Abstract
Objectives
The purpose of this study is to compare the effects of cognitive-behavioral therapy delivered by telephone (CBT-T) and telephone-delivered nondirective supportive therapy (NST-T) on sleep, health-related quality of life, and physical disability in rural older adults with Generalized Anxiety Disorder.
Design
Secondary analyses of a randomized clinical trial
Setting
Participants’ homes
Participants
141 rural-dwelling adults 60 years and older diagnosed with Generalized Anxiety Disorder
Intervention
CBT-T vs. NST-T
Measurements
Sleep was assessed with the Insomnia Severity Index. Health-related quality of life was assessed with the SF-36. Physical disability was assessed with the Pepper Center Tool for Disability. Assessments occurred at baseline, 4 months, 9 months, and 15 months.
Results
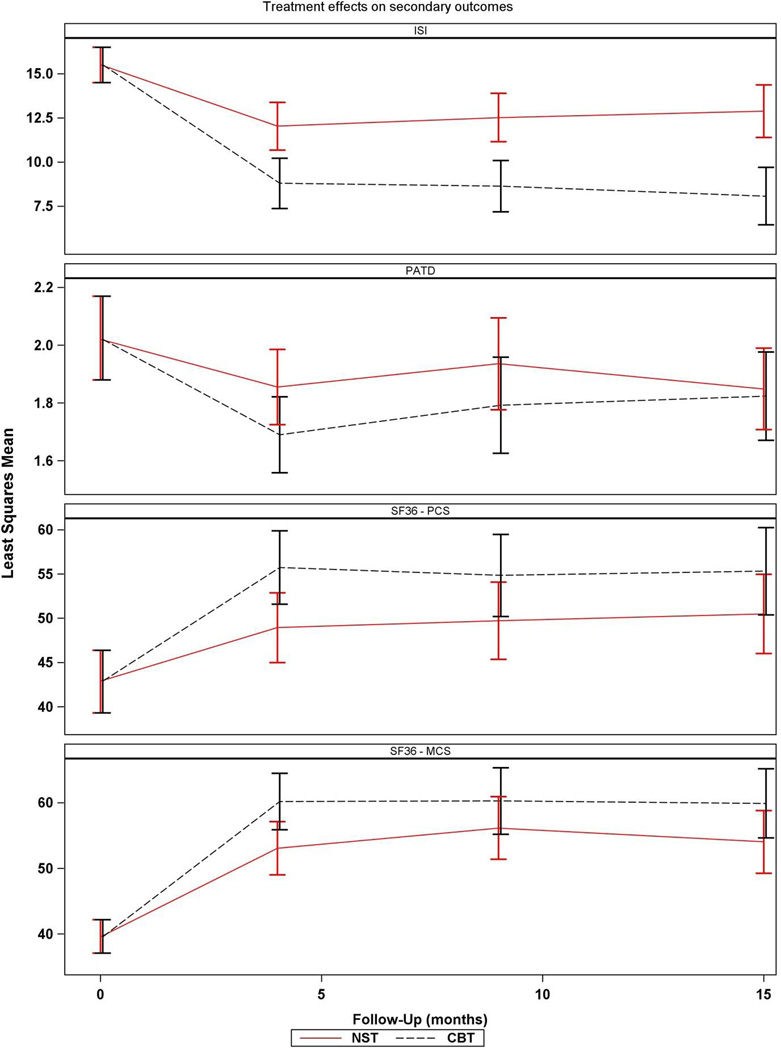
Insomnia declined in both groups from baseline to 4 months, with a significantly greater improvement among participants who received CBT-T. Similarly, Mental and Physical Component Summaries of the SF-36 declined in both groups, with a differential effect favoring CBT-T. Participants in both interventions reported declines in physical disability although there were no significant differences between the 2 interventions. Improvements in insomnia were maintained at the 15-month assessment, while between-group differences shrank on the Mental and Physical Component Summaries of the SF-36 by the 15-month assessment.
Conclusions
Telephone-delivered cognitive-behavioral therapy was superior to nondirective supportive therapy in reducing insomnia and improving health-related quality of life. The effects of CBT-T on sleep were maintained up to 1 year after completing the treatment.
Keywords: cognitive-behavioral therapy, supportive therapy, psychotherapy, GAD, late-life anxiety, RCT
Introduction
Generalized Anxiety Disorder (GAD) is characterized by excessive and uncontrollable worry and is accompanied by restlessness, fatigue, poor concentration, irritability, muscle tension, and/or sleep disturbance (1). GAD is one of the more common anxiety disorders, affecting up to 7.3% of older adults (2). The impact of GAD extends beyond mental health; late-life GAD is associated with worse physical health and even mortality (3–5).
One of the associated symptoms of GAD is sleep disturbance. Thus, it is not surprising that older adults with GAD report a greater degree of insomnia than older adults without GAD (6). Further, older adults with GAD report greater sleep disturbance than younger adults with GAD (6). Yet, few studies have reported on sleep as an outcome of psychotherapy trials, and those that do report conflicting outcomes. Brenes and colleagues (7) found that cognitive-behavioral therapy (CBT) for GAD was superior to enhanced usual care in reducing insomnia among older adults. Similarly, Bush and colleagues (8) found that CBT for late-life GAD was associated with greater improvements in the ability to fall asleep compared with enhanced usual care. However, others have found that sleep difficulties often remain after the treatment of GAD (9).
Late-life GAD is also associated with poor health-related quality of life (HRQL) even after controlling for medical conditions and comorbid depression (10–11). The greatest differences between older adults with GAD and healthy older adults are in the HRQL constructs of social functioning, energy, and role limitations due to emotional health (11). Compared with other anxiety disorders, GAD is associated with the greatest impairment in HRQL (12).
Disability within the context of GAD has often focused on work productivity. However, physical disability and self-care are more relevant to older adults. Older adults with GAD report more difficulty with activity limitations and impaired ability to participate in activities as frequently as they would like (10). GAD has also been associated with impairments in self-care activities and participating in non-work activities such as hobbies (13).
This study is based on data from a randomized clinical trial comparing telephone-delivered cognitive-behavioral therapy (CBT-T) with nondirective supportive therapy (NST-T) for the treatment of GAD in rural elders (14). We found that CBT-T was superior to NST-T in reducing worry, GAD symptoms, and depressive symptoms. The purpose of this report is to examine the effects of treatment on the non-mood-related secondary outcomes of sleep, HRQL, and disability in older adults with GAD. It was hypothesized that CBT-T would be superior to NST-T in improving sleep, HRQL, and disability.
Methods
Participants
Participants were part of a randomized clinical trial comparing CBT-T and NST-T for the treatment of rural-dwelling older adults with GAD. For more information about recruitment procedures, please see Brenes et al. (15). In brief, participants were adults aged 60 years and older with a principal or co-principal diagnosis of GAD who lived in rural counties within North Carolina. Participants were randomized to receive either CBT-T or NST-T. Data from this study are based on the baseline assessment conducted prior to randomization and the post-intervention (4, 9, and 15 months after baseline) assessments.
Measures
The Insomnia Severity Index (ISI; 16) is a 7-item self-report measure of type and severity of insomnia symptoms, including problems with sleep onset, sleep maintenance, or early morning awakening; satisfaction with current sleep pattern; interference with daily functioning; noticing impairment attributed to sleep problems; and level of concern or distress caused by the sleep problem. Responses are summed, with higher scores indicating greater sleep impairment. The ISI has demonstrated good reliability, validity, and sensitivity to change with treatment in older adults (16).
The SF-36 (17) is a self-report measure of HRQL consisting of 36 items that form 8 subscales: physical functioning, role limitations due to physical health problems, role limitations due to emotional health problems, social functioning, freedom from pain, energy, emotional well-being, and general health perceptions. These 8 subscales are also combined into two domains: the Physical Component Summary (PCS) and the Mental Component Summary (MCS). All of these scales range from 0 (maximum impairment) to 100 (no impairment).
The Pepper Center Tool for Disability (PCT-D; 18–19) is a 19-item self-report measure of perceived difficulties with mobility and performing basic and advanced activities of daily living during the last month. Participants rate how much difficulty they had performing each of the activities on a 5-point Likert scale ranging from 1 “no difficulty” to 5 “unable to do” the task, and items are averaged to yield the final score. This measure has been validated in four large samples of older adults and has demonstrated response to change in physical activity interventions (18).
Interventions
CBT-T consisted of up to 11 weekly psychotherapy sessions that addressed recognition of anxiety symptoms, relaxation, cognitive restructuring and the use of coping statements, problem solving, worry control, behavioral activation, exposure therapy, and relapse prevention. Optional sessions focused on sleep and pain. Each session was accompanied by a workbook chapter. NST-T consisted of 10 weekly sessions of supportive therapy described as “high-quality therapeutic relationship that provides a warm, genuine, and accepting atmosphere through the use of supportive and reflective communications” (p. 9; 20–21). Booster sessions were provided for both interventions at 2, 4, 8, and 12 weeks after completion of the weekly sessions. Ten percent of therapy sessions were reviewed by 2 independent evaluators to assess therapist adherence and competence in delivery of the interventions. Mean ratings for all therapists were above the a priori minimum of 6.0 (good) on 9-point scales of adherence and competence.
Statistical Analyses
Demographic and health characteristics were summarized with means, standard deviations, counts, and percentages. Comparison of the outcomes between intervention groups was made using constrained mixed-model repeated measures analysis of covariance with an unstructured covariance matrix to account for the fact that the multiple measurements (at baseline, 4, 9, and 15 months post-randomization) from participants are not independent. The models contained terms for therapist (a factor to which participants were randomized), baseline presence/absence of a depressive disorder (used to stratify randomization), use of psychotropic medications at baseline (used to stratify randomization), and intervention effects that were specific to each follow-up time. Because this was a randomized trial, pre-randomization intervention-specific outcome means were constrained to be the same (22). For randomized trials, constrained mixed-models can provide more efficient estimates of post-randomization treatment differences when either baseline or post-randomization measures are missing (23). A contrast was used to test the effect of the intervention on all four outcomes at the 4-month visit, the pre-specified time point for which the primary outcomes were tested. Standardized intervention differences were calculated at 4-months using the t-statistic from the contrast to obtain an estimate of the standard difference. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
To control the false discovery rate at the 0.05 level, we used the multiple-comparison procedure of Benjamini and Hochberg (24) across all four hypothesis tests (for the four tests of hypotheses at the 4-month visit). Results for the 8 subscales of the SF-36 and results for other outcomes collected at the 9- and 15-month visits are presented as differences between intervention groups with 95% confidence intervals.
Results
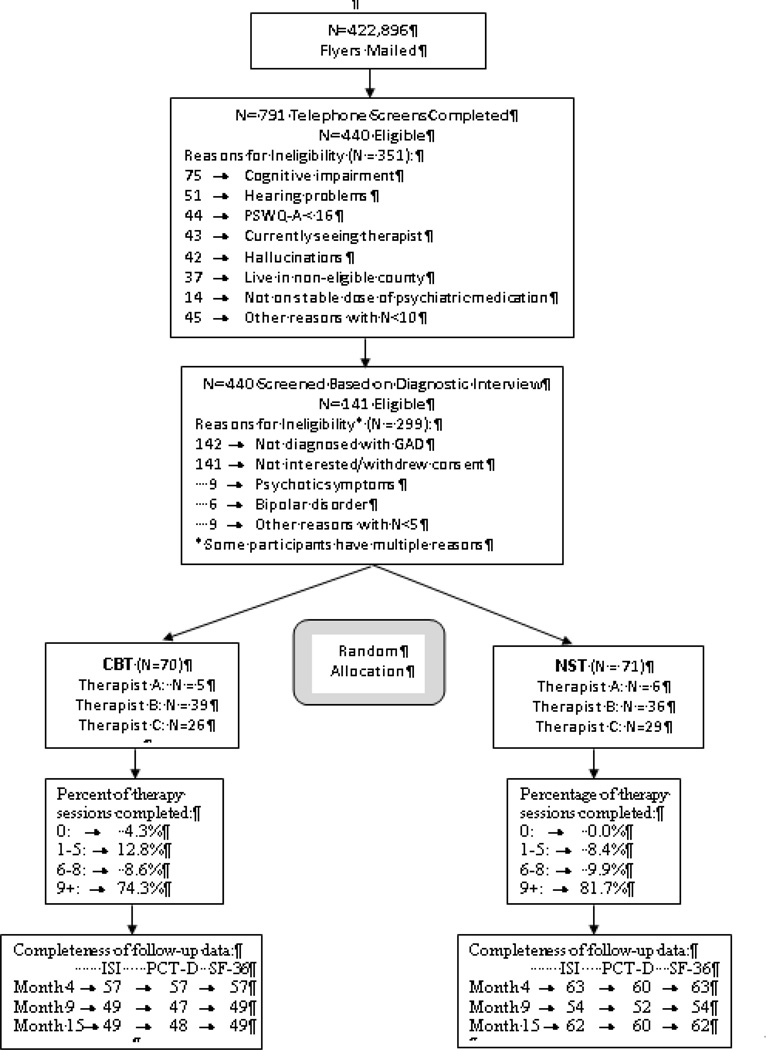
Participants ranged in age from 60 to 87 years with a mean age of 66.8 (SD=6.2). The sample consisted of largely white (90.8% white, 5.7% black, 3.5% Native American), well-educated (37.6% had some college, 44.7% had a college degree) women (81.6%). Demographics, health-related characteristics, and baseline values of the secondary outcomes for the sample are presented in Table 1. Figure 1 presents participant flow from randomization through the 15-month follow-up.
Table 1.
Baseline Characteristics of Randomized Participants
| Characteristic | Total (N=141) | CBT (N=70) | NST (N=71) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 26 (18.4) | 12 (17.1) | 14 (19.7) |
| Female | 115 (81.6) | 58 (82.9) | 57 (80.3) |
| Race, n (%) | |||
| Black or African American | 8 (5.7) | 4 (5.7) | 4 (5.6) |
| Caucasian or White | 128 (90.8) | 64 (91.4) | 64 (90.1) |
| Other | 5 (3.5) | 2 (2.9) | 3 (4.2) |
| Age, n (%), years | |||
| 60–64 | 66 (46.8) | 29 (41.4) | 37 (52.1) |
| 65–69 | 38 (27.0) | 20 (28.6) | 18 (25.4) |
| 70–74 | 19 (13.5) | 8 (11.4) | 11 (15.5) |
| 75+ | 18 (12.8) | 13 (18.6) | 5 (7.0) |
| Education, n (%) | |||
| Less than high school (HS) | 7 (5.0) | 4 (5.7) | 3 (4.2) |
| HS grad or GED | 18 (12.8) | 9 (12.9) | 9 (12.7) |
| Some college | 53 (37.6) | 19 (27.1) | 34 (47.9) |
| College degree | 63 (44.7) | 38 (54.3) | 25 (35.2) |
| Income, n (%) | |||
| Less than $24,999 | 33 (23.4) | 12 (17.1) | 21 (29.6) |
| $25,000 to $49,999 | 41 (29.1) | 21 (30.0) | 20 (28.2) |
| $50,000 to $74,999 | 19 (13.5) | 10 (14.3) | 9 (12.7) |
| More than $75,000 | 16 (11.4) | 7 (10.0) | 9 (12.7) |
| Missing | 32 (22.7) | 20 (28.6) | 12 (16.9) |
| Marital status, n (%) | |||
| Never been married | 1 (0.7) | 1 (1.4) | 0 (0.0) |
| Married or living with someone | 75 (53.2) | 37 (52.9) | 38 (53.5) |
| Divorced | 27 (19.2) | 13 (18.6) | 14 (19.7) |
| Separated | 9 (6.4) | 4 (5.7) | 5 (7.0) |
| Widowed | 29 (20.6) | 15 (21.4) | 14 (19.7) |
| Currently employed, n (%) | 38 (27.0) | 17 (24.3) | 21 (29.6) |
| Living status, n (%) | |||
| With others | 87 (61.7) | 41 (58.6) | 46 (64.8) |
| Alone | 54 (38.3) | 29 (41.4) | 25 (35.2) |
| Smoking status, n (%) | |||
| Never | 68 (48.2) | 37 (52.9) | 31 (43.7) |
| Current | 14 (9.9) | 6 (8.6) | 8 (11.3) |
| Former | 59 (41.8) | 27 (38.6) | 32 (45.1) |
| Current psychotropic medication usage, n (%) | |||
| Anxiolytics | 38 (27.0) | 22 (31.4) | 16 (22.5) |
| Hypnotics | 12 (8.5) | 5 (7.1) | 7 (9.9) |
| Antidepressants | 54 (38.3) | 29 (41.4) | 25 (35.2) |
| Antipsychotics/neuroleptics | 3 (2.1) | 1 (1.4) | 2 (2.8) |
| Stimulants | 1 (0.7) | 0 (0.0) | 1 (1.4) |
| History of self-reported health problems, n (%) | |||
| Hypertension | 92 (65.7) | 43 (62.3) | 49 (69.0) |
| Myocardial infarction | 9 (6.4) | 4 (5.8) | 5 (7.0) |
| Congestive heart failure | 8 (5.7) | 4 (5.7) | 4 (5.6) |
| Stroke | 11 (7.9) | 5 (7.2) | 6 (8.5) |
| Diabetes | 29 (20.6) | 9 (12.9) | 20 (28.2) |
| ISI score, mean (SD) | 15.5 (5.59) | 15.3 (5.17) | 15.7 (5.98) |
| SF-36 MCS, mean (SD) | 39.7 (13.89) | 42.1 (12.36) | 37.4 (14.88) |
| SF-36 PCS, mean (SD) | 42.9 (19.70) | 45.6 (19.13) | 40.4 (20.0) |
| PCT-D score, mean (SD) | 2.0 (0.76) | 1.9 (0.65) | 2.2 (0.84) |
| Presence of comorbid depression diagnosis, n (%) Total Current Past |
102 (72.3) 54 (38.3) 48 (34.0) |
51 (72.9) 23 (32.9) 28 (40.0) |
51 (71.8) 31 (43.7) 20 (28.2) |
| Presence of comorbid anxiety diagnosis, n (%) | 72 (51.1) | 31 (44.3) | 41 (57.8) |
Note: CBT = Cognitive-behavioral therapy; ISI = Insomnia Severity Index; NST = Nondirective Supportive Therapy; PCT-D = Pepper Center Tool for Disability; SF-36-MCS = SF-36 Mental Component Score; SF-36-PCS = SF-36 Physical Component Score.
Figure 1.
CONSORT Diagram
Note: CBT = Cognitive-behavior therapy; GAD = Generalized Anxiety Disorder; ISI = Insomnia Severity Index; NST = Nondirective supportive therapy; PCT-D = Pepper Center Tool for Disability; PSWQ-A = Penn State Worry Questionnaire-Abbreviated.
ISI and SF-36 measures were missing for 15% of participants at the 4-month visit, 27% at the 9-month visit, and 21% at the 15-month visit. PCT-D was missing for 17% of participants at the 4-month visit, 30% at the 9-month visit, and 23% at the 15-month visit. Missingness was similar between groups at 4 and 9 months, however, it is significantly higher for the CBT-T group at the 15-month visit (13% vs 30% for ISI and SF-36, Chi-Square=6.32, df=1, p=0.01; 15% vs 31% for PCT-D, Chi-Square=4.99, df=1, p=0.03).
The modelling results are shown in Table 2 and Figure 2. Using the Benjamini and Hochberg multiple comparisons procedure to control the false discovery rate at the 0.05 level resulted in declaring 4-month intervention differences significant for the ISI and both SF-36 domains, but not the PCT-D. In summary, symptoms of insomnia (ISI) declined on average among participants in both interventions at the 4-month visit, but participants in the CBT-T intervention experienced significantly greater improvement (F=10.21; df=1,136; nominal p=0.002). Similarly, on average, all participants reported improvements in HRQL (SF-36) at 4 months on both the PCS and the MCS. Further, participants in the CBT-T intervention experienced significantly greater improvements on both subscales (PCS: F=5.43; df=1,136; nominal p=0.021; MCS: F=5.58; df=1,136; nominal p=0.020). Although participants in both interventions reported, on average, declines in physical disability symptoms (PCT-D) from baseline to 4 month follow-up, there was no differential effect by intervention (F=2.60; df=1,134; nominal p=0.109). Scores on the individual subscales of the SF-36 are presented in the supplement (Data, Supplemental Digital Content 1 and 2). In order to estimate the clinical significance of these findings, we computed the standardized effects (ES) of treatment on the outcomes. The differential effects of CBT-T and NST-T on sleep (ES = 0.27) and HRQL (PCS component ES = 0.20; MCS component ES = 0.20) were moderate in size. The between-group estimates and confidence intervals for the ISI scores at 9 and 15 months post-intervention are also shown in Table 2 and Figure 2. They are consistent with that observed at the immediate (4-month) post-intervention assessment. There is some shrinkage of the between-group differences at the 9- and 15-month follow-up assessments for both the PCS and the MCS. Nine and 15-month follow-up findings for the PCT-D were similar to the immediate post-intervention findings.
Table 2.
Summary of Secondary Results through All Follow-Up
| Outcome | Month | CBT | NST | Difference in Means Between Groups (95% CI) |
P value for Intervention Effect* |
||
|---|---|---|---|---|---|---|---|
| Mean (95% CI) |
Mean Change from Baseline (95% CI) |
Mean (95% CI) |
Mean Change from Baseline (95% CI) |
||||
| ISI | 4 | 8.70 (7.10, 10.3) | −6.69 (−8.16, −5.23) | 11.9 (10.4, 13.4) |
−3.48 (−4.87, −2.09) | 3.21 (1.23, 5.20) | 0.002 |
| 9 | 8.60 (7.01, 10.2) | −6.79 (−8.27, −5.32) | 12.4 (10.9, 14.0) |
−2.94 (−4.34, −1.55) | 3.85 (1.86, 5.84) | ||
| 15 | 8.00 (6.30, 9.70) | −7.39 (−9.04, −5.73) | 12.8 (11.2, 14.4) |
−2.59 (−4.11, −1.07) | 4.80 (2.62, 6.98) | ||
| SF36 – MCS | 4 | 60.5 (55.9, 65.1) | 20.29 (15.99, 24.60) |
53.5 (49.1, 57.9) |
13.27 (9.19, 17.35) |
−7.03 (−12.91, 1.15) |
0.020 |
| 9 | 60.6 (55.2, 65.9) | 20.38 (15.24, 25.51) |
56.4 (51.4, 61.5) |
16.25 (11.40, 21.09) |
−4.13 (−11.14, 2.88) |
||
| 15 | 60.3 (54.7, 65.8) | 20.08 (14.74, 25.41) |
54.4 (49.3, 59.4) |
14.17 (9.32, 19.01) |
−5.91 (−13.06, 1.24) |
||
| SF36 – PCS | 4 | 55.0 (50.1, 60.0) | 12.53 (8.38, 16.67) |
48.3 (43.5, 53.1) |
5.82 (1.89, 9.75) | −6.71 (−12.40, − 1.02) |
0.021 |
| 9 | 54.2 (48.8, 59.6) | 11.67 (7.04, 16.31) |
49.0 (43.9, 54.1) |
6.50 (2.13, 10.87) | −5.17 (−11.51, 1.17) |
||
| 15 | 54.7 (49.2, 60.3) | 12.22 (7.29, 17.16) |
49.8 (44.6, 55.0) |
7.29 (2.82, 11.75) | −4.94 (−11.56, 1.68) |
||
| PCT-D | 4 | 1.71 (1.55, 1.87) | −0.30 (−0.44, −0.17) | 1.86 (1.70, 2.01) |
−0.16 (−0.29, −0.03) | 0.15 (−0.03, 0.33) | 0.109 |
| 9 | 1.82 (1.63, 2.02) | −0.19 (−0.36, −0.02) | 1.96 (1.77, 2.15) |
−0.05 (−0.21, 0.11) | 0.14 (−0.10, 0.37) | ||
| 15 | 1.83 (1.65, 2.01) | −0.18 (−0.33, −0.03) | 1.87 (1.70, 2.04) |
−0.14 (−0.28, 0.00) | 0.04 (−0.17, 0.25) | ||
P-values are based on F tests from constrained mixed-model repeated measures analysis of covariance, adjusting for therapist, baseline presence of depressive disorder, and baseline use of psychotropic medications. DF=136 for ISI and SF-36 scales, and DF=134 for PCT-D.
Note: ISI = Insomnia Severity Index; PCT-D = Pepper Center Tool for Disability; SF-36-MCS = SF-36 Mental Component Score; SF-36-PCS = SF-36 Physical Component Score.
Figure 2.
Least squares means and 95% confidence intervals obtained from constrained mixed models.
Note: CBT = Cognitive-behavior therapy; ISI = Insomnia Severity Index; MCS = Mental Component Summary; NST-Nondirective supportive therapy; PCS = Physical Component Summary; PCT-D = Pepper Center Tool for Disability.
Discussion
We examined the effects of telephone-delivered CBT and NST on sleep, HRQL, and physical disability among rural elders with GAD. While we found that both treatments were associated with improvements in each of these domains at the 4-month follow-up, participants who received CBT-T demonstrated significantly greater declines in insomnia and improvement in HRQL. Thus, this study provides further support that the impact of CBT-T for the treatment of late-life GAD extends beyond mood symptoms.
Sleep is a common complaint among anxious older adults (6), and reports of insomnia at baseline were high in the current sample. There was a differential effect favoring CBT-T for reducing symptoms of insomnia. This difference may be due to the inclusion of one CBT-T session specifically focused on sleep. We also found CBT-T had a significantly greater impact on worry and depressive symptoms (14) which may account for sleep improvements. One meta-analysis of GAD treatment studies found that CBT for GAD had a large effect on sleep (9); however, the included trials did not include studies of late-life GAD. In fact, few studies of older adults with GAD have reported on sleep as an outcome of psychotherapy treatment trials. Two studies that have included it found that CBT was superior to enhanced usual care for improving sleep (7–8). Insomnia in older adults has been associated with a number of negative health outcomes, including greater risk of falls (25), poorer physical functioning (26), and increased risk of developing depression (27). Thus, improvements in insomnia may have other health benefits.
HRQL was also significantly improved, with a greater improvement experienced by participants who received CBT-T. Examination of subscale scores showed improvement in both physical and emotional domains of HRQL. Other studies of late-life GAD have found improvements in HRQL, generally with respect to the MCS (28–29) or other mental component subscales (30–31). To our knowledge, this study is the first to report significant improvements in the PCS of HRQL with treatment of late-life GAD. The PCS includes general health perceptions, pain, physical functioning, and role limitations due to physical health problems. As part of the CBT-T intervention, some participants received an optional session on pain if they reported pain at baseline or during the course of the intervention. Further, CBT-T encouraged participants to increase pleasant activities and face feared situations, both of which could have resulted in improved scores on physical functioning and role limitations due to physical health problems. In spite of significant improvements in HRQL, post-intervention scores on the SF-36 subscales were closer to scores of older adults with GAD than scores of healthy older adults without GAD (10). This suggests that even with treatment, HRQL does not improve to the levels of those without GAD.
There is a dearth of information on the impact of late-life GAD on physical disability, as most studies focus on work and social disabilities. Hendriks and colleagues (13) found that GAD was associated with significant limitations in mobility, self-care, life activities and responsibilities, and participating in community events; however, their sample was limited to adults under the age of 65 years. Porensky and colleagues (10) found that older adults with GAD report increased physical disability compared with older adults without GAD. In the current study, while we found that participants in both interventions demonstrated improvement from baseline, there were no differential effects of the interventions on physical disability. This may be due to the high level of functioning of participants at baseline, leaving little room for improvement. Inclusion of age-appropriate measures of functioning, including physical disability, in studies of late-life GAD is needed.
In addition to the immediate effect of the interventions, we also examined the effects 6 months and 1 year after completion of the intervention. We found that the effects of CBT-T on insomnia were maintained through the 1-year follow-up. However, improvements in HRQL diminished from baseline to 1 year follow-up by 17% for the MCS component and almost 30% for the PCS component. Thus, CBT-T produced a sustained reduction in insomnia, and a partially sustained improvement in HRQL at 1 year post intervention.
The findings of this study must be interpreted in light of the following limitations. The sample consisted of predominantly well-educated, white women, limiting generalizability. Further, measures of insomnia are self-reported and do not include any objective measures. Nonetheless, these findings suggest that CBT-T is superior to NST-T for reducing insomnia and improving HRQL in rural-dwelling older adults with GAD. Further, the effects on insomnia are maintained up to 1 year after completing treatment.
Supplementary Material
Acknowledgments
Sources of Support: R01 MH083664 from the National Institute of Mental Health (NIMH)
This research is funded by grant number R01 MH083664 from the NIMH. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No disclosures to report
Supplemental Digital Content 1. doc
Supplemental Digital Content 2. Doc
Contributor Information
Gretchen A. Brenes, Department of Psychiatry and Behavioral Medicine, Wake Forest School of Medicine
Suzanne C. Danhauer, Department of Social Sciences and Health Policy, Wake Forest School of Medicine
Mary F. Lyles, Department of Internal Medicine-Geriatrics, Wake Forest School of Medicine
Andrea Anderson, Department of Biostatistics, Wake Forest School of Medicine
Michael E. Miller, Department of Biostatistics, Wake Forest School of Medicine
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 2000. text rev. [Google Scholar]
- 2.Beekman AT, Bremmer MA, Deeg DJ, et al. Anxiety disorders in later life: a report from the Longitudinal Aging Study Amsterdam. Int J Geriatr Psychiatry. 1998;13:717–726. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Butnoriene J, Bunevicius A, Saudargiene A, et al. Metabolic syndrome, major depression, generalized anxiety disorder, and ten-year all-cause and cardiovascular mortality in middle aged and elderly patients. Int J Cardiol. 2015;190:360–366. doi: 10.1016/j.ijcard.2015.04.122. [DOI] [PubMed] [Google Scholar]
- 4.Martens EJ, deJonge P, Na B, et al. Scared to death? Generalized anxiety disorder and cardiovascular events in patients with stable coronary heart disease: The Heart and Soul Study. Arch Gen Psychiatry. 2010;67:750–758. doi: 10.1001/archgenpsychiatry.2010.74. [DOI] [PubMed] [Google Scholar]
- 5.Phillips AC, Batty GAD, Gale CR, et al. Generalized anxiety disorder, major depressive disorder, and their comorbidity as predictors of all-cause and cardiovascular mortality: the Vietnam experience study. Psychosom Med. 2009;71:395–403. doi: 10.1097/PSY.0b013e31819e6706. [DOI] [PubMed] [Google Scholar]
- 6.Brenes GA, Miller ME, Stanley MA, et al. Insomnia in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry. 2009;17:465–472. doi: 10.1097/jgp.0b013e3181987747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenes GA, Miller ME, Williamson JD, et al. A randomized controlled trial of telephone-delivered cognitive-behavioral therapy for late-life anxiety disorders. Am J Geriatr Psychiatry. 2012;20:707–716. doi: 10.1097/JGP.0b013e31822ccd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bush AL, Armento ME, Weiss BJ, et al. The Pittsburgh Sleep Quality Index in older primary care patients with generalized anxiety disorder: psychometrics and outcomes following cognitive behavioral therapy. Psych Res. 2012;199:24–30. doi: 10.1016/j.psychres.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belleville G, Cousineau H, Levrier K, et al. The impact of cognitive-behavior therapy for anxiety disorders on concomitant sleep disturbances: A meta-analysis. J Anx Disord. 2010;24:379–386. doi: 10.1016/j.janxdis.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Porensky EK, Dew MA, Karp JF, et al. The burden of late life generalized anxiety disorder: effects on disability, health-related quality of life, and healthcare utilization. Am J Geriatr Psychiatry. 2009;17:473–482. doi: 10.1097/jgp.0b013e31819b87b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackenzie CS, Reynolds K, Chou KL, et al. Prevalence and correlates of generalized anxiety disorder in a national sample of older adults. Am J Geriatr Psychiatry. 2011;19:305–315. doi: 10.1097/JGP.0b013e318202bc62. [DOI] [PubMed] [Google Scholar]
- 12.Comer JS, Blanco C, Hasin DS, et al. Health-related quality of life across the anxiety disorders: results from the national epidemiologic survey on alcohol and related conditions (NERSARC) J Clin Psychiatry. 2011;72:45–50. doi: 10.4088/JCP.09m05094blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendriks SM, Licht CM, Spijker J, et al. Disorder-specific cognitive profiles in major depressive disorder and generalized anxiety disorder. BMC Psychiatry. 2014;14:96. doi: 10.1186/1471-244X-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenes GA, Danhauer SC, Lyles MF, et al. Telephone-delivered CBT and telephone-delivered nondirective supportive therapy for rural older adults with GAD: A randomized clinical trial. JAMA Psychiatry. 2015;72:1012–1020. doi: 10.1001/jamapsychiatry.2015.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenes GA, Danhauer SC, Lyles MF, et al. Telephone-delivered psychotherapy for rural-dwelling older adults with generalized anxiety disorder: study protocol of a randomized controlled trial. BMC Psychiatry. 2014;14:34. doi: 10.1186/1471-244X-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30:47383. [PubMed] [Google Scholar]
- 18.Ip EH, Rejeski WJ, Marsh AP, et al. Measuring disability in older adults: The ICF framework. Geriatr Gerontol Int. 2008;8:48–54. doi: 10.1111/j.1447-0594.2008.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rejeski WJ, Ettinger WH, Jr, Shumaker S, et al. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthritis Cartilage. 1995;3:157–167. doi: 10.1016/s1063-4584(05)80050-0. [DOI] [PubMed] [Google Scholar]
- 20.Borkovec TD, Costello E. Efficacy of applied relaxation and cognitive-behavioral therapy in the treatment of generalized anxiety disorder. J Consult Clin Psychol. 1993;61:611–619. doi: 10.1037//0022-006x.61.4.611. [DOI] [PubMed] [Google Scholar]
- 21.Borkovec TD, Newman MG, Pincus AL, et al. A component analysis of cognitive-behavioral therapy for generalized anxiety disorder and the role of interpersonal problems. J Consult Clin Psychol. 2002;70:288–298. [PubMed] [Google Scholar]
- 22.Liang KY, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post design. Sankha: Indian J Stat (Series B) 2000;62:134–148. [Google Scholar]
- 23.Lu K. On efficiency of constrained longitudinal data analysis versus longitudinal analysis of covariance. Biometrics. 2012;66:891–896. doi: 10.1111/j.1541-0420.2009.01332.x. [DOI] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and power approach to multiple testing. J.R Statist. Coc B. 1995;57:289–300. [Google Scholar]
- 25.Stone KL, Ancoli-Israel S, Blackwell T, et al. Poor sleep is associated with increased risk of falls in older women. Arch Intern Med. 2008;168:1768–1775. doi: 10.1001/archinte.168.16.1768. [DOI] [PubMed] [Google Scholar]
- 26.Dam TL, Ewing SK, Ancoli-Israel, et al. Association between sleep and physical function in older men: The Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2008;56:1665–1673. doi: 10.1111/j.1532-5415.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Mendoza J, Shea S, Vgontzas AN, et al. Insomnia and incident depression: Role of objective sleep duration and natural history. J Sleep Res. 2015:24390–24398. doi: 10.1111/jsr.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanley MA, Wilson NL, Amspoker AB, et al. Lay providers can deliver effective cognitive behavior therapy for older adults with Generalized Anxiety Disorder: a randomized trial. Depress Anxiety. 2014;31:391–401. doi: 10.1002/da.22239. [DOI] [PubMed] [Google Scholar]
- 29.Stanley MA, Wilson N, Novy DM, et al. Cognitive behavior therapy for Generalized Anxiety Disorder; a randomized clinical trial. JAMA. 2009;301:1460–1467. doi: 10.1001/jama.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanley MA, Beck JG, Novy DM, et al. Cognitive-behavioral treatment of late-life generalized anxiety disorder. J Consult Clin Psychol. 2003;71:309–319. doi: 10.1037/0022-006x.71.2.309. [DOI] [PubMed] [Google Scholar]
- 31.Wetherell JL, Gatz M, Craske MG. Treatment of generalized anxiety disorder in older adults. J Consult Clin Psychol. 2003;71:31–40. doi: 10.1037//0022-006x.71.1.31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.