Abstract
There is a large body of work demonstrating that infants are sensitive to the distinction between human and mechanical entities from the early months of life, and have different expectations for the way these entities move and interact. The current work investigates the extent to which the functional organization of the immature brain reflects these early emerging sensitivities. Infants aged 8 months watched two kinds of hands (human or mechanical) engage in two kinds of events (one with a functional outcome and one without). Using functional near-infrared spectroscopy (fNIRS), we assessed hemodynamic activation in the left and right temporal and temporal-occipital cortex in response to these events. The neuroimaging data revealed a significantly greater increase in activation in the right middle-posterior temporal cortex to events executed by the human than the mechanical hand; the event in which the hand engaged (function or non-function) did not significantly influence hemodynamic responses. In comparison, the left middle-temporal cortex showed significantly greater activation to events executed by the human than mechanical hand, but only when the events were functionally relevant. That is, the left middle-posterior temporal cortex responded selectively to human (as compared to mechanical) agents, but only in the context of functionally relevant actions on objects. These results reveal that the immature brain is functionally specialized to support infants’ processing of human and non-human agents as distinct entities. These results also shed light on the cognitive and cortical mechanisms that guide infants’ learning about agentive action and object function.
Keywords: Infants, fNIRS, temporal cortex, object processing, human hands, functional events
1. Introduction
1.1 Background
The visual world is dynamic and complex and filled with a myriad of objects. From the early days of life we organize objects into broad categories (e.g., biological/non-biological, animate/inanimate, social/mechanical) and hold expectations for how objects should move and interact (Fox & McDaniel, 1982; Gelman & Opfer, 2002; Leslie, 1994; Luo & Baillargeon, 2010; Setoh, Wu, Baillargeon, & Gelman, 2013; Spelke & Kinzler, 2007). These expectations guide our interpretation of events involving familiar objects and facilitate learning about new objects. In the mature brain, there are dedicated networks that support processing of objects on the basis of the category to which they belong. Much of what we know about these networks comes from functional neuroimaging studies, which have significantly enhanced our understanding of how the mature human mind organizes, processes, and represents object-related information. In contrast, little is known about the functional organization of these cortical networks in the immature brain. To narrow this gap in knowledge the current research focuses on the cortical bases of two distinctions to which infants are sensitive: that between (a) human/non-human agents and (b) the functional/non-functional use of tools.
1.1.0 Biological and non-biological motion
Over the last 35+ years a great deal of research has been conducted on infants’ sensitivity to biological motion. Most of this research employed point displays of human action and revealed that from the early months of life infants are sensitive to biological motion (Bertenthal, Proffitt, & Cutting, 1984; Fox & McDaniel, 1982; Hirai & Hiraki, 2005; for a review, see Bertenthal, 1993). At the same time, “biological” is a broad term that has been used to encompass a wide range of entities and it is difficult to ascertain from early studies whether the distinction that best characterizes the findings is that between “biological and non-biological” or that between “human and non-human.” Subsequent research has revealed that infants, children, and adults respond selectively to human as compared to non-human biological (e.g., spider, dog) motion, just as they respond selectively to human as compared to non-human non-biological (e.g., robot, mechanical) motion (e.g., Carter & Pelphrey, 2006; Chouchourelou, Golden, & Shiffrar, 2013; Kaiser, Shiffrar, & Pelphrey, 2012; Pyles, Garcia, Hoffman, & Grossman, 2007). Most relevant to the current research is that infants perceive entities with human-like motion patterns as possessing goal-directed, agentive behavior (Csibra, Bíró, Koós, & Gergely, 2003; Csibra, Gergely, Bíró, Koós, & Brockbank, 1999; Schlottmann & Ray, 2010). The extent to which infants extend these characteristics to non-human entities is more complex and discussed below.
1.1.1 Human and Non-Human Agents
One of the most salient agents to which infants are exposed from birth are humans and, more particularly, the hands of humans. We define “agent” as an entity that moves on its own accord (is self-propelled) and changes the state of another entity. We do not necessarily attribute volition, intention, or sentience (i.e., mental states) to an agent, but many agents do possess these characteristics. Infants 6 months and older are sensitive to action sequences performed by human hands: infants parse sequences into meaningful unites, imitate novel actions on objects and, eventually, extrapolate the end state of incomplete sequences (Baldwin & Baird, 2001; Bellagamba & Tomasello, 1999; Meltzoff, 1988a, 1988b, 1995). In seminal studies investigating infants’ understanding of the extent to which the behavior of human hands is goal-directed, Woodward (1998), habituated 6- and 9-month-old infants to an event in which a human hand repeatedly acted on an object. In test trials, infants saw the hand act on either (a) the same object at a different location or (b) a different object at the same location. Infants in both age groups looked longer during test trials when the hand acted on a different object at the same location than the same object at a different location, suggesting that by at least 6 months infants are more sensitive to a change in a hands’ goal than its path of motion. Subsequent studies revealed that by 9 months infants represent goal-directed actions as specific to an individual agent (Buresh & Woodward, 2007; Woodward, 1998, see Woodward, 2009 for a review) and that infants are more likely to make a goal attribution if the action on an object results in a salient change of state (e.g., the hand moves or manipulates an object, as compared to touches an object), if the action sequence is one with which they are familiar, or if the action is one in which they have themselves engaged (Biro & Leslie, 2007; Jovanovic et al., 2007; Király, Jovanovic, Prinz, Aschersleben, & Gergely, 2003; Sommerville, Woodward, & Needham, 2005).
In contrast, when Woodward (1998) replaced the human hand with a mechanical (clawed) hand, 6- and 9-month-old infants showed no goal-related expectations: they looked about equally to the same object/different location and different object/same location test events. There are now several reports confirming that infants younger than 12 months are more sensitive to goal-directed actions executed by human agents than mechanical devices (Gerson & Woodward, 2012; Hofer, Hauf, & Aschersleben, 2005; Jovanovic et al., 2007) and are more likely to simulate and imitate actions of human hands than those of mechanical devices (Boyer, Samantha Pan, & Bertenthal, 2011; Legerstee & Markova, 2007; Meltzoff, 1995). At the same time, with sufficient cues to establish goal intention, such as self-propulsion, clear action-effects, and equifiniality (Biro & Leslie, 2007; Király et al., 2003) or with experience with the claw (Boyer et al., 2011; Gerson & Woodward, 2012; Hofer et al., 2005) infants as young as 7 to 9 months will attribute goal-directedness to non-human agents. These and related findings suggest that infants have a bias to assign intentional agency to humans but not mechanical devices (Fields, 2014), a bias that is malleable with salient goal-related cues and motor experience.
1.1.2 Object Function
One type of behavior in which human hands often engage is that of the functional use of tools. We define “function” as an agent-produced action on an object that the object affords and/or for which it was intended, either by design or through conventional use (Wilcox, Woods, & Chapa, 2008; for related definitions, see Booth & Waxman, 2002; Booth, 2006; Casler & Kelemen, 2007; Kemler Nelson, 1995, 1999). Object function, as an event, contains a causal structure: the features of the object provide a mechanism by which to achieve a goal (i.e., perform the function) and aid the agent in the completion of that goal.
From an early age infants are sensitive to the functional properties of tools. By at least 6 months infants detect the functional relation between object parts and surfaces and interact with objects in ways that are consistent with these relations (Bourgeois, Khawar, Neal, & Lockman, 2005; Palmer, 1989; Ruff, 1984). Young infants also recognize the affordances of tools and tailor their actions accordingly, gradually becoming more sophisticated at manipulating objects on the basis of the functions they afford (Barrett, Davis, & Needham, 2007; Clifton, Rochat, Litovsky, & Perris, 1991; Freeman, Lloyd, & Sinha, 1980; Lockman, Ashmead, & Bushnell, 1984; McCarty, Clifton, Ashmead, Lee, & Goubet, 2001; Pieraut-Le Bonniec, 1985; von Hofsten & Fazel-Zandy, 1984; von Hofsten & Rönnqvist, 1988). Furthermore, infants distinguish between functional and non-functional use of tools (Wilcox & Chapa, 2004; Wilcox, Smith, & Woods, 2011; Wilcox et al., 2008), generalize functional properties to objects similar in appearance or that share important characteristics (Baldwin, Markman, & Melartin, 1993; Booth & Waxman, 2002), and attend to novel ways objects can be used and imitate those actions (Meltzoff, 1988a, 1988b). Older infants and young children use object function as the basis for which to categorize objects, make inferences about the function of an object based on category membership, and extend labels to novel objects that function in a similar way (Booth & Waxman, 2002; Booth, 2000, 2006; Kemler Nelson, Russell, Duke, & Jones, 2000; Kemler Nelson, Frankenfield, Morris, & Blair, 2000; Madole & Cohen, 1995).
Collectively, this body of work suggests that infants are not only sensitive to object function across a wide range of situations and tasks, but that they use function-related information to make inferences about what physical properties an object should possess, how it will be acted on and used as a tool, and the ontological category to which it belongs. Function is not just a salient object property: it is a learning mechanism.
1.1.3 Cortical networks
There is reason to believe that early emerging knowledge evident in the infant behavioral work reviewed above reflects organizing principles of the human cortex. For example, there is a large body of research indicating that the superior temporal sulcus (STS) is critical to the analysis of human motion in children and adults (Carter & Pelphrey, 2006; Grossman, Battelli, & Pascual-Leone, 2005; Martin & Weisberg, 2003; Morris, Pelphrey, & McCarthy, 2008; Pelphrey et al., 2003; Peuskens, Vanrie, Verfaillie, & Orban, 2005; Pyles et al., 2007), showing greater sensitivity to point-light and animated displays containing upright human motion, as compared to displays containing robot motion, motion of mechanical objects, or disjointed or inverted human motion (Carter & Pelphrey, 2006; Grossman et al., 2005; Martin & Weisberg, 2003). However, because in many of these studies human and biological motion were confounded (just as we saw in early behavioral work), it was not clear whether the observed effects reflected a biological/non-biological distinction or a human/non-human distinction (Chouchourelou et al., 2013; Han et al., 2013). More recent work using human biological motion (e.g., human running), non-human biological motion (e.g., animal jumping), and mechanical motion (robot or mechanical device) stimuli suggests that the posterior temporal sulcus, particularly in the right hemisphere, responds more robustly to human motion than to either non-human biological motion or mechanical motion (Han et al., 2013; Kaiser et al., 2012; Morris et al., 2008; Pyles et al., 2007). Finally, there is evidence that posterior STS (pSTS) is critical to the analysis of intentional, goal-directed actions (Carter & Pelphrey, 2006; Martin & Weisberg, 2003; Pelphrey et al., 2003; Peuskens et al., 2005; Pyles et al., 2007; for reviews see Adolphs, 2009; Cunningham, Johnson, Gatenby, Gore, & Banaji, 2003; Cunningham & Zelazo, 2007) and that pSTS responses are more robust in the right than left hemisphere.
In contrast, movement of mechanical, robot, or animate objects typically leads to activation in the middle temporal gyrus (MTG) (Beauchamp, Lee, Haxby, & Martin, 2002, 2003; Beauchamp & Martin, 2007; Han et al., 2013; Martin & Weisberg, 2003), or post-central gyrus (PCG) (Morris et al., 2008), and these patterns of activation are dissociated from those obtained in response to human motion. For example, viewing vignettes in which objects with human-like movement patterns leads to activation in the STS, whereas viewing vignettes in which objects with mechanical movements engage in automated actions leads to greater activation in the MTG (Martin & Weisberg, 2003). Although there are no neuroimaging studies of which we are aware that investigated cortical responses to functional and non-functional use of tools, there is evidence that viewing videotapes or point light displays of a tool undergoing motion leads to activation in MTG, often left lateralized (Beauchamp et al., 2002, 2003; Beauchamp & Martin, 2007)..
Very little is known about the origins and development of the patterns of activation reported in infant populations. In one of a limited number of studies, Lloyd-Fox and colleagues (Lloyd-Fox et al., 2009; see also Farroni et al., 2013) investigated the extent to which the infant cortex responds differentially to social and mechanical stimuli using functional near-infrared spectroscopy (fNIRS). In these studies 5-month-olds were presented with a video of a woman engaged in actions (e.g., moved her eyes, opened her mouth, or moved her hands to play peek-a-boo), and a video of inanimate objects undergoing mechanical movements (e.g., machine cogs, pistons, or a moving mechanical toy), on alternating test trials. Bilateral activation was obtained in posterior temporal cortex in response to the dynamic social stimuli. In contrast, activation was obtained in left middle-anterior temporal channels in response to the dynamic mechanical stimuli. Although these studies reveal distinct patterns of activation to the social and mechanical events, the stimuli varied on many different dimensions, making it difficult to draw firm conclusions about which differences elicited the responses observed.
Taking a slightly different approach, Grossman and colleagues (Grossmann, Lloyd-Fox, & Johnson, 2013) assessed hemodynamic responses to human and robotic motion in 4-month-olds. Infants saw events in which the form of an object (human or robot) was crossed with the motion that the object displayed (human or robot). Two main findings emerged: (1) areas in the right premotor cortex responded selectively to robot as compared to human motion (regardless of whether the motion was seen on a human or robot form), and (2) left temporal cortex responded selectively to congruent (human-human/robot-robot) as compared to incongruent (human-robot/robot-human) form-motion pairings. Unlike Lloyd-Fox and colleagues (2009), actions of human and non-human entities did not elicit distinct patterns of activation in temporal cortex.
1.2 Current Research
In summary, it is clear that infants are sensitive to the distinction between human and non-human entities, and have different expectations for whether these entities engage in agentive, goal-directed behavior. Infants are also sensitive to the functional use of tools, and are selective about the conditions under which object-directed actions are perceived as functionally relevant. However, we know little about the cortical architecture that supports processing of these distinctions to which infants are sensitive. The purpose of the current research is to identify the extent to which the immature brain is organized in accordance with these behavioral sensitivities. If infants’ processing of human and non-human agents is supported by different cognitive networks, we would expect different patterns of cortical activation to events involving human as compared to non-human (claw) hands. Likewise, if infants process events involving tools engaged in functionally relevant as compared to non-functionally relevant actions differently, then distinct cortical systems/structures should be invoked. Finally, we tested the hypothesis that infants are more sensitive to human hands, as compared to mechanical hands, as agents of function. If so, infants’ response to object function may differ for human as compared to non-human agents.
2. Experiment
This experiment assessed cortical activation during infants’ processing of events in which agents acted on tools. Infants aged 6 to 10 months were shown events in which one of two agent types, human hand or mechanical hand, engaged in one of two actions with a tool, functionally relevant or functionally irrelevant (Figure 1). We assessed activation bilaterally in temporal and temporal-occipital cortex. If infants are sensitive to the distinction between human and mechanical hands, and the processing of these two types of agents are mediated by different cognitive and cortical networks, we would expect to see this reflected in hemodynamic responses. Similarly, if infants are sensitive to the distinction between function and non-function events, we would expect hemodynamic responses to reflect this difference. Finally, if infants have different expectations for whether human and mechanical hands engage in agentive tool use, we would expect an interaction between agent and function in patterns of cortical activation.
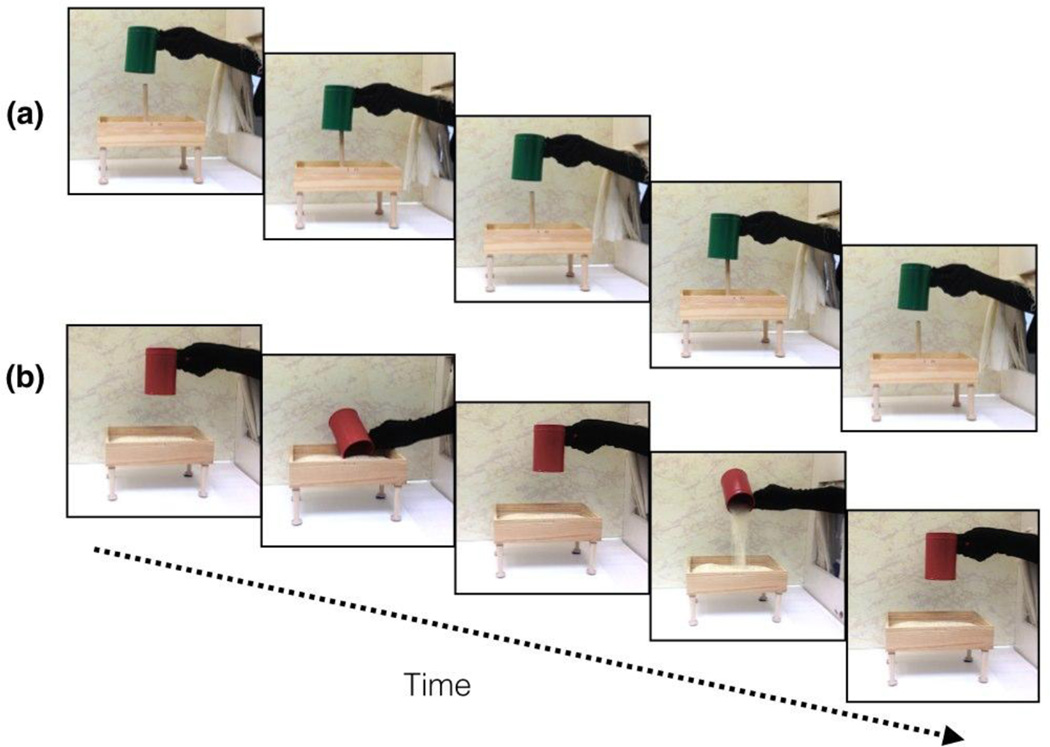
Figure 1.
The (a) pound and (b) pour test events of the human hand, function condition. The figure depicts one cycle of the test event; two cycles of the event were seen in each test trial. Infants saw pound and pour events on alternating trials. The trial type seen first (pound or pour) was counterbalanced across infants.
2.1 Methods
2.1.1 Participants
Infants aged 6 to 10 months participated (N = 69; M = 249 days; SD = 29 days; F = 29; range = 186–312 days). Thirty-eight additional infants were tested but eliminated from the sample due to procedural problems (n = 3), inability to complete at least 6 out of the 12 total trials (n = 14), difficulty obtaining optical signal (n = 18), or fussiness (n = 3). The percentage of infants excluded here is typical for fNIRS studies with infant populations (Sarah Lloyd-Fox, Blasi, & Elwell, 2010). Infants were pseudo-randomly assigned to one of four conditions formed by crossing agent (human hand or mechanical hand) and event type (function or non-function), with the stipulation that similar number of males and females be assigned to each condition: human hand, function (n = 19); hand, non-function (n = 16), mechanical hand, function (n = 16); mechanical hand non-function (n = 18).
Parents were recruited primarily by social media and commercially available lists, and given $5 or a lab t-shirt for their participation.
2.1.2 Task and Procedure
Crossing the two independent factors - agent and event type - formed the four conditions describe below.
Infants in the human hand, function condition were presented with three pairs of test trials. Each pair of trials consisted of a pound trial and a pour trial (Figure 1). Each trial was 15 s in duration, during which infants watched two complete cycles of the test event (pound or pour) appropriate for that trial. Each pair of pound and pour trials was seen with a different pair of green and red containers (Figure 2). The green container always pounded a nail box and the red container always scooped and poured from a rice box, so the object color predicted the function event in which the object would engage. A human hand, covered with a black glove, performed all of the events. Because more than one experimenter helped conduct the study, the human hand (and arm to which it was attached) was covered in a black glove to control for individual differences in skin color and other visible features (e.g., hair, moles, or blemishes).1 A curtain was raised to begin and lowered to end each trial.
Figure 2.
The three pairs of cups used in the three pairs of test events. In each pair, the green cup was used in the pound event and the red cup in the pour event.
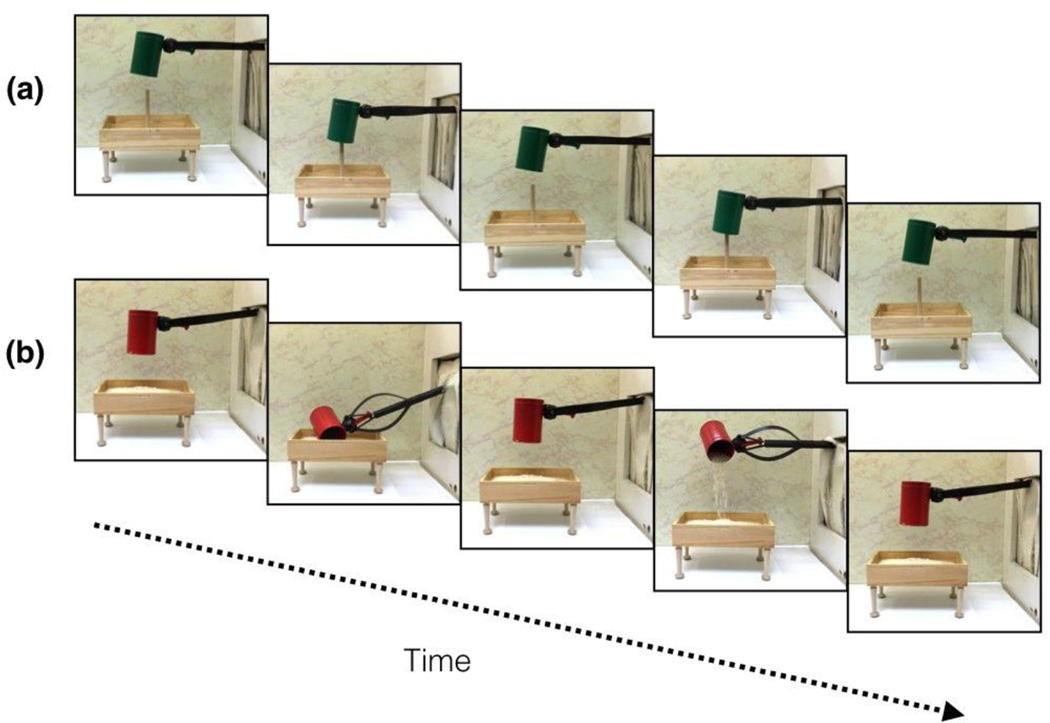
Infants in the mechanical hand, function condition were presented with the same events as infants in the human hand, function condition except that instead of seeing the events performed by a human hand wearing a black glove, all of the events were performed by a mechanical grabber claw, painted black (Figure 3). The grabber claw (and rod to which it was attached) was painted black to equate the perceptual cues in the human hand and mechanical hand conditions as much as possible.
Figure 3.
The (a) pound and (b) pour test events of the mechanical hand, function condition. The figure depicts one cycle of the test event; two cycles of the event were seen in each test trial. Infants saw pound and pour events on alternating trials. The trial type seen first (pound or pour) was counterbalanced across infants.
Infants in the human hand, non-function condition saw the same events as infants in the human hand, function condition, except the nail box (pound trials) and rice box (pour trials) were moved 21 cm to the left, toward the center of the stage. Hence, in the pound trials the green container moved up and down without coming in contact with the nail, and in the pour trials the red container made scooping and pouring motions without scooping or pouring rice.
Infants in the mechanical hand, non-function condition saw the same events as infants in the human hand, non-function condition, except that the black mechanical grabber claw also performed them instead of the gloved human hand.
The size of both of the pound and pour boxes was 23.5 cm × 13 cm, including the legs; however, the nail box had an additional 12 cm wooden nail that protruded from the top. The test events seen here are similar to priming trials seen in previous work (Wilcox & Chapa, 2004; Wilcox, Hirshkowitz, Hawkins, & Boas, 2014). Infants saw pound and pour events on alternating trials. The order in which infants saw the events (pound and pour) was constant across all three pairs of trails and was randomly assigned. Because analysis of optical imaging data requires a baseline interval, each trial was preceded by a 10 s baseline, during which the screen was lowered, covering the apparatus and stage; no other visual or auditory stimuli were presented during this time.
Infants sat in a Bumbo or their parents lap in a dark, quiet room and watched the test events appropriate for their condition on a puppet-stage apparatus. Trained experimenters produced the events with a precise, timed script. Two observers, naive to the condition to which infants were assigned, monitored infants’ looking behavior through peepholes in the frames on either side of the apparatus. Each observer held a game controller connected to a Dell computer, and pressed a button when the infants attended to the event. The looking times by the primary observer were used in data analysis. Inter-observer agreement was calculated and averaged 94% (per trial and infant).
Total duration of looking (i.e., cumulative looking) to each test trial was obtained. Trials in which infants looked < 8 s (constituting one full cycle of the event) were excluded from analysis. This ensures that group differences in hemodynamic responses could not be attributed to group differences in overall time spent attending to the events.
2.1.3 Instrumentation
The imaging equipment contained eight fiber optical cables that delivered near-infrared light to the scalp of the participant (emitters), eight fiber optical cables that detected the diffusely reflected light at the scalp (detectors), and a control box that served as the source of the near-infrared light and the receiver of the reflected light. The control box produced light at wavelengths of 690 nm, which is more sensitive to deoxygenated blood, and 830 nm, which is more sensitive to oxygenated blood, with two laser-emitting diodes (TechEn Inc.).
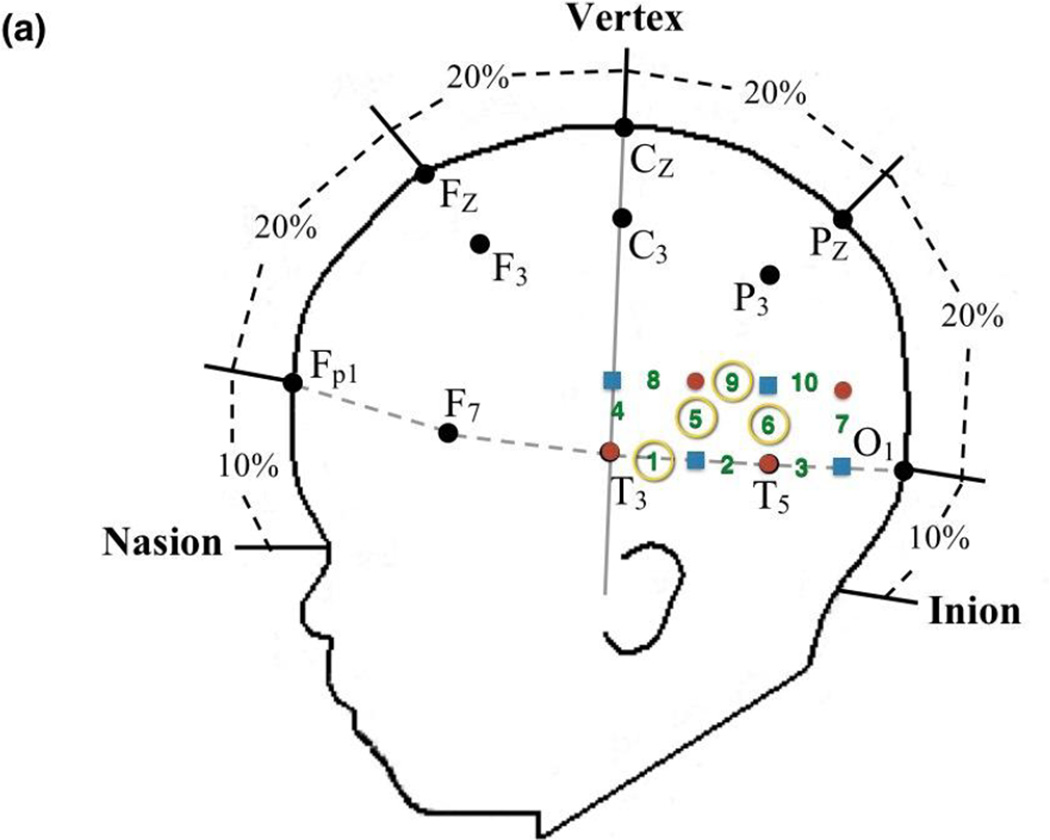
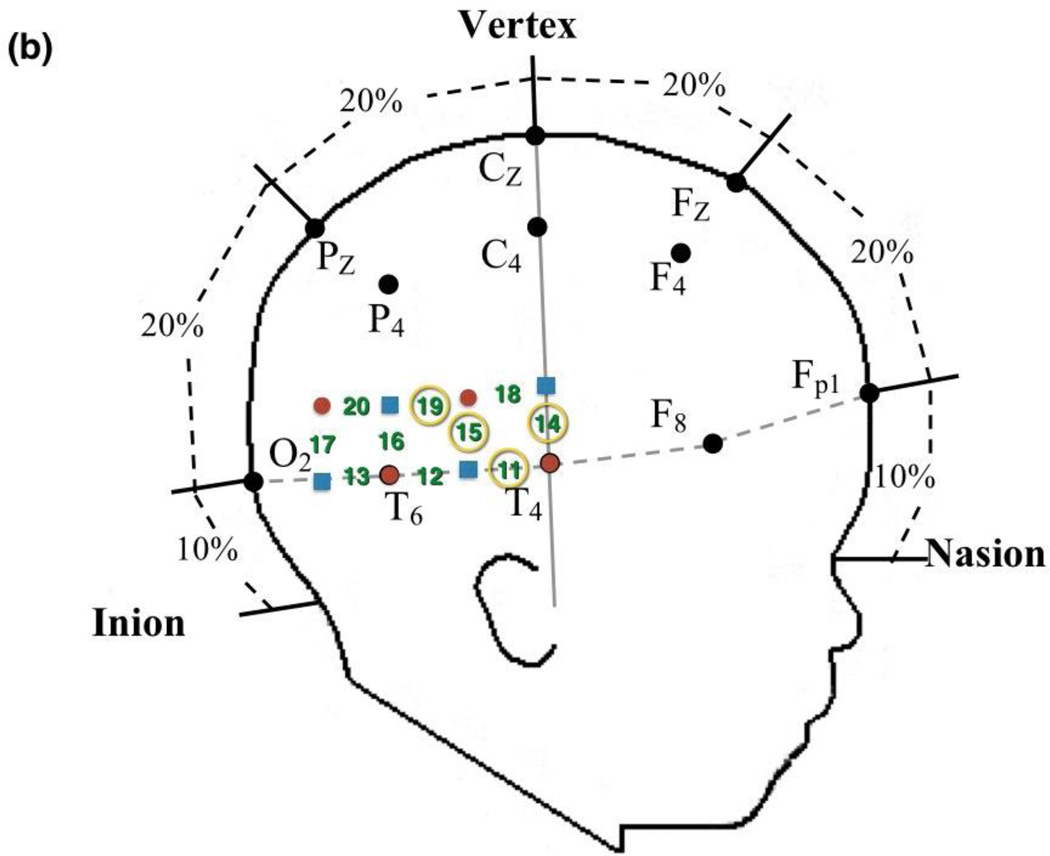
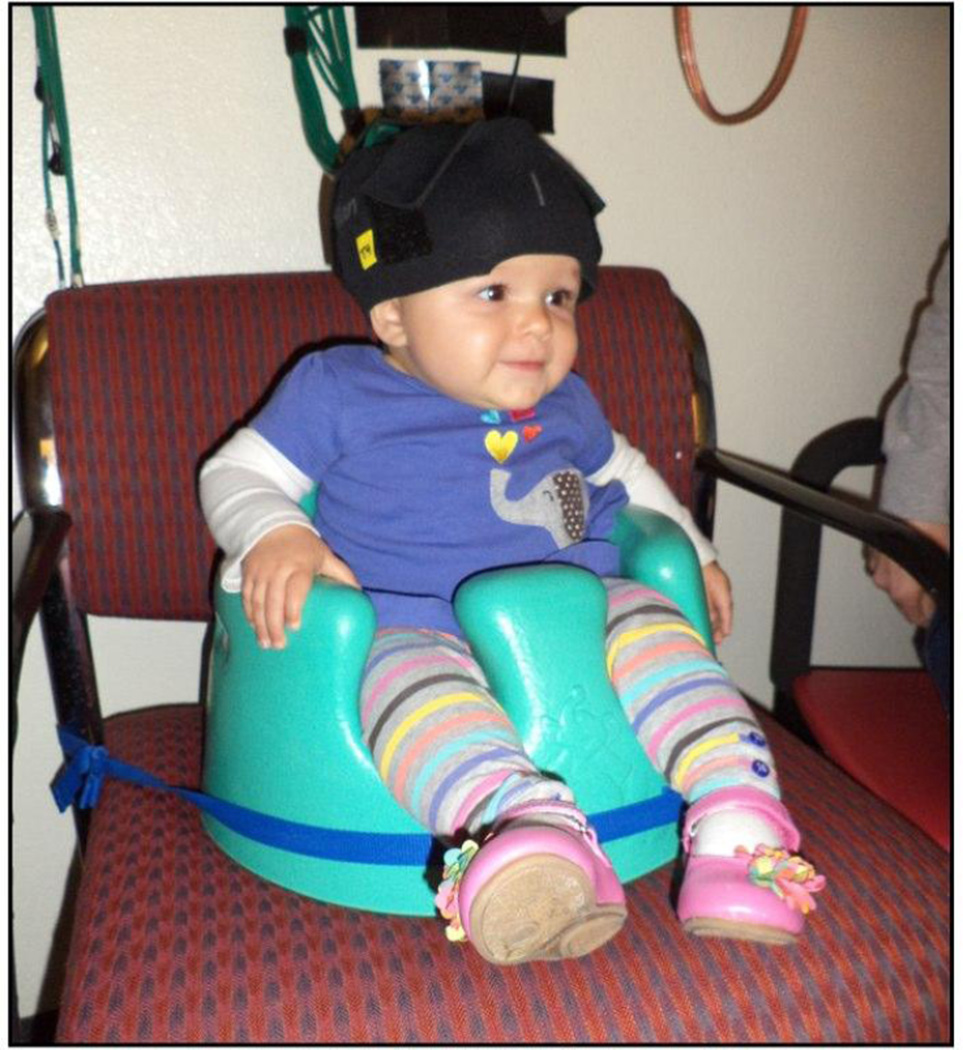
Prior to the experimental session, infants were fitted with a custom-made headgear that secured the fiber optics to the scalp. The headgear consisted of two pads, each containing 4 sources and 4 detectors, which covered temporal and temporal-occipital regions of the left and right hemispheres, respectively. Configuration of the sources and detectors within the headgear, location of corresponding channels, and placement of the headgear on the infant’s head are illustrated in Figures 4 and 5. Source-detector separation was 2 cm. The pads in which the sources and detectors were embedded were not elastic so the distance between the sources and detectors within each pad remained fixed even when head circumference varied. The bands connecting the two pads were elastic. The headgear was placed on the infant’s head using T3 and T4 as the primary anchors in left and right hemisphere, respectively. The mean head circumference measurements did not differ significantly by condition, F (3,69) = 0.874, p > 0.05 (Human Function: M = 45.34 cm, SD = 1.41 cm; Human Motion: M = 44.84 cm, SD = 1.55 cm; Mechanical Function: M = 44.56 cm, SD = 1.23 cm; Mechanical Motion: M = 44.94 cm, SD = 1.69 cm). Although the head circumference of the infants tested ranged from 41.5 cm to 49 cm, the difference in the amount of skull covered by the left and right segments of the headgear, each, differed by 1.69 cm between the smallest and largest head circumference, which is less than the source-detector distance.
Figure 4.
Headgear configuration and placement. The headgear consisted of two pads, placed over temporal and temporal-occipital regions of the (a) left and (b) right hemisphere, respectively. Four emitters (red circles) and four detectors (black squares) were embedded in each pad and the left and right pads were anchored at T3 and T4, respectively, of the 10–20 International EEG system. Emitter-detector distances were all 2 cm. The numbers represent channels. The circled numbers are those channels that were included in the ROI for that hemisphere.
Figure 5.
An infant wearing the headgear, while participating in the study. Infants sat in a supportive seat to restrain excess movement. The headgear was secured onto the infant’s head by an elastic chinstrap.
2.1.4 Processing of fNIRS Data
The fNIRS data were processed for each source-detector pairing and event separately, similar to the procedure to that of Wilcox and colleagues (Wilcox et al., 2014). Briefly, the raw signals were acquired at a rate of 200 samples per second, digitally low-pass filtered at 3 Hz, a principal components analysis was used to design a filter for systemic physiology and motion artifacts, and the data were converted to relative concentrations of oxygenated (HbO) blood using the modified Beer-Lambert Law.
For the test trials, changes in HbO were examined using the following time epochs: the 2 s prior to the onset of the test event, the 15 s test event, and the 10 s following the test event. The mean optical signal from −2 to 0 s (baseline) was subtracted from the signals and other segments of the time epoch were interpreted relative to this zeroed baseline. Optical signals were averaged across trials and then infants for each test condition. Trials objectively categorized as containing motion artifacts (a change in optical density intensity greater than 1 unit within 0.5 s during the 2 s baseline and the test event) were eliminated from the mean. Although each infant was presented with 12 total trials, each infant must have contributed 6 or more trials to the total to be included in the final sample.
On the basis of this criterion, and looking time criteria, 198 of 809 (24.5%) possible trials were eliminated from the analysis. The total percent eliminated did not vary across the four conditions: Human hand, function condition, 46 of 228 (20.2%) possible trials as compared to human hand, non-function condition, 49 of 188 (26.1%) possible trials, z-score = −1.4239, p > .05; or mechanical hand, function condition, 46 of 179 (25.7%) possible trials as compared to mechanical hand, non-function condition, 57 of 214 (26.6%) possible trials), z-score = −.2104, p > .05; or human hand, function condition as compared to mechanical hand, function condition, z-score = −1.322, p > .05.
2.2 Results
2.2.1 Looking time data
Duration of looking time data, in seconds, were averaged across trials and infants for each event condition, and a univariate ANOVA was conducted with agent and event as the between-subjects factors (Table 1). The main effect of agent, F (1,68) = 1.282, p > .05, and event, F (1,68) = 1.090, p > .05, were not significant. However, the interaction of agent × event was significant, F (1,68) = 8.705, p = .004, η2 = 0.828. Paired comparisons revealed that infants in the human hand, function condition looked significantly longer at the display than infants in the human hand, non-function condition, t(33) = 3.800, p < .001, Cohen’s d = 1.323 (Cohen, 1988). Infants in the human hand, function and the mechanical hand, function conditions did not differ significantly in their mean looking times, t(33) = 1.223, p > .05, Cohen’s d = .403, nor did the infants in the mechanical hand, function and the mechanical hand, non-function conditions, t(32) = −1.111, p > .05, Cohen’s d = .37. Typically we do not find significant differences in looking times to human, function and human, non-function events (Wilcox & Chapa, 2004; Wilcox et al., 2008), so this outcome was unexpected. These differences will be considered in our analysis of hemodynamic responses.
Table 1.
Total looking time (in seconds) per condition.
| Human Hand | Mechancial Hand | |||
|---|---|---|---|---|
| Function | Non- Function |
Function | Non- Function |
|
| Mean | 13.49 | 12.49 | 13.04 | 13.51 |
| Std. Deviation |
0.82 | 0.71 | 1.35 | 1.15 |
2.2.2 Optical imaging data
For each of the 20 channels (10 channels within each hemisphere) responses were averaged over 8–15 s. Averaging over this time epoch captured hemodynamic responses following completion of one full event cycle until the end of the trial. Responses were then averaged over trial and infant, for each of the four conditions separately, to obtain a grand average. Mean hemodynamic responses, including HbO and HbR, are reported in Appendix A and Appendix B, respectively. However, because HbO responses are typically more robust than HbR responses (Strangman, Franceschini, & Boas, 2003), we focused our analyses on HbO.
To test our prediction that condition-specific patterns of activation would be obtained in temporal cortex, mean HbO responses at each channel were subjected to 2 × 2 ANOVA with agent (human/mechanical) and event (function/non-function) as the between-subjects factors for each channel within each hemisphere separately. We sought to identify spatially continuous channels that could be grouped together, on the basis of activation patterns, to form Regions of Interest (ROIs). For each of the ten channels in each hemisphere, the corresponding ten p-values were corrected for multiple comparisons using the False Discovery Rate (FDR) analysis (Benjamini & Hochberg, 1995).
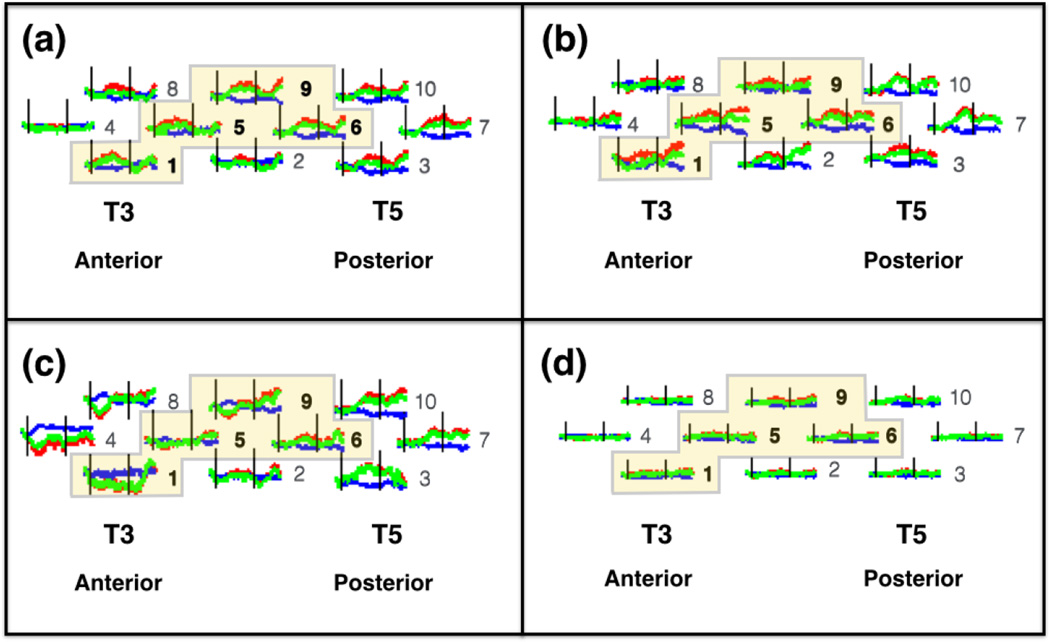
In the left hemisphere, four spatially contiguous channels (1, 5, 6, and 9) showed a significant agent × event interaction (Table 2); no significant main effects were obtained. These four neighboring channels were grouped together to form an ROI, which was located over left middle-posterior temporal cortex (Figure 6). A grand mean for the ROI was computed by averaging the responses obtained at the four channels. The ROI grand mean was subjected to a 2 (agent) × 2 (event) ANOVA. The outcome of this analysis mirrored that obtained in the individual channels. The main effects of agent (F(1,66) = 2.253, p > .05) and event (F(1,66) = 2.209, p > .05) failed to reach significance, but a significant interaction was obtained between agent × event, F(1,66) = 15.112, p < .001, η2 = 0.969. Paired comparisons revealed that the human hand, function event elicited significantly greater activation than the mechanical hand, function event, t(33) = 3.577, p < .001, Cohen’s d = 1.245. In contrast, activation obtained in response to the human hand, non-function event and the mechanical hand, non-function event did not differ significantly, t(29) = −1.858, p > .05, nor did activation obtained in response to the human hand, function event and the human hand, non-function event, t(33) = 1.626, p > .05. In other words, left hemisphere responses were driven by the difference between human and mechanical hand, but only when the actions were functionally relevant.
Table 2.
HbO responses for left hemisphere channels (univariate ANOVA), and follow-up comparisons for left hemisphere univariate ANOVA analyses. The otcome of these analyses, which were corrected for multiple comparisons (Benjamini & Hochberg, 1995), revealed four spatially contiguous channels in each hemisphere that were sensitive to the categorical distinctions under investigation.
| Interaction | Follow Up Comparisons | |||||||
|---|---|---|---|---|---|---|---|---|
| Human Function > Robot Function |
Human Motion > Robot Motion |
Human Function > Human Motion |
||||||
| Channel | F-value (df) | p-value | t-value (df) | p-value | t-value (df) | p-value | t-value (df) | p-value |
| 1 | 9.919 (1,53) | 0.003 | 2.740 (26) | 0.006 | −1.699 (23) | 0.052 | 1.131 (24) | 0.269 |
| 5 | 4.474 (1,47) | 0.040 | 2.444 (25) | 0.011 | −0.670 (18) | 0.256 | 0.800 (22) | 0.432 |
| 6 | 4.579 (1,58) | 0.037 | 1.822 (30) | 0.039 | −1.235 (24) | 0.115 | 0.752 (32) | 0.457 |
| 9 | 11.126 (1,60) | 0.002 | 3.590 (32) | <.001 | −1.284 (24) | 0.211 | 2.227 (31) | 0.033 |
Figure 6.
Mean hemodynamic responses in the left hemisphere of infants in each condition: (a) human hand, function; (b) human hand, non-function; (c) mechanical hand, function; and (d) mechanical hand, non-function. T3 and T5 correspond to the International 10–20 coordinates, and T3 served as our left hemisphere anchor point when securing the headgear on the infant. The red curves indicate change in oxyhemoglobin concentration (HbO), the blue curves indicate change in de-oxyhemoglobin concentration (HbR), and the green curves indicate the sum total of HbO and HbR (HbT). The black vertical lines indicate time points 0 s and 15 s, the onset and offset of the trial, respectively. The horizontal axis indicates time (−2 s to 25 s), and the vertical axis indicates change in optical density units (ΔOD, in µM cm). The numbers to the right of each waveform indicate the channel (see Figure 4a for reference). The highlighted channels indicate the four spatially contiguous channels (see text) that were averaged to obtain a grand mean for a left region of interest (ROI). The response obtained in the left ROI differed significantly for conditions (a) and (c). In contrast, the left ROI response obtained in conditions (b) and (d), and conditions (a) and (b), did not differ significantly.
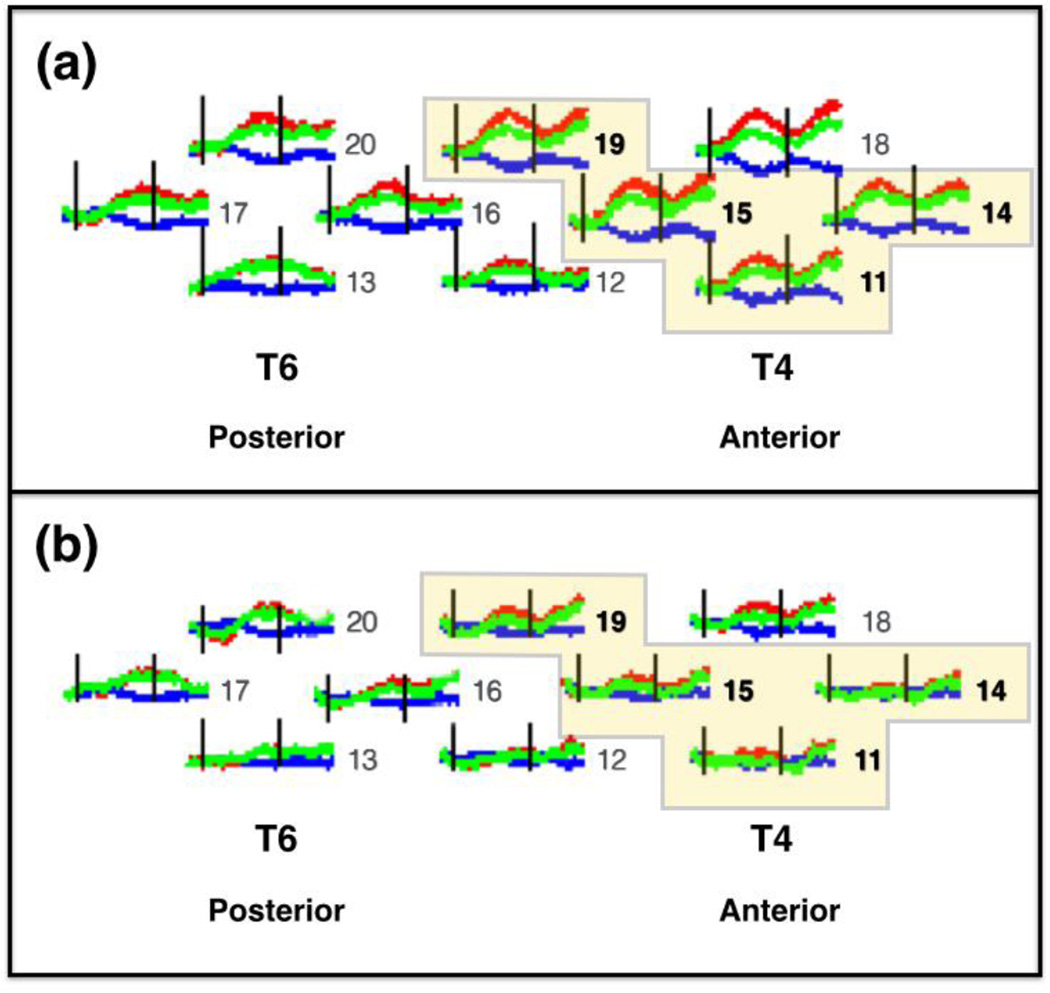
In the right hemisphere, four spatially contiguous channels (11, 14, 15, and 19) showed a significant main effect of agent (Table 3). The main effect of event and the agent × event interaction were not significant. The four neighboring channels were grouped together to form an ROI, which was located over right middle-posterior temporal cortex (Figure 7). A grand mean for the ROI was computed by averaging the responses obtained at the four channels. The ROI grand mean was subjected to a 2 (agent) × 2 (event) ANOVA. A significant main effect of agent was obtained, F(1,65) = 14.502, p < .001, η2 = 0.963, revealing that the response to the human hand was greater than that to the mechanical hand. The main effect of event (F(1,65) = 0.111, p > .05), and the interaction between agent × event (F(1,65) = 1.351, p > .05), failed to reach significance.
Table 3.
HbO responses for right hemisphere channels (univariate ANOVA), corrected for multiple comparisons (Benjamini & Hochberg, 1995).
| Agent | Event | Interaction | ||||
|---|---|---|---|---|---|---|
| Channel |
F-value (df) |
p-value |
F-value (df) |
p-value |
F-value (df) |
p-value |
| 11 | 4.367 (1,56) |
0.042 | .042 (1,56) |
0.839 | .870 (1,56) |
0.355 |
| 14 | 8.354 (1,60) |
0.005 | .399 (1,60) |
0.530 | 2.635 (1,60) |
0.110 |
| 15 | 8.827 (1,55) |
0.005 | .043 (1,55) |
0.836 | .180 (1,55) |
0.673 |
| 19 | 6.136 (1,63) |
0.016 | 1.947 (1,63) |
0.168 | .000 (1,63) |
0.996 |
Figure 7.
Mean hemodynamic responses in the right hemisphere of infants, collapsed across event, for each of the two agent types: (a) human hand and (b) mechanical hand. T4 and T6 correspond to the International 10–20 coordinates, and T4 served as our right hemisphere anchor point when securing the headgear on the infant. The red curves indicate change in oxyhemoglobin concentration (HbO), the blue curves indicate change in de-oxyhemoglobin concentration (HbR), and the green curves indicate the sum total of HbO and HbR (HbT). The black vertical lines indicate time points 0 s and 15 s, the onset and offset of the trial, respectively. The horizontal axis indicates time (−2 s to 25 s), and the vertical axis indicates change in optical density units (ΔOD, in µM cm). The numbers to the right of each waveform indicate the channel (see Figure 4b for reference). The highlighted channels indicate the four spatially contiguous channels (see text) that were averaged to obtain a right region of interest (ROI). The response obtained in the right ROI differed significantly for (a) and (b).
Recall that analysis of the looking time data revealed that infants attended more to the human function than the mechanical function event. One might be concerned that differences in attention to the visual displays could have contributed to the hemodynamic responses observed. Although we view this unlikely, mostly because a different pattern of results was obtained in the looking time and HbO data, to control for this possibility we conduced the same analyses on the right and left ROIs as described above, but added looking time as a covariate. We found the same pattern of results. In the left hemisphere, the main effects of agent (F(1,66) = 2.646, p > .05) and event (F(1,66) = 2.345, p > .05) failed to reach significance, but a significant interaction was obtained between agent × event, F(1,66) = 12.498, p < .001, η2 = 0.936. Follow-up comparisons revealed that the human hand, function event elicited significantly greater activation than the mechanical hand, function event, p < .001. In contrast, activation obtained in response to the human hand, non-function event and the mechanical hand, non-function event did not differ significantly, p > .05. Likewise, activation obtained in response to the human hand, function event and the human hand, non-function event did not differ significantly, p > .05. In the right hemisphere, a significant main effect of agent was obtained, F(1,65) = 13.224, p < .001, η2 = 0.947. The main effect of event (F(1,65) = 0.298, p > .05), and the interaction between agent × event (F(1,65) = 2.102, p > .05), failed to reach significance.
3. Discussion
There is a large body of behavioral work indicating that infants organize objects into ontological categories and information about these categories guide infants’ expectations for how objects should move and interact (e.g., Gelman & Opfer, 2002; Gervain et al., 2011; Leslie, 1994; Luo & Baillargeon, 2010; Setoh et al., 2013; Spelke & Kinzler, 2007). The current studies assessed cortical activation in response to events that differed on one of two critical dimensions: whether the event was (a) produced by a human hand or a mechanical hand and (b) composed of actions on objects that were functionally relevant or not functionally relevant. In all other ways the events were identical. Hence, differences in patterns of activation could be attributed to one of these two factors, or an interaction between these two factors, and not to other characteristics of the events. Two main findings emerged.
3.1 Effect of agent: Human hand and mechanical hand
In the right hemisphere a ROI in the middle-posterior temporal cortex, which included channels 11, 14, 15, and 19, evidenced significantly greater activation to the human hand than to the mechanical hand. The event in which the hand engaged, function or non-function, did not significantly influence hemodynamic responses. These findings reveal, for the first time, that the infant brain is sensitive to the distinction between human and mechanical hands, and that the right middle-posterior temporal cortex is specialized for processing events involving actions of the human hand. This outcome is consistent with the outcome of fMRI studies conducted with adult participants, which have reported greater responses in pSTS, typically on the right, to the movement of human hands as compared to mechanical devices (Kaiser et al., 2012; Morris et al., 2008) and suggest that these responses become lateralized early in life. At the same time, we did not find selective responses to the mechanical hand as predicted. Functional imaging studies with adults have reported dissociation of responses to human and mechanical actions. Whereas the movement of human hands generates (typically right lateralized) responses in pSTS, mechanical motion generates (typically left lateralized) responses in MTG (Beauchamp et al., 2002, 2003; Beauchamp & Martin, 2007; Han et al., 2013; Martin & Weisberg, 2003). Why did we find selective, lateralized responses to events involving the human hand but not events involving the mechanical hand?
One possibility is that this outcome reflects infants’ greater experience with human than mechanical hands. Whereas infants have extensive and repeated exposure to actions of human hands from birth – parent’s pick them up, change their diaper, dangle toys for them to look at – they have few, if any, experiences with mechanical hands. In support of an experience hypothesis, there is evidence that experience with human hands and the actions they perform, as well as an infant’s own motor skill level, facilitates behavioral and cortical responses to action patterns (Biro & Leslie, 2007; Hunnius & Bekkering, 2014; Jovanovic et al., 2007; Király et al., 2003; Sarah Lloyd-Fox, Wu, Richards, Elwell, & Johnson, 2013; Sommerville et al., 2005). Similarly, behavioral and cortical responses to novel and more complex action patterns in adults are associated with adult’s experience with those action patterns (Blake & Shiffrar, 2007; Chouchourelou et al., 2013; Servos, Osu, Santi, & Kawato, 2002).
Another possibility is that the right middle-posterior temporal response reflects sensitivity to agency rather than human motion. That is, infants perceived the human hand but not the mechanical hand as an agent. Given the large body of research indicating that infants attribute agency to non-human entities, including mechanical devices, across a wide range of experimental contexts (Biro & Leslie, 2007; Hunnius & Bekkering, 2014; Jovanovic et al., 2007; Király et al., 2003), it is unlikely that the infants in the current study did not grant agency to the mechanical hand.
Another possible explanation for this pattern of results is that it reflects infants’ differential attribution of intention to human as compared to mechanical agents. Typically infants perceive human action as volitional and intentional in nature, and are less likely to perceive the actions of non-human entities as intentional (Fields, 2014). Although it is possible that the infants in the present study interpreted the actions of the human hand, but not the mechanical hand, as intentional in nature we consider this explanation unlikely, for the following reason. Infants’ percept of intentionality is more robust if the event in which the non-human entity is involved includes a salient change of state (e.g., an action on an object as compared to touching an object) or if the action sequence is one in which infants are familiar or have themselves engaged. In the current studies, the events involved a salient change of state (e.g., movement of the containers to make contact with other surfaces/substances) and actions (pounding/pouring) in which infants are familiar and often engage (Biro & Leslie, 2007; Jovanovic et al., 2007; Király et al., 2003; Sommerville et al., 2005). Hence, it is more likely that the pattern of results reported here reflects infants’ sensitivity to the distinction between human and mechanical hands, than the distinction between intentional and non-intentional behavior. However, we acknowledge that this hypothesis warrants further testing.
3.2 Interaction effect: agent and event
In the ROI in the left middle-posterior temporal cortex, which included channels 1, 5, 6, and 9, a significant interaction between agent and event was obtained. Follow-up comparisons revealed that cortical responses to the human-function and mechanical-function events differed significantly. That is, greater activation was obtained to the function event when a human as compared to a mechanical hand performed it. In contrast, cortical responses to human-function and human-non-function events, and to mechanical-function and mechanical-non-function events, did not differ significantly. In other words, the left posterior temporal cortex responded selectively to human as compared to mechanical agents, but only in the context of functionally relevant actions on objects. These data provide some insight into the cognitive and cortical architecture that supports infants reasoning about functionally relevant events.
There is a large body of behavioral work showing that infants are sensitive to the functional properties of objects from the early months of life. For example, by 6 to 8 months infants recognize the functional relation between an object and its parts, use objects in ways that are consistent with these relations, and form event categories on the basis the functions in which objects engage (Bourgeois et al., 2005; Gibson & Walker, 1984; Molina & Jouen, 1998; Palmer, 1989; Ruff, 1984; Wilcox & Chapa, 2004; Wilcox et al., 2008). Just as infants’ behavioral responses to object function are robust and selective, the present research demonstrates that cortical responses to object function are robust and selective. What is most novel about these results is that the response to function was selective to events involving human hands. So, whereas the right hemisphere showed greater sensitivity to actions of human than mechanical agents, the left hemisphere showed this sensitivity only within the context of events in which the actions performed on the objects were functionally relevant, a finding that has not emerged so clearly in infant behavioral work.
We were puzzled that the distinction between function and non-function, alone, was not reflected in cortical responses. Given the large body of research demonstrating that infants are sensitive to the functional properties of objects, and that infants use functionally-relevant, but not functionally-irrelevant, information to guide their apprehension of objects (Wilcox & Chapa, 2004; Wilcox et al., 2008), we expected cortical responses in the temporal cortex to reflect this conceptual distinction. Although it is difficult to interpret null results, it is possible that agent type (i.e., whether the agent was a human hand or a mechanical hand) was more salient to infants than whether the agent engaged in functionally relevant events on objects. Of course, it is also possible that cortical areas from which we did not measure, such as frontal or premotor cortex, would respond more sensitively to this distinction.
3.3 Concluding Comments
The outcome of this study contributes significantly to our understanding of the cortical basis of infants’ processing of agents, physical objects, and functionally relevant events. These findings shed light on the cognitive architecture that underlies infants’ acquisition of object knowledge and allows us to begin to build a picture of the developing cortical networks that support this knowledge acquisition. At the same time, there is a significant amount of work left to do. As we and others move forward in this field, care must be taken to design studies that allow for strong inferences about localized and process-specific patterns of cortical activation. It is also important to test additional age groups in order to gain better insight into developmental processes. Although challenging, continued work along these lines is exciting because it has the potential to address fundamental questions about the cognitive and neural architecture that supports the development of human knowledge.
Supplementary Material
Acknowledgments
We thank Melissa Wallace Klapuch, Amy Hirshkowitz, Laura Hawkins, and the staff of the Infant Cognition Lab at Texas A&M University for help with data collection and management, and the infants and parents who so graciously participated in the research. Preparation of this manuscript was supported by grant R01-HD057999 to TW and grant P41-RR14075 to DAB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In Woodward (1998) the arm but not the hand of the experimenter was covered. Guajardo & Woodward (2004) reported that when the arm and hand are covered, as compared to having only the arm covered, infants are less likely to perceive the actions as goal directed (and by extension as produced by a human agent). In Guajardo & Woodward, the hand reached along a direct path and grasped the toy, a relatively simple motor sequence. In the present experiments, the action sequences in which the hand engaged were more complex (e.g., the arm/hand followed a unique path - and were seen from different perspectives - as the pound/pour events were produced), providing a rich set of visual cues by which to identify the hand as human. Given this rich set of visual cues, evidence that additional visual cues support infants’ perception of the hand/arm as a human agent (Guajardo & Woodward, 2004), and the fact that the human and mechanical hand conditions elicited different patterns of cortical activation, we are confident that the infants in the present experiment perceived the human hand as uniquely human.
CONFLICT OF INTEREST STATEMENT
There are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. DAB is an inventor on a technology licensed to TechEn, a company whose medical pursuits focus on noninvasive, optical brain monitoring. DAB’s interests were reviewed and managed by Massachusetts General Hospital and Partners Health Care in accordance with their conflict of interest policies.
REFERENCES
- Adolphs R. The social brain: neural basis of social knowledge. Annual Review of Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DA, Baird JA. Discerning intentions in dynamic human action. Trends in Cognitive Sciences. 2001;5(4):171–178. doi: 10.1016/s1364-6613(00)01615-6. [DOI] [PubMed] [Google Scholar]
- Baldwin DA, Markman EM, Melartin RL. Infants’ ability to draw inferences about nonobvious object properties: evidence from exploratory play. Child Development. 1993;64(3):711–728. [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel Visual Motion Processing Streams for Manipulable Objects and Human Movements. Neuron. 2002;34:149–159. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. FMRI responses to video and point-light displays of moving humans and manipulable objects. Journal of Cognitive Neuroscience. 2003;15(7):991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Martin A. Grounding object concepts in perception and action: Evidence from fMRI studies of tools. Cortex. 2007;43(3):461–468. doi: 10.1016/s0010-9452(08)70470-2. [DOI] [PubMed] [Google Scholar]
- Bellagamba F, Tomasello M. Re-enacting intended acts: Comparing 12- and 18-month-olds. Infant Behavior and Development. 1999;22(2):277–282. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57(1):289–300. [Google Scholar]
- Bertenthal B. Infants’ perception of biomechanical motions: Intrinsic image and knowledge-based constraints. In: Granrud C, editor. Carnegie Symposium on Cognition: Visual Perception and Cognition in Infancy. Hillsdale, NJ: Erlbaum; 1993. pp. 175–214. [Google Scholar]
- Bertenthal BI, Proffitt DR, Cutting JE. Infant sensitivity to figural coherence in biomechanical motions. Journal of Experimental Child Psychology. 1984;37(2):213–230. doi: 10.1016/0022-0965(84)90001-8. [DOI] [PubMed] [Google Scholar]
- Biro S, Leslie AM. Infants’ perception of goal-directed actions: Development through cue-based bootstrapping. Developmental Science. 2007;10(3):379–398. doi: 10.1111/j.1467-7687.2006.00544.x. [DOI] [PubMed] [Google Scholar]
- Blake R, Shiffrar M. Perception of Human Motion. Annual Review of Psychology. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- Booth AE. The facilitative effect of agent-produced motions on categorization in infancy. Infant Behavior and Development. 2000;23(2):153–174. [Google Scholar]
- Booth AE. Object function and categorization in infancy: Two mechanisms of facilitation. Infancy. 2006;10(2):145–169. [Google Scholar]
- Booth AE, Waxman S. Object names and object functions serve as cues to categories for infants. Developmental Psychology. 2002;38(6):948–957. doi: 10.1037//0012-1649.38.6.948. [DOI] [PubMed] [Google Scholar]
- Bourgeois KS, Khawar AW, Neal SA, Lockman JJ. Infant Manual Exploration of Objects, Surfaces, and Their Interrelations. Infancy. 2005;8(3):233–252. [Google Scholar]
- Boyer TW, Samantha Pan J, Bertenthal BI. Infants’ understanding of actions performed by mechanical devices. Cognition. 2011;121(1):1–11. doi: 10.1016/j.cognition.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Buresh JS, Woodward AL. Infants track action goals within and across agents. Cognition. 2007;104(2):287–314. doi: 10.1016/j.cognition.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Carter EJ, Pelphrey KA. School-aged children exhibit domain-specific responses to biological motion. Social Neuroscience. 2006;1(3–4):396–411. doi: 10.1080/17470910601041382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casler K, Kelemen D. Reasoning about artifacts at 24 months: The developing teleo-functional stance. Cognition. 2007;103:120–130. doi: 10.1016/j.cognition.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Chouchourelou A, Golden A, Shiffrar M. What does “Biological Motion” really mean? Differentiating visual percepts of human, animal, and non-biological motions. In: Johnson K, Shiffrar M, editors. People Watching: Social, Perceptual, and Neurophysiological Studies of Body Perception. New York: Oxford University Press; 2013. pp. 63–81. [Google Scholar]
- Clifton RK, Rochat P, Litovsky RY, Perris EE. Object representation guides infants’ reaching in the dark. Journal of Experimental Psychology. Human Perception and Performance. 1991;17(2):323–329. doi: 10.1037//0096-1523.17.2.323. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Statistical Power Analysis for the Behavioral Sciences. 1988 [Google Scholar]
- Csibra G, Bíró S, Koós O, Gergely G. One-year-old infants use teleological representations of actions productively. Cognitive Science. 2003;27(1):111–133. [Google Scholar]
- Csibra G, Gergely G, Bíró S, Koós O, Brockbank M. Goal attribution without agency cues: The perception of “pure reason” in infancy. Cognition. 1999;72:237–267. doi: 10.1016/s0010-0277(99)00039-6. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Gatenby JC, Gore JC, Banaji MR. Neural components of social evaluation. Journal of Personality and Social Psychology. 2003;85(4):639–649. doi: 10.1037/0022-3514.85.4.639. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Zelazo PD. Attitudes and evaluations: a social cognitive neuroscience perspective. Trends in Cognitive Sciences. 2007;11(3):97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Fields C. Motion, identity and the bias toward agency. Frontiers in Human Neuroscience. 2014 Aug;8:597. doi: 10.3389/fnhum.2014.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R, McDaniel C. The perception of biological motion by human infants. Science (New York, N.Y.) 1982;218(4571):486–487. doi: 10.1126/science.7123249. [DOI] [PubMed] [Google Scholar]
- Gelman SA, Opfer JE. Development of the Animate – Inanimate Distinction. Blackwell Handbook of Childhood Cognitive Development. 2002;1985:151–166. [Google Scholar]
- Gerson SA, Woodward AL. A claw is like my hand: comparison supports goal analysis in infants. Cognition. 2012;122(2):181–192. doi: 10.1016/j.cognition.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J, Mehler J, Werker JF, Nelson Ca, Csibra G, Lloyd-Fox S, Aslin RN. Near-infrared spectroscopy: A report from the McDonnell infant methodology consortium. Developmental Cognitive Neuroscience. 2011;1(1):22–46. doi: 10.1016/j.dcn.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EJ, Walker AS. Development of Knowledge of Visual-Tactual Affordances of Substance. Child Development. 1984;55(2):453–460. [PubMed] [Google Scholar]
- Grossman ED, Battelli L, Pascual-Leone A. Repetitive TMS over posterior STS disrupts perception of biological motion. Vision Research. 2005;45(22):2847–2853. doi: 10.1016/j.visres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Grossmann T, Lloyd-Fox S, Johnson MH. Brain responses reveal young infants’ sensitivity to when a social partner follows their gaze. Developmental Cognitive Neuroscience. 2013;6:155–161. doi: 10.1016/j.dcn.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guajardo JJ, Woodward AL. Is Agency Skin Deep? Surface Attributes Influence Infants’ Sensitivity to Goal-Directed Action. Infancy. 2004;6(3):361–384. [Google Scholar]
- Han Z, Bi Y, Chen J, Chen Q, He Y, Caramazza A. Distinct Regions of Right Temporal Cortex Are Associated with Biological and Human-Agent Motion: Functional Magnetic Resonance Imaging and Neuropsychological Evidence. Journal of Neuroscience. 2013;33(39):15442–15453. doi: 10.1523/JNEUROSCI.5868-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer T, Hauf P, Aschersleben G. Infant’s perception of goal-directed actions performed by a mechanical device. Infant Behavior and Development. 2005;28(4):466–480. [Google Scholar]
- Hunnius S, Bekkering H. What are you doing? How active and observational experience shape infants’ action understanding. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1644):20130490. doi: 10.1098/rstb.2013.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic B, Király I, Elsner B, Gergely G, Prinz W, Aschersleben G. The role of effects for infants’ perception of action goals. Psychologia. 2007;50(4):273–290. [Google Scholar]
- Kaiser MD, Shiffrar M, Pelphrey KA. Socially tuned: Brain responses differentiating human and animal motion. Social Neuroscience. 2012;7(3):301–310. doi: 10.1080/17470919.2011.614003. [DOI] [PubMed] [Google Scholar]
- Kemler Nelson DG. Principle-based inferences in young children’s categorization: Revisiting the impact of function on the naming of artifacts. Cognitive Development. 1995;10(3):347–380. [Google Scholar]
- Kemler Nelson DG. Attention to functional properties in toddlers’ naming and problem-solving. Cognitive Development. 1999;14(1):77–100. [Google Scholar]
- Kemler Nelson DG, Frankenfield A, Morris C, Blair E. Young children’s use of functional information to categorize artifacts: Three factors that matter. Cognition. 2000;77(2):133–168. doi: 10.1016/s0010-0277(00)00097-4. [DOI] [PubMed] [Google Scholar]
- Kemler Nelson DG, Russell R, Duke N, Jones K. Two-year-olds will name artifacts by their functions. Child Development. 2000;71(5):1271–1288. doi: 10.1111/1467-8624.00228. [DOI] [PubMed] [Google Scholar]
- Király I, Jovanovic B, Prinz W, Aschersleben G, Gergely G. The early origins of goal attribution in infancy. Consciousness and Cognition. 2003 Nov;12:752–769. doi: 10.1016/s1053-8100(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Legerstee M, Markova G. Intentions make a difference: infant responses to still-face and modified still-face conditions. Infant Behavior & Development. 2007;30(2):232–250. doi: 10.1016/j.infbeh.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Leslie AM. Pretending and believing: issues in the theory of ToMM. Cognition. 1994;50(1–3):211–238. doi: 10.1016/0010-0277(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Elwell CE. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neuroscience and Biobehavioral Reviews. 2010;34:269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Volein A, Lloyd-Fox S, Blasi A, Volein A, Johnson MH. Social perception in infancy: A near infrared spectroscopy study. Child Development. 2009;80(4):986–999. doi: 10.1111/j.1467-8624.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Wu R, Richards JE, Elwell CE, Johnson MH. Cortical Activation to Action Perception is Associated with Action Production Abilities in Young Infants. Cerebral Cortex. 2013 Feb;:1–9. doi: 10.1093/cercor/bht207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman JJ, Ashmead DH, Bushnell EW. The development of anticipatory hand orientation during infancy. Journal of Experimental Child Psychology. 1984;37:176–186. doi: 10.1016/0022-0965(84)90065-1. [DOI] [PubMed] [Google Scholar]
- Luo Y, Baillargeon R. Toward a Mentalistic Account of Early Psychological Reasoning. Current Directions in Psychological Science. 2010;19(5):301–307. doi: 10.1177/0963721410386679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madole KL, Cohen LB. The role of object parts in infants’ attention to form-function correlations. Developmental Psychology. 1995;31(4):637–648. [Google Scholar]
- Martin A, Weisberg J. Neural Foundations for Understanding Social and Mechanical Concepts. Cognitive Neuropsychology. 2003;20(3-6):575–587. doi: 10.1080/02643290342000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty ME, Clifton RK, Ashmead DH, Lee P, Goubet N. How infants use vision for grasping objects. Child Development. 2001;72(4):973–987. doi: 10.1111/1467-8624.00329. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. Infant imitation after a 1-week delay: Long-term memory for novel acts and multiple stimuli. Developmental Psychology. 1988a;24(4):470–476. doi: 10.1037/0012-1649.24.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Infant imitation and memory: nine-month-olds in immediate and deferred tests. Child Development. 1988b;59(1):217–225. doi: 10.1111/j.1467-8624.1988.tb03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Understanding the intentions of others: Re-enactment of intended acts by 18-month-old children. Developmental Psychology. 1995;31(5):838–850. doi: 10.1037/0012-1649.31.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina M, Jouen F. Modulation of the palmar grasp behavior in neonates according to texture property. Infant Behavior and Development. 1998;21:659–666. [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Perceived causality influences brain activity evoked by biological motion. Social Neuroscience. 2008;3(1):16–25. doi: 10.1080/17470910701476686. [DOI] [PubMed] [Google Scholar]
- Palmer CF. The discriminating nature of infants’ exploratory actions. Developmental Psychology. 1989;25(6):885–893. [Google Scholar]
- Pelphrey KA, Mitchell TV, McKeown MJ, Goldstein J, Allison T, McCarthy G. Brain activity evoked by the perception of human walking: controlling for meaningful coherent motion. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(17):6819–6825. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuskens H, Vanrie J, Verfaillie K, Orban GA. Specificity of regions processing biological motion. European Journal of Neuroscience. 2005;21(10):2864–2875. doi: 10.1111/j.1460-9568.2005.04106.x. [DOI] [PubMed] [Google Scholar]
- Pyles JA, Garcia JO, Hoffman DD, Grossman ED. Visual perception and neural correlates of novel “biological motion”. Vision Research. 2007;47(21):2786–2797. doi: 10.1016/j.visres.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Ruff HA. Infants’ manipulative exploration of objects: Effects of age and object characteristics. Developmental Psychology. 1984;20(1):9–20. [Google Scholar]
- Schlottmann A, Ray E. Goal attribution to schematic animals: do 6-month-olds perceive biological motion as animate? Developmental Science. 2010;13(1):1–10. doi: 10.1111/j.1467-7687.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- Servos P, Osu R, Santi A, Kawato M. The neural substrates of biological motion perception: an fMRI study. Cerebral Cortex (New York, N.Y.: 1991) 2002;12(7):772–782. doi: 10.1093/cercor/12.7.772. [DOI] [PubMed] [Google Scholar]
- Setoh P, Wu D, Baillargeon R, Gelman R. Young infants have biological expectations about animals. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(40):15937–15942. doi: 10.1073/pnas.1314075110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognition. 2005;96:1–11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelke ES, Kinzler KD. Core knowledge. Developmental Science. 2007;10:89–96. doi: 10.1111/j.1467-7687.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- Strangman G, Franceschini MA, Boas DA. Factors affecting the accuracy of near-infrared spectroscopy concentration calculations for focal changes in oxygenation parameters. NeuroImage. 2003;18(4):865–879. doi: 10.1016/s1053-8119(03)00021-1. [DOI] [PubMed] [Google Scholar]
- von Hofsten C, Fazel-Zandy S. Development of visually guided hand orientation in reaching. Journal of Experimental Child Psychology. 1984;38(2):208–219. doi: 10.1016/0022-0965(84)90122-x. [DOI] [PubMed] [Google Scholar]
- von Hofsten C, Rönnqvist L. Preparation for grasping an object: a developmental study. Journal of Experimental Psychology. Human Perception and Performance. 1988;14(4):610–621. doi: 10.1037//0096-1523.14.4.610. [DOI] [PubMed] [Google Scholar]
- Wilcox T, Chapa C. Priming infants to attend to color and pattern information in an individuation task. Cognition. 2004;90:265–302. doi: 10.1016/s0010-0277(03)00147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Hirshkowitz A, Hawkins L, Boas DA. The effect of color priming on infant brain and behavior. NeuroImage. 2014;85:302–313. doi: 10.1016/j.neuroimage.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Smith T, Woods R. Priming infants to use pattern information in an object individuation task: the role of comparison. Developmental Psychology. 2011;47(3):886–897. doi: 10.1037/a0021792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Woods R, Chapa C. Color-function categories that prime infants to use color information in an object individuation task. Cognitive Psychology. 2008;57:220–261. doi: 10.1016/j.cogpsych.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AL. Infants selectively encode the goal object of an actor’s reach. Cognition. 1998;69:1–34. doi: 10.1016/s0010-0277(98)00058-4. [DOI] [PubMed] [Google Scholar]
- Woodward AL. Infants’ grasp of others’ intentions. Current Directions in Psychological Science. 2009;18(1):53–57. doi: 10.1111/j.1467-8721.2009.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.