Abstract
Phenotypically and functionally diverse regulatory T cell (Tr cell) subsets populate lymphoid and non-lymphoid tissues where their maintenance and function are governed by unique homeostatic signals. Whereas Tr cells resident in non-lymphoid tissues depend on continual TCR signaling for their survival and function, phenotypically naïve Tr cells occupying secondary lymphoid organs (SLOs) are largely supported by paracrine Il-2 signaling. Crucially, the absence of either of these distinct Tr cell subsets results in pathogenic autoimmunity, underscoring their non-redundant roles in the preservation of self-tolerance. However, the cellular and molecular factors precipitating Il-2 release and subsequent maintenance of SLO-resident Tr cells are still poorly understood. Here we report that Il-2-dependent Tr cells in the spleen compete for a limiting supply of paracrine Il-2 generated by auto-reactive CD4+ T cells in response to MHCII-restricted auto-antigen activation by 33D1+ CD11bint DCs. Manipulating this cellular circuit culminating in Il-2 production could have clinical benefits in settings where diminished Tr cell abundance is desired.
Introduction
The adaptive immune system provides protection and immunologic memory to a diverse array of foreign antigens. This must be achieved while remaining non-responsive to self-antigens, innocuous environmental antigens, and components of the commensal microbiota that inhabit mucosal surfaces. The generation and selection of αβT cells which fit these criteria occurs in the thymus where T cells somatically recombine a series of germ line encoded gene segments to generate a unique T cell receptor (TCR) that is then evaluated on its ability to bind to major histocompatibility complexes (positive selection) without recognizing MHC bearing self-peptides (negative selection). Cells which fail to meet these conditions are eliminated within the thymus. Despite the culling of non- or auto-reactive cells during T cell development, a smaller number of auto-reactive cells escapes negative selection and egress from the thymus where they can clonally expand after recognizing cognate self-antigen. Therefore, scarce auto-reactive T cells have the potential to cause devastating autoimmunity if left unregulated. However, a second non-deletional mechanism of T cell development has evolved by which a portion of CD4+ T cells bearing self-reactive TCRs survive negative selection and seed the periphery as regulatory cells. These regulatory T cells (Tr cells) express the master transcription factor Foxp3 and suppress aberrant auto-reactive T cell responses through a variety of mechanisms including sequestration of key T cell growth factors and metabolites, production of anti-inflammatory cytokines, and modulation of dendritic cell (DC) function (1, 2). The critical importance of Tr cells is best exemplified in the fatal multi-organ lymphoproliferative disease which develops in their absence due to non-functional or hypomorphic alleles of the Foxp3 gene (3, 4).
Like phenotypically and functionally diverse effector T cells, Tr cell subsets exist in different tissues with unique homeostatic maintenance requirements (5, 6). Most broadly, Tr cells can be subdivided based on localization within lymphoid or non-lymphoid tissues. Whereas pro-survival signals downstream of Il-2 engagement maintain Tr cells within T cell zones of secondary lymphoid organs (SLOs) (7, 8), maintenance of Tr cells resident in non-lymphoid tissues is largely Il-2-independent, and distinct signals including TCR signaling (9), ICOS-mediated co-stimulation (10, 11), and Il-7 (12, 13), can modulate their abundance and function. In addition to regulating their abundance, the ability of Tr cells to sequester Il-2 helps inhibit the priming of auto-reactive T cells in SLOs. However, Tr cells cannot produce Il-2 themselves due to transcriptional repression at the Il-2 locus by Foxp3 (14, 15), and are therefore dependent on paracrine sources of Il-2 for their survival. As such, the consumption of Il-2 by SLO-resident Tr cells is both indispensable for their survival and essential to their function. Il-2 production by conventional T cells requires their interaction with antigen-presenting cells (APC) bearing cognate antigen and appropriate co-stimulatory molecules. Therefore the maintenance of Il-2 dependent Tr cells requires a tripartite circuit consisting of an antigen-bearing APC, an antigen-specific T cell, and a proximally located Tr cell. To date, the cellular and molecular factors which comprise this circuit and how they operate to maintain Il-2 dependent Tr cells is SLOs under homeostatic conditions has not been fully elucidated. Here we show that Tr cells resident in the spleen are under continual competition for a limiting supply of Il-2 and that subtle changes in Il-2 availability can profoundly influence immune activation. Moreover, we find that due to their potent ability to induce Il-2 release from conventional CD4+ Foxp3− T cells through the presentation of MHCII-restricted auto-antigens, 33D1+ CD11bint DCs are key cellular players in the homeostatic maintenance of Il-2-dependent Tr cells.
MATERIALS AND METHODS
Mice
C57BL/6 (B6), B6.CD4−/−, B6.RAG−/−, B6.Il-2−/−, OT-II, Balb.c and D011.10 mice were purchased from The Jackson Laboratory. CD11c-DTR-Tg mice, B6.Foxp3gfp mice, BATf3−/−, and sOVA mice were provided by the following: CD11c-DTR-Tg mice; S. Zeigler (Benaroya Research Institute, Seattle WA), B6.Foxp3gfp mice; A. Rudensky (MSKCC, New York NY), BATf3−/− mice; K. Urdahl (CIDR, Seattle WA), sOVA mice; A. Abbas (University of California, San Francisco, CA). M. Pepper (UW, Seattle WA) and D. Raulet (UC, Berkley CA) supplied MHCII−/− and β2M−/− bones for the generation of chimeric mice, respectively. Bone marrow chimeras were generated by reconstituting irradiated recipient mice (2 x 600 RAD separated by > 4 hours) with ≥ 2x106 RBC-depleted bone marrow cells of the appropriate genotype. Chimeric mice were rested ≥ 8–10 weeks before experiments unless otherwise indicated. All mice were bred and maintained at Benaroya Research Institute and experiments were pre-approved by the Office of Animal Care and use Committee of Benaroya Research Institute. Non-chimeric mice used in experiments were between 7–14 weeks of age at time of sacrifice.
Flow cytometry and cell-sorting
For DC isolations, minced whole spleens were digested in PBS supplemented with 25μg/ml (final) Blenzme (Roche Liberase TM) for 30 minutes at 37°C under agitation. Cell suspensions were then passed through a 70 μm cell strainer into PBS-EDTA (2μM). Erythrocytes were lysed in ACK lysis buffer (Gibco) and the remaining leukocytes were washed in PBS-EDTA. For cell sorting or when otherwise required, DCs were enriched using CD11c-microbeads (Miltenyi – positive selection) according to the manufacturers protocol. Cell surface staining for flow cytometry was performed in FACS buffer (PBS-1% FCS) using the following antibody clones: TCRβ (H57-597), MHCII (M5-114), CD11c (N418), CD11b (M1/70), DC marker (33D1), CD4 (RM4-5), CD8α (53-6.7), CD44 (IM7), CD62L (MEL-14), DO11.10 (KJ1-26), CD25 (PC61.5). Cells were incubated in the appropriate antibody cocktail for 15 minutes at 4°C, then washed in FACS buffer before collecting events on an LSR II (BD Biosciences). For intracellular staining, surface antigens were pre-labeled before permeabilization with eBioscience FixPerm buffer. Cells were then washed and stained with antibodies against Foxp3 (FJK-16s) and/or Ki67 (Sol15). Sorting was performed using a FACS Aria (BD Biosciences) when experiments necessitated pure cell populations. Flow cytometry data was analyzed using FlowJo software (Treestar).
Ex vivo staining
To assess pSTAT5 levels directly ex vivo, spleens were immediately disrupted between glass slides into BD Cytofix/Cytoperm buffer. Cells were then incubated for 20 minutes at room temperature, washed in FACS buffer, resuspended in 500 μl 90% methanol, and incubated on ice for ≥ 30 min. Cells were stained for surface and intracellular antigens including pSTAT5 (pY694; BD Biosciences), for 45 min at room temperature. Due to intra-experiment variability in ex vivo pSTAT5 staining (typically ranging from 7–11% pSTAT5+ for control Tr cells), Tr cell pSTAT5 data has been normalized to un-manipulated control Tr cells (set to 1) within individual experiments where multiple data sets are pooled.
In vitro DC/T cell co-cultures
For autologous DC/T cell co-cultures, 7.5x104 CD11c-enriched or sorted DC subsets were co-incubated with 3x105 purified GFP− CD4+ or CD8+ T cells from FoxP3GFP mice in ½ surface area 96 well plates (Costar) in a final volume of 100 μl. The media used for cell culture (RPMI, 1% HEPES, 1% L-Glut, 1% Penn/Strep, 1% Sodium Pyruvate, 0.5% Gentamycin, βME (1X of 1000X stock)) was supplemented with 10% autologous mouse serum sourced from FoxP3GFP donors. Serum was heat inactivated at 57°C for 30 minutes before adding to media. Following 72H cultures, cell-free supernatants were diluted 5X in RP-10 (RPMI-10% FCS) and added to an equal volume suspension of 5x105 CD4+ T cells (Invitrogen – negative isolation) of which ~10–15% were FoxP3+ Tr cells. Control cells received an equal volume of RP-10. Following 30 minute incubations at 37°C, cells were resuspended in BD Cytofix/Cytoperm buffer as previously described. pSTAT5 was measured amongst CD4+ FoxP3+ CD25hi cells as a surrogate measurement of Il-2 production within each DC/T cell co-culture. Il-2−/− CD4+ T cells were used as the T cell source for initial co-cultures where indicated. In some experiments, 5 μl of αMHCII antibody (M5-114) was added to culture media to block MHCII-mediated antigen presentation. For in vitro CD4+ T cell activation and proliferation assays using model antigen, sorted DC subsets from Balb.c or sOVA were incubated in flat bottom 96 well tissue-culture plates (Cellstar) for 20H (early activation) or 72H (proliferation) with CD4-enriched DO11.10 cells at the indicated T cell:DC ratios. For proliferation assays, DO11.10 cells were pre-labeled with the cell tracking dye CFSE prior to culture.
DC depletions and adoptive transfers
CD11c-DTR-Tg or CD11c-DTR-chimeric mice were given an initial I.P. injection of 200 ng diphtheria toxin (Calbiochem), then additional 200ng injections every other day until sacrifice. Where indicated, sorted 33D1+ CD11bint or 33D1− CD11blo DCs of the indicated genotype and frequency were adoptively transferred into CD11c-DTR-Tg mice retro-orbitally directly following the first DTx treatment.
Antibody treatments and pan-GPCR inhibition
For Il-2 blocking experiments, mice were given 300 μg of αIl-2 (S4B6-1) or an equivalent amount of rat IgG (Sigma) by retro-orbital injection at minutes 0, 30, 60, 120, and 240 prior to sacrifice. For CD80/86 blocking experiments, mice were given a single injection of 100 ug αCD80 (16-10A1), αCD86 (GL1), both, or an equivalent amount of rat IgG (Sigma) by retro-orbital injection 48 hours prior to sacrifice. For pan-GPCR inhibition, CD11c-enriched cells were incubated for 1 hour at 37°C in RP-10 with/without 1 ug/ml pertussis toxin (List Biologics). Cells were washed 2X before i.v. injection into DC-depleted recipient mice.
In vivo DC labeling
Five micrograms of PE conjugated anti-CD45.2 (104-2) was injected into mice retro-orbitally 5 minutes prior to sacrifice. Splenocytes were prepared for flow cytometry as described and localization of adoptively transferred cells was determined by degree of PE-CD45.2 staining amongst CD45.1+ (A20) DCs within the MHCIIhi CD11chi gate.
Statistical analysis
All data are presented as the mean values ± SEM unless otherwise indicated. Comparisons between groups were analyzed using unpaired Student’s t tests or one-way ANOVA as indicated in the figure legends.
Supplementary Information
There is no supplementary information.
RESULTS
Homeostatic Il-2 limits the frequency and function of splenic Tr cells
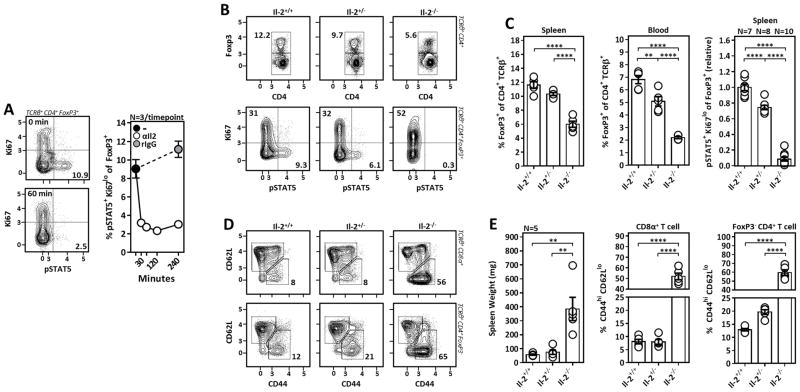
Il-2−/−, Il-2Rα−/− and Il-2Rβ−/− mice developed spontaneous lymphoproliferative autoimmune disease characterized by expansion and hyper-activation of T cells demonstrating that Il-2 is dispensable as a T cell growth factor but critical for the preservation of self-tolerance through its ability to support Tr cells (16–20). Il-2 activation in Tr cells leads to the phosphorylation of signal transducer and activator of transcription 5 (STAT5), culminating in expression of pro-survival genes including Bcl-2 and Mcl-1. This sustains lymphoid-resident Tr cells and when present in excess can drive Tr cell proliferation and population expansion (21), thereby ensuring that the number of Tr cells is calibrated to the amount of Il-2 produced by effector T cells. To understand the kinetics of STAT5-phosphorylation (pSTAT5) downstream of Il-2 activation in Tr cells, Il-2 blocking antibodies (S4B6-1) were administered into mice i.v. where we saw the disappearance of Tr cell pSTAT5 by 30 minutes post-treatment. Thus, pSTAT5 characterizes recent Il-2 activation in Tr cells (Fig. 1A). Similarly, we used Il-2+/+, Il-2+/−, and Il-2−/− mice to show that pSTAT5 correlates with the degree of Il-2 availability and is virtually absent in Il-2−/− mice (Fig. 1B, 1C). Thus, additional γC cytokines such as Il-7 and Il-15 which utilize STAT5 for signaling appear to have minimal roles in the homeostatic maintenance Tr cells within SLOs. Consistent with its requirement for the prevention of spontaneous autoimmunity, Il-2-deficient mice developed massive splenomegaly and displayed hyper-activation of conventional CD4+ and CD8+ T cells by four weeks of age. Additionally, although they do not display any overt immunopathology, CD4+ T cells from age-matched Il-2+/− mice also displayed a hyper-activated phenotype (Fig. 1D, 1E) with diminished frequencies of splenic and circulating Tr cells (Fig. 1C). Collectively, these data suggest the homeostatic reservoir of Tr cell-supportive Il-2 is limiting, and that small perturbations in Il-2 availability can substantially influence Tr cell abundance and immune activation. This is consistent with the identification of polymorphisms in the genes encoding IL-2 and CD25, where subtle changes in the production or function of these proteins is nonetheless associated with an increased risk of developing multiple autoimmune diseases including type-1 diabetes (22, 23), multiple sclerosis (24, 25), juvenile idiopathic arthritis (26), and systemic lupus erythematosus (27).
Figure 1. Tr cells compete for a limiting supply of Il-2.
(A) Rat IgG or Il-2 blocking antibody (S4B6-1) was administered to mice I.V. at the indicated time-points prior to sacrifice to assess Il-2 activation kinetics in splenic Tr cells by phosphorylation of STAT5 (pSTAT5). (B and D) Representative flow cytometry plots assessing Tr cell frequencies and pSTAT5 (B), and activation of FoxP3− T cells (D), in WT (Il-2+/+) Il-2 heterozygous (Il-2 +/−) and Il-2 knockout (Il-2−/−) mice. (C) Tr cell frequencies amongst TCRβ+ CD4+ T cells in the spleen (left) and blood (middle) of Il-2+/+, +/−, and −/− mice (N = 5). (Right) Compiled data for Tr cell pSTAT5 in mice of the indicated genotype (N = replicate mice per group). (E) Spleen weight (left) and frequency of activated (CD44hi CD62Llo) CD4+ and CD8+ T cells as gated in D (N=5). Data representative of at least 2 independent experiments. Error bars in all panels represent mean ±SEM. **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001, as calculated by one-way ANOVA with Tukey’s post hoc test.
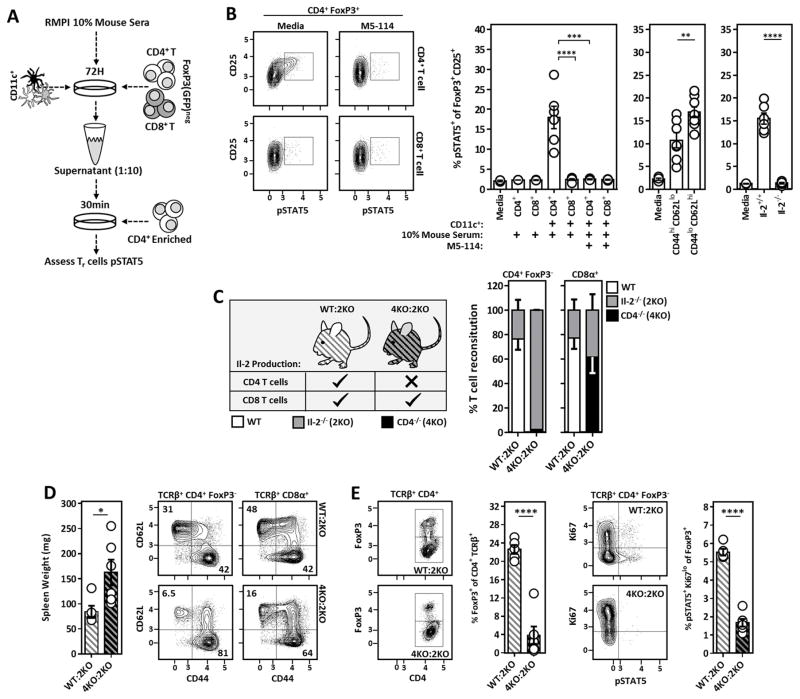
CD4+ T cell-derived Il-2 is critical for the maintenance of Il-2-dependent Tr cells and the prevention of spontaneous autoimmunity
Il-2 can be produced by activated CD4+ and CD8+ T cells. Therefore, to better understand the contribution of CD4+ vs. CD8+ T cell-derived Il-2 in the homeostatic maintenance of Il-2-dependent Tr cells, bulk CD11c-enriched cells (60% MHCIIhi CD11chi) were cultured with autologous polyclonal FoxP3− CD4+ or CD8+ T cells in the presence of 10% autologous mouse serum. After 3 days, aliquots of each supernatant were briefly added to freshly isolated CD4+ T cells and STAT5 phosphorylation was measured in FoxP3+ CD25+ Tr cells as a surrogate for soluble Il-2 within each culture (Fig. 2A). Importantly, given that all cell isolations/enrichments were performed in buffer devoid of foreign protein (e.g. from bovine calf serum or bovine serum albumin), Il-2 produced under these conditions is likely the result of activation of self-reactive T cells. Supernatants from CD4+ T cells co-cultured with CD11c+ cells in the presence of autologous serum led to robust phosphorylation of STAT5 amongst FoxP3+ CD25+ Tr cells, while supernatants from similar cultures containing CD8+ T cells did not. Additionally, Il-2 generation in CD11c+/CD4+ T cell co-cultures was MHC-Class II (MHCII) and DC-dependent as provision of an α-MHCII antibody (M5-114) or cultures devoid of DCs abolished Il-2 production (Fig. 2B). Using this culture system, naïve (CD44lo CD62Lhi) CD4+ T cells produced more Il-2 than activated (CD44hi CD62Llo) CD4+ T cells in response to autologous DCs, although this difference was subtle (Fig. 2B). Whereas DCs have been reported to produce Il-2 upon activation (28), supernatants from co-cultures containing wild-type DCs and Il-2−/− CD4+ T cells failed to induce pSTAT5 in Tr cells, confirming that CD4+ T cells and not DCs are the principal source of Tr cell-supportive Il-2. (Fig. 2B). To determine if CD4+ T cell-derived Il-2 supports Tr cell development and maintenance in vivo, mixed bone marrow chimeras were generated by reconstituting RAG−/− mice with T-cell depleted bone marrow from WT:Il-2−/− or CD4−/−:Il-2−/− donors. Thus, within our CD4−/−:Il-2−/− mixed chimeras, all CD4+ T cells (including Tr cells) develop from Il-2−/− donor cells whereas other T cell populations derived from the CD4−/− donor cells remained Il-2-sufficient. By contrast, WT:Il-2−/− mixed chimeras retained a population of CD4+ T cells capable of Il-2 production (Fig. 2C). Around week 5 post-reconstitution, coinciding with the emergence of donor-derived T cells from the recipient thymus, we noticed a striking deterioration in the physical appearance of our CD4−/−:Il-2−/− mixed chimeras while control chimeras remained healthy. Upon sacrifice, we observed varying degrees of splenomegaly, hyper-activation of conventional CD4+ and CD8+ T cells (Fig. 2D), and a dearth of Tr cells within CD4−/−:Il-2−/− mixed chimeras compared to WT:Il-2−/− controls (Fig. 2E). Furthermore, those Tr cells recovered were absent of pSTAT5 to a degree reminiscent of Il-2−/− mice (Fig. 2E). Thus, these experiments demonstrate CD4+ T cell-derived Il-2 is critical for the maintenance of Il-2-dependent Tr cells and the prevention of autoimmune-like disease, whereas CD8+ T cells have a minimal contribution to Tr cell maintenance despite their ability to produce Il-2.
Figure 2. CD4+ T cell-derived Il-2 sustains SLO resident Tr cells and prevents the development of spontaneous autoimmunity.
(A) Experimental design to assess qualitative Il-2 production in DC/T cell co-cultures via bio-assay. (B) Representative flow cytometry plots (left) and compiled data (right) assessing pSTAT5 amongst freshly isolated CD4+ FoxP3+ T cells stimulated with 1:10 diluted co-culture supernatants generated as outlined in A. Anti-MHCII (M5-114) antibodies were included in the original co-cultures where indicated. (Middle) Experiments were repeated using sorted CD44hi CD62Llo and CD44lo CD62Lhi CD4+ T cell subsets. (Right) Similar experiments were performed utilizing unfractionated Il-2−/− CD4+ T cells as the co-culture T cell source. The frequency of pSTAT5+ cells amongst CD25+ FoxP3+ T cells is quantified. (C) Design of WT:Il-2−/− and CD4−/−:Il-2−/− chimeras used to assess the requirement of CD4+ vs. CD8+ T cell-derived Il-2 in the maintenance of Il-2 dependent Tr cells in vivo. (Right) Reconstitution analysis of CD4+ and CD8+ T cells from WT:Il-2−/− and CD4−/−:Il-2−/− chimeras 5–6 weeks post-reconstitution. ~98% of the CD4+ T cells within CD4−/−:Il-2−/− chimeras were Il-2-deficient. (D) Spleen weight and naïve vs. activated phenotype of splenic CD4+ and CD8+ T cells from WT:Il-2−/− and CD4−/−:Il-2−/− chimeras at time of sacrifice. (E) Analysis of Tr cell frequencies and Il-2 activation (pSTAT5) in splenic Tr cells of the indicated chimeric mice at time of sacrifice. Chimera data is pooled from two independent experiments with at least 2 mice per group. Error bars in all panels represent mean ±SEM. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001, as calculated by unpaired students Student’s t-test (D) or one-way ANOVA with Tukey’s post hoc test (B).
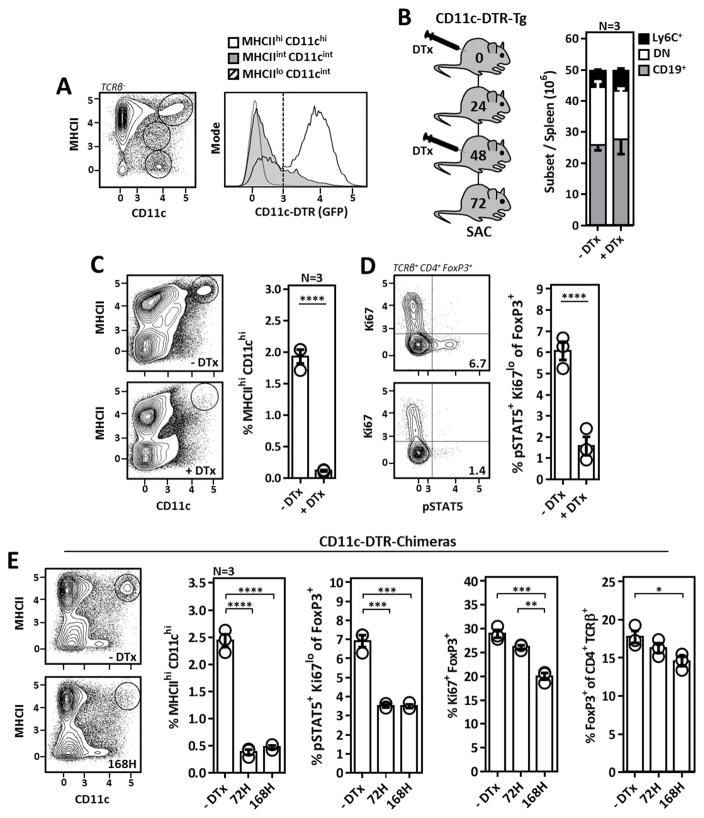
DCs are required for Tr cell Il-2 activation in vivo
DC abundance can be experimentally manipulated through provision or Ab-mediated depletion of DC growth and survival factors including GM-CSF and Flt3L (29, 30). While such experiments have revealed Tr cell frequencies are intimately linked to the size of the DC niche, whether DCs directly modulate Tr cell frequencies via antigen presentation/co-stimulation, indirectly through Il-2 production, or both remains unclear. Therefore we next wanted to address a general role for DCs in the maintenance of Il-2-dependent and -independent Tr cells. Utilizing mice expressing the diphtheria toxin receptor (DTR) downstream of the CD11c promoter and containing an additional GFP reporter cassette (CD11c-DTR-Tg mice) (31), we observed DTR expression predominantly in MHCIIhi CD11chi cDCs (Fig. 3A). Accordingly, following three days of DTx treatment in CD11c-DTR-Tg mice, we achieved rapid and highly specific elimination of MHCIIhi CD11chi cDCs with minimal impact on the frequencies of non-DC cell subsets (Fig. 3B, 3C). Tr cell pSTAT5 was markedly diminished upon DC depletion demonstrating DCs were required for Il-2 activation in Tr cells (Fig. 3D). We next wanted to observe how Tr cell Il-2 activation and proliferation, respective hallmarks of Il-2-dependent and -independent Tr cells (32), were influenced by prolonged DC depletion. However, due to expression of CD11c on cell populations in the CNS, off-target DTx toxicity limits the duration of DTx exposure to < 96 hours in CD11c-DTR-Tg mice. Therefore we generated CD11c-DTR chimeric mice to restrict DTR expression to CD11c-expressing cells of hematopoietic origin. Following three or seven days of DTx treatment in chimeric mice, we observed a similar loss of Tr cell pSTAT5 following acute DC ablation which was sustained at our one week time-point (Fig. 3E). Additionally, the overall frequency, as well as the percentage of actively proliferating Tr cells were reduced upon DC depletion, although both occurred after loss of Tr cell pSTAT5. Together these experiments demonstrate in addition to stimulating Tr cells directly through antigen presentation/co-stimulation, DCs are crucially required for support of Il-2-dependent Tr cells via their ability to stimulate paracrine Il-2 production.
Figure 3. DCs are required for the homeostatic maintenance of Il-2-dependent Tr cells.
(A) Flow cytometric analysis of GFP (DTR) expression in various splenic cell populations from CD11c-DTR-Tg mice. (B) Strategy for the elimination of DCs in CD11c-DTR-Tg mice (left) and broad cellular subset composition of splenocytes from CD11c-DTR-Tg mice ±DTx treatment (right). (C) Analysis of MHCIIhi CD11chi DCs in CD11c-DTR-Tg mice ±DTx treatment. (D) Il-2 activation (pSTAT5) in Tr cells from CD11c-DTR-Tg mice ±DTx treatment. (E) Analysis of the DC and Tr cell compartment in CD11c-DTR-Chimeric mice treated ±DTx for 3 or 7 days. Tr cell Il-2 activation (pSTAT5), proliferation (Ki67), and frequency amongst TCRβ+ CD4+ T cells are plotted. Error bars in all panels represent mean ±SEM. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001, as calculated by unpaired students Student’s t-test (C, D) or one-way ANOVA with Tukey’s post hoc test (E).
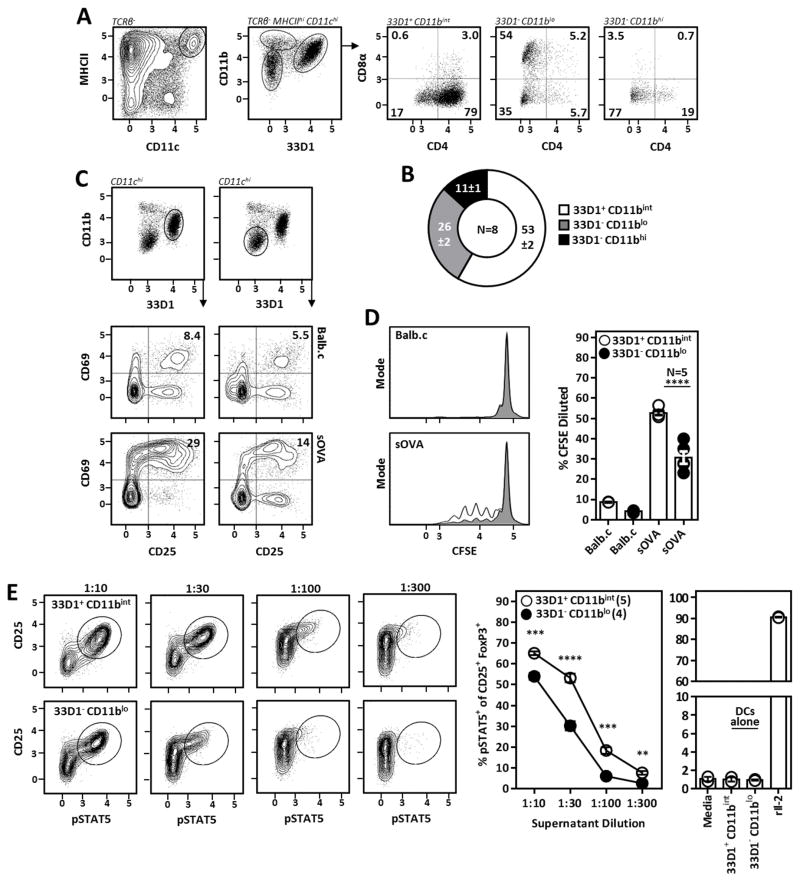
33D1+ CD11bint DCs are potent activators of auto-reactive CD4+ T cells
cDCs encompass a phenotypic and functional heterogeneous group of antigen presenting cells which cannot be evaluated on an individual basis in pan-DC depletion experiments. Therefore, to identify the relevant DC subsets supporting Il-2-dependent Tr cells, we assessed the ability of various DC subsets to present self-antigens to conventional CD4+ T cells in vitro. Although classically defined using the markers CD4 and CD8α (33, 34), we identified three distinct cDC subsets in the spleen using the myeloid marker CD11b and the monoclonal antibody 33D1 (which recognizes the putative inhibitory receptor DCIR2) (35). 33D1+ CD11bint DCs comprise the majority of cDCs in the spleen and correspond with canonical “CD4 DCs”. The second most abundant DC subset, 33D1− CD11blo DCs, contain a mixed population of CD8α+ and CD8α− cells (Fig. 4A, 4B) and are BATf3- and Flt3L-dependent; collectively arguing these cells represent canonical “CD8α DCs” despite heterogeneity of CD8α expression (data not shown). A third population of 33D1− CD11bhi CD4lo/− CD8αlo/− DCs (Fig. 4A, 4B) was excluded as the candidate DC subset supporting Il-2-dependent Tr cells after observing these DCs survived DTx-mediated killing despite a profound loss of Tr cell pSTAT5 in their presence (data not shown). In order to assess the ability of 33D1+ CD11bint and 33D1− CD11blo (CD8α) DCs to activate CD4+ T cells in response to a model self-antigen, DCs were sorted from mice expressing soluble OVA (sOVA) and co-cultured overnight with OVA-specific TCR-transgenic CD4+ T cells. Consistent with published reports using targeted DC antigen delivery (36), 33D1+ CD11bint DCs proved to be the most potent CD4+ T cell activators as measured by upregulation of the early T cell activation markers CD25 and CD69, whereas 33D1− CD11blo DCs had intermediate CD4+ T cell activation potential (Fig. 4C). Additionally, 33D1+ CD11bint DCs induced greater CD4+ T cell proliferation compared to their 33D1− CD11blo counterparts as measured by CFSE dilution (Fig. 4D). To investigate the ability of these subsets to drive Il-2 production from CD4+ T cells, 33D1+ CD11bint and 33D1− CD11blo DCs were cultured with autologous polyclonal FoxP3− CD4+ T cells for 72H and supernatants were used to stimulate STAT5 phosphorylation in Tr cells from freshly isolated CD4+ T cells as outlined in Fig. 2A. Here, 33D1+ CD11bint DCs induced significantly more Il-2 from polyclonal CD4+ T cells compared to 33D1− CD11blo DCs (Fig. 4E), while Il-2 was undetectable when either DC subset was cultured alone demonstrating DCs do not spontaneously secrete Il-2.
Figure 4. 33D1+ CD11bint DCs are potent activators and inducers of Il-2 production from auto-reactive CD4+ T cells.
(A) Gating strategy used to identify splenic DC subsets and how they relate to canonical “CD4” and “CD8α” DCs. (B) Relative abundance of individual splenic DC subsets identified as in A. Numbers indicate mean ±Standard Deviation. (C) Early T cell activation as assessed by CD25 and CD69 upregulation on OVA-specific CD4+ T cells following overnight culture with 33D1+ CD11bint or 33D1− CD11blo DCs from Balb.c or sOVA mice; 3:1 (T cell:DC ratio). (D) Proliferation of OVA-specific CD4+ T cells cultured for 72H as in E; 10:1 (T cell:DC ratio). (E) (Left) Il-2 activation (pSTAT5) amongst Tr cells from freshly isolated CD4+ T cells briefly cultured in supernatant diluted as indicated from CD4+ T cell/33D1+ CD11bint DC or CD4+ T cell/33D1− CD11blo DC co-cultures as outlined in Fig. 2A. (Right) Supernatants from 33D1+ CD11bint or 33D1− CD11blo DCs cultured alone failed to stimulate Tr cell pSTAT5 demonstrating DCs do not serve as a direct source of Il-2 in this culture system. Recombinant mouse Il-2 (rIl-2) was used as a positive control. Error bars in all panels represent mean ±SEM. **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001, as calculated by unpaired students Student’s t-test (D, G) or one-way ANOVA with Tukey’s post hoc test (F).
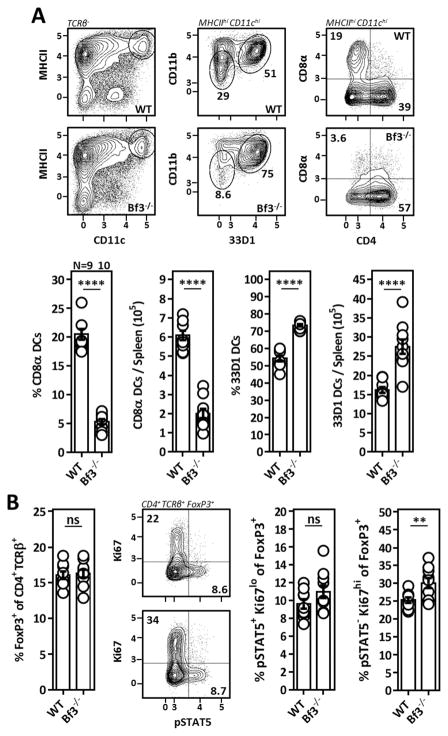
While our data indicated that 33D1− CD11blo (CD8α) DCs are relatively poor CD4+ T cell activators and inducers of Il-2 production in vitro, these cells express high levels of MHCII and therefore should be capable of presenting MHCII-restricted antigens to CD4+ T cells in vivo. Therefore, to further evaluate their role in the homeostatic maintenance of Tr cells, we examined mice lacking BATf3, a basic leucine zipper transcription factor that is required for the development of CD8α DCs (37). Here we observed a profound reduction in the frequency and absolute number of 33D1− CD11blo DCs which mirrored the overall reduction in CD8α-expressing DCs reported for these mice. Furthermore, 33D1+ CD11bint DCs were expanded in BATf3−/− spleens, presumably the result of enhanced availability of shared DC growth- and survival factors such as Flt3L which may be efficiently consumed by 33D1− CD11blo DCs (Fig. 5A). Despite the substantial reduction in 33D1− CD11blo (CD8α) DCs, Tr cell Il-2 activation was unchanged in BATf3−/− mice demonstrating this subset is not necessary for the homeostatic maintenance of Il-2-dependent Tr cells. Furthermore, Tr cells from BATf3−/− spleens were more actively proliferative (based on Ki-67 staining) than those from WT mice suggesting enhanced antigen presentation to auto-reactive CD4+ T cells by 33D1+ CD11bint DCs in the absence of 33D1− CD11blo DCs (Fig. 5B). In combination with our in vitro observations, these data collectively demonstrate that although multiple DC populations are capable of inducing Il-2 production from auto-reactive CD4+ T cells, 33D1+ CD11bint DCs are the most potent CD4+ T cell activators and likely play a key role in the maintenance of Il-2-dependent Tr cells.
Figure 5. 33D1− CD11blo (CD8α) DCs are dispensable for the maintenance of Il-2 dependent Tr cells.
(A) Representative flow cytometry (top) and compiled data (bottom) identifying splenic DC subsets from 7–8 week old WT or BATf3−/− (Bf3−/−) mice. (B) Tr cell frequencies, Il-2 activation (pSTAT5), and proliferation (Ki67) amongst Tr cells from WT or BATf3−/− spleens in A. Data pooled from two independent experiments with at least 4 mice per group. Error bars in all panels represent mean ±SEM. **, P ≤ 0.01; ****, P ≤ 0.0001, as calculated by unpaired students Student’s t-test. ns, not significant.
33D1+ CD11bint and 33D1− CD11blo (CD8α) DCs are both sufficient for the generation of Tr cell supportive Il-2 through antigen presentation on MHCII and CD80/86 co-stimulation
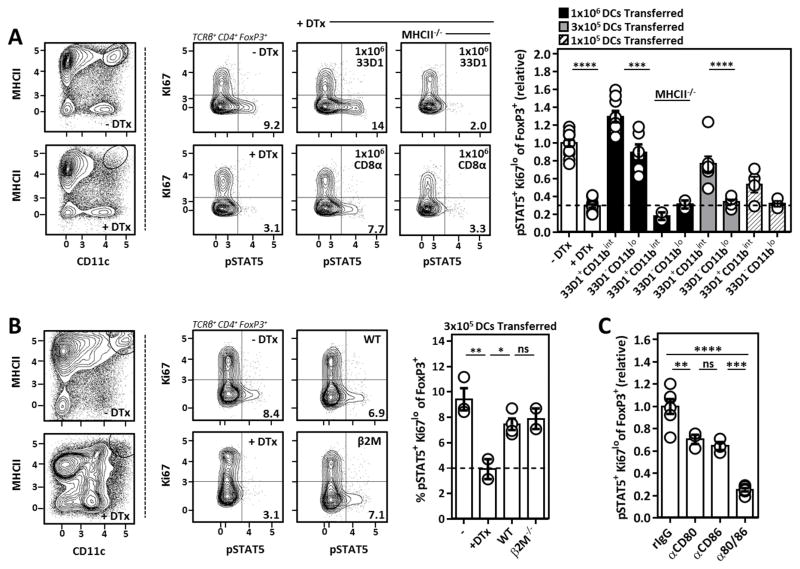
33D1− CD11blo DCs are not necessary for the homeostatic maintenance of Il-2 dependent Tr cells (Fig. 5). However, given their ability to activate and drive Il-2 production from CD4+ T cells in vitro, we next examined if either 33D1+ CD11bint or 33D1− CD11blo DCs were sufficient for the generation of Tr cell supportive Il-2 in vivo. For this, sorted DCs were adoptively transferred into DTx treated CD11c-DTR-Tg mice to evaluate their ability to rescue Il-2 activation in Tr cells from DC-depleted animals (Fig. 6A). At a high transfer dose, both 33D1+ CD11bint and 33D1− CD11blo DCs rescued Il-2 activation in Tr cells from DC-depleted mice, and this required MHCII-restricted antigen presentation. Titrating down the number of adoptively transferred cells, 33D1+ CD11bint DCs showed a clear superiority over 33D1− CD11blo DCs in their ability to rescue Tr cell pSTAT5 in DC-depleted mice (Fig. 6A). Furthermore, 33D1+ CD11bint DCs incapable of presenting MHCI-restricted antigens to CD8+ T cells due to loss of β2-microglobulin (β2M−/−) were equally capable of rescuing Il-2 activation in Tr cells from DC-depleted mice compared to their WT counterparts (Fig. 6B), supporting previous in vitro and in vivo observations that MHCII-mediated antigen presentation to CD4+ T cells represents the principal source of homeostatic Tr cell-supportive Il-2. Reconciling these observations with those made in BATf3−/− mice, 33D1− CD11blo DCs are sufficient but not necessary for Tr cell Il-2 activation. Finally, we addressed the role of CD80/86 co-stimulation in the maintenance of Il-2-dependent Tr cells by treating mice individually or in tandem with blocking antibodies against CD80 and/or CD86. Here we observed an essential but redundant role for CD80 and CD86 in the support of Il-2-dependent Tr cells, likely through the ability of CD28-mediated co-stimulation to promote Il-2 production from conventional CD4+ T cells (Fig. 6C).
Figure 6. Sufficiency of DC subsets and their molecular requirements for the maintenance of Il-2-dependent Tr cells in vivo.
(A) Representative flow cytometry and compiled data comparing Tr cell Il-2 activation (pSTAT5) from DC-deplete mice receiving varying amounts of adoptively transferred DCs of the indicated subset and genotype. Numbers listed above bar graphs indicate number of DCs used for the adoptive transfer. Data pooled from 4 independent experiments and pSTAT5 normalized to the untreated control Tr cells within each experiment. (B) Il-2 activation (pSTAT5) in Tr cells from DC-depleted mice receiving 3x105 WT or β2M−/− 33D1+ CD11bint DCs. (C) Mice were treated with a single dose of 100μg of the indicated antibodies 48H prior to sacrifice. Histogram represents Tr cell Il-2 activation (pSTAT5) in mice receiving CD80/CD86 antibodies individually or in tandem. Error bars in all panels represent mean ±SEM. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001, as calculated one-way ANOVA with Tukey’s post hoc test. ns, not significant.
DC migration to the splenic white pulp is critical for the homeostatic maintenance of Il-2 dependent Tr cells
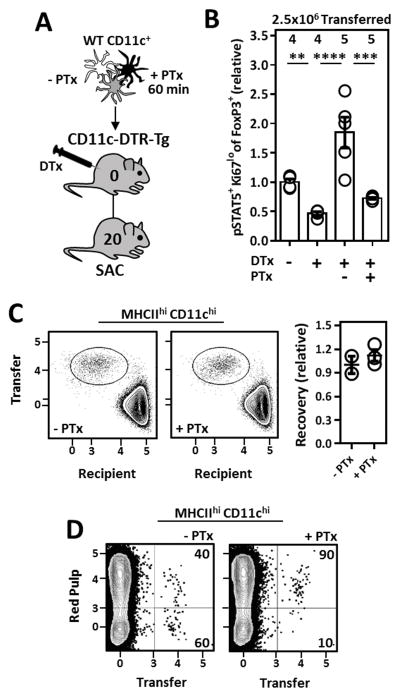
33D1+ CD11bint DCs primarily reside within splenic marginal zone bridging channels (MZBCs); specialized regions at the nexus of the B cell follicle, T cell zone, and red pulp (38, 39). In this location 33D1+ CD11bint DCs are thought to capture particulate antigens in the blood and ferry them across the marginal sinus into T cell zones of the spleen. G-protein coupled receptors (GPCRs) including EBI2 (40, 41), S1P1–5 (42), and CCR7 (43, 44) are critically important for DC positioning within MZBCs and essential for chemotaxis of migratory DCs into secondary lymphoid tissues. Pertussis toxin (PTx) is a Gαi inhibitor and can be used to antagonize Gαi-linked GPCR function. Therefore, to address how inhibition of Gαi-dependent GPCR signaling in DCs would influence DC positioning and subsequent Il-2-dependent Tr cell homeostasis in the spleen, CD11c+ cells were treated with/without PTx in vitro and extensively washed prior to transfer into DC-depleted mice (Fig. 7A) where we assessed their ability to rescue STAT5 phosphorylation in splenic Tr cells. Compared to those receiving un-manipulated CD11c+ cells, PTx pre-treatment dramatically compromised the ability of transferred DCs to rescue Il-2 activation in Tr cells from DC-depleted mice (Fig. 7B), without jeopardizing the survival of transferred cells (Fig. 7C). By injecting recipient mice intravenously with a PE-conjugated αCD45 antibody which differentially labels cells in the red/white pulp (45), we confirmed PTx pre-treatment blocked the ability of transferred DCs to access the white pulp as ~90% of recovered PTx pre-treated DCs stained positive for the injected label (Fig. 7D). Collectively, these experiments emphasize the critical importance of DC migration into the splenic white pulp for the efficient generation of homeostatic Tr cell-supportive Il-2.
Figure 7. DC access to the splenic white pulp is required for the maintenance of Il-2-dependent Tr cells.
(A) Strategy to assess the ability of GPCR-inhibited DCs to rescue Il-2 activation (pSTAT5) in Tr cells from DC-depleted mice. CD11c-enriched cells were incubated for 60 minutes in RP-10 ±1 μg/ml pertussis toxin (PTx) prior to adoptive transfer. (B) Il-2 activation (pSTAT5) in Tr cells from mice receiving 2.5x106 CD11c+ cells treated as in A. Data pooled from two independent experiments with at least 2 mice per group. (C) Recovery of adoptively transferred DCs ±PTx pretreatment 20H post-transfer into congenically marked recipients. (D) Localization of adoptively transferred DCs within recipient mice as assessed by in vivo antibody labeling. 2.5x106 CD11c+ cells ±PTx pretreatment were transferred i.v. Error bars in all panels represent mean ±SEM. **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001, as calculated by one-way ANOVA with Tukey’s post hoc test
DISCUSSION
It is now well established that phenotypic and functionally heterogeneous Tr cell subsets exist in lymphoid and non-lymphoid tissues, where they exhibit a differential dependence on Il-2 signaling or TCR/co-stimulatory receptor activation for their homeostatic maintenance and function, respectively (6). Because Foxp3 directly inhibits their Il-2 production, Tr cells within SLOs depend on paracrine Il-2 downstream of cellular and molecular interactions which are largely Tr cell-extrinsic. Yet to date a clear understanding of the cellular and molecular factors which comprise this circuit and how they operate to maintain Il-2-dependent Tr cells at homeostasis is critically lacking. For instance, although DCs and Tr cells are known to be linked in a ‘homeostatic loop’ (30), the mechanisms linking DCs and Tr cells have not been fully defined, and the relative abilities of different DC subsets to modulate Tr cell abundance is not known. In the present study we provide a comprehensive analysis of the precise cellular and molecular factors maintaining Il-2-dependent Tr cells in the spleen at homeostasis. In short, we found that the frequency and function of Il-2-dependent Tr cells in the spleen is contingent on the presentation of MHCII-restricted auto-antigens to self-reactive CD4+ T cells, largely by CD80/86-bearing 33D1+ CD11bint DCs within the splenic white pulp. Deviations or malfunctions in this circuit likely impair the suppressive capacity of Tr cells through the inability to efficiently produce Il-2. Thus, we have provided empirical evidence supporting the notion that Tr cell frequencies are commensurate with the degree of T cell auto-reactivity. To this we add new insights that Il-2 released from 33D1+ CD11bint DC-activated auto-reactive CD4+ T cells represents the major homeostatic signal calibrating the frequency and function of splenic Tr cells. Interestingly, 33D1+ DCs in the spleen were recently shown to promote the generation of CD4+ follicular helper T (Tfh) cells by upregulating CD25 and sequestering IL-2 (46). Thus, depending on the context, these cells can either promote Il-2 signaling to support Tr cell maintenance, or inhibit Il-2 signaling to generate Tfh cells, and through these reciprocal mechanisms influence both the regulation of autoimmunity and the generation of protective antibody responses.
SLO-resident Tr cells constitutively express high levels of CD25, inhibiting auto-reactive T cell priming in part by sequestering Il-2 from neighboring conventional T cells to establish an insurmountable threshold for complete T cell activation in the absence of additional inflammatory stimuli. Il-2 signaling has the supplementary effect of reinforcing Tr cell lineage stability through FoxP3 induction and co-opting bystander suppression by promoting CTLA-4 and CD39/73 expression (47, 48). Our lab has previously shown the acquisition of Il-2 by SLO resident Tr cells is largely dependent on the chemokine receptor CCR7 suggesting paracrine Il-2 is spatially constrained within SLOs (32). Indeed, Tr cells have recently been shown to perceive Il-2 within T cell/CD11clo DC clusters found in para-cortical regions of lymph nodes (48). This is consistent with our own observation that PTx pre-treatment of adoptively transferred DCs inhibited their ability to promote Il-2 activation in splenic Tr cells from DC-depleted mice; presumably a reflection of their inability to localize with and activate Il-2 producing CD4+ T cells inside T cell zones of the spleen. Interestingly, inducible genetic ablation of TCR expression on Tr cells does not alter lineage stability or the frequency of Il-2-dependent Tr cells within SLOs, but instead is required for the maintenance of non-lymphoid tissue-resident Tr cells (9). This is likely due to the fact that upon TCR engagement in the presence of inflammatory signals, Tr cells up-regulate tissue homing receptors and redistribute out of SLOs into peripheral tissues where they undergo successive rounds of TCR-dependent proliferation - establishing a repertoire of Tr cells specific for antigens typically encountered within those tissues (49–51). Tr cell which fail to engage their TCRs undergo programmed cell death, no longer supported by anti-apoptotic signals downstream of Il-2 (21). Therefore, SLO-resident Tr cells transition from naïve-like, Il-2 consuming “passive” suppressors to actively suppressive, TCR/co-stimulation dependent tissue-resident cells. Despite their unique homeostatic maintenance requirements, continual involvement from both Il-2-dependent and -independent Tr cells is necessary for the prevention of autoimmunity.
Given the observation that Il-2+/− mice have reduced Tr cell frequencies and enhanced effector T cell activation, our data strongly suggests Tr cells are under continual competition for a limiting supply of Il-2. Consequently, altering the Il-2 reservoir, either directly or through manipulation of the cells involved in its production, could dramatically enhance T cell-mediated immunity. These insights may help reconcile the somewhat paradoxical observation that mice constitutively lacking DCs (ΔDC) develop spontaneous fatal Th1/17 mediated autoimmunity (52). While Tr cell frequencies from ΔDC mice were shown to be normal, it was suggested a critical sub-population of DC-dependent Tr cells may be absent in these mice allowing B cells or macrophages to compensatorily activate conventional CD4+ T cells, driving autoimmunity. We speculate this sub-population of Tr cells is in fact SLO-resident Il-2-dependent Tr cells no longer supported in the absence of 33D1+ CD11bint DCs in the spleen and equivalent CD103− CD11b+ DCs in lymph nodes.
The relevant antigens driving basal Il-2 production supporting Il-2-dependent Tr cells could in theory be derived from two major sources; commensal microorganisms and/or self. While critically important for intestinal Tr cell homeostasis (53), enhanced frequencies and intact Il-2 activation amongst circulating Tr cells from gnotobiotic mice demonstrate microbial-derived antigens are not required for the production of Tr cell-supportive Il-2 (54). Interrogating the role of “self” antigens in the generation of homeostatic Il-2 has relied heavily on TCR-transgenic mice in which model antigens are experimentally introduced or ectopically expressed (55). While this approach has been instrumental to our understanding of how cognate antigen recognition shapes conventional and regulatory T cell homeostasis and function, the supra-physiological levels of antigen and high precursor frequencies of antigen-specific T cells typically employed in these systems may misrepresent what occurs for self-antigen recognition at the steady-state. In contrast, by co-culturing cells in media devoid of any foreign protein, we have directly shown CD4+ T cells generate Il-2 in response to bona fide self-antigens. Although activated CD8+ T cells are known to produce Il-2, we show using CD4−/−:Il-2−/− mixed bone marrow chimeras that CD8+ T cell-derived Il-2 is insufficient for the maintenance of Il-2-dependent Tr cells. CD8+ T cells may be inherently less auto-reactive than CD4+ T cells and therefore fewer of the cells may contribute to base-line Il-2 production. Alternatively, production of Il-2 by CD8+ T cells may be mechanistically or spatially segregated in a way that precludes its access to CD25hi Tr cells. Although DCs have been reported to produce Il-2 upon activation (28), our data indicate that DCs are not a significant source of the Il-2 that acts on Tr cells. For instance, MHCII-deficient DCs failed to rescue Il-2 activation in Tr cells from DC-depleted mice despite Il-2-sufficiency. Furthermore, supernatants from DCs cultured alone or DCs cultured with Il-2-deficient CD4+ T cells failed to stimulate pSTAT5 in freshly isolated Tr cells demonstrating CD4+ T cells and not DCs are the critical source of Tr cell-supportive Il-2.
Given their propinquity to the blood-filtering red pulp, the presentation of serum proteins by 33D1+ CD11bint DCs to CD4+ T cells likely represents the major antigenic source stimulating the production of homeostatic Tr cell-supportive Il-2. Indeed, a recent report has demonstrated 33D1+ DCs within MZBCs can prime CD4+ T cell responses to red blood cell antigens, and this response is abolished if recipient mice are given LPS prior to transfusion (39). We posit the redistribution of 33D1+ CD11bint DC into T cell zones of the spleen, which has been widely reported upon LPS injection (40, 56), is to blame for this tempered response. Therefore, 33D1+ CD11bint DCs must be optimally positioned within splenic MZBCs to acquire the particulate blood antigens they subsequently present to auto-reactive CD4+ T cells to induce Tr cell supportive Il-2 release from CD4+ T cells.
Recently, the manipulation of Tr cell abundance and/or function has become an attractive therapeutic strategy to either boost or inhibit immune responses in a variety of clinical settings. We propose that, in addition to directly targeting Tr cells, similar modulation of specific DC lineages could synergize existing Tr cell-based therapeutics. For example, while inferior at activating CD4+ T cells, 33D1− CD11blo (CD8α) DCs mediate the cross-presentation of antigens to CD8+ T cells (57). Accordingly, BATf3−/− mice show increased susceptibility to viral infections (58), impaired syngeneic tumor rejection (37), and defective intracellular pathogen clearance (59) – all consequences of diminished CD8+ T cell function in the absence of CD8α DCs. While we have demonstrated CD8+ T cells do not generate Tr cell-supportive Il-2, they do produce robust IFNγ down-stream of Il-12 release from CD8α DCs (59, 60). As immunotherapies aimed at dampening Tr cell responses are currently used for the treatment of solid tumors, bolstering DCs responsible for cross-priming CD8+ T cell responses could promote cytotoxic anti-tumor immunity at the expense of those encouraging Il-2 production (and subsequent Tr cell maintenance) from CD4+ T cells. Likewise, given that Il-2 controls the frequency of Il-2-dependent Tr cells in the spleen and is of limiting supply, manipulating the Il-2 reservoir, either directly or through the cells or molecules which stimulate its release could be therapeutically beneficial in augmenting immune responses naturally constrained by Tr cells.
Acknowledgments
Funding: This work was funded by grants from the NIH (AR055695, AI067750, AI085130, HL098067) to D.J.C., and the University of Washington immunology training grant (T32AI106677) awarded to J.M.S.
We would like to thank K. Arumuganathan (Aru) for assistance in flow cytometry and cell sorting. Drs. Marion Pepper and David Raulet donated MHCII−/− and β2M−/− bones for the generation of mixed chimeras. Drs. Steve Zeigler, Kevin Urdahl, Abul Abbas, and Alexander Rudensky graciously provided CD11c-DTR-Tg mice, BATf3−/− mice, sOVA mice, and Foxp3gfp mice respectively. We thank Drs. Jessica Hamerman, Estelle Bettelli, Keith Elkon, Marion Pepper, and Edward Clark for their comments regarding the manuscript. Samantha Motley provided laboratory support and helped maintain animal colonies.
Footnotes
Disclosures
The authors declare no competing financial interests.
References
- 1.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 4.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gratz IK, Campbell DJ. Organ-specific and memory treg cells: specificity, development, function, and maintenance. Front Immunol. 2014;5:333. doi: 10.3389/fimmu.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74:961–965. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 8.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev. 2014;259:40–59. doi: 10.1111/imr.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redpath SA, van der Werf N, Cervera AM, MacDonald AS, Gray D, Maizels RM, Taylor MD. ICOS controls Foxp3(+) regulatory T-cell expansion, maintenance and IL-10 production during helminth infection. Eur J Immunol. 2013;43:705–715. doi: 10.1002/eji.201242794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busse M, Krech M, Meyer-Bahlburg A, Hennig C, Hansen G. ICOS mediates the generation and function of CD4+CD25+Foxp3+ regulatory T cells conveying respiratory tolerance. J Immunol Baltim Md 1950. 2012;189:1975–1982. doi: 10.4049/jimmunol.1103581. [DOI] [PubMed] [Google Scholar]
- 12.Simonetta F, Gestermann N, Martinet KZ, Boniotto M, Tissières P, Seddon B, Bourgeois C. Interleukin-7 influences FOXP3+CD4+ regulatory T cells peripheral homeostasis. PloS One. 2012;7:e36596. doi: 10.1371/journal.pone.0036596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gratz IK, Truong HA, Yang SHY, Maurano MM, Lee K, Abbas AK, Rosenblum MD. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J Immunol Baltim Md 1950. 2013;190:4483–4487. doi: 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 15.Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, Miyachi Y, Tsukada T, Sakaguchi S. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 16.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 17.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Duncan GS, Takimoto H, Mak TW. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor beta chain. J Exp Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol Baltim Md 1950. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 20.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 21.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, Humblet-Baron S, Schönefeldt S, Herold MJ, Hildeman D, Strasser A, Bouillet P, Lu LF, Matthys P, Freitas AA, Luther RJ, Weaver CT, Dooley J, Gray DHD, Liston A. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3+ regulatory T cells. Nat Immunol. 2013;14:959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vella A, Cooper JD, Lowe CE, Walker N, Nutland S, Widmer B, Jones R, Ring SM, McArdle W, Pembrey ME, Strachan DP, Dunger DB, Twells RCJ, Clayton DG, Todd JA. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am J Hum Genet. 2005;76:773–779. doi: 10.1086/429843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fichna M, Zurawek M, Fichna P, Ziółkowska-Suchanek I, Januszkiewicz D, Nowak J. Polymorphic variant at the IL2 region is associated with type 1 diabetes and may affect serum levels of interleukin-2. Mol Biol Rep. 2013;40:6957–6963. doi: 10.1007/s11033-013-2815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matesanz F, Fedetz M, Collado-Romero M, Fernández O, Guerrero M, Delgado C, Alcina A. Allelic expression and interleukin-2 polymorphisms in multiple sclerosis. J Neuroimmunol. 2001;119:101–105. doi: 10.1016/s0165-5728(01)00354-x. [DOI] [PubMed] [Google Scholar]
- 25.Alcina A, Fedetz M, Ndagire D, Fernández O, Leyva L, Guerrero M, Abad-Grau MM, Arnal C, Delgado C, Lucas M, Izquierdo G, Matesanz F. IL2RA/CD25 gene polymorphisms: uneven association with multiple sclerosis (MS) and type 1 diabetes (T1D) PloS One. 2009;4:e4137. doi: 10.1371/journal.pone.0004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinks A, Ke X, Barton A, Eyre S, Bowes J, Worthington J, Thompson SD, Langefeld CD, Glass DN, Thomson W UK Rheumatoid Arthritis Genetics Consortium, and British Society of Paediatric and Adolescent Rheumatology Study Group. Association of the IL2RA/CD25 gene with juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:251–257. doi: 10.1002/art.24187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieberman LA, Tsokos GC. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J Biomed Biotechnol. 2010;2010:740619. doi: 10.1155/2010/740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granucci F, Vizzardelli C, Pavelka N, Feau S, Persico M, Virzi E, Rescigno M, Moro G, Ricciardi-Castagnoli P. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol. 2001;2:882–888. doi: 10.1038/ni0901-882. [DOI] [PubMed] [Google Scholar]
- 29.Hanada K, Tsunoda R, Hamada H. GM-CSF-induced in vivo expansion of splenic dendritic cells and their strong costimulation activity. J Leukoc Biol. 1996;60:181–190. doi: 10.1002/jlb.60.2.181. [DOI] [PubMed] [Google Scholar]
- 30.Darrasse-Jèze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao K, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung S, Unutmaz D, Wong P, Sano G-I, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, Campbell DJ. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211:121–136. doi: 10.1084/jem.20131142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol Baltim Md 1950. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 34.McLellan AD, Kapp M, Eggert A, Linden C, Bommhardt U, Bröcker EB, Kämmerer U, Kämpgen E. Anatomic location and T-cell stimulatory functions of mouse dendritic cell subsets defined by CD4 and CD8 expression. Blood. 2002;99:2084–2093. doi: 10.1182/blood.v99.6.2084. [DOI] [PubMed] [Google Scholar]
- 35.Nussenzweig MC, Steinman RM, Witmer MD, Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc Natl Acad Sci U S A. 1982;79:161–165. doi: 10.1073/pnas.79.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 37.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chappell CP, Draves KE, Giltiay NV, Clark EA. Extrafollicular B cell activation by marginal zone dendritic cells drives T cell-dependent antibody responses. J Exp Med. 2012;209:1825–1840. doi: 10.1084/jem.20120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calabro S, Gallman A, Gowthaman U, Liu D, Chen P, Liu J, Krishnaswamy JK, Nascimento MSL, Xu L, Patel SR, Williams A, Tormey CA, Hod EA, Spitalnik SL, Zimring JC, Hendrickson JE, Stowell SR, Eisenbarth SC. Bridging channel dendritic cells induce immunity to transfused red blood cells. J Exp Med. 2016;213:887–896. doi: 10.1084/jem.20151720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gatto D, Wood K, Caminschi I, Murphy-Durland D, Schofield P, Christ D, Karupiah G, Brink R. The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nat Immunol. 2013;14:446–453. doi: 10.1038/ni.2555. [DOI] [PubMed] [Google Scholar]
- 41.Yi T, Cyster JG. EBI2-mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. eLife. 2013;2:e00757. doi: 10.7554/eLife.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Czeloth N, Schippers A, Wagner N, Müller W, Küster B, Bernhardt G, Förster R. Sphingosine-1 phosphate signaling regulates positioning of dendritic cells within the spleen. J Immunol Baltim Md 1950. 2007;179:5855–5863. doi: 10.4049/jimmunol.179.9.5855. [DOI] [PubMed] [Google Scholar]
- 43.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Förster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, Tohya K, Guo Z, Umemoto E, Ebisuno Y, Yang BG, Seoh JY, Lipp M, Kiyono H, Miyasaka M. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol Baltim Md 1950. 2006;176:803–810. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 45.Cinamon G, Zachariah MA, Lam OM, Foss FW, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Lu E, Yi T, Cyster JG. EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature. 2016;533:110–114. doi: 10.1038/nature17947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 Controls the Stability of Foxp3 expression in TGF-β-induced Foxp3+ T cells in vivo. J Immunol Baltim Md 1950. 2011;186:6329–6337. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Z, Gerner MY, Van Panhuys N, Levine AG, Rudensky AY, Germain RN. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature. 2015;528:225–230. doi: 10.1038/nature16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenblum MD, I, Gratz K, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14:1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohnmacht C, Pullner A, King SBS, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Min B, Thornton A, Caucheteux SM, Younes SA, Oh K, Hu-Li J, Paul WE. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur J Immunol. 2007;37:1916–1923. doi: 10.1002/eji.200737236. [DOI] [PubMed] [Google Scholar]
- 55.Attridge K, Walker LSK. Homeostasis and function of regulatory T cells (Tregs) in vivo: lessons from TCR-transgenic Tregs. Immunol Rev. 2014;259:23–39. doi: 10.1111/imr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cyster JG. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J Exp Med. 1999;189:447–450. doi: 10.1084/jem.189.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torti N, Walton SM, Murphy KM, Oxenius A. Batf3 transcription factor-dependent DC subsets in murine CMV infection: differential impact on T-cell priming and memory inflation. Eur J Immunol. 2011;41:2612–2618. doi: 10.1002/eji.201041075. [DOI] [PubMed] [Google Scholar]
- 59.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, Sher A, Ploegh HL, Murphy TL, Sibley LD, Murphy KM. CD8α(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35:249–259. doi: 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martínez-López M, Iborra S, Conde-Garrosa R, Sancho D. Batf3-dependent CD103+ dendritic cells are major producers of IL-12 that drive local Th1 immunity against Leishmania major infection in mice. Eur J Immunol. 2015;45:119–129. doi: 10.1002/eji.201444651. [DOI] [PMC free article] [PubMed] [Google Scholar]