Abstract
Pretransplant conditioning regimens critically determine outcomes in the setting of allogeneic (allo) stem cell transplantation (SCT). The use of nucleoside analogs such as fludarabine (Flu) in combination with intravenous (IV) busulfan (Bu) has been shown to be highly effective as a pretransplant conditioning regimen in acute (AML), chronic (CML) myeloid leukemia, and myelodysplastic syndrome (MDS). Because leukemia relapse remains the leading cause of death following allo-SCT, we studied whether clofarabine (Clo), a nucleoside analog with potent anti-leukemia activity, can be used to complement Flu. In a preliminary report, we previously showed the safety and efficacy of Clo ± Flu with IV Bu in 51 patients with high risk AML, CML and MDS. The study has now been completed and we present long-term follow-up data on the entire 70-patient population, which included 49 (70%), 8 (11%) and 13 (19%) patients with AML, MDS and CML, respectively. Thirteen patients (19%) were in complete remission and 41 patients (59%) received matched unrelated donor grafts. Engraftment was achieved in all patients. Sixty-three (90%) patients achieved CR. There were no deaths reported at day +30 and the 100-day non-relapse mortality was 4% (n=3). Thirty-one percent of patients (n=22) developed grade II-IV aGvHD, and the median OS and PFS times were 2.4 years and 0.9 years, respectively. Our results confirm the safety and OS and PFS advantage of the arms with higher Clo doses and lower Flu doses, which was most prominent in the AML/MDS group.
Keywords: Conditioning regimen, Clofarabine, Busulfan, Fludarabine, Allogeneic Stem Cell Transplantation
Introduction
The conditioning regimens used in the allogeneic (allo) stem cell transplantation (SCT) setting are critical for reducing disease burden and establishing an immunosuppressed environment that allows engraftment of donor cells. Intravenous (IV) busulfan (Bu) combined with the nucleoside analog fludarabine (Flu) has become one of the principal conditioning regimens used in the allo-SCT setting [1-7]. Factors that contribute to the safety and efficacy of IV Bu-Flu include the non-overlapping end organ toxicities of Flu and Bu and the predictability of systemic Bu levels with the use of IV Bu [8-11].
The cytoreductive capability of the conditioning regimen has a significant bearing on long-term disease outcomes, especially in high-risk patients [12, 13]. This observation underscores the critical need for conditioning regimens with potent anti-leukemia activity that would eradicate residual leukemia and provide ample time to establish donor immunity for the graft versus leukemia effect of the allo-SCT [12-15]. We introduced clofarabine (Clo) in combination with Flu and IV Bu as one strategy to improve the anti-leukemia activity of the conditioning regimen and demonstrated its safety and efficacy in 51 patients with AML (n=42) and CML (n=9) [16]. Clo has a potent anti-leukemia activity, and it synergizes with Flu and Bu in vitro [16, 17], hence providing the rationale to test it in the allo-SCT setting.
Using a Bayesian model, in our original study [16], we randomized patients adaptively to one of 4-arms in which Flu was complemented with Clo in different proportions; all patients received IV Bu. The four arms were as follows: Arm 1 -Clo:Flu 10:30 mg/m2, Arm 2 - 20:20 mg/m2, Arm 3 - 30:10 mg/m2, and Arm 4 – single-agent Clo at 40 mg/m2. Addition of Clo had minimal toxicities with no major organ toxicities or graft versus host disease (GvHD) that were attributed to this nucleoside analog. We reported a 2-year OS and PFS of 48% and 41%, respectively, with a median OS of 23 months. A multivariate analysis suggested a trend for improved OS and EFS for the AML patients treated in the arms with higher Clo doses, including patients treated in Arm 4 who received Clo without Flu.
In this report, we present a long-term follow-up on the entire group of 70 patients. Patient covariates were balanced between all treatment arms. Our results confirm the safety of this new double nucleoside analog + IV Bu regimen and demonstrate an advantage for better disease control in the patients conditioned with higher Clo doses.
Patients and Methods
Patient Eligibility
We studied 70 patients with AML (n=49), MDS (n=8) or CML (n=13) transplanted at The University of Texas M. D. Anderson Cancer Center (MDACC) between October 2006 and October 2011. Patients provided written informed consent for their treatment and were treated in accordance with the Declaration of Helsinki.
The inclusion criteria for AML patients were induction chemotherapy failure, or high-risk disease in first complete remission (CR1), characterized by cytogenetics other than translocation (t) (8;21), inversion (inv) 16, or t(15;17), and/or by the need for more than one cycle of chemotherapy to achieve CR [18]. Patients with AML beyond CR1 were also eligible, and were prioritized to include those with active leukemia at the time of transplantation. For MDS, eligibility criteria included patients with an International Prognostic Score System (IPSS) score of ≥2 [19], or if they progressed after previous chemotherapy. CML patients were eligible for this trial if they failed to achieve a cytogenetic CR to tyrosine-kinase inhibitor (TKI)-based therapy, and completed TKI-therapy at least 10 days prior to the start of preparative treatment to avoid a serious hepatic interaction with busulfan metabolism. The patients enrolled in this study were eligible for standard myeloablative conditioning regimens.
In addition to the above-listed disease-specific criteria, eligibility criteria included acceptable renal function (creatinine ≤1.5 mg); adequate hepatic function (normal bilirubin and SGPT ≤ 3 times the upper normal limit); ZUBROD performance status ≤2; absence of uncontrolled infection including negative serology for hepatitis B and C, and HIV; adequate cardiac function (LVEF ≥45%); acceptable pulmonary function (FEV1, FVC and DLCO ≥50% of predicted); and no chemotherapy within 30 days of study entry.
Stem cell grafts were obtained from human leukocyte antigen (HLA)-compatible related (fully matched or one antigen mismatched) donors or matched unrelated donors (MUD). HLA matching was assessed using high-resolution DNA-typing. The NIH Common Terminology Criteria v3.0 was used to assess clinical serious adverse events. All patients signed informed consent according to institutional guidelines. PCR-based technology was used to document engraftment and chimerism from blood and marrow [4].
Pretransplant Conditioning Program
The treatment regimens used are considered myeloablative and were based on a backbone of IV Bu-Flu. Regimens consisted of Flu (Fludara®, Genzyme Corporation, Cambridge, MA) infused over 60 minutes daily for four days (days -6 to -3). Each Flu dose was followed by Clo (Clolar®, Genzyme Corp.), also infused over 60 minutes daily for four days and then by IV Bu (IV Busulfex® [busulfan] Injection, Otsuka America Pharmaceuticals Inc., Princeton, NJ), over 3 hours once daily for four days. The Bu dose was calculated to target an average daily systemic exposure dose, represented by the area under the concentration vs. time curve (AUC) of 6,000 μMol-min ±10%, or total course AUC of 24,000 μMol-min over four days. PK-parameters derived from a Bu test dose of 32 mg/m2 administered 48 hours before the start of the therapeutic conditioning program (day -8) was used to calculate the Bu dose. The clinical study was designed as an adaptively randomized 4-arm trial:
Arm 1) Flu 30 mg/m2/day + Clo 10 mg/m2/day
Arm 2) Flu 20 mg/m2/day + Clo 20 mg/m2/day
Arm 3) Flu 10 mg/m2/day + Clo 30 mg/m2/day
Arm 4) Clo alone at 40 mg/m2/day
In addition to the fixed dose of IV Bu and the above four Flu-Clo chemotherapy schedules, a total dose of 4 mg/kg of rabbit antithymocyte globulin (rATG) (Thymoglobulin®, Genzyme, Corp.) was infused to patients who had a one-antigen-mismatched related donor or a matched unrelated donor using the following schedule: 0.5 mg/kg on day -3, 1.5 mg/kg on day -2, and 2.0 mg/kg IV on day -1. The stem cell products were infused on day 0. Tacrolimus and mini-dose methotrexate were used for graft-vs-host disease prophylaxis [2, 20].
Regimen Stopping Rule
Stopping rule would terminate an experimental arm for safety if, given the observed 30-day TRM data, it was likely that the rate of 30-day TRM in that arm was higher than the rate of 1% seen historically. Specifically, denoting the probability of TRM within 30 days in arm j=1,2,3,4 by pj, and the historical probability of this event by pH, the safety rule would stop accrual to the jth arm if Pr(pj > pH | data) > 0.95, where it was assumed that pH followed an informative beta prior with effective sample size 120 and mean 0.01, and that p1, p2, p3, p4 followed non-informative beta priors with effective sample size 1 and mean 0.01.
Statistical Analyses
Unadjusted distributions of OS and PFS were estimated by the method of Kaplan and Meier [21]. The log rank test was used to test differences in OS or PFS between subgroups [22]. Exact tests for association were carried out using the exact Fisher-Freeman-Halton test [23]. Bayesian survival time regression was used to assess the relationship between OS, PFS, and patient covariates and treatment arms, assuming a log-normal distribution for flexibility and numerical stability [24]. In the fitted model, for each variable the posterior probability of a beneficial effect (pbe) = Pr(β>0 | data) where β denotes the coefficient of the variable in the model's linear term. Values of pbe > 0.99 or < 0.01 may be interpreted as highly significant, and pbe >0.95 or < 0.05 as significant. All statistical analyses were performed using SAS 9.3 for Windows.
Results
Patient Characteristics
Seventy patients with AML (n=49), MDS (n=8) or CML (n=13) received allo-SCT between April 2006 and October 2011. The median age at the time of transplant was 46 (range, 6-59) years; 25 patients (36%) were >50 years. Thirty-one (44%) patients were female, and 39 (56%) were male. Only thirteen (19%) patients were in CR at the time of SCT. Forty-one patients (57%) received MUD SCTs; 19 (46%) of these MUD patients received bone marrow (BM) as the stem cell graft. Twenty-nine patients received their graft from a related donor and of these patients, 27 (93%) were fully matched related donor (MRD), while 2 (7%) were one-antigen mismatched. Of the patients who received a MRD graft, 24 (89%) patients received a peripheral blood progenitor cell (PBPC) graft. BM was used as the graft source for the two patients who received a one-antigen mismatched SCT. Pretransplant characteristics for this subgroup of patients are presented in Table 1.
Table 1.
Characteristics of patients who achieved CR following allo-SCT (n=70).
| Variable | Value | Number (%) |
|---|---|---|
| Gender | Male | 39 (56%) |
| Female | 31 (44%) | |
| Age (years) | >50 | 25 (36%) |
| ≤50 | 45 (64%) | |
| Disease | AML | 49 (70%) |
| MDS | 8 (11%) | |
| CML | 13 (19%) | |
| Disease status at transplantation | CR | 13 (19%) |
| Active disease | 57 (81%) | |
| Cytogenetics | Good | 6 (9%) |
| Intermediate | 27 (38%) | |
| Poor | 16 (23%) | |
| Donor type | MRD | 27 (38%) |
| MUD | 41 (59%) | |
| Mismatched | 2 (3%) | |
| Stem cell source | Bone Marrow | 24 (34%) |
| Peripheral Blood | 46 (66%) |
Abbreviations: AML, acute myeloid leukemia; myelodysplastic syndrome, MDS; chronic myeloid leukemia, CML; MRD, matched related donor; MUD, matched unrelated donor; CR, complete remission.
Patient Characteristics According to the Treatment Arm
Eighteen patients were randomized to treatment Arm 1 (Flu 30 mg/m2, Clo 10 mg/m2), including 11 patients (61%) with AML, 2 patients (11%) with MDS and 5 patients (28%) with CML. The median age of these patients at the time of transplant was 36.5 (range 6-59) years. Seven patients were randomized to treatment Arm 2 (Flu 20 mg/m2, Clo 20 mg/m2), which included 4 patients (57%) with AML, 1 patient (14 %) with MDS and 2 patients (29%) with CML. The median age of these patients at the time of transplant was 54 (range, 11-59) years. Twenty-nine patients were randomized to treatment Arm 3 (Flu 10 mg/m2, Clo 30 mg/m2), which included 23 patients (79%) with AML, 2 patients (7%) with MDS and 4 patients (14%) with CML. The median age of these patients at the time of transplant was 42 (range, 14-59) years. Sixteen patients were randomized to treatment Arm 4 (Clo 40 mg/m2), and these included 11 patients (69%) with AML, 3 patients (19%) with MDS and 2 patients (12%) with CML. The median age of these patients at the time of transplant was 49 (range, 7-59) years. Patients in CR in each group were 3 (17%) in Arm 1, 1 (14%) in Arm 2, 6 (21%) in Arm 3 and 3 (19%) in Arm 4 at the time of SCT (P >0.05). There were 9 patients (50%) in Arm 1 that received MUD SCT and 9 patients (50%) that received MRD SCT; 3 patients (43%) in Arm 2 that received MUD SCT and 4 patients (57%) that received MRD SCT; 21 patients (72%) in Arm 3 that received MUD SCT, 7 patients (24%) that received MRD SCT and 1 patient (4%) who received a one-antigen mismatched related SCT; and 8 patients (50%) in Arm 4 that received MUD SCT and 7 patients (44%) that received MRD SCT and 1 patient (6%) who received a one-antigen mismatched related SCT (P>0.05). Of the AML patients in Arm 1, 1 (9%), 6 (55%) and 4 (36%) patients had good, intermediate and poor risk cytogenetics, respectively. Of the AML patients in Arm 2, 2 (50%) and 2 (50%) patients had intermediate and poor risk cytogenetics, respectively. Of the AML patients in Arm 3, 3 (3%), 13 (57%) and 7 (30%) patients had good, intermediate and poor risk cytogenetics, respectively. Of the AML patients in Arm 4, 6 (55%), 2 (18%) and 3(27%) patients had good, intermediate and poor risk cytogenetics, respectively. Patient characteristics by treatment arm are shown in Table 2.
Table 2.
Characteristics by treatment arm of patients who achieved CR following allo-SCT.
| Arm Number (%) | Variable | Value | Number (%) |
|---|---|---|---|
| Arm 1 18 (26%) | Gender | Male | 8 (44%) |
| Female | 10 (56%) | ||
| Age (years) | >50 | 3 (17%) | |
| ≤50 | 15 (83%) | ||
| Disease | AML | 11 (61%) | |
| MDS | 2 (11%) | ||
| CML | 5 (28%) | ||
| Disease status at transplantation | CR | 3 (17%) | |
| Active disease | 67 (83%) | ||
| Cytogenetics | Good | 1(9%) | |
| Intermediate | 6(55%) | ||
| Poor | 4(36%) | ||
| Donor type | MRD | 9 (50%) | |
| MUD | 9 (50%) | ||
| Mismatched | 0 | ||
| Arm 2 7 (10%) | Gender | Male | 6 (86%) |
| Female | 1 (14%) | ||
| Age (years) | >50 | 5 (71%) | |
| ≤50 | 2 (29%) | ||
| Disease | AML | 4 (57%) | |
| MDS | 1 (14%) | ||
| CML | 2 (29%) | ||
| Disease status at transplantation | CR | 1 (14%) | |
| Active disease | 6 (86%) | ||
| Cytogenetics | Good | 0 | |
| Intermediate | 2(50 %) | ||
| Poor | 2(50 %) | ||
| Donor type | MRD | 4 (57%) | |
| MUD | 3 (43%) | ||
| Mismatched | 0 | ||
| Arm 329 (41%) | Gender | Male | 15 (52%) |
| Female | 14 (48%) | ||
| Age (years) | >50 | 10 (34%) | |
| ≤50 | 19 (66%) | ||
| Disease | AML | 23 (79%) | |
| MDS | 2 (7%) | ||
| CML | 4 (14%) | ||
| Disease status at transplantation | CR | 6 (21%) | |
| Active disease | 23 (79%) | ||
| Cytogenetics | Good | 3(3%) | |
| Intermediate | 13(57%) | ||
| Poor | 7(30%) | ||
| Donor type | MRD | 7 (24%) | |
| MUD | 21 (72%) | ||
| Mismatched | 1 (4%) | ||
| Arm 4 16 (23%) | Gender | Male | 10 (63%) |
| Female | 6 (37%) | ||
| Age (years) | >50 | 7 (44%) | |
| ≤50 | 9 (56%) | ||
| Disease | AML | 11 (69%) | |
| MDS | 3 (19%) | ||
| CML | 2 (12%) | ||
| Disease status at transplantation | CR | 3 (19%) | |
| Active disease | 13 (81%) | ||
| Cytogenetics | Good | 2(18%) | |
| Intermediate | 6(55%) | ||
| Poor | 3(27%) | ||
| Donor type | MRD | 7 (44%) | |
| MUD | 8 (50%) | ||
| Mismatched | 1 (6%) |
Abbreviations: AML, acute myeloid leukemia; myelodysplastic syndrome, MDS; chronic myeloid leukemia, CML; MRD, matched related donor; MUD, matched unrelated donor; CR, complete remission.
Transplant Outcomes
Engraftment was achieved in all patients (n=70) with median time of 12 (range, 10-22) days. Full donor chimerism was achieved in 57 (81%) patients at a median of 31 (range, 27-580) days. Sixty-three (90%) patients stayed in remission or achieved CR following transplant, and of these patients, 27 (43%) subsequently progressed. There were no deaths reported at day +30. One hundred-day non-relapse mortality (NRM) was 4% (n=3) and was caused by infection (n=1), acute GvHD (aGvHD) (n=1) and hemorrhage (n=1). Thirty-one percent of patients (n=22) developed grade II-IV aGvHD, 6% (n= 4) developed grade III-IV aGvHD, and 40% (n=28) developed chronic GvHD. There were no significant renal or hepatic toxicities reported.
During the study, 9 patients died without relapse (13%). Among the 9 patients, 2 patients (22%) died due to aGvHD, 2 patients (22%) died due to chronic GvHD, 1 patient (11%) died due to hemorrhage, 1 patient (11%) died due to secondary malignancy, 1 patient (11%) died due to a fungal infection, 1 patient (11%) died due to a protozoal infection and 1 patient (11%) died due to other organ failure. The overall relapse rate was 41% (n=29) and the relapse rate in each arm was 33% (n=6) in Arm 1, 57% (n=4) in Arm 2, 48% (n=14) in Arm 3, and 31% (n=5) in Arm 4. Outcomes by treatment arm are shown in Table 3. Fisher exact test was used to compare the relapse rates among arms, and no statistically significant difference in relapse rate was found (P=0.52), although this data should be cautiously interpreted due to the small number of patients in each subgroup.
Table 3.
Outcomes by treatment arm for patients who achieved CR following allo-SCT.
| Arm Number (%) | Variable | Value | Number (%) |
|---|---|---|---|
| Arm 1 18 (26%) | NRM | Yes | 2 (11%) |
| No | 16 (89%) | ||
| Progression | Yes | 6 (33%) | |
| No | 12 (67%) | ||
| Death | Yes | 8 (44%) | |
| No | 10 (56%) | ||
| Arm 2 7 (10%) | NRM | Yes | 1 (14%) |
| No | 6 (86%) | ||
| Progression | Yes | 4 (57%) | |
| No | 3 (43%) | ||
| Death | Yes | 6 (86%) | |
| No | 1 (14%) | ||
| Arm 3 29 (41%) | NRM | Yes | 5 (17%) |
| No | 24 (83%) | ||
| Progression | Yes | 14 (48%) | |
| No | 15 (52%) | ||
| Death | Yes | 16 (55%) | |
| No | 13 (45%) | ||
| Arm 4 16 (23%) | NRM | Yes | 1 (6%) |
| No | 15 (94%) | ||
| Progression | Yes | 5 (31%) | |
| No | 11 (69%) | ||
| Death | Yes | 6 (38%) | |
| No | 10 (62%) |
OS and DFS
The median follow-up period for all patients and those who achieved CR following allo-SCT was 36.3 (range, 1.3 -102.5) months and 50.8 (range, 2.4-102.5) months, respectively. The median OS time over all four arms was 2.4 years. The median OS times by arm were Arm 1: not reached; Arm 2: 0.4 year; Arm 3: 2.2 years; Arm 4: not reached. The median PFS time over all four arms was 0.9 year. The median PFS times for arms 1-4 were 2.0 years, 0.2 year, 0.8 year, and not reached, respectively.
The 5-year OS rate was 48% (95% confidence interval [CI]: 38%-62%). The 5-year OS rates of the arms 1-4 were 56% (95% CI: 37%-84%), 14% (95% CI: 2%-88%), 44% (95% CI: 29%-67%) and 62% (95% CI: 43%-91%), respectively. The 5-year PFS rate was 41% (95% CI: 31%-54%). The 5-year PFS rates for arms 1-4 were 49% (95% CI: 31%-79%), 14% (95% CI: 2%-88%), 34% (95% CI: 20%-57%) and 56% (95% CI: 37%-87%), respectively.
Kaplan-Meier plots demonstrated better OS and PFS in patients who received the highest dose of Clo, irrespective of the disease status at the time of allo-SCT (Figures 1 and 2). Furthermore, since 81% (n=57) of the patients treated in our study had AML or MDS, we also determined how the Clo dosing affected the survival outcomes in this subgroup of patients. Our results showed that patients who received the highest doses of Clo (40 mg/m2/day) had the longest OS and PFS (Figures 3A and 3B). To determine whether these observations were due to the dose of Clo, we compared the outcomes of the patients who received the higher doses of Clo (arms 3 and 4) with those who received the lower doses of Clo (Arms 1 and 2). Kaplan-Meier plots (Figure 3) showed higher OS and PFS in patients who received the higher Clo doses, suggesting that the improved survival effect was indeed due to Clo (P=0.07 for OS and P=0.12 for PFS). Furthermore, a multivariate Bayesian survival regression model assessing post-transplant OS (Table 4), shows a strong trend in which a higher Clo dose is associated with improved OS. As seen in many previous studies, AML/MDS (versus CML) was associated with worse OS. Similar results were seen for PFS (data not shown).
Figure 1.
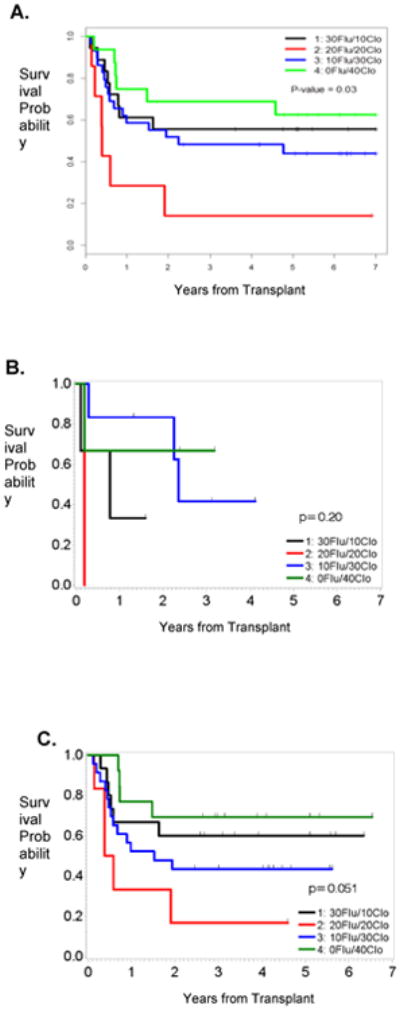
OS by treatment arm for the entire cohort (A), patients in CR (B) or with active disease (C).
Figure 2.
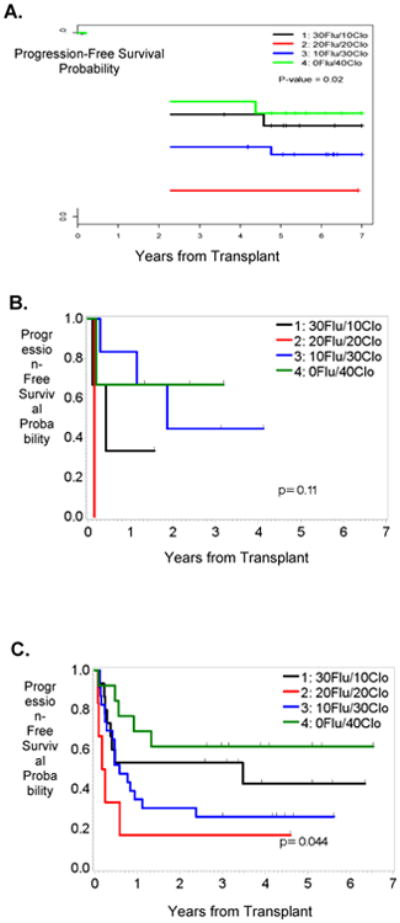
PFS by treatment arm for the entire cohort (A), patients in CR (B) or with active disease (C).
Figure 3.
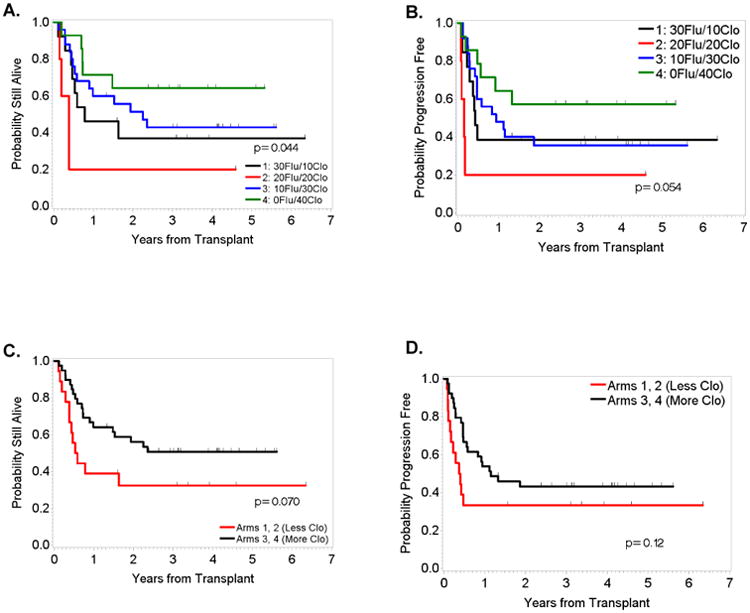
OS (left) and PFS (right) for patients with AML/MDS by treatment arm (A and B) and combined arms (C and D).
Table 4.
Fitted multivariate Bayesian lognormal survival regression model assessing effects of treatment arms and covariates on post-transplant overall survival (N=70, Deaths=35). Dose arm effects are compared to baseline arm Flu 30 mg/m2 + Clo 10 mg/m2. All doses are mg/m2/day.
| Posterior Quantities | |||
|---|---|---|---|
|
| |||
| Variable | Mean Effect (SD) | Posterior 95% Credible Interval | Probability of a Beneficial Effect* |
| Intercept | 9.23 (2.29) | 4.91, 13.92 | -- |
| Flu 20 + Clo 20 | -1.90 (1.08) | -4.12, 0.16 | 0.04 |
| Flu 10 + Clo 30 | -0.21 (0.77) | -1.78, 1.27 | 0.39 |
| Flu 0 + Clo 40 | 1.15 (0.94) | -0.67, 3.01 | 0.90 |
| Age at SCT | -0.22 (0.60) | -1.41, 0.95 | 0.36 |
| MUD donor type | 0.44 (0.64) | -0.82, 1.73 | 0.76 |
| In CR at SCT | -0.28 (0.79) | -1.83, 1.31 | 0.36 |
| AML/MDS disease | -1.67 (0.89) | -3.52, 0.02 | 0.03 |
Abbreviations: Flu = fludarabine, Clo= clofarabine; SCT = stem cell transplantation; MUD = matched unrelated donor; CR, = complete remission; SD = standard deviation.
This is the Probability of a Beneficial Effect, not the P value.
Patient covariates were balanced between the treatment arms. The various Kaplan-Meier plots suggest OS and PFS advantages in the arms with higher Clo dose and lower Flu dose. This observation is most prominent in the AML/MDS patients (Figure 3). Arm 2, “20-20” arm, had the worst patient outcomes, which included 6 deaths: 5 deaths (83%) were caused by disease recurrence/persistence, and 1 death (17%) was caused by the chronic GvHD. Although the comparisons showed that this arm had the worst OS and PFS, the sample size in this arm was very small, since this group met the early stopping criteria (detailed in the “Patients and Methods” section), and therefore it is difficult to make definitive conclusions.
Discussion
In this report of 70 patients with AML, MDS, and CML, we saw improved efficacy of Clo in combination with Flu and IV Bu as part of a pretransplant conditioning regimen. Our data demonstrate that adding Clo to Flu in the IV Bu ± ATG regimen appears to be highly active in patients with aggressive and relapsed/refractory myeloid leukemia. In addition to its direct anti-leukemia activities, the immunosuppressive effects of Clo are sufficient to allow for adequate stem cell engraftment. Moreover, the 4% NRM in this study is similar to that expected with Flu + IV Bu in a similar a patient population [1-4].
A number of published studies demonstrated the efficacy of IV Bu containing regimens. In one study of 1,483 patients with myeloid malignancies, IV Bu was shown to be superior to total body irradiation in pretransplant conditioning regimens, demonstrating superior survival with no increased risk of TRM or relapse [25]. In another study of 1,230 patients with AML, patients who received IV Bu/cyclophosphamide (Cy) demonstrated a lower rate of TRM and relapse, and longer PFS and OS in contrast with patients who received Cy/TBI [26]. With this proven superiority of IV Bu in patients with myeloid malignancies, a number of studies then focused on avoiding the use of two alkylating agents (i.e. Cy) in order to minimize conditioning regimen toxicity. Thus, a nucleoside analog, most commonly Flu, has been shown to be an effective adjunct to IV Bu in pretransplant regimens and is currently one of the mainstay drugs used with IV Bu [1-4, 27, 28]. However, even with Flu + IV Bu, leukemia relapse remains the primary cause of death in patients with myeloid malignancies following allo-SCT. Clo has potent anti-leukemia activity and was shown to synergize in vitro with busulfan and Clo [16, 29], hence prompting this study to test its efficacy in the pretransplant setting.
There are few published studies that support the use of IV Bu+ Clo in pretransplant conditioning regimens. In a phase 1/2 study, 46 patients with various hematologic malignancies, including 31 patients with active AML, received IV Bu (AUC=4800 μMol-min) and Clo at 20 mg/m2 (n=6), 30 mg/m2 (n=21) and 40 mg/m2 (n=19). CR was achieved in 80% of the patients and the two-year NRM and OS for all patients was 31% and 28%, respectively [30]. The major toxicities reported in that study included transient transaminitis (50%), mucositis (24%), hand-foot syndrome (13%), transient hypoxia (13%), nausea/vomiting (9%), and diarrhea (9%), but notably no serious renal or hepatic toxicity. This may invite a challenge of the Clo dose of 40 mg/m2 once daily for four days since there was a strong association in our study between leukemia control and delivered Clo dose. Chevallier et al. [31] reported the results of a phase 2 study combining IV Bu with Clo (30 mg/m2) and antithymocyte globulin in 30 patients with AML (n=11), ALL (n=13), MDS (n=5) and bi-phenotypic leukemia (n=1). Most of the patients (n=27) included in that study were in CR. The 1-year OS, DFS and NRM rates were 63%, 57% and 3.3%, respectively. A subgroup analysis showed that OS, DFS and leukemia relapse favored the patients with AML/MDS over those with ALL. A recently published phase 2 study further confirmed the safety and efficacy of IV Bu+ Clo at the higher Clo dose (40 mg/m2) [32]. In that study, which included patients with AML (n = 25), MDS (n=5) and ALL (n=4), 2-year OS and PFS were 56% and 50%, respectively, and the cumulative incidences of acute and chronic GVHD were 21% and 44%, respectively. As predicted, OS and PFS survival rates were highest for the AML patients in CR1, both at 82%. In addition to myeloid leukemia, our results here can also be extrapolated to patients with ALL and are supported by the aforementioned trials and data from an earlier study we conducted in patients with ALL undergoing allo-SCT. In that study, 51 patients received MRD (n=24), MUD (n=25) or syngeneic (n = 2) SCT after a pretransplant conditioning regimen that consisted of Clo (40 mg/m2)+ IV Bu given once daily for 4 days. The regimen was well tolerated, with a 100-day NRM rate of 6% and 1-year OS, DFS and TRM rates of 67%, 54% and 32%, respectively [33].
To date, using a nucleoside analog with Bu has been shown to be the safest approach in pretransplant conditioning regimens [1-4, 27, 28], although one study reported inferior efficacy outcomes with Flu in comparison with Cy [5]. Since leukemia relapse remains the leading cause of death in patients with myeloid leukemia after allo-SCT, novel approaches for pretransplant conditioning regimens are constantly being examined. This is especially true in patients with active disease at the time of allo-SCT, where relapse related mortality is high, irrespective of the addition of a nucleoside analog (i.e. Flu) or a second alkylating agent (i.e. Cy) to pretransplant conditioning regimens [26, 34, 35]. In patients at high risk for relapse, a combination of two nucleoside analogs including Flu and Clo, which have potent synergistic anti-leukemia activities [29, 36-41], may be the ideal partner(s) for IV Bu, as our data indicate in this report.
Finally, we recognize that a randomized trial is the ideal approach to determine the superiority of Clo when substituted for Flu in combination with IV Bu. However the Bayesian continuous reassessment approach that we employed in this study is adequate to determine the value of the Clo substitution. Because of the encouraging results that we observed with our initial study [16], our data in this expanded cohort, along with the studies that have been published since our original report, together show 1) the safety of Clo in the pretransplant setting, 2) its immunosuppressive activity that allows for adequate engraftment, and 3) its efficacy in patients with myeloid leukemia, including patients with active disease at the time of allo-SCT. These data could be used in clinical decision making until definitive data are obtained from larger randomized trials.
Highlights.
Clofarabine is safe when used with IV Bu+Flu in pretransplant conditioning regimens
Pretransplant conditioning with IV Bu+Clo+Flu has potent anti-leukemia activity
IV Bu+Clo+Flu conditioning is efficacious in patients with aggressive AML/MDS
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (CA16672), by the Stephen L. and Lavinia Boyd Fund for Leukemia Research, and by funds donated by grateful patients.
Footnotes
Financial Disclosure Statement: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Russell JA, Tran HT, Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468–476. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 2.de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 3.Russell JA, Savoie ML, Balogh A, et al. Allogeneic transplantation for adult acute leukemia in first and second remission with a novel regimen incorporating daily intravenous busulfan, fludarabine, 400 CGY total-body irradiation, and thymoglobulin. Biol Blood Marrow Transplant. 2007;13:814–821. doi: 10.1016/j.bbmt.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Andersson BS, de Lima M, Thall PF, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14:672–684. doi: 10.1016/j.bbmt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JH, Joo YD, Kim H, et al. Randomized trial of myeloablative conditioning regimens: busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol. 2013;31:701–709. doi: 10.1200/JCO.2011.40.2362. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Zhai X, Song Z, et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. Journal of hematology & oncology. 2013;6:15. doi: 10.1186/1756-8722-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rambaldi A, Grassi A, Masciulli A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. The Lancet Oncology. 2015;16:1525–1536. doi: 10.1016/S1470-2045(15)00200-4. [DOI] [PubMed] [Google Scholar]
- 8.Kashyap A, Wingard J, Cagnoni P, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8:493–500. doi: 10.1053/bbmt.2002.v8.pm12374454. [DOI] [PubMed] [Google Scholar]
- 9.Kim SE, Lee JH, Choi SJ, Lee JH, Ryu SG, Lee KH. Morbidity and non-relapse mortality after allogeneic bone marrow transplantation in adult leukemia patients conditioned with busulfan plus cyclophosphamide: a retrospective comparison of oral versus intravenous busulfan. Haematologica. 2005;90:285–286. [PubMed] [Google Scholar]
- 10.Lee JH, Choi SJ, Lee JH, et al. Decreased incidence of hepatic veno-occlusive disease and fewer hemostatic derangements associated with intravenous busulfan vs oral busulfan in adults conditioned with busulfan + cyclophosphamide for allogeneic bone marrow transplantation. Annals of hematology. 2005;84:321–330. doi: 10.1007/s00277-004-0982-4. [DOI] [PubMed] [Google Scholar]
- 11.Madden T, de Lima M, Thapar N, et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biol Blood Marrow Transplant. 2007;13:56–64. doi: 10.1016/j.bbmt.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 12.de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 13.Shimoni A, Hardan I, Shem-Tov N, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20:322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 14.Shimoni A, Hardan I, Shem-Tov N, Yerushalmi R, Nagler A. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: long-term follow-up. Leukemia. 2010;24:1050–1052. doi: 10.1038/leu.2010.12. [DOI] [PubMed] [Google Scholar]
- 15.Wong R, Shahjahan M, Wang X, et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11:108–114. doi: 10.1016/j.bbmt.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Andersson BS, Valdez BC, de Lima M, et al. Biol Blood Marrow Transplant. 2010. Clofarabine+/-Fludarabine with Once Daily IV Busulfan as Pretransplant Conditioning Therapy for Advanced Myeloid Leukemia and MDS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdez BC, Andersson BS. Interstrand crosslink inducing agents in pretransplant conditioning therapy for hematologic malignancies. Environmental and molecular mutagenesis. 2010;51:659–668. doi: 10.1002/em.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating MJ, Smith TL, Gehan EA, et al. Factors related to length of complete remission in adult acute leukemia. Cancer. 1980;45:2017–2029. doi: 10.1002/1097-0142(19800415)45:8<2017::aid-cncr2820450806>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 20.Przepiorka D, Khouri I, Ippoliti C, et al. Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after HLA-mismatched marrow or blood stem cell transplantation. Bone marrow transplantation. 1999;24:763–768. doi: 10.1038/sj.bmt.1701983. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimaion from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 22.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 23.Fisher R. On the interpretation of χ2 from contingency tables, and the calculation of P. J Roy Statist Soc. 1922;85:87–94. [Google Scholar]
- 24.Ibrahim JG, Chen MH, Sinha D. Bayesian Survival Analysis. New York: Springer; 2001. [Google Scholar]
- 25.Bredeson C, LeRademacher J, Kato K, et al. Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood. 2013;122:3871–3878. doi: 10.1182/blood-2013-08-519009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122:3863–3870. doi: 10.1182/blood-2013-07-514448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chae YS, Sohn SK, Kim JG, et al. New myeloablative conditioning regimen with fludarabine and busulfan for allogeneic stem cell transplantation: comparison with BuCy2. Bone marrow transplantation. 2007;40:541–547. doi: 10.1038/sj.bmt.1705770. [DOI] [PubMed] [Google Scholar]
- 28.Bredeson CN, Zhang MJ, Agovi MA, et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:993–1003. doi: 10.1016/j.bbmt.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valdez BC, Li Y, Murray D, Champlin RE, Andersson BS. The synergistic cytotoxicity of clofarabine, fludarabine and busulfan in AML cells involves ATM pathway activation and chromatin remodeling. Biochemical pharmacology. 2011;81:222–232. doi: 10.1016/j.bcp.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magenau J, Tobai H, Pawarode A, et al. Clofarabine and busulfan conditioning facilitates engraftment and provides significant antitumor activity in nonremission hematologic malignancies. Blood. 2011;118:4258–4264. doi: 10.1182/blood-2011-06-358010. [DOI] [PubMed] [Google Scholar]
- 31.Chevallier P, Labopin M, Socie G, et al. Results from a clofarabine-busulfan-containing, reduced-toxicity conditioning regimen prior to allogeneic stem cell transplantation: the phase 2 prospective CLORIC trial. Haematologica. 2014;99:1486–1491. doi: 10.3324/haematol.2014.108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Jawahri A, Li S, Ballen KK, et al. Phase II Trial of Reduced-Intensity Busulfan/Clofarabine Conditioning with Allogeneic Hematopoietic Stem Cell Transplantation for Patients with Acute Myeloid Leukemia, Myelodysplastic Syndromes, and Acute Lymphoid Leukemia. Biol Blood Marrow Transplant. 2016;22:80–85. doi: 10.1016/j.bbmt.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Kebriaei P, Basset R, Ledesma C, et al. Clofarabine combined with busulfan provides excellent disease control in adult patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1819–1826. doi: 10.1016/j.bbmt.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alatrash G, Pelosini M, Saliba RM, et al. Platelet recovery before allogeneic stem cell transplantation predicts posttransplantation outcomes in patients with acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2011;17:1841–1845. doi: 10.1016/j.bbmt.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warlick ED, Cioc A, Defor T, Dolan M, Weisdorf D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant. 2009;15:30–38. doi: 10.1016/j.bbmt.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Faderl S, Ferrajoli A, Wierda W, et al. Clofarabine combinations as acute myeloid leukemia salvage therapy. Cancer. 2008;113:2090–2096. doi: 10.1002/cncr.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faderl S, Gandhi V, Keating MJ, Jeha S, Plunkett W, Kantarjian HM. The role of clofarabine in hematologic and solid malignancies--development of a next-generation nucleoside analog. Cancer. 2005;103:1985–1995. doi: 10.1002/cncr.21005. [DOI] [PubMed] [Google Scholar]
- 38.Faderl S, Gandhi V, O'Brien S, et al. Results of a phase 1-2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105:940–947. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- 39.Kantarjian HM, Erba HP, Claxton D, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28:549–555. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 40.Kantarjian HM, Gandhi V, Kozuch P, et al. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J Clin Oncol. 2003;21:1167–1173. doi: 10.1200/JCO.2003.04.031. [DOI] [PubMed] [Google Scholar]
- 41.Kantarjian HM, Jeha S, Gandhi V, Wess M, Faderl S. Clofarabine: past, present, and future. Leukemia & lymphoma. 2007;48:1922–1930. doi: 10.1080/10428190701545644. [DOI] [PubMed] [Google Scholar]


