Abstract
Lactic acid (LA) is present in tumors, asthma, and wound healing, environments with elevated IL-33 and mast cell infiltration. While IL-33 is a potent mast cell activator, how LA affects IL-33-mediated mast cell function is unknown. To investigate this, mouse bone marrow-derived mast cells (BMMC) were cultured with or without LA and activated with IL-33. LA reduced IL-33-mediated cytokine and chemokine production. Using inhibitors for monocarboxylate transporters (MCT) or replacing LA with sodium lactate revealed that LA effects are MCT-1- and pH-dependent. LA selectively altered IL-33 signaling, suppressing TAK1, JNK, ERK, and NFκB phosphorylation, but not p38 phosphorylation. LA effects in other contexts have been linked to HIF-1α, which was enhanced in BMMC treated with LA. Since HIF-1α has been shown to regulate the microRNA miR-155 in other systems, LA effects on miR-155-5p and -3p species were measured. In fact, LA selectively suppressed miR-155-5p in a HIF-1α-dependent manner. Moreover, overexpressing miR-155-5p, but not miR-155-3p, abolished LA effects on IL-33-induced cytokine production. These in vitro effects of reducing cytokines were consistent in vivo, since LA injected intraperitoneally into C57BL/6 mice suppressed IL-33-induced plasma cytokine levels. Lastly, IL-33 effects on primary human mast cells were suppressed by LA in an MCT-dependent manner. Our data demonstrate that LA, present in inflammatory and malignant microenvironments, can alter mast cell behavior to suppress inflammation.
Keywords: Lactic acid, IL-33, mast cell, hypoxia-inducible factor, inflammation
Introduction
Mast cells are sentinels of the innate immune system, guarding the body against select bacterial and parasitic infections. However, mast cells are best known for the major role they play in allergies and allergic asthma. The interaction of allergen with mast cell-bound IgE and subsequent signaling through the IgE receptor, FcεRI, result in a signaling cascade provoking release of early and late phase mediators (1–5). The early phase mediators, released within minutes of activation, include tryptases, chymases, histamine, prostaglandins, leukotrienes and platelet-activating factor, whereas the late phase mediators, released hours later, consist of cytokines and chemokines, including IL-1β, IL-4, IL-5, IL-6, IL-10, IL-13, TNF, MIP-1α, and MCP-1. These factors yield the clinical symptoms of immediate hypersensitivity, including the wheal-and-flare response, itching, and vasodilation/edema. Mast cells can also promote chronic diseases such as asthma upon repeated antigen exposure, which results in airway remodeling due to sustained inflammation (1–5).
While IgE crosslinking is the best-studied form of mast cell activation, many stimuli elicit a mast cell response. IL-33 is a recently discovered alarmin in the IL-1 family. Produced by endothelial cells, epithelial cells, fibroblasts, mast cells, and keratinocytes in response to damage or stress, IL-33 promotes a TH2 response (6–9). Binding to the ST2/IL-1RacP receptor on mast cells results in the release of cytokines, chemokines and lipid mediators (6, 7). IL-33 has also been shown to promote mast cell survival, maturation and adhesion (8, 10). While it is a poor inducer of degranulation, IL-33 augments degranulation triggered through the IgE receptor (6, 7). IL-33 has beneficial effects in atherosclerosis, cardiac remodeling and helminth infection (8). However, it has been linked to asthma, rheumatoid arthritis, multiple sclerosis, Type I diabetes, and skin inflammation (7).
Inflammation causes important changes in the cellular microenvironment, which can be beneficial if temporally and spatially controlled, but pathological when chronic. A well-known example of chronic inflammation is the tumor microenvironment (TME). Tumors are known to preferentially undergo anaerobic glycolysis, even in the presence of sufficient oxygen, resulting in a hypoxic microenvironment with high (40mM) lactic acid (LA) concentrations (11–19). These unique environmental factors can alter cellular responses, allowing tumors to escape immune surveillance (16, 19–21). There is evidence that the TME uses LA to promote tumor-associated macrophages (TAM) to take on an M2 phenotype, which is anti-inflammatory and has reduced antigen presentation ability (12, 19). Tumor-derived LA has also been shown to inhibit dendritic cell function, resulting in decreased proliferation and reduced antigen presentation (14). Among cytotoxic T cells, LA decreases proliferation and inhibits their cytotoxic function (16). Other examples of altered microenvironments that have increased lactate levels are obesity, hypertension, and Type II diabetes, as well as tissues suffering injury, infection, or ischemia (20–23).
Since LA is known to alter cellular responses in inflammatory environments, we tested the effects of physiological LA concentrations on mast cell function. LA exposure for 24 hours was sufficient to suppress IL-33-mediated cytokine secretion, effects that were pH- and monocarboxylate transporter-1 (MCT-1)-dependent. Suppression of IL-33-induced inflammatory cytokine and chemokine secretion was accompanied by reduced activation of several signaling intermediates. Expectedly, LA treatment enhanced HIF-1α expression. This correlated with a decrease in pro-inflammatory miR-155-5p, which was reversed by HIF-1α blockade. miR-155-5p overexpression abolished LA suppressive effects, demonstrating the critical nature of the HIF-1α-miR-155 cascade. These results provide insight into microenvironmental effects during wound healing, chronic inflammation, and tumorigenesis.
Materials and Methods
Animals
C57BL/6 male and female mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and used at a minimum of 6 weeks old, with approval from the Virginia Commonwealth University institutional animal care and use committee (IACUC).
Mouse Mast Cell Cultures
Mouse bone marrow-derived mast cells (BMMC) were derived by harvesting bone marrow from C57BL/6 mouse femurs, followed by culture in complete RPMI (cRPMI) 1640 medium (Invitrogen Life Technologies, Carlsbad, CA) containing 10% FBS, 2mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 1mM sodium pyruvate, and 1mM HEPES (all from Corning, Corning, NY), supplemented with IL-3-containing supernatant from WEHI-3B cells and SCF-containing supernatant from BHK-MKL cells. The final concentrations of IL-3 and SCF were adjusted to 1.5ng/ml and 15ng/ml, respectively, as measured by ELISA. BMMC were used after 3 weeks of culture, at which point these primary populations were >90% mast cells, based on staining for c-Kit and FcεRI expression. Mouse peritoneal mast cells were obtained by collecting peritoneal lavage from C57BL/6 mice, which cultured in cRPMI supplemented with recombinant mouse IL-3 and SCF at 10ng/mL each. Peritoneal mast cells were used after 10–12 days, at which these ex vivo expanded cells were approximately 85% mast cells, based on staining for c-Kit and FcεRI expression.
Cytokines and Reagents
Recombinant mouse IL-3, SCF, and IL-33, recombinant human IL-33, as well as mouse IL-6, TNF, and MCP-1 (CCL-2) ELISA kits were purchased from BioLegend (San Diego, CA). Mouse MIP-1α (CCL-3) and VEGF ELISA kits were purchased from PeproTech (Rocky Hill, NJ). Mouse IL-13 ELISA kits were purchased from eBioscience (San Diego, CA). L-(+)-lactic acid and Sodium L-lactate were purchased from Sigma-Aldrich (St. Louis, MO). Human IL-6, TNF, and MCP-1 ELISA kits were purchased from BD OptEIA (BD Biosciences; Franklin Lakes, NJ).
Cell Culture Conditions
For IL-33 activation, BMMC (2×106 cells/mL) were cultured in 20ng/mL of IL-3 and SCF in cRPMI. An equal volume of 25mM LA in cRPMI was added to the cell suspension, resulting in a final cell concentration of 1×106 cells/ml, 10ng/mL of IL-3 and SCF, and 12.5mM LA. Control conditions received cRPMI in place of LA. After 24 hours of pretreatment in LA media, cells then received 100ng/mL of IL-33 for 16 hours, after which supernatants were collected. pH was measured for media alone, lactic acid, and lactate-conditioned media using the Beckman Phi 45 pH meter.
Western Blot Analysis
Cells were cultured at 2×106/ml and lysed in Lysis Buffer (Cell Signaling Technology, Danvers, MA) supplemented with 1.5X ProteaseArrest (G-Biosciences, Maryland Heights, MO). Protein concentration was determined using the Pierce BCA protein assay kit (Thermo Scientific). Proteins were resolved by SDS-PAGE using 30 μg of total protein per sample on 4–20% Mini-Protean TGX Gels (Bio-Rad, Hercules, CA). Transfer was made onto nitrocellulose membranes, which were then blocked for 1 hour at room temperature with 2% BSA in PBS. Membranes were rinsed in PBS and then incubated overnight at 4°C in PBS-T containing 2% BSA and primary antibody diluted 1:1000. Membranes were probed with antibodies purchased from Cell Signaling Technology (Danvers, MA), including: anti phospho-TAK1 (Thr-184/187, catalog no. 4508), total TAK1 (catalog no. 5206), phospho-NFκB p65 (Ser-536, catalog no. 13346), total NFκB p65 (catalog no. 4764), phospho-JNK (Thr-183/Tyr-185, catalog no. 9251), total JNK (catalog no. 9258), phospho-p38 (Thr-180/Tyr-182, catalog no. 9216), total p38 (catalog no. 9212), phospho-ERK1/2 (Thr-202/Tyr-204, catalog no. 9101), total ERK1/2 (catalog no. 4695), and GAPDH (catalog no. 2118). Membranes were washed the next day with PBS-T every 5 minutes for a total of 30 minutes, then incubated with a 1:15,000 dilution of either goat anti-rabbit DyLight800 (catalog no. 5151) or goat anti-mouse DyLight680 (catalog no. 5470) infrared-labeled secondary antibodies (Cell Signaling Technology, Danvers, MA). Membranes were rinsed a final time before being analyzed with an Odyssey CLx infrared scanner (Li-Cor, Lincoln, Nebraska). Normalization was done using Image Studio 4.0 software (Li-Cor, Lincoln, Nebraska).
Inhibitors
TAK1 inhibitor (5Z)-7-Oxozeanol (5 μM; Tocris Bioscience, Bristol, UK), JNK inhibitor SP600125 (10 μM; EMD Millipore, Billerica, MA), NFκB inhibitor BAY 11-7085 (2 μM; Tocris Bioscience, Bristol, UK), ERK inhibitor FR180204 (25 μM; Cayman Chemical, Ann Arbor, MI) and monocarboxylate transporter (MCT) inhibitors α-cyano-4-hydroxycinnamic acid (CHC; 5 mM; Sigma Aldrich, St. Louis, MO) and AR-C155858 (100 nM; Tocris Bioscience, Bristol, UK), were solubilized in dimethyl sulfoxide (DMSO). Under the conditions used, none of these inhibitors caused significant cell death. Inhibitors were added to culture one hour prior to activation with IL-33 (100 ng/mL). Supernatants were collected 16 hours later, and ELISAs used to determine cytokine production.
mRNA and microRNA qPCR
After BMMC were treated in 12.5mM LA, total RNA was extracted with TRIzol reagent (Life Technologies, Grand Island, NY) and later measured using the Thermo Scientific NanoDrop™ 1000 UV–vis Spectrophotometer (Thermo Scientific, Waltham, MA) according to manufacturer’s recommended protocol. For RNA extract that would be used to measure microRNA expression, polyadenylation was done prior to cDNA synthesis using the qScript microRNA cDNA synthesis kit (Quanta Biosciences, Gaithersburg, MD). For samples used to measure mRNA expression, cDNA was synthesized using the qScript cDNA Synthesis Kit (Quanta Biosciences, Gaithersburg, MD) following the manufacturer’s protocol. qPCR analysis was performed with Bio Rad CFX96 Touch™ Real-Time PCR Detection System (Hercules, CA) and SYBR® Green detection using a relative Livak Method (24). Each reaction was performed according to the manufacturer’s protocol using 10ng of sample cDNA, with the following primers: mmu-miR-155-5p, 5′-UUAAUGCUAAUUGUGAUAGGGGU-3′ (cat no. MMIR-0155, Quanta Biosciences); mmu-miR-155-3p, 5′-CUCCUACCUGUUAGCAUUAAC-3′ (cat no. MMIR-0155*, Quanta Biosciences); HIF-1α forward, 5′-TGAGGCTCACCATCAGTTAT – 3′; HIF-1α reverse, 5′-TAACCCCATGTATTTGTTC-3′; MCT-1 forward, 5′-GCTGGAGGTCCTATCAGCAG-3′; MCT-1 reverse, 5′-CGGACAGCTTTTCTCCTTTG-3′; MCT-2 forward, 5′-TTACCGTATCTGGGCCTTTG-3′; MCT-2 reverse, 5′-CCAAAGCAGTTTCGAAGGAG-3′; GAPDH forward, 5′-GATGACATCAAGAAGGTGGTG-3′; GAPDH reverse, 5′-GCTGTAGCCAAATTCGTTGTC-3′; SNORD47, 5′-GUGAUGAUUCUGCCAAAUGAUACAAAGUGAUAUCACCUUUAAACCGUUCAUUUU AUUUCUGAGG-3′ (cat no. MM-SNORD47, Quanta Biosciences); β-Actin forward, 5′-GATGACGATATCGCTGCGC-3′; and β-Actin reverse, 5′-CTCGTCACCCACATAGGAGT-3′. Amplification conditions for microRNA detection were set to heat-activation at 95 °C for 2 min followed by 40 cycles of denaturation at 95 °C for 5s, annealing at 60 °C for 15s, and extension at 70 °C for 15s. All other reactions consisted of a heat-activation step at 95 °C for 10 min followed by 40 cycles of 95 °C for 15s, 55 °C for 30s and 60 °C for 1 min. Fluorescence data were collected during the extension step of the reaction.
siRNA and miR-mimic Transfection
BMMC were transfected with 100nM HIF-1α siRNA (cat no. GS15251) and scrambled FlexiTube siRNA (cat no. 1027280) from Qiagen (Valencia, CA). miR-155-5p (cat no. 470919), miR-155-3p (cat no. 471999), and negative control/mock (cat no. 479903) microRNA mimics were transfected at 50nM and purchased from Exiqon (Woburn, MA). Transfections were done with the Amaxa Nucleofactor from Lonza (Allendale, NJ) using program T5 in the following transfection media: Dulbecco’s modified Eagle’s medium (DMEM), 20% FBS, and 50 mM HEPES Buffer. BMMC were incubated in IL-3 and SCF (10ng/mL each) and used 48 hours after transfection.
Intraperitoneal Injections
C57BL/6 mice (12–16 weeks old were first injected with 1mg/kg of ketoprofen from Spectrum Chemical (New Brunswick, NJ), then 30 minutes later injected with 4mg/kg of 4%(w/v) of LA. 16 hours later, mice were then injected with 1μg of recombinant mouse IL-33 from eBioscience (San Diego, CA). Four hours later, mice were euthanized and blood was collected via cardiac puncture and plasma prepared from collected blood. All animal protocols were approved by the VCU Insititutional Animal Care and Use Committee.
Human Skin Mast Cell Culture
As approved by the Internal Review Board at the University of South Carolina, surgical skin samples were collected from the Cooperative Human Tissue Network of the National Cancer Institute. Skin mast cells were harvested and cultured from 5 human donors as previously described (25). After 6–10 weeks, mast cells were used at which time purity was nearly 100%, as confirmed with toluidine blue staining.
Statistical Analysis
Data were presented as mean ± SE and analyzed using GraphPad Prism 6 software (GraphPad, La Jolla, CA). Comparisons between two groups were done using unpaired Student’s t test and comparisons between multiple groups were done using one-way analysis of variance with Tukey’s post-hoc test. All p values <0.05 were deemed significant.
Results
Lactic acid suppresses IL-33-mediated mast cell inflammatory cytokine production
In a normal physiological state, LA is present in peripheral tissue at 1–2 mM (26), whereas in pathological conditions LA concentrations often exceed 10 mM (19, 27). Elevated levels of LA are present in tissues where pathological conditions also promote mast cell infiltration and IL-33 expression (7, 13, 14, 28). Therefore, we decided to first investigate how LA alters IL-33-mediated activation of mast cells by measuring the impact on cytokine secretion. The timing and dose of LA treatment were determined by measuring changes in IL-33-induced cytokine and chemokine production. LA exposure greatly reduced IL-33-mediated IL-6 secretion, with maximal effects at 6–24 hours of pre-exposure. Adding LA simultaneously or 48 hours prior to IL-33 yielded no inhibition (Figure 1A). In response to varying doses of LA, an IC50 between 6–12.5 mM was observed for different cytokines (Figure 1B). While 25 mM LA yielded further inhibition of some cytokines, it also resulted in approximately 30% cell death (data not shown). Hence, we employed 12.5 mM LA, which elicited no changes in cell viability (data not shown). The inhibitory effect of LA was consistent among pro-inflammatory cytokines, since BMMC pretreated in 12.5 mM LA for 24 hours prior to IL-33 activation showed significantly decreased IL-6, TNF, MCP-1, MIP-1α, and IL-13 production, yet increased VEGF production (Figure 1C). Lastly, peritoneal mast cells harvested from C57BL/6 mice were also pretreated with LA then activated with IL-33. As shown in Figure 1D, LA significantly suppressed the IL-33-induced production of IL-6, MIP-1α, and MCP-1 by peritoneal mast cells as well. These findings demonstrate that LA can selectively suppress inflammatory cytokines from in vitro differentiated as well as ex vivo expanded primary mast cells.
FIGURE 1. Lactic acid suppresses IL-33-mediated cytokine production in mast cells.
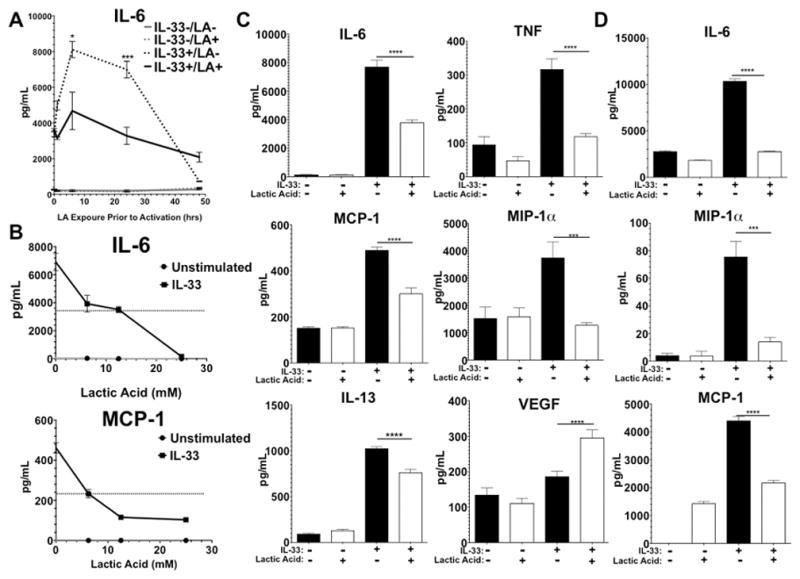
Mast cells were pretreated with 12.5 mM lactic acid prior to IL-33 activation. Supernatants were collected 16 hrs after IL-33 activation. A) Time-course and B) dose-response experiments were done to determine kinetics of lactic acid effects on BMMC cytokine production. C) BMMC treated with 12.5mM LA for 24 hours were activated for 16 hours with IL-33, and supernatants were assessed by ELISA. D) Peritoneal mast cells harvested from C57BL/6 mice were treated and activated as in (C). Results are expressed as mean ±SEM. Results of A), B), and D) are representative of three independent experiments conducted in triplicate. Results in C) are three independent experiments conducted in triplicate. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; NS, not significant.
Lactic acid-mediated suppression is pH- and MCT-1-dependent
Previous papers have demonstrated that pH plays a critical role in the ability of LA to alter cellular function. For example, LA has been shown to suppress LPS-induced TNF secretion and delay NFκB activation in monocytes, while promoting an anti-inflammatory M2 phenotype in macrophages (19, 29, 30). However, lactate, the salt form of LA which does not lower pH, enhances LPS-mediated inflammatory responses in macrophages (21). We noted that media pH decreased to 6.5 immediately after adding LA, and returned to 7.2 within 6–8 hours (data not shown). To investigate the importance of pH on LA effects in mast cells, BMMC were cultured in 12.5 mM LA or sodium lactate for 24 hours prior to IL-33 activation. The results showed a clear difference, as LA suppressed IL-33-mediated IL-6, TNF, IL-13, and MCP-1 production, while sodium lactate had no effect (Figure 2A), indicating the importance of pH on cytokine production.
FIGURE 2. LA effects are pH- and MCT-1-dependent.
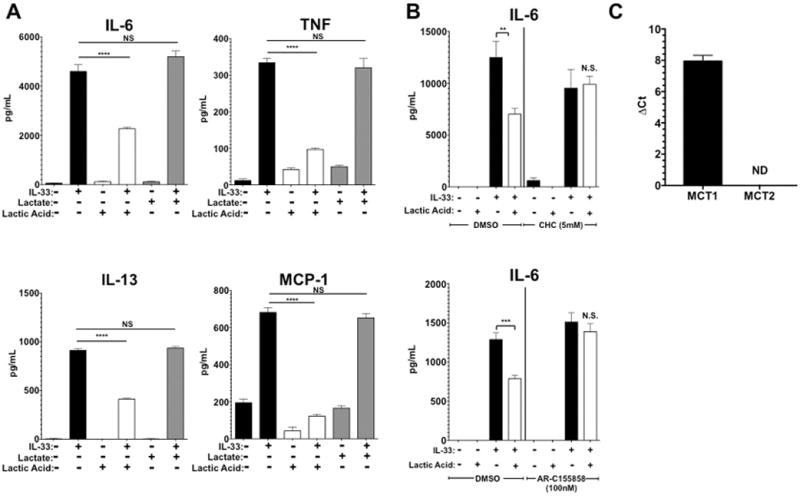
A) BMMC were cultured in media alone, 12.5 mM lactic acid, or sodium lactate media for 24 hours prior to IL-33 activation. ELISA was used to measure IL-6, TNF, IL-13, and MCP-1 in culture supernatants. B) BMMC were treated with either vehicle (DMSO) or the indicated MCT inhibitors, α-cyano-4-hydroxycinnamic acid (CHC) or AR-C155858 for 1 hour prior to lactic acid or media treatment for 24 hours. Cells were then activated with 100ng/mL of IL-33 for 16 hours, supernatants collected, and IL-6 measured using ELISA. C) MCT-1 and MCT-2 expression was measured during 40 cycles of RT-qPCR using RNA harvested from BMMC. β-actin was used as the housekeeping gene for MCT-1 and GAPDH for MCT-2, based on primer optimization and melting point similarities. ND means “not detectable”. Results are expressed as mean ±SEM and are representative of 3 independent experiments conducted in triplicate. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; NS, not significant.
Other work has demonstrated that cellular entry and egress of LA is controlled by MCT, a group of transporters within the solute carrier family (SLC16). MCT-1 and -2 can transport lactate into the cell (31–33). While MCT-2 expression is largely restricted to the testis, heart, and brain, MCT-1 is widely expressed including in hematopoietic cells (31, 34, 35). To determine if MCT-1 is important for LA effects on mast cells, we treated BMMC with two different MCT inhibitors, α-cyano-4-hydroxycinnamic acid (CHC; a pan-MCT inhibitor) and AR-C155858 (an MCT-1 & -2 inhibitor), and measured IL-33-induced cytokine production. In the presence of these inhibitors, LA did not suppress IL-33-mediated production of IL-6 (Figure 2B). To determine MCT-1 and -2 expression in mast cells, RNA was harvested from BMMC and analyzed via qPCR. Functionality of MCT-1 and -2 primers were confirmed by using kidney tissue cDNA with these primers (data not shown). While MCT-1 transcripts were easily detected in BMMC within 40 cycles, MCT-2 transcripts were not detectable at all (Figure 2C). Thus these data demonstrate that LA effects on IL-33 activation are likely dependent upon acid pH- and the MCT-1 carrier.
Lactic acid selectively alters IL-33 signaling
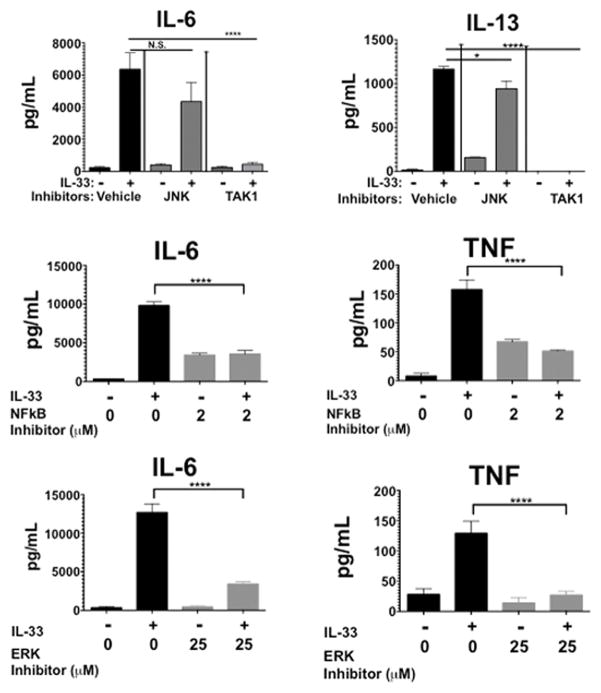
We next sought to elucidate how LA alters IL-33 signaling. Flow cytometry revealed a modest (approximately 17%) decrease in surface ST2 expression after 24 hours of LA treatment, which seemed unlikely to explain the inhibitory effects (data not shown). Therefore we examined LA-mediated alterations in downstream signaling events. BMMC were pretreated in 12.5 mM LA for 24 hours and activated with IL-33 before lysis. These lysates were then used to assess changes in TAK1, JNK, p38, NFκB p65, and ERK phosphorylation, pathways suggested to be important for IL-33-mediated mast cell function (36). LA suppressed IL-33-induced activation of NFκB p65, JNK, ERK, and TAK1, without altering p38 phosphorylation (Figure 3). To determine if blocking the suppressed pathways alone is sufficient to mimic the effects of LA on cytokine production, we treated BMMC with JNK, TAK1, ERK, or NFκB chemical inhibitors. While the JNK inhibitor did not reproduce the same level of suppression, TAK1, NFκB, or ERK inhibition completely abolished IL-33-mediated cytokine production, mimicking LA effects (Figure 4). Thus LA inhibits multiple IL-33 signaling cascades, yielding redundant suppression of the pro-inflammatory stimulus.
FIGURE 3. IL-33 signaling is suppressed by lactic acid treatment.
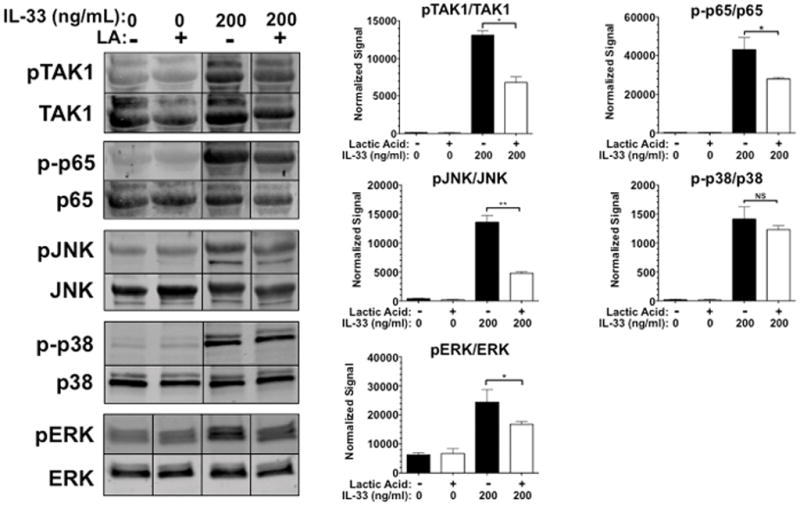
BMMC were pretreated for 24 hours with media or lactic acid then activated with IL-33 at 200ng/mL for 5 minutes. Lysates were analyzed by western blotting to measure expression and phosphorylation of the indicated proteins. Representative blots are shown on the left, while bar charts show mean ± SEM of phospho-protein:total protein ratios calculated using the LI-Cor Odyssey software Image Studio 4.0. Results shown are representative of three experiments done in triplicate. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; NS, not significant.
FIGURE 4. Signal transduction inhibitors reproduce lactic acid-mediated suppression.
BMMC were pretreated with inhibitors (JNK: SP600125, 10 μM; TAK1: (5Z)-7-Oxozeaenol, 5 μM; NFκB: BAY 11-7085, 2 μM; ERK: FR180204, 25 μM) for 1 hour. Cells were then activated with 100ng/mL of IL-33 for 16 hours, supernatants were collected and analyzed via ELISA. Data are shown as mean ± SEM of three independent experiments conducted in triplicate. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; NS, not significant.
miR-155-5p blockade is required for LA-mediated suppression
The microRNA miR-155 has recently emerged as a powerful pro-inflammatory regulator, by virtue of its ability to target and suppress inhibitory proteins (37). Since LA is known to promote HIF-1α production (19, 38, 39) and HIF-1α can control miR-155 expression (40–42), we hypothesized that LA-induced HIF-1α might suppress miR-155, yielding a net negative effect on IL-33 signaling. After 6 hours of LA treatment, HIF-1α mRNA increased nearly 3-fold, while miR-155-5p was suppressed >50% in BMMC. Interestingly, the miR-155-3p species was unaffected, indicating selectivity of these effects (Figure 5A). HIF-1α siRNA transfection prevented LA-mediated miR-155-5p suppression (Figure 5B), demonstrating that this process is HIF-1α-dependent. To test the functional importance of miR-155-5p suppression, BMMC were transfected with miR-155-5p or miR-155-3p mimics (Figure 5C). BMMC transfected with a miR-155-3p mimic prior to LA treatment still showed suppression of IL-33-induced cytokine production (Figure 5D, upper graphs). However, transfecting the miR-155-5p mimic eliminated LA-mediated suppression, suggesting that miR-155-5p is critical for LA effects (Figure 5D, lower graphs).
FIGURE 5. Lactic acid effects require HIF-1α-dependent miR-155-5p suppression.
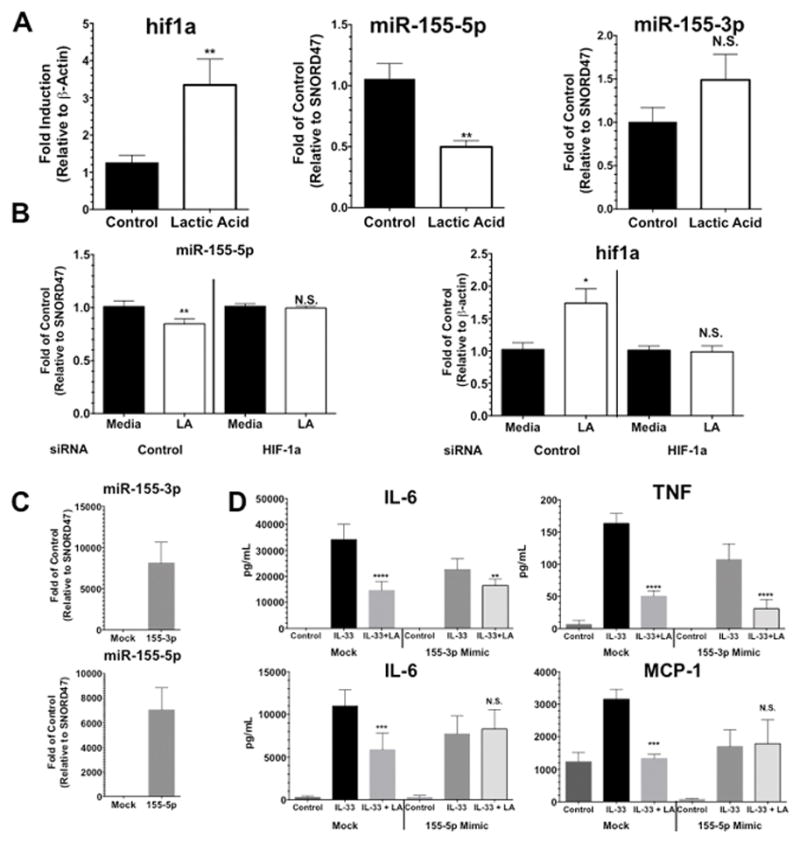
A) BMMC were treated with lactic acid for 6 hours followed by collecting RNA to measure HIF-1α and miR-155 product. B) Cells were transfected with HIF-1α siRNA, incubated for 24 hours, treated with lactic acid for 6 hours, and then microRNA and mRNA were collected to measure miR-155-5p, as well as HIF-1α (to confirm knockdown). C) and D) BMMC were transfected with miR-155 mimics or a control/Mock transcript. After 48 hours of culture, cells were treated with LA for 24 hours, activated with IL-33 for 16 hours, and cytokines were measured by ELISA. Confirmation of transfection is shown in C) and examining the ability of LA to suppress IL-33-mediated cytokine production is shown in D). Results are expressed as mean ±SEM. Results in A) are 3 independent experiments conducted in triplicate and results in B), C), and D) are representative of 2 independent experiments done in triplicate. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; NS, not significant.
Lactic acid suppresses IL-33-mediated inflammatory responses in vivo
Thus far, we have shown that LA suppresses IL-33-mediated mast cell inflammatory responses in vitro and ex vivo. Next we investigated LA effects on IL-33-mediated inflammatory responses in vivo. Intraperitoneal IL-33 injection has been shown to elicit mast cell-mediated inflammation coupled with cytokine production (43). Mice were first injected intraperitoneally with LA, then 16 hours later injected with IL-33. IL-33 greatly elevated plasma MCP-1 and IL-13 levels, an effect that was nearly completely reversed by LA pretreatment (Figure 6). Thus LA antagonizes the pro-inflammatory effects of IL-33 in vivo as well as in vitro.
FIGURE 6. Lactic acid suppresses IL-33-mediated inflammation in vivo.
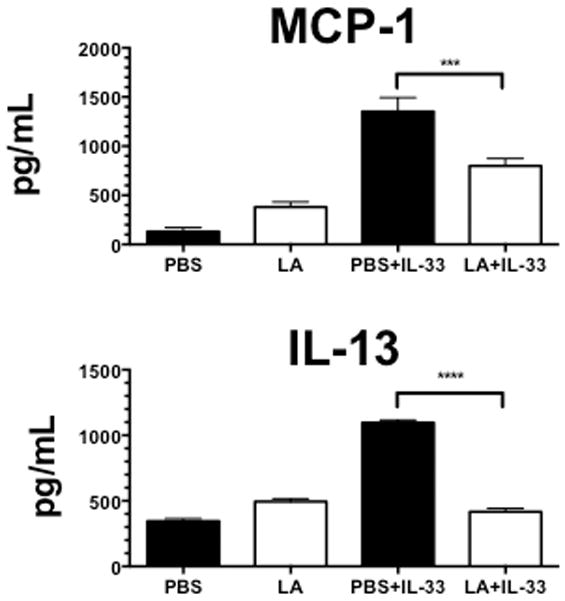
C57BL/6 mice were first injected subcutaneously with ketoprofen (1mg/kg in PBS) as an analgesic. 30 minutes later, mice were injected with either LA (4mg/kg in 4% (w/v) solution in PBS) or PBS alone. 16 hours later, mice were injected with either PBS or 1μg of IL-33. After 4 hours, mice were euthanized, blood plasma was collected from cardiac puncture, and samples analyzed by ELISA. Results are expressed as mean ±SEM and n=7. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; NS, not significant.
Lactic acid suppresses primary human skin mast cells in an MCT-dependent manner
In order to determine if our mouse data are consistent in human mast cells, we assessed the effects of LA on IL-33-induced cytokine production using primary human skin mast cells (SkMC). SkMC from 5 healthy donors were harvested and cultured in the presence of LA for 24 hours. Because SkMC respond poorly to IL-33 alone, we measured IL-33-mediated enhancement of IgE responses (7). LA pretreatment suppressed both IgE-mediated cytokine production and the enhancing effects of IL-33. Furthermore, LA effects were completely or partially reversed by the MCT inhibitor CHC (Figure 7). These data suggest that LA consistently suppresses IL-33 signaling, and mediates these effects via MCT transporters in both murine and human mast cells.
Figure 7. Primary human skin mast cell mediator release is decreased by lactic acid in an MCT-dependent manner.
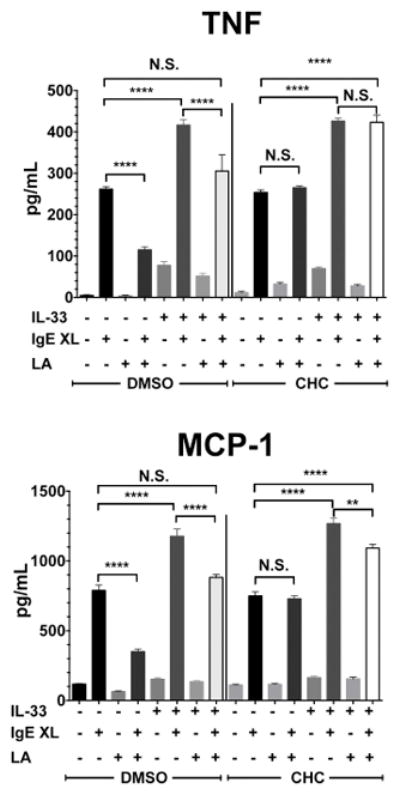
Primary human skin mast cells cultured from 5 donors were treated with either DMSO or an MCT inhibitor (CHC; 2.5mM), along with media alone or 12.5 mM lactic acid for 24 hours, then activated with either IL-33, IgE-Ag cross linking (IgE XL), or both. Supernatants were collected after 16 hours and analyzed by ELISA. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; NS, not significant.
Discussion
LA is a byproduct of anaerobic glycolysis and is known to be increased in a variety of pathological states including cancer, obesity, type II diabetes and wound healing (20–23). LA concentrations, which are 1–2mM in plasma at rest, can reach 20mM during acute exercise and 40mM at tumor sites (19, 26). While LA has been shown to inhibit cytotoxic T cell-mediated killing, promote M2 macrophage differentiation, and prevent dendritic cell antigen presentation, its effects on mast cell function have not been investigated (14–16, 19). Mast cells are known to participate in allergic disease, parasitic infection, and resistance to bacteria. They have less defined roles in cancer and wound healing, but are known to participate in both (2–5, 44–47). Our data indicate that LA alters inflammatory cytokine production in mast cells stimulated with IL-33, a cytokine elevated in many pathological conditions. In the context of cancer, this could promote tumor escape from immune surveillance, as is true for tumor-associated macrophages in response to tumor-derived LA (19). In wound healing, it may prevent a destructive chronic inflammatory response, but might also reduce pathogen clearance.
BMMC exhibited decreased inflammatory cytokine production when exposed to LA prior to IL-33 activation. Similar to reports on other cell types, the inhibitory effects of LA in our assays were tightly linked to acidity (15). While sodium lactate increases NFκB signaling and transcription in macrophages stimulated with LPS (21, 48), LA decreases LPS-mediated signaling in macrophages (29, 30). Sodium lactate is the salt of LA, with the deprotonated carboxyl group linked to sodium via an ionic bond. Our results show that without the decrease in pH, IL-33-induced cytokine and chemokine production was unaltered. Additionally, we demonstrated that the MCT-1 transporter is critical for LA-mediated suppression. Others have shown that MCT-1 employs proton co-transport with lactate (49), supporting our data showing LA but not its salt is suppressive. Interestingly, MCT-1 was recently shown to require a chaperone protein, CD147, for its function (32). This warrants further investigation of mast cell CD147 expression and how this protein contributes to LA effects.
LA has been shown to suppress TLR4-mediated signaling and cytokine production (29, 30). IL-33 signaling is still being unraveled, but shares common molecular pathways with TLRs. IL-33 activates the MAP3K TAK1 as an apical kinase, with resulting downstream activation of MAP kinases, NFκB, and AP-1 in mast cells (36). Our data show that while p38 phosphorylation is not affected, TAK1, NFκB p65, ERK and JNK phosphorylation were significantly diminished, and this correlated with decreased cytokine production. Chemical inhibitors of TAK1, ERK, or NFκB each mimicked LA effects on IL-33 signaling, while JNK inhibition had no effect. Whether LA effects on multiple signaling proteins are simply due to apical TAK1 blockade yielding multiple downstream consequences, and how LA inhibits phosphorylation are issues that require further study.
In response to LA treatment, HIF-1α expression was elevated in mast cells, as predicted by previous studies (19, 38, 39). A known molecule targeted by HIF-1α in other systems is miR-155, which is pro-inflammatory in many lineages (41). Studies show that miR-155 possesses a hypoxia response element in its promoter region, further connecting HIF-1α and miR-155 (42). We demonstrated that LA specifically suppresses miR-155-5p, while leaving miR-155-3p unaltered. This selectivity appears consequential, since miR-155-5p but not -3p overexpression reversed LA effects. Despite originating from the same transcribed pri-miRNA, it is not unusual for 3p and 5p strands from the same pri-miRNA to be expressed differently in mature form in response to different stimuli. By nature of being complimentary to one another, 3p and 5p strands can have very different targets, eliciting effects with negative or positive feedback on the degradation of these strands (50–52). Additionally, HIF-1α silencing restored miR-155-5p levels, indicating that in mast cells, HIF-1α negatively regulates miR-155-5p expression. This is another example of lineage-restricted effects, since HIF-1α induces miR-155 in populations such as epithelial cells (42). Clearly our understanding of hypoxia and tissue acidity will need to account for variations in lineage, allowing for nuanced effects.
The functional relevance of these findings is supported by consistent effects in vivo and on primary human mast cells. Several features of these experiments warrant further discussion and investigation. First, a recent paper showed that reduced pH enhances IgE-mediated mouse mast cell cytokine production (53). We incidentally noted the opposite while stimulating human mast cells with IgE (Figure 7). The differences between these studies could be due to the acids employed, assay parameters, or species variation. Kamide et al. did not specify the acid they employed. A strong acid might have different effects than LA (pKa=3.86) and may not be transported by MCT-1. The previous study also employed a 3-hour incubation, whereas we cultured for 24 hours. However, our IL-33 studies showed no enhancing effects of inflammatory cytokines at time points between 0–48 hours. More work should be done to examine the effect of LA on IgE-signaling. It is also important to state that our in vivo assay did not limit LA effects to mast cells, though mast cells are activated by systemic IL-33 (43). A variety of cytokine-producing cells respond to IL-33. This list continues to grow and currently includes mast cells, basophils, eosinophils, ILC2, some TH2 cells, and macrophages (54–58). Therefore our results demonstrate that LA can antagonize IL-33-induced systemic cytokine production in vivo, but do not restrict these effects to a specific lineage. While it is striking to find nearly complete suppression of cytokines in vivo, further study is needed to reveal how LA acts on various lineages.
In conclusion, LA is able to suppress inflammatory responses among IL-33-activated mast cells in a pH- and MCT-1-dependent manner. This suppression requires a HIF-1α-dependent blockade of miR-155-5p. LA’s ability to suppress IL-33-mediated inflammatory responses was reproduced in vivo and in human mast cells. These data provide fundamental insight into how tissue microenvironments, especially in pathological conditions, can greatly alter mast cell responses. Given our expanding comprehension of myriad activities played by IL-33 and mast cells, understanding and intervening in these signaling cascades is likely to be clinically important.
Acknowledgments
This work was supported by National Institutes of Health grants 1R01AI59638 and 1R01AI101153 to JJR and 1R01AI095494 to CAO
Abbreviations
- LA
lactic acid
- MCT
monocarboxylate transporter
- MCT-1
monocarboxylate transporter-1
- BMMC
bone marrow derived-mast cells
- HIF-1α
hypoxia induced factor 1-α
- TME
tumor microenvironment
- TAM
tumor associated macrophages
- SCF
stem cell factor
- BCA
bicinchoninic acid assay
- TAK1
TGF-β activated kinase-1
- CHC
α-Cyano-4-hydroxycinnamic acid
- SLC
solute carrier family
- SkMC
skin mast cells
- ILC2
group 2 innate lymphoid cells
References
- 1.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012;106:9–14. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Burton OT, Oettgen HC. Beyond immediate hypersensitivity: evolving roles for IgE antibodies in immune homeostasis and allergic diseases. Immunol Rev. 2011;242:128–143. doi: 10.1111/j.1600-065X.2011.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124:639–648. doi: 10.1016/j.jaci.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, Okayama Y, Akira S, Saito H, Galli SJ, Nakae S. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 7.Silver MR, Margulis A, Wood N, Goldman SJ, Kasaian M, Chaudhary D. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm Res. 2010;59:207–218. doi: 10.1007/s00011-009-0088-5. [DOI] [PubMed] [Google Scholar]
- 8.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 9.Hsu CL, Neilsen CV, Bryce PJ. IL-33 Is Produced by Mast Cells and Regulates IgE- Dependent Inflammation. PLoS ONE. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, Galli SJ, Nakae S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 11.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maturu P, Overwijk WW, Hicks J, Ekmekcioglu S, Grimm EA, Huff V. Characterization of the inflammatory microenvironment and identification of potential therapeutic targets in wilms tumors. Transl Oncol. 2014;7:484–492. doi: 10.1016/j.tranon.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 15.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 17.Semenza GL. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol. 2009;19:12–16. doi: 10.1016/j.semcancer.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Yang P, Wang XF. Microenvironmental regulation of cancer metastasis by miRNAs. Trends Cell Biol. 2014;24:153–160. doi: 10.1016/j.tcb.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen JC, Buresh C, Norton JA. Lactic acidosis increases tumor necrosis factor secretion and transcription in vitro. J Surg Res. 1990;49:350–353. doi: 10.1016/0022-4804(90)90036-2. [DOI] [PubMed] [Google Scholar]
- 21.Samuvel DJ, Sundararaj KP, Nareika A, Lopes-Virella MF, Huang Y. Lactate boosts TLR4 signaling and NF-kappaB pathway-mediated gene transcription in macrophages via monocarboxylate transporters and MD-2 up-regulation. J Immunol. 2009;182:2476–2484. doi: 10.4049/jimmunol.0802059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beghdadi W, Madjene LC, Benhamou M, Charles N, Gautier G, Launay P, Blank U. Mast cells as cellular sensors in inflammation and immunity. Front Immunol. 2011;2:37. doi: 10.3389/fimmu.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiGirolamo M, Newby FD, Lovejoy J. Lactate production in adipose tissue: a regulated function with extra-adipose implications. FASEB J. 1992;6:2405–2412. doi: 10.1096/fasebj.6.7.1563593. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Kambe N, Kambe M, Kochan JP, Schwartz LB. Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood. 2001;97:2045–2052. doi: 10.1182/blood.v97.7.2045. [DOI] [PubMed] [Google Scholar]
- 26.Withers RT, Sherman WM, Clark DG, Esselbach PC, Nolan SR, Mackay MH, Brinkman M. Muscle metabolism during 30, 60 and 90 s of maximal cycling on an air-braked ergometer. European Journal of Applied Physiology. 1991;63:354–362. doi: 10.1007/BF00364462. [DOI] [PubMed] [Google Scholar]
- 27.Ruan GX, Kazlauskas A. Lactate Engages Receptor Tyrosine Kinases Axl, Tie2, and Vascular Endothelial Growth Factor Receptor 2 to Activate Phosphoinositide 3-Kinase/Akt and Promote Angiogenesis. J Biol Chem. 2013;288:21161–21172. doi: 10.1074/jbc.M113.474619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostroukhova M, Goplen N, Karim MZ, Michalec L, Guo L, Liang Q, Alam R. The role of low-level lactate production in airway inflammation in asthma. AJP: Lung Cellular and Molecular Physiology. 2012;302:L300–L307. doi: 10.1152/ajplung.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peter K, Rehli M, Singer K, Renner-Sattler K, Kreutz M. Lactic acid delays the inflammatory response of human monocytes. Biochemical and Biophysical Research Communications. 2015:1–7. doi: 10.1016/j.bbrc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Dietl K, Renner K, Dettmer K, Timischl B, Eberhart K, Dorn C, Hellerbrand C, Kastenberger M, Kunz-Schughart LA, Oefner PJ, Andreesen R, Gottfried E, Kreutz MP. Lactic Acid and Acidification Inhibit TNF Secretion and Glycolysis of Human Monocytes. The Journal of Immunology. 2010;184:1200–1209. doi: 10.4049/jimmunol.0902584. [DOI] [PubMed] [Google Scholar]
- 31.Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate Influx through the Endothelial Cell Monocarboxylate Transporter MCT1 Supports an NF- B/IL-8 Pathway that Drives Tumor Angiogenesis. Cancer Research. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 32.Le Floch R, Chiche J, Marchiq I, Naiken T, Ilc K, Murray CM, Critchlow SE, Roux D, Simon MP, Pouyssegur J. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proceedings of the National Academy of Sciences. 2011;108:16663–16668. doi: 10.1073/pnas.1106123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortes-Campos C, Elizondo R, Carril C, Martínez F, Boric K, Nualart F, Garcia-Robles MA. MCT2 Expression and Lactate Influx in Anorexigenic and Orexigenic Neurons of the Arcuate Nucleus. PLoS ONE. 2013;8:e62532–15. doi: 10.1371/journal.pone.0062532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin RY, Vera JC, Chaganti RSK, Golde DW. Human Monocarboxylate Transporter 2 (MCT2) Is a High Affinity Pyruvate Transporter. J Biol Chem. 1998;273:28959–28965. doi: 10.1074/jbc.273.44.28959. [DOI] [PubMed] [Google Scholar]
- 35.Hosoya KI, Kondo T, Tomi M, Takanaga H, Ohtsuki S, Terasaki T. MCT1-Mediated Transport of L-Lactic Acid at the Inner Blood–Retinal Barrier: A Possible Route for Delivery of Monocarboxylic Acid Drugs to the Retina. Pharmaceutical Research. 2001;18:1669–1676. doi: 10.1023/a:1013310210710. [DOI] [PubMed] [Google Scholar]
- 36.Andrade MV, Iwaki S, Ropert C, Gazzinelli RT, Cunha-Melo JR, Beaven MA. Amplification of cytokine production through synergistic activation of NFAT and AP-1 following stimulation of mast cells with antigen and IL-33. Eur J Immunol. 2011;41:760–772. doi: 10.1002/eji.201040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji Y, Wrzesinski C, Yu Z, Hu J, Gautam S, Hawk NV, Telford WG, Palmer DC, Franco Z, Sukumar M, Roychoudhuri R, Clever D, Klebanoff CA, Surh CD, Waldmann TA, Restifo NP, Gattinoni L. miR-155 augments CD8+ T-cell antitumor activity in lymphoreplete hosts by enhancing responsiveness to homeostatic γc cytokines. Proc Natl Acad Sci USA. 2015;112:476–481. doi: 10.1073/pnas.1422916112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Saedeleer CJ, Copetti T, Porporato PE, Verrax J, Feron O, Sonveaux P. Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PLoS ONE. 2012;7:e46571–12. doi: 10.1371/journal.pone.0046571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuo RJ, Gu XW, Qi QR, Wang TS, Zhao XY, Liu JL, Yang ZM. Warburg-like Glycolysis and Lactate Shuttle in Mouse Decidua during Early Pregnancy. J Biol Chem. 2015;290:21280–21291. doi: 10.1074/jbc.M115.656629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babar IA, Czochor J, Steinmetz A, Weidhaas JB, Glazer PM, Slack FJ. Inhibition of hypoxia-induced miR-155 radiosensitizes hypoxic lung cancer cells. Cancer Biology & Therapy. 2014;12:908–914. doi: 10.4161/cbt.12.10.17681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu R, Zhang Y, Yang X, Yan J, Sun Y, Chen Z, Jiang H. Isoflurane attenuates LPS-induced acute lung injury by targeting miR-155-HIF1-alpha. Front Biosci (Landmark Ed) 2015;20:139–156. doi: 10.2741/4302. [DOI] [PubMed] [Google Scholar]
- 42.Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, Scholz CC, Simpson DA, Leonard MO, Tambuwala MM, Cummins EP, Taylor CT. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Molecular and Cellular Biology. 2011;31:4087–4096. doi: 10.1128/MCB.01276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enoksson M, Möller-Westerberg C, Wicher G, Fallon PG, Forsberg-Nilsson K, Lunderius-Andersson C, Nilsson G. Intraperitoneal influx of neutrophils in response to IL-33 is mast cell-dependent. Blood. 2013;121:530–536. doi: 10.1182/blood-2012-05-434209. [DOI] [PubMed] [Google Scholar]
- 44.Marichal T, Tsai M, Galli SJ. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol Res. 2013;1:269–279. doi: 10.1158/2326-6066.CIR-13-0119. [DOI] [PubMed] [Google Scholar]
- 45.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benítez-Bribiesca L, Wong A, Utrera D, Castellanos E. The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J Histochem Cytochem. 2001;49:1061–1062. doi: 10.1177/002215540104900816. [DOI] [PubMed] [Google Scholar]
- 47.Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Crit Rev Immunol. 2011;31:475–529. doi: 10.1615/critrevimmunol.v31.i6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nareika A, He L, Game BA, Slate EH, Sanders JJ, London SD, Lopes-Virella MF, Huang Y. Sodium lactate increases LPS-stimulated MMP and cytokine expression in U937 histiocytes by enhancing AP-1 and NF-kappaB transcriptional activities. Am J Physiol Endocrinol Metab. 2005;289:E534–42. doi: 10.1152/ajpendo.00462.2004. [DOI] [PubMed] [Google Scholar]
- 49.Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H+ symporters. J Mol Med. 2015:1–17. doi: 10.1007/s00109-015-1307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang CJ, Nguyen PNN, Choo KB, Sugii S, Wee K, Cheong SK, Kamarul T. Frequent Co-Expression of miRNA-5p and -3p Species and Cross-Targeting in Induced Pluripotent Stem Cells. Int J Med Sci. 2014;11:824–833. doi: 10.7150/ijms.8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choo KB, Soon YL, Nguyen PNN, Hiew MSY, Huang CJ. MicroRNA-5p and -3p co-expression and cross-targeting in colon cancer cells. J Biomed Sci. 2014;21:892–14. doi: 10.1186/s12929-014-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuchenbauer F, Mah SM, Heuser M, McPherson A, Ruschmann J, Rouhi A, Berg T, Bullinger L, Argiropoulos B, Morin RD, Lai D, Starczynowski DT, Karsan A, Eaves CJ, Watahiki A, Wang Y, Aparicio SA, Ganser A, Krauter J, Dohner H, Dohner K, Marra MA, Camargo FD, Palmqvist L, Buske C, Humphries RK. Comprehensive analysis of mammalian miRNA* species and their role in myeloid cells. Blood. 2011;118:3350–3358. doi: 10.1182/blood-2010-10-312454. [DOI] [PubMed] [Google Scholar]
- 53.Kamide Y, Ishizuka T, Tobo M, Tsurumaki H, Aoki H, Mogi C, Nakakura T, Yatomi M, Ono A, Koga Y, Sato K, Hisada T, Dobashi K, Yamada M, Okajima F. Acidic environment augments FcεRI-mediated production of IL-6 and IL-13 in mast cells. Biochemical and Biophysical Research Communications. 2015;464:949–955. doi: 10.1016/j.bbrc.2015.07.077. [DOI] [PubMed] [Google Scholar]
- 54.Lu J, Kang J, Zhang C, Zhang X. The role of IL-33/ST2L signals in the immune cells. Immunology Letters. 2015;164:11–17. doi: 10.1016/j.imlet.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Rak GD, Osborne LC, Siracusa MC, Kim BS, Wang K, Bayat A, Artis D, Volk SW. IL-33-Dependent Group 2 Innate Lymphoid Cells Promote Cutaneous Wound Healing. Journal of Investigative Dermatology. 2016;136:487–496. doi: 10.1038/JID.2015.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 57.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. Journal of Allergy and Clinical Immunology. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moulin D, Donzé O, Talabot-Ayer D, Mézin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40:216–225. doi: 10.1016/j.cyto.2007.09.013. [DOI] [PubMed] [Google Scholar]