Abstract
AIM
To describe a series of patients with aberrant polypoid nodule scar developed after gastric endoscopic submucosal dissection (ESD), and to discuss its pathogenesis and clinical management.
METHODS
We reviewed retrospectively the endoscopic database of two academic institutions located in Brazil and Japan and searched for all patients that underwent ESD to manage gastric neoplasms from 2003 to 2015. The criteria for admission in the study were: (1) successful en bloc ESD procedure with R0 and curative resection confirmed histologically; (2) postoperative endoscopic examination with identification of a polypoid nodule scar (PNS) at ESD scar; (3) biopsies of the PNS with hyperplastic or regenerative tissue, reviewed by two independent experienced gastrointestinal pathologists, one from each Institution. Data were examined for patient demographics, Helicobacter pylori status, precise neoplastic lesion location in the stomach, tumor size, histopathological assessment of the ESD specimen, and postoperative information including medical management, endoscopic and histological findings, and clinical outcome.
RESULTS
A total of 14 patients (10 men/4 women) fulfilled the inclusion criteria and were enrolled in this study. One center contributed with 8 cases out of 60 patients (13.3%) from 2008 to 2015. The second center contributed with 6 cases (1.7%) out of 343 patients from 2003 to 2015. Postoperative endoscopic follow-up revealed similar findings in all patients: A protruded polypoid appearing nodule situated in the center of the ESD scar surrounded by convergence of folds. Biopsies samples were taken from PNS, and histological assessment revealed in all cases regenerative and hyperplastic tissue, without recurrent tumor or dysplasia. Primary neoplastic lesions were located in the antrum in 13 patients and in the angle in one patient. PNS did not develop in any patient after ESD undertaken for tumors located in the corpus, fundus or cardia. All patients have been followed systematically on an annual basis and no malignant recurrence in the ESD scar has been identified (mean follow-up period: 45 mo).
CONCLUSION
PNS may occur after ESD for antral lesions and endoscopically look concerning, especially for the patient or the family doctor. However, as long as curative R0 resection was successfully achieved and histology demonstrates only regenerative and hyperplastic tissue, PNS should be viewed as a benign alteration that does not require any type of intervention, other than endoscopic surveillance.
Keywords: Endoscopic submucosal dissection, Early gastric cancer, Endoscopic treatment, Healing, Scar
Core tip: Endoscopic submucosal dissection is the treatment of choice for superficial gastric neoplasms. After curative endoscopic submucosal dissection (ESD), postoperative scar is expected to look consolidated and homogeneous. We describe a series of 14 patients that underwent curative gastric ESD with R0 resection and surprisingly developed an aberrant polypoid nodule at the ESD scar. We denominated this new entity as polypoid nodule scar (PNS). It is noteworthy that PNS occurred only after ESD undertaken for tumors located in the antrum. We reviewed the hypothesis and pathogenic factors that could explain the occurrence of this unusual phenomenon, and discuss propositions about patient’s postoperative clinical management.
INTRODUCTION
Endoscopic submucosal dissection (ESD) is considered by current guidelines as the treatment of choice for patients with superficial gastric neoplasms with little or no risk of lymph nodes metastasis[1-3]. It permits en bloc resection of tumors and reliable histological assessment of the resected specimen to determine the potential curability of the endoscopic resection. Particularly for lesions situated in the antrum, ESD is technically easier and highly effective to proportionate cure of intramucosal cancers removed with free margins. Postoperative endoscopic examination is recommended to all patients after curative ESD with two main purposes: (1) inspection of the scar to rule out residual tumor or recurrence; and (2) surveillance for metachronous neoplastic lesions.
After a curative ESD, postoperative scar usually looks consolidated and homogeneous without residual tumor, infiltration or polypoid formation. Interestingly, we have been observing that a subset of patients after curative ESD, particularly for lesions located in the antrum, may develop anomalous and bizarre postoperative scars, with relatively huge and protruded polypoid nodular neoformation, an entity that has not been described until our first report[4]. Biopsy specimens taken from these scars have demonstrated regenerative mucosa without recurrent neoplastic cells. However, in our practice, such intriguing findings can make both patients and physician concern about the reliability of the endoscopic curative resection, and may imply a request for closer follow-up or discussion about endoscopic, or even surgical, reintervention due to fear of tumor recurrence.
The objectives of this study are to describe a series of cases with aberrant polypoid nodule scar (PNS) after gastric ESD experienced in two referral centers in Latin America (Center 1) and Asia (Center 2), and to discuss the pathogenesis and propositions about the clinical management.
MATERIALS AND METHODS
The study was carried out in accordance with the Helsinki Declaration. All patients that underwent ESD provided informed consent preoperatively. Clinical information was extracted retrospectively from the endoscopy database of both institutions, which register all patients with gastric neoplasms managed by ESD.
Inclusion criteria
Eligibility for ESD was assessed preoperatively by means of white-light endoscopy, digital chromoendoscopy, magnifying observation, indigo carmine staining and endoscopic ultrasound (in selected cases). The following criteria were utilized for patients enrollment in this study: (1) successful en bloc ESD procedure with confirmatory histology of R0 and curative resection; (2) postoperative endoscopic examination with identification of a polypoid nodule scar corresponding to the site where ESD was undertaken; and (3) biopsies of the PNS with histological assessment demonstrating hyperplastic or regenerative tissue. Two independent experienced gastrointestinal pathologists, one from each center, reviewed PNS biopsies. Data were examined for patient demographics, Helicobacter pylori (H. pylori) status, precise neoplastic lesion location in the stomach, tumor and specimen size, histopathological assessment of the ESD specimen, postoperative information including medical management, endoscopic and histological findings, and clinical outcome.
ESD procedure
ESD technique has been described in detail elsewhere[5,6]. Briefly, markings were placed at least 2 mm beyond the borders of the lesion after careful endoscopic assessment by chromoendoscopy and/or magnifying endoscopy with narrow band imaging (NBI) or Fuji intelligent chromoendoscopy (FICE). Viscous solutions such as 0.4% hyaluronic acid (Muco-up®, Johnsons and Johnsons, Japan) or 0.4% hydroxypropyl-methylcellulose[7] were used for submucosal (SM) injection. ESD was undertaken with 2.5 Flush-Knife Ball Tipped (Fujifilm Co., Japan) in Center 1 or ceramic-ball insulated tip knife (IT knife, Olympus Co., Japan) in Center 2. Mucosal incision was undertaken around the tumor in a circumferential or semi-circumferential manner. SM dissection was performed in the deep submucosa, just above the proper muscle layer, with identification and hemostasis of the penetrating vessels. After complete tumor resection, the ulcer site was assessed and visible vessels were coagulated with a hemostatic forceps. The specimen was stretched and fixed in a styrofoam plate, immersed in 10% formaldehyde solution and sent to the pathology department.
Histological assessment and definitions
After being embedded in 10% paraffin, the specimens were cut into 2-mm slices and stained with hematoxylin and eosin. Additional immunochemistry studies with D2-40 and CD34 were carried out for lymphatic and vascular invasion assessment, at the discretion of the pathologist. Tumor size, depth of invasion, lymphatic and vascular invasion, grade of differentiation, and resection margins were histopathologically examined[8]. En bloc resection was defined endoscopically as the complete removal of the tumor including the markings into one non-fragmented piece[2]. R0 resection was defined histologically as complete tumor removal with both lateral and deep margins free of neoplastic cells. Endoscopic resection was considered curative when pathology report demonstrated adenoma with low or high-grade dysplasia, well or moderately well differentiated adenocarcinoma, depth of invasion restricted to mucosa or superficial submucosal (SM1), with free vertical and radial margins and no lymphatic or vascular invasion[2,3,9]. ESD was considered non-curative according to the following criteria[2,3,5]: Undifferentiated cancer greater than 2 cm, deep submucosal tumor invasion (SM2), tumor compromise of lateral or profound borders, and lymph-vascular invasion. Patients with non-curative resection were not included in this study. PNS was defined as a protuberant polypoid appearing nodule situated exactly in the post ESD scar site, with or without converging folds and with histological assessment demonstrating only regenerative or hyperplastic tissue growth without any residual or recurrent neoplastic tissue, confirmed by two experienced gastrointestinal pathologists, one from each center.
Postoperative care
Patients remained hospitalized for postoperative observation ranging from 2 to 7 d. Intravenous proton pump inhibitors (PPI) were administered to all patients during the first postoperative days followed by an 8-wk course of oral PPI after hospital discharge. If ESD procedure was considered curative, first follow-up endoscopy was scheduled in between 3 and 6 mo, and annually thereafter. ESD scar was inspected carefully for any abnormality such as residual tumor or polypoid nodule growth and multiple forceps biopsies were performed.
RESULTS
A total of 14 patients (10 men/4 women) fulfilled the inclusion criteria and were enrolled in this series. One center contributed with 8 cases (13.3%) out of 60 patients that underwent ESD for gastric tumors from 2008 to 2015. The second center contributed with 6 cases (1.7%) out of 343 patients from 2003 to 2015. Table 1 demonstrates the total number of cases performed in each center, and the incidence of PNS according to the region of the stomach. A total of 8 patients (57%) tested positive for H. pylori and received eradication therapy ahead of the procedure. The remaining 6 patients were negative for H. pylori infection.
Table 1.
Endoscopic submucosal dissection procedures distribution in Centers 1 and 2 and incidence of polypoid nodule scar according to region of the stomach n (%)
| ESD procedures | Center 1 (Brazil) | Center 2 (Japan) |
| Total number of gastric ESD (n) | 60 | 343 |
| ESD in antrum | 37 (62%) | 158 (46%) |
| ESD in proximal stomach | 23 (36%) | 185 (54%) |
| Total number of PNS cases | 8 (13.3%) | 6 (1.7%) |
| Number of PNS in antrum lesions | 8 (21.6%) | 6 (3.8%) |
| Number of PNS in proximal stomach | 0 (0%) | 0 (0%) |
ESD: Endoscopic submucosal dissection; PNS: Polypoid nodule scar.
Postoperative endoscopic follow-up revealed similar findings in all 14 patients: A protruded polypoid appearing nodule situated in the center of the ESD scar surrounded or not by convergence of folds. Biopsies were taken from the nodular part of the scar and histological assessment showed a similar pattern in all cases characterized by hyperplastic regenerative mucosa on the fibrotic tissue in the submucosa, without any signs of residual or recurrent dysplasia or tumor. Table 2 summarizes clinical and histological information of the 14 cases. Primary neoplastic lesions were located in the antrum, except for one patient that presented a lesion situated in the angle. Specimen size ranged from 20 mm to 82 mm (mean size of 36 mm). All patients have been followed periodically on an annual basis and no malignant recurrence in the ESD scar has been identified (mean follow-up period of 45 mo; range: 6 to 144 mo). Figures 1 and 2 are illustrative of two cases of PNS, one from Japan and the other from Brazil respectively, with the characteristic endoscopic and histologic findings.
Table 2.
Characteristics of tumors and follow-up data
| Case list | Gastric region | Location | Tumor size (mm) | H. pylori status before ESD | Specimen size (mm) | Histology | Tumor depth | Post-ESD treatment | Follow-up (yr) |
| 1 | Antrum | Anterior wall | 8 | Positive | 30 | Moderately differentiated adenocarcinoma | M | Rabeprazole | 8 |
| 2 | Antrum | Greater curvature | 13 | Positive | 37 | Well differentiated adenocarcinoma | M | Omeprazole | 11 |
| 3 | Antrum | Lesser curvature | 25 | Positive | 50 | Well differentiated adenocarcinoma | M | Rabeprazole | 13 |
| 4 | Antrum | Greater curvature | 15 | Positive | 32 | Well differentiated adenocarcinoma | M | Rabeprazole | 5 |
| 5 | Antrum | Lesser curvature | 8 | Negative | 20 | Well differentiated adenocarcinoma | M | Rabeprazole | 2 |
| 6 | Antrum | Greater curvature | 10 | Negative | 20 | High-grade dysplasia | M | Omeprazole | 7 |
| 7 | Antrum | Lesser curvature | 25 | Positive | 40 | Well differentiated adenocarcinoma | M | Omeprazole + sucralfate | 4 |
| 8 | Antrum | Greater curvature | 20 | Positive | 40 | High-grade dysplasia | M | Esomeprazole + sucralfate | 4 |
| 9 | Antrum | Anterior wall | 12 | Positive | 22 | High-grade dysplasia | M | Omeprazole + sucralfate | 4 |
| 10 | Antrum | Greater curvature | 25 | Negative | 40 | Inflammatory lesion indefinite for dysplasia | M | Esomeprazole + sucralfate | 4 |
| 11 | Antrum | Anterior wall | 20 | Negative | 35 | Inflammatory fibroid polyp | SM | Omeprazole + sucralfate | 2 |
| 12 | Antrum | Greater curvature | 30 | Positive | 40 | High-grade dysplasia | M | Omeprazole + sucralfate | 1 |
| 13 | Angle | Lesser curvature | 45 | Negative | 82 | Well differentiated adenocarcinoma | M | Rabeprazole | 2 |
| 14 | Antrum | Posterior wall | 20 | Negative | 32 | High-grade dysplasia | M | Omeprazole + sucralfate | 1 |
ESD: Endoscopic submucosal dissection; H. pylori: Helicobacter pylori; M: Mucosa; SM: Submucosa.
Figure 1.
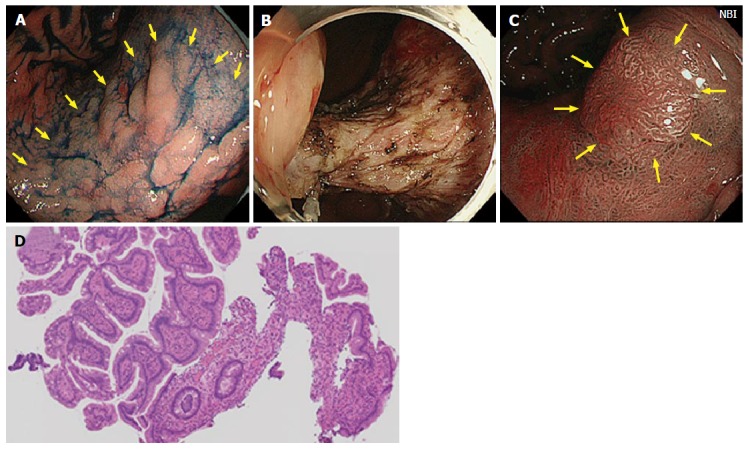
Case from Japan. A: A large superficial elevated lesion was found at the lesser curvature of the gastric angle (yellow arrows); B: The lesion was removed by endoscopic submucosal dissection technique. The lesion was diagnosed as well differentiated adenocarcinoma confined to the mucosa and resection margin was free from the tumor; C: One year later, a polypoid nodule was noted at the center of the scar (yellow arrows). Narrow band image suspected irregular surface structure on the surface of the nodule; D: Biopsy specimens were taken from the polypoid nodule. Histological examination showed hyperplastic change of the foveolar epithelium and increased capillaries and inflammatory cell infiltration in the lamina propria.
Figure 2.
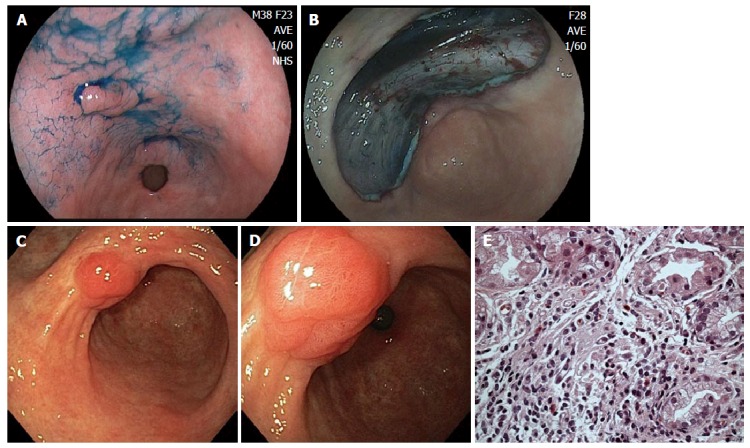
Case from Brazil. A: A depressed lesion (0IIc) was found at the lesser curvature of antrum; B: The lesion was removed by endoscopic submucosal dissection technique. The lesion was diagnosed as well differentiated adenocarcinoma confined to the muscularis mucosae and resection margins were free of tumor; C: Patient developed a polypoid nodule at the center of the scar. Three years later, polypoid nodule scar (PNS) with convergence of folds is still present; D: Closer view of PNS, demonstrating irregular surface and suspicious appearance on white-light image; E: Biopsy specimens were taken from the polypoid nodule. Histological examination showed similar findings to case illustrated in Figure 1: Regenerative hyperplastic tissue with inflammatory cell infiltration.
DISCUSSION
The healing process of a post ESD ulcer is still not completely understood. In general, after a successful curative endoscopic resection, follow-up endoscopy is supposed to demonstrate a homogeneous and flat epithelized scar covered by a regular appearing mucosa with some grade of fibrosis. In the present study we originally report a series of 14 patients with gastric lesions located predominantly in the antrum, that underwent a curative ESD R0 resection confirmed by histological criteria, and that developed an aberrant polypoid nodule in the post ESD scar. Histological assessment of the tissue growth, examined independently by two experienced gastrointestinal pathologists, were all very similar among the 14 cases, and revealed regenerative and hyperplastic tissue growth, without residual or recurrent neoplasia.
Ito et al[10] reported that polypoid nodule at ulcer scar was observed in 12 (6%) of 200 patients with gastric ulcer. Interestingly all lesions were located in the antrum[10]. In old days, some of these patients underwent gastric resection because these alterations were suspected to be malignant[11]. For development of polypoid nodule at ulcer scar, Kato et al[12] investigated the gastric ulcer healing process by endoscopy and indicated that, in some patients, granulation tissue protruded in healing ulcer. This is more frequently observed in patients that received histamine-2 receptor antagonist compared to those treated with drugs other than acid suppressant (22.0% vs 9.7%). The protruded granulation tissue develops in 17.5%-66.6% of patents with gastric ulcer treated with PPI[13,14]. The protruded granulation tissue tends to disappear after scaring, while in some patients it may remain at the center of the scar for a long time[13,15], a finding that we also noted in our series and is illustrated in Figure 2 (images show PNS still present 3 years after ESD). Histological finding of the polypoid nodule at ulcer scar is indicated as hyperplastic regenerative mucosa on the fibrotic tissue[11].
In our series all patients received PPI in the postoperative period to speed up the healing process, a clinical management that is adopted universally in ESD referral centers[16]. PPI accelerates ulcer healing mainly due to potent gastric acid secretion inhibition. However, PPI also increases the cyclooxigenase-2 (COX-2) expression and prostaglandin E synthases in the ulcerated mucosa[17]. COX-2 generated Prostaglandin E2 stimulates the expression of growth factors in the mucosa, such as vascular endothelial growth factor[18], hepatocyte growth factor[19], basic fibroblast growth factor[20]. This accelerated mucosal repair and angiogenesis may contribute to nodular overgrowth of the regenerative mucosa.
There still remain some questions unanswered concerning the occurrence and pathogenesis of PNS. A unique characteristic of PNS is that we noted this finding only after ESD performed in the distal stomach (antrum or incisure). In both centers, we did not notice PNS after ESD for lesions located in the gastric body, fundus or cardia. Likewise we did not observe this finding after esophageal or colorectal ESD. The reason for this phenomenon is unclear. We postulate that the frequent gastric peristalsis may enhance development of PNS in the antrum. Moreover, submucosal layer in the antrum is thicker; therefore inflammatory or regenerative reaction in the submucosa can be more obvious in the antrum than in the corpus of fundus. Another interesting question is whether PNS may also occur after EMR. Although in the present study we did not look specifically for patients that underwent EMR, data in the literature support that even peptic ulcer causes PNS, therefore it seems fair to assume that PNS may develop after EMR. The importance of H. pylori infection is also undetermined. Our data do not show a clear association between PNS and H. pylori status, as 8 patients (57%) tested positive and the other 6 (43%) were negative for H. pylori infection. However, more investigation in needed to draw firm conclusions about predisposing factors involved with PNS development.
Endoscopists should acknowledge the occurrence of aberrant polypoid nodules at ESD scar, particularly in antral lesions. Such occurrence, to our knowledge, has only been reported recently and we proposed to adopt the terminology PNS to describe this phenomenon[4]. It is of paramount importance to distinguish PNS from residual carcinoma or submucosal tumor recurrence. PNS is composed of granulation tissue or regenerative mucosa, and the surface structure and vasculature are as irregular as those of intramucosal carcinoma. Therefore, the first priority is to make sure that the endoscopic resection was R0 and curative by histologic criteria, ruling out a residual carcinomatous tumor. Secondly, to distinguish PNS from submucosal recurrence is not so difficult because surface structure of PNS is irregular, in contrast to submucosal recurrence that tends to present a smooth and regular surface, covered with normal gastric mucosa. Image enhanced endoscopy with magnifying endoscopy associated with indigo carmine and digital chromoendoscopy with NBI or FICE potentially are useful tools to facilitate the differential diagnosis.
The incidence of PNS post ESD is still undetermined, though expected to be rare. Apparently the size of the lesion or the size of the resected area, do not seem to be directly involved in PNS development, since we noted a wide variation in tumor size (8 mm to 82 mm), and even small lesions under 10 mm developed PNS. In this study, the incidence of PNS was significantly different between the two centers (Center 1%-13.3%; Center 2%-1.7%). This difference can be justified, at least in part, because Center 1 performed ESD more frequently for tumors located in the antrum (62%) in comparison to Center 2 (46%). Perhaps, the ESD technique could also influence the occurrence of PNS. There was a difference between the 2 centers in terms of ESD knives (Center 1 - needle type knife; Center 2 - insulated tip knife), settings of electrosurgical unit and operator’s experience. Moreover, because this was a retrospective study, the incidence of PNS may be underestimated, due to cases lost for follow-up or unavailability of the endoscopic images. A prospective large-scale multicenter study enrolling multiple ESD centers is needed to assess the true incidence of PNS.
PNS endoscopically looks concerning, especially for the patient and the family doctor. Nevertheless, as long as the ESD procedure is considered curative, with R0 resection confirmed by a standardized histological evaluation, and multiple biopsies taken from the scar rule out tumor recurrence and reveals only hyperplastic changes, PNS should be viewed as a regenerative lesion with an expected benign behavior. Over time PNS may become less protruded, as we noted in some of our patients, or even disappear. Most importantly, endoscopists when facing a PNS should refrain to indicate any type of invasive measure such as endoscopic or surgical reintervention, and recommend annual endoscopic surveillance.
In summary, we report the first series of aberrant polypoid nodule scars observed after gastric ESD that corresponds to a regenerative healing process and that requires no additional treatment other than periodic endoscopic follow-up.
COMMENTS
Background
Endoscopic submucosal dissection (ESD) is considered by current guidelines as the treatment of choice for patients with superficial gastric neoplasms with little or no risk of lymph nodes metastasis. It permits en bloc resection of tumors and reliable histological assessment of the resected specimen to determine the potential curability of the endoscopic resection. Postoperative endoscopic examination is recommended to all patients after curative ESD with two main purposes: (1) inspection of the scar to rule out residual tumor or recurrence; and (2) surveillance for metachronous neoplastic lesions.
Research frontiers
After a curative ESD, postoperative scar is expected to look consolidated and homogeneous without residual tumor, infiltration or polypoid formation. However, there is scarce data about the healing process of post-ESD defects and ulcers.
Innovations and breakthroughs
In this study, the authors report the first series of 14 patients from two Academic Institutions from Brazil and Japan, that developed aberrant polypoid nodule scars after curative gastric ESD, undertaken for neoplastic lesions located in the distal stomach (antrum and incisure). They denominated this new entity as polypoid nodule scar (PNS). PNS endoscopically looks concerning, especially for the patient and the family doctor. Nevertheless, as long as the ESD procedure was curative, with R0 resection confirmed by a standardized histological evaluation, and multiple biopsies taken from the scar rule out tumor recurrence and reveals only hyperplastic changes, PNS should be viewed as a regenerative lesion with an expected benign behavior, that requires no additional treatment other than periodic endoscopic follow-up.
Applications
ESD has been increasingly utilized to treat early gastric neoplasms all over the world. This study brings new concepts about the healing process of ESD defects, particularly for antral lesions. The understanding and knowledge of this new entity by endoscopists involved with ESD procedure is crucial to prevent unnecessary and aggressive reintervention to manage a benign hyperplastic tissue reaction that may be confounded with tumor recurrence.
Terminology
PNS refers to polypoid nodule scar, an aberrant and protuberant nodular scar that develops after ESD and has no histological evidence of tumor recurrence or dysplasia. PNS corresponds to a hyperplastic regenerative healing process, already know in the past to occur after the healing of gastric peptic ulcers.
Peer-review
Available papers dedicated to understand the healing process of ESD defects are scarce. The authors in this study reported a new entity named PNS that occurs after gastric ESD for lesions located mainly in the antrum. Although, the occurrence of this phenomenon is supposed to be rare, the true incidence of PNS remains to be determined. Large-scale multicenter and prospective study are needed to better investigate this newly described finding.
Footnotes
Institutional review board statement: The data was extracted retrospectively from the endoscopy database. Our Ethics and Research Committee does not require IRB submission for such kind of study. Patients signed a consent form for the procedure and the study was conducted according to Helsinque Declaration.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: There are no conflicts of interest to report.
Data sharing statement: No data were created so no data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
Peer-review started: March 23, 2016
First decision: April 20, 2016
Article in press: July 13, 2016
P- Reviewer: Aoyagi K, Kim JJ, Morgagni P, Muguruma N, Skok P S- Editor: Qiu S L- Editor: A E- Editor: Li D
References
- 1.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 3.Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3–15. doi: 10.1111/den.12518. [DOI] [PubMed] [Google Scholar]
- 4.Arantes V, Uedo N, Pedrosa MS. Endoscopic management of bariatric surgery complications: what the gastroenterologist should know. Rev Gastroenterol Mex. 2016;81:35–47. doi: 10.1016/j.rgmx.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Piñeros EAF, Arantes V, Toyonaga T. Endoscopic submucosal dissection of early gastric cancer: State of the art. Rev Col Gastroenterol. 2012;27:194–214. [Google Scholar]
- 6.Arantes V, Albuquerque W, Freitas Dias CA, Demas Alvares Cabral MM, Yamamoto H. Standardized endoscopic submucosal tunnel dissection for management of early esophageal tumors (with video) Gastrointest Endosc. 2013;78:946–952. doi: 10.1016/j.gie.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Arantes V, Albuquerque W, Benfica E, Duarte DL, Lima D, Vilela S, Lima G, Sakai P, Filho FM, Artifon E, et al. Submucosal injection of 0.4% hydroxypropyl methylcellulose facilitates endoscopic mucosal resection of early gastrointestinal tumors. J Clin Gastroenterol. 2010;44:615–619. doi: 10.1097/MCG.0b013e3181d6bd8e. [DOI] [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto H. Endoscopic submucosal dissection--current success and future directions. Nat Rev Gastroenterol Hepatol. 2012;9:519–529. doi: 10.1038/nrgastro.2012.97. [DOI] [PubMed] [Google Scholar]
- 10.Ito S, Kishi S, Ishikawa K, Uragami K, Seki H. A case of gastric ulcer scar with type IIa-like elevation in center of the lesion. Gastroenterol Endosc. 1974;16:194–197. [Google Scholar]
- 11.Ito S, Kishi S, Mori H, Akagi G. An elevated type of gastric ulcer scar. Gastrointest Endosc. 1979;25:58–60. doi: 10.1016/s0016-5107(79)73361-x. [DOI] [PubMed] [Google Scholar]
- 12.Kato H, Hattori K, Nishikawa H, Hayashi R, Kawamoto M, Fujiwara N. Endoscopic study on healing of gastric ulcer by the treatment with H2-blocker. Gastroenterol Endosc. 1986;28:2291–2296. [Google Scholar]
- 13.Nakamura T, Tsukamoto Y, Yamanaka T, Hayashi S. A study on the whitish protrusion appearing in the base of peptic ulcer during the administration of proton pump inhibitor. Gastroenterol Endosc. 1992;34:1548–1554. [Google Scholar]
- 14.Ashida K, Osaka N, Tei H, Takiuchi H, Sakaguchi M, Tanaka M, Okumura Y, Asada S, Irata I, Oshiba S. Studies on the mechanism of the protrusion of the base of the ulcer during the administration of omeprazole. Gastroenterol Endosc. 1989;31:1776–1782. [Google Scholar]
- 15.Tanaka T, Kimura M, Akiyama T, Suzuki S. Long follow-up study of elevated scar of acute antral kissing ulcers. Gastroenterol Endosc. 1984;26:1534–1537. [Google Scholar]
- 16.Uedo N, Takeuchi Y, Yamada T, Ishihara R, Ogiyama H, Yamamoto S, Kato M, Tatsumi K, Masuda E, Tamai C, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007;102:1610–1616. doi: 10.1111/j.1572-0241.2007.01197.x. [DOI] [PubMed] [Google Scholar]
- 17.Okazaki M, Shimizu I, Ishikawa M, Fujiwara S, Yamamoto H, Shiraishi T, Horie T, Iuchi A, Ito S. Gastric mucosal levels of prostaglandins and leukotrienes in patients with gastric ulcer after treatment with rabeprazole in comparison to treatment with ranitidine. J Med Invest. 2007;54:83–90. doi: 10.2152/jmi.54.83. [DOI] [PubMed] [Google Scholar]
- 18.Wallace JL, Devchand PR. Emerging roles for cyclooxygenase-2 in gastrointestinal mucosal defense. Br J Pharmacol. 2005;145:275–282. doi: 10.1038/sj.bjp.0706201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Schuppan D, Drozdowicz D, Ptak A, Pawlik M, Nakamura T, Hahn EG. Involvement of cyclooxygenase (COX)-2 products in acceleration of ulcer healing by gastrin and hepatocyte growth factor. J Physiol Pharmacol. 2000;51:751–773. [PubMed] [Google Scholar]
- 20.Sakai Y, Fujita K, Sakai H, Mizuno K. Prostaglandin E2 regulates the expression of basic fibroblast growth factor messenger RNA in normal human fibroblasts. Kobe J Med Sci. 2001;47:35–45. [PubMed] [Google Scholar]


