Abstract
AIM
To investigate the prognostic effect of a delayed interval between neoadjuvant chemoradiotherapy (CRT) and surgery in locally advanced rectal cancer.
METHODS
We evaluated 87 patients with locally advanced mid- or distal rectal cancer undergoing total mesorectal excision following an interval period after neoadjuvant CRT at Şişli Hamidiye Etfal Training and Research Hospital, Istanbul between January 2009 and January 2014. Patients were divided into two groups according to the interval before surgery: < 8 wk (group I) and ≥ 8 wk (group II). Data related to patients, cancer characteristics and pathological examination were collected and analyzed.
RESULTS
When the distribution of timing between group I (n = 45) and group II (n = 42) was viewed, comparison of interval periods (median ± SD) of groups showed a significant difference of as 5 ± 1.28 wk in group I and 10.1 ± 2.2 wk in group II (P < 0.001). The median follow-up period for all patients was 34.5 (9.9-81) mo. group II had significantly higher rates of pathological complete response (pCR) than group I had (19% vs 8.9%, P = 0.002). Rate of tumor regression grade (TRG) poor response was 44.4% in group I and 9.5% in group II (P < 0.002). A poor pathological response was associated with worse disease-free survival (P = 0.009). The interval time did not show any association with local recurrence (P = 0.79).
CONCLUSION
Delaying the neoadjuvant CRT-surgery interval may provide nodal down-staging, improve pCR rate, and decrease the rate of TRG poor response.
Keywords: Rectal carcinoma, Pathological tumor response, Neoadjuvant chemoradiotherapy, Interval timing, Tumor down-staging
Core tip: Delaying the neoadjuvant chemoradiotherapy (CRT)-surgery interval for treatment of locally advanced rectal carcinoma may improve pathological complete response rates by providing nodal down-staging, as well as decreasing the rate of tumor regression grade (TRG) poor response. TRG may be an important predictive factor for disease-free survival. Extending the interval between CRT and surgery may improve the survival through tumor down-staging without increasing the rate of surgical complications.
INTRODUCTION
Locally advanced distal and mid-rectal tumors are commonly treated with preoperative combined chemoradiotherapy (CRT) followed by total mesorectal excision (TME)[1-3]. Previously conducted studies have recommended a treatment interval time between preoperative neoadjuvant CRT and surgery for the treatment of locally advanced rectal cancer[4,5]. The first prospective trial (The Lyon Trial R90-01), which assigned patients randomly to have surgery at two different time intervals following CRT, was conducted in 1999. That trial showed that a 6-8-wk treatment interval between radiotherapy and surgery improved tumor down-staging and yielded a higher pathological response rate compared with a 2-wk interval. Since then, a 6-8-wk interval has been accepted as the appropriate treatment interval between neoadjuvant therapy and surgery[6]. However, a definite definition for an optimum interval period is still lacking in the medical literature. In addition, even though the known effect of an extended interval on pathological complete response (pCR), the impact of pCR on disease-free survival (DFS) and overall survival (OS) has not been clearly described[7].
The aim of this study was to determine whether the interval time between preoperative neoadjuvant CRT and surgery affected the rates of pCR, perioperative surgical complications, sphincter-saving surgery, DFS and OS in locally advanced mid-or distal rectal cancer.
MATERIALS AND METHODS
Patients
This was a retrospective review of a series of 113 consecutive patients who underwent preoperative neoadjuvant CRT followed by radical resection with TME for curative intent of locally advanced mid- or distal rectal cancer between January 2009 and January 2014 at Şişli Hamidiye Etfal Training and Research Hospital, General Surgery and Oncology Departments. The study was approved by the local ethics committee. Written informed consent was obtained from all study participants, or their legal guardian for being included in the study. All patients included in the study (1) were aged ≥ 18 years; (2) had pathological diagnosis of adenocarcinoma of mid-rectal (located between 5 and 10 cm from the anal verge) and distal rectal (situated in the first 5 cm from the anal verge, excluding the anal canal) tumors by endoscopic biopsy; (3) had tumors with T3/T4 stage or N (+) as demonstrated on pelvic phased-array magnetic resonance imaging; and (4) underwent TME after neoadjuvant CRT. Study parameters including interval period between neoadjuvant CRT and surgery, operation time and type, intraoperative and early postoperative morbidity and mortality, and hospital stay were recorded. Reports from pathological examinations were interrogated to extract data on total and metastatic lymph node numbers and surgical margins. Data on local recurrence, organ metastases that occurred during postoperative follow-up period, DFS and OS rates were also recorded. Postoperative anastomotic complications were defined according to severity grading of anastomotic leakage of the International Study Group of Rectal Cancer[8]. Twenty-six patients, including those who had widespread metastasis at the time of diagnosis (n = 3), patients who underwent short-term radiotherapy (5 × 5 Gy) (n = 7), individuals with synchronized tumors or had treatment due to other malignancies with rectal cancer as secondary (n = 5), patients who could not tolerate neoadjuvant chemotherapy (n = 3), and patients who were lost to follow-up (n = 8) were excluded. The remaining 87 patients constituted the study population. The study patients were divided into two groups according to the interval time between neoadjuvant CRT and surgery: < 8 wk (group I) and ≥ 8 wk (group II).
In all patients, tumor localization and pathological diagnosis were made by using procto-sigmoidoscopy and endoscopic biopsy, respectively. Systemic staging was performed using thoracic and abdominal computed tomography (CT), while local staging was performed using phased-array magnetic resonance imaging. Preoperative neoadjuvant CRT was given for a total of 5 wk and it included 45-50.4 Gy radiotherapy (5 × 1.8-2.0 Gy/wk) and concomitant 5-fluorouracil (180 mg/m2 per day) for 5 d/wk. Surgery was performed in all patients at the earliest interval of 4 wk after neoadjuvant chemotherapy. The interval between neoadjuvant therapy and surgery varied according to logistics and scheduling preferences of the attending surgeon.
Postoperative follow-up protocol was performed every 3-4 mo during the first year, once every 6 mo during the second year, and once every year after the second year. During follow-up, local recurrences were determined using thoracoabdominal CT, positron emission tomography-CT or colonoscopy.
Pathological examination
Histopathological examination of the resected specimens including the mesorectum was performed to identify as many lymph nodes as possible. Evaluation of tumor regression grade (TRG) after CRT in the primary tumor of the rectal wall was performed by experienced pathologists according to the Ryan scheme for tumor regression score[9], which was suggested by the College of American Pathologists protocol. The absence of viable cancer cells or acellular pools of mucin in resected specimens were considered as complete response (TRG 1). Single cells or microscopic foci of cancer cells in samples were assessed as near complete response (TRG 2). Residual cancer outgrown by fibrosis was considered as the minimal response (TRG 3). Minimal or no tumor kill, or extensive residual cancer in specimens were found as a poor response (TRG 4). T stage 0 was considered as complete tumor response in the rectal wall, while near complete, minimal or inadequate responses were examined at any T stage. In the present study, tumoral down-staging or pathological tumor response was expressed by TNM classification, according to American Joint Committee on Cancer, and pCR was defined as T stage 0 in the rectal wall without metastatic lymph node in the mesorectum.
Statistical analysis
Data were analyzed using SPSS for Windows, version 17.0 (SPSS, Chicago IL, United States) by the official biostatistician of the hospital. Continuous variables are represented as mean ± SD and categorical variables as numbers and percentages. Intergroup analyses were performed using Students’ t test and Mann-Whitney U test for continuous variables and χ2 test and Fisher’s exact test for categorical variables. The Spearman correlation test was used to investigate the relationship between continuous variables and intervals between CRT and surgery. Paired t and Wilcoxon tests were used to compare dependent groups. Oncological outcomes of patients were classified as 2-year and 5-year DFS and OS. The Kaplan-Meier test, log-rank test, and Cox regression analyses were used to determine the relationship between potential risk factors and DFS and OS. OS was defined as the period between diagnosis of the disease until death that occurred as a result of the disease. DFS was defined as the time between diagnosis of the disease until local recurrence or far-organ metastasis. Patients who died from other causes or died within the early postoperative period were censored. Results were evaluated between 95%CIs, and the level of statistical significance was set at P < 0.05.
RESULTS
A total of 87 patients who had locally advanced mid- or distal rectal cancer underwent surgical resection with TME after neoadjuvant CRT. Of these 45 (group I) had a treatment interval < 8 wk and 42 (group II) had an interval ≥ 8 wk. Patient demographics and clinical characteristics were comparable in both groups (Table 1). When the distribution of timing in both groups was viewed, comparison of interval periods (median ± SD) between the groups showed a significant difference; as 5 ± 1.28 (2-7.8) wk in group I and 10.1 ± 2.2 (8.2-20.2) wk in group II (P < 0.001) (Figure 1).
Table 1.
Demographic and clinical characteristics of patients
| Group I (n = 45) | Group II (n = 42) | P value | |
| Age (mean ± SD) | 53.7 ± 13.4 | 58 ± 13.2 | 0.82 |
| Sex (male/female) | 32/13 | 31/11 | 0.62 |
| Localization of tumor from the anal verge (cm) (mean ± SD) | 5.6 ± 3 | 6.1 ± 2.8 | 0.39 |
| T stage (2-4) | 8/28/9 | 6/32/4 | 0.56 |
| Stage (II/III) | 4/41 | 5/37 | 0.53 |
| Preoperative radiation dose (Gy) (mean) | 49.5 ± 1.99 | 49.5 ± 2 | 0.78 |
| Follow-up time (mo) (mean ± SD) | 37.2 ± 19.6 | 31.1 ± 20.7 | 0.51 |
Figure 1.
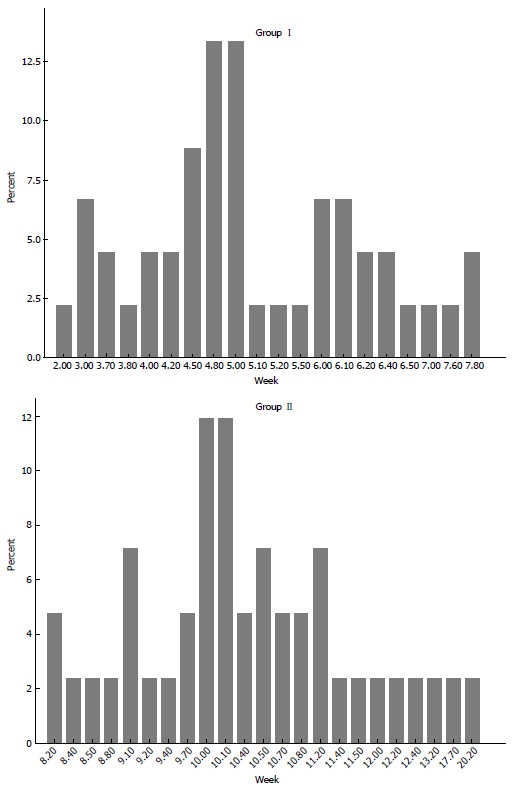
Distributions of groups with regard to interval time between neoadjuvant therapy and surgery. Median interval periods ± SD were 5 ± 1.28 (2-7.8) wk in group I and 10.1 ± 2.2 (8.2-20.2) wk in group II (P < 0.001).
Effect of interval period on preoperative variables
Statistical analysis did not show any correlation between treatment interval and respective preoperative and postoperative variables (operation time and type, diverting ileostomy dehiscence rate, intraoperative and postoperative complication rate, hospital stay and early postoperative mortality rate) (Table 2). Despite all patients being informed about surgical approach and necessity of diverting stoma, six had not given consent to have a diverting loop stoma. Therefore, 42 patients (93.3%) in group I and 39 patients (92.9%) in group II were diverted at the time of TME.
Table 2.
Effect of interval time on the perioperative variables n (%)
| Group I | Group II | P value | |
| (n = 45) | (n = 42) | ||
| Procedure type | |||
| LAR | 28 (62.2) | 31 (73.8) | 0.06 |
| ULAR | 11 (24.4) | 7 (16.7) | 0.09 |
| APR | 6 (13.3) | 4 (9.5) | 0.50 |
| Diverting ileostomy | 42 (93.3) | 39 (92.9) | 0.90 |
| Operative time (min) (mean ± SD) | 134.2 ± 19.9 | 133.4 ± 23.5 | 0.62 |
| Intraoperative complications | 8 (8.9) | 3 (7.1) | 0.48 |
| Postoperative complications | 13 (28.9) | 11 (26.1) | 0.42 |
| Early postoperative mortality | 1 (2.3) | 2 (4.7) | 0.37 |
| Hospital stay (d) (mean ± SD) | 11 ± 10.5 | 10 ± 9.3 | 0.32 |
APR: Abdominoperineal resection; LAR: Low anterior resection; ULAR: Ultralow anterior resection.
For patients who had distally located tumors, sphincter-saving surgery was performed in 17 (64.7%) in group I and 11 (63.6%) in group II (P = 0.86). Intraoperative complications occurred in four patients from group I, left ureter injuries in two patients (4.4%), and presacral significant bleeding in the other two (4.4%). In group II, there was right ureter injuries in two patients (4.7%) and bladder injury in one patient (2.3%) (P = 0.48). During the postoperative period, complications occurred in 13 patients (28.9%) in group I and 11 patients (26.1%) in group II. Complications that occurred in group I were anastomotic leakage in five patients (11.2%) and wound infection in eight (17.8%). Surgical complications in both groups were comparable (P = 0.42).
Anastomotic complications were classified based on the severity grading of anastomotic complications of the International Study Group of Rectal Cancer. In group I, three cases with diverting stoma developed perianastomotic abscess in the pelvis. These were classified as Grade B anastomotic complications, and managed successfully with percutaneous abscess drainage and antibiotics. Two cases that were not diverted at the time of TME required reoperation for Grade C anastomotic leakages. Mortality occurred in 1 (2.3%) of the patients who had Grade C anastomotic leakage on postoperative day 18.
In group II, anastomotic leakage was observed in 8 patients (19%), wound infection in 3 (7.1%) and mortality in 2 (4.7%). Mortality was due to postoperative pulmonary emboli and myocardial infarction. Grade A anastomotic complications appeared in four patients with diverting ileostomy who were treated with antibiotics, without the need for invasive interventions or surgical procedures. Grade B anastomotic complications occurred in three cases, and one of them did not have a diverting stoma. These patients underwent percutaneous abscess drainage and were treated with antibiotics. One patient who was not diverted with a stoma at the time of TME developed Grade C anastomotic leakage, and surgery was performed in this case. There was no mortality associated with anastomotic leakage in group II patients. There were no significant differences between the groups in terms of anastomotic complications or early postoperative mortality (P = 0.07 and P = 0.37, respectively).
Effect of interval period on stage and pathological response
In both groups, there were no significant differences in terms of postoperative T stage (P = 0.17). However, histopathological examination of TME specimens showed different nodal complete response in both groups (46.7% vs 81%, P = 0.001) (Table 3). The pathological tumor down-staging was found to be related to a decreased number of metastatic lymph nodes in the mesorectum. Tumor down-staging rate was 57.4%, as the pCR [Stage 0 (T0N0)] rate was 13.8%. In group I, tumor down-staging occurred in 22 patients (48.9%), while no down-staging was obtained in 23 patients (51.1%); of whom 22 patients had Stage 3 disease and the remaining one had Stage 2 disease. The pCR rate in group I was 8.9%. In group II, 33 patients (78.5%) developed tumor down-staging and nine (21.4%) showed no down-staging. Eight of the patients who showed no down-staging had Stage 3 disease and the other one had Stage 2 disease. The pCR rate in group II was 19%. A significant decrease in postoperative stage was seen in patients who had a longer interval period. Patients in group II had significantly higher rates of pCR than their counterparts in group I (19% vs 8.9%, P = 0.002).
Table 3.
Comparison of pre- and post-treatment stages in both groups n (%)
|
Group I (n = 45) |
Group II (n = 42) |
Comparison of groups I and II | |||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | P value | |
| T stage | 0.17 | ||||
| T0 | - | 9 (18.9) | - | 8 (19) | |
| T1 | - | 2 (4.4) | - | 2 (4.8) | |
| T2 | 8 (17.8) | 14 (31.7) | 6 (14.3) | 14 (33.4) | |
| T3 | 28 (62.2) | 19 (42.8) | 32 (76.2) | 16 (38.1) | |
| T4 | 9 (20) | 1 (2.2) | 4 (9.5) | 2 (4.8) | |
| Stage | 0.002 | ||||
| Stage 0 | - | 4 (8.9) | - | 8 (19) | |
| Stage 1 | - | 11 (24.4) | - | 15 (35.7) | |
| Stage 2 | 4 (8.9) | 8 (17.8) | 2 (11.9) | 11 (26.2) | |
| Stage 3 | 41 (91.1) | 22 (48.9) | 37 (88.1) | 8 (19) | |
| Postop LN without metastasis | 21 (46.7) | 34 (81) | 0.001 | ||
Significant predictors of pathological response such as age, gender, tumor localization, preoperative stage, preoperative T stage, and interval time were investigated by univariate and multivariate analyses. Except for pathological TRG and interval time, no positive correlation was noted between other predictive factors and pathological response. However, while rate of poor response TRG (TRG-4) was found to be 44.4% in group I, it was 9.5% in group II (P < 0.002). Although, an extended interval between CRT and surgery was found to increase rates of complete or near-complete TRG response, this rate was not significant when both groups were compared (Table 4).
Table 4.
Analysis of the effect of factors on pathological tumor regression grade
| TRG |
Relationship between demographics and TRG (p) and OR with 95%CI |
Distribution and comparison of TRG rates in both groups |
||||||
| Age | Sex | Tumor localization | Preop T stage | Preop stage | Group I (n = 45) | Group II (n = 42) | P value | |
| % | % | |||||||
| Complete response | (0.46) | (0.84) | (0.17) | (0.24) | (0.48) | (n = 4) | (n = 8) | 0.36 |
| 2.19, 95%CI: 0.55-8.72 | 0.54, 95%CI: 0.12-2.29 | 0.66, 95%CI: 0.15-2.92 | 1.00, 95%CI: 0.90-1.50 | 1.21, 95%CI: 0.12-11.8 | 8.9 | 19 | ||
| Near complete response | (0.91) | (0.79) | (0.38) | (0.75) | (0.80) | (n = 9) | (n = 14) | 0.35 |
| 1.02, 95%CI: 0.37-2.79 | 1.11, 95%CI: 0.36-3.38 | 1.93, 95%CI: 0.64-5.8 | 0.50, 95%CI: 0.12-2.57 | 0.36, 95%CI: 0.10-1.51 | 20 | 33.3 | ||
| Minimal response | (0.79) | (0.59) | (0.12) | (0.66) | (0.38) | (n = 12) | (n = 16) | 0.15 |
| 0.67, 95%CI: 0.25-1.76 | 1.25, 95%CI: 0.43-3.63 | 0.45, 95%CI: 0.16-1.20 | 2.25, 95%CI: 0.36-13.8 | 2.02, 95%CI: 0.37-10.9 | 26.7 | 38.1 | ||
| Poor response | (0.48) | (0.95) | (0.11) | (0.19) | (0.70) | (n = 20) | (n = 4) | 0.002 |
| 0.98, 95%CI: 0.35-2.76 | 0.94, 95%CI: 0.31-2.82 | 1.65, 95%CI: 0.56-4.84 | 3.22, 95%CI: 0.92-11.2 | 1.34, 95%CI: 0.23-7.62 | 44.4 | 9.5 | ||
P < 0.05 is statistical significance. OR: Odds ratio; TRG: Tumor regression grade.
A total of 60 patients who were diagnosed with Stage 2 or 3 disease after histopathological examination of TME specimens, including 30 patients in each group, were recommended for postoperative adjuvant therapy. However, 57 patients eventually received adjuvant therapy after surgery due to early postoperative mortality in three patients.
Factors predicting local recurrence, DFS and OS
The median follow-up period for all patients was 34.5 (9.9-81) mo. Median follow-up time for group I was 37.5 (9.9-74.5) mo and group II was 31.2 (10.7-81) mo. Median follow-up was comparable in both groups (P = 0.59).
Analysis of OS in group I showed median survival duration of 62.8 (95%CI: 55.8-69.7) mo, and a 24-mo survival rate of 91.5%, and 60-mo survival rate of 79.1%. For group II, the median OS was 77.9 (95%CI: 72-81) mo. Twenty-four-month survival rate was 100%, and 60-mo survival rate was 94.4%. OS showed a significant difference when both groups were compared (P = 0.02). The median DFS duration in group I was 50.8 (95%CI: 43.4-58.2) mo. DFS rates were 76.4% at 24 mo and 55.3% at 60 mo. For group II, median DFS was 71.2 (95%CI: 63.1-79.2) mo. DFS rate was 85.1% at 24 mo and remained unchanged at 85.1% until 60 mo in group II. DFS rates differed significantly when both groups were compared (P = 0.01) (Figure 2).
Figure 2.
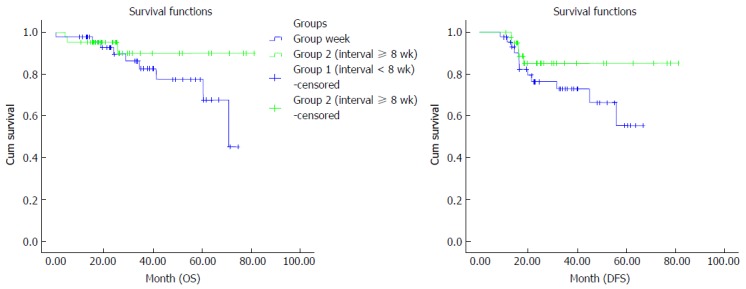
Comparison of overall survival and disease-free survival between the groups by Kaplan–Meier curves. The median DFS duration in group II was better than in group I (P = 0.01). DFS: Disease-free survival; OS: Overall survival.
When potential factors affecting OS and DFS were analyzed, nodal down-staging was found to have a positive correlation with OS and DFS (Table 5). OS and DFS were better in patients who achieved nodal down-staging (OS: 78% vs 52.1%, P = 0.001; DFS: 72.3% vs 43.1%, P = 0.001) (Figure 3).
Table 5.
Effect of factors on overall survival and disease-free survival
|
OS |
DFS |
|||
| P value | HR with 95%CI | P value | HR with 95%CI | |
| Sex | 0.61 | 0.97, 95%CI: 0.19-4.99 | 0.69 | 0.50, 95%CI: 0.46-4.46 |
| Age | 0.57 | 1.01, 95%CI: 0.95-1.08 | 0.60 | 1.00, 95%CI: 0.94-1.06 |
| Tumor localization | 0.53 | 0.97, 95%CI: 0.72-1.30 | 0.88 | 1.17, 95%CI: 0.80-1.70 |
| Pre-treatment stage | 0.94 | 0.77, 95%CI: 0.80-7.50 | 0.45 | 0.90, 95%CI: 0.80-1.50 |
| Pre-treatment T stage | 0.59 | 1.08, 95%CI: 0.21-5.48 | 0.39 | 0.39, 95%CI: 0.15-3.02 |
| Post-treatment stage | 0.01 | 18.07, 95%CI: 0.60-53.9 | 0.007 | 0.82, 95%CI: 0.10-6.23 |
| Post-treatment T stage | 0.13 | 0.62, 95%CI: 0.34-11.3 | 0.07 | 0.25, 95%CI: 0.19-8.54 |
| Postoperative metastatic lymph node (+) | 0.001 | 0.91, 95%CI: 0.69-1.20 | 0.001 | 1.25, 95%CI: 0.93-1.67 |
| Pathologic TRG | 0.11 | 0.90, 95%CI: 1.28-6.35 | 0.04 | 1.19, 95%CI: 0.17-8.41 |
DFS: Disease-free survival; OS: Overall survival; TRG: Tumor regression grade.
Figure 3.

Effect of presence of tumor in lymph nodes and its correlation with overall survival (A) and disease-free survival (B). Survival rates were better in patients who achieved nodal down-staging (P = 0.001). OS: Overall survival; DFS: Disease-free survival.
After investigating the correlation between survival rates and pathological TRG, lower and moderate pathological regression grades (TRG 1-3) provided similar survival benefit, but only a poor pathological response (TRG 4) was associated with worse DFS (P = 0.009). However, TRG scores did not show any association with OS and local recurrence (P = 0.06 and P = 0.39, respectively) (Figure 4).
Figure 4.

Correlation between level of the pathological tumor responses and disease-free survival. Only a poor pathologic response (TRG 4) was associated with worse DFS (P = 0.009). TRG: Tumor regression grade; DFS: Disease-free survival.
Local recurrence was found to be 8.9% after a mean duration of 71.1 ± 2.3 mo in group I. Group II had a local recurrence rate of 7.1% after a mean duration of 72.4 ± 4.6 mo. The interval time did not show any association with local recurrence (P = 0.79) (Figure 5).
Figure 5.
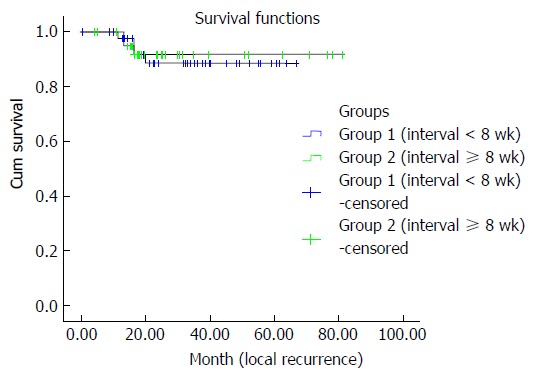
Local recurrences were similar in both interval groups. The interval duration did not show any association with local recurrence (P = 0.79).
DISCUSSION
Since the initial description of the TME technique for rectal cancer by Heald, TME has become the standard surgical treatment for mid- and distal rectal cancer[10-12]. Previous prospective studies have shown that surgery alone is not sufficient for local disease control, but its combination with preoperative CRT reduces local recurrence and increases DFS[3,13-19]. The Lyon R90-01 Trial was the first randomized prospective study to compare the effects of short and long intervals after neoadjuvant therapy on pathological tumor down-staging, and found that a 6-8-wk treatment interval between radiotherapy and surgery improved tumor down-staging and pCR. Since then, a 6-8-wk interval has been accepted as an appropriate interval between preoperative neoadjuvant therapy and surgery[6]. In contrast, there have been rising concerns among surgeons regarding radiation-induced pelvic fibrosis that may occur as a result of a longer waiting period. This is because fibrosis may cause operative difficulty and lead to an increased rate of surgical complications. Therefore, the issue of surgical timing has encouraged further studies to investigate the optimal interval time for surgical treatment in terms of oncological outcomes.
Several studies that have examined the effect of different intervals after neoadjuvant CRT on tumor response, pCR, local tumor control and survival have reported conflicting results (Table 6). Our study showed a higher rate of pCR among patients who had an interval > 8 wk. pCR was associated with a decreased number of metastatic lymph nodes in the mesorectum, which led to tumor down-staging. The only factor providing nodal down-staging was found to be interval period. In contrast, a shorter or longer interval time did not show any effect on T stage of the rectal wall. Specifically, a longer interval time was associated with a significant reduction in TRG 4 (poor response) rates. High TRG 4 rate (44%) in the shorter interval group in our study may be explained by performing surgery at 5 wk after neoadjuvant CRT in most patients.
Table 6.
Studies comparing the effects of the interval periods between neoadjuvant therapy and surgery on oncological outcome in locally advanced rectal cancer
| Ref. | Total no. of patients | Design | Interval time (wk) | pCR | Local recurrence | OS |
| Francois et al[6] | 201 | Prospective, randomized | 2/6-8 | 7%/14%2 | 13%/10%2 | 69%/66%2 |
| Wolthuis et al[20] | 356 | Retrospective | ≤ 7/> 7 | 16%/28%2 | 6%/3%2 | NA |
| Kalady et al[21] | 306 | Prospective | < 8/≥ 8 | 16.3%/28%2 | NA | NA |
| Garcia-Aguilar et al[22] | 136 | Prospective, nonrandomized | 6/11 | 18%/25%2 | NA | NA |
| de Campos-Lobato et al[23] | 177 | Retrospective | < 8/≥ 8 | 16.5%/30.8%2 | 1.2%/10.5%2 | NA |
| Tulchinsky et al[24] | 132 | Retrospective | ≤ 7/> 7 | 17%/35%2 | 6%/4%1 | 81%/93%2 |
| Sloothaak et al[25] | 1593 | Prospective | < 13/13-14/15-16 | 10%/13%/18%1 | NA | NA |
| Saglam et al[31] | 153 | Prospective, randomized | 4/8 | 19.7%/14.3%2 | 11.8%/10.3%2 | 76.5%/74.2%2 |
| Rödel et al[36] | 385 | Prospective | > 6 | 10.4% | 3% | 85% |
| Kerr et al[42] | 189 | Retrospective | Median 76 d | 15.9% | 21% | NA |
| (6-215 d) |
Significant difference statistically;
Not significant difference statistically. pCR: Pathological complete response; NA: Not available; OS: Overall survival.
It is questionable whether the poor tumor response also reduces DFS, while pCR is assumed as an indicator of improved DFS. Our findings established a negative correlation between TRG 4 (poor response) and DFS; an outcome that has not been mentioned in previous studies. Its clinical significance represents poor prognosis in terms of disease recurrence. Thus, the possibility of early recurrence of the disease should be considered in follow-up of patients who had TRG 4.
In a retrospective study by Wolthuis et al[20], patients who underwent surgery after a treatment interval > 7 wk showed better pathological tumor response. However, pathological response in this study was evaluated based on the final pathological T staging of the rectal wall. In the Lyon R90-01 Trial by Francois et al[6], despite the exclusion of preoperative chemotherapy, a longer interval time was again associated with better clinical tumor response. In another study, Kalady et al[21] reported a pCR of 31.8% among patients who waited for > 8 wk before surgery and 16% in patients who had an interval < 8 wk. The authors further documented that an extended interval between completion of neoadjuvant therapy and surgery was the single most important determinant of better tumor response regarding final T stage in rectal wall as well as lymph nodes. Garcia-Aguilar et al[22] in their prospective multicenter study investigated the effect of an extended interval between CRT and surgery on tumor response, CRT-related toxicity, and surgical complications. The authors also examined the impact of intense chemotherapy that was given during this interval period on pCR rates. They found that intense CRT in addition to increasing the time interval between neoadjuvant therapy and surgery may increase the pCR rate without significantly increasing CRT-related complications, operative difficulty or postoperative complications. Similarly, de Campos-Lobato et al[23] found an interval > 8 wk to be associated with a higher rate of pCR. In a recent study of predictive factors affecting pCR by Tulchinsky et al[24], neoadjuvant-surgery interval time was an independent predictive factor of tumor down-staging. In the largest and one of the most recent studies by the Dutch Surgical Colorectal Audit, a longer CRT–surgery interval of approximately 11 wk was related to the highest chance of pCR[25]. Also, meta-analyses of several studies confirmed that an interval > 8 wk before surgery resulted in more tumor regression[26,27].
In contrast, there have been studies reporting no correlation between duration of treatment interval and pCR. Findings from these studies have associated pCR with the longer period needed for a CRT response[5,19,28-32]. In a study by Pucciarelli et al[33], tumor response was associated with preoperative chemotherapy regimen. A surgical interval > 6 wk was identified as a favorable prognostic factor for OS, although no differences were observed in pCR or DFS.
Our rates of tumor responses were lower than in the other studies. This was because of failure of tumor down-staging in half of the patients in group I. However, the longer interval group still had a better pCR than the shorter interval group had in the present study, and pCR rate was 19%, which was comparable with other studies. In the present study, tumor down-staging was related to a decrease in metastatic lymph nodes in the mesorectum, and a longer interval was needed for nodal down-staging.
A pCR is deemed to increase the chances of sphincter-saving surgery, decreases local recurrence, and has a positive prognostic impact on survival. Our study showed a positive correlation between pathological tumor response and OS and DFS. Patients who had an extended interval time had higher OS and DFS rates. These findings are in agreement with the study of Yeo et al[34]. In their multicenter retrospective study, which examined mesorectal nodal status in patients with T0 stage in the rectal wall after neoadjuvant therapy, nodal status was the most efficient independent prognostic factor for DFS and OS. Similarly, Abdul-Jalil et al[35] reported that pCR and nodal status after neoadjuvant therapy were important predictive factors for survival. Pucciarelli et al[33] showed that preoperative T staging was the only independent prognostic factor of DFS and OS; however, they reported that pathological tumor response did not affect survival. In the study by Francois et al[6] an extended interval time did not have any effect on local recurrence and short-term survival. Also, in our study, rate of poor response TRG (TRG 4) was found to have negative effect on DFS, and was significantly lower in patients who had an extended interval before surgery. This shows the contribution of the prolonged interval to longer DFS. However, these factors were not associated with local recurrence. Similarly, Rödel et al[36] found that poor TRG was associated with worse DFS rate (63%), but not with local recurrence. In contrast, they found an association between TRG and presence of residual tumors in lymph nodes, but not between TRG and interval before surgery[36]. In another study by Abdul-Jalil et al[35], TRG had no prognostic effect on survival.
Although it is suggested that neoadjuvant CRT increases the chances of sphincter-saving surgery, the benefit of extending the interval time from neoadjuvant therapy to surgery in reducing rates of abdominoperineal resection is controversial. Most studies investigating sphincter preservation rates by neoadjuvant therapy-surgery interval have reported no correlation between them[5,6,22,24-26,29-31,37,38]. Our sphincter-saving surgery rates were similar in both groups, indicating no influence of neoadjuvant therapy-surgery interval. Also, its rate of 64% was comparable with the results of the other studies[2,24,39].
One of the major concerns regarding extending the interval after neoadjuvant CRT is radiotherapy-induced fibrosis, which can lead to operative difficulty and an increased risk of intraoperative and postoperative morbidity. Current data on the effect of interval time and perioperative morbidity have varied. Buie et al[40] in their retrospective study of 246 patients, who underwent TME after neoadjuvant CRT, noted a significant increase in the rate of pelvic sepsis. In contrast, Martel et al[41] reported factors such as smoking, difficult anastomosis, and anastomosis located in the first 4 cm from the anal verge as significant predictors of pelvic sepsis, and not neoadjuvant CRT. In a recent prospective randomized study by Saglam et al[31], although overall surgical complication rate was higher in the short interval group, similar individual postoperative complications in both groups were observed. Similar findings were reported by Kerr et al[42] in another retrospective study that included 189 patients. Postoperative complication rates in that study were higher in patients operated on after < 8 wk delay, while the interval was not related to mortality. In contrast, several studies have shown no significant increase in postoperative complications by delaying surgery after neoadjuvant CRT[6,20,22-24,30,32,43-45]. The findings of the present study have also shown similar postoperative complication rates in both short and long neoadjuvant therapy-surgery interval. Besides, we report a surgical complication rate of 26%-28%, which is considerably lower than the 40%-43% that previously reported[24,39]. Our short-interval group had an anastomotic leakage rate of 11.1% compared with 19% in the long-interval group. The overall anastomotic complication rate in the present study was 14%, and did not differ significantly between the short- and long-interval groups (11.1% and 19%, respectively). These findings were comparable with the results of other studies reporting an anastomotic leakage rate of 10%-20%[4,6,24,40,46]. Our early postoperative morbidity rates also showed no significant difference between the groups.
The current study had several limitations. First, the retrospective nature of the study did not allow a comprehensive appraisal of the reported findings, and may account for the significant effect of the interval time on poor response TRG (TRG 4) and its effect on DFS. The other limitation was that the study did not allow a full analysis of long-term oncological results in all patients, because of the patients who did not complete the 5-year follow-up period.
The findings of the present study suggest that delaying the neoadjuvant CRT-surgery interval provides nodal down-staging and improves pCR rates, as well as decreasing the rate of TRG poor response. TRG may be an important predictive factor for DFS. Extending the interval between CRT and surgery may improve survival through tumor down-staging without increasing the rate of surgical complications. Studies investigating the optimal time between neoadjuvant CRT and surgery and its effect on pre- and postoperative outcomes should be encouraged for better oncological outcomes and lowest morbidity.
COMMENTS
Background
Today, locally advanced distal and mid-rectal tumors are commonly managed with preoperative combined chemoradiotherapy (CRT) followed by a waiting time before total mesorectal excision (TME). Previous studies have shown that a 6-8-wk interval between CRT and surgery improves tumor down-staging and provides a higher pathological response rate. However, the optimum interval is still lacking in the medical literature. In addition, even though the effect of an extended interval on pathological complete response (pCR) is known, the impact of pCR on disease-free survival (DFS) and overall survival (OS) has not been clearly described. Thus, interval before surgery is important in terms of tumor response and survival. The aim of this study was to determine whether the interval time between preoperative neoadjuvant CRT and surgery affects the rates of pCR, perioperative surgical complications, sphincter-saving surgery, DFS and OS in locally advanced mid- or distal rectal cancer.
Research frontiers
Although the concept of interval between CRT and surgery in the management of locally advanced rectal cancer is generally accepted, a clear and explicit waiting period before surgery is not defined. However, previous studies have shown that an extended interval time between CRT and surgery improve survival and tumor response rates without increasing surgical complications. Based on the current results, there is a trend towards extension of the interval to > 8 wk before surgery for locally advanced rectal cancer.
Innovations and breakthroughs
In this study, longer interval time was related to nodal down-staging and better pathological tumor response. pCR rate was 19% in the longer-interval group, comparable with other studies. Also, patients who had an extended interval had higher OS and DFS rates. However, in this study, poor response tumor regression grade (TRG) 4 had a negative effect on DFS and was significantly lower in patients who had an extended interval before surgery. This finding emphasizes the importance of the negative effect of poor TRG on DFS.
Applications
The present study suggests that an interval > 8 wk between CRT and surgery provides tumor down-staging and higher tumor response rates, and improves survival. Poor TRG is associated with shorter interval before surgery and related to worse DFS. If a patient has TRG 4 in his/her histopathological examination of the resected specimen, the possibility of early recurrence should be considered in follow-up of the patient.
Terminology
TME is a gold standard surgical technique for treatment of rectal cancer, first described by Bill Heald in 1982. A significant length of the bowel around the tumor together with mesorectum involving metastatic lymph nodes is removed en bloc. Neoadjuvant CRT is the administration of chemotherapeutic agents before surgery for locally advanced rectal cancer. The goals of neoadjuvant CRT are to reduce tumor size before radical surgical intervention, and provide local control of the disease. DFS is the length of disease-free time until the first relapse of the disease after curative treatment. OS is the length of time that patient is still alive, from the date of diagnosis or the start of treatment. TRG is a scoring system evaluating the response of the primary tumor of the rectal wall to CRT in resected specimens.
Peer-review
The authors have concluded that studies investigating the optimal time between neoadjuvant CRT and surgery and its effect on pre and postoperative outcomes should be encouraged for better oncological outcomes and lowest morbidity.
Footnotes
Institutional review board statement: The clinical study entitled “Delaying surgery after neoadjuvant chemoradiotherapy improves prognosis of rectal cancer” is acceptable for clinical research and approved by the Ethical Committee of Sisli Hamidiye Etfal Training and Research Hospital.
Informed consent statement: Informed written consent was obtained from all study participants, or their legal guardian for being included in the study.
Conflict-of-interest statement: Mehmet Mihmanlı, Esin Kabul Gürbulak, İsmail Ethem Akgün, Mustafa Fevzi Celayir, Pınar Yazıcı, Deniz Tunçel, Tuna Tülin Bek, Ayhan Öz and Sinan Ömeroğlu declare that they have no conflicts of interest.
Data sharing statement: Technical appendix, statistical code and dataset available from the corresponding author at (ekabul@gmail.com). Participants gave informed consent for data sharing. No additional data are available.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Peer-review started: May 3, 2016
First decision: June 6, 2016
Article in press: July 18, 2016
P- Reviewer: Hua BJ, Park SC S- Editor: Qi Y L- Editor: Kerr C E- Editor: Zhang FF
References
- 1.Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumdar M, Enker W, Paty PB, Weiser MR, Klimstra D, Saltz L, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241:829–836; discussion 836-838. doi: 10.1097/01.sla.0000161980.46459.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, Allen PK, Lynch PM, Glober G, Wolff R, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44:1027–1038. doi: 10.1016/s0360-3016(99)00099-1. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Glehen O, Chapet O, Adham M, Nemoz JC, Gerard JP. Long-term results of the Lyons R90-01 randomized trial of preoperative radiotherapy with delayed surgery and its effect on sphincter-saving surgery in rectal cancer. Br J Surg. 2003;90:996–998. doi: 10.1002/bjs.4162. [DOI] [PubMed] [Google Scholar]
- 5.Moore HG, Gittleman AE, Minsky BD, Wong D, Paty PB, Weiser M, Temple L, Saltz L, Shia J, Guillem JG. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47:279–286. doi: 10.1007/s10350-003-0062-1. [DOI] [PubMed] [Google Scholar]
- 6.Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, Souquet JC, Adeleine P, Gerard JP. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 7.Bujko K. Timing of surgery following preoperative therapy in rectal cancer: there is no need for a prospective randomized trial. Dis Colon Rectum. 2012;55:e31; author reply e31–e32. doi: 10.1097/DCR.0b013e31823f86cb. [DOI] [PubMed] [Google Scholar]
- 8.Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, O’Donoghue DP, Moriarty M, Fennelly D, Sheahan K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47:141–146. doi: 10.1111/j.1365-2559.2005.02176.x. [DOI] [PubMed] [Google Scholar]
- 10.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 11.Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894–899. doi: 10.1001/archsurg.133.8.894. [DOI] [PubMed] [Google Scholar]
- 12.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–460. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 13.Local recurrence rate in a randomised multicentre trial of preoperative radiotherapy compared with operation alone in resectable rectal carcinoma. Swedish Rectal Cancer Trial. Eur J Surg. 1996;162:397–402. [PubMed] [Google Scholar]
- 14.Kapiteijn E, Kranenbarg EK, Steup WH, Taat CW, Rutten HJ, Wiggers T, van Krieken JH, Hermans J, Leer JW, van de Velde CJ. Total mesorectal excision (TME) with or without preoperative radiotherapy in the treatment of primary rectal cancer. Prospective randomised trial with standard operative and histopathological techniques. Dutch ColoRectal Cancer Group. Eur J Surg. 1999;165:410–420. doi: 10.1080/110241599750006613. [DOI] [PubMed] [Google Scholar]
- 15.Cammà C, Giunta M, Fiorica F, Pagliaro L, Craxì A, Cottone M. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA. 2000;284:1008–1015. doi: 10.1001/jama.284.8.1008. [DOI] [PubMed] [Google Scholar]
- 16.Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 17.Bosset JF, Horiot JC. [Prevention of pelvic recurrence by preoperative radiochemotherapy and total mesorectal excision of rectal carcinoma?] Praxis (Bern 1994) 2001;90:581–586. [PubMed] [Google Scholar]
- 18.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Cummings B, Catton P, Dawson L, Kim J, Ringash J, Wong R, Yi QL, Brierley J. Primary radical external beam radiotherapy of rectal adenocarcinoma: long term outcome of 271 patients. Radiother Oncol. 2005;77:126–132. doi: 10.1016/j.radonc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Wolthuis AM, Penninckx F, Haustermans K, De Hertogh G, Fieuws S, Van Cutsem E, D’Hoore A. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol. 2012;19:2833–2841. doi: 10.1245/s10434-012-2327-1. [DOI] [PubMed] [Google Scholar]
- 21.Kalady MF, de Campos-Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery IC, Fazio VW. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250:582–589. doi: 10.1097/SLA.0b013e3181b91e63. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Aguilar J, Smith DD, Avila K, Bergsland EK, Chu P, Krieg RM. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg. 2011;254:97–102. doi: 10.1097/SLA.0b013e3182196e1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Campos-Lobato LF, Geisler DP, da Luz Moreira A, Stocchi L, Dietz D, Kalady MF. Neoadjuvant therapy for rectal cancer: the impact of longer interval between chemoradiation and surgery. J Gastrointest Surg. 2011;15:444–450. doi: 10.1007/s11605-010-1197-8. [DOI] [PubMed] [Google Scholar]
- 24.Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval & gt; 7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15:2661–2667. doi: 10.1245/s10434-008-9892-3. [DOI] [PubMed] [Google Scholar]
- 25.Sloothaak DA, Geijsen DE, van Leersum NJ, Punt CJ, Buskens CJ, Bemelman WA, Tanis PJ. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg. 2013;100:933–939. doi: 10.1002/bjs.9112. [DOI] [PubMed] [Google Scholar]
- 26.Foster JD, Jones EL, Falk S, Cooper EJ, Francis NK. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum. 2013;56:921–930. doi: 10.1097/DCR.0b013e31828aedcb. [DOI] [PubMed] [Google Scholar]
- 27.Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann Surg. 2016;263:458–464. doi: 10.1097/SLA.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 28.Dolinsky CM, Mahmoud NN, Mick R, Sun W, Whittington RW, Solin LJ, Haller DG, Giantonio BJ, O’Dwyer PJ, Rosato EF, et al. Effect of time interval between surgery and preoperative chemoradiotherapy with 5-fluorouracil or 5-fluorouracil and oxaliplatin on outcomes in rectal cancer. J Surg Oncol. 2007;96:207–212. doi: 10.1002/jso.20815. [DOI] [PubMed] [Google Scholar]
- 29.Stein DE, Mahmoud NN, Anné PR, Rose DG, Isenberg GA, Goldstein SD, Mitchell E, Fry RD. Longer time interval between completion of neoadjuvant chemoradiation and surgical resection does not improve downstaging of rectal carcinoma. Dis Colon Rectum. 2003;46:448–453. doi: 10.1007/s10350-004-6579-0. [DOI] [PubMed] [Google Scholar]
- 30.Lim SB, Choi HS, Jeong SY, Kim DY, Jung KH, Hong YS, Chang HJ, Park JG. Optimal surgery time after preoperative chemoradiotherapy for locally advanced rectal cancers. Ann Surg. 2008;248:243–251. doi: 10.1097/SLA.0b013e31817fc2a0. [DOI] [PubMed] [Google Scholar]
- 31.Saglam S, Bugra D, Saglam EK, Asoglu O, Balik E, Yamaner S, Basaran M, Oral EN, Kizir A, Kapran Y, et al. Fourth versus eighth week surgery after neoadjuvant radiochemotherapy in T3-4/N0+ rectal cancer: Istanbul R-01 study. J Gastrointest Oncol. 2014;5:9–17. doi: 10.3978/j.issn.2078-6891.2013.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calvo FA, Morillo V, Santos M, Serrano J, Gomez-Espí M, Rodriguez M, Del Vale E, Gracia-Sabrido JL, Ferrer C, Sole C. Interval between neoadjuvant treatment and definitive surgery in locally advanced rectal cancer: impact on response and oncologic outcomes. J Cancer Res Clin Oncol. 2014;140:1651–1660. doi: 10.1007/s00432-014-1718-z. [DOI] [PubMed] [Google Scholar]
- 33.Pucciarelli S, Toppan P, Friso ML, Russo V, Pasetto L, Urso E, Marino F, Ambrosi A, Lise M. Complete pathologic response following preoperative chemoradiation therapy for middle to lower rectal cancer is not a prognostic factor for a better outcome. Dis Colon Rectum. 2004;47:1798–1807. doi: 10.1007/s10350-004-0681-1. [DOI] [PubMed] [Google Scholar]
- 34.Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, Choi DH, Nam H, Kim JS, Cho MJ, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01) Ann Surg. 2010;252:998–1004. doi: 10.1097/SLA.0b013e3181f3f1b1. [DOI] [PubMed] [Google Scholar]
- 35.Abdul-Jalil KI, Sheehan KM, Kehoe J, Cummins R, O’Grady A, McNamara DA, Deasy J, Breathnach O, Grogan L, O’Neill BD, et al. The prognostic value of tumour regression grade following neoadjuvant chemoradiation therapy for rectal cancer. Colorectal Dis. 2014;16:O16–O25. doi: 10.1111/codi.12439. [DOI] [PubMed] [Google Scholar]
- 36.Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 37.Pach R, Kulig J, Richter P, Gach T, Szura M, Kowalska T. Randomized clinical trial on preoperative radiotherapy 25 Gy in rectal cancer--treatment results at 5-year follow-up. Langenbecks Arch Surg. 2012;397:801–807. doi: 10.1007/s00423-011-0890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sirohi B, Barreto SG, Patkar S, Gupta A, DeSouza A, Talole S, Deodhar K, Shetty N, Engineer R, Goel M, et al. Down-staging following neoadjuvant chemo-radiotherapy for locally advanced rectal cancer: Does timing of surgery really matter? Indian J Med Paediatr Oncol. 2014;35:263–266. doi: 10.4103/0971-5851.144986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alves A, Panis Y, Mathieu P, Kwiatkowski F, Slim K, Mantion G. Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509–514. doi: 10.1016/s0399-8320(05)82121-9. [DOI] [PubMed] [Google Scholar]
- 40.Buie WD, MacLean AR, Attard JA, Brasher PM, Chan AK. Neoadjuvant chemoradiation increases the risk of pelvic sepsis after radical excision of rectal cancer. Dis Colon Rectum. 2005;48:1868–1874. doi: 10.1007/s10350-005-0154-1. [DOI] [PubMed] [Google Scholar]
- 41.Martel G, Al-Suhaibani Y, Moloo H, Haggar F, Friedlich M, Mamazza J, Poulin EC, Stern H, Boushey RP. Neoadjuvant therapy and anastomotic leak after tumor-specific mesorectal excision for rectal cancer. Dis Colon Rectum. 2008;51:1195–1201. doi: 10.1007/s10350-008-9368-3. [DOI] [PubMed] [Google Scholar]
- 42.Kerr SF, Norton S, Glynne-Jones R. Delaying surgery after neoadjuvant chemoradiotherapy for rectal cancer may reduce postoperative morbidity without compromising prognosis. Br J Surg. 2008;95:1534–1540. doi: 10.1002/bjs.6377. [DOI] [PubMed] [Google Scholar]
- 43.Veenhof AA, Kropman RH, Engel AF, Craanen ME, Meijer S, Meijer OW, van der Peet DL, Cuesta MA. Preoperative radiation therapy for locally advanced rectal cancer: a comparison between two different time intervals to surgery. Int J Colorectal Dis. 2007;22:507–513. doi: 10.1007/s00384-006-0195-5. [DOI] [PubMed] [Google Scholar]
- 44.Glimelius B. Optimal Time Intervals between Pre-Operative Radiotherapy or Chemoradiotherapy and Surgery in Rectal Cancer? Front Oncol. 2014;4:50. doi: 10.3389/fonc.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng WG, Zhou ZX, Liang JW, Wang Z, Hou HR, Zhou HT, Zhang XM, Hu JJ. Impact of interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer on surgical and oncologic outcome. J Surg Oncol. 2014;110:463–467. doi: 10.1002/jso.23665. [DOI] [PubMed] [Google Scholar]
- 46.Alberts JC, Parvaiz A, Moran BJ. Predicting risk and diminishing the consequences of anastomotic dehiscence following rectal resection. Colorectal Dis. 2003;5:478–482. doi: 10.1046/j.1463-1318.2003.00515.x. [DOI] [PubMed] [Google Scholar]


