Abstract
The Brucella abortus general stress response (GSR) system regulates activity of the alternative sigma factor, σE1, which controls transcription of approximately 100 genes and is required for persistence in a BALB/c mouse chronic infection model. We evaluated the host response to infection by a B. abortus strain lacking σE1 (ΔrpoE1), and identified pathological and immunological features that distinguish ΔrpoE1-infected mice from wild-type (WT), and that correspond with clearance of ΔrpoE1 from the host. ΔrpoE1 infection was indistinguishable from WT in terms of splenic bacterial burden, inflammation and histopathology up to 6 weeks post-infection. However, Brucella-specific serum IgG levels in ΔrpoE1-infected mice were 5 times higher than WT by 4 weeks post-infection, and remained significantly higher throughout the course of a 12-week infection. Total IgG and Brucella-specific IgG levels peaked strongly in ΔrpoE1-infected mice at 6 weeks, which correlated with reduced splenomegaly and bacterial burden relative to WT-infected mice. Given the difference in immune response to infection with wild-type and ΔrpoE1, we tested whether ΔrpoE1 confers protective immunity to wild-type challenge. Mice immunized with ΔrpoE1 completely resisted WT infection and had significantly higher serum titers of Brucella-specific IgG, IgG2a and IFN-γ after WT challenge relative to age-matched naïve mice. We conclude that immunization of BALB/c mice with the B. abortus GSR pathway mutant, ΔrpoE1, elicits an adaptive immune response that confers significant protective immunity against WT infection.
Keywords: antibody, ecfG, phyR, brucellosis, general stress response, rpoE1, vaccine
Introduction
Brucella spp. are Gram-negative intracellular pathogens that inflict a significant burden on agricultural production and human health on a global scale (1). During the course of intracellular infection, Brucella must adapt to changes in the host environment including oxidative burst, low pH, and nutrient limitation. Brucella and other species in the class Alphaproteobacteria employ a conserved regulatory system, known as the general stress response (GSR), to adapt to such non-optimal growth conditions (2, 3). The GSR has been functionally characterized in Alphaproteobacteria that inhabit a range of ecological niches including freshwater (2, 4-7), plant roots and soil (8-10), plant leaf surfaces (8, 11-16), the interior of mammalian cells (17, 18), arthropod vectors and mammalian reservoir hosts (19, 20). In the case of animal- and plant-associated Alphaproteobacteria, the GSR has been implicated in establishment or maintenance of host-microbe interactions (8, 17, 19).
In B. abortus, the GSR is directly regulated by the LovhK-PhyR two-component signaling system. LovhK is a soluble histidine kinase that responds to variable environmental cues: it binds a flavin mononucleotide (FMN) cofactor, autophosphorylates in response to blue light in vitro (21), and controls transcription and cellular adaptation to a range of acute stress conditions (18, 22). Phospho-LovhK (LovhK~P) transfers its phosphoryl group to the anti-anti-σ factor, PhyR, which increases PhyR affinity for the anti-σE1 factor NepR (17). Binding of PhyR~P to NepR is proposed to release the GSR σ-factor, σE1, allowing its association with RNA polymerase (RNAP) to regulate transcription of genes required for acute stress survival and infection (17, 18, 22) (Figure 1). We have previously reported that B. abortus mutants missing the GSR regulatory genes encoding σE1 or PhyR are attenuated specifically during the chronic phase of infection in a BALB/c mouse model (17, 18). However, these studies only measured bacterial burden in the spleen over an 8-12 week period; the corresponding effects of infection on host pathophysiology and immune response remain uncharacterized. This study focuses on defining features of murine host pathology and immune response associated with infection by a B. abortus strain missing the core GSR regulator, σE1 (ΔrpoE1). We have further assessed the efficacy of the B. abortus ΔrpoE1 mutant as a live attenuated vaccine.
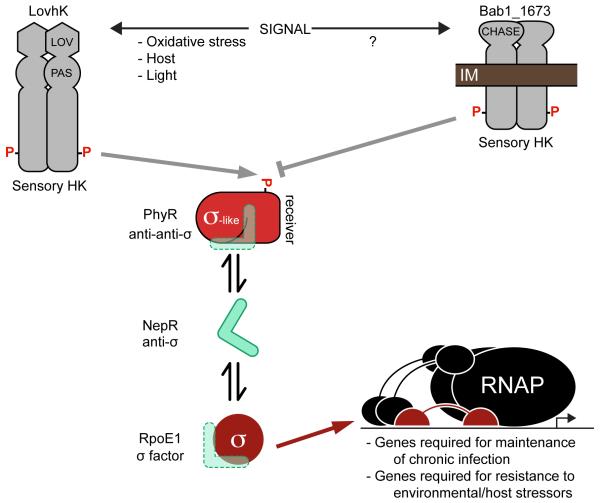
Figure 1. Model of the B. abortus general stress response (GSR) regulatory system.
The stress-activated sensor histidine kinase (HK), LovhK, autophosphorylates and transmits a phosphoryl group to the anti-anti-σ factor, PhyR. The HK, Bab1_1673, represses PhyR activation. Phosphorylated PhyR (PhyR~P) binds the anti-σ factor, NepR. When released from NepR, the σ-factor σE1 associates with RNA polymerase (RNAP) to activate transcription of the GSR regulon.
Mice infected with ΔrpoE1 had less spleen inflammation in the chronic phase (>6 weeks post-infection) than mice infected with WT B. abortus. This reduced splenomegaly correlated with a ≈3 log decrease in the number of splenic bacteria and decreased immunohistochemical staining of Brucella antigen in fixed spleens. Host clearance of B. abortus ΔrpoE1 strongly correlated with increased Brucella-specific IgG production relative to WT-infected mice, providing evidence that the ΔrpoE1 strain elicits a more robust humoral response than WT. Mice immunized with a single dose of 5×104 colony forming units (CFU) of ΔrpoE1 were completely protected from a WT challenge at 9 weeks post-immunization, demonstrating that ΔrpoE1 is a live attenuated vaccine. Vaccinated mice had higher total IgG, IgG2a, Brucella-specific IgG, and IFN-y levels than aged-matched naïve mice after WT challenge.
Our data provide evidence that B. abortus ΔrpoE1 elicits a robust immune response that corresponds with reduced splenic inflammation and clearance of ΔrpoE1 from the host. We further demonstrate that the B. abortus ΔrpoE1 strain is an effective live attenuated vaccine in a mouse model of infection. There remains a need for more safe and effective brucellosis vaccines (23, 24), and the data presented here illustrate the potential of ΔrpoE1 for development as a new brucellosis vaccine.
Materials and Methods
Bacterial Strains and Culture Conditions
Experiments conducted with live B. abortus strain 2308 were performed at the University of Chicago, Howard Taylor Ricketts Laboratory on the campus of Argonne National Laboratory. Experiments were performed at Biosafety Level 3 (BSL3) conditions as required by the Centers of Disease Control rules and regulations governing the use of Select Agents. Growth and maintenance of strains is as previously described (25).
Animal Infection
All animal experiments followed protocols approved by the University of Chicago Institutional Animal Care and Use Committee and the Institutional Biosafety Committee. For infections, we used the wild-type B. abortus strain 2308 and the B. abortus ΔrpoE1 strain (17). Bacterial strains were grown for 2 days on Schaedler Blood Agar (SBA) at 37°C before being suspended in sterile phosphate-buffered saline (PBS; pH 7.2, GE Healthcare), to a concentration of 5 × 105 CFU/mL. Bacterial titers were first estimated spectrophotometrically at 600 nm (OD600); the CFU per inoculum was confirmed post-infection by serial dilution plating on tryptic soy agar (TSA). Eight week old female BALB/c mice (Harlan Laboratories, Inc.) were infected by intraperitoneal (IP) injection with 100 μL of bacteria at a concentration of 5 × 105 CFU/mL (for a total infectious dose of 5 × 104 CFU per mouse). At 1, 2, 4, 6, 9, 12 weeks post-infection, animals (n = 8 per time point) were asphyxiated with CO2 prior to collection of blood via cardiac-puncture and aseptic removal of spleens. Serum from 8 mice for each strain and time point was separated from blood using a serum separation tube (Sarstedt). After serum was collected, samples were subsequently stored at −20°C until the end of the experiments. For each sample, five mouse spleens were weighed and splenic bacterial burden (CFU/spleen) was measured by homogenizing the spleens in 5 mL PBS with 0.1% Triton X-100, before serial dilution and plating on TSA. The three remaining mouse spleens were fixed and prepared for histology (described below).
Histology
Spleens prepared for histology (n = 3) were first fixed with formalin for 7 days. Formalin was removed and replaced with fresh formalin and fixed for another 7 days before washing and subsequently transferring spleens to 70% ethanol. Whole spleens were submitted for tissue embedding, hematoxylin and eosin (H & E) staining, and immunohistochemistry to Nationwide Histology (Veradale, Washington). For immunohistochemistry, goat anti-Brucella IgG was used (Tetracore, Inc). We subsequently analyzed and scored slides at the University of Chicago. Pictures of fixed mouse spleens were taken at the University of Chicago Integrated Light Microscopy Facility on a Cambridge Research and Instrumentation whole slide scanner fitted with an Allied Vision Technologies Stingray F146C color camera.
Determination of Antibody and Cytokine Responses
Mouse serum immunoglobulin G (IgG) and interferon gamma (IFN-γ) titers were measured using an enzyme-linked immunosorbent assay (ELISA) in 96-well ELISA plates (Nunc-immuno MaxiSorb, Sigma). Total mouse serum IgG, IgG1, and IgG2a titers were measured using mouse-specific ELISA kits by following manufacturer's instructions (eBioscience). To determine Brucella-specific IgG titers, ELISA plates were coated with 100 μL of heat-killed bacterial suspensions prepared in PBS (OD600=2.0) from B. abortus grown for 2 days on SBA. Cultures were heat-killed at 65°C for 1 hour, cooled to room temperature, and treated with kanamycin (50 μg/mL) and gentamycin (50 μg/mL) to prevent bacterial growth. Serum IFN-γ titers were measured using the OptiEIA mouse IFN-γ ELISA kit (BD Biosciences) following the manufacturer’s instructions. The signal from all ELISA plates were measured at 450 nm with a 570 nm background correction using a Tecan Infinite M200 PRO fluorescence plate reader. All values presented are the mean ± the standard error of the mean (SEM) from at least 5 mice for each time-point and condition.
Vaccination Studies
For vaccine studies, eight-week-old female BALB/c mice were vaccinated by IP injection (100 μL) with 5 × 105 CFU/mL of the B. abortus ΔrpoE1 strain in PBS. After 9 weeks, naïve age-matched mice and ΔrpoE1-vaccinated mice were challenged with 100 μL of 5 × 105 CFU/mL of WT B. abortus. Three weeks post-challenge, bacterial burden, spleen weight, and immune responses were measured as described above. Primers specific to the rpoE1 locus were used to differentiate WT from ΔrpoE1 bacteria isolated from the spleens of vaccinated mice. WT bacteria gave a PCR product of 1141 base pairs (bp) while the ΔrpoE1 strain gave a PCR product of 568 bp, using the primers ΔrpoE1_confirm_up 5′-CCTTTCGAGTGCTGATGCAT-3′ and ΔrpoE1_confirm_dn 5′ – GCTGACAGCAAGACCAACC-3′.
Results
Mice infected with Brucella abortus ΔrpoE1 have reduced chronic splenomegaly, which correlates with reduced splenic bacterial burden
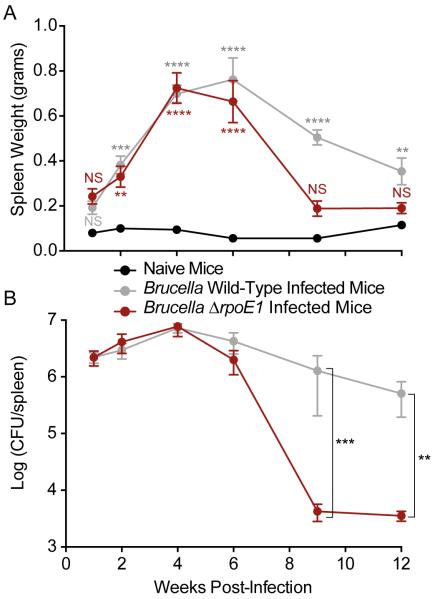
Mice infected with WT B. abortus had pronounced spleen swelling (i.e. splenomegaly, assessed by measuring spleen weight), which peaked at 4 weeks post-infection (wpi) and slowly decreased during the chronic phase of infection (approximately 4 – 12 wpi). Peak spleen weight at this time point was 0.70 ± 0.09 grams; peak bacterial burden was ≈107 colony forming units per spleen (CFU/spleen). As previously described, mouse splenomegaly correlates with an increase in splenic bacterial burden (26).
Through the first four weeks of infection, WT- and ΔrpoE1-infected mice were statistically indistinguishable in terms of splenomegaly and bacterial burden (Figure 2). Beginning 6 wpi, mice infected with ΔrpoE1 exhibited reduced splenomegaly and bacterial burden compared to WT-infected mice, though mean values were not statistically distinct at this time point (Figure 2). By 9 wpi we observed statistically significant reductions in both spleen weight (P<0.0001) and bacterial burden (P<0.001). ΔrpoE1-infected mice had a 2.5-fold reduction in spleen weight and a 3.0 log reduction in splenic bacterial burden relative to WT-infected mice (Figure 2). Both spleen weight and bacterial burden remained constant between 9 and 12 wpi in ΔrpoE1-infected mice, but decreased slightly in this period in WT-infected mice (Figure 2). These results demonstrate that host splenomegaly is reduced in ΔrpoE1-infected mice during the chronic phase relative to WT-infected mice. Reduced splenomegaly in the chronic phase is correlated with clearance of B. abortus ΔrpoE1 from the murine host.
Figure 2. ΔrpoE1-infected mice have reduced splenic bacterial burden and splenomegaly during the chronic phase of infection.
Female BALB/c mice were infected intraperitoneally with WT or ΔrpoE1 B. abortus and (A) spleen weights and (B) bacterial burden were measured at 1, 2, 4, 6, 9, and 12 weeks post-infection. Data presented are the mean ± the standard error of the mean (n = 5). Graphs represent data from naïve mice (black circle), WT-infected mice (grey circle), and ΔrpoE1-infected mice (red circle). One-way ANOVA was followed by a Bonferroni post-test to assess the significance in the mean differences between naïve mice and WT- or ΔrpoE1-infected mice (A) or between WT- and ΔrpoE1-infected mice (B). NS = not-significant, (*) = P<0.05, (**) = P<0.01, (***) = P<0.001, (****) = P < 0.0001.
Spleens of ΔrpoE1-infected mice have reduced pathologic features and contain less Brucella antigen
To assess differences in pathology of mice infected with WT and ΔrpoE1 strains in the early and chronic phase, we harvested spleens at 1 and 9 wpi. Spleens were fixed, mounted, and subjected to hematoxylin and eosin (H&E) staining. We scored spleens for white to red pulp ratio, average number of lymphoid follicles, germinal center size, marginal zone thickness, extramedullary hematopoiesis, histiocytic proliferation and presence of granulomas. Our results are summarized in Table 1 (data are scored on a scale of 0-3, with 0 being no apparent pathology). Naïve (i.e. uninfected) spleens scored 0 in all categories. Spleens of ΔrpoE1- and WT-infected mice were inflamed and contained Brucella granulomas at 1 wpi, consistent with what has been previously reported for BALB/c infection with WT B. abortus (27). However, there were modest differences in scored histopathology between strains at this early time point. Though spleen weight and total CFU per spleen were statistically equivalent at 1 wpi (Figure 2), ΔrpoE1-infected mice had lower extramedullary hematopoiesis, histiocytic proliferation and fewer granulomas than WT (Table 1, Figure S1). These data provide evidence of differences in interaction of ΔrpoE1 and WT Brucella strains with the murine host in the acute phase, though infections look similar with respect to spleen colonization and bulk inflammation.
Table 1.
Summary of histopathologic scoring of naïve mouse spleens, and spleens of mice infected with wild-type or ΔrpoE1 B. abortus.
| Naïve | B. abortus WT | B. abortus ΔrpoE1 | ||||
|---|---|---|---|---|---|---|
| Week Post-infection (wpi) |
1 | 9 | 1 | 9 | 1 | 9 |
|
white pulp to red
pulp ratio |
Normal (≈ 1:1) |
Normal (≈ 1:1) |
Decrease Score 1 |
Decrease Score 3 |
Decrease Score 1 |
Decrease Score 0.5 |
|
Average lymphoid
follicles per field |
8 to 12 | 8 to 12 | Decrease Score 1 |
Decrease Score 2 |
Decrease Score 1 |
Decrease Score 0.5 |
| Size of follicles | normal | normal | Decrease Score 1 |
Decrease Score 1 |
Decrease Score 1 |
Score 0 |
|
Marginal zone
depletion |
normal | normal | Increase Score 1 |
Increase Score 2 |
Increase Score 1 |
Increase Score 0-1 |
|
Extramedullary
hematopoiesis |
minimal | minimal | Increase Score 2 |
Increase Score 2 |
Increase Score 1 |
Increase Score 0-1 |
|
Histiocytic
proliferation |
none | none | Increase Score 2 |
Increase Score 3 |
Increase Score 1 |
Increase Score 0-1 |
| Granulomas | none | none | Increase Score 1 |
Increase Score 3 |
Increase Score 0-1 |
Increase Score 0-1 |
Scores range from 0 to 3, and are based on masked evaluation of sectioned and stained spleen tissue. The presence of Brucella in tissue was confirmed by immunohistochemistry. 0 = normal pathology (all naïve spleens scored 0 in all categories); 1 = mild pathology, 2 = moderate pathology, 3 = severe pathology relative to uninfected (naïve) control.
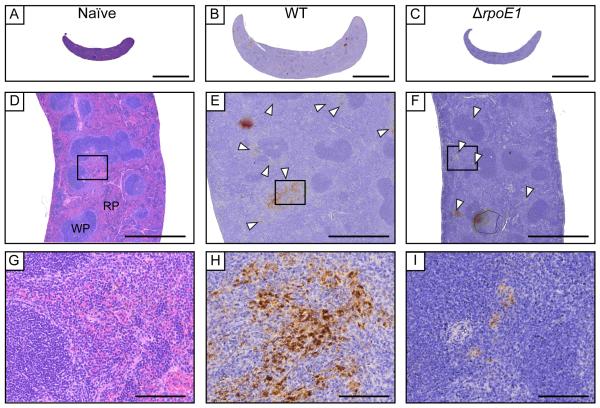
By 9 wpi, spleens of ΔrpoE1-infected mice had reduced pathologic features in all scored categories relative to 1 wpi (Table 1, Figure 3). In contrast, spleens of mice infected with WT had significantly reduced white to red pulp ratio due to disruption of lymphoid follicles (follicular lysis) and red pulp expansion while also displaying significantly increased marginal zones. In addition, spleens of WT-infected mice had higher extramedullary hematopoiesis, histiocytic proliferation, granulomas and presence of Brucella immunoreactivities than ΔrpoE1 at 9 wpi (Table1, Figure 3). Images of naïve, WT- and ΔrpoE1-infected mouse spleens at 9 wpi are presented with histological features labeled in Figure 3.
Figure 3. Spleen histology of BALB/c mice infected with wild-type and ΔrpoE1 B. abortus strains.
At 9 weeks post-infection spleens were harvested, fixed, and slides were prepared. H&E staining was performed on all samples. Uninfected (A, D, G), WT-infected (B, E, H), or ΔrpoE1-infected (C,F,I) mouse spleen pictures were taken at 10X (A-C), 50X (D-F) or 400X magnification (G-I). Brucella antigen was visualized by immunohistochemistry with an anti-Brucella antibody (D-I). White arrowheads point to areas of extensive Brucella antigen staining. Scale bars are 5 mm (A), 1 mm (B), and 100 μm (C). WP = white pulp, RP = red pulp.
ΔrpoE1 infection elicits an increased IgG antibody response relative to WT infection
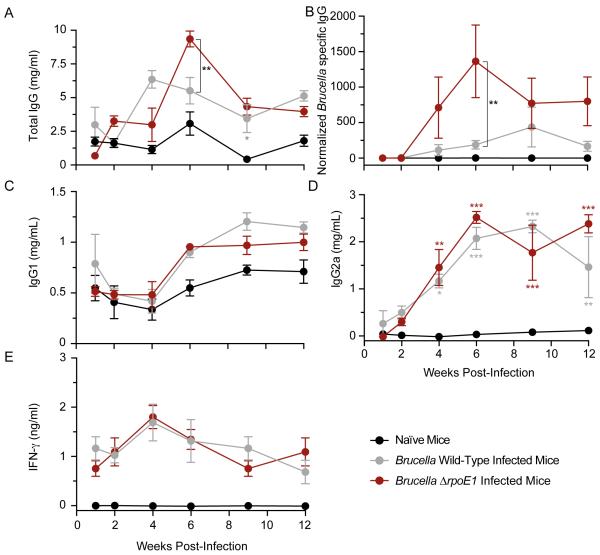
To test whether there are differences in antibody or cytokine responses in mice infected with ΔrpoE1 and WT, we harvested serum from uninfected (naïve) control mice and infected mice at 1, 2, 4, 6, 9 and 12 wpi. We measured serum levels of total IgG, Brucella-specific IgG, IgG1, IgG2a, and IFN-γ by enzyme-linked immunosorbent assays (ELISA). Total serum IgG was higher across the 12-week experiment in both WT- and ΔrpoE1-infected mice relative to uninfected control (Figure 4A). WT- and ΔrpoE1-infected mice had similar total IgG levels with the exception of 6 wpi when total IgG serum levels were ≈2 times higher in ΔrpoE1 (9.4 mg/mL versus 5.5 mg/mL; Figure 4A). We next measured IgG specific to B. abortus. Serum harvested from WT- and ΔrpoE1-infected mice was incubated in ELISA plates coated with a suspension of heat-killed B. abortus; measurements were normalized to uninfected control serum. The level of Brucella-specific IgG was ≈6 times higher in ΔrpoE1-infected mice by 4 wpi (Figure 4B). Levels peaked at 6 wpi when antibodies specific to B. abortus antigen(s) were 7 times higher in ΔrpoE1-infected compared to WT-infected mice (one-way ANOVA with Bonferroni correction; P<0.001) (Figure 4B). Thus the difference in total IgG levels we observe between strains is largely attributable to a response to B. abortus-specific antigen(s).
Figure 4. Antibody and cytokine quantification in wild-type and ΔrpoE1-infected mouse sera.
Mouse serum was harvested at 1, 2, 4, 6, 9, and 12 weeks post-infection from naïve-control mice, WT-, or ΔrpoE1-infected mice. Amounts of total IgG (A) Brucella-specific IgG (B) IgG1 (C) IgG2a (D) and IFN-γ (E) were determined by ELISA (see Materials and Methods for details). Data presented are from naïve mice (black circle), WT-infected mice (grey circle), or ΔrpoE1-infected mice (red circle). Each data point (n = 5) is the mean ± the standard error of the mean. Unless otherwise indicated, one-way ANOVA was followed by a Bonferroni post test to assess the significance in the mean differences between naïve mice and WT- or ΔrpoE1-infected mice. NS = not significant, (*) = P<0.05, (**) = P<0.01, (***) = P<0.001, (****) = P < 0.0001.
WT B. abortus infection is reported to stimulate a robust CD4+ T helper 1 (Th1) response (28, 29) without a significant Th2 response (30). Though BALB/c is regarded as a Th2-dominant mouse strain (31), BALB/c can mount a Th1 response against bacterial and parasitic infections (32, 33); there is evidence that BALB/c do not actually present a Th2 bias following WT Brucella infection (34). Given the specific defect of the ΔrpoE1 strain in the chronic phase of infection, and the known role of the Th1 cytokine IFN-γ in control of chronic infection (35), we sought to test whether BALB/c infected with WT and ΔrpoE1 may show a differential skew in Th1/Th2 responses. To this end, we profiled IgG sub-classes IgG1 and IgG2a and the cytokine IFN-γ in harvested sera. The ratio of IgG1 to IgG2a can serve as an indicator of Th1 versus Th2 responses in the host: high IgG2a/IgG1 ratios indicate a Th1 response, and a low ratio indicates a Th2 response (36, 37). IFN-γ is an established Th1 cytokine, and a marker for Th1 response (38).
Both WT- and ΔrpoE1-infected mice displayed similar increases in IgG1 and IgG2a across the 12-week experiment with levels of IgG1 peaking at approximately 9 wpi and IgG2a peaking at 6 wpi (Figure 4C,D). The IgG2a/IgG1 ratio suggests a mixed Th1/Th2 response, though relative increase in IgG2a was much larger than IgG1 after infection. IFN-γ levels increased by a factor of ≈2 over the first four weeks of infection, and decreased gradually back to levels measured at 1 wpi by the end of the 12-week experiment (Figure 4E). We observed no significant differences between WT- and ΔrpoE1-infected mice for IgG1, IgG2a, or IFN-γ at any time point. Together, these data provide evidence that ΔrpoE1 elicits a stronger antibody response than WT B. abortus in BALB/c mice (Figure 4A-B), while the Th1/Th2 profile of ΔrpoE1-infected mice does not appear to differ from WT infection (Figure 4C-E).
Vaccination with ΔrpoE1 protects mice against WT B. abortus challenge
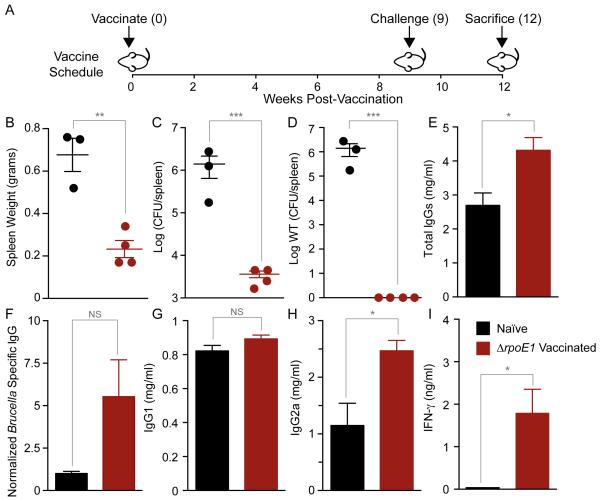
Brucella infection in humans is correlated with the incidence of infection of their ungulate livestock, which can range above 70% at the herd level in certain regions of the world (39-42). Current efforts to control disease in livestock, and by extension, humans, are limited by the availability of broadly effective and safe animal vaccines (24). The observation that B. abortus ΔrpoE1 elicits a strong humoral response (Figure 4B) that corresponds with clearance of infection from the host (Figure 2) suggested that ΔrpoE1 may be an effective live-attenuated vaccine. To test this hypothesis, we vaccinated mice with the ΔrpoE1 strain via IP injection (5 × 105 CFU/mL). At 9 weeks post-vaccination, we challenged ΔrpoE1 vaccinated and naïve age-matched mice with 5 × 104 CFU of WT B. abortus. We measured spleen weights and bacterial burden in the spleen at 3 weeks post-challenge (wpc) (Figure 5A).
Figure 5. Analysis of splenomegaly, bacterial burden, antibody, and cytokine profiles in ΔrpoE1-vaccinated mice.
Naïve age-matched control mice or ΔrpoE1-vaccinated mice were infected with WT Brucella 9 weeks post-vaccination (A). Spleen weights (B), total Brucella burden (C), and WT Brucella (D) were determined for each spleen. Quantitative ELISAs were performed to analyze serum levels of total IgG (E), Brucella-specific IgG (F), IgG1 (G) IgG2a (H), and IFN-γ (I). For vaccination studies, naïve mice (n = 3) are colored black and ΔrpoE1-vaccinated mice (n = 4) are colored red. Data presented are the mean ± SEM. T-tests were performed between naïve and ΔrpoE1-vaccinated mice. NS = not-significant, (*) = P<0.05, (**) = P<0.01, (***) = P<0.001, (****) = P < 0.0001.
Naïve mice exhibited pronounced splenomegaly with a mean spleen weight of 0.68 ± 0.14 grams at 3 wpc (Figure 5B). ΔrpoE1-vaccinated mice had significantly lower spleen weight of 0.23 ± 0.08 grams (t-test; P<0.01). Reduced splenomegaly corresponded with reduced bacterial burden: ΔrpoE1 vaccinated mice had ≈3 logs less splenic bacteria than naïve mice at 3 wpc (t-test; <0.001) (Figure 5C). As presented above, the ΔrpoE1 strain persists at low levels in the spleen at 12 wpi (Figure 2B). Thus, it was necessary to determine whether B. abortus isolated from the spleens of vaccinated mice at 3 wpc was ΔrpoE1, WT, or a mixture of both strains. To this end, we PCR amplified the rpoE1 locus of all bacterial colonies isolated from a spleen section of vaccinated mice. All bacteria isolated from plated spleen homogenates of vaccinated mice (n ≥ 48) were confirmed to be the ΔrpoE1 genotype. Thus, vaccination with ΔrpoE1 provided complete protection against WT B. abortus challenge in the context of this assay.
Mice vaccinated with ΔrpoE1 have higher antibody and IFN-y responses than naïve mice upon WT challenge
We next assessed whether naïve and ΔrpoE1-vaccinated mice exhibited differences in antibody or IFN-γ production upon WT challenge. Total IgG serum levels were significantly higher in ΔrpoE1-vaccinated mice at 3 wpc (t-test; P<0.0001) (Figure 5E). Brucella-specific IgG levels, measured as described above, were ≈5 times higher in ΔrpoE1-vaccinated mice than naïve control mice (Figure 5F). There was no difference in total IgG1 levels between naïve and ΔrpoE1-vaccinated mice at 3 wpc (Figure 5G). Total IgG2a serum levels were ≈2 times higher in ΔrpoE1-vaccinated mice (t-test; P=0.020), which is consistent with a stronger Th1 response (Figure 5H). Finally, IFN-y response was significantly higher in ΔrpoE1-vaccinated mice than unvaccinated naïve mice (t-test; P=0.019) (Figure 5I). We conclude that vaccination with ΔrpoE1 results in significantly increased antibody production and IFN-y production relative to naïve mice upon WT challenge.
Discussion
Genes encoding the GSR system (Figure 1) are determinants of chronic brucellosis in a BALB/c mouse model of disease (17). A goal of this study was to define features of the host response that distinguish wild-type (WT) B. abortus infection from infection with a strain harboring a null allele of the GSR sigma factor, rpoE1 (ΔrpoE1). Deletion of rpoE1 had no effect on splenic bacterial burden or spleen inflammation in the acute phase (up to 6 wpi). However, histological analysis of ΔrpoE1 and WT infected spleens at 1 wpi revealed pathologic features that differ at this time point including lower histiocytic proliferation and extramedullary hematopoiesis, and fewer granulomas in ΔrpoE1 at this early time point (Table 1). As previously reported (17, 18), deletion of rpoE1 resulted in significant reduction of splenic bacteria and decreased splenomegaly in the chronic phase (> 6 wpi) of infection relative to WT (Figure 2).
Reduced splenic bacterial burden of ΔrpoE1-infected mice during the chronic phase of infection suggested that this strain may elicit a stronger host immune response than WT B. abortus. To test this hypothesis, we measured serum levels of specific cytokines and antibodies known to be important in host-mediated clearance of Brucella infection (26, 35, 43, 44). We observed no significant difference in IFN-γ levels between WT- and ΔrpoE1-infected mice over a 12-week experiment. We did observe a peak in total IgG and Brucella-specific IgG in the serum of ΔrpoE1-infected mice at 6 wpi, which corresponds with clearance of ΔrpoE1 from the spleen. This IgG peak was significantly reduced in WT-infected mice. These data provide evidence that ΔrpoE1 elicits a more robust humoral response than WT B. abortus. At this time, we cannot discern whether clearance of the ΔrpoE1 strain is mediated or enhanced by these antibodies, or if antibody production is simply a consequence of antigen release triggered by host clearance of ΔrpoE1 by innate or cell-mediated mechanisms known to control Brucella spp. infection in mice (43, 45-48).
While a number of Brucella live vaccines have been developed (26, 49-52), there is a need for more effective vaccines (23, 24). Vaccines in current use are documented to cause spontaneous abortion and arthritis in livestock and have varying levels of protective efficacy (23, 50, 53). Accordingly, we tested the efficacy of B. abortus ΔrpoE1 as a live attenuated vaccine. Though a low number of ΔrpoE1 brucellae remained in the spleen at the time of vaccination, ΔrpoE1-vaccinated mice showed no signs of spleen inflammation and completely resisted splenic colonization by WT brucellae. This stands in contrast to BALB/c mice vaccinated with some commercially available vaccines; vaccinated mice are not significantly protected against splenic colonization upon WT challenge (54). Serum from vaccinated mice and naïve mice after WT challenge revealed significantly higher levels of total IgG, Brucella-specific IgG, IgG2a, and IFN-γ in ΔrpoE1-vaccinated mice. The increase in IgG2a, but not IgG1, and the significantly higher IFN-γ levels in vaccinated mice post-challenge (Figure 5) suggests that ΔrpoE1 elicits Th1-mediated protection (30, 55).
We do not currently understand the mechanism by which the B. abortus GSR regulatory system mediates bacterial survival in the host during the chronic phase of infection. Transcriptional profiling approaches have defined the GSR regulon in B. abortus (17, 18), and these studies have demonstrated that a variety of outer membrane and other cell envelope proteins, stress response proteins, and genes of unknown function are under direct or indirect transcriptional control of RpoE1 (σE1). The roles of these GSR-regulated genes in maintenance of chronic infection remain largely undefined, though several including rpoH1 (56), ba14K (18, 57), pgm (58, 59), wrpA (60), cydAB (61), cydX (62), the urease gene cluster (63), and flagellar genes (64) have been linked to B. abortus virulence or host-Brucella interaction. Alterations in the B. abortus envelope due to dysregulation of the GSR in the chronic phase of infection may reveal B. abortus antigens that are otherwise repressed. This could explain the increased antibody response elicited by ΔrpoE1, and subsequent clearance from the host (Figure 2; Figure 4 A-B). Alternatively, it is possible that B. abortus encounters particular physical or chemical stressors during the chronic phase of infection that result in death of ΔrpoE1, which has known acute stress survival defects in vitro (17). The exact nature of such a chronic phase stress in this scenario is not obvious given that ΔrpoE1 has no deficiency in entry or replication in activated macrophages in vitro, or in infection and colonization of mouse spleens over the first 6 weeks of infection (Figure 2) (17). Future studies will elucidate the specific links between ΔrpoE1 clearance from the host, activation of host immunity, and ΔrpoE1-mediated protection against wild-type B. abortus infection.
Supplementary Material
Acknowledgements
We thank members of the Crosson lab for helpful discussions, Dr. Lauriane Quenee and Dr. Lois Zitzow for help with animal experiments, the University of Chicago Integrated Light Microscopy Core Facility, and Heather Marlatt at Nationwide Histology.
Funding Information.
Funding for this project was provided in part by National Institutes of Health grant numbers U19AI107792 and R01AI107159 (S.C.). J.W.W. was supported by an NIH Ruth Kirschstein Postdoctoral Fellowship F32GM109661.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- 1.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 2.Fiebig A, Herrou J, Willett J, Crosson S. General Stress Signaling in the Alphaproteobacteria. Annu Rev Genet. 2015;49:603–625. doi: 10.1146/annurev-genet-112414-054813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francez-Charlot A, Kaczmarczyk A, Fischer HM, Vorholt JA. The general stress response in Alphaproteobacteria. Trends Microbiol. 2015;23:164–171. doi: 10.1016/j.tim.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Foreman R, Fiebig A, Crosson S. The LovK-LovR two-component system is a regulator of the general stress pathway in Caulobacter crescentus. J Bacteriol. 2012;194:3038–3049. doi: 10.1128/JB.00182-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrou J, Foreman R, Fiebig A, Crosson S. A structural model of anti-anti-sigma inhibition by a two-component receiver domain: the PhyR stress response regulator. Mol Microbiol. 2010;78:290–304. doi: 10.1111/j.1365-2958.2010.07323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrou J, Rotskoff G, Luo Y, Roux B, Crosson S. Structural basis of a protein partner switch that regulates the general stress response of alpha-proteobacteria. Proc Natl Acad Sci U S A. 2012;109:E1415–E1423. doi: 10.1073/pnas.1116887109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrou J, Willett JW, Crosson S. Structured and Dynamic Disordered Domains Regulate the Activity of a Multifunctional Anti-sigma Factor. MBio. 2015;6:e00910. doi: 10.1128/mBio.00910-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gourion B, Sulser S, Frunzke J, Francez-Charlot A, Stiefel P, Pessi G, Vorholt JA, Fischer HM. The PhyR-sigma(EcfG) signalling cascade is involved in stress response and symbiotic efficiency in Bradyrhizobium japonicum. Mol Microbiol. 2009;73:291–305. doi: 10.1111/j.1365-2958.2009.06769.x. [DOI] [PubMed] [Google Scholar]
- 9.Bastiat B, Sauviac L, Bruand C. Dual control of Sinorhizobium meliloti RpoE2 sigma factor activity by two PhyR-type two-component response regulators. J Bacteriol. 2010;192:2255–2265. doi: 10.1128/JB.01666-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastiat B, Sauviac L, Picheraux C, Rossignol M, Bruand C. Sinorhizobium meliloti sigma factors RpoE1 and RpoE4 are activated in stationary phase in response to sulfite. PLoS One. 2012;7:e50768. doi: 10.1371/journal.pone.0050768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaczmarczyk A, Campagne S, Danza F, Metzger LC, Vorholt JA, Francez-Charlot A. Role of Sphingomonas sp. strain Fr1 PhyR-NepR-sigmaEcfG cascade in general stress response and identification of a negative regulator of PhyR. J Bacteriol. 2011;193:6629–6638. doi: 10.1128/JB.06006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaczmarczyk A, Hochstrasser R, Vorholt JA, Francez-Charlot A. Complex two-component signaling regulates the general stress response in Alphaproteobacteria. Proc Natl Acad Sci U S A. 2014;111:E5196–5204. doi: 10.1073/pnas.1410095111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaczmarczyk A, Hochstrasser R, Vorholt JA, Francez-Charlot A. Two tiered histidine kinase pathway involved in heat shock and salt sensing in the general stress response of Sphingomonas melonis Fr1. J Bacteriol. 2015;197:1466–1477. doi: 10.1128/JB.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francez-Charlot A, Frunzke J, Reichen C, Ebneter JZ, Gourion B, Vorholt JA. Sigma factor mimicry involved in regulation of general stress response. Proc Natl Acad Sci U S A. 2009;106:3467–3472. doi: 10.1073/pnas.0810291106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gourion B, Francez-Charlot A, Vorholt JA. PhyR is involved in the general stress response of Methylobacterium extorquens AM1. J Bacteriol. 2008;190:1027–1035. doi: 10.1128/JB.01483-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metzger LC, Francez-Charlot A, Vorholt JA. Single-domain response regulator involved in the general stress response of Methylobacterium extorquens. Microbiology. 2013;159:1067–1076. doi: 10.1099/mic.0.066068-0. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Caswell CC, Foreman R, Roop RM, Crosson S. The Brucella abortus General Stress Response System Regulates Chronic Mammalian Infection and Is Controlled by Phosphorylation and Proteolysis. J Biol Chem. 2013;288:13906–13916. doi: 10.1074/jbc.M113.459305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HS, Willett JW, Jain-Gupta N, Fiebig A, Crosson S. The Brucella abortus virulence regulator, LovhK, is a sensor kinase in the general stress response signalling pathway. Mol Microbiol. 2014;94:913–925. doi: 10.1111/mmi.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abromaitis S, Koehler JE. The Bartonella quintana extracytoplasmic function sigma factor RpoE has a role in bacterial adaptation to the arthropod vector environment. J Bacteriol. 2013;195:2662–2674. doi: 10.1128/JB.01972-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tu N, Lima A, Bandeali Z, Anderson B. Characterization of the general stress response in Bartonella henselae. Microb Pathog. 2015;92:1–10. doi: 10.1016/j.micpath.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swartz TE, Tseng TS, Frederickson MA, Paris G, Comerci DJ, Rajashekara G, Kim JG, Mudgett MB, Splitter GA, Ugalde RA, Goldbaum FA, Briggs WR, Bogomolni RA. Blue-light-activated histidine kinases: two-component sensors in bacteria. Science. 2007;317:1090–1093. doi: 10.1126/science.1144306. [DOI] [PubMed] [Google Scholar]
- 22.Sycz G, Carrica MC, Tseng TS, Bogomolni RA, Briggs WR, Goldbaum FA, Paris G. LOV Histidine Kinase Modulates the General Stress Response System and Affects the virB Operon Expression in Brucella abortus. PLoS One. 2015;10:e0124058. doi: 10.1371/journal.pone.0124058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen SC, Stoffregen WS. Essential role of vaccines in brucellosis control and eradication programs for livestock. Expert Rev Vaccines. 2005;4:915–928. doi: 10.1586/14760584.4.6.915. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Wu Q. Research progress in live attenuated Brucella vaccine development. Curr Pharm Biotechnol. 2013;14:887–896. doi: 10.2174/1389201014666131226123016. [DOI] [PubMed] [Google Scholar]
- 25.Willett JW, Herrou J, Briegel A, Rotskoff G, Crosson S. Structural asymmetry in a conserved signaling system that regulates division, replication, and virulence of an intracellular pathogen. Proc Natl Acad Sci U S A. 2015;112:E3709–3718. doi: 10.1073/pnas.1503118112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grillo MJ, Blasco JM, Gorvel JP, Moriyon I, Moreno E. What have we learned from brucellosis in the mouse model? Veterinary Research. 2012;43 doi: 10.1186/1297-9716-43-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enright FM, Araya LN, Elzer PH, Rowe GE, Winter AJ. Comparative histopathology in BALB/c mice infected with virulent and attenuated strains of Brucella abortus. Vet Immunol Immunopathol. 1990;26:171–182. doi: 10.1016/0165-2427(90)90065-z. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira SC, Zhu Y, Splitter GA. Recombinant L7/L12 ribosomal protein and gamma-irradiated Brucella abortus induce a T-helper 1 subset response from murine CD4+ T cells. Immunology. 1994;83:659–664. [PMC free article] [PubMed] [Google Scholar]
- 29.Street NE, Schumacher JH, Fong TA, Bass H, Fiorentino DF, Leverah JA, Mosmann TR. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990;144:1629–1639. [PubMed] [Google Scholar]
- 30.Agranovich I, Scott DE, Terle D, Lee K, Golding B. Down-regulation of Th2 responses by Brucella abortus, a strong Th1 stimulus, correlates with alterations in the B7.2-CD28 pathway. Infect Immun. 1999;67:4418–4426. doi: 10.1128/iai.67.9.4418-4426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22:460–466. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- 32.Barbi J, Brombacher F, Satoskar AR. T cells from Leishmania major-susceptible BALB/c mice have a defect in efficiently up-regulating CXCR3 upon activation. J Immunol. 2008;181:4613–4620. doi: 10.4049/jimmunol.181.7.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Pelayo MC, Bachy VS, Kaveh DA, Hogarth PJ. BALB/c mice display more enhanced BCG vaccine induced Th1 and Th17 response than C57BL/6 mice but have equivalent protection. Tuberculosis (Edinb) 2015;95:48–53. doi: 10.1016/j.tube.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Vitry MA, De Trez C, Goriely S, Dumoutier L, Akira S, Ryffel B, Carlier Y, Letesson JJ, Muraille E. Crucial role of gamma interferon-producing CD4+ Th1 cells but dispensable function of CD8+ T cell, B cell, Th2, and Th17 responses in the control of Brucella melitensis infection in mice. Infect Immun. 2012;80:4271–4280. doi: 10.1128/IAI.00761-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy EA, Sathiyaseelan J, Parent MA, Zou BX, Baldwin CL. Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology. 2001;103:511–518. doi: 10.1046/j.1365-2567.2001.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci U S A. 1998;95:15553–15558. doi: 10.1073/pnas.95.26.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 38.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed MO, Elmeshri SE, Abuzweda AR, Blauo M, Abouzeed YM, Ibrahim A, Salem H, Alzwam F, Abid S, Elfahem A, Elrais A. Seroprevalence of brucellosis in animals and human populations in the western mountains region in Libya, December 2006-January 2008. Euro Surveill. 2010;15 [PubMed] [Google Scholar]
- 40.Gwida M, Al Dahouk S, Melzer F, Rosler U, Neubauer H, Tomaso H. Brucellosis - regionally emerging zoonotic disease? Croat Med J. 2010;51:289–295. doi: 10.3325/cmj.2010.51.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDermott JJ, Arimi SM. Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Vet Microbiol. 2002;90:111–134. doi: 10.1016/s0378-1135(02)00249-3. [DOI] [PubMed] [Google Scholar]
- 42.Osoro EM, Munyua P, Omulo S, Ogola E, Ade F, Mbatha P, Mbabu M, Ng'ang'a Z, Kairu S, Maritim M, Thumbi SM, Bitek A, Gaichugi S, Rubin C, Njenga K, Guerra M. Strong Association Between Human and Animal Brucella Seropositivity in a Linked Study in Kenya, 2012-2013. Am J Trop Med Hyg. 2015;93:224–231. doi: 10.4269/ajtmh.15-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldwin CL, Parent M. Fundamentals of host immune response against Brucella abortus: what the mouse model has revealed about control of infection. Vet Microbiol. 2002;90:367–382. doi: 10.1016/s0378-1135(02)00222-5. [DOI] [PubMed] [Google Scholar]
- 44.Vitry MA, Mambres DH, De Trez C, Akira S, Ryffel B, Letesson JJ, Muraille E. Humoral Immunity and CD4(+) Th1 Cells Are Both Necessary for a Fully Protective Immune Response upon Secondary Infection with Brucella melitensis. Journal of Immunology. 2014;192:3740–3752. doi: 10.4049/jimmunol.1302561. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira SC, de Almeida LA, Carvalho NB, Oliveira FS, Lacerda TL. Update on the role of innate immune receptors during Brucella abortus infection. Vet Immunol Immunopathol. 2012;148:129–135. doi: 10.1016/j.vetimm.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 46.Pei J, Ding X, Fan Y, Rice-Ficht A, Ficht TA. Toll-like receptors are critical for clearance of Brucella and play different roles in development of adaptive immunity following aerosol challenge in mice. Front Cell Infect Microbiol. 2012;2:115. doi: 10.3389/fcimb.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss DS, Takeda K, Akira S, Zychlinsky A, Moreno E. MyD88, but not toll-like receptors 4 and 2, is required for efficient clearance of Brucella abortus. Infect Immun. 2005;73:5137–5143. doi: 10.1128/IAI.73.8.5137-5143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Byndloss MX, Tsolis RM. Brucella spp. Virulence Factors and Immunity. Annu Rev Anim Biosci. 2016;4:111–127. doi: 10.1146/annurev-animal-021815-111326. [DOI] [PubMed] [Google Scholar]
- 49.Arenas-Gamboa AM, Rice-Ficht AC, Fan Y, Kahl-McDonagh MM, Ficht TA. Extended safety and efficacy studies of the attenuated Brucella vaccine candidates 16 M(Delta)vjbR and S19(Delta)vjbR in the immunocompromised IRF-1−/− mouse model. Clin Vaccine Immunol. 2012;19:249–260. doi: 10.1128/CVI.05321-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avila-Calderon ED, Lopez-Merino A, Sriranganathan N, Boyle SM, Contreras-Rodriguez A. A history of the development of Brucella vaccines. Biomed Res Int. 2013;2013:743509. doi: 10.1155/2013/743509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conde-Alvarez R, Arce-Gorvel V, Gil-Ramirez Y, Iriarte M, Grillo MJ, Gorvel JP, Moriyon I. Lipopolysaccharide as a target for brucellosis vaccine design. Microb Pathog. 2013;58:29–34. doi: 10.1016/j.micpath.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 52.Godfroid J, Scholz HC, Barbier T, Nicolas C, Wattiau P, Fretin D, Whatmore AM, Cloeckaert A, Blasco JM, Moriyon I, Saegerman C, Muma JB, Al Dahouk S, Neubauer H, Letesson JJ. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev Vet Med. 2011;102:118–131. doi: 10.1016/j.prevetmed.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Moriyon I, Grillo MJ, Monreal D, Gonzalez D, Marin C, Lopez-Goni I, Mainar-Jaime RC, Moreno E, Blasco JM. Rough vaccines in animal brucellosis: structural and genetic basis and present status. Vet Res. 2004;35:1–38. doi: 10.1051/vetres:2003037. [DOI] [PubMed] [Google Scholar]
- 54.Jimenez de Bagues MP, Elzer PH, Jones SM, Blasco JM, Enright FM, Schurig GG, Winter AJ. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect Immun. 1994;62:4990–4996. doi: 10.1128/iai.62.11.4990-4996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clapp B, Skyberg JA, Yang X, Thornburg T, Walters N, Pascual DW. Protective live oral brucellosis vaccines stimulate Th1 and th17 cell responses. Infect Immun. 2011;79:4165–4174. doi: 10.1128/IAI.05080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delory M, Hallez R, Letesson JJ, De Bolle X. An RpoH-like heat shock sigma factor is involved in stress response and virulence in Brucella melitensis 16M. J Bacteriol. 2006;188:7707–7710. doi: 10.1128/JB.00644-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vemulapalli TH, Vemulapalli R, Schurig GG, Boyle SM, Sriranganathan N. Role in virulence of a Brucella abortus protein exhibiting lectin-like activity. Infect Immun. 2006;74:183–191. doi: 10.1128/IAI.74.1.183-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ugalde JE, Comerci DJ, Leguizamon MS, Ugalde RA. Evaluation of Brucella abortus phosphoglucomutase (pgm) mutant as a new live rough-phenotype vaccine. Infect Immun. 2003;71:6264–6269. doi: 10.1128/IAI.71.11.6264-6269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ugalde JE, Czibener C, Feldman MF, Ugalde RA. Identification and characterization of the Brucella abortus phosphoglucomutase gene: role of lipopolysaccharide in virulence and intracellular multiplication. Infect Immun. 2000;68:5716–5723. doi: 10.1128/iai.68.10.5716-5723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herrou J, Czyz DM, Willett JW, Kim HS, Chhor G, Babnigg G, Kim Y, Crosson S. WrpA is an atypical flavodoxin-family protein under regulatory control of the Brucella abortus general stress response system. J Bacteriol. 2016;198:1281–1293. doi: 10.1128/JB.00982-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Endley S, McMurray D, Ficht TA. Interruption of the cydB locus in Brucella abortus attenuates intracellular survival and virulence in the mouse model of infection. J Bacteriol. 2001;183:2454–2462. doi: 10.1128/JB.183.8.2454-2462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun YH, de Jong MF, den Hartigh AB, Roux CM, Rolan HG, Tsolis RM. The small protein CydX is required for function of cytochrome bd oxidase in Brucella abortus. Front Cell Infect Microbiol. 2012;2:47. doi: 10.3389/fcimb.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sangari FJ, Seoane A, Rodriguez MC, Aguero J, Garcia Lobo JM. Characterization of the urease operon of Brucella abortus and assessment of its role in virulence of the bacterium. Infect Immun. 2007;75:774–780. doi: 10.1128/IAI.01244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fretin D, Fauconnier A, Kohler S, Halling S, Leonard S, Nijskens C, Ferooz J, Lestrate P, Delrue RM, Danese I, Vandenhaute J, Tibor A, DeBolle X, Letesson JJ. The sheathed flagellum of Brucella melitensis is involved in persistence in a murine model of infection. Cell Microbiol. 2005;7:687–698. doi: 10.1111/j.1462-5822.2005.00502.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.