
Photomicrography and figure preparation
Immunohistochemistry photomicrographs were taken with a digital camera and imported into Adobe Photoshop for mounting. Fluorescent and immunofluorescent microscopy was performed on a Delta Vision microscope (Applied Precision, Issaquah, WA) using a 20X air or a 60x oil immersion objective, a sCMOS camera, and 2×2 binning. Image acquisition, deconvolution, and analysis were performed using softWoRx 5.0 software (GE, Issaquah, WA). To optimize visualization of staining or immunofluorescence, photomicrographs were modified, when necessary, by adjusting brightness and contrast.
Results
Calcineurin binds and dephosphorylates the phosphorylated C-terminus of TDP-43
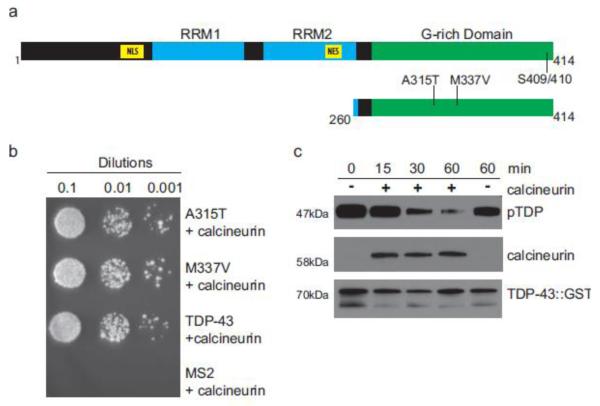
TDP-43 protein contains distinct functional domains, including two RNA recognition motifs, nuclear localization and export signals, and a prion-like G-rich C-terminal domain important for protein-protein interactions (Fig. 1a). The C-terminus of TDP-43 is the site of the majority of ALS-causing mutations identified in human disease, indicating this region of the protein may mediate pathological protein modifications and aggregation. Phosphorylation at the C-terminal serines 409 and 410 (S409/410) are a pathological signature of ALS, FTLD-TDP, and other TDP-43 proteinopathies.
Fig. 1.
Calcineurin interacts with and dephosphorylates TDP-43. (a) Full length human TDP-43 contains both nuclear localization (NLS) and export (NES) signals, RNA recognition motifs (RRM), and a C-terminal G-rich domain. The portion of TDP-43 used as bait in the yeast two-hybrid assay, amino acids 260-414, is depicted below. (b) Wild-type TDP-43 (TDP-43) and fALS-mutants A315T TDP-43 (A315T) and M337V TDP-43 (M337V) were assayed for interaction with calcineurin A/ PPP3CC. Serial dilutions of transformed colonies were plated on selective media to assay specificity and the relative strength of interactions. The bacteriophage coat protein MS2 was used as a negative control for calcineurin binding. (c) A time dependent decrease in pTDP is observed in an in vitro dephosphorylation assay. Full-length recombinant hyperphosphorylated TDP-43 was incubated in the presence (+) or absence (−) of purified calcineurin for the indicated length of time, up to 60 minutes. pTDP is detected by immunoblotting with a phospho-S409/410 pTDP specific antibody. Calcineurin and total TDP-43 immunoblots are included to demonstrate load and protein stability during the course of the experiment.
To identify proteins that interact with the C-terminus of TDP-43, we performed a yeast two-hybrid screen using human TDP-43 amino acids 260-414 as bait (Fig. 1a). We surveyed a human fetal brain library for TDP-43 C-terminus binding proteins. From this screen, we identified PPP3CC as a protein-protein interaction partner of both wild-type TDP-43 and familial ALS mutated TDP-43 (A315T, M337V) (Fig. 1b). PPP3CC is the catalytic subunit of protein phosphatase 3 heterodimer, better known as calcineurin [43]. Calcineurin is a Ca2+/calmodulin-dependent protein phosphatase highly expressed in the mammalian brain [29]. Calcineurin has been extensively studied, and has numerous regulatory roles impacting synaptic plasticity, membrane receptors, ion channels, neuritogenesis, and response to ischemia [5, 6, 42].
To test whether calcineurin can bind and dephosphorylate TDP-43 directly, we used an in vitro dephosphorylation assay incubating purified calcineurin with phosphorylated full-length wild type TDP-43. We observed that TDP-43 was robustly dephosphorylated by calcineurin (Fig. 1c), demonstrating a functional enzyme/substrate relationship between calcineurin and TDP-43 phosphorylated at S409/410 (pTDP).
Calcineurin regulates pTDP levels in vivo in C. elegans
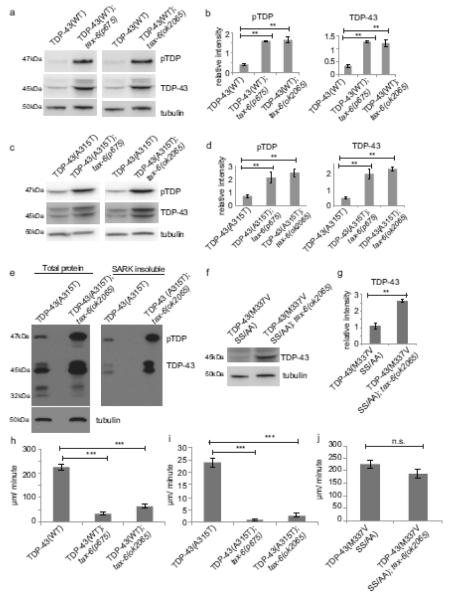
Given calcineurin’s observed activity on pTDP in vitro, it may regulate TDP-43 phosphorylation levels in vivo. To test this hypothesis, we utilized a C. elegans model of ALS that expresses full-length human TDP-43 pan-neuronally. This model exhibits progressive age-dependent movement defects and neurodegeneration, as well as accumulation of insoluble phosphorylated, ubiquitinated, and C-terminal truncated species of TDP-43 [32]. The C. elegans calcineurin A homolog TAX-6 plays important roles in behavioral modulation, but knockout of worm tax-6 is viable [30]. We crossed two different genetic loss-of-function alleles of tax-6 to our TDP-43 transgenic animals and assayed changes in TDP-43 phosphorylation by immunoblot. In the absence of functional tax-6, we saw an increase in the amount of total and phosphorylated human TDP-43 in transgenic animals expressing either WT or fALS mutant A315T TDP-43 (Fig. 2a-e), consistent with a conserved role for calcineurin as a TDP-43 phosphatase. We and others have observed a link between total TDP-43 and pTDP levels; when pTDP is increased, total TDP-43 protein also increases [7, 26, 33, 62]. This may be due to changes in protein stability or pTDP’s blockade of normal TDP-43 protein degradation pathways.
Fig. 2.
The C. elegans calcineurin A homolog, tax-6, regulates TDP-43 phosphorylation in vivo. (a) Two different tax-6 loss of function alleles, p675 and ok2065, increase phosphorylation (pTDP) and total protein levels of WT TDP-43 transgenic C. elegans. (b) Bar graphs represent measurement of 3 independent replicate immunoblots. Significance was evaluated using one-way analysis of variance with Tukey’s multiple comparison test among TDP-43(WT), TDP-43(WT); tax-6(p675), and TDP-43(WT); tax-6(ok2065). p=0.001 comparing relative pTDP band intensities. p=0.005 comparing relative total TDP-43 band intensities. (c) tax-6 loss of function alleles increase pTDP and total TDP in A315T TDP-43 transgenic C. elegans. (d) Bar graphs represent measurement of 4 independent replicate immunoblots. Significance was evaluated using one-way analysis of variance with Tukey’s multiple comparison test among TDP-43(A315T), TDP-43(A315T); tax-6(p675), and TDP-43(A315T); tax-6(ok2065). p=0.008 comparing relative pTDP band intensities. p=0.0002 comparing total TDP-43 band intensities. (e) TDP-43(A315T) and TDP-43(A315T); tax-6(ok2065) were subjected to sequential protein extraction with detergents of increasing solubilizing strengths. Levels of sarkosyl-insoluble (SARK) TDP-43 and pTDP are increased with loss of tax-6. (f) tax-6(ok2065) increases levels of total TDP-43 in non-phosphorylatable TDP-43(M337V SS/AA); tax-6(ok2065). (g) Bar graphs represent measurement of 4 independent replicate immunoblots. Significance was evaluated using Student’s t-test between TDP-43(M337V SS/AA) and TDP-43(M337V SS/AA); tax-6(p675), p=0.0002. (h-i) Calcineurin loss of function significantly worsens TDP-43 tg motor dysfunction. Dispersal velocity of developmentally staged L4 larvae was measured by calculating the radial distance traveled from a designated central starting point over 30 minutes. (h) N=166 for TDP-43(WT), N=144 for TDP-43(WT); tax-6(p675), N=149 for TDP-43(WT); tax-6(ok2065). Significance was evaluated using one-way analysis of variance with Tukey’s multiple comparison test among TDP-43(WT), TDP-43(WT); tax-6(p675), and TDP-43(WT); tax-6(ok2065). p<0.0001 versus TDP-43(WT). (i) N=364 for TDP-43(A315T), N=177 for TDP-43(A315T); tax-6(p675), N=140 for TDP-43(A315T); tax-6(ok2065). Significance was evaluated using one-way analysis of variance with Tukey’s multiple comparison test among TDP-43(A315T), TDP-43(A315T); tax-6(p675), and TDP-43(A315T); tax-6(ok2065). p<0.0001 versus TDP-43(A315T). (j) TDP-43(M337V) with mutations of serines 409/410 to non-phosphorylatable alanines (SS/AA) do not exhibit exacerbated motor phenotypes with calcineurin loss of function. N=210 for TDP-43(M337V SS/AA), N=118 for TDP-43(M337V SS/AA); tax-6(ok2065). Significance was evaluated using Student’s t-test between TDP-43(M337V SS/AA) and TDP-43(M337V SS/AA); tax-6(ok2065). n.s. is not significant.
In both mammals and C. elegans, the absence of calcineurin’s regulatory subunit results in greatly reduced catalytic activity [4, 54]. Therefore, we also tested the effect of a loss of function mutation in the calcineurin B regulatory subunit, cnb-1. We observed a similar increase in total TDP-43 and pTDP in both wild-type TDP-43(WT); cnb-1(−) and fALS mutant TDP-43(M337V); cnb-1(−) animals (Supplemental Fig. 1a-e). Consequently, decreasing calcineurin activity by depletion of either catalytic or regulatory subunits allows pTDP accumulation, as a result of its failure to dephosphorylate TDP-43.
Detergent insoluble pTDP occurs in the neuronal inclusions of ALS and FTLDTDP. Our C. elegans TDP-43 transgenic animals also accumulate detergent insoluble TDP-43 [32]. To determine whether tax-6(−) promotes accumulation of insoluble pTDP, we treated TDP-43(A315T); tax-6(−) animals with sequential protein extractions with detergents of increasing solubilizing strengths. We observed that both pTDP and total TDP-43 accumulate in the sarkosyl-insoluble fraction, and loss of functional tax-6 increases insoluble TDP-43 and pTDP (Fig. 2e). We also assayed TDP-43(M337V); cnb-1(−) animals, and saw a similar accumulation of insoluble pTDP and total TDP-43 (Supplemental Fig. 1e).
tax-6(−) animals move normally, while TDP-43 transgenic animals have obvious and progressive motor dysfunction leading to paralysis and death (Supplemental Fig. 1f, [32]). However, in TDP-43(WT); tax-6(−) and TDP-43(A315T); tax-6(−) animals, we observed dramatic exacerbation of the TDP-43 induced motor dysfunction relative to TDP-43 animals alone (Fig. 2h-i). cnb-1(−) animals are hyperactive relative to wild-type animals (Supplemental Fig. 1f); however, TDP-43(M337V); cnb-1(−) animals also displayed significantly worsened motor dysfunction (Supplemental Fig. 1g). To test whether the tax-6(−) enhancement of TDP-43 phenotypes is due to phosphorylation at S409/410, we crossed tax-6(−) or cnb-1(−) with TDP-43 animals containing non-phosphorylatable serine to alanine substitutions at S409/410 (SS/AA) in addition to M337V. We found that loss of tax-6 increased levels of total TDP-43 (Fig. 2f-g). However, neither TDP-43(M337V SS/AA); tax-6(−) nor TDP-43(M337V SS/AA); cnb-1(−) animals have any enhancement of locomotion dysfunction (Fig. 2j and Supplemental Fig. 1h), indicating the synthetic toxicity of calcineurin loss-of-function and the TDP-43 transgene occurs through regulation of S409/410 phosphorylation and not through changes in levels of total TDP-43 protein.
Loss of calcineurin promotes TDP-43 mediated neurodegeneration in C. elegans
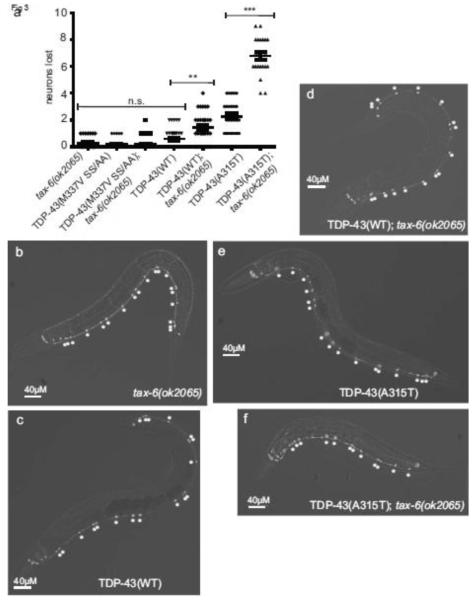
TDP-43 tg animals display degeneration of specific neuronal types with aging, including gamma-aminobutyric acid (GABA) positive inhibitory motor neurons [32]. Loss of calcineurin may promote neuronal dysfunction or increased neurodegeneration. C. elegans neuronal integrity can be assessed in vivo using a GABAergic D-type motor neuron reporter, Punc-47::GFP, which drives expression of GFP in all 19 D-type motor neurons [39]. To determine whether motor neurons are lost at a higher rate in TDP-43 tg; tax-6(−) animals, we scored intact GABAergic motor neurons in vivo using the fluorescent reporter. We found that although TDP-43(WT) animals alone did not exhibit significant neuronal loss at day 1 of adulthood, TDP-43(WT); tax-6(−) animals lost an average of nearly 7% of total D-type GABAergic neurons. TDP-43(A315T) animals lost an average of 12%, but TDP-43(A315T); tax-6(−) animals lost 36% of GABAergic neurons (Fig. 3a-f). These animals also displayed dystrophic neurites, axonal defasciculation, and frank degeneration of axons (Supplemental Fig. 2). Therefore in C. elegans the loss of calcineurin promotes neuronal dysfunction and death. To test whether phosphorylation at S409/410 is necessary for tax-6 loss of function mediated neurodegeneration, we tested TDP-43(M337V SS/AA); tax-6(−) animals. These animals had no appreciable neurodegeneration and were identical to control animals without TDP-43 transgenes (Figure 3a). Taken together, the biochemical, behavioral, and imaging data above demonstrate calcineurin functions in vivo to regulate TDP-43 phosphorylation levels and subsequent neurotoxicity.
Fig. 3.
Loss of calcineurin function promotes TDP-43 dependent neurodegeneration. (a) GFP-labeled D-type GABAergic motor neurons were counted at day 1 of adulthood in vivo in living worms. tax-6(ok2065) had an average of 0.3 neurons lost out of a normal potential complement of 19 total (13VD + 6DD type neurons) (N=25). TDP-43(M337V SS/AA) averaged 0.2 neurons lost per animal (N=30), TDP-43(M337V SS/AA); tax-6(ok2065) averaged 0.2 (N=29), TDP-43(WT) averaged 0.6 (N=32), TDP-43(WT); tax-6(ok2065) averaged 1.5 (N=30), TDP-43(A315T) averaged 2.2 (N=25), and TDP-43(A315T); tax-6(ok2065) animals averaged 6.8 neurons lost (N=22). Significance was evaluated using one-way analysis of variance with Tukey’s multiple comparison test among strains tested. n.s. is not significant. **p<0.05. ***p<0.0001. (b-d) GFP fluorescence images of GABAergic motor neurons. Neuron cell bodies are marked with asterisks. Animals are oriented with head left and ventral down. (b) tax-6(ok2065), (c) TDP-43(WT), (d) TDP-43(WT); tax-6(ok2065), (e) TDP-43(A315T), (f) TDP-43(A315T); tax-6(ok2065).
Inhibition of calcineurin promotes accumulation of pTDP in human cells without altering TDP-43 RNA splicing functions
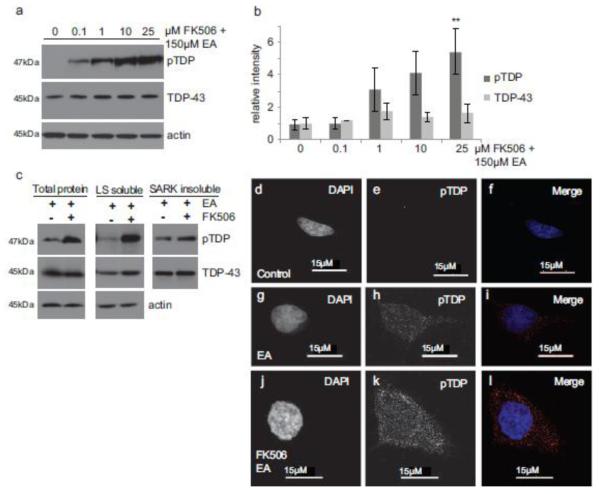
We have shown human calcineurin acts to directly dephosphorylate TDP-43 in vitro. Thus calcineurin may normally prevent the accumulation of pTDP in vivo and inhibition of calcineurin could provoke accumulation of pTDP in mammals. To test this hypothesis, we treated HEK293 cells with the calcineurin inhibitor FK506. FK506 binds the immunophilin FKBP12, forming a drug-immunophilin complex that interacts with and inhibits calcineurin activity [36]. Cells were incubated with FK506, followed by induction of full-length phosphorylated TDP-43 using a chemical trigger, ethacrynic acid [21]. We observed a dose-dependent increase in pTDP with calcineurin inhibition (Fig. 4a-b). Interestingly, levels of total TDP-43 remain constant, perhaps due to the relatively rapid experimental time course. To determine whether FK506 treatment results in accumulation of detergent insoluble pTDP, we performed a sequential extraction using buffers with increasing solubilizing strengths [Low Salt< Triton x-100< Sarkosyl< Urea]. We find that pTDP is present in all fractions including both soluble and sarkosyl-insoluble fractions (Fig. 4c).
Fig. 4.
Calcineurin regulates TDP-43 phosphorylation in mammalian cells. (a) HEK293 cells show a dose-dependent increase in full-length pTDP following treatment with the calcineurin inhibitor FK506 and induction of pTDP with a chemical trigger. Cells were treated with the indicated concentrations of the calcineurin inhibitor FK506 for 1.5 hours, followed by induction of phosphorylated TDP-43 with 150μM ethacrynic acid (EA) for 3 hours. pTDP and TDP-43 were detected by immunoblot. (b) Bar graphs represent measurement of 3 independent replicate immunoblots for pTDP and total TDP-43. Significance was evaluated using repeated measures analysis of variance with Dunnett’s multiple comparison test. **p=0.0342 comparing control treated cells versus 25μM FK506 treated cells. (c) HEK293 cells treated with EA or EA/FK506 were subjected to sequential protein extraction with detergents of increasing solubilizing strengths, and immunoblotted for pTDP and total TDP-43. pTDP and total TDP-43 are present in both soluble (low salt buffer (LS) soluble) and insoluble (sarkosyl-insoluble (SARK)) protein fractions. (d-f) Control HEK293 cells immunostained for pTDP have minimal pTDP immunoreactivity. (g-i) HEK293 cells treated with EA have moderate pTDP immunoreactivity. (j-l) HEK293 cells treated with FK506 to inhibit calcineurin prior to EA treatment have strong pTDP immunoreactivity throughout the cytoplasm and nucleus. DAPI staining (d,g,j) indicates the nucleus. pTDP staining (e,h,k). Merge of DAPI (blue) and pTDP (red) staining (f,i,l).
Cell immunofluorescence following FK506 treatment and ethacrynic acid induction of pTDP shows increased pTDP staining compared to control cells (Fig. 4d-l). This pTDP staining is distributed throughout the cytoplasm and nucleus, and includes areas of higher intensity staining that may represent pre-aggregates.
Cells incubated with FK506 in the absence of ethacrynic acid accumulated a lower molecular weight 35kDa pTDP species (TDP-35) detectable by immunoblot (Supplemental Fig. 3a). TDP-35 has been previously observed in human disease, and has been demonstrated to promote aggregation and recruit TDP-43 into inclusions [8, 9, 22]. To determine whether activation of calcineurin is protective, we treated HEK293 cells with the metal ion Ni2+. Ni2+ is a robust calmodulin-independent activator of calcineurin in vitro and in vivo [28, 48, 51]. Incubating HEK293 cells with Ni2+ treatment resulted in clearance of pTDP-35 (Supplementary Fig. 3b), indicating that increased calcineurin activity can decrease pTDP species. Taken together, our data suggest calcineurin dephosphorylates TDP-43 in human cultured cells, and inhibition of calcineurin activity allows pTDP to accumulate under conditions of cellular stress.
Accumulation of pTDP may cause changes in gene expression or splicing profiles of TDP-43 regulated genes. We tested whether the expression of HDAC6, a TDP-43 regulatory target [27], changed following FK506 treatment. However, we did not see any significant alteration of HDAC6 expression as measured by quantitative reverse transcription PCR (qRT-PCR) (Supplemental Fig. 3c). We also evaluated splicing in TDP-43 target genes POLDIP3/SKAR, STAG2, and FNIP1 [11, 14, 55], but found no changes in their respective splicing profiles (Supplemental Fig. 3c). Thus pTDP accumulation observed following FK506 treatment does not appear to alter TDP-43 splicing functions for multiple known target genes.
Calcineurin co-localizes with pTDP-positive inclusions in FTLD-TDP and ALS
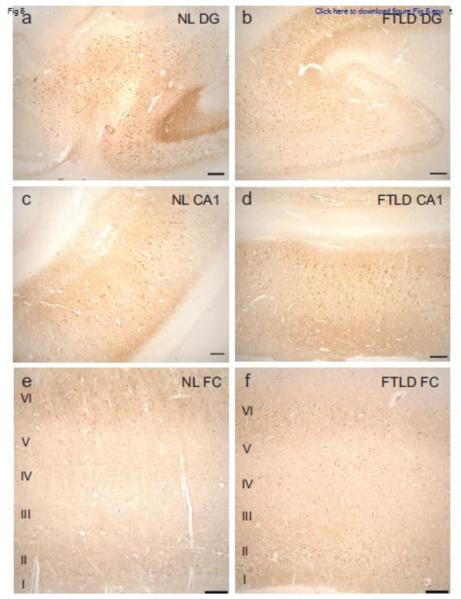
Calcineurin is a highly expressed neuronal phosphatase present in many regions of the brain, including the hippocampus and cerebral cortex [49]. To determine whether changes in calcineurin distribution pattern or localization were apparent in disease affected brain regions containing pTDP aggregates, we immunostained tissue from patients with FTLD-TDP and unaffected controls (normal, NL) (Fig. 5 and Table 1). We observed similar distributions of calcineurin in the hippocampus and frontal cortex in both FTLD-TDP and control tissue. In the hippocampus, calcineurin was abundant in the dentate granule cells as well as CA pyramidal neurons, while in the frontal cortex, calcineurin immunoreactivity was most prominent in cortical layers II, IV, and VI (Fig. 5). FTLD-TDP cases and unaffected controls were qualitatively ranked for staining intensity in cortical layers II, IV, and VI (Table 2). In both cases and controls calcineurin staining was consistent in cortical layers II and VI. Interestingly, in cortical layer IV, calcineurin staining was mild (N=3) or undetectable (N=5) in all of the controls evaluated. However, calcineurin staining was detected in cortical layer IV in all FTLD-TDP cases evaluated. In FTLD-TDP cases, layer IV staining was ranked as moderate (N=8) or robust (N=1), with only one case ranked as mild. It is possible that increased calcineurin expression in FTLD-TDP cases is protective for neurons in cortical layer IV, as these neurons are typically spared pTDP pathology and neurodegeneration in FTLD-TDP.
Fig. 5.
Calcineurin distribution in the hippocampus and frontal cortex is similar in FTLDTDP and normal subjects. Representative photomicrographs depicting calcineurin immunoreactivity in the dentate gyrus (a,b), hippocampal CA1 field (c,d), and frontal cortex (e,f) of a normal control subject (a,c,e) and a subject with FTLD-TDP (b,d,f). Scale bar: 250 μm.
Table 1.
Clinical details from evaluated FTLD-TDP cases
| Type | Family History |
Mutation | Sex | Age | Onset | Duration | Brain Weight (g) |
|---|---|---|---|---|---|---|---|
| A | + | F | 64 | 59 | 5 | 880 | |
| A | + | M | 68 | 62 | 6 | 1040 | |
| A | + | GRN | M | 69 | 62 | 7 | 1000 |
| B | U | F | 58 | U | U | 1110 | |
| B | + | F | 54 | 49 | 5 | 1100 | |
| B | − | F | 85 | 82 | 3 | 980 | |
| B | − | M | 49 | 44 | 5 | 1188 | |
| B | U | M | 67 | 61 | 6 | 1140 | |
| C | − | F | 65 | 53 | 12 | 990 | |
| C | + | F | 81 | 68 | 13 | 1040 |
Age age at death, Duration length of disease course, Family History other affected family members with FTLD-TDP, GRN progranulin gene, Mutation identified genetic cause of FTLD-TDP, Onset age at diagnosis of FTLD, Type harmonized FTLD-TDP classification [37], U unknown, + positive, − negative
Table 2.
Ranking of calcineurin immunostaining
| Controls | ||
|---|---|---|
|
Layer
II |
Layer
IV |
Layer
VI |
| M | ND | M |
| R | L | R |
| R | L | R |
| M | ND | M |
| M | L | M |
| L | ND | L |
| M | ND | L |
| M | ND | M |
| FTLD-TDP Cases | ||
|
Layer
II |
Layer
IV |
Layer
VI |
| M | M | M |
| R | M | R |
| L | R | M |
| R | M | R |
| R | M | R |
| M | M | M |
| M | M | M |
| R | M | R |
| M | M | M |
| M | L | M |
Layer cortical layer, L mild, M moderate, ND not detected, R robust
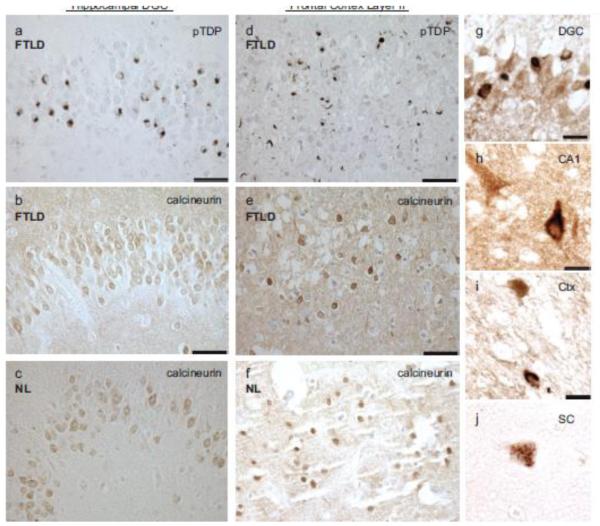
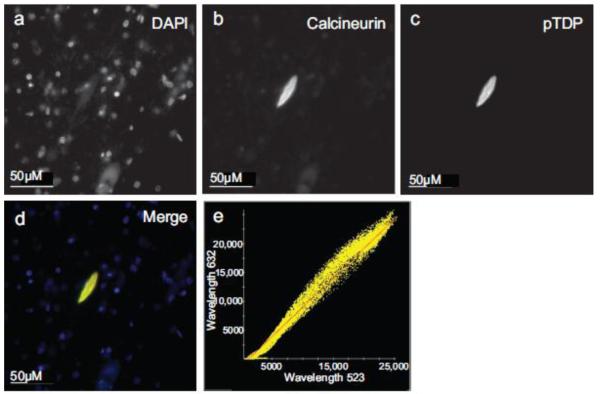
FTLD-TDP affected hippocampal and cerebral cortical regions rich in pTDP deposition were robustly immunoreactive for calcineurin (Fig. 6a-f). To evaluate whether calcineurin is co-expressed in neurons with pTDP aggregates, we performed double-label immunohistochemistry for calcineurin and pTDP. We observed co-expression of calcineurin in cells bearing pTDP-positive lesions in hippocampal dentate granule cells, CA1 pyramidal cells, and frontal cortex layer II (Fig. 6g-i). Motor neurons accumulate pTDP aggregates in ALS. To assess whether calcineurin is co-expressed with pTDP in disease affected spinal cord motor neurons, we performed double-label immunohistochemistry for calcineurin and pTDP. We found that calcineurin and pTDP staining also overlaps in these cells in cytoplasmic aggregates (Fig. 6j). We did not observe calcineurin and pTDP co-localizaed in dystrophic neurites. To confirm co-localization of calcineurin and pTDP, we performed double-label immunofluorescence for calcineurin and pTDP in FTLD-TDP hippocampal dentate granule cells (Fig. 7, Supplemental Fig. 4) and frontal cortex layer II (Fig. 8, Supplemental Fig. 5). We observed significant co-localization of calcineurin with pTDP-positive cytoplasmic aggregates in both brain regions in FTLD-TDP cases (Fig. 7d-e; Fig. 8d-e). We also performed double-label immunofluorescence for calcineurin and pTDP on spinal cord sections from ALS patients (Fig. 9 and Supplemental Fig. 7). In these neurons, calcineurin and pTDP proteins are abundant and significantly co-localize. The co-occurrence of pTDP and calcineurin in neurons of degenerating regions is consistent with a role for calcineurin regulation of pTDP accumulation in ALS and FTLD-TDP.
Fig. 6.
Calcineurin is expressed in brain regions exhibiting pTDP pathology, and co-expressed with pTDP pathology. Representative photomicrographs depicting pTDP (a,d) and calcineurin (b,c,e,f) in the hippocampal dentate granule cells (DGC) (a,b,c) and layer II of the frontal cortex (d,e,f) in an FTLD-TDP (a,b,d,e) and a normal (c,f) case. The distribution of pTDP immunoreactivity in the hippocampus and cortex of the FTLD case overlaps calcineurin (a,b,d,e). The distribution of calcineurin immunoreactivity in normal and FTLD-TDP cases appears similar (b,c,e,f). Scale bars: 50 μm. Calcineurin (brown) is co-expressed with pTDP (black) in (g) hippocampal dentate granule cells (DGC), (h) hippocampal CA1 pyramidal neurons (CA1), (i) cortical neurons (Ctx), and (j) ALS spinal cord motor neurons (SC). Scale bars: 25 μm.
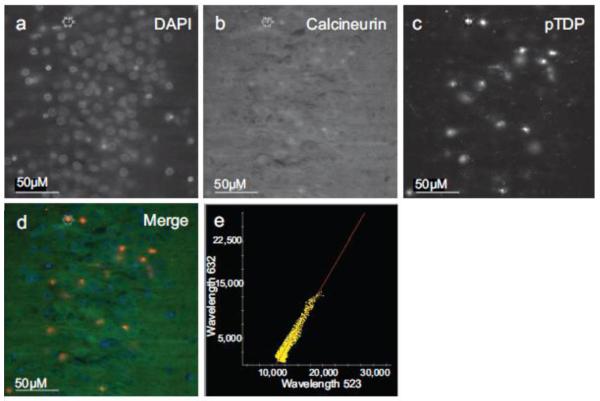
Fig. 7.
Calcineurin co-localizes with pTDP pathology in FTLD-TDP hippocampus. Immunofluorescence of (a) DAPI, (b) calcineurin, (c) pTDP. (d) Merge image of DAPI, calcineurin, and pTDP immunofluorescence. (e) Plot of pTDP and calcineurin colocalization (Pearson’s coefficient of correlation = 0.9527).
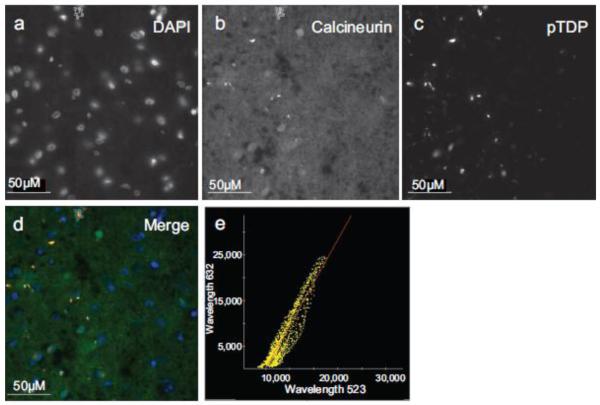
Fig. 8.
Calcineurin co-localizes with pTDP pathology in FTLD-TDP frontal cortex. Immunofluorescence of (a) DAPI, (b) calcineurin, (c) pTDP. (d) Merge image of DAPI, calcineurin, and pTDP immunofluorescence. (e) Plot of pTDP and calcineurin colocalization (Pearson’s coefficient of correlation = 0.9328).
Fig. 9.
Calcineurin co-localizes with pTDP pathology in ALS spinal cord motor neurons. Immunofluorescence of (a) DAPI, (b) calcineurin, (c) pTDP. (d) Merge image of DAPI, calcineurin, and pTDP immunofluorescence. (e) Plot of pTDP and calcineurin colocalization (Pearson’s coefficient of correlation = 0.9779).
Discussion
We have demonstrated that the phosphatase calcineurin can directly interact with and dephosphorylate TDP-43. This is the first report of a phosphatase with specificity for pS409/410 TDP-43. Our data indicate regulation of TDP-43 phosphorylation is critical to maintaining neuronal function and viability. We have shown purified recombinant human calcineurin catalyzes dephosphorylation of purified human pTDP in vitro. In a C. elegans model of TDP-43 proteinopathy, genetic loss of calcineurin function dramatically worsens motor phenotypes, pTDP accumulation, and neurodegeneration. In mammalian cell culture, pharmacological inhibition of calcineurin drives accumulation of phosphorylated TDP-43, indicating calcineurin has a conserved role in regulating pTDP. In disease affected brain regions from patients with FTLDTDP, we observe overlap and co-localization of calcineurin protein and pTDP-positive lesions in both the frontal cortex and hippocampus. We observe a similar co-localization of calcineurin and pTDP in spinal cord motor neurons from patients with ALS. The results presented here suggest calcineurin activity catalyzes the conversion of pathological pTDP back to normal non-phosphorylated TDP-43.
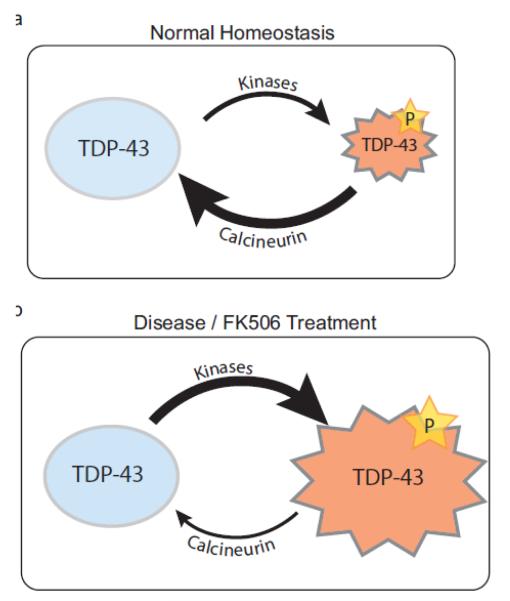
TDP-43 phosphorylation may be a regulated cellular event balanced by the activities of specific kinases and phosphatases. While pTDP is not detected in normal human brain, it is possible that it is transiently produced at low levels and rapidly dephosphorylated. We do observe a low level of 35kDa pTDP in HEK293 cells under normal culture conditions (Supplementary Fig. 3b). pTDP may influence aspects of TDP-43 protein metabolism or function, possibly in response to specific or general cellular stresses. While the endogenous or environmental triggers of neuronal TDP-43 phosphorylation remain unclear, in mammalian cell culture full-length pTDP is induced under conditions of oxidative stress [21, 26]. If cellular homeostasis is disrupted, an imbalance between phosphate addition and removal on TDP-43 could lead to accumulation of pTDP and subsequent neurotoxicity (Fig. 10). This hypothesis is consistent with our data showing Ni2+ mediated activation of calcineurin decreases endogenous pTDP-35 present in HEK293 cells (Supplementary Fig. 3b).
Fig. 10.
Calcineurin protects against the accumulation of neurotoxic pTDP. (a) Under normal cellular conditions, low levels of pTDP are generated and rapidly cleared through the action of the phosphatase calcineurin. (b) During an acute cellular stress or following dysregulation or inhibition of calcineurin, the balance between non-phosphorylated and phosphorylated TDP-43 is shifted towards production of pTDP, leading to neuronal dysfunction and eventual cell death.
Our hypothesis is supported by another neuropathological observation: while calcineurin staining intensity appeared consistent between FTLD-TDP cases and controls in cortical layers II and VI, staining in cortical layer IV was stronger in patients than in controls. Layer IV is typically spared TDP-43 pathology and neuronal loss in FTLD-TDP. It is possible that upregulation of calcineurin in these neurons protects against the accumulation of pTDP.
This study demonstrates normal calcineurin activity antagonizes the activity of TDP-43 kinases. Taken together our data have potentially clinically relevant implications. Calcineurin inhibitors have clinical utility as immunosuppressant drugs to prevent transplanted organ rejection [38]. Clearly calcineurin inhibition on its own does not trigger ALS or other TDP-43 proteinopathies in treated patients. However, lymphocytes from patients with ALS have decreased calcineurin activity levels compared to controls [13], perhaps indicating changes in calcineurin activity are playing a role in the genesis or progression of disease. A subset of patients who have received calcineurin inhibitors following organ transplant have adverse neurological and psychological effects, including tremor, neuralgia, peripheral neuropathy, psychosis, hallucinations, seizures, cerebellar ataxia, motor weakness, or reversible encephalopathy [60]. In some cases, neurotoxic symptoms persist after discontinuation of calcineurin inhibitors [12]. Suppressing calcineurin activity may unmask latent TDP-43 proteinopathy or exacerbate progression of TDP-43 pathology in TDP-43 proteinopathy patients. The relationship between calcineurin inhibitor use and TDP-43 proteinopathy warrants further study. Calcineurin inhibitors have been proposed as a therapeutic strategy for ALS on the basis of immunosuppressive/anti-neuroinflammatory mechanisms, and a clinical trial using FK506 in ALS has been initiated [16]. Our data demonstrate that calcineurin inhibition can trigger pTDP accumulation in some contexts, and suggest caution should be taken in these approaches to therapy.
Supplementary Material
Supplemental Fig. 1 The C. elegans calcineurin B homolog, cnb-1, regulates TDP-43 phosphorylation in vivo. (a) A loss of function mutation in the calcineurin regulatory subunit cnb-1 increases phosphorylation and total protein levels of WT TDP-43 transgenic C. elegans. (b) Bar graphs represent measurement of 3 independent replicate immunoblots. Significance was evaluated using Student’s t-test between TDP-43(WT) and TDP-43(WT); cnb-1(jh103). p=0.04 comparing pTDP band intensities. p=0.04 comparing total TDP-43 band intensities. (c) cnb-1 loss of function increases pTDP and total TDP in M337V TDP-43 transgenic C. elegans. (d) Bar graphs are quantitation of 3 independent replicate immunoblots. Significance was evaluated using Student’s t-test between TDP-43(M337V) and TDP-43(M337V); cnb-1(jh103), p=0.001 comparing pTDP band intensities. p=0.003 comparing total TDP-43 band intensities. (e) TDP-43(M337V) and TDP-43(M337V); cnb-1(jh1035) were subjected to sequential protein extraction with detergents of increasing solubilizing strengths. Levels of sarkosyl-insoluble (SARK) pTDP are increased with loss of cnb-1. (f) Dispersal velocities of normal and mutant control worm strains were measured by calculating the radial distance traveled from a designated central starting point over 30 minutes. N>100 for each genotype tested. cnb-1(jh103) is significantly hyperactive relative to wild-type worms, p<0.01. (g) TDP-43(M337V); cnb-1(jh103) animals have significantly reduced locomotion velocity compared to TDP-43(M337V) alone. N>130 per genotype, p=0.01. (c) TDP-43(M337V SS/AA); cnb-1(jh103) move better than TDP-43(M337V SS/AA) alone, comparable to the cnb-1(jh103) hyperactivity observed in (f). N>200, p<0.001.
Supplemental Fig. 2 C. elegans TDP-43 tg; tax-6(−) animals exhibit nerve cord defasciculation and GFP foci. (a-c) High-magnification images of identical posterior segments of the ventral nerve cord and axonal processes. (a) tax-6(ok2065) have normal fasciculation of axonal bundles and commissures that extend from the dorsal to the ventral nerve cord. (b) TDP-43(A315T) have nodes of higher-intensity GFP-positive foci and lose axonal commissures between the dorsal and ventral nerve cords. (c) TDP-43(A315T); tax-6(ok2065) have nodes of higher-intensity GFP-positive foci, loss of axonal processes, and exhibit axonal defasciculation. (d) High-magnification image of a segment of the TDP-43(A315T); tax-6(ok2065) ventral nerve cord near the vulva. TDP-43(A315T); tax-6(ok2065) display severe axonal defasciculation, dystrophic neurites, and nodes of higher-intensity GFP-positive foci.
Supplemental Fig. 3 Calcineurin inhibition and activation have opposite effects on pTDP. (a) HEK293 cells treated with the calcineurin inhibitor FK506 accumulate pTDP-35. (b) HEK293 cells treated with NiCl2 have reduced pTDP-35. (c) HDAC6 mRNA levels do not significantly change in response to FK506 or ethacrynic acid. Expression levels of HDAC6 were tested by quantitative reverse transcription PCR (qRT-PCR). Three independent experiments were evaluated and combined for the average relative expression change. All values were normalized to the housekeeping gene GAPDH. Error bars are standard deviation. Significance was evaluated using one-way analysis of variance with Tukey’s multiple comparison test, but no significant differences were found between experimental conditions. (d) Splicing of TDP-43 regulated genes FNIP, POLDIP3/SKAR, and STAG2 was evaluated using RT-PCR. Relative intensities of mRNA splice isoforms were unchanged between experimental samples.
Supplementary Fig. 4 Calcineurin co-localizes with pTDP pathology in FTLD-TDP hippocampus. (a) 60X fluorescent panel image of DAPI (Blue), calcineurin (Green), pTDP (Red). (b) Colocalization of calcineurin and pTDP. (c) Plot of pTDP and calcineurin colocalization (Pearson’s coefficient of correlation = 0.5502). (d) DAPI. (e) Calcineurin. Peripheral 50μM or larger filamentous bodies observed here and in (a-b) are green autofluorescent artifacts from tissue preparation (See Supplementary Fig. 6 for single label calcineurin immunostaining). (f) pTDP.
Supplementary Fig. 5 Calcineurin co-localizes with pTDP pathology in FTLD-TDP frontal cortex. (a) 60x fluorescent panel image of DAPI (Blue), calcineurin (Green), pTDP (Red). (b) Colocalization of calcineurin and pTDP. (c) Plot of pTDP and calcineurin colocalization (Pearson’s coefficient of correlation = 0.6433). (d) DAPI. (e) Calcineurin. (f) pTDP.
Supplemental Fig. 6 Single label calcineurin immunofluorescence immunostaining is consistent with double label immunostaining data from Supplemental Figs. 4 and 5. Immunostaining in FTLD-TDP hippocampus (a) calcineurin, (b) DAPI, (c) merge image, and in FTLD-TDP frontal cortex (d) Calcineurin, (e) DAPI, and (f) merge image.
Supplemental Fig. 7 Calcineurin co-localizes with pTDP pathology in ALS spinal cord motor neurons. Immunofluorescence of (a) DAPI, (b) calcineurin, (c) pTDP. (d) Merge image of DAPI, calcineurin, and pTDP immunofluorescence. (e) Colocalization of calcineurin and pTDP. (f) Plot of pTDP and calcineurin colocalization (Pearson’s coefficient of correlation = 0.8785).
Acknowledgements
We thank the reviewers for helpful comments and suggestions. We thank Michael Gitcho for constructive discussion about calcineurin and TDP-43. We thank Elaine Loomis, John Kushleika, Kaili Chickering, Susan Danner, and Samantha Rice for outstanding technical assistance and Allison Beller for outstanding administrative support. We thank Virginia Lee and Manuela Neumann for antibodies and the Developmental Studies Hybridoma Bank (NICHD) for the β-tubulin antibody E7. We thank WormBase (WS251) for C. elegans genetic information. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440), and other strains were provided by the National Bioresource Project (Japan). This work was supported by grants from the Department of Veterans Affairs [Merit Review Grant #I01BX002619 to B.K., Career Development Award 2 #I01BX007080 to N.L.] and National Institutes of Health [R01NS064131 to B.K and P50AG05136 for T.J.M. and C.D.K. for the UW ADRC for A.L.O and B.G.].
References
- 1.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. doi:S0006-291X(06)02318-7 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Ayala YM, Zago P, D'Ambrogio A, Xu YF, Petrucelli L, Buratti E, Baralle FE. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008;121:3778–3785. doi: 10.1242/jcs.038950. doi:jcs.038950 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Bandyopadhyay J, Lee J, Lee JI, Yu JR, Jee C, Cho JH, Jung S, Lee MH, Zannoni S, Singson A, Kim DH, Koo HS, Ahnn J. Calcineurin, a calcium/calmodulin-dependent protein phosphatase, is involved in movement, fertility, egg laying, and growth in Caenorhabditis elegans. Mol Biol Cell. 2002;13:3281–3293. doi: 10.1091/mbc.E02-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumgärtel K, Mansuy IM. Neural functions of calcineurin in synaptic plasticity and memory. Learn Mem. 2012;19:375–384. doi: 10.1101/lm.027201.112. [DOI] [PubMed] [Google Scholar]
- 6.Bolsover SR. Calcium signalling in growth cone migration. Cell Calcium. 2005;37:395–402. doi: 10.1016/j.ceca.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Brady OA, Meng P, Zheng Y, Mao Y, Hu F. Regulation of TDP-43 aggregation by phosphorylation and p62/SQSTM1. J Neurochem. 2011;116:248–259. doi: 10.1111/j.1471-4159.2010.07098.x. [DOI] [PubMed] [Google Scholar]
- 8.Che MX, Jiang LL, Li HY, Jiang YJ, Hu HY. TDP-35 sequesters TDP-43 into cytoplasmic inclusions through binding with RNA. FEBS Lett. 2015;589:1920–1928. doi: 10.1016/j.febslet.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Che MX, Jiang YJ, Xie YY, Jiang LL, Hu HY. Aggregation of the 35-kDa fragment of TDP-43 causes formation of cytoplasmic inclusions and alteration of RNA processing. FASEB J. 2011;25:2344–2353. doi: 10.1096/fj.10-174482. [DOI] [PubMed] [Google Scholar]
- 10.Cohen TJ, Hwang AW, Restrepo CR, Yuan CX, Trojanowski JQ, Lee VM. An acetylation switch controls TDP-43 function and aggregation propensity. Nat Commun. 2015;6:5845. doi: 10.1038/ncomms6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Conti L, Akinyi MV, Mendoza-Maldonado R, Romano M, Baralle M, Buratti E. TDP-43 affects splicing profiles and isoform production of genes involved in the apoptotic and mitotic cellular pathways. Nucleic Acids Res. 2015;43:8990–9005. doi: 10.1093/nar/gkv814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiMartini A, Fontes P, Dew MA, Lotrich FE, de Vera M. Age, model for end-stage liver disease score, and organ functioning predict posttransplant tacrolimus neurotoxicity. Liver Transpl. 2008;14:815–822. doi: 10.1002/lt.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferri A, Nencini M, Battistini S, Giannini F, Siciliano G, Casali C, Damiano MG, Ceroni M, Chiò A, Rotilio G, Carrì MT. Activity of protein phosphatase calcineurin is decreased in sporadic and familial amyotrophic lateral sclerosispatients. J Neurochem. 2004;90:1237–1242. doi: 10.1111/j.1471-4159.2004.02588.x. [DOI] [PubMed] [Google Scholar]
- 14.Fiesel FC, Weber SS, Supper J, Zell A, Kahle PJ. TDP-43 regulates global translational yield by splicing of exon junction complex component SKAR. Nucleic Acids Res. 2012;40:2668–2682. doi: 10.1093/nar/gkr1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujishiro H, Uchikado H, Arai T, Hasegawa M, Akiyama H, Yokota O, Tsuchiya K, Togo T, Iseki E, Hirayasu Y. Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol. 2009;117:151–158. doi: 10.1007/s00401-008-0463-2. [DOI] [PubMed] [Google Scholar]
- 16.Glass JD. Immunosuppression in Amyotrophic Lateral Sclerosis (ALS) (NIPALS2013) ClinicalTrials.gov. 2013 p NCT01884571. [Google Scholar]
- 17.Guthrie CR, Schellenberg GD, Kraemer BC. SUT-2 potentiates tau-induced neurotoxicity in Caenorhabditis elegans. Hum Mol Genet. 2009;18:1825–1838. doi: 10.1093/hmg/ddp099. doi:ddp099 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer's disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. S0006-8993(07)02224-X [pii] [DOI] [PubMed] [Google Scholar]
- 20.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iguchi Y, Katsuno M, Takagi S, Ishigaki S, Niwa J, Hasegawa M, Tanaka F, Sobue G. Oxidative stress induced by glutathione depletion reproduces pathological modifications of TDP-43 linked to TDP-43 proteinopathies. Neurobiol Dis. 2012;45:862–870. doi: 10.1016/j.nbd.2011.12.002. doi:S0969-9961(11)00377-9 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Inukai Y, Nonaka T, Arai T, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle FE, Akiyama H, Hisanaga S, Hasegawa M. Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS. FEBS Lett. 2008;582:2899–2904. doi: 10.1016/j.febslet.2008.07.027. doi:S0014-5793(08)00618-2 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, Rademakers R, Boeve BF, Parisi JE, Smith GE, Ivnik RJ, Petersen RC, Jack CR, Jr., Dickson DW. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008 doi: 10.1212/01.wnl.0000304041.09418.b1. doi:01.wnl.0000304041.09418.b1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, Petrucelli L, Senjem ML, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Jack CR, Parisi JE, Petersen RC, Dickson DW. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol. 2014;127:811–824. doi: 10.1007/s00401-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, Pradat PF, Camu W, Meininger V, Dupre N, Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. doi: 10.1038/ng.132. doi:ng.132 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Kabuta C, Kono K, Wada K, Kabuta T. 4-Hydroxynonenal induces persistent insolubilization of TDP-43 and alters its intracellular localization. Biochem Biophys Res Commun. 2015;463:82–87. doi: 10.1016/j.bbrc.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Shanware NP, Bowler MJ, Tibbetts RS. Amyotrophic lateral sclerosis-associated proteins TDP-43 and FUS/TLS function in a common biochemical complex to co-regulate HDAC6 mRNA. J Biol Chem. 2010;285:34097–34105. doi: 10.1074/jbc.M110.154831. doi:M110.154831 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King MM, Huang CY. Activation of calcineurin by nickel ions. Biochem Biophys Res Commun. 1983;114:955–961. doi: 10.1016/0006-291x(83)90653-8. [DOI] [PubMed] [Google Scholar]
- 29.Klee CB, Crouch TH, Krinks MH. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979;76:6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhara A, Inada H, Katsura I, Mori I. Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron. 2002;33:751–763. doi: 10.1016/s0896-6273(02)00607-4. [DOI] [PubMed] [Google Scholar]
- 31.Kuhnlein P, Sperfeld AD, Vanmassenhove B, Van Deerlin V, Lee VM, Trojanowski JQ, Kretzschmar HA, Ludolph AC, Neumann M. Two German kindreds with familial amyotrophic lateral sclerosis due to TARDBP mutations. Arch Neurol. 2008;65:1185–1189. doi: 10.1001/archneur.65.9.1185. doi:65/9/1185 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liachko NF, Guthrie CR, Kraemer BC. Phosphorylation Promotes Neurotoxicity in a Caenorhabditis elegans Model of TDP-43 Proteinopathy. J Neurosci. 2010;30:16208–16219. doi: 10.1523/JNEUROSCI.2911-10.2010. doi:30/48/16208 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liachko NF, McMillan PJ, Guthrie CR, Bird TD, Leverenz JB, Kraemer BC. CDC7 inhibition blocks pathological TDP-43 phosphorylation and neurodegeneration. Ann Neurol. 2013;74:39–52. doi: 10.1002/ana.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liachko NF, McMillan PJ, Strovas TJ, Loomis E, Greenup L, Murrell JR, Ghetti B, Raskind MA, Montine TJ, Bird TD, Leverenz JB, Kraemer BC. The tau tubulin kinases TTBK1/2 promote accumulation of pathological TDP-43. PLoS Genet. 2014;10:e1004803. doi: 10.1371/journal.pgen.1004803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling H, Holton JL, Lees AJ, Revesz T. TDP-43 pathology is present in most post-encephalitic parkinsonism brains. Neuropathol Appl Neurobiol. 2014;40:654–657. doi: 10.1111/nan.12067. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 37.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masuda S, Inui K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther. 2006;112:184–198. doi: 10.1016/j.pharmthera.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 39.McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- 40.McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, Perl DP, Hedley-Whyte ET, Price B, Sullivan C, Morin P, Lee HS, Kubilus CA, Daneshvar DH, Wulff M, Budson AE. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69:918–929. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT, Aging NIo, Association As National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morioka M, Hamada J, Ushio Y, Miyamoto E. Potential role of calcineurin for brain ischemia and traumatic injury. Prog Neurobiol. 1999;58:1–30. doi: 10.1016/s0301-0082(98)00073-2. [DOI] [PubMed] [Google Scholar]
- 43.Muramatsu T, Kincaid RL. Molecular cloning and chromosomal mapping of the human gene for the testis-specific catalytic subunit of calmodulin-dependent protein phosphatase (calcineurin A) Biochem Biophys Res Commun. 1992;188:265–271. doi: 10.1016/0006-291x(92)92379-c. [DOI] [PubMed] [Google Scholar]
- 44.Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, Woltjer R, Lopez OL, Hamilton R, Tsuang DW, Galasko D, Masliah E, Kaye J, Clark CM, Montine TJ, Lee VM, Trojanowski JQ. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114:221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 45.Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, Forman MS, Troost D, Kretzschmar HA, Trojanowski JQ, Lee VM. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. doi:314/5796/130 [pii] [DOI] [PubMed] [Google Scholar]
- 47.Ng AS, Rademakers R, Miller BL. Frontotemporal dementia: a bridge between dementia and neuromuscular disease. Ann N Y Acad Sci. 2015;1338:71–93. doi: 10.1111/nyas.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pallen CJ, Wang JH. Regulation of calcineurin by metal ions. Mechanism of activation by Ni2+ and an enhanced response to Ca2+/calmodulin. J Biol Chem. 1984;259:6134–6141. [PubMed] [Google Scholar]
- 49.Polli JW, Billingsley ML, Kincaid RL. Expression of the calmodulin-dependent protein phosphatase, calcineurin, in rat brain: developmental patterns and the role of nigrostriatal innervation. Brain Res Dev Brain Res. 1991;63:105–119. doi: 10.1016/0165-3806(91)90071-p. [DOI] [PubMed] [Google Scholar]
- 50.Rutherford NJ, Zhang YJ, Baker M, Gass JM, Finch NA, Xu YF, Stewart H, Kelley BJ, Kuntz K, Crook RJ, Sreedharan J, Vance C, Sorenson E, Lippa C, Bigio EH, Geschwind DH, Knopman DS, Mitsumoto H, Petersen RC, Cashman NR, Hutton M, Shaw CE, Boylan KB, Boeve B, Graff-Radford NR, Wszolek ZK, Caselli RJ, Dickson DW, Mackenzie IR, Petrucelli L, Rademakers R. Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet. 2008;4:e1000193. doi: 10.1371/journal.pgen.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savoia CP, Liu QH, Zheng YM, Yadav V, Zhang Z, Wu LG, Wang YX. Calcineurin upregulates local Ca(2+) signaling through ryanodine receptor-1 in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2014;307:L781–790. doi: 10.1152/ajplung.00149.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwab C, Arai T, Hasegawa M, Yu S, McGeer PL. Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol. 2008;67:1159–1165. doi: 10.1097/NEN.0b013e31818e8951. [DOI] [PubMed] [Google Scholar]
- 53.Seyfried NT, Gozal YM, Dammer EB, Xia Q, Duong DM, Cheng D, Lah JJ, Levey AI, Peng J. Multiplex SILAC analysis of a cellular TDP-43 proteinopathy model reveals protein inclusions associated with SUMOylation and diverse polyubiquitin chains. Mol Cell Proteomics. 2010;9:705–718. doi: 10.1074/mcp.M800390-MCP200. doi:M800390-MCP200 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shibasaki F, Hallin U, Uchino H. Calcineurin as a multifunctional regulator. J Biochem. 2002;131:1–15. doi: 10.1093/oxfordjournals.jbchem.a003063. [DOI] [PubMed] [Google Scholar]
- 55.Shiga A, Ishihara T, Miyashita A, Kuwabara M, Kato T, Watanabe N, Yamahira A, Kondo C, Yokoseki A, Takahashi M, Kuwano R, Kakita A, Nishizawa M, Takahashi H, Onodera O. Alteration of POLDIP3 splicing associated with loss of function of TDP-43 in tissues affected with ALS. PLoS One. 2012;7:e43120. doi: 10.1371/journal.pone.0043120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. doi:1154584 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, Neumann M. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, Clay D, Wood EM, Chen-Plotkin AS, Martinez-Lage M, Steinbart E, McCluskey L, Grossman M, Neumann M, Wu IL, Yang WS, Kalb R, Galasko DR, Montine TJ, Trojanowski JQ, Lee VM, Schellenberg GD, Yu CE. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson RS, Yu L, Trojanowski JQ, Chen EY, Boyle PA, Bennett DA, Schneider JA. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol. 2013;70:1418–1424. doi: 10.1001/jamaneurol.2013.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Q, Marescaux C, Wolff V, Jeung MY, Kessler R, Lauer V, Chen Y. Tacrolimus-associated posterior reversible encephalopathy syndrome after solid organ transplantation. Eur Neurol. 2010;64:169–177. doi: 10.1159/000319032. [DOI] [PubMed] [Google Scholar]
- 61.Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Hasegawa M, Akiyama H, Sikkink S, Pickering-Brown S, Mann DM. Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol. 2010;120:55–66. doi: 10.1007/s00401-010-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang YJ, Gendron TF, Xu YF, Ko LW, Yen SH, Petrucelli L. Phosphorylation regulates proteasomal-mediated degradation and solubility of TAR DNA binding protein-43 C-terminal fragments. Mol Neurodegener. 2010;5:33. doi: 10.1186/1750-1326-5-33. doi:1750-1326-5-33 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1 The C. elegans calcineurin B homolog, cnb-1, regulates TDP-43 phosphorylation in vivo. (a) A loss of function mutation in the calcineurin regulatory subunit cnb-1 increases phosphorylation and total protein levels of WT TDP-43 transgenic C. elegans. (b) Bar graphs represent measurement of 3 independent replicate immunoblots. Significance was evaluated using Student’s t-test between TDP-43(WT) and TDP-43(WT); cnb-1(jh103). p=0.04 comparing pTDP band intensities. p=0.04 comparing total TDP-43 band intensities. (c) cnb-1 loss of function increases pTDP and total TDP in M337V TDP-43 transgenic C. elegans. (d) Bar graphs are quantitation of 3 independent replicate immunoblots. Significance was evaluated using Student’s t-test between TDP-43(M337V) and TDP-43(M337V); cnb-1(jh103), p=0.001 comparing pTDP band intensities. p=0.003 comparing total TDP-43 band intensities. (e) TDP-43(M337V) and TDP-43(M337V); cnb-1(jh1035) were subjected to sequential protein extraction with detergents of increasing solubilizing strengths. Levels of sarkosyl-insoluble (SARK) pTDP are increased with loss of cnb-1. (f) Dispersal velocities of normal and mutant control worm strains were measured by calculating the radial distance traveled from a designated central starting point over 30 minutes. N>100 for each genotype tested. cnb-1(jh103) is significantly hyperactive relative to wild-type worms, p<0.01. (g) TDP-43(M337V); cnb-1(jh103) animals have significantly reduced locomotion velocity compared to TDP-43(M337V) alone. N>130 per genotype, p=0.01. (c) TDP-43(M337V SS/AA); cnb-1(jh103) move better than TDP-43(M337V SS/AA) alone, comparable to the cnb-1(jh103) hyperactivity observed in (f). N>200, p<0.001.
Supplemental Fig. 2 C. elegans TDP-43 tg; tax-6(−) animals exhibit nerve cord defasciculation and GFP foci. (a-c) High-magnification images of identical posterior segments of the ventral nerve cord and axonal processes. (a) tax-6(ok2065) have normal fasciculation of axonal bundles and commissures that extend from the dorsal to the ventral nerve cord. (b) TDP-43(A315T) have nodes of higher-intensity GFP-positive foci and lose axonal commissures between the dorsal and ventral nerve cords. (c) TDP-43(A315T); tax-6(ok2065) have nodes of higher-intensity GFP-positive foci, loss of axonal processes, and exhibit axonal defasciculation. (d) High-magnification image of a segment of the TDP-43(A315T); tax-6(ok2065) ventral nerve cord near the vulva. TDP-43(A315T); tax-6(ok2065) display severe axonal defasciculation, dystrophic neurites, and nodes of higher-intensity GFP-positive foci.
Supplemental Fig. 3 Calcineurin inhibition and activation have opposite effects on pTDP. (a) HEK293 cells treated with the calcineurin inhibitor FK506 accumulate pTDP-35. (b) HEK293 cells treated with NiCl2 have reduced pTDP-35. (c) HDAC6 mRNA levels do not significantly change in response to FK506 or ethacrynic acid. Expression levels of HDAC6 were tested by quantitative reverse transcription PCR (qRT-PCR). Three independent experiments were evaluated and combined for the average relative expression change. All values were normalized to the housekeeping gene GAPDH. Error bars are standard deviation. Significance was evaluated using one-way analysis of variance with Tukey’s multiple comparison test, but no significant differences were found between experimental conditions. (d) Splicing of TDP-43 regulated genes FNIP, POLDIP3/SKAR, and STAG2 was evaluated using RT-PCR. Relative intensities of mRNA splice isoforms were unchanged between experimental samples.
Supplementary Fig. 4 Calcineurin co-localizes with pTDP pathology in FTLD-TDP hippocampus. (a) 60X fluorescent panel image of DAPI (Blue), calcineurin (Green), pTDP (Red). (b) Colocalization of calcineurin and pTDP. (c) Plot of pTDP and calcineurin colocalization (Pearson’s coefficient of correlation = 0.5502). (d) DAPI. (e) Calcineurin. Peripheral 50μM or larger filamentous bodies observed here and in (a-b) are green autofluorescent artifacts from tissue preparation (See Supplementary Fig. 6 for single label calcineurin immunostaining). (f) pTDP.
Supplementary Fig. 5 Calcineurin co-localizes with pTDP pathology in FTLD-TDP frontal cortex. (a) 60x fluorescent panel image of DAPI (Blue), calcineurin (Green), pTDP (Red). (b) Colocalization of calcineurin and pTDP. (c) Plot of pTDP and calcineurin colocalization (Pearson’s coefficient of correlation = 0.6433). (d) DAPI. (e) Calcineurin. (f) pTDP.
Supplemental Fig. 6 Single label calcineurin immunofluorescence immunostaining is consistent with double label immunostaining data from Supplemental Figs. 4 and 5. Immunostaining in FTLD-TDP hippocampus (a) calcineurin, (b) DAPI, (c) merge image, and in FTLD-TDP frontal cortex (d) Calcineurin, (e) DAPI, and (f) merge image.
Supplemental Fig. 7 Calcineurin co-localizes with pTDP pathology in ALS spinal cord motor neurons. Immunofluorescence of (a) DAPI, (b) calcineurin, (c) pTDP. (d) Merge image of DAPI, calcineurin, and pTDP immunofluorescence. (e) Colocalization of calcineurin and pTDP. (f) Plot of pTDP and calcineurin colocalization (Pearson’s coefficient of correlation = 0.8785).